Abstract
Background and Purpose
Acute kidney injury (AKI) is a rapid renal dysfunctional disease, for which no effective drugs or therapies are available to improve prognosis. Loganetin is a natural product with unknown bioactivities. Here, we identified a new protective effect and mechanism of Loganetin in a mouse model of AKI induced by rhabdomyolysis.
Experimental Approach
AKI was induced using glycerol by i.m. injection in mice models. Thirty minutes and 24 and 48 hr after injection of glycerol, the mice received 2 and 18 mg·kg−1 of Loganetin i.p. respectively. Then mice blood and kidney were collected for various biochemical and histopathological studies. Mechanistic studies on modulation of AKI by Loganetin were performed using HK‐2 cells and Toll‐like receptor 4 (TLR4) knockout mice.
Key Results
In the Loganetin treated group, kidney damage and mortality rate were declined, and blood urea nitrogen and serum creatinine were much lower. Loganetin prevented damage to the tubular structures induced by glycerol and decreased apoptotic cells at the corticomedullary junction. In HK‐2 cells, Loganetin could inhibit NF‐κB pathway and pro‐apoptotic genes expression. However, TLR4 was silenced by a specific shRNA, and the inhibitory effect of Loganetin in HK‐2 cells vanished. Loganetin also down‐regulated the expression of inflammation factors by suppressing TLR4 activity.
Conclusion and Implications
All the results suggested that TLR4 plays a critical role in AKI development, and Loganetin ameliorates AKI by inhibiting TLR4 activity and blocking the JNK/p38 pathway, which provides a new strategy for AKI treatment.
Abbreviations
- AKI
acute kidney injury
- ATN
acute tubular necrosis
- BUN
blood urea nitrogen
- C‐cas‐3
cleaved caspase‐3
- CKD
chronic kidney disease
- CREA
serum creatinine
- PAS
periodic acid‐Schiff
- RIAKI
rhabdomyolysis‐induced AKI
- TLR4
Toll‐like receptor 4
What is already known
Loganetin is a natural small molecular product that has been shown to inhibit inflammation and protect the kidney from diabetic nephropathy.
What this study adds
Loganetin displays anti‐inflammatory and anti‐apoptosis effects in the mouse kidney both in vitro and in vivo, and these are mediated by its suppression of the function of TLR4.
What is the clinical significance
TLR4 has a critical role in the development of AKI and this study provides important data for the rational use of Loganetin for treating AKI in the clinic.
1. INTRODUCTION
Acute kidney injury (AKI) is a rapid renal dysfunctional disease with severe tubular damage and is a global health problem, with more than 10 million people affected annually (Bellomo, Kellum, & Ronco, 2012). There are many causes of this disease, including ischaemic, obstructive, toxic, and infectious pathogens (Bellomo et al., 2012). One important cause of AKI is sepsis (Uchino et al., 2005). LPS plays an important role in the pathogenesis of sepsis‐induced AKI (Xu et al., 2014). Therefore, LPS is used in models for experimental sepsis‐induced AKI. AKI is a common complication and affects approximately 20% of hospitalized patients in both developed and developing countries (Mehta et al., 2016). Recently, it was reported that almost 2 million people die of AKI every year, and the survivors have a high risk of chronic kidney disease (CKD; Li, Burdmann, & Mehta, 2013).
Although the pathophysiology of AKI is complex, we know that mitochondrial dynamics play important roles in AKI development. Mitochondrial dysfunction results in ROS generation, causing cellular damage via chemical modification of proteins, lipids, and nucleic acids (Valko, Jomova, Rhodes, Kuca, & Musilek, 2016). Patients with CKD or diabetes mellitus have increased steady‐state levels of oxidative stress and are thus at high risk of developing AKI (Arora & Singh, 2014; Okamura & Pennathur, 2015). Therefore, oxidative stress has been implicated as one of the principal drivers of renal injury of AKI. It has been reported that increasing mitochondrial function can improve AKI (Jesinkey et al., 2014). Another characteristic of AKI is nephron loss via tubular epithelial cell programmed cell death triggering an immune response. Renal ischaemia is one of the major causes of AKI. An acute inflammatory process leads to cytokine and chemokine production (Kim et al., 2013). Both innate and adaptive immune cells are involved in renal ischaemic‐induced AKI. During AKI, the expression of toll‐like receptor 2 (TLR2) and TLR4 are elevated in renal tubular epithelial cells (Jang, Ko, Wasowska, & Rabb, 2009).
TLRs, which belong to a superfamily of transmembrane receptors, recognize their respective ligands by molecular pattern recognition, which plays a key role in the innate immune system. Activation of TLRs induces the release of a series of cytokines and chemokines that attract inflammatory cells. TLR4, the receptor for endotoxin, is expressed on kidney microvascular endothelial cells and kidney tubular epithelial cells (El‐Achkar et al., 2006; Wu et al., 2007). Importantly, it has been demonstrated that activation of TLR4 on both kidney microvascular endothelial cells and kidney tubular epithelial cells increases acute tubular injury and kidney dysfunction in ischaemic AKI (Chen et al., 2011; Pulskens et al., 2008; Wu et al., 2007; Wu et al., 2010). The function of TLR4 is involved not only in the acute phase of injury in kidney disease but also in regulating kidney fibrosis and CKD (Souza et al., 2015). Inhibition of TLR4 signalling relieves symptoms in mouse models of unilateral ureteral obstruction (Campbell et al., 2011).
Loganetin is a natural product from cornus fruits. It is well known that Loganetin can be obtained from Loganin enzymatically (Nagatoshi, Terasaka, Nagatsu, & Mizukami, 2011). Although the biological functions of Loganetin have been little investigated, the activities of Loganin have been studied extensively. Loganin can attenuate oxidative stress in SH‐SY5Y cells by regulating JNK/p38 and ERK1/2 pathways (Kwon et al., 2011). It has also been shown that Loganin has neuroprotective effects in Parkinson's disease models (Yao et al., 2017). Recent studies have shown that Loganin inhibits inflammation and protects the kidney (Kim et al., 2015; Li et al., 2016; Liu et al., 2015; Zhao et al., 2016). However, whether Loganetin can modulate inflammation in AKI is not known.
There is currently no effective treatment for AKI. In the present study, we evaluated the effects of Loganetin on an AKI mouse model and explored its effects on TLR4 expression and the inflammation‐associated NF‐κB pathway. This research offers a new strategy for AKI treatment.
