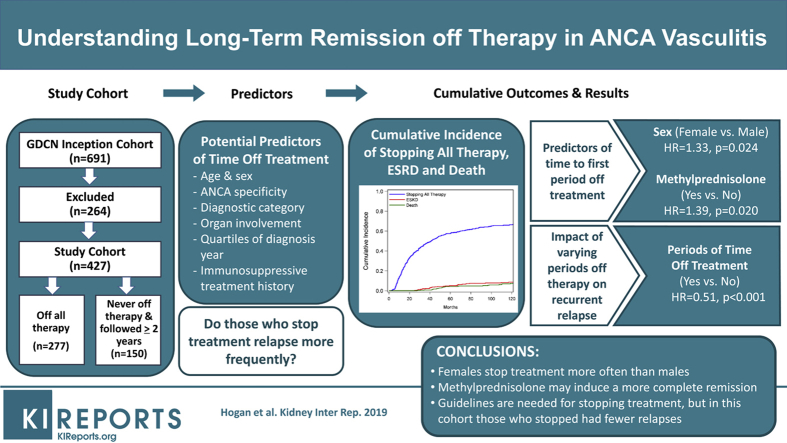
Abstract
Introduction
In antineutrophil cytoplasmic antibody-associated (ANCA) vasculitis, relapse risk and long-term immunosuppressive therapy are problematic. Stopping immunotherapy has not been well described.
Methods
The Glomerular Disease Collaborative Network ANCA vasculitis inception cohort was evaluated. Patients who stopped all immunotherapy and those continuously on immunotherapy (≥2 years) were included. Time to first period off therapy was modeled with end-stage kidney disease and death as competing risks to understand influences of stopping therapy. Cause-specific hazard ratios (HRs) with 95% confidence intervals (CI) and P values are reported. Models controlled for age, sex, ANCA specificity, organ involvement, diagnosis era, and treatments (yes/no). Repeated events analysis was used to assess the time-dependent variable of time off treatment on recurrent relapse with HRs, 95% CIs, and P values are reported (same control variables without treatments).
Results
In 427 patients, 277 (65%) stopped therapy (median 20 months from initial induction); 14% for ≥2 different periods of time and 23% for periods ≥5 years. In multivariable models of time to discontinuation of treatment, women (HR 1.33; 95% CI 1.04–1.70; P = 0.024) and those treated with pulse methylprednisolone (HR 1.39; 95% CI 1.05–1.84; P = 0.020) were more likely to stop. The time-dependent variable of time off treatment was associated with fewer recurrent relapses (HR 0.51; 95% CI 0.41–0.63; P < 0.001).
Conclusions
Stopping immunotherapy was common. Women and those treated with methylprednisolone stop treatment more often, but underlying mechanisms are unknown. Stopping treatment was associated with fewer relapses, suggesting that even without guidelines there may be benefits without an untoward detriment of relapse.
Keywords: ANCA, relapse, stopping therapy, time off therapy, vasculitis
Graphical abstract
Decades of clinical experience and evidence-based treatments for ANCA-associated small vessel vasculitis (ANCA vasculitis) have led to frequent remission and reduced organ damage from the initial phase of the disease.1, 2 Maintenance therapy has evolved to include more frequent use of rituximab and azathioprine in recent years. The impact of these treatments outside of a trial is not reflected within our own cohort, which has a predominance of renal disease in which the risk of relapse has remained steady.1 Use of long-term immunosuppressive medications comes with the weighty consideration of keeping disease activity from returning in the face of serious and potentially fatal risks of infection and malignancy.3
Discontinuation of therapy is generally not considered a viable option for patients with primary vasculitis,4 although there is a common patient-driven question of when all immunosuppressive therapy can be stopped that is discussed in our own clinics. In a recent qualitative study of perspectives of glucocorticoid use in this disease, patients noted its benefits in quelling active disease, while also acknowledging the difficulty in balancing fears: relapse from withdrawal versus the substantial emotional, physical, and social burdens when taking this drug.5 Remission off therapy has been reported for long-term cohort studies and clinical trials,6, 7, 8, 9, 10, 11 but there is a paucity of research into understanding the frequency, patient characteristics, and impact on outcomes among those stopping maintenance immunosuppression.
It is likely that any discontinuation would provide a reprieve from the many risks of continued immunosuppressive therapy. Operating under this hypothesis, and bolstered by patients’ pleas, many clinicians seek opportunities to stop therapy. We aimed to describe patients in our cohort who came off immunotherapy and to understand when in the disease course this was achieved and for how long. Given this was an observational study without documentation of why treatment decisions were made, we sought to identify baseline characteristics for stopping therapy. We also aimed to understand the impact of durations of time off therapy on recurrent relapse. Finally, we aimed to describe details on a subset of patients who have experienced 5 or more years of remission off therapy.
Materials and Methods
Subjects and Definitions
The Glomerular Disease Collaborative Network (GDCN) inception cohort of patients with ANCA vasculitis diagnosed between April 1978 and May 2014 was used for this study. Subsets of this cohort have been evaluated in previous studies.1, 7, 12, 13, 14, 15, 16, 17, 18 The GDCN is a longitudinal, glomerular disease patient registry and bio bank repository that has been ongoing for more than 35 years with patients primarily from the southeastern United States. The GDCN primarily identifies patients diagnosed by a renal biopsy evaluated by the University of North Carolina nephropathology service. This includes patients followed at University of North Carolina hospitals and from private practice nephrologists throughout the southeastern United States. Patients referred to any GDCN physician who meet the biopsy requirements are also invited to participate in the cohort even if their biopsy was not evaluated at the University of North Carolina (<10% of the cohort). Patients with biopsy-proven pauci-immune glomerulonephritis or small vessel vasculitis in any other organ(s) with or without granulomatous inflammation were eligible for inclusion in this study. Patients were followed from diagnosis until end-stage kidney disease (ESKD) or death. ANCA positivity was required as evaluated by immunofluorescence microscopy or antigen-specific, enzyme-linked immunosorbent assay, and classified as cytoplasmic ANCA and/or proteinase 3-ANCA (collectively noted as PR3 ANCA), or perinuclear ANCA and/or myeloperoxidase-ANCA (collectively noted as MPO ANCA). A positive P-ANCA alone required concurrent negative antinuclear antibody test. Diagnostic disease categories were defined using the modified Chapel Hill Consensus Conference nomenclature,19 and designated as granulomatosis with polyangiitis (GPA), eosinophilic GPA, microscopic polyangiitis (MPA), or pauci-immune necrotizing and/or crescentic glomerulonephritis without overt signs of systemic vasculitis.20, 21
Once patients signed consent to participate in the cohort, medical records were obtained, updated over time, and reviewed approximately annually (RJF or PHN with SLH, CJP, or LNB). Review of records included collection of demographics, organ system involvement, and diagnostic categorization, as well as changes over time in disease status (active, remission) and vasculitis-related treatments with start and end dates. Development of ESKD and death were documented. ESKD was presumed to be from ANCA disease, with additional potential contributing causes not known. Treatments were not mandated by a specific protocol. Medical records rarely indicated why maintenance therapy was continued or stopped. Induction and maintenance therapy commonly included a combination of methylprednisolone, oral prednisone, cyclophosphamide, azathioprine, mycophenolate mofetil, and/or rituximab. The last date of therapy was defined as the date the following drugs were stopped: prednisone, oral cyclophosphamide, azathioprine, or mycophenolate mofetil. The end date of treatment for i.v. cyclophosphamide was 30 days after therapy. If treated with rituximab, the last day of therapy was considered as the date CD19+ B cells were detected in circulation or 1 year after the last dose if B-cell measures were not available. Retreatment with any of these medications ended the time off therapy. Short courses (<2 weeks) of prednisone ≤30 mg per day for nonvasculitis indications (e.g., gout) were not considered retreatment.
