Abstract
Introduction
Studies in antineutrophil cytoplasmic autoantibody (ANCA)–associated vasculitis (AAV) consistently show that the months following diagnosis have the greatest impact on the long-term renal function. Yet, it remains uncertain how much early gain should be expected with treatment. We sought to determine the factors associated with the change in glomerular filtration rate (GFR) throughout the first year.
Methods
We retrospectively reviewed patients from 3 university hospitals who received treatments. We assessed the proportions of glomeruli with crescents, with global sclerosis, the AAV glomerulonephritis classification, the severity of chronic vascular and tubulo-interstitial disease, and the presence of acute tubular injury (ATI). We used repeated-measures analyses of variance (ANOVAs) to determine factors associated with the change in GFR throughout the first year.
Results
There were 162 individuals with AAV identified, 96 with a valid renal biopsy and 82 with at least 12 months of follow-up. The initial GFR of 30 ± 25 ml/min per 1.73 m2 rose by 15 ± 20 during the first year. The severity of pathology findings, myeloperoxidase positivity, and those with kidney- and lung-limited disease presented with a lower GFR. Younger patients with a lower initial GFR and the presence of ATI correlated with a greater increase in GFR by 12 months. A higher proportion of crescents did not predict the change in GFR, contrary to global glomerulosclerosis, where each 10% increase added a loss of 2.7 ± 1.3 ml/min per 1.73 m2 per year (P = 0.03). These factors remained independent of each other.
Conclusion
Multiple factors influence renal recovery during the first year of therapy. Estimating the change in GFR early on will help identify and reassess outliers.
Keywords: ANCA-associated vasculitis, crescentic glomerulonephritis, immunosuppression, pathology
Crescentic necrotizing glomerulonephritis (GN) in AAV requires prompt initiation of immunosuppressive therapy for optimal outcome. Studies reporting the long-term renal survival in AAV repeatedly illustrate that the initial months following diagnosis are the most defining1, 2, 3, 4, 5, 6; yet, few have focused on how to predict the change in GFR during this period.7, 8
The induction and maintenance protocols available in AAV have evolved in recent years. The advent of rituximab as an alternative to cyclophosphamide and azathioprine now gives clinicians an additional option and reinforces the need to correctly identify an appropriate response.9, 10 Contrary to relapses, which are easier to identify, no clear definition of early resistance exists. Intuitively, clinicians expect a greater gain in renal function with cellular lesions and fewer with sclerotic ones. They also anticipate greater recovery when ATI is present.
The AAV GN classification proposed 4 simple pathological subtypes (sclerotic, crescentic, mixed, and focal) that were found predictive of the change in GFR over time, with most studies focusing at 12 months or later.1, 2, 3, 4, 5, 6 Whether using exact proportion of glomeruli with global sclerosis or crescents would give a more precise estimate of the change in GFR is uncertain. Other findings, such as ATI, are perhaps also important.11, 12, 13, 14 Finally, it is unknown when an increase in GFR can be expected with current regimens.
Our main objective was to identify independent clinical and pathological factors associated with the change in GFR during the first year of treatment. We hypothesized that gains will be limited to the first months and will correlate with the proportion of glomeruli with crescents and inversely to the proportion of those with global sclerosis.
Materials and Methods
Study Design and Patient Selection
This retrospective cohort study included all available subjects with AAV GN identified between 2001 and January 2017 at 3 large hospitals affiliated with the University of Montréal (Hôpital du Sacré-Coeur de Montréal, Centre Hospitalier de l'Université de Montréal, and Hôpital Maisonneuve-Rosemont).15 Of the prevalent patients found, the oldest renal biopsy dated back to 1989. If more than one biopsy was available, we considered the first one. The ethics committee of all 3 hospitals approved this study. The authors adhered to the Declaration of Helsinki.
We reviewed laboratory ANCA measurements and archive files to find patients with AAV with biopsy-proven renal involvement, excluding any concomitant anti–glomerular basement membrane disease. All cases identified are presented. For the follow-up analyses, and to avoid studying patients in whom little or no treatment was given (e.g., renal-limited AAV with advanced sclerotic lesions), we considered only individuals who received corticosteroids, a second agent, and had at least 12 months of follow-up. We did not require a specific length of treatment and considered the intent to treat, as clinical assessment during follow-up could justify modifications (e.g., stopping immunosuppression in an anuric patient at 3 months).
Data Collection
We collected the following from medical records: demographics, preexisting comorbidities (hypertension, coronary artery disease, and diabetes), organ involvement at disease presentation, and immunosuppressive agents, including pulse methylprednisolone and plasma exchange. The GFR was estimated using the Modification of Diet in Renal Disease formula. Because it was not possible to calculate the GFR in individuals receiving dialysis, the minimally assigned GFR was 10 ml/min per 1.73 m2. We assessed renal function at the time of biopsy, and at 1, 3, 6, and 12 months and, when available, 24 months. We calculated for each of these time points, a delta GFR by subtracting the GFR at the time of biopsy. The quantification of proteinuria, based on either 24-hour urine collection or spot urinary protein-to-creatinine ratio (expressed in g/g) and the degree of hematuria (<2, 2–5, 6–10, and >10 red blood cells/high-powered field [RBC/HPF]) were assessed at time of biopsy, and 3, 6, and 12 months. Causes of death also were recorded.