2. METHODS
2.1. Animals and rhabdomyolysis‐induced AKI model
C57/BL6J mice (RRID:IMSR_JAX:000664, male, 6–8 weeks old, 18–22 g, n = 40) were purchased from Vital River Laboratory (Vital River Laboratory Animal Technology, Beijing, China). These mice were randomly divided into four groups, 10 mice per group. Thirty TLR4 knockout (TLR4‐KO) mice C57BL/10ScNJ (RRID:IMSR_JAX:003752, male, 8 weeks old, 18–22 g) and 30 TLR4 wild‐type (TLR4‐WT) mice C57BL/10ScSn (RRID:IMSR_JAX:000476, male, 8 weeks old, 18–22 g) were obtained from the Model Animal Research Centre of Nanjing University (Nanjing, Jiangsu, China). Mice were randomly divided into three groups, 10 mice per group. All animals were housed in the centre for new drug safety evaluation of Lunan Pharmaceutical (Lunan Pharmaceutical Group Co., Ltd., Linyi, China) under a pathogen‐free, temperature‐controlled environment with a 12‐hr light/dark cycle. The animals were supplied with pellet chow and water ad libitum. Mice were kept group‐housed at five per cage in specific pathogen free (SPF) with corncob bedding. During the whole experiment, animal welfare was ensured. All experiments were in accordance with the experimental protocols previously approved by the Institute's Animal Ethics Committee at the Pharmacological centre of Lunan Pharmaceutical Group Co., Ltd., and were performed in accordance with the U.S. National Institutes of Health Guidelines for Care and Use of Laboratory Animals (National Research Council [U.S.] Committee for the Update of the Guide for the Care and Use of Laboratory Animals). Humane endpoints were deployed according to the report of Ashall and Millar (2014). Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010) and with the recommendations made by the British Journal of Pharmacology.
To achieve the rhabdomyolysis‐induced AKI (RIAKI) model in mice, 50% glycerol was i.m. injected (10 ml·kg−1) into the hind limbs of Balb/c mice. To investigate the possible protective effect of Loganetin against AKI, 40 mice were randomly divided into four groups, in the control (a) and model (b) groups, and 10 mice in each group underwent i.m. injected 50% glycerol or equal‐volume saline respectively. In treatment group, 2 (c) or 18 mg·kg−1 (d) of Loganetin was administered i.p. 30 min and 24 and 48 hr after glycerol injection, and mice were anaesthetized using isoflurane (Lunan Pharmaceutical Group Co., Ltd., Linyi, Shan Dong Province, China) with an anaesthetic machine (model: SN‐487, SHINANO MFG Co., Ltd., Japan) and killed at 24, 48, and 72 hr respectively. Blood and kidney tissues were collected for subsequent analysis. Finally, the animals were killed by abdominal aorta bleeding. The experimental protocol is summarized in Figure 1. To determine whether it is possible protective effects of Loganetin on the kidney occurred via TLR4, C57BL/10ScNJ (TLR4‐KO, n = 30) and C57BL/10ScSn (TLR4‐WT, n = 30) mice were administered saline as control group or 50% glycerol with or without Loganetin (18 mg·kg−1) treatment, 10 mice per group, and samples were collected at 72 hr after glycerol injection.
Figure 1.
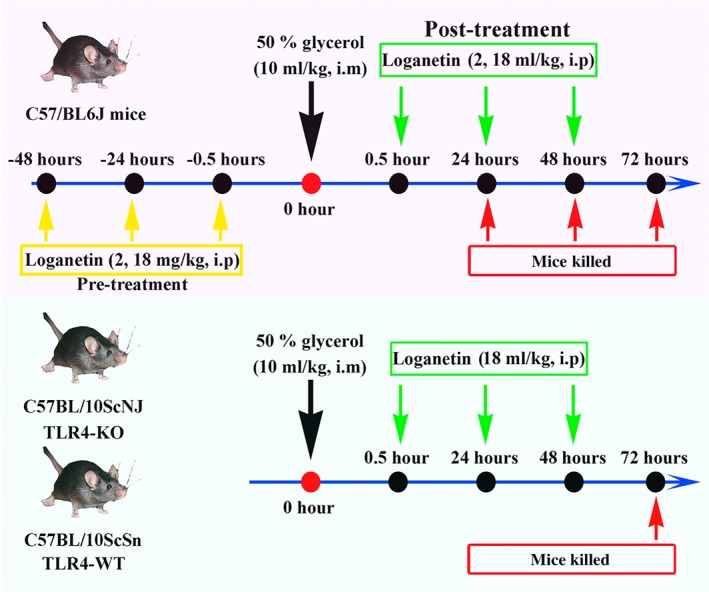
Experimental protocol for the induction of rhabdomyolysis‐induced acute kidney injury in mice; 50% glycerol was administered i.m. into the hind limbs of mice at a dose of 10 ml·kg−1. Saline‐injected mice were used as controls. To determine the protective effect of Loganetin, 2 or 18 mg·kg−1 of body weight of Loganetin or PBS was administered i.p. at the indicated time points (0.5, 24, and 48 hr) post‐acute kidney injury; 24, 48, and 72 hr after glycerol injection, mice were killed for blood and kidney samples
2.2. Renal function, histology, and TUNEL assay
To determine the kidney injury induced by glycerol and protective effects of Loganetin, serum creatinine (CREA) and blood urea nitrogen (BUN), indicators of kidney function, were evaluated using QuantiChrom Creatinine and UREA Assay Kits (BioAssay Systems, Hayward, CA) according to methods described previously (Press et al., 2017). To detect tissue injury, mice kidney tissues were fixed in 4% paraformaldehyde buffer for 24 hr and embedded in paraffin. Subsequently, paraffin blocks were cut into 2‐μm sections and then subjected to routine H&E, PAS, and TUNEL staining according to previously described protocols (Wang, Li, et al., 2017; You et al., 2017; Zhang, Liu, et al., 2017). Histological differences among the groups were recorded. For H&E staining, five different fields from each group were randomly selected, and an acute tubular necrosis (ATN) score was used to indicate the tubular injury graded from 0 to 5 according to necrosis, dilation, and cast formation (0, none; 1, <15%; 2, 15% to 30%; 3, 31% to 50%; 4, 51% to 75%; 5, >75%). To quantify the PAS stain, the % area of brush border was calculated using image software. For TUNEL‐stain semiquantitative analysis, 10 different fields from each group were randomly selected to count the TUNEL‐positive cells.mm‐1.
