Abstract
Topical ophthalmic formulations of analogues of the endogenous arachidonic acid cyclooxygenase metabolite, PGF2α, are the standard of care treatment for the blinding disease glaucoma. These are the most potent and efficacious medical therapies for lowering intraocular pressure (IOP), the most important risk factor identified for disease progression. They have few side effects and offer the convenience of once‐a‐day dosing. It was initially believed that endogenous PGs raised IOP and caused substantial ocular surface adverse effects. However, carefully designed experiments demonstrated that esterification of the carboxylic acid afforded potent and efficacious topical ocular hypotensive activity. The final hurdle to be overcome was improvement of the side effect profile. A hypothesis was advanced that the IOP‐lowering effect of PGF2α isopropyl ester was due to activation of its cognate PG‐FP receptor, while side effects were largely due to promiscuous interaction with other PG receptors. This hypothesis was validated by modification of the ω chain (carbons 13–20) to a phenyl group. This provided the first marketed FP‐class PG agonist analogue (FP‐PGA) ocular hypotensive agent, latanoprost. Since the introduction of latanoprost into clinical medicine to lower and control IOP, a number of additional FP‐PGAs have been discovered, characterized and marketed, including travoprost, tafluprost, unoprostone isopropyl ester and bimatoprost (an amide).
Linked Articles
This article is part of a themed section on Eicosanoids 35 years from the 1982 Nobel: where are we now? To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v176.8/issuetoc
Abbreviations
- ACG
angle‐closure glaucoma
- AQH
aqueous humour
- FP‐PGA
FP‐class PG agonist analogue
- IOP
intraocular pressure
- PGA
PG agonist
- POAG
primary open‐angle glaucoma
- RGC
retinal ganglion cell
- TM
trabecular meshwork
Introduction
Glaucoma is a blinding disease characterized by the death of retinal ganglion cells (RGCs) and their axons, which are retinal neurons that transmit visual information from photoreceptors to the visual cortex of the brain (Sharif, 2017). The majority of patients suffer from primary open‐angle glaucoma (POAG): ‘primary’ because the cause is idiopathic and ‘open‐angle’ because there is sufficient space between the base of the iris and the trabecular meshwork (TM) (the iridocorneal angle) for aqueous humour (AQH) to drain from the anterior chamber of the eye. Secondary open‐angle glaucoma describes a disease developed secondary to readily identified factors, such as injury, iatrogenic intervention (e.g. steroid use) or pigment dispersion into the TM. Angle‐closure glaucoma (ACG) is characterized by blockage of AQH outflow due to contact of the iris root with the TM and cornea. Although many patients with chronic angle closure are asymptomatic, if angle closure becomes completely manifest, it is typically an acute‐onset medical crisis requiring immediate intervention. ACG is more prevalent in patients of East Asian heritage (Jonas et al., 2017).
Open‐angle glaucoma patients typically experience a gradual loss of peripheral vision until only a small area of central vision remains. A change in the appearance of the optic nerve head, formed in the back of the eye by RGC axons that exit the globe to form the optic nerve, is frequently associated with vision deterioration. An increase in the ratio of the diameter of the centre of the optic disc relative to the diameter of the entire disc (cupping) indicates a higher risk for disease progression (Jonas et al., 2017). Since an elevated intraocular pressure (IOP) – usually defined as a sustained measured IOP of ≥21 mmHg (Kniestedt et al., 2008) – has historically been the risk factor most strongly identified with disease progression, the standard of care has long been IOP reduction (Kass et al., 2002). This is even the case in normal‐tension glaucoma patients, who have IOP values within the normal range but still exhibit glaucomatous visual field loss (Mallick et al., 2016). IOP is determined by the balance between AQH production (also called inflow) in the ciliary body and its outflow through the TM (conventional outflow) and the uveoscleral tract (uveoscleral outflow). The uveoscleral tract is constituted by spaces between bundles of ciliary muscle. In normal human eyes, conventional outflow is responsible for most of the aqueous outflow facility (Carreon et al., 2017). IOP lowering can be achieved by either suppressing inflow or facilitating outflow. Since the AQH is the primary source of nutrition and oxygenation for the avascular cornea and lens and since high IOP is usually due to decreased outflow through the TM, it might be supposed that therapies increasing outflow would be preferred a priori. However, there is extensive clinical experience with several inflow‐reducing agents, several of which are the mainstays in current glaucoma treatment (see Table 1).
Table 1.
Current drug classes used for treating glaucoma/ocular hypertension
| Pharmacological class | Drug examples | Mechanism of action | Comments |
|---|---|---|---|
| α‐adrenoceptor agonist | Adrenaline, apraclonidine, brimonidine | AQH Inflow suppression and increase uveoscleral outflow | Adrenaline used historically; brimonidine widely used today |
| β‐adrenoceptor antagonists (β blockers) | Timolol, betaxolol, levobunolol | AQH Inflow suppression | Widely used; can induce bradycardia; contra‐indicated in asthmatics |
| Carbonic anhydrase inhibitors | Dorzolamide, brinzolamide | AQH Inflow suppression | Systemic acetazolamide and methazolamide used historically, currently used for acute IOP control instead of chronic therapy |
| ACh muscarinic receptor agonists | Pilocarpine, carbachol | Increase conventional outflow of AQH | The oldest medical therapy for glaucoma, use limited by four times a day dosing and ocular side effects |
| PG analogues | Latanoprost, travoprost, bimatoprost, tafluprost | Increase uveoscleral, and also conventional, outflow of AQH | The most widely used, most potent, and most efficacious drug class, additionally enabling once‐a‐day dosing |
Current drug therapies suppress AQH inflow and facilitate its outflow. Table 1 below lists the major pharmacological classes of topical ocular hypotensives (Realini, 2011). Topical application is preferred to systemic dosing in order to deliver the maximum concentration to the target tissue while minimizing systemic exposure and, thus, the risk of systemic adverse events. As with any self‐administered drug therapy, the lack of patient compliance is a major problem limiting medication effectiveness (Tsai, 2009). Minimal side effects and low required dosing frequency (ideally, once a day or less often) are two factors that should improve compliance (vide infra). FP‐class PG agonist analogues (FP‐PGAs) were a major advance in glaucoma medical therapy because of their once‐a‐day dosing, superior efficacy and minimal side effects.