Remission on or off therapy required the absence of dysmorphic urinary red blood cells and no evidence of vasculitic lesions or symptoms in any organ, with a Birmingham Vasculitis Activity Score of zero.22 Patients often used urinary dipsticks regularly at home, and were seen immediately if the presence of blood was noted. Relapse was defined as the appearance of dysmorphic urinary red blood cells or active vasculitic lesions in any organ deemed severe enough to warrant a change in therapy with a Birmingham Vasculitis Activity Score greater than zero. Persistent proteinuria alone was not indicative of active glomerulonephritis.
Statistical Methods
Categorical variables were expressed as frequencies and percentages and compared using Fisher’s exact tests. Continuous variables were expressed as medians and interquartile ranges (IQRs) and compared using Wilcoxon rank tests. Discontinuing immunotherapy could occur more than once over the disease course; therefore, percentage of follow-up time off therapy was calculated for each patient using total months off therapy over total follow-up time.
A competing risk model was used to evaluate the influence of demographics and disease characteristics on time to the first period off all treatment, with ESKD and death used as competing risks. Univariate and multivariable models for both cause-specific HR and subdistribution HR models are reported.23 Era of treatment was explored using quartiles for the distribution of the date of diagnosis of all patients enrolled in the cohort over time (before 1993, 1993 to 1999, 2000–2004, and after 2004). For modeling, we explored the covariates of ANCA specificity and disease category in separate models, and elected to present results by categories: PR3 ANCA and GPA, MPO ANCA and GPA, PR3 ANCA and MPA, and MPO ANCA and MPA. Patients with renal limited and eosinophilic GPA were included with MPA. Final base models included age, because it was deemed clinically important, sex, ANCA specificity with diagnosis group, and pulmonary and upper respiratory disease involvement because they had P values less than or equal to 0.10 in univariate models.
Although detailed information on dose schedules of the numerous treatments used were not available, we looked at whether patients were ever treated with a variety of immunosuppressives, including prednisone, methylprednisolone, cyclophosphamide (oral or i.v.), plasmapheresis, mycophenolate mofetil, and azathioprine. For competing risk models, each treatment was evaluated as ever or never being used. This was truncated at the time of coming off therapy for the first time for those who stopped all therapy and was over the entire disease course for those who never stopped therapy. All treatments were added together into the base model. Treatments were removed for the final multivariable model if the P value was greater than 0.10. The models with treatments were explored with and without oral corticosteroids because this was used by 97% of the sample. If 2 treatments were correlated, models were explored using a combined treatment variable and then also with each treatment separately. The cumulative incidence function of the first time that patients stop therapy, ESKD, and death over 10 years was calculated.
A conditional model for recurrent events was used to assess the impact of the time-dependent measure of being off therapy on recurrent relapses.24, 25 In this type of model, time intervals are defined between each relapse, with subjects assumed not to be at risk for a subsequent relapse until a prior relapse has occurred. The model controlled for demographic variables, era of treatment as described previously, and consistent clinical risk factors for relapse, ANCA specificity, and the presence of pulmonary and upper respiratory involvement seen in earlier versions of this cohort.13, 18 Models were also explored using ANCA specificity and disease category groups as described previously for modeling.
A minimum of 2 years of follow-up beyond the start of induction therapy was required for those who never came off treatment. This was to ensure patients had the opportunity to come off treatment and was chosen because this was the approximate median time to coming off therapy for the first time among those who stopped treatment (20 months). P values were reported, with a 2-sided value of <0.05 considered statistically significant. Analyses were conducted using SAS software (version 9.4; SAS Institute, Cary, NC).
This study was approved by the University of North Carolina Institutional Review Board, with informed consent provided by all patients.
Results
Summary of Who Stopped Therapy
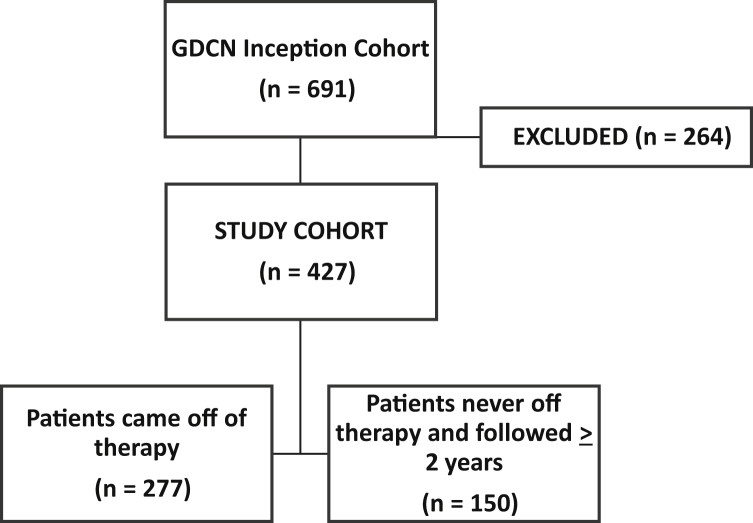
The GDCN inception cohort included 691 patients (Figure 1). A total of 264 patients were excluded because they did not respond to induction therapy (n = 117), were still being treated with induction therapy (n = 20), were not offered therapy (n = 27), or were on therapy and not followed for a minimum of 2 years (n = 100). Those never treated (n = 27) were typically from early in our cohort when they were considered at a late stage of disease for effective therapy. A total of 427 patients were included in this study. Of these, 86 (20%) were followed until they reached ESKD, 104 (24%) until they died, and 237 (56%) until their last follow-up date.
Figure 1.
Study inclusion numbers. GDCN, Glomerular Disease Collaborative Network.
Of 427 patients, 277 (65%) stopped all immunosuppressive therapy at some point during follow-up; 86% for 1 period of time (n = 327), 13% for 2 (n = 37), and 1% for 3 or more (n = 3) periods of time. These patients were off therapy over their entire course of follow-up for a median of 45 months (IQR 16, 91 months), which represented 60% of the total time they were followed (IQR 32%, 81%). A total of 142 patients (51%) who stopped therapy experienced a period of 2 or more years of consecutive time off therapy, and 63 (23%) of these patients were off therapy for 5 or more consecutive years.