2.3. elisa, immunohistochemistry, immunofluorescence, and immunoblot analysis
To determine the expression levels of inflammation factors (IFN‐γ, TNF‐α, IL‐17A, IL‐6, and IL‐1β), mice sera were collected at different time points from each group and analysed by elisa following methods from previous studies (Ren et al., 2016; Zhao et al., 2014). To investigate the levels of proteins in the TLR4‐related signalling pathway, such as TLR4, phospho‐NF‐κB p65 (p‐p65) or nonphospho‐NF‐κB p65 (p65), JNK, p‐JNK, p38, p‐p38, p21, c‐Jun, Bax, and cleaved caspase‐3, kidney‐paraffin blocks and tissues were subjected to immunohistochemistry, immunofluorescence, and immunoblot analysis. As in previous studies (Wang, Zhang, et al., 2017; Zhang, Wang, et al., 2017), the kidney‐paraffin sections were rehydrated through a graded series of ethanol and then incubated in 0.3% H2O2 overnight at 4°C to block endogenous peroxidase activity. Specific primary antibodies were incubated for 12 hr at 4°C in different dilutions (p‐p65, p65, JNK, p38, 1:100; p‐JNK, p‐p38, p21, 1:150; c‐Jun, Bax, cleaved caspase‐3, 1:200). After DAB or fluorescence‐conjugated antibody staining, five fields of the slides were collected from each animal at 20× and 40× magnification. For immunoblot assays, the procedures were based on previous studies (Li, Wan, et al., 2015; Li, Xie, et al., 2015). Briefly, kidney extracts were resuspended in a storage solution containing 100 mM Tris (pH 7.4), 0.1 mM EDTA, 0.1 mM dithiothreitol, 1.15% (w.v‐1) KCl, and 20% (v.v‐1) glycerol. To determine protein expression, the proteins (30 μg) were separated by SDS‐PAGE. After transfer to PVDF membranes, samples were incubated overnight at 4°C with the respective antibodies at different dilutions (p‐p65, p65, JNK, p38, 1:2000; p‐JNK, p‐p38, p21, 1:1500; c‐Jun, Bax, cleaved caspase‐3, 1:2500). All the blots were incubated with peroxidase‐conjugated antibody (1:6000, Millipore, Billerica, MA) at room temperature for 1 hr. β‐Actin (1:4000) was used as a loading control. The blots were visualized using chemiluminescence and imaged by the AutoChemi Imaging System (UVP LLC, Upland, CA). The blots were analysed using the MCID Elite software (InterFocus Imaging Ltd., Linton, UK). And the grey intensity of the phosphorylated protein was quantified and normalized to β‐actin levels and expressed as phosphorylated protein/non‐phosphorylated protein/β‐actin. The immuno‐related procedures used comply with the recommendations made by the British Journal of Pharmacology.
2.4. Real‐time PCR analysis
To evaluate mRNA expressions, total RNA was extracted from kidney tissues and cultured cells using the TRIzol reagent according to the manufacturer's protocol. cDNA was synthesized using a cDNA Synthesis Kit. All real‐time PCR reactions with SYBR Green were performed and analysed with the ABI Prism 7500 instrument (Applied Biosystems, Foster City, CA). GAPDH was used for normalization of relative expression levels.
2.5. Cell culture and treatments
Human renal proximal tubular epithelial (HK‐2) cells were obtained from ATCC and maintained in DMEM/F12 medium containing 10% FBS. HEK293 cells stably expressing TLR4, MD‐2, and CD14 were purchased from InvivoGen (HEK293‐hTLR4/MD2‐CD14, Catalog #293‐htlr4md2cd14, RRID:CVCL_Y395, InvivoGen, San Diego, CA) and cultured in DMEM/F12 medium containing 10% FBS, blasticidin (10 μg·ml−1), HygroGold (50 μg·ml−1), and Normocin (100 μg·ml−1).
Ice‐cold RPMI 1640 medium was used to lavage the mice peritoneally to obtain macrophages, which were plated in 96‐well plates at a density of 5 × 105 cells per well in RPMI complete medium.
Mice macrophages were pre‐incubated with TAK‐242 (10 or 100 nM) or Loganetin (50, 100, or 200 nM) for 0, 15, 30, 45, 60, 75, or 90 min, and then the cells were washed and stimulated with 1 ng·ml−1 LPS or myoglobin (Sigma) for 6 hr. The expression levels of TNF‐α and IL‐6 in culture supernatants were determined by elisa.
HEK293‐hTLR4/MD2‐CD14 cells were seeded in a 96‐well plate at a density of 2 × 104 cells per well and cultured overnight. Cells were transfected with 10‐ng phRL‐TK as internal control and with 20‐ng pNiFty or 30‐ng pISRE‐TA‐Luc plasmid. At 6 hr after transfection, a series of graded concentrations of LPS, TAK‐242, or Loganetin (from 10−4 to 106 nM) was added to the medium and incubated overnight. Subsequently, luciferase activities were determined by the Dual‐Glo Luciferase Assay System. Experiments were performed in triplicate for each group, and the results are presented as mean + SD.
HK‐2 cells (RRID:CVCL_0302) with or without shTLR4 plasmid transfection were pretreated with Loganetin (100 nM) for 0, 3, 6, 12, and 24 hr then washed and incubated with LPS (1 ng·ml−1) for 6 hr. Then cells were fixed in 4% paraformaldehyde for immunofluorescence assays or lysed in cell lysis buffer for immunoblot assays.
In order to further identify the function of Loganetin in AKI, HK‐2 cells or peritoneal macrophages were treated with 250 μM of myoglobin for 24 hr after Loganetin pretreatment. A TLR4‐related signalling pathway in HK‐2 cells was detected (Huang et al., 2018). And TNF‐α and IL‐6 levels in macrophages were determined by elisa.
To thoroughly evaluate the effect of Loganetin, HK‐2 cells were stimulated with LPS (1 ng·ml−1) for 6 hr and then washed and incubated with Loganetin (100 nM) for 24 hr. Cell viability and apoptosis were determined.
2.6. Flow cytometry and Annexin V/propidium iodide assay
Intact kidney tissues were decapsulated, diced, and digested in collagenase Type IV (ThermoFisher Scientific Inc. Waltham, MA) for 30 min at 37°C. The kidney tissue suspension was then passed through 40‐μm Falcon meshes followed by red cell lysis. Single‐cell suspensions were layered onto 34/51% discontinuous Percoll (GE Healthcare) density gradients and then centrifuged at 4,000× g for 30 min. Cells at the interface of the gradient were collected and washed twice with PBS. The cells were counted and incubated with anti‐mouse CD3‐APC, CD4‐FITC, CD45‐Per‐CP, CD11b‐PEcy5, F4/80‐APC‐Cy7, IFN‐γ‐PE or TNF‐α‐PE, and IL‐17A‐PE (Biolegend, San Diego, CA) for 1 hr at 4°C and detected on a BD Accuri C6 (BD Biosciences, San Jose, CA).