Pathology Review
Pathology reports were reviewed. We only included biopsies with at least 7 glomeruli and at least 1 showing a crescent or a necrotic lesion within the floculus. We recorded the total number of glomeruli, those with global sclerosis, with crescents, and whether necrosis was observed. We did not consider from reports complex lesions such as the type of crescents. When describing the tubulo-interstitial compartment, we noted whether ATI was reported and the severity of tubular atrophy and interstitial fibrosis (0–5, absent; +1, mild or 6%–24%; +2, moderate or 25% to 49%; +3, severe or ≥50%). When a separate score existed for tubular atrophy and interstitial fibrosis, we reported the higher of the two. The same approach was used to score the severity of arterial and arteriolar sclerosis. Using these variables, we derived the AAV GN classification.1
All available pathology slides were rescored by either 1 of 2 nephropathologists (VR and FG). During review, crescents were defined by extracapillary lesions composed of cells or collagenous matrix that involve >10% of the circumference of Bowman’s capsule. Cellular crescents required ≥50% of the lesion occupied by cells, fibrocellular crescents had <50% of the lesion occupied by cells and <90% occupied by matrix, and fibrous crescents had ≥90% of the lesion composed of matrix.16 ATI was defined by the presence of tubular epithelial simplification with flattening and loss of brush border to overt necrosis and denudation of tubular basement membrane on at least 10% of cortex.17 Tubular atrophy, interstitial fibrosis, arterial sclerosis, and arteriolar hyalinosis were scored as detailed previously.
Statistical Analysis
Variables are expressed as mean ± SD, median with interquartile range, or percentages, appropriately. Univariate analyses were performed using the Student t test, 1-way ANOVA, and Pearson correlation. We used the Pearson correlation to perform a trend test for ordinal variables (e.g., 0, 1+, 2+, 3+). Associations with proteinuria were tested using the Spearman Rho test. Proportions were compared using the Pearson χ2.
We assessed the agreement between the pathology report and the revisions using the intraclass correlation coefficient, a measure of reproducibility applicable to dichotomous, ordinal, and continuous variables. By convention, variables with an intraclass correlation coefficient <0.4, 0.4 to 0.6, and >0.6 have a poor, moderate, and good reproducibility, respectively.18 For ATI, we performed the McNemar test to see if the reviewers were more, or less, likely to call this lesion.
We present univariate associations between each variable of interest and (i) the initial GFR, (ii) the change in GFR at the 12-month time-point (ΔGFR12months), and (iii) the change in GFR over time using repeated-measures ANOVAs, to test how potential risk factors influence the trajectory of GFR over time (“within-subject variable”). Post hoc tests with Bonferroni correction were used to see which time points differed statistically. We then ran mixed repeated-measures ANOVAs by adding a “between-subject variable” (e.g., proportion of glomeruli with crescents) in addition to the time effect.19 We repeated this separately for each variable of interest. We report the F and partial eta (ηp2) scores. Because the Mauchly tests of sphericity were always <0.001, we report the F test using the Greenhouse-Geisser correction for the degree of freedom. A significant interaction between the GFR estimates in time and another variable indicated that the change in GFR over time was influenced by that variable.
Finally, we performed 2 multivariate models: a mixed repeated-measures ANOVA and a linear regression with ΔGFR12months using all factors that were statistically associated by univariate analyses. Analyses were carried out using SPSS software (IBM Corp., Chicago, IL). All P values were 2-tailed and values ≤0.05 defined statistical significance.
Results
Patient Selection and Missing Data
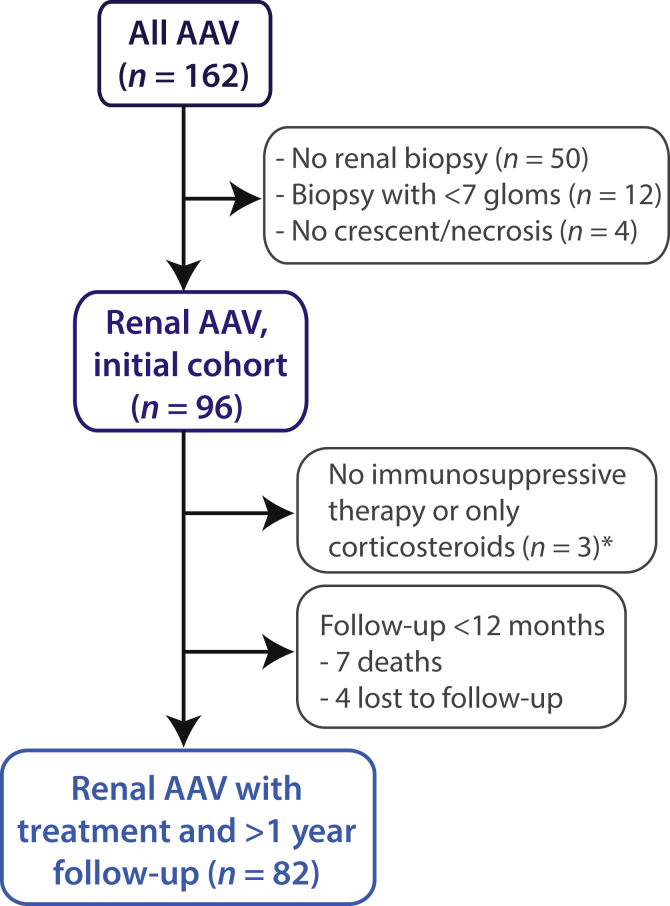
We reviewed 162 records of individuals with AAV and excluded 50 without a renal biopsy, 12 with less than 7 glomeruli, and 4 with no crescent or necrosis found (Figure 1). The 96 remaining individuals constituted our initial cohort. Two individuals received no treatment and 1 received only steroids, 7 died within the first year (4 from severe pulmonary AAV, 2 from pneumonia, and 1 from pulmonary emboli), and 4 were lost to follow-up. Once removed, we were left with 82 patients for our follow-up cohort, which was assessed at multiple time points.