Patients who came off all therapy did so for the first time in a median of 20 months (IQR 12, 40 months) from the start of immunotherapy for induction treatment. They then remained off therapy for a median of 36 months (IQR 13, 88). Most patients had never relapsed before stopping therapy for the first time (228/277 = 82%), whereas the remaining 18% had at least 1 disease relapse before coming off therapy (1 prior relapse in 30/277 = 11%, 2 prior relapses in 13/277 = 5%), and 3 or more prior relapses in 6/277 = 2%).
Compared with those continuously on therapy (n = 150, Table 1), those who stopped therapy were more likely women (50% vs. 39%, P = 0.025), MPO ANCA positive (60% vs. 47%, P = 0.015), to have glomerulonephritis only (21% vs. 13%, P = 0.008), and less likely to have pulmonary involvement (44% vs. 58%, P = 0.008). Age, race, upper respiratory tract involvement, and renal involvement were not statistically different between those who did and did not come off therapy.
Table 1.
ANCA vasculitis patients who came off therapy compared with those who never came off therapy
| Variables: n (%) or median (IQR) | Patients who came off therapy, n = 277 | Patients who never came off therapy, n = 150 | Pa |
|---|---|---|---|
| Age at diagnosis | 58 (45, 69) | 58 (44, 69) | 0.96 |
| Female sex | 139 (50) | 58 (39) | 0.025 |
| White race | 237 (86) | 131 (87) | 0.66 |
| MPO (or P) ANCA | 165 (60) | 71 (47) | 0.015 |
| Diagnostic category: | |||
| MPA | 145 (52) | 74 (49) | 0.008 |
| GPA | 72 (26) | 51 (34) | |
| GN only | 59 (21) | 20 (13) | |
| EGPA | 1 (<1) | 5 (3) | |
| Organ involvement (at diagnosis): | |||
| Pulmonary | 123 (44) | 87 (58) | 0.008 |
| Upper respiratory | 108 (39) | 68 (45) | 0.22 |
| Renal | 261 (94) | 139 (93) | 0.54 |
| Era of diagnosis (quartiles): | |||
| <1993 | 72 (26) | 29 (19) | 0.044 |
| 1993–1999 | 65 (23) | 51 (34) | |
| 2000–2004 | 65 (23) | 40 (27) | |
| >2004 | 75 (27) | 30 (20) | |
| Treatment history: | |||
| Corticosteroids (oral) | 267 (96) | 149 (99) | 0.11 |
| Pulse methylprednisolone | 197 (71) | 93 (62) | 0.07 |
| Cyclophosphamide (oral or i.v.) | 249 (90) | 130 (87) | 0.33 |
| Plasmapheresis | 54 (19) | 22 (15) | 0.23 |
| Azathioprine | 87 (31) | 52 (35) | 0.52 |
| Mycophenolate mofetil | 86 (31) | 56 (37) | 0.20 |
| Rituximab | 67 (24) | 21 (14) | 0.013 |
ANCA, antineutrophil cytoplasmic antibody-associated; EGPA, eosinophilic granulomatosis with polyangiitis; GN, pauci-immune necrotizing and/or crescentic glomerulonephritis without other organ involvement; GPA, granulomatosis with polyangiitis; IQR, interquartile range; MPA, microscopic polyangiitis; MPO, myeloperoxidase.
P values were calculated by Fisher’s exact test for categorical variables and Wilcoxon 2-sample test for continuous variables.
Looking within each quartile of diagnosis time, the percentage of patients who came off therapy varied, with 71% coming off therapy among those diagnosed before 1993 (72/101) and after 2004 (75/105), compared with 56% (65/116) and 62% (65/105) among those diagnosed between 1993 and 1999, and 2000 and 2004, respectively (P = 0.044).
The types of immunosuppressive drugs patients were treated with before coming off therapy were similar to what patients were prescribed over their full course of disease, with the exception of rituximab, which was more commonly used among those who stopped therapy (24%) than those who did not stop (14%, P = 0.013).
Those Off Therapy for 5 or More Years
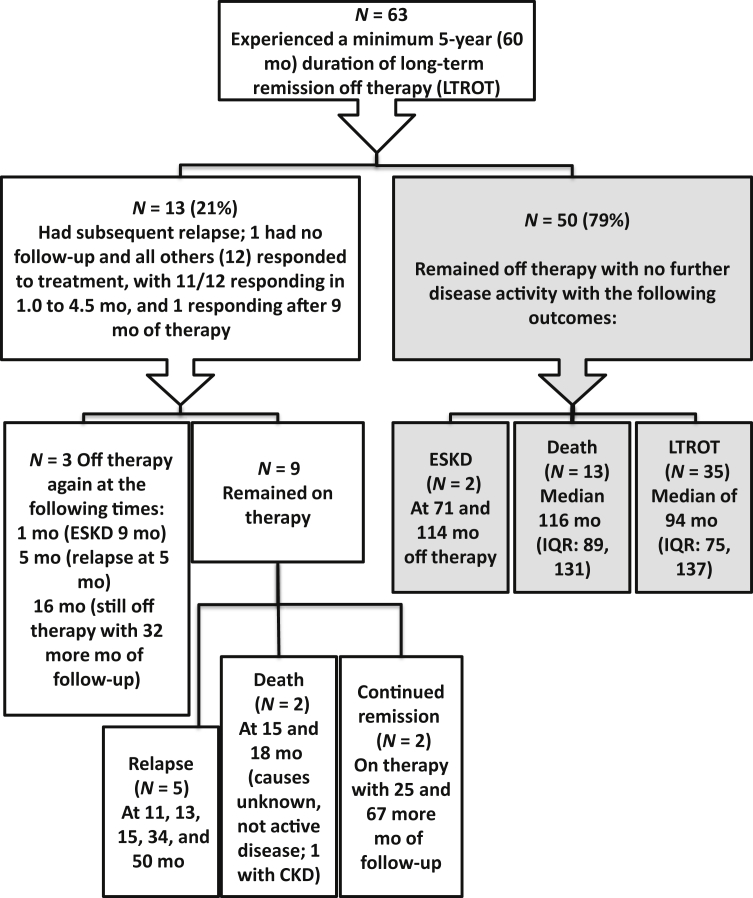
Of the 277 who stopped therapy, a subset of 63 patients were off therapy for 5 or more years (Figure 2), for a median time off therapy of 92 months (IQR 76, 134) which is 7.7 years. Among these patients, 54% were female, 35% had PR3 ANCA specificity, 38% had pulmonary involvement, and 38% had upper respiratory involvement. This long stretch of time off treatment occurred even in patients who had previously experienced a disease relapse (13/63 = 21%).
Figure 2.
A flowchart of the long-term outcomes in the subset of patients off therapy for >5 years. CKD, chronic kidney disease (as documented in the physician’s notes); ESKD, end-stage kidney disease; IQR, interquartile range; LTROT, long-term remission off therapy.