Cell death was analysed using the Annexin V‐FITC/propidium iodide (PI) apoptosis detection kit (BD Biosciences, San Jose, CA). Briefly, cells were collected and washed with cold PBS twice and then resuspended in 1× binding buffer at a concentration of 1 × 106 cells·ml−1. A 100 μl volume of this cell suspension was incubated with 5 μl Annexin V‐FITC reagent and 5 μl PI reagent for 15 min. Binding buffer (400 μl) was added to each sample, and cells were analysed by flow cytometry. Annexin V−/PI− represents live cells; Annexin V+/PI− represents early apoptotic cells; Annexin V−/PI+ reflects necrosis; and double positivity indicates both late apoptosis and necrosis.
2.7. Statistical analysis
Data are expressed as the mean ± SD. One‐way ANOVA followed by Tukey's post hoc test was used to compare multiple treatment groups. Two‐way ANOVA was used to assess the statistical significance of the differences between multiple treatment groups at different time points. Statistical significance was set at P < 0.05. The data and statistical analysis comply with the recommendations of the British Journal of Pharmacology on experimental design and analysis in pharmacology (Curtis et al., 2018).
2.8. Chemicals, reagents, kits, and antibodies
High purity Loganetin (>99%) was obtained from the State Key Laboratory of Generic Manufacture Technology of Chinese Traditional Medicine (Lunan Pharmaceutical Group Co., Ltd., Linyi, Shan Dong Province, China). Glycerol (for molecular biology, >99%), LPS (#L2630), and myoglobin were purchased from Sigma (Sigma‐Aldrich, St Louis, MO). TAK‐242 (#243984‐11‐4) and phalloidin‐FITC conjugate were purchased from Millipore (MerckMillipore, Billerica, MA). SYBR Green was from Toyobo (Cat. K1621, TOYOBO, Osaka, Japan).
The TLR4 shRNA (h) and control shRNA plasmids were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). TRIzol reagent was from Invitrogen (Carlsbad, CA), and the M‐MLV reverse transcriptase was from Promega (Madison, WI). Ethanol and xylene (chromatographic grade) were purchased from Fisher Scientific Co. (Fisher Scientific, Houston, TX). Ultrapure HPLC‐grade water was prepared using the Milli‐Q® system.
Haematoxylin and eosin (H&E) stain kits (#C0105) and TUNEL kits (#C1098) were purchased from Beyotime Biotechnology (Beyotime Biotechnology, Shanghai, China). Periodic acid‐Schiff (PAS) stain kits were obtained from Solarbio (#G1285, Solarbio Life Sciences, Beijing, China). Mouse IFN‐γ, TNF‐α, IL‐17A, IL‐6, and IL‐1β elisa kits were supplied by R&D Systems (Boston, MA). The ECL detection kit was from Thermo Scientific (#32106, Waltham, MA), the cDNA Synthesis Kit was from TOYOBO (Osaka, Japan), and the Dual‐Glo Luciferase Assay System was purchased from Promega (#E2920, Madison, WI).
TLR4 rabbit mAb (#14358), NF‐κB p65 rabbit mAb (#8242, RRID:AB_10859369), phospho‐NF‐κB p65 (Ser536) rabbit mAb (#3033, RRID:AB_331284), JNK rabbit mAb (#9252, RRID:AB_2250373), phospho‐JNK (Thr183/Tyr185) rabbit mAb (#4668, RRID:AB_823588), p38 rabbit mAb (#2387, RRID:AB_11178801), Phosphor‐p38 (Thr180/Tyr182) rabbit mAb (#4511, RRID:AB_2139682), p21 rabbit mAb (#2947, RRID:AB_823586), c‐Jun rabbit mAb (#9165, RRID:AB_2130165), Bax rabbit mAb (#14796, RRID:AB_2716251), cleaved caspase‐3 (C‐cas‐3; Asp175) rabbit mAb (#9664, RRID:AB_2070042), and β‐actin mouse mAb (#3700, RRID:AB_2242334) were purchased from CST (Cell Signaling Technology, Boston, MA).
2.9. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander, Fabbro et al., 2017a,b; Alexander, Kelly et al., 2017).
3. RESULTS
3.1. Loganetin protected against rhabdomyolysis‐induced kidney injury in mice
Initially, we investigated and confirmed the protective effects of Loganetin against RIAKI in C57/BL6J mice. The protocols are summarized in Figure 1. Loganetin (2 or 18 mg·kg−1) was i.p. injected 0.5, 24, and 48 hr after 50% glycerol injection. Mice were killed at 24, 48, and 72 hr, and kidneys and blood were collected for the following experiments.