Figure 1.
Patient selection. AAV, antineutrophil cytoplasmic autoantibody–associated vasculitis.
*Three patients with renal-limited AAV and advanced sclerotic lesions had standard treatment regimens withheld.
Sixteen of the follow-up GFRs were missing (<4%) and were extrapolated using the GFR before and after. Proteinuria and urinary RBC/HPF were missing at different time points in up to 52% and 40% of cases, respectively. All but 2 reports had a mention of the interstitium. Nine had no description of the tubules, and thus, only for this variable, we considered missing values to have absent ATI. The severity of arterial or arteriolar sclerosis was not mentioned in 9 cases. All other variables were complete.
Baseline Characteristics
The initial cohort was predominantly white, with 52% men and an age at biopsy of 60 ± 14 years (Table 1). The GFR at diagnosis was 30 ± 25 ml/min per 1.73 m2, and 19 (23%) of patients initially required dialysis with an additional 2 by the end of the first month of follow-up. Myeloperoxidase-ANCAs were slightly more prevalent and 7.3% presented double ANCA positivity. Pulmonary and ear, nose, and throat involvement existed in approximately one-half and one-third of the patients, respectively. Proteinuria was 1.4 (0.8–2.4) g per day.
Table 1.
Baseline characteristics patients with ANCA-associated vasculitis with renal active lesions (n = 96)
| Age | 60 ± 14 |
|---|---|
| Male, % | 52 |
| White ethnicity, % | 85 |
| Preexisting comorbidities, % | |
| Diabetes | 12 |
| Hypertension | 44 |
| Coronary artery disease | 11 |
| ANCA Anti-proteinase 3/myeloperoxidase, %a | 49 / 51 |
| Double ANCA, % | 7.3 |
| Other systems affected, % | |
| Lungs/Alveolar hemorrhage | 50/20 |
| Ear, nose, and throat | 36 |
| Rheumatology | 40 |
| Nervous system | 13 |
| Skin | 19 |
| Ophthalmology | 10 |
| GFR, ml/min per 1.73 m2 | 30 ± 25 |
| Proteinuria, g/db | 1.4 (0.8–2.4) |
ANCA, antineutrophil cytoplasmic autoantibody.
Values are expressed as mean ± SD, median (Q1–Q3), or percentages.
When both ANCA were present, the predominant value is reported.
Initial proteinuria was missing in 33 individuals. All other variables were complete.
Pathology Assessment
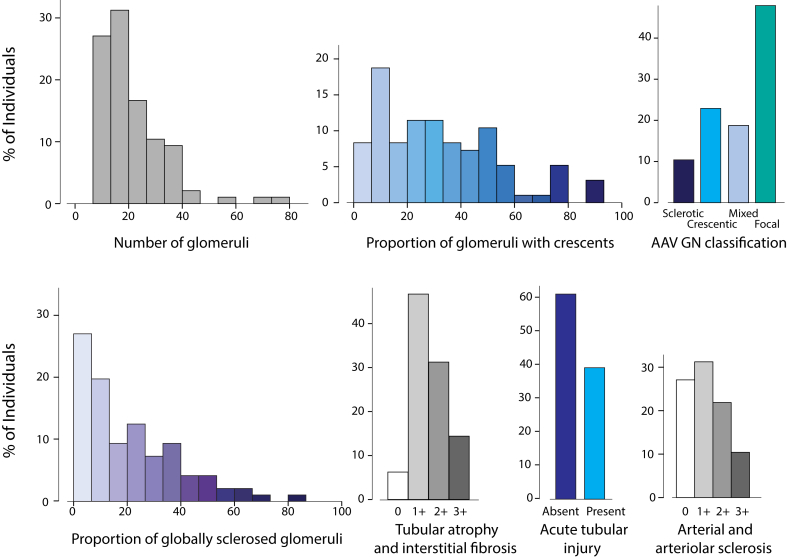
Biopsy findings obtained from records are illustrated in Figure 2. There was a median of 19 (12–27) glomeruli and a proportion of global glomerulosclerosis and glomeruli with crescents of 21% ± 19% and 33% ± 23%, respectively. We applied the AAV GN classification and obtained 48% focal, 19% mixed, 23% crescentic, and 10% sclerotic classes. ATI was noted in 38% of biopsies, and the median tubulo-interstitial and median vascular chronicity scores were 1+ (1–2) and 1+ (0–2), respectively. Slides were reviewed in 54 of 96 cases. The intraclass correlation coefficients were good for the proportion of globally sclerosed glomeruli (0.84), the proportion with crescents (0.66), and the AAV GN classification (0.67); moderate for the severity of tubular atrophy and interstitial fibrosis (0.57); and poor for ATI (0.18) and the severity of chronic vascular disease (0.38). The reviewers identified significantly more ATI and 19 of 21 of the discordant scores had an initial report with no mention of ATI contrary to the review (P < 0.001, McNemar test). From the reviewed cases, 41% had only cellular crescents, 35% had both cellular + fibrocellular crescents, and 24% had some fibrous crescents.
Figure 2.
Pathology findings (n = 96). AAV GN, antineutrophil cytoplasmic autoantibody–associated vasculitis glomerulonephritis.
Treatments and Outcomes
Therapies given are detailed in Table 2. Pulse of methylprednisolone was given in 77%, plasma exchange in 18%, and most received cyclophosphamide for induction therapy, orally (52%), or i.v. (37%). Two patients received induction without a cyclophosphamide or rituximab-based regimen; they had mild disease at presentation (secondary analyses excluding them did not change the results). Azathioprine was the most commonly used drug during maintenance therapy (59%).