Typically, an ophthalmologist starts a patient on an FP‐PGA to lower IOP. If the patient responds poorly, a second agent in a different pharmacological class is usually prescribed as an adjunctive therapy. The rationale behind combining classes with two different mechanisms is that the probability of additive efficacy is increased. Frequently, the adjunctive agent includes a fixed combination drug product where two inflow‐suppressing drugs of different classes (e.g. brimonidine + brinzolamide in Simbrinza®) are contained in a single formulation. Outside of the United States, there is the additional option of a fixed combination of a PG analogue with timolol; for example, a fixed combination of travoprost and timolol (Duotrav®) is available in the EU. The prescribing of a fixed combination helps to decrease treatment burden, reduce ocular side effects and thus increase patient compliance (Hollo et al., 2014; Newman‐Casey et al., 2015).
Patients who are inadequately controlled by topical ocular drug therapy usually progress to surgical intervention to improve outflow facility, which is beyond the scope of this discussion, but to which the reader is referred to several reviews (Jones et al., 2005; Vinod and Gedde, 2017; Zhou and Aref, 2017). Recently, miniature AQH outflow‐enhancing devices have allowed for more minimally invasive glaucoma surgeries (MIGS) and may be used in conjunction with cataract surgery to lower IOP (Richter and Coleman, 2016; Lavia et al., 2017).
Preclinical models used for FP‐PGA characterization
Typically, the first test systems used for evaluation of an FP‐PGA as a potential ocular hypotensive is testing for FP receptor affinity, functional potency and intrinsic activity (‘efficacy’) in vitro (Hellberg et al., 2001). Cells natively expressing FP receptors, such as A7r5 rat vascular smooth muscle cells (Griffin et al., 1998) or Swiss mouse 3T3 fibroblasts (Griffin et al., 1997; Sharif et al., 1998), are conveniently used. Functional efficacy can be determined by measuring the accumulation of [3H]‐inositol phosphates in the extracellular medium induced by a substance relative to that induced by a positive control full agonist such as fluprostenol or cloprostenol. Alternatively, mobilization of intracellular Ca2+ can also be used as a functional readout allowing rapid screening of compounds (Kelly et al., 2003). Note that the FP‐PGA carboxylic acids, not the prodrugs, are most usefully screened in these assays. Those FP‐PGA carboxylic acids with appropriate affinity and efficacy at the receptor are converted to the prodrug of interest and their ability to lower IOP and evoke ocular side effects are evaluated in animal models. Typically, topical ocular formulations are initially screened for in vivo potency and efficacy in a cat miosis model (Stjernschantz and Resul, 1992; Hellberg et al., 2001). Contraction of the cat iris sphincter is a well‐known FP receptor activation‐linked biological activity and isolated tissues can also be utilized for determining relative potency and efficacy in vitro (Sharif et al., 2008). These assays and models can provide a relatively quick readout for predicting potency in IOP‐lowering monkey models and thus for rank‐ordering preference for evaluation in them.
The side effect profile is often investigated in rabbits, due to their ready availability and sensitivity to ocular irritation. For example, a rabbit acute ocular irritation response model has been reported in which topical formulations of FP‐PGAs are rank ordered for their potency and efficacy in eliciting ocular adverse effects, such as conjunctival hyperaemia (red eye), swelling and discharge (Hellberg et al., 2001). These can be combined for a composite score or analysed separately. Conjunctival hyperaemia is the most typically reported ocular adverse effect of FP‐PGAs and so is frequently the basis for differentiating potential drug candidates for advancement. The final in vivo preclinical efficacy model used is typically IOP reduction in monkeys. Rabbits are apparently insensitive to the IOP‐lowering effects of FP‐PGAs and so are not useful for efficacy screening (Stjernschantz, 2001). Both sedated, normotensive (Resul et al., 1993) and unsedated (conscious) ocular hypertensive (Hellberg et al., 2001) monkeys have been used. In the latter group, ocular hypertension is induced by lasering the TM to induce a scarring response and so decrease outflow facility. This has the advantage of a larger signal‐to‐noise ratio to detect an IOP effect, as opposed to normotensive animals. Furthermore, since the monkeys are trained to accept the administration of eye drops and applanation tonometry for IOP measurement, there are fewer potential confounding effects than with the IOP values obtained during sedation.