As shown in Figure 2, kidney H&E staining demonstrated that glycerol injection induced significant kidney tissue damage in C57/BL6J mice, especially in the kidney tubular structures. This damage was significantly ameliorated in Loganetin‐treated mice in both 2 and 18 mg·kg−1 treatment groups. The injury (glycerol‐induced) and protection (Loganetin‐mediated) were time‐ and dose‐dependent (Figure 2a). The hind limb muscle tissues of mice from each group were collected, and H&E staining results showed that neither 2 nor 18 mg·kg−1 Loganetin ameliorated the degree of rhabdomyolysis compared with glycerol + PBS (Figure 2b). To quantitfy tubular injury, ATN scores of 72‐hr‐treated mice were calculated according to a series evaluation standard that included tubular necrosis, tubular dilation, and cast formation (Figure 2c). Glycerol treatment resulted in dramatically more damage (ATN score 4.0 ± 1.0) compared with the control group, but this damage is significantly less with Loganetin treatment at both dosages (2 mg·kg−1, 2.9 ± 0.5; 18 mg·kg−1, 1.5 ± 0.5). The survival status in each group (n = 10 in each group) were recorded by electronic monitoring equipment, and survival rates were calculated using GraphPad Prism 5 software (GraphPad Software, La Jolla, CA; Figure 2d). There were no mice in the control group that died throughout the course of the experiment (72 hr). However, a 50% (n = 5) mortality rate was observed in the glycerol‐treated group, and these deaths occurred in the early stage (from the 30th to 65th hour). In contrast, Loganetin treatment resulted in a lower mortality rate at both 2‐ and 18‐mg·kg−1 dosages (30% and 10%, respectively) and lower death incidence at the end of this study (from the 58th to 66th hour). Meanwhile, the long‐term protective effects of Loganetin on mortality were evaluated when mice were pre or post‐treated with Loganetin and using the following procedure. Mice received an i.p. injection for three consecutive days. After the last injection, 50% glycerol was i.m. injected (10 ml·kg−1) into the hind limbs of mice, and the mortality rates were recorded for 15 days. The results are shown in Supporting Information Figure S1g; no death occurred in the control group. Compared with the control group, a 90% mortality rate (n = 9) in glycerol‐treated group occurred during the early stage (from the second to the fifth day). Post‐treatment with Loganetin significantly reduced the mortality compared with the model group such that the mortality rates were 70% and 50% in 2‐ and 18‐mg·kg−1 treatment respectively. Furthermore, it is noticeable that pre‐Loganetin treatment exhibited a more efficient protective effect, further reducing the mortality rates (50% and 30% in Loganetin 2‐ and 18 mg·kg−1, respectively) and prolonging the life span. In the 2 mg·kg−1 Loganetin pretreated group, three deaths occurred in the early stage (two in Day 3 and one in Day 5), and two in the end‐stage (one in Day 9 and one in Day 11) of this study. In contrast, one animal died in the early (Day 4), mid‐ (Day 8), and end‐stage (Day 12) in the 18 mg·kg−1 Loganetin pretreatment group respectively. In addition, BUN and serum CREA, which are biomarkers for kidney function, were evaluated using respective kits. The results showed that, compared with control mice, the glycerol injection caused obvious increases in serum CREA and BUN. In contrast, treatment with Loganetin significantly improved kidney function (Figure 2e,f). Subsequently, to precisely evaluate the tubular changes after glycerol with or without Loganetin administration, PAS staining was performed to clearly show intratubular cast and brush border loss. As shown in Figure 3a, glycerol caused significant damage to the tubular structure, characterized by swelling and degeneration of the tubular epithelial cells, disappearance of the brush border, necrosis of part of the epithelial cells, and formation of myoglobin tubes. Loganetin inhibited this pathological process in a dose‐dependent manner; Loganetin treatment preserved the brush borders and reduced the occurrence of intraluminal casts (Figure 3a). To further evaluate apoptosis in kidney tissues during RIAKI, TUNEL staining was performed, and the results showed that there were significantly more apoptotic cells at the corticomedullary junction in mice subjected to glycerol injection. Consistent with kidney function and morphology, administration of Loganetin at both dosages suppressed this apoptosis markedly (Figure 3b). Semiquantitative analyses of PAS and TUNEL assays are presented in Figure 3c,d respectively.
Figure 2.
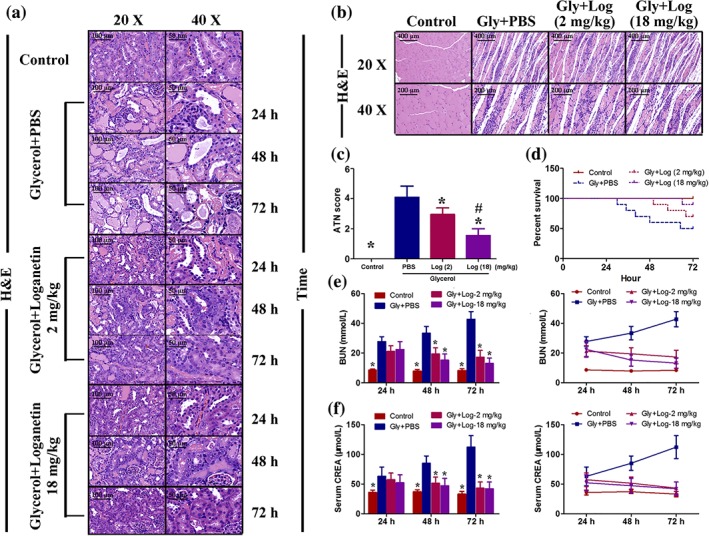
Loganetin ameliorated renal dysfunctions in rhabdomyolysis‐induced acute kidney injury. Mice subjected to different treatments were killed at the indicated time points. Representative haematoxylin and eosin staining images of kidney (a) and muscle (b) tissue in each group are shown, and the acute tubular necrosis scores at 72 hr (c) were calculated. (d) Representative survival curves of each group. Serum levels of (e) creatinine (CREA) and (f) blood urea nitrogen (BUN) were measured, and the results are presented as bars (left) and curves (right). Bar = 100 μm. Data are expressed as the means ± SD, n = 10. *P < 0.05 versus Gly + PBS group; #P < 0.05 versus Loganetin 2 mg·kg−1 group
Figure 3.
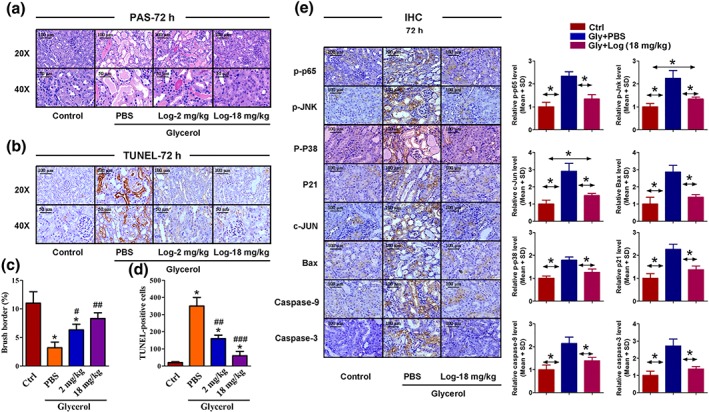
Loganetin inhibited apoptosis of tubular epithelial cells and regulated TLR4‐related protein expression in rhabdomyolysis‐induced acute kidney injury. Representative images of periodic acid‐Schiff staining (a) and TUNEL staining (b) and respective semi‐quantification of brush borders (c) and TUNEL‐positive cells (d). Representative images of immunohistochemistry assays of kidney tissues with p65, JNK, p38, and phospho‐p65, ‐JNK, ‐p38, p21, c‐Jun, Bax, caspase‐9, and cleaved caspase‐3 (e). Bar = 100 μm. Data are expressed as the means ± SD, n = 10.*P < 0.05 versus Gly + PBS group; #P < 0.05 versus Loganetin 2 mg·kg−1 group
To further investigate the role of Loganetin alone on kidney function, 2 or 18 mg·kg−1 of Loganetin were injected i.p. 0.5, 24, and 48 hr after saline injection. H&E, PAS, and TUNEL staining were performed after 72 hr. Compared with the control group, both the 2 and 18 mg·kg−1 Loganetin groups showed a potential trend but no significant effects on the kidney function and morphology including tubular injury (ATN score), kidney function (BUN and CREA), brush border, and TUNEL‐positive cells (Supporting Information Figure S1a–f).