Table 2.
Treatment and outcomes at 1 year (n = 82)
| Induction therapy, n (%) | |
| Prednisone | 82 (100) |
| Pulse methylprednisolone | 63 (77) |
| Plasma exchange | 15 (18) |
| Additional immunosuppressive treatments | 82 (100) |
| Cyclophosphamide | 73 (89) |
| Cyclophosphamide and Rituximab | 3 (3.6) |
| % i.v./p.o. cyclophosphamide | 37, 52 |
| Duration of cyclophosphamide (mo) | 6 (4–10) |
| Rituximab | 4 (4.9) |
| Methotrexate | 1 (1.2) |
| Azathioprine | 1 (1.2) |
| Maintenance therapy, n (%) | |
| Rituximab/cyclophosphamide → azathioprine | 3 (3.6) |
| Rituximab → azathioprine | 2 (2.4) |
| Cyclophosphamide → methotrexate | 5 (6.1) |
| Cyclophosphamide → azathioprine | 42 (51) |
| Cyclophosphamide → rituximab | 2 (2.4) |
| Cyclophosphamide → mycophenolate mofetil | 1 (1.2) |
| No change | 27 (33) |
| Outcomes | |
| Change in GFR at 12 months (ml/min per 1.73 m2) | +15 ± 20 |
| Relapse, n (%) | 1 (2.4) |
| Required dialysis during the 1st year, n (%) | 21 (26) |
| Recovery from dialysis, n (% of those with dialysis) | 12 (57) |
All received corticosteroids and an additional immunosuppressant and completed at least 1 year of follow-up.
→, changed to; GFR, glomerular filtration rate.
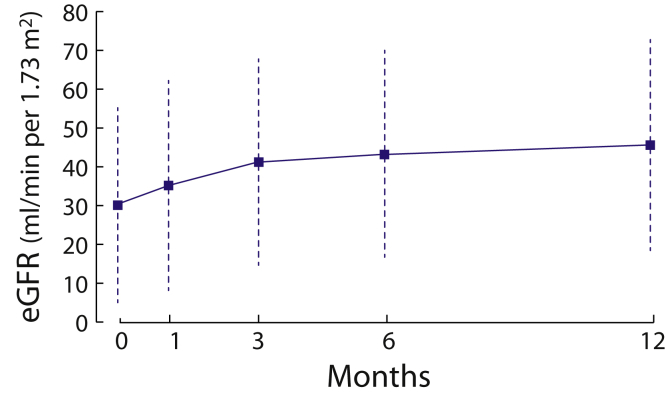
Overall, the cohort gained 15 ± 20 ml/min per 1.73 m2 during the first year of therapy and 12 of 21 recovered sufficiently to stop dialysis. Improvement in GFR occurred mostly during the first 3 months of therapy (Figure 3, repeated-measures ANOVA for different time points: F(2.4,196.1) = 26.6, P < 0.001, ηp2 = 0.247). All time-point comparisons were statistically different, except between the third and sixth months. In a subgroup with an available GFR assessment at 24 months, there existed a small but statistically significant increase between the 12th and 24th months (ΔGFR12 months +18 ± 20 vs. ΔGFR24 months +19 ± 20, P < 0.001, paired t test, n = 57). Proteinuria dropped by 0.8 (0.2–1.8) g per day at 12 months. For those with available urine microscopy, the proportion of individuals with >5 RBC/HPF was 48%, 22%, and16% at 3, 6, and 12 months, respectively. The median level of hematuria fell to <2 RBC/HPF at 1 year.
Figure 3.
Change in estimated glomerular filtration rate (eGFR) during the first year of therapy in antineutrophil cytoplasmic autoantibody–associated vasculitis (n = 82). Using a repeated-measures analysis of variance showed that there was a significant effect of time on the glomerular filtration rate, F(2.4,196.1) = 26.6, P < 0.001, ηp2 = 0.247. Post hoc tests with Bonferroni correction showed that all time-point differences were statistically significant, except between the third and sixth months of follow-up.
Univariate Factors Associated With the Initial GFR and ΔGFR12 months
The correlations between the initial GFR and clinico-pathological characteristics are shown in Table 3. Individuals with ear nose and throat, neurologic, dermatologic, rheumatologic, and ophthalmologic organ involvement each presented with a greater GFR compared with those with renal ± pulmonary disease only. Individuals with proteinase 3–AAV had an initial GFR of 38 ± 30 compared with 21 ± 16 ml/min per 1.73 m2 for myeloperoxidase-AAV (P < 0.001, t test). A lower GFR at presentation was associated with a greater proteinuria. The severities of glomerular and tubulo-interstitial lesions were also associated with a reduced initial GFR, whereas the severity of vascular sclerosis lesions was not.
Table 3.