The discovery and development of FP‐PGA therapy
The potential of PGs as therapeutic agents for glaucoma was suggested by the discovery of the ocular hypotensive effects of topically applied endogenous PGs, especially PGF2α and PGE2, in cats and monkeys (Stern and Bito, 1982). This was followed by several reports of significant IOP‐lowering effects of PGs in humans, using both topical (Giuffrè, 1985; Flach and Eliason, 1988; Villumsen et al., 1989) and systemic (Zajacz et al., 1976) administration. However, concerns about side effects deterred the clinical use of these compounds. Firstly, there was apprehension about the risk of blood‐aqueous barrier breakdown from chronic topical application of classic inflammatory mediators such as PGE2 (Vegge et al., 1975). Secondly, topical PG application typically produced a foreign‐body sensation and a feeling of grittiness in the eye (Giuffrè, 1985; Flach and Eliason, 1988; Villumsen et al., 1989), which would limit patient compliance. Thirdly, a pronounced initial IOP spike was frequently observed in both humans (Zajacz et al., 1976; Flach and Eliason, 1988) and rabbits (Green and Kim, 1975), which would be ill‐advised in glaucoma patients. Fourthly, PGs usually induce substantial conjunctival hyperaemia (Giuffrè, 1985; Flach and Eliason, 1988; Villumsen et al., 1989).
Pioneering investigations (Bito, 2001; Stjernschantz, 2001) provided insights that were key in progressing the promising but flawed PGF2α to the launching of the first FP‐PG analogue drug latanoprost. The first of these insights was the elucidation that in many early reports the ocular hypertensive and pro‐inflammatory effects of PGs were likely confounded by the invasive techniques used to introduce these agents (frequently, via a cannula inserted into the anterior chamber) and by the supra‐physiological concentrations used (Bito, 2001). In the rabbit (the most commonly used species in these studies) especially, these would induce a profound inflammatory effect with blood‐aqueous barrier breakdown that did not represent the clinical situation in which these agents might be used.
Second was the recognition that the carboxylic acid moiety of the endogenous PGs had a poor absorption, disposition and metabolism profile for penetration from the ocular surface to the AQH and then to ciliary muscle and TM (the presumed sites of action) due to the ionization of the acid at physiological pH. This necessitated the use of topical ocular doses as large as 0.5 mg in order to obtain an AQH PG concentration sufficient to lower IOP. At these relatively high doses, activation of PG receptors in the corneal epithelium (the most highly innervated tissue in the body) and in conjunctival blood vessels was likely responsible for the ocular neurosensory and vasodilator side effects. Use of an ester prodrug greatly improved the PG corneal penetration to the AQH, improving the potency while maintaining efficacy. The much lower concentration of the active pharmaceutical ingredient (API) used caused fewer ocular side effects (Bito, 2001; Stjernschantz, 2001).
Third was the appreciation that the side effects of the endogenous PGs and their ester prodrugs were likely due in a large part to binding to multiple PG receptor subtypes. For example, PGF2α has been reported to bind with similar affinity to the EP3 as to its cognate FP receptor (Sharif et al., 2003a). In a series of key medicinal chemistry and pharmacology studies, refashioning of the carbon number 18‐carbon number 20 n‐propyl termination of PGF2α isopropyl ester to a phenyl group and saturation of the double bond between carbons 13 and 14 afforded a compound, latanoprost, which induced potent constriction of the cat iris pupil, sustained IOP‐lowering in monkeys and dramatically reduced conjunctival hyperaemia in rabbits and ocular irritation in cats. Additionally, the free acid form of latanoprost was reportedly as potent and efficacious as PGF2α in an FP receptor‐linked ex vivo functional assay, but with reduced potency and efficacy at EP PG subtype receptors (EP1, EP2 and EP3). Confirmation of its efficacy and benign side effect profile in humans led to the approval and launch of Xalatan® as the first marketed PG analogue‐containing drug product in the United States (Stjernschantz, 2001). Figure 1 summarizes these structural features of latanoprost that enhance its therapeutic utility compared to PGF2α.
Figure 1.
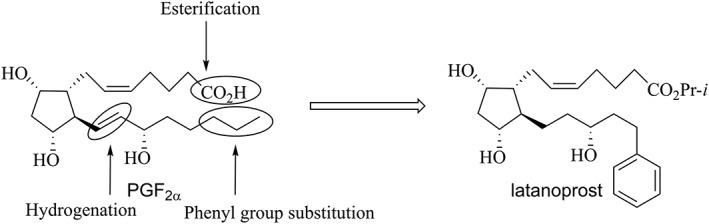
Structural modification of PGF2α leading to latanoprost
The clinical utility of PG analogues
The introduction of PG analogues starting in the mid‐1990's revolutionized glaucoma therapy, and these are now the first‐line agents representing the standard of care for combating ocular hypertension associated with POAG. As compared to other drugs widely used to lower IOP, such as the β‐adrenoceptor blocker timolol and the α‐adrenoceptor agonist brimondine, they have demonstrated superior average IOP‐lowering efficacy (25–35% vs. 20–25% IOP reduction for other classes), higher potency (0.0015–0.03% vs. 0.1–2%) and longer duration of action allowing once‐a‐day topical ocular dosing (vs. twice to four times a day) (Cheema et al., 2016). To illustrate this last point, PG analogue treatment has been reported to lower IOP for as long as 84 h after cessation of dosing in humans (Dubiner et al., 2004). Minimization of the large diurnal IOP variation frequently observed in glaucoma patients, which is an important disease progression risk factor, has been confirmed in other studies (Parrish et al., 2003). The profound impact of PG analogue therapy on medical practice is suggested by a report that an increase in the dispensing of PG analogues strongly correlated with an almost 20% reduction in glaucoma filtration surgery rate in Ontario, Canada, from 1996 through to 2004 (Rachmiel et al., 2006).