3.2. The TLR4/JNK/p38 pathway was involved in the protective effects of Loganetin
To investigate the factors probably involved in the protective role of Loganetin in RIAKI mice, protein levels of the kidney tissues of each group were analysed by immunohistochemistry (IHC) staining. As shown in Figure 3e, phospho‐p65, ‐JNK, ‐p38, p21, c‐Jun, Bax, caspase‐9, and C‐cas‐3 were increased in the glycerol injection group compared with the control group. Loganetin showed an inhibitory effect on the expression of these proteins (Figure 3e, left). IHC staining was quantified by Panoramic Scan (3DHISTECH Ltd., Budapest, Hungary) and calculated by Caseviewer Software (QuantCenter version, 2.0.0.61665, 3DHISTECH Ltd., Budapest, Hungary), and the results are shown in Figure 3e (right). To further confirm these differences in protein levels, proteins were extracted from the kidney tissues of each group, and the relative expression levels were evaluated by immunoblot (Figure 4a,b). These results were consistent with the IHC staining. The relative mRNA expression levels in mouse kidney tissues were detected by qRT‐PCR, and the primer sequences and results are shown in Supporting Information Figure S2.
Figure 4.
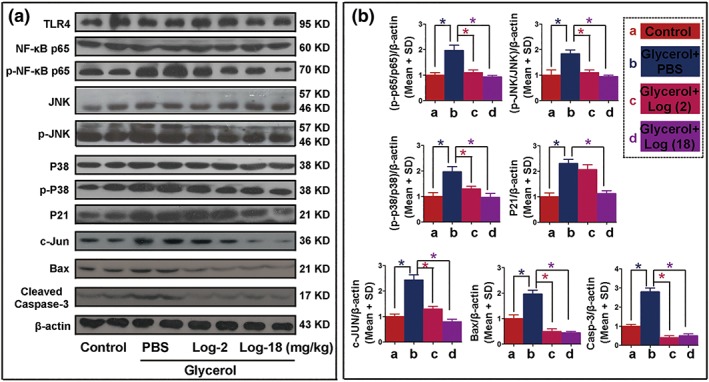
Loganetin regulated the protein expressions of TLR4/JNK/p38 pathway in rhabdomyolysis‐induced acute kidney injury. Seventy‐two hours after glycerol or saline injection, kidney tissue proteins were extracted from the control group and rhabdomyolysis‐induced acute kidney injury mice with or without Loganetin treatment. The samples were analysed for TLR4, p65, p‐p65, JNK, p‐JNK, p38, p‐p38, p21, c‐Jun, Bax, cleaved caspase 3, and β‐actin using specific antibodies. (a) Representative immunoblot analysis. (b) Immunoblot signals were quantified by densitometry and normalized with β‐actin. The (p‐p65/p65)/β‐actin, (p‐JNK/JNK)/β‐actin, (p‐p38/p38)/β‐actin, p21/β‐actin, C‐Jun/β‐actin, BAX/β‐actin, and cleaved caspase 3/β‐actin ratios are shown. Shown are representative blots and expressed as the means ± SD from series with tissues from n = 10; the bars with different superscripts in each panel were significantly different. *P < 0.05 versus Gly + PBS group
3.3. Loganetin ameliorated RIAKI‐associated renal inflammation
Flow cytometry was performed to evaluate the inflammatory state by detecting cytokines in kidney tissues. The levels of pro‐inflammatory cytokines, including IFN‐γ, TNF‐α, and IL‐17A (Figure 5a–f), and inflammatory cells including CD3+ T cells, CD45+ immune cells, and CD11b + F4/80+ macrophages (Figure 5l–n) were all significantly higher in mice injected with glycerol compared to the control mice. Loganetin treatment partially reduced this increase (Figure 5a–i). Similar consequences were observed for IL‐6, IL‐1β, TNF‐α, IFN‐γ, and IL‐17A in mouse serum by elisa assays (Figure 5g–k). However, TNF‐α and IL‐6 concentrations in peritoneal macrophages stimulated with myoglobin were significantly decreased after Loganetin treatment (Supporting Information Figure S3). Relative mRNA levels were determined by qRT‐PCR, and the primer sequences and results are shown in Supporting Information Figure S4. All these results were consistent with kidney function and histological results and indicated that Loganetin has the ability to act as a potential immunomodulator.
Figure 5.
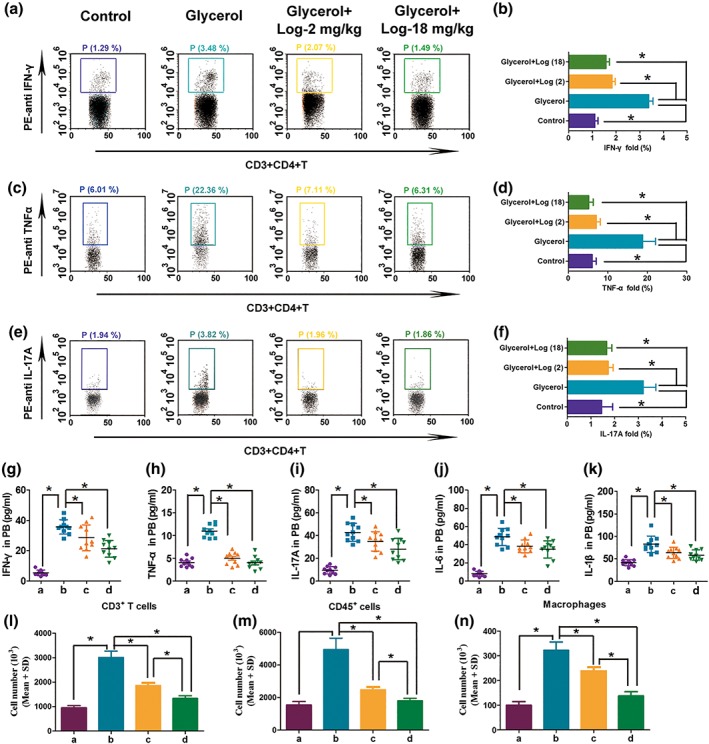
Loganetin ameliorated inflammation in rhabdomyolysis‐induced acute kidney injury mice. (a,b) IFN‐γ, (c,d) TNF‐α, and (e,f) IL‐17A were assayed by Flow cytometry. (g–k) IFN‐γ, TNF‐α, IL‐17A, IL‐6 and IL‐1β in peripheral blood were analysed by elisa. (l–n) CD3 + T cells, CD45+ immune cells, and CD11b + F4/80+ macrophages in kidney tissue were detected by Flow cytometry. Data are expressed as the means ± SD, n = 10. *P < 0.05