Univariate factors associated with the initial GFR and change in GFR at 12 months in AAV renal disease
| Initial GFR (n = 96) | P | ΔGFR12 months (n = 82) | P | |
|---|---|---|---|---|
| Age (10-year increase) | β: −5.3 ± 1.8 | 0.004 | β: −4.0 ± 1.6 | 0.02 |
| Initial GFR (10 ml/min per 1.73 m2 increase) | - | β: -2.4 ± 0.9 | 0.007 | |
| Initial proteinuria (g/d) | Spearman's rho: −0.35 | 0.006 | Spearman's rho: −0.05 | 0.73 |
| Only renal ± pulmonary diseasea | 20 ± 12 | <0.001 | +13 ± 17 | 0.46 |
| Other organ involvement | 35 ± 29 | +16 ± 21 | ||
| Proteinase 3–ANCA | 38 ± 30 | <0.001 | +15 ± 21 | 0.74 |
| Myeloperoxidase-ANCA | 21 ± 16 | +16 ± 19 | ||
| 10% increase in sclerotic glomeruli | β: −4.4 ± 1.3 | 0.001 | β: −2.7 ± 1.3 | 0.03 |
| 10% increase in glomeruli with crescents | β: −3.7 ± 1.1 | 0.001 | β: +1.0 ± 1.0 | 0.32 |
| Absence of acute tubular injury | 32 ± 26 | 0.24 | +11 ± 16 | 0.008 |
| Presence of acute tubular injury | 26 ± 24 | +23 ± 24 | ||
| AAV GN classification | <0.001 | 0.20b | ||
| Sclerotic | 17 ± 9 | Focal>other | +6 ± 12 | |
| Mixed | 21 ± 15 | +9 ± 18 | ||
| Crescentic | 20 ± 15 | +21 ± 17 | ||
| Focal | 41 ± 30 | +16 ± 23 | ||
| Tubular atrophy or interstitial fibrosis | <0.001 | 0.18 | ||
| Absent | 73 ± 46 | trend test | +1 ± 25 | trend test |
| 1+ | 32 ± 23 | +21 ± 22 | ||
| 2+ | 24 ± 20 | +9 ± 15 | ||
| 3+ | 18 ± 8 | +10 ± 11 |
Results are presented as mean ± SD or by unstandardized β (ml/min per 1.73 m2).
AAV GN, ANCA-associated vasculitis glomerulonephritis; ANCA, antineutrophil cytoplasmic autoantibody; GFR, glomerular filtration rate.
Ear nose throat, neurologic, dermatologic, rheumatologic, and ophthalmologic involvement were each individually associated with a greater initial GFR, but not with a different ΔGFR12 months.
Taken together, sclerotic and mixed classes had less gain in GFR at 12 months compared with crescentic and focal classes (P = 0.05). Sex, ethnicity, preexisting conditions (diabetes, coronary artery disease, and hypertension), change in proteinuria at 12 months, the presence of pulmonary disease, and the severity of vascular sclerosis were not associated with the initial GFR or a change in GFR at 12 months.
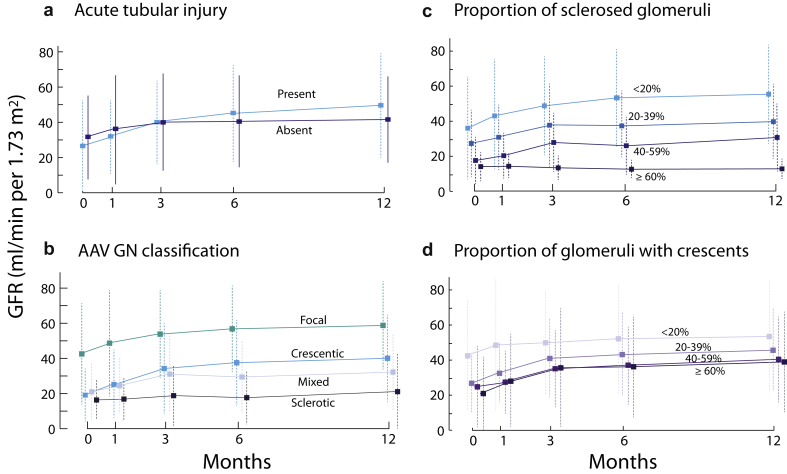
Older individuals and those with a greater initial renal function gained less GFR by 12 months (Table 3). Sex, ethnicity, ANCA type, extrarenal organ involvement, preexisting comorbidities, and initial or change over a year in proteinuria and hematuria were not associated ΔGFR12 months. Those with ATI had a +23 ± 24 ml/min per 1.73 m2 ΔGFR12 months compared with only +11 ± 16 when absent (P = 0.008, t test). Furthermore, patients who exited dialysis were much more likely to have ATI compared with those who did not recover (73% vs. 13% with ATI, P = 0.006, χ2). Each increase of 10% in global glomerulosclerosis was associated with a 2.7 ± 1.3 ml/min per 1.73 m2 lower ΔGFR12 months (P = 0.03, Pearson) but the proportion of crescents did not correlate with ΔGFR12 months. However, in the subset of individuals with an assessment of the type of crescents, we found that individuals with any fibrous crescents gained significantly less GFR at 12 months compared with those without (ΔGFR12 months: +7 ± 17 ml/min per 1.73 m2 with any fibrous crescents compared with +25 ± 22 without, P = 0.02, t test). Finally, we were not able to show a statistically different ΔGFR12 months with the AAV glomerulonephritis classification, although sclerotic and mixed classes, merged together, did show a lower ΔGFR12months compared with crescentic and focal classes (P = 0.05, t test). These findings were similar when we considered simultaneously all time points using mixed repeated-measures ANOVAs (Figure 4).
Figure 4.
Influence of pathology findings on the change of glomerular filtration rate (GFR) during the first year of therapy in antineutrophil cytoplasmic autoantibody (ANCA)–associated vasculitis (AAV). There was a significant interaction between (a) acute tubular injury and time, F(2.4,199) = 4.55, P = 0.007, ηp2 = 0.054, and (c) a significant interaction between the proportion of sclerosed glomeruli and time, F(2.4,196) = 2.85, P = 0.05, ηp2 = 0.03. These effects tell us that the change in GFR over time was influenced by these 2 findings. (b) The AAV GN classification and (d) the proportion of crescents did not statistically influence the degree of change in GFR throughout the first year of follow-up. AAV GN, ANCA-associated vasculitis glomerulonephritis.