The four FP‐PGAs approved for use in the United States, the EU and Japan as of late 2017 are latanoprost (Digiuni et al., 2012), travoprost (Quaranta et al., 2015), bimatoprost (Lee et al., 2017) and tafluprost (Keating, 2016) (Figure 2). These are widely thought to be prodrugs, with their biomolecular mechanism of action believed to be activation of the PG FP receptor by their derivative, free carboxylic acid moiety. However, there is controversy with respect to the mechanism of action for bimatoprost, with some hypothesizing that it per se activates a new cognate ‘prostamide’ receptor (Woodward et al., 2013). Others have contended that like an isopropyl ester, the amide functions as a prodrug, with the carboxylic acid liberated from enzymatic hydrolysis (a well‐known potent FP agonist) being the active species (Camras et al., 2008; Sharif et al., 2002; Sharif and Klimko, 2009).
Figure 2.
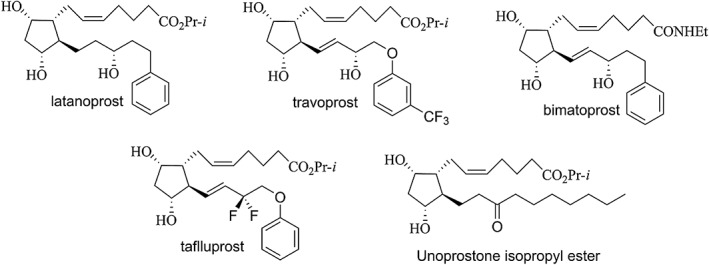
Structures of PG analogues used clinically for reducing IOP.
A fifth FP‐PGA‐like compound, unoprostone isopropyl ester (Figure 2), was approved in Japan in 1994 and in the United States in 2000, albeit with drug products having two different API concentrations: 0.12% in Japan and 0.15% in the United States. Although it is structurally similar to the PG analogues, it is less efficacious in humans (Jampel et al., 2002), and its carboxylic acid derivative has significantly lower functional potency and efficacy at the FP receptor, being a low‐efficacy partial agonist (Sharif et al., 2003b). Activation of calcium‐activated potassium channels, possibly by both the ester per se and by its carboxylic acid derivative, has been suggested as the major biomolecular mechanism of action of unoprostone (Cuppoletti et al., 2007).
Four of the five FP‐PGAs used clinically are also available outside of the United States as a fixed combination with the β‐blocker timolol: Xalacom® or Xalcom® (contains latanoprost), Duotrav® (contains travoprost), Ganfort® (contains bimatoprost) and Tapcom® (Japan)/Taptiqom® (EU) (contains tafluprost). As mentioned previously, these fixed combinations aim to improve compliance for those patients requiring two medications to adequately control their IOP. Additional clinical trials have investigated fixed combinations of PG analogues with approved ocular hypotensive agents from other classes as shown in Table 2; however, as of late 2017, none have yet been approved in any jurisdiction.
Table 2.
PG analogue‐containing fixed‐dose combination product evaluated in clinical trials
| PG analogue | Other ocular hypotensive (Class) | Phase | Trial number at www.clinicaltrials.gov website |
|---|---|---|---|
| Bimatoprost | Brimonidine (α adrenoceptor agonist)) | 2 | NCT01863953 |
| Travoprost | Brinzolamide (carbonic anhydrase inhibitor) | 3 | NCT00767494 |
| Travoprost | AR‐12286 (rho kinase inhibitor) | 2 | NCT01789736 |
| Latanoprost | Dorzolamide (carbonic anhydrase inhibitor) | 2 | NCT01896180 |
Future directions in PG‐based therapy
One future direction under clinical investigation is a dual pharmacophore PG‐FPA, where affinity at an additional PG receptor is rationally added. The hypothesis is that activation of the additional PG receptor can afford additional efficacy without increasing side effects. The most advanced example in this class is ONO‐9054 (Sepetaprost; Figure 3), a prodrug whose free carboxylic acid derivative is a dual FP/EP3 receptor agonist. Results from phase 1 single‐ and multiple‐dose escalation (Harris et al., 2016) and phase 2a (Miller‐Ellis et al., 2017) studies suggest possible enhanced efficacy accompanied by increased conjunctival hyperemia incidence, as compared to Xalatan®. A phase 2b study started in mid‐2017 (NCT03216902 at clinicaltrials.gov).
Figure 3.
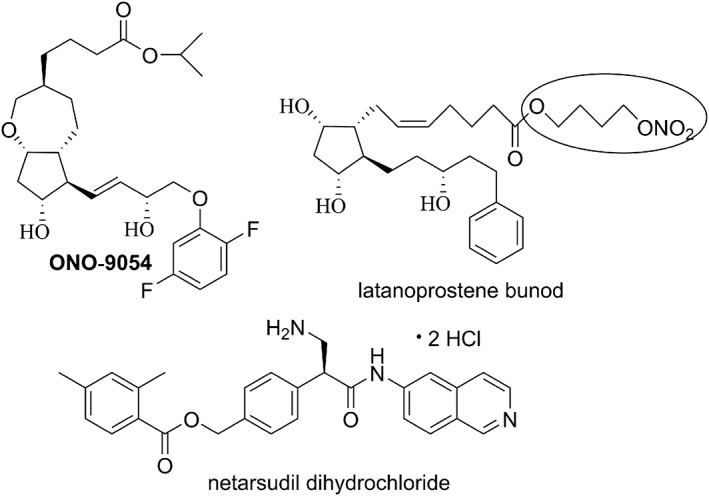
Structures of ONO‐9054, latanoprostene bunod (highlighting the NO‐donating group) and netarsudil dihydrochloride
Another direction being explored is the addition of a novel IOP‐lowering mechanism, either in the molecule itself or in a fixed combination (two separate molecules), to one of the marketed PG analogues. An example of the former is latanoprostene bunod (Figure 3), in which the isopropyl ester of latanoprost is replaced with a 4‐nitroxybutyl ester. After topical ocular dosing, ocular esterases liberate latanoprost acid plus 4‐nitroxybutanol (instead of isopropyl alcohol). The latanoprost acid liberated is thought to bind to FP receptors in the target tissue (likely the ciliary muscle, the TM and the sclera), up‐regulate matrix metalloproteinase release and enhance extracellular matrix digestion to improve uveoscleral outflow. The 4‐nitroxybutanol component is metabolized to nitric oxide, a gaseous molecule thought to have an endogenous role in maintaining IOP homeostasis (Dismuke et al., 2009). It is thought that NO increases conventional outflow by activating soluble guanyl cyclase, thus increasing production of cGMP, with downstream cytoskeletal relaxation in the TM and Schlemm's canal (Kaufman, 2017). Thus, in principle, the two different outflow pathways can be enhanced simultaneously. In phase 3 trials, a drug product containing 0.024% API dosed once a day was superior at all but one of the time points to the 0.5% timolol drug product dosed twice a day (Medeiros et al., 2016; Weinreb et al., 2016). The FDA approved this drug product, brand name Vyzulta™, in late 2017. It is interesting that timolol was used as comparator, given that Xalatan® was used as comparator in a phase 2b trial, in which the 0.024% dose of latanprostene bunod was reported to afford a significantly greater reduction in mean diurnal IOP at day 28 (the end of the study) (Weinreb et al., 2015).