Among those who maintained remission off therapy for 5 or more years, 13 relapsed; 8 in the sixth year of follow-up and the other 5 in 6.4 to 16.0 years after stopping therapy. Retreatment of relapses was successful, with 11 of 13 patients going into remission in 1.0 to 4.5 months. Retreatment included mycophenolate mofetil (n = 6) or cyclophosphamide (n = 5), usually in conjunction with pulse methylprednisolone and oral prednisone; with 2 also given rituximab. The remaining 2 patients were treated for 9 to 12 months (both with mycophenolate mofetil), with 1 having smoldering disease until eventually attaining a complete remission at 9 months and the other responding at 12 months after rituximab was added. Follow-up of these 13 patients beyond the relapse that occurred after being off therapy for >5 years is shown in Figure 2.
The remaining 50 patients off treatment for at least 5 years remained in stable remission until they reached ESKD from slowly progressive chronic kidney disease (n = 2; at 6.0 and 9.5 years), died (n = 12, median 9.6 years, IQR 8.4, 11.59), or until last follow-up (n = 36; median 7.5 years, IQR 6.6, 11.5) (Figure 2).
We attempted to understand ANCA titer changes in the cohort of patients off therapy for 5 or more years, in whom 41 had MPO ANCA (65%) and 22 had PR3 ANCA (35%). Among the 13 who relapsed (7 MPO, 6 PR3), 4 (31%) had negative titers while off therapy and when they relapsed (3 MPO, 1 PR3), and 9 had negative titers while off therapy with increasing titers at relapse (4 MPO, 5 PR3). Among the 50 patients who remained in remission off therapy, 15 had no information on ANCA titers during their time off therapy; but limited information was attainable in 35 patients. Among these 35 patients, 21 (60%) had negative titers before coming off therapy and throughout follow-up (10 MPO, 11 PR3), 6 (17%) had positive titers when therapy was stopped and throughout their follow-up (3 MPO, 3 PR3), and 8 (23%) showed no pattern with both positive/negative titers when stopping therapy and throughout follow-up (5 MPO, 3 PR3).
Predictors of Stopping Therapy
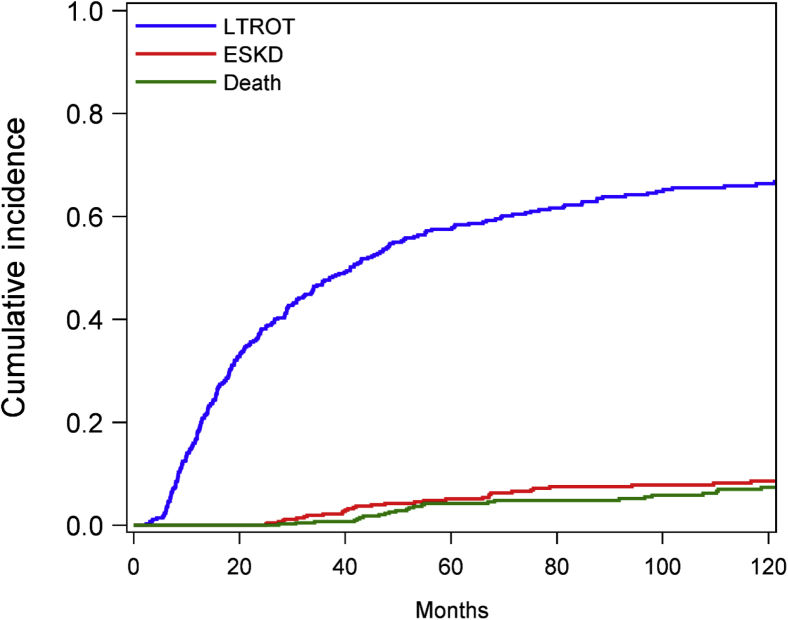
The cumulative incidence function of stopping therapy for the first time is shown in Figure 3. Cause-specific hazards models show the relative effect of covariates on the occurrence of the primary event of stopping therapy (Table 2). In the multivariable base model, women were 30% more likely to stop treatment than men (HR 1.33; 95% CI 1.04–1.70; P = 0.024), and there was an era effect (lower likelihood of coming off treatment in 1993–2004 compared with before 1993, HR 0.66; 95% CI 0.46–0.94; P = 0.023).
Figure 3.
Cumulative incidence function plot of the first time that patients stop therapy or reach the competing risks of end-stage kidney disease (ESKD) or death. LTROT, long-term remission off therapy.
Table 2.
Competing risk models of time to the first period off all treatment, with ESKD and death as competing risks
| Variables | Cause-specific hazard model |
Subdistribution hazard model |
||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariable | Univariate | Multivariable | |||||
| Base model: | HR (95% CI) | P | HR (95% CI) | P | SHR (95% CI) | P | SHR (95% CI) | P |
| Age (per yr) | 1.01 (1.00–1.01) | 0.15 | 1.00 (1.00–1.01) | 0.29 | 1.00 (1.00–1.01) | 0.41 | 1.00 (1.00–1.01) | 0.47 |
| Sex (female vs. male) | 1.31 (1.03–1.66) | 0.026 | 1.33 (1.04–1.70) | 0.024 | 1.29 (1.02–1.63) | 0.033 | 1.28 (0.99–1.65) | 0.06 |
| ANCA and diagnosis (reference GPA and PR3): | ||||||||
| GPA and MPO | 1.31 (0.78–2.20) | 0.30 | 0.96 (0.56–1.67) | 0.89 | 1.30 (0.83–2.06) | 0.26 | 0.95 (0.56–1.61) | 0.86 |
| MPA and MPO | 1.72 (1.25–2.37) | <0.001 | 1.33 (0.91–1.93) | 0.14 | 1.68 (1.24–2.28) | <0.001 | 1.28 (0.88–1.85) | 0.20 |
| MPA and PR3 | 1.33 (0.92–1.94) | 0.13 | 1.13 (0.76–1.68) | 0.56 | 1.27 (0.89–1.81) | 0.19 | 1.07 (0.74–1.57) | 0.71 |
| Pulmonary (yes vs. no) | 0.77 (0.61–0.98) | 0.03 | 0.89 (0.69–1.16) | 0.39 | 0.73 (0.58–0.92) | <0.001 | 0.82 (0.62–1.07) | 0.14 |
| Upper respiratory (yes vs. no) | 0.74 (0.58–0.94) | 0.02 | 0.91 (0.69–1.20) | 0.48 | 0.77 (0.61–0.98) | 0.031 | 0.93 (0.70–1.22) | 0.58 |
| Era (reference <1993) | ||||||||
| 1993–1999 | 0.60 (0.43–0.84) | 0.003 | 0.66 (0.46–0.94) | 0.023 | 0.59 (0.41–0.85) | 0.004 | 0.65 (0.44–0.97) | 0.033 |
| 2000–2004 | 0.74 (0.52–1.03) | 0.08 | 0.91 (0.63–1.31) | 0.60 | 0.72 (0.50–1.04) | 0.07 | 0.88 (0.58–1.33) | 0.54 |
| >2004 | 0.87 (0.63–1.21) | 0.42 | 1.08 (0.75–1.58) | 0.67 | 0.89 (0.65–1.24) | 0.49 | 1.09 (0.72–1.63) | 0.69 |
| Treatments added to base modela (yes vs. no) | ||||||||
| Corticosteroids | 0.61 (0.32–1.14) | 0.12 | 0.55 (0.30–1.02) | 0.06 | ||||
| Pulse methylprednisolone | 1.22 (0.94–1.58) | 0.14 | 1.39 (1.05–1.84) | 0.020 | 1.22 (0.94–1.58) | 0.14 | 1.36 (1.01–1.82) | 0.041 |
| Cyclophosphamide (oral or i.v.) | 1.30 (0.88–1.92) | 0.19 | 1.26 (0.86–1.85) | 0.24 | ||||
| Plasmapheresis | 1.09 (0.81–1.47) | 0.55 | 1.12 (0.85–1.49) | 0.42 | ||||
| Azathioprine | 0.76 (0.59–0.98) | 0.03 | 0.71 (0.54–0.94) | 0.018 | 0.80 (0.63–1.01) | 0.06 | 0.75 (0.57–0.99) | 0.038 |
| Mycophenolate mofetil | 0.63 (0.49–0.82) | <0.001 | 0.63 (0.48–0.84) | 0.002 | 0.67 (0.52–0.85) | <0.001 | 0.67 (0.51–0.87) | 0.003 |
| Rituximab | 0.90 (0.68–1.19) | 0.47 | 1.02 (0.81–1.29) | 0.85 | ||||
ANCA, antineutrophil cytoplasmic antibody-associated; CI, confidence interval; GPA, granulomatosis with polyangiitis; HR, hazard ratio; MPA, microscopic polyangiitis, which also includes the small number of eosinophilic granulomatosis with polyangiitis (EGPA) patients for this grouping; MPO, myeloperoxidase; PR3, proteinase 3; SHR, subdistribution hazard ratio,
All treatments were added together into the base model, then removed for the final multivariable model if the P value was greater than 0.10. However, the models shown in the table include each treatment separately in the base model (univariate columns), then with the base model and other treatments that met the criteria for being included in the models (multivariable columns). Treatments were those ever given before coming off treatment for those who stopped, and ever given over the entire follow-up for those continually on treatment.