Multivariate Analyses
Using the 4 significant factors associated by univariate analysis with the change in renal function over 1 year (initial age and GFR, the presence of ATI, and the proportion of globally sclerosed glomeruli), we performed multivariate analyses using both a mixed model repeated-measures ANOVA (data not shown) and linear regression (Table 4), and found each remained statistically significant. Adding clinically relevant risk factors not found predictive in this cohort, such as proteinuria, did not modify the significance level of these 4 factors.
Table 4.
Independent factors associated with the gain in eGFR at 12 months
| Unstandardized β ± SE | P | |
|---|---|---|
| (Constant) | 61 ± 10 | <0.001 |
| Age (10-year increase) | −5.2 ± 1.4 | 0.001 |
| Initial GFR (10 ml/min per 1.73 m2 increase) | −3.8 ± 0.7 | <0.001 |
| 10% increase in sclerotic glomeruli | −3.9 ± 1.1 | 0.001 |
| Presence of acute tubular injury | +12.8 ± 3.7 | 0.001 |
The R2 for the model was 0.40.
eGFR, estimated glomerular filtration rate; GFR, glomerular filtration rate.
Discussion
This retrospective study of newly diagnosed AAV renal disease showed that the greatest gain in GFR occurs in the first 3 months of treatment, with smaller additional gains seen up to 2 years. We identified younger age, a lower initial GFR, fewer glomeruli with global sclerosis, and ATI as characteristics independently associated with a greater gain in GFR during the first year of treatment. We also demonstrated that fewer gains in GFR should be expected with the presence of fibrous crescents. Knowing these will help clinicians determine if a patient's renal function has improved as expected and, if it has not, compel them to rapidly reassess treatments or the need for a new biopsy.
The treatment of renal AAV can arguably be separated into 3 distinct periods: (i) induction therapy and addressing resistance to it, (ii) maintaining remission and the prevention of progression of chronic kidney disease, and (iii) the treatment of relapses. Clumping these together makes it harder to draw conclusions on how to improve outcomes. Although the definition of a relapse can vary, it remains easier to identify (e.g., rise in creatinine with a newly active urine sediment) compared with a resistance to initial therapy (e.g., should an increase in GFR of 8 ml/min per 1.73 m2 at 3 months be considered satisfactory?). We showed that the increase in GFR beyond 3 months is modest, supporting that adequate initial therapy is critical. Others recognized this period in which response was associated with better patient and renal survival.20 The advent of treatment alternatives, such as rituximab, reinforces the need to identify outliers.9, 10 Recent trials use a Birmingham Vasculitis Activity Score of 0 as the definition of a remission.9, 21, 22, 23 The renal components of this score include the absolute initial value or a >30% rise in creatinine (or a drop of >25% of GFR), hypertension, hematuria ≥ 10 RBC/HPF, or proteinuria >1+ by dipstick analysis. This score is insufficient to determine if gains in GFR during treatment are adequate.
In light of this, Berden et al. proposed a simple predictive classification based on the predominance of the glomerular lesions. Its univariate predictive value at 1 and 5 years has repeatedly been validated1, 2, 3, 4, 5, 6, 14, 24; however, clinical and pathological predictors within the first few months have been reported infrequently.20 Studies mostly report kidney survival, thus giving a disproportionate weight on the initial GFR as a predictor of end-stage renal disease.25 Considering the change in GFR, such as gains in function, gives additional information.
As others have found,11, 20, 26, 27, 28 we were able to show that younger patients, and those with a low initial GFR, are expected to have a greater gain in GFR during the first year of treatment. We were unable to show a prognostic value of proteinuria and hematuria, perhaps underpowered by the missing values. However, severe reductions in GFR inevitably lower proteinuria and can influence its predictive value. These may be more important predictors during the maintenance phase and management of chronic kidney disease, or when assessing the possibility of a relapse.8 We also could not address in this study other important risk factors of chronic kidney disease progression, such as blood pressure control, obesity, and dyslipidemia management. Finally, ANCA type did not influence the change in renal function over time and the higher initial GFR in patients with proteinase-3 probably reflects earlier presentation, because those with greater extrarenal disease are thus likely to seek medical assistance sooner, rather than a less aggressive disease.29, 30
As expected, many pathology findings correlated with the initial GFR but only 2 were associated with the change in GFR over time. Our findings do not contradict previous publications using the AAV GN classification, but they show that the impact of global glomerulosclerosis should be considered as a continuous process. We were possibly underpowered to conclude that more crescents are associated with greater gains in renal function, although we found that the type of crescent identified is important. Others were also unable to link the proportion of crescents with the change in GFR at 12 months.11 By contrast, the irreversibility of global glomerulosclerosis facilitates the study of its predictive value.
We assessed from reports the numbers of total glomeruli, sclerosed glomeruli, and glomeruli with crescents, as well as the severities of interstitial fibrosis, tubular atrophy, and chronic vascular changes. These measurements, standard to all biopsy reports, were made by trained nephropathologists, and the agreements we obtained with slide review were similar to large prospective multirater studies.16, 25, 31 We found a high proportion of subjects with ATI. We realize that its intraclass correlation coefficient was poor, as in other series.32, 33 Interestingly, discordant cases were almost all in situations in which the report had no mention of ATI and the review, done specifically to see if it was present, did. Acute and chronic tubulo-interstitial lesions have been found to be independently predictive of renal outcomes in AAV.11, 12, 13, 14, 25 They encompass acute tubular necrosis, tubulo-interstitial infiltrates, and tubulitis. ATI was also frequent in a cohort of 149 subjects with variable GN, and its presence strongly correlated with an initially reduced GFR.34 It is possible that pathologists attribute less importance in reporting small amounts of ATI in the setting of crescentic GN, as found in this study. Our findings support the need for a quantitative approach when reporting tubulo-interstitial lesions in glomerular diseases. Finally, we did not rely on the type of crescents found in the reports, given their complex nature, and considered only the subgroup with a standardized assessment.