Note that the increased concentration of latanoprostene bunod in the drug product investigated (0.024%) compared to that for latanoprost in Xalatan® (0.005%) translates to about a fourfold increased concentration of the bioactive carboxylic acid. This may have been designed to maximize the probability that the concentration of 4‐nitroxybutanol liberated upon enzymatic hydrolysis would be high enough to release sufficient NO for a reduction in IOP to be observed. Intriguingly, a diminished IOP response has been reported when the daily dose of latanoprost is increased (achieved by increasing the API concentration or increasing the dose frequency) from the clinically used 0.005% once‐a‐day regimen (Lindèn and Alm, 1998). This may be due to desensitization of the FP receptors. A more widespread use of Vyzulta™ in clinical practice will determine whether the increased latanoprost concentration in this medication changes its well‐known efficacy and side effect profile, seen with Xalatan®.
An example of a combination drug using an agent with a novel mechanism of action is the fixed combination drug product RoclatanTM, a fixed combination of 0.02% of the rho kinase inhibitor/noradrenaline transport inhibitor netarsudil dihydrochloride (Figure 3) and 0.005% latanoprost. It was reported to afford superior efficacy in phase 3 trials compared to its constituents dosed as monotherapies. However, a disadvantageous increased adverse event rate versus latanoprost monotherapy was reported, including an increased rate of hyperaemia and conjunctival haemorrhage (Aerie Pharmaceuticals, 2017). These adverse events might be expected based on the well‐known vasodilator properties of rho kinase inhibitors. Additional conjugates of FP‐PGAs and other IOP‐lowering agents have recently been described (Ellis et al., 2017).
Another approach that is being investigated is sustained‐release therapy. Since, as mentioned previously, lack of patient compliance (Newman‐Casey et al., 2015) with daily dosing of eye drops is likely a major contributor to suboptimal effectiveness of glaucoma drug therapy, sustained release therapies aim to relieve the drug administration burden for the patient and provide continuous drug exposure. In principle, these can be delivered in an ophthalmologist's office and replaced on a regular basis. Several clinically evaluated approaches are listed in Table 3. As of late 2017, the most advanced therapy in development appears to be a bimatoprost‐loaded biodegradable rod that is injected intracamerally, which is currently recruiting for phase 3 trials. Results from a phase 2 trial have been published (Lewis et al., 2017), showing it has sustained efficacy in a majority of patients up to 6 months after implantation.
Table 3.
PG analogue sustained release combinations evaluated clinically
| PG analogue | Sustained release platform | Highest phase (www.clinicaltrials.gov trial number) | Comment |
|---|---|---|---|
| Bimatoprost | Intracameral insert | Phase 3 recruiting (NCT02250651 and NCT02247804, timolol comparator; NCT02507687 and NCT02636946, laser trabeculoplasty comparator) | Phase 2 results published (Lewis et al., 2017) |
| Bimatoprost | Peri‐ocular ring | Phase 2 completed (NCT02358369) | – |
| Latanoprost | Subconjunctival insert | Phase 1 terminated (NCT01180062) | – |
| Latanoprost | Subconjunctival insert | Phase 2 ongoing (NCT02129673) | – |
| Latanoprost | Punctal plug | Phase 2 completed (NCT01481077) | – |
| Travoprost | Intracameral implant | Phase 2 recruiting (NCT02371746) | – |
| Travoprost | Punctal plug | Phase 3 recruiting (NCT02914509) | Phase 2 feasibility study Asian cohort results published (Perera et al., 2016) |
| Travoprost | Intraocular implant | Phase 2 ongoing (NCT02754596) | – |
Conclusions
Appropriate chemical modifications of the endogenous FP receptor agonist, PGF2α, have been necessary in order to produce FP‐PGAs with much reduced ocular side effects without losing ocular hypotensive efficacy. This drug discovery campaign required almost two decades of medicinal chemistry and ocular biology research. However, the fruits of such dedicated efforts have culminated in FP‐PGA drugs that are now first‐line therapeutics for treating ocular hypertension and POAG. Whilst much progress has been made to reduce the visual impairment caused by this ocular disease, preservation of peripheral vision requires more effort, and more potent and efficacious IOP‐lowering drugs are indicated. Sadly, since even the ocular normotensive patients continue to experience vision loss, the need to directly protect the RGCs and their axon by so‐called neuroprotective agents is warranted and exceedingly important. The next generation of drugs should endeavour to have poly‐pharmacology embedded in their chemical structures (or be novel conjugates) whereby both IOP‐lowering and appropriate neuroprotective drug activities can be delivered to the patients' eyes. Such drugs are eagerly awaited by physicians and POAG patients around the world.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017a, 2017b, 2017c, 2017d).