When all treatments were included in the competing risk model of stopping therapy for the first time, oral corticosteroids, pulse methylprednisolone, cyclophosphamide, azathioprine, and mycophenolate mofetil all reached the required P value of 0.10 for retention in the model. However, use of pulse methylprednisolone and cyclophosphamide were highly associated (P = 0.0048). Categories of those using pulse methylprednisolone without cyclophosphamide or using neither of these drugs were small (n = 24 for each); therefore, models were evaluated removing each of these treatments one at a time. Estimates for other variables and treatments in the base model were consistent in all models explored, and the final model includes the 3 consistent treatments that were associated with stopping therapy: pulse methylprednisolone, azathioprine, and mycophenolate mofetil.
In the final model, controlling for these base factors, those treated with pulse methylprednisolone were more likely to stop therapy (HR 1.39; 95% CI 1.05–1.84; P = 0.020), whereas those treated with azathioprine or mycophenolate mofetil were less likely to stop therapy (HR 0.71; 95% CI 0.54–0.94; P = 0.018 and HR 0.63; 95% CI 0.48–0.84; P = 0.002, respectively, Table 2). Subdistribution models reveal the effect of each covariate on the cumulative incidence of stopping therapy, and in these models only era was a statistically significant factor (1993–2004 compared with before 1993, subdistribution HR 0.65; 95% CI 0.44–0.97; P = 0.033). Adding treatments to the same base model, the treatment impacts were similar to the cause-specific model. These models were unchanged when controlling for either ANCA specificity or disease category separately.
Impact of Times Off Therapy on Relapse
Over the entire follow-up of the study cohort (n = 427), 194 patients never relapsed and this was not different for those who did and did not ever stop treatment (42% vs. 53%, respectively, P = 0.11). Among the remaining 233 patients, there were 452 disease relapses; 147 associated with a time off treatment and 305 when patients were on therapy.
The multivariable model assessing the recurrent event of relapse revealed that the time-dependent variable of time off treatment was associated with half as many relapses (HR 0.51; 95% CI 0.41–0.63; P < 0.0001) (Table 3). Covariates included PR3 ANCA specificity, a well-established risk factor for disease relapse, which was associated with relapse in this model. Younger age and female sex were also associated with relapse. There were more relapses in patients diagnosed in the most recent era (>2004). Diagnosis was also modeled in the same multivariable model (taking ANCA specificity out), but did not reach statistical significance (GPA vs. all others, HR 1.24; 95% CI 1.24–1.57; P = 0.08). When modeled with ANCA specificity and diagnosis as groups, GPA with PR3 ANCA specificity was more likely to relapse than MPA with MPO ANCA specificity (HR 1.44; 95% CI 1.07–1.93; P = 0.015), but there were no other differences between groups. Other variables in the model were essentially unchanged when controlling for diagnosis or ANCA specificity and diagnosis groups together.
Table 3.
Recurrent relapse survival model with time off treatment as a time-dependent covariate
| Variables | Estimate | HR (95% CI) | P |
|---|---|---|---|
| Age (per yr) | −0.0075 | 0.99 (0.99–1.00) | 0.008 |
| Sex (female vs. male) | 0.279 | 1.32 (1.07–1.63) | 0.010 |
| ANCA specificity (PR3 vs. MPO) | 0.2539 | 1.29 (1.03–1.62) | 0.03 |
| Pulmonary (yes vs. no) | 0.153 | 1.17 (0.95–1.43) | 0.15 |
| Upper respiratory (yes vs. no) | 0.0025 | 1.00 (0.81–1.24) | 0.98 |
| Era (reference < 1993) | |||
| 1993–1999 | 0.2313 | 1.26 (0.93–1.71) | 0.14 |
| 2000–2004 | 0.1837 | 1.20 (0.87–1.66) | 0.26 |
| >2004 | 0.6172 | 1.85 (1.37–2.51) | <0.001 |
| Periods of time off treatment (yes vs. no for each new treatment time over follow-up) | −0.6813 | 0.51 (0.41–0.63) | <0.001 |
ANCA, antineutrophil cytoplasmic antibody-associated; CI, confidence interval; HR, hazard ratio; MPO, myeloperoxidase; PR3, proteinase 3.
Discussion
In our ANCA vasculitis cohort, 65% of patients who had an initial response to therapy stopped therapy, with most doing so within 2 years of their initial diagnosis. A number of studies6, 7, 8, 9, 10, 11, 26, 27, 28 support ours in showing that 31% to as many as 80% of patients stop all therapy while in complete remission. Follow-up times among these studies vary greatly, with means of 2.0 to 11.9 years, and neither duration of time off therapy nor outcomes are described.
There are no evidence-based guidelines for when to stop treatment.29 Although given the lowest evidence grade of D, the British Society for Rheumatology and British Health Professionals for Rheumatology do provide a statement for withdrawal of all treatment.30, 31 They recommend patients in remission for 1 year on maintenance therapy be considered for tapering off oral prednisone; then, once withdrawn from prednisone, tapered off other immunosuppressive therapy after another 6 months.