Some methodological decisions merit comments. We considered all patients with AAV with the fewest exclusion criteria to avoid overselection; there were only 2 with mild disease who did not receive a cyclophosphamide- or rituximab-based regimen; excluding these did not change the results. Our retrospective design has limitations. Intention to treat includes those who may have not received a full treatment. The standard of care has also changed over the study period. It is possible that older patients and those with severe glomerulosclerosis had their treatment reduced or terminated too early by fear of greater complications or the preemptive belief that the patient would not respond. We were not able to reliably assess the impacts on the GFR of infections, treatment inobservance, or the uses of plasma exchanges or pulses of methylprednisolone. Finally, any delay from the time treatments were started to the moment the renal biopsy was performed also may have influenced our results.
In conclusion, this study identified determinants of early renal recovery during the first year of therapy. It highlights the importance of identifying within 3 months outliers who should rapidly be reassessed and will help refine the definition of a renal remission in AAV.
Disclosure
All the authors declared no competing interests. The study protocol has been approved by the research institute’s committee on human research. Due to the retrospective nature of the study, patients did not give written consent
Acknowledgments
The research efforts of LPL and ST were supported by scholarships from the Fonds de Recherche en Santé du Québec. FL was supported by a scholarship by the Consortium de Recherche en Néphrologie de l'Université de Montréal.
References
- 1.Berden A.E., Ferrario F., Hagen E.C. Histopathologic classification of ANCA-associated glomerulonephritis. J Am Soc Nephrol. 2010;21:1628–1636. doi: 10.1681/ASN.2010050477. [DOI] [PubMed] [Google Scholar]
- 2.Hilhorst M., Wilde B., van Breda Vriesman P. Estimating renal survival using the ANCA-associated GN classification. J Am Soc Nephrol. 2013;24:1371–1375. doi: 10.1681/ASN.2012090912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bjorneklett R., Sriskandarajah S., Bostad L. Prognostic value of histologic classification of ANCA-associated glomerulonephritis. Clin J Am Soc Nephrol. 2016;11:2159–2167. doi: 10.2215/CJN.04800516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iwakiri T., Fujimoto S., Kitagawa K. Validation of a newly proposed histopathological classification in Japanese patients with anti-neutrophil cytoplasmic antibody-associated glomerulonephritis. BMC Nephrol. 2013;14:125. doi: 10.1186/1471-2369-14-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang D.Y., Wu L.H., Liu G. Re-evaluation of the histopathologic classification of ANCA-associated glomerulonephritis:a study of 121 patients in a single center. Nephrol Dial Transplant. 2012;27:2343–2349. doi: 10.1093/ndt/gfr643. [DOI] [PubMed] [Google Scholar]
- 6.Quintana L.F., Perez N.S., De Sousa E. ANCA serotype and histopathological classification for the prediction of renal outcome in ANCA-associated glomerulonephritis. Nephrol Dial Transplant. 2014;29:1764–1769. doi: 10.1093/ndt/gfu084. [DOI] [PubMed] [Google Scholar]
- 7.Noone D.G., Twilt M., Hayes W.N. The new histopathologic classification of ANCA-associated GN and its association with renal outcomes in childhood. Clin J Am Soc Nephrol. 2014;9:1684–1691. doi: 10.2215/CJN.01210214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cordova-Sanchez B.M., Mejia-Vilet J.M., Morales-Buenrostro L.E. Clinical presentation and outcome prediction of clinical, serological, and histopathological classification schemes in ANCA-associated vasculitis with renal involvement. Clin Rheumatol. 2016;35:1805–1816. doi: 10.1007/s10067-016-3195-z. [DOI] [PubMed] [Google Scholar]
- 9.Stone J.H., Merkel P.A., Spiera R. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med. 2010;363:221–232. doi: 10.1056/NEJMoa0909905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Specks U., Merkel P.A., Seo P. Efficacy of remission-induction regimens for ANCA-associated vasculitis. N Engl J Med. 2013;369:417–427. doi: 10.1056/NEJMoa1213277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Lind van Wijngaarden R.A., Hauer H.A., Wolterbeek R. Clinical and histologic determinants of renal outcome in ANCA-associated vasculitis: A prospective analysis of 100 patients with severe renal involvement. J Am Soc Nephrol. 2006;17:2264–2274. doi: 10.1681/ASN.2005080870. [DOI] [PubMed] [Google Scholar]
- 12.Bajema I.M., Hagen E.C., Hermans J. Kidney biopsy as a predictor for renal outcome in ANCA-associated necrotizing glomerulonephritis. Kidney Int. 1999;56:1751–1758. doi: 10.1046/j.1523-1755.1999.00758.x. [DOI] [PubMed] [Google Scholar]
- 13.Berden A.E., Jones R.B., Erasmus D.D. Tubular lesions predict renal outcome in antineutrophil cytoplasmic antibody-associated glomerulonephritis after rituximab therapy. J Am Soc Nephrol. 2012;23:313–321. doi: 10.1681/ASN.2011040330. [DOI] [PubMed] [Google Scholar]