Conflict of interest
The authors declare no conflict of interest.
Klimko, P. G. , and Sharif, N. A. (2019) Discovery, characterization and clinical utility of prostaglandin agonists for the treatment of glaucoma. British Journal of Pharmacology, 176: 1051–1058. 10.1111/bph.14327.
References
- Aerie Pharmaceuticals (2017). Aerie Pharmaceuticals Reports Positive Roclatan™ (netarsudil/latanoprost ophthalmic solution) 0.02%/0.005% phase 3 12‐month topline safety results. Available at http://investors.aeriepharma.com/releasedetail.cfm?ReleaseID=1033691 (accessed 28th July 2017).
- Alexander SPH, Christopoulos A, Davenport AP, Kelly E, Marrion NV, Peters JA et al (2017a). The Concise Guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. Br J Pharmacol 174: S17–S129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Striessnig J, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017b). The Concise Guide to PHARMACOLOGY 2017/18: voltage‐gated ion channels. Br J Pharmacol 174: S160–S194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Marrion NV, Peters JA, Faccenda E, Harding SD et al (2017c). The Concise Guide to PHARMACOLOGY 2017/18: transporters. Br J Pharmacol 174: S360–S446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017d). The Concise Guide to PHARMACOLOGY 2017/18: enzymes. Br J Pharmacol 174: S272–S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bito LZ (2001). A new approach to the medical management of glaucoma, from the bench to the clinic, and beyond: the proctor lecture. Invest Ophthalmol Vis Sci 42: 1126–1133. [PubMed] [Google Scholar]
- Camras CB, Sharif NA, Wax MB, Stjernshantz J (2008). Bimatoprost, the prodrug of a prostaglandin analogue. Br J Ophthalmol 92: 862–863. [PubMed] [Google Scholar]
- Carreon TA, Edwards G, Wang H, Bhattacharya SK (2017). Segmental outflow of aqueous humor in mouse and human. Exp Eye Res 158: 59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheema A, Chang RT, Shrivastava A, Singh K (2016). Update on the medical treatment of primary open‐angle glaucoma. Asia‐Pacific J Ophthalmol (Philadelphia) 5: 51–58. [DOI] [PubMed] [Google Scholar]
- Cuppoletti J, Malinowska DH, Tewari KP, Chakrabarti J, Ueno R (2007). Cellular and molecular effects of unoprostone as a BK channel activator. Biochim Biophys Acta 1768: 1083–1092. [DOI] [PubMed] [Google Scholar]
- Digiuni M, Fogagnolo P, Rossetti L (2012). A review of the use of latanoprost for glaucoma since its launch. Exp Opin Pharmacother 13: 723–745. [DOI] [PubMed] [Google Scholar]
- Dismuke WM, Sharif NA, Ellis DZ (2009). Human trabecular meshwork cell volume decrease by NO‐independent soluble guanylate cyclase activators YC‐1 and BAY‐58‐2667 involves the BKCa ion channel. Invest Ophthalmol Vis Sci 50: 3353–3359. [DOI] [PubMed] [Google Scholar]
- Dubiner HB, Sircy MD, Landry T, Bergamini MV, Silver LH, Darell Turner F et al (2004). Comparison of the diurnal ocular hypotensive efficacy of travoprost and latanoprost over a 44‐hour period in patients with elevated intraocular pressure. Clin Ther 26: 84–91. [DOI] [PubMed] [Google Scholar]
- Ellis D, Scheibler L, Sharif NA (2017). Prostaglandin conjugates and derivatives for treating glaucoma and ocular hypertension. US Patent 9604949 B2
- Flach AJ, Eliason JA (1988). Topical prostaglandin E2 effects on normal human intraocular pressure. J Ocul Pharmacol 4: 13‐18 (Erratum in J Ocul Pharmacol 1991: 7: 189). [DOI] [PubMed]
- Giuffrè G (1985). The effects of prostaglandin F2 alpha in the human eye. Graefes Arch Clin Exp Ophthalmol 222: 139–141. [DOI] [PubMed] [Google Scholar]
- Green K, Kim K (1975). Pattern of ocular response to topical and systemic prostaglandin. Invest Ophthalmol 14: 36–40. [PubMed] [Google Scholar]
- Griffin BW, Williams GW, Crider JY, Sharif NA (1997). FP prostaglandin receptors mediating inositol phosphates generation and calcium mobilization in Swiss 3T3 Cells: a pharmacological study. J Pharmacol Exp Ther 281: 845–854. [PubMed] [Google Scholar]
- Griffin BW, Magnino P, Pang I‐H, Sharif NA (1998). Pharmacological characterization of an FP prostaglandin receptor on rat vascular smooth muscle cells (A7r5) coupled to phosphoinositide turnover and intracellular calcium mobilization. J Pharmacol Exp Ther 286: 411–418. [PubMed] [Google Scholar]
- Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S et al (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A, Ward CL, Rowe‐Rendleman CL, Ouchi T, Wood A, Fujii A et al (2016). Ocular hypotensive effect of ONO‐9054, an EP3/FP receptor agonist: results of a randomized, placebo‐controlled, dose escalation study. J Glaucoma 25: e826–e833. [DOI] [PubMed] [Google Scholar]