Withdrawal from immunosuppressive treatment was recently described in a small study of 18 patients.32 In this study, patients stopped therapy after approximately 24 months of maintenance therapy, but only 12 patients were completely weaned off prednisone. Over a mean follow-up of 64 months, only 3 of these patients relapsed. Attention to stopping therapy was also explored in a study of combined clinical trials that found that azathioprine maintenance therapy beyond 18 months was ineffective in preventing relapse in ANCA-associated vasculitis compared with its use for <18 months.33 Two trials of 2 versus 4 years of azathioprine for maintenance therapy have been reported. One showed no benefit of the longer duration of therapy, although slow recruitment and early termination limited statistical power for detecting a difference.34 In the second trial, glucocorticoid and azathioprine therapy beyond 2 years was associated with a 3-fold reduction of relapse compared with those who stopped treatment at 2 years.28 However, those treated longer had worrisome, albeit not statistically significant, increases in total adverse events (P = 0.07), cytopenia (P = 0.07), and cardiovascular events (P = 0.06).
It is generally accepted that infections and malignancy impact morbidity and mortality in this disease,35, 36 so developing a risk-benefit formula for chronic maintenance versus discontinuation of treatment after remission is considered an unmet need.37 Discontinuation of therapy has generally been considered unachievable, at least for a few years after onset,4 but the studies described previously and our study show a drive by patients and providers to consider this option. Clearly, some patients can stay off and do well for long periods, so identifying patients likely to do well off therapy is critical in future studies. Unfortunately, our study revealed there are no demographics or current clinical phenotypes that are helpful in determining who can stop therapy. Women were approximately 28% more likely to stop therapy than men in our cohort, but without understanding more about communication styles and health literacy, this is not likely useful in predicting who can come off treatment.
The differences in stopping treatment across quartiles of time spanning more than 3 decades in this study were likely related to changes in both treatments and practice patterns. In the earliest quarter of time (before 1993), there were likely fewer options for maintenance therapies, whereas across time, mycophenolate mofetil and azathioprine, then later rituximab, were used more regularly. Suggestions from our modeling that fewer stopped therapy with a recorded use of mycophenolate mofetil and azathioprine is likely due to the lack of guidelines for stopping therapy. The ease of filling these oral medications and continued use among those with few or no adverse events may also encourage continued use. In contrast, it is not clear why those treated with pulse methylprednisolone, typically used as part of induction therapy, were more likely to stop treatment. Data on the benefits of pulse methylprednisolone in this disease are limited,29 but longstanding research suggests glucocorticoids may decrease the ability of neutrophils to gain access to the interstitium and increase inflammation, thus limiting their release of damaging effects on the vascular wall.38, 39, 40, 41 Furthermore, and specific to pulse methylprednisolone, this drug can decrease the T-cell repertoire and limit T-cell differentiation, leading to a decrease in the proinflammatory response of the adaptive immune system.42, 43 Perhaps, then, patients who have been treated with this drug have had a more thorough response and really do feel better. This ultimately leads to discussion and then action regarding stopping therapy. Better biomarkers of subclinical treatment responses could help us understand whether there is certainty behind this finding.
A number of studies show that relapse is a concern when stopping therapy8 or with shorter maintenance therapies.9, 28 Our study suggests that time off therapy, when evaluated as a time-dependent covariate, is associated with approximately half as many relapses than when on continuous therapy. We acknowledge that there are many biases in selecting who stopped therapy in our cohort, but we expected a higher relapse rate. This result suggests that even in the absence of guidelines, the process of selecting who can stop therapy in the practical clinical setting is not putting patients at increased risk of relapse. And although it clearly needs to be studied in more controlled settings, it is encouraging and in support of pursuing high-level evidence-based guidelines for stopping therapy.
Reasons patients stopped therapy were not available, although they likely want to curtail risks of infections and malignancy, which are the established major events associated with long-term immunosuppressive therapy.35, 36 Patients’ hopes to be free of the cost and day-to-day impacts of taking these medications may have also influenced these attempts, as the disease and its treatments can diminish quality of life (i.e., fatigue, depression, anxiety, and sleep disturbances), and cause treatment-specific effects (i.e., weight gain and mood and cognitive changes).44, 45, 46 Future studies should delineate all reasons surrounding for termination of immunosuppressive therapy.
There are several limitations to our study. There were no predefined therapeutic guidelines for when to stop immunosuppressive therapy; therefore, there are inherent biases in who did or did not stop therapy, which limits inferences that can be made from statistical modeling. Also, those who never stopped therapy may have been on low or inadequate doses, perhaps toward tapering off, that contributed to their risk of relapse. These issues suggest that there may well be unmeasured confounding, particularly with respect to the time-period around the decision to stop therapy. Therefore, these results need to be validated and expanded using time-varying treatment and other decision-related data that are not available in this cohort. Established risk factors for relapse, including PR3 ANCA serotype and lung involvement, likely influenced physicians in deciding whether a patient could try coming off therapy, but this was not absolute, with many patients having these risk factors included among those who stopped therapy. What remains important is that stopping all immunotherapy was attempted in well over half of patients followed for at least 2 years. In some patients, the time off therapy was maintained for years. In the absence of a protocol for stopping treatment, future research must prospectively gather ongoing details on influences for stopping and continuing treatment. In addition, changes in treatment practices over the decades of this study made it difficult and impractical to study the important details for the various induction and maintenance therapies, including dose, duration, and tapering schedules associated with onset and each separate disease relapse. Also, although adverse events of long-term immunosuppressive therapy are well described,47, 48 this study was not designed to evaluate if duration of the absence of therapy affected patient-reported outcomes or long-term adverse events. A shift of perspective to include collection of details on this important subgroup of patients is needed to better understand the influence of time off therapy on these outcomes.
Overall, this work informs our vision for a cure; a goal that must be pursued to be realized. But until a cure is found, breaks in therapy may help the immune system recover and reduce long-term risks of treatment. It is likely that duration off therapy of 2 or more years in 50% and 5 or more years among 22% of those who stopped all immunosuppressive therapy in our cohort represents a significant advantage. Although this study generates more questions than answers, it underscores the need to understand who can stop therapy and then continue to do well off therapy for extended periods. There may be differences in the underlying cascade of events that caused the disease or in genetics that affect treatment response. In the meantime, it is encouraging that some patients can have a successful period off therapy, some for more than 5 years. With close cooperation between patients and providers, stopping immunotherapy can be considered if the patient has been in remission on maintenance therapy. We make this recommendation with caution and note that patient involvement is critical given they are likely to provide the best and earliest prediction of emerging disease activity.49 Any hint of disease activity must be evaluated swiftly to quell relapses, which our study shows can be without untoward consequences, as seen in our cohort off therapy for 5 or more years.
Disclosure
All the authors declared no competing interests.
Acknowledgements
We acknowledge the altruistic participation of those with ANCA vasculitis who participate in our cohort and contribute to our research efforts through their willingness to share their medical information.