3.4. Loganetin is a TLR4 antagonist and blocked JNK/p38 pathway in vitro
Loganetin is known to inhibit pro‐inflammatory factors and multiple molecules involved in the TLR4 signal pathway. Based on these clues, we hypothesize that this chemical modulates the activity of TLR4. To explore this hypothesis, NF‐κB and ISRE Luciferase‐report plasmids (pNiFty and pISRE‐TA‐Luc) were used to transfect HEK293‐hTLR4/MD2‐CD14 cells, which were subsequently stimulated by LPS with or without Loganetin or TAK‐242 pretreatment. The results demonstrated that LPS, the classic TLR4 ligand, dramatically induced the activity of both pNiFty and pISRE‐TA‐Luc plasmids at extremely low concentrations (EC50 were 6.09 ± 1.13 and 6.41 ± 1.45 nM, respectively). In contrast, TAK‐242 and Loganetin treatment resulted in lower pNiFty (IC50 of TAK‐242, 8.08 ± 1.23 nM; IC50 of Loganetin, 25.4 ± 3.12 nM) and pISRE‐TA‐Luc (IC50 of TAK‐242, 3.09 ± 0.53 nM; IC50 of Loganetin, 4.75 ± 0.76 nM) activity (Figure 6a,b). In addition, mouse peritoneal macrophages were lavaged from each group, cultured and pre‐incubated with TAK‐242 or Loganetin in a concentration series for different times, and then stimulated with LPS. TNF‐α and IL‐6 were detected by elisa. Pretreatment with Loganetin (200 nM) for 60 min showed significant inhibitory effects on the production of TNF‐α and IL‐6 (Figure 6c,d). Even at lower concentrations (100 or 50 nM), longer pre‐incubations increased the inhibitory efficacy.
Figure 6.
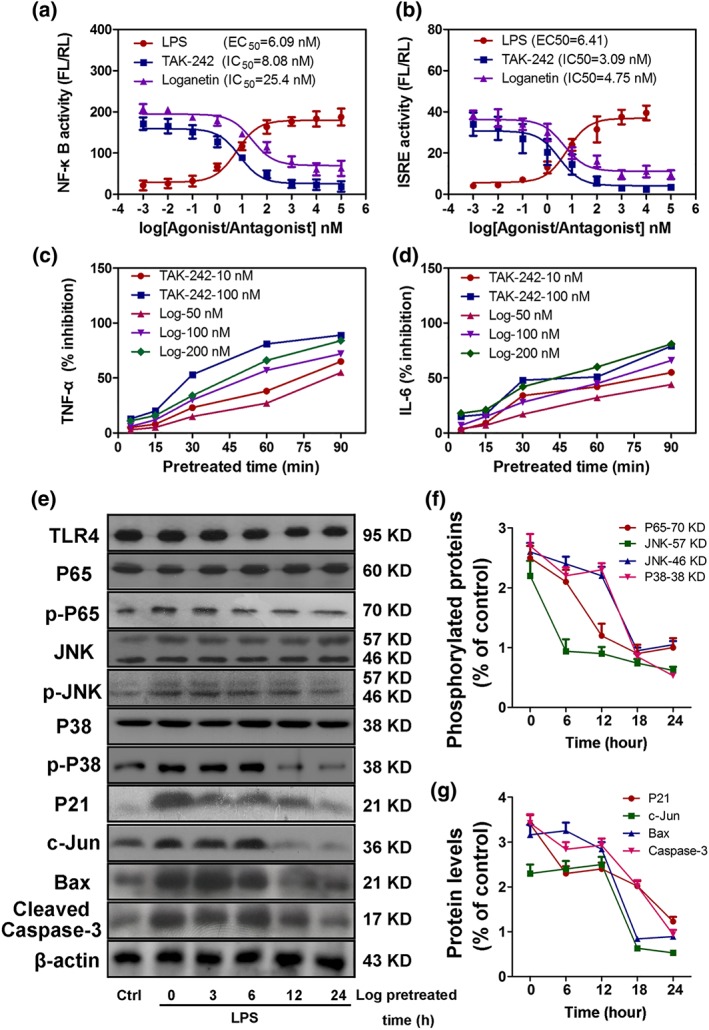
Loganetin is a potential TLR4 antagonist and regulates TLR4‐related protein expression in vitro. The inhibitory effects of Loganetin on NF‐κB and ISRE activation in HEK293‐hTLR4/MD2‐CE14 cells (a,b). Cells were transfected with 10‐ng phRL‐TK as an internal control, and 20‐ng pNiFty (a) or 30‐ng pISRE‐TA‐Luc (b) plasmid. Six hours after transfection, a series of graded LPS, TAK‐242, or Loganetin concentrations (from 10−4 to 106 nM) was added to the medium and treated overnight. Subsequently, luciferase activities were determined. Time‐ and concentration‐dependence of the inhibitory effect of Loganetin on the production of TNF‐α and IL‐6 in mouse peritoneal macrophages stimulated with LPS were determined. Cells were incubated with TAK‐242 or Loganetin at the indicated times, washed, and stimulated with LPS. The concentrations of TNF‐α (c) and IL‐6 (d) in the culture supernatants were determined using specific elisa kits. (e–g) HK‐2 cells pretreated with Loganetin (100 nM) at the indicated times, washed, and stimulated with LPS (1 ng·ml−1) for 6 hr were lysed using cell lysis buffer for immunoblots. Experiments were performed in triplicate for each group
To determine the time course of the inhibitory effect of Loganetin on the production of TLR4‐related proteins in vitro, HK‐2 cells were incubated with Loganetin (100 nM) from 0 to 24 hr and then replaced by LPS. A total of 11 proteins were detected by immunoblot and are shown in Figure 6e. LPS (1 ng·ml−1) significantly elevated the expression of seven proteins (p‐p65, p‐JNK, p‐p38, P21, c‐Jun, Bax, and C‐cas‐3). Loganetin exhibited rapid inhibitory effects on p‐p65, p‐p38, p‐JNK, and C‐cas‐3 expression as early as 3 hr, followed by a subsequent decrease in inhibition thereafter (Figure 6e). However, non‐phosphorylated proteins, such as p65, JNK, p38, and even TLR4, were not affected by either LPS or Loganetin. All semiquantitative results are shown in Figure 6f,g. Relative mRNA levels were determined by qRT‐PCR and are shown in Supporting Information Figure S5.