- 14.Tanna A., Guarino L., Tam F.W. Long-term outcome of anti-neutrophil cytoplasm antibody-associated glomerulonephritis: evaluation of the international histological classification and other prognostic factors. Nephrol Dial Transplant. 2015;30:1185–1192. doi: 10.1093/ndt/gfu237. [DOI] [PubMed] [Google Scholar]
- 15.Goupil R., Brachemi S., Nadeau-Fredette A.C. Lymphopenia and treatment-related infectious complications in ANCA-associated vasculitis. Clin J Am Soc Nephrol. 2013;8:416–423. doi: 10.2215/CJN.07300712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Working Group of the International IgA Nephrology Network and the Renal Pathology Society. Roberts I.S., Cook H.T. The Oxford classification of IgA nephropathy: pathology definitions, correlations, and reproducibility. Kidney Int. 2009;76:546–556. doi: 10.1038/ki.2009.168. [DOI] [PubMed] [Google Scholar]
- 17.Barisoni L., Nast C.C., Jennette J.C. Digital pathology evaluation in the multicenter Nephrotic Syndrome Study Network (NEPTUNE) Clin J Am Soc Nephrol. 2013;8:1449–1459. doi: 10.2215/CJN.08370812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bartko J.J. The intraclass correlation coefficient as a measure of reliability. Psychol Rep. 1966;19:3–11. doi: 10.2466/pr0.1966.19.1.3. [DOI] [PubMed] [Google Scholar]
- 19.Krueger C., Tian L. A comparison of the general linear mixed model and repeated measures ANOVA using a dataset with multiple missing data points. Biol Res Nurs. 2004;6:151–157. doi: 10.1177/1099800404267682. [DOI] [PubMed] [Google Scholar]
- 20.Lee T., Gasim A., Derebail V.K. Predictors of treatment outcomes in ANCA-associated vasculitis with severe kidney failure. Clin J Am Soc Nephrol. 2014;9:905–913. doi: 10.2215/CJN.08290813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mukhtyar C., Lee R., Brown D. Modification and validation of the Birmingham Vasculitis Activity Score (version 3) Ann Rheum Dis. 2009;68:1827–1832. doi: 10.1136/ard.2008.101279. [DOI] [PubMed] [Google Scholar]
- 22.Guillevin L., Pagnoux C., Karras A. Rituximab versus azathioprine for maintenance in ANCA-associated vasculitis. N Engl J Med. 2014;371:1771–1780. doi: 10.1056/NEJMoa1404231. [DOI] [PubMed] [Google Scholar]
- 23.Jones R.B., Tervaert J.W., Hauser T. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med. 2010;363:211–220. doi: 10.1056/NEJMoa0909169. [DOI] [PubMed] [Google Scholar]
- 24.Nohr E., Girard L., James M. Validation of a histopathologic classification scheme for antineutrophil cytoplasmic antibody-associated glomerulonephritis. Hum Pathol. 2014;45:1423–1429. doi: 10.1016/j.humpath.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 25.Ford S.L., Polkinghorne K.R., Longano A. Histopathologic and clinical predictors of kidney outcomes in ANCA-associated vasculitis. Am J Kidnedy Dis. 2014;63:227–235. doi: 10.1053/j.ajkd.2013.08.025. [DOI] [PubMed] [Google Scholar]
- 26.Vergunst C.E., van Gurp E., Hagen E.C. An index for renal outcome in ANCA-associated glomerulonephritis. Am J Kidnedy Dis. 2003;41:532–538. doi: 10.1053/ajkd.2003.50115. [DOI] [PubMed] [Google Scholar]
- 27.Ellis C.L., Manno R.L., Havill J.P. Validation of the new classification of pauci-immune glomerulonephritis in a United States cohort and its correlation with renal outcome. BMC Nephrol. 2013;14:210. doi: 10.1186/1471-2369-14-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lionaki S., Hogan S.L., Jennette C.E. The clinical course of ANCA small-vessel vasculitis on chronic dialysis. Kidney Int. 2009;76:644–651. doi: 10.1038/ki.2009.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poulton C.J., Nachman P.H., Hu Y. Pathways to renal biopsy and diagnosis among patients with ANCA small-vessel vasculitis. Clin Exp Rheumatol. 2013;31:S32–S37. [PMC free article] [PubMed] [Google Scholar]
- 30.de Joode A.A., Sanders J.S., Stegeman C.A. Renal survival in proteinase 3 and myeloperoxidase ANCA-associated systemic vasculitis. Clin J Am Soc Nephrol. 2013;8:1709–1717. doi: 10.2215/CJN.01020113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bellur SS, Roberts ISD, Troyanov S, et al. Reproducibility of the Oxford classification of immunoglobulin A nephropathy, impact of biopsy scoring on treatment allocation and clinical relevance of disagreements:evidence from the VALidation of IGA study cohort [e-pub ahead of print]. Nephrol Dial Transplant.https://doi.org/10.1093/ndt/gfy337. Accessed December 2018. [DOI] [PubMed]
- 32.Royal V., Nasr S.H., Ecotiere L. Histologic predictors of renal outcome in myeloma cast nephropathy: a multicenter study. J Am Soc Nephrol. 2018;29:19A. [Google Scholar]
- 33.Glassford N.J., Skene A., Guardiola M.B. Interobserver agreement for post mortem renal histopathology and diagnosis of acute tubular necrosis in critically ill patients. Crit Care Resusc. 2017;19:337–343. [PubMed] [Google Scholar]
- 34.Tavares M.B., Chagas de Almeida Mda C., Martins R.T. Acute tubular necrosis and renal failure in patients with glomerular disease. Ren Fail. 2012;34:1252–1257. doi: 10.3109/0886022X.2012.723582. [DOI] [PMC free article] [PubMed] [Google Scholar]