This work was supported in part by federal grant P01 DK058335, ANCA Glomerulonephritis: from Molecules to Man (Principal Investigator: R.J. Falk) from the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases.
The results presented in this paper have not been published previously in whole or part, except in abstract format.
Author Contributions
Conception and design (SLH, PHN, and RJF), analysis and interpretation of data (SLH, YH, and RJF), drafting and revising the article (SLH, PHN, CJP, and RJF), providing intellectual content of critical importance to the work described (SLH, PHN, CJP, YH, LNB, MEF, JCJ, and RJF), final approval of manuscript submission (SLH, PHN, CJP, YH, LNB, MEF, JCJ, and RJF).
References
- 1.Rhee R.L., Hogan S.L., Poulton C.J. Trends in long-term outcomes among patients with antineutrophil cytoplasmic antibody-associated vasculitis with renal disease. Arthritis Rheumatol. 2016;68:1711–1720. doi: 10.1002/art.39614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kallenberg C.G. Pathogenesis and treatment of ANCA-associated vasculitides. Clin Exp Rheumatol. 2015;33:S11–S14. [PubMed] [Google Scholar]
- 3.Flossmann O. Risks of treatments and long-term outcomes of systemic ANCA-associated vasculitis. Presse Med. 2015;44:e251–e257. doi: 10.1016/j.lpm.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 4.Luqmani R.A. Discontinuation of therapies in vasculitis. Clin Exp Rheumatol. 2013;31:S93–S97. [PubMed] [Google Scholar]
- 5.Robson J.C., Dawson J., Cronholm P.F. Patient perceptions of glucocorticoids in anti-neutrophil cytoplasmic antibody-associated vasculitis. Rheumatol Int. 2018;38:675–682. doi: 10.1007/s00296-017-3855-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fauci A.S., Haynes B.F., Katz P. Wegener's granulomatosis: prospective clinical and therapeutic experience with 85 patients for 21 years. Ann Intern Med. 1983;98:76–85. doi: 10.7326/0003-4819-98-1-76. [DOI] [PubMed] [Google Scholar]
- 7.Nachman P.H., Hogan S.L., Jennette J.C. Treatment response and relapse in antineutrophil cytoplasmic autoantibody-associated microscopic polyangiitis and glomerulonephritis. J Am Soc Nephrol. 1996;7:33–39. doi: 10.1681/ASN.V7133. [DOI] [PubMed] [Google Scholar]
- 8.Faurschou M., Westman K., Rasmussen N. Brief Report: long-term outcome of a randomized clinical trial comparing methotrexate to cyclophosphamide for remission induction in early systemic antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum. 2012;64:3472–3477. doi: 10.1002/art.34547. [DOI] [PubMed] [Google Scholar]
- 9.Springer J., Nutter B., Langford C.A. Granulomatosis with polyangiitis (Wegener's): impact of maintenance therapy duration. Medicine (Baltimore) 2014;93:82–90. doi: 10.1097/MD.0000000000000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samson M., Puechal X., Devilliers H. Long-term follow-up of a randomized trial on 118 patients with polyarteritis nodosa or microscopic polyangiitis without poor-prognosis factors. Autoimmun Rev. 2014;13:197–205. doi: 10.1016/j.autrev.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Puechal X., Pagnoux C., Perrodeau E. Long-term outcomes among participants in the WEGENT trial of remission-maintenance therapy for granulomatosis with polyangiitis (Wegener's) or microscopic polyangiitis. Arthritis Rheumatol. 2016;68:690–701. doi: 10.1002/art.39450. [DOI] [PubMed] [Google Scholar]
- 12.Hogan S.L., Nachman P.H., Wilkman A.S. Prognostic markers in patients with antineutrophil cytoplasmic autoantibody-associated microscopic polyangiitis and glomerulonephritis. J Am Soc Nephrol. 1996;7:23–32. doi: 10.1681/ASN.V7123. [DOI] [PubMed] [Google Scholar]
- 13.Hogan S.L., Falk R.J., Chin H. Predictors of relapse and treatment resistance in antineutrophil cytoplasmic antibody-associated small-vessel vasculitis. Ann Intern Med. 2005;143:621–631. doi: 10.7326/0003-4819-143-9-200511010-00005. [DOI] [PubMed] [Google Scholar]
- 14.Lionaki S., Hogan S.L., Jennette C.E. The clinical course of ANCA small-vessel vasculitis on chronic dialysis. Kidney Int. 2009;76:644–651. doi: 10.1038/ki.2009.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGregor J.G., Hogan S.L., Hu Y. Glucocorticoids and relapse and infection rates in anti-neutrophil cytoplasmic antibody disease. Clin J Am Soc Nephrol. 2012;7:240–247. doi: 10.2215/CJN.05610611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee T., Gasim A., Derebail V.K. Predictors of treatment outcomes in ANCA-associated vasculitis with severe kidney failure. Clin J Am Soc Nephrol. 2014;9:905–913. doi: 10.2215/CJN.08290813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGregor J.G., Negrete-Lopez R., Poulton C.J. Adverse events and infectious burden, microbes and temporal outline from immunosuppressive therapy in antineutrophil cytoplasmic antibody-associated vasculitis with native renal function. Nephrol Dial Transplant. 2015;30(Suppl 1):i171–i181. doi: 10.1093/ndt/gfv045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pagnoux C., Hogan S.L., Chin H. Predictors of treatment resistance and relapse in antineutrophil cytoplasmic antibody-associated small-vessel vasculitis: comparison of two independent cohorts. Arthritis Rheum. 2008;58:2908–2918. doi: 10.1002/art.23800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jennette J.C., Falk R.J., Bacon P.A. 2012 revised international chapel hill consensus conference nomenclature of vasculitides. Arthritis Rheum. 2013;65:1–11. doi: 10.1002/art.37715. [DOI] [PubMed] [Google Scholar]
- 20.Falk R.J., Jennette J.C. ANCA disease: where is this field heading? J Am Soc Nephrol. 2010;21:745–752. doi: 10.1681/ASN.2009121238. [DOI] [PubMed] [Google Scholar]
- 21.Jennette J.C., Falk R.J., Andrassy K. Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum. 1994;37:187–192. doi: 10.1002/art.1780370206. [DOI] [PubMed] [Google Scholar]
- 22.Luqmani R.A., Bacon P.A., Moots R.J. Birmingham Vasculitis Activity Score (BVAS) in systemic necrotizing vasculitis. QJM. 1994;87:671–678. [PubMed] [Google Scholar]
- 23.Austin P.C., Lee D.S., Fine J.P. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133:601–609. doi: 10.1161/CIRCULATIONAHA.115.017719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prentice R.L., Williams B.J., Peterson A.V. On the regression analysis of multivariate failure time data. Biometrika. 1981;68:373–379. [Google Scholar]