- Hellberg MR, Sallee VL, McLaughlin MA, Sharif NA, DeSantis L, Dean TR et al (2001). Preclinical efficacy of travoprost, a potent and selective FP prostaglandin receptor agonist. J Ocul Pharmacol Ther 17: 421–432. [DOI] [PubMed] [Google Scholar]
- Hollo G, Topouzis F, Fechtner RD (2014). Fixed‐combination intraocular pressure‐lowering therapy for glaucoma and ocular hypertension: advantages in clinical practice. Expt Opin Pharmacother 15: 1737–1747. [DOI] [PubMed] [Google Scholar]
- Jampel HD, Bacharach J, Sheu WP, Wohl LG, Solish AM, Christie W et al (2002). Randomized clinical trial of latanoprost and unoprostone in patients with elevated intraocular pressure. Am J Ophthalmol 134: 863–871. [DOI] [PubMed] [Google Scholar]
- Jonas JB, Aung T, Bourne RR, Bron AM, Ritch R, Panda‐Jonas S (2017). Glaucoma. Lancet 390: 2183–2193. [DOI] [PubMed] [Google Scholar]
- Jones E, Clarke J, Khaw PT (2005). Recent advances in trabeculectomy technique. Curr Opin Ophthalmol 16: 107–113. [DOI] [PubMed] [Google Scholar]
- Kass MA, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP et al (2002). The ocular hypertension treatment study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open‐angle glaucoma. Arch Ophthamol 120: 701–713. [DOI] [PubMed] [Google Scholar]
- Kaufman PL (2017). Latanoprostene bunod ophthalmic solution 0.024% for IOP lowering in glaucoma and ocular hypertension. Exper Opin Pharmacother 18: 433–444. [DOI] [PubMed] [Google Scholar]
- Keating GM (2016). Tafluprost ophthalmic solution 0.0015%: a review in glaucoma and ocular hypertension. Clin Drug Invest 36: 499–508. [DOI] [PubMed] [Google Scholar]
- Kelly CR, Williams GW, Sharif NA (2003). Real‐time intracellular Ca2+‐mobilization by travoprost acid, bimatoprost, unoprostone and other analogs via endogenous mouse, rat and cloned human FP prostaglandin receptors. J Pharmacol Exp Ther 304: 238–245. [DOI] [PubMed] [Google Scholar]
- Kniestedt C, Punjabi O, Lin S, Stamper RL (2008). Tonometry through the ages. Surv Ophthamol 53: 568–591. [DOI] [PubMed] [Google Scholar]
- Lavia C, Dallorto L, Maule M, Ceccarelli M, Fea AM (2017). Minimally invasive glaucoma surgeries (MIGS) for open angle glaucoma: a systematic review and meta‐analysis. PLoS One 12: e0183142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D, Mantravadi AV, Myers JS (2017). Patient considerations in ocular hypertension: role of bimatoprost ophthalmic solution. Clin Ophthalmol 11: 1273–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindèn C, Alm A (1998). Latanoprost twice daily is less effective than once daily: indication of receptor subsensitivity? Curr Eye Res 17: 567–572. [PubMed] [Google Scholar]
- Lewis RA, Christie WC, Day DG, Craven ER, Walters T, Bejanian M et al (2017). Bimatoprost sustained‐release implants for glaucoma therapy: 6‐month results from a phase I/II clinical trial. Am J Ophthalmol 175: 137–147. [DOI] [PubMed] [Google Scholar]
- Mallick J, Devi L, Malik PK (2016). Update on normal tension glaucoma. J Ophthalmol Vis Res 11: 204–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros FA, Martin KR, Peace J, Scassellati Sforzolini B, Vittitow JL, Weinreb RN et al (2016). Comparison of latanoprostene bunod 0.024% and timolol maleate 0.5% in open‐angle glaucoma or ocular hypertension: the LUNAR study. Am J Ophthalmol 168: 250–259. [DOI] [PubMed] [Google Scholar]
- Miller‐Ellis EG, Berlin MS, Ward CL, Sharpe JA, Jamil A, Harris A (2017). Ocular hypotensive effects and tolerability of the novel dual EP3/FP receptor agonist ONO‐9054 vs. Xalatan®: results of a 28‐day, double‐masked, randomized study. Br J Ophthalmol 101: 796–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman‐Casey PA, Robin AL, Blachley T, Farris K, Heisler M, Resnicow K et al (2015). The most common barriers to glaucoma medication adherence: a cross‐sectional survey. Ophthalmology 122: 1308–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish RK, Palmberg P, She WP, for the XLT Study Group (2003). A comparison of latanoprost, bimatoprost, and travoprost in patients with elevated intraocular pressure: a 12‐week, randomized, masked‐evaluator multicenter study. Am J Ophthalmol 135: 688–703. [DOI] [PubMed] [Google Scholar]
- Perera SA, Ting DS, Nongpiur ME, Chew PT, Aquino MC, Sng C et al (2016). Feasibility study of sustained‐release travoprost punctum plug for intraocular pressure reduction in an Asian population. Clin Ophthalmol 10: 757–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaranta L, Riva I, Katsanos A, Floriani I, Centofanti M, Konstas AG (2015). Safety and efficacy of travoprost solution for the treatment of elevated intraocular pressure. Clin Ophthamol 9: 633–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachmiel R, Trope GE, Chipman ML, Gouws P, Buys YM (2006). Effect of medical therapy on glaucoma filtration surgery rates in Ontario. Arch Ophthalmol 124: 1472–1477. [DOI] [PubMed] [Google Scholar]