- 25.Hosmer D.W., Lemeshow S., May S. Applied Survival Analysis: Regression Modeling of Time-to-Event Data. 2nd ed. Wiley; Hoboken, NJ: 2008. Recurrent event models; p. 9. [Google Scholar]
- 26.Alberici F, Smith RM, Jones RB, et al. Long-term follow-up of patients who received repeat-dose rituximab as maintenance therapy for ANCA-associated vasculitis. In: Balding DJ, Cressie NAC, Fitzmaurice GM, et al., eds. Rheumatology (Oxford). 2015;54:1153–1160. [DOI] [PMC free article] [PubMed]
- 27.Sada K.E., Yamamura M., Harigai M. Different responses to treatment across classified diseases and severities in Japanese patients with microscopic polyangiitis and granulomatosis with polyangiitis:a nationwide prospective inception cohort study. Arthritis Res Ther. 2015;17:305. doi: 10.1186/s13075-015-0815-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karras A., Pagnoux C., Haubitz M. Randomised controlled trial of prolonged treatment in the remission phase of ANCA-associated vasculitis. Ann Rheum Dis. 2017;76:1662–1668. doi: 10.1136/annrheumdis-2017-211123. [DOI] [PubMed] [Google Scholar]
- 29.Geetha D., Jin Q., Scott J. Comparisons of guidelines and recommendations on managing antineutrophil cytoplasmic antibody-associated vasculitis. Kidney Int Rep. 2018;3:1039–1049. doi: 10.1016/j.ekir.2018.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lapraik C., Watts R., Bacon P. BSR and BHPR guidelines for the management of adults with ANCA associated vasculitis. Rheumatology (Oxford) 2007;46:1615–1616. doi: 10.1093/rheumatology/kem146a. [DOI] [PubMed] [Google Scholar]
- 31.Ntatsaki E., Carruthers D., Chakravarty K. BSR and BHPR guideline for the management of adults with ANCA-associated vasculitis. Rheumatology (Oxford) 2014;53:2306–2309. doi: 10.1093/rheumatology/ket445. [DOI] [PubMed] [Google Scholar]
- 32.Gapud E.J., Manno R., Seo P. Long-term clinical course of antineutrophil cytoplasmic antibody-associated vasculitis patients off maintenance therapy. Cureus. 2018;10:e2372. doi: 10.7759/cureus.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Joode A.A.E., Sanders J.S.F., Puechal X. Long term azathioprine maintenance therapy in ANCA-associated vasculitis:combined results of long-term follow-up data. Rheumatology (Oxford) 2017;56:1894–1901. doi: 10.1093/rheumatology/kex281. [DOI] [PubMed] [Google Scholar]
- 34.Sanders J.S., de Joode A.A., DeSevaux R.G. Extended versus standard azathioprine maintenance therapy in newly diagnosed proteinase-3 anti-neutrophil cytoplasmic antibody-associated vasculitis patients who remain cytoplasmic anti-neutrophil cytoplasmic antibody-positive after induction of remission: a randomized clinical trial. Nephrol Dial Transplant. 2016;31:1453–1459. doi: 10.1093/ndt/gfw211. [DOI] [PubMed] [Google Scholar]
- 35.Westman K., Flossmann O., Gregorini G. The long-term outcomes of systemic vasculitis. Nephrol Dial Transplant. 2015;30(Suppl 1):i60–i66. doi: 10.1093/ndt/gfu392. [DOI] [PubMed] [Google Scholar]
- 36.Kronbichler A., Jayne D.R., Mayer G. Frequency, risk factors and prophylaxis of infection in ANCA-associated vasculitis. Eur J Clin Invest. 2015;45:346–368. doi: 10.1111/eci.12410. [DOI] [PubMed] [Google Scholar]
- 37.Hoffman G.S. What does the future hold for clinical studies in vasculitis? Clin Exp Immunol. 2011;164(Suppl 1):35–38. doi: 10.1111/j.1365-2249.2011.04365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fauci A.S., Dale D.C., Balow J.E. Glucocorticosteroid therapy: mechanisms of action and clinical considerations. Ann Intern Med. 1976;84:304–315. doi: 10.7326/0003-4819-84-3-304. [DOI] [PubMed] [Google Scholar]
- 39.Peters W.P., Holland J.F., Senn H. Corticosteroid administration and localized leukocyte mobilization in man. N Engl J Med. 1972;286:342–345. doi: 10.1056/NEJM197202172860703. [DOI] [PubMed] [Google Scholar]
- 40.Ignarro L.J., Cech S.Y. Lysosomal enzyme secretion from human neutrophils mediated by cyclic CMP: inhibition of cyclic GMP accumulation and neutrophil function by glucocorticosteroids. J Cyclic Nucleotide Res. 1975;1:283–292. [PubMed] [Google Scholar]
- 41.Ignarro L.J. Glucocorticosteroid inhibition of nonphagocytic discharge of lysosomal enzymes from human neutrophils. Arthritis Rheum. 1977;20:73–83. doi: 10.1002/art.1780200114. [DOI] [PubMed] [Google Scholar]
- 42.Luther C., Adamopoulou E., Stoeckle C. Prednisolone treatment induces tolerogenic dendritic cells and a regulatory milieu in myasthenia gravis patients. J Immunol. 2009;183:841–848. doi: 10.4049/jimmunol.0802046. [DOI] [PubMed] [Google Scholar]
- 43.Momcilovic M., Miljkovic Z., Popadic D. Methylprednisolone inhibits interleukin-17 and interferon-gamma expression by both naive and primed T cells. BMC Immunol. 2008;9:47. doi: 10.1186/1471-2172-9-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Basu N., McClean A., Harper L. The characterisation and determinants of quality of life in ANCA associated vasculitis. Ann Rheum Dis. 2014;73:207–211. doi: 10.1136/annrheumdis-2012-202750. [DOI] [PubMed] [Google Scholar]
- 45.Tesar V., Hruskova Z. Limitations of standard immunosuppressive treatment in ANCA-associated vasculitis and lupus nephritis. Nephron Clin Pract. 2014;128:205–215. doi: 10.1159/000368569. [DOI] [PubMed] [Google Scholar]
- 46.McClean A., Morgan M.D., Basu N. Physical fatigue, fitness, and muscle function in patients with antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Care Res (Hoboken) 2016;68:1332–1339. doi: 10.1002/acr.22827. [DOI] [PubMed] [Google Scholar]
- 47.Niethammer D., Kummerle-Deschner J., Dannecker G.E. Side-effects of long-term immunosuppression versus morbidity in autologous stem cell rescue:striking the balance. Rheumatology (Oxford) 1999;38:747–750. doi: 10.1093/rheumatology/38.8.747. [DOI] [PubMed] [Google Scholar]
- 48.Wall N., Harper L. Complications of long-term therapy for ANCA-associated systemic vasculitis. Nat Rev Nephrol. 2012;8:523–532. doi: 10.1038/nrneph.2012.107. [DOI] [PubMed] [Google Scholar]
- 49.Tomasson G., Davis J.C., Hoffman G.S. Brief report: The value of a patient global assessment of disease activity in granulomatosis with polyangiitis (Wegener's) Arthritis Rheumatol. 2014;66:428–432. doi: 10.1002/art.38248. [DOI] [PubMed] [Google Scholar]