- Realini T (2011). A history of glaucoma pharmacology. Optomet Vis Sci 88: 36–38. [DOI] [PubMed] [Google Scholar]
- Resul B, Stjernschantz J, No K, Liljebris C, Selén G, Astin M et al (1993). Phenyl‐substituted prostaglandins: potent and selective antiglaucoma agents. J Med Chem 36: 243–248. [DOI] [PubMed] [Google Scholar]
- Richter GM, Coleman AL (2016). Minimally invasive glaucoma surgery: current status and future prospects. Clin Ophthalmol 10: 189–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif NA, Klimko P (2009). Update and commentary on the prodrug bimatoprost and a putative prostamide receptor. Expert Rev Ophthalmol 4: 477–489. [Google Scholar]
- Sharif NA, Williams GW, Xu SX, Crider JY, Griffin BW, Davis TL (1998). Pharmacological analysis of [3H]PGE1 / [3H]PGE2 and [3H]PGF2α binding in bovine corpus luteum: identification of EP3 and FP prostaglandin receptors and correlation with functional data. J Pharmacol Exp Ther 286: 1094–1102. [PubMed] [Google Scholar]
- Sharif NA, Kelly CR, Crider JY (2002). Agonist activity of bimatoprost, travoprost, latanoprost, unoprostone isopropyl ester and other prostaglandin analogs at the cloned human ciliary body FP prostaglandin receptor. J Ocular Pharmacol Ther 18: 313–324. [DOI] [PubMed] [Google Scholar]
- Sharif NA, Kelly CR, Crider JY (2003a). Human trabecular meshwork cell responses induced by bimatoprost, travoprost, unoprostone, and other FP prostaglandin receptor agonist analogues. Invest Ophthalmol Vis Sci 44: 715–721. [DOI] [PubMed] [Google Scholar]
- Sharif NA, Kelly CR, Crider JY, Williams GW, Xu SX (2003b). Ocular hypotensive FP prostaglandin (PG) analogs: PG receptor subtype binding affinities and selectivities, and agonist potencies at FP and other PG receptors in cultured cells. J Ocular Pharmacol Ther 19: 501–515. [DOI] [PubMed] [Google Scholar]
- Sharif NA, Kaddour‐Djebbar I, Abdel‐Latif AA (2008). Cat iris sphincter smooth‐muscle contraction: comparison of FP‐class prostaglandin analog agonist activities. J Ocul Pharmacol Ther 24: 152–163. [DOI] [PubMed] [Google Scholar]
- Sharif NA (2017). Ocular hypertension and glaucoma: a review and current perspectives. Int J Ophthalmol Vis Sci 2: 22–36. [Google Scholar]
- Stern FA, Bito LZ (1982). Comparison of the hypotensive and other ocular effects of prostaglandins E2 and F2, on cat and rhesus monkey eyes. Invest Ophthalmol Vis Sci 22: 588–598. [PubMed] [Google Scholar]
- Stjernschantz J, Resul B (1992). Phenyl substituted prostaglandin analogs for glaucoma treatment. Drugs Fut 17: 691–704. [Google Scholar]
- Stjernschantz JW (2001). From PGF2α‐isopropyl ester to latanoprost: a review of the development of Xalatan®: the Proctor Lecture. Invest Ophthalmol Vis Sci 42: 1134–1145. [PubMed] [Google Scholar]
- Tsai JC (2009). A comprehensive perspective on patient adherence to topical glaucoma therapy. Ophthalmol 116 (Supplement): S30–S36. [DOI] [PubMed] [Google Scholar]
- Vegge T, Neufeld AH, Sears ML (1975). Morphology of the breakdown of the blood‐aqueous barrier in the ciliary processes of the rabbit eye after prostaglandin E2 . Invest Ophthalmol 14: 33–36. [PubMed] [Google Scholar]
- Villumsen J, Alm A, Söderström M (1989). Prostaglandin F2 alpha‐isopropyl ester eye drops: effect on intraocular pressure in open‐angle glaucoma. Br J Ophthalmol 73: 975–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinod K, Gedde SJ (2017). Clinical investigation of new glaucoma procedures. Curr Opin Ophthalmol 28: 187–193. [DOI] [PubMed] [Google Scholar]
- Weinreb RN, Ong T, Scassellati SB, Vittitow JL, Singh K, Kaufman PL et al (2015). A randomised, controlled comparison of latanoprostene bunod and latanoprost 0.005% in the treatment of ocular hypertension and open angle glaucoma: the VOYAGER study. Ophthalmol 99: 738–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreb RN, Scassellati S, Forzolin IB, Vittitow J, Liebmann J (2016). Latanoprostene bunod 0.024% versus timolol maleate 0.5% in subjects with open‐angle glaucoma or ocular hypertension: the APOLLO study. Ophthalmol 123: 965–973. [DOI] [PubMed] [Google Scholar]
- Woodward DF, Wang JW, Poloso NJ (2013). Recent progress in prostaglandin F2α ethanolamide (prostamide F2α) research and therapeutics. Pharmacol Rev 65: 1135–1147. [DOI] [PubMed] [Google Scholar]
- Zajacz M, Torok M, Mocsary P (1976). Effect on human eye of prostaglandin and a prostaglandin analog used to induce abortion. IRCS Medical Science: Clinical Medicine: Library Compendium 4: 316 Chemical Abstracts Accession Number 85:72796. [Google Scholar]
- Zhou Y, Aref AA (2017). A review of selective laser trabeculoplasty: recent findings and current perspectives. Ophthalmol Ther 6: 19–32. [DOI] [PMC free article] [PubMed] [Google Scholar]