In contrast, to verify the protective effects of Loganetin, HK‐2 cells were post‐treated with Loganetin (100 nM) for 24 hr after LPS (1 ng·ml−1) stimulation (6 hr). From the results shown in Supporting Information Figure S6, compared with the LPS‐treated group, Loganetin significantly reduced cell apoptosis rate and enhanced cell viability in a time‐dependent manner.
To further confirm that TLR4 could mediate the protective effects of Loganetin, the shTLR4 plasmid was used to transfect HK‐2 cells, and then immunoblot and immunofluorescence assays were used to compare related proteins expression with TLR4‐WT HK‐2 cells after both cell lines had been pretreated with or without Loganetin for 12 hr during LPS stimulation. Immunoblot and immunofluorescence assays demonstrated that TLR4 was almost completely inhibited by the shTLR4 plasmid (Figure 7a). Compared with the control, TLR4‐KO showed significantly lower p‐p65, p‐JNK, p‐p38, p21, c‐Jun, Bax, and C‐cas‐3 expression. These protein levels were higher in TLR4‐WT cells stimulated with LPS but lower with Loganetin pre‐incubation for 12 hr. However, in TLR4‐KO cells, no inhibitory effects of Loganetin on protein expression were observed, which indicates that TLR4 mediated Loganetin's effects on protein levels (Figure 7b). The results of a semiquantitative analysis are presented. To clearly demonstrate these mechanisms, immunofluorescence assays with p‐JNK, p‐p38, p21, and c‐Jun antibodies were performed, and the results were consistent with those of the immunoblot assays (Figure 7c–f). Relative immunofluorescence values are shown in Supporting Information Figure S7.
Figure 7.
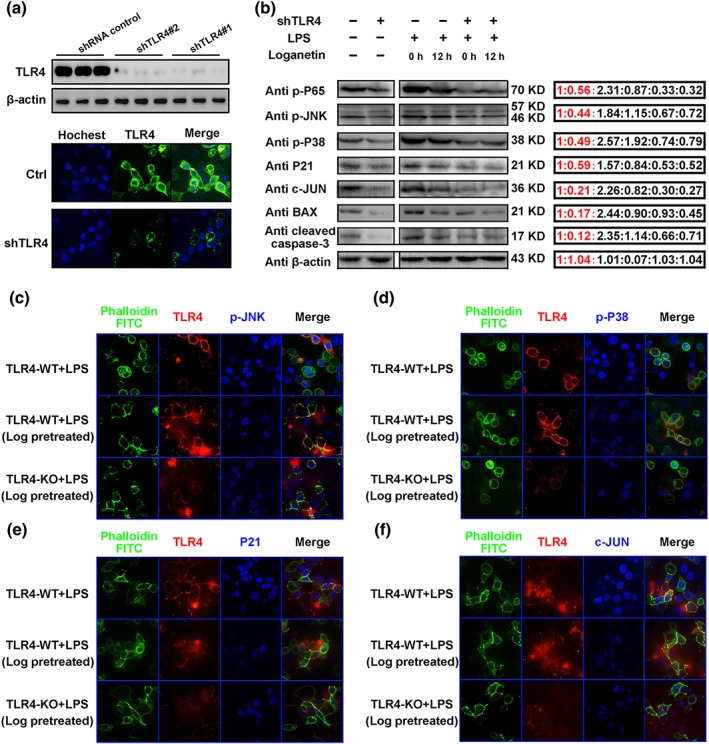
The protective mechanisms of Loganetin on HK‐2 cells were mediated by the TLR4. TLR4 expression in HK‐2 cells transfected with shTLR4 plasmid was determined by Western blot and immunofluorescence (a). HK‐2 cells with or without shTLR4 plasmid transfection were pretreated with Loganetin (100 nM) for 0 and 12 hr, then washed and incubated with LPS (1 ng·ml−1) for 6 hr, and lysed in cell lysis buffer for immunoblots (b) or fixed in 4% paraformaldehyde for immunofluorescence assays (c–f) respectively. Experiments were performed in triplicate for each group
3.5. Loganetin inhibited the apoptosis of tubular epithelial cells in response to LPS
Although necrosis of epithelial cells has long served as an important indicator of AKI, one group has reported that this feature is more suitable for the pathognomonic lesions of ischaemic AKI (Press et al., 2017). Thereafter, researchers have found that apoptosis plays a crucial role in the pathogenesis of AKI, which even outweighs necrosis (Wang, Li, et al., 2017; Zhang, Liu, et al., 2017; Zhao et al., 2014). Therefore, we explored whether Loganetin was involved in modulating apoptosis of kidney epithelial cells in RIAKI. First, the effects of Loganetin on HK‐2 cell apoptosis and viability were tested by morphology and MTT assays respectively. In TLR4‐WT cells, Loganetin pretreatment ameliorated LPS‐induced tubular cell apoptosis (from 18.5 ± 3.2% to 9.46 ± 2.14%) and reductions in viability (Figure 8a,b; from 41.3 ± 9.6% to 87.4.3 ± 11.3%). However, when TLR4 was depleted by the shTLR4 plasmid, neither single LPS treatment nor LPS combined with Loganetin had any effect on apoptosis and viability (Figure 8a,b), which confirmed that Loganetin could inhibit apoptosis, and this activity was mediated by TLR4. Subsequently, flow cytometry analysis based on Annexin V/PI (AV/PI) staining was performed to detect different types of cell death. The staining results can be divided into four quadrants: AV−/PI− and AV+/PI+ represent live and late apoptosis plus necroptosis cells, respectively, and AV+/PI− and AV−/PI+ reflect early apoptosis and necroptosis cells respectively. Consistent with previous results, exposure to LPS caused a significant decrease in cell viability, especially in early apoptosis of cells, compared to vehicle‐treated TLR4‐WT‐HK‐2 cells. Moreover, addition of Loganetin obviously reduced early apoptosis rates. However, in TLR4‐KO‐HK‐2 cells, LPS and Loganetin did not influence cell apoptosis rates. Compared with WT‐HK‐2 cells, TLR4 depletion reduced LPS‐induced pro‐apoptosis and Loganetin‐mediated anti‐apoptotic effects (Figure 8c). The relative quantitative analyses are presented in Supporting Information Figure S8a. Overall, these data suggested that Loganetin could suppress apoptosis of tubular epithelial cells, which is mediated by TLR4.
Figure 8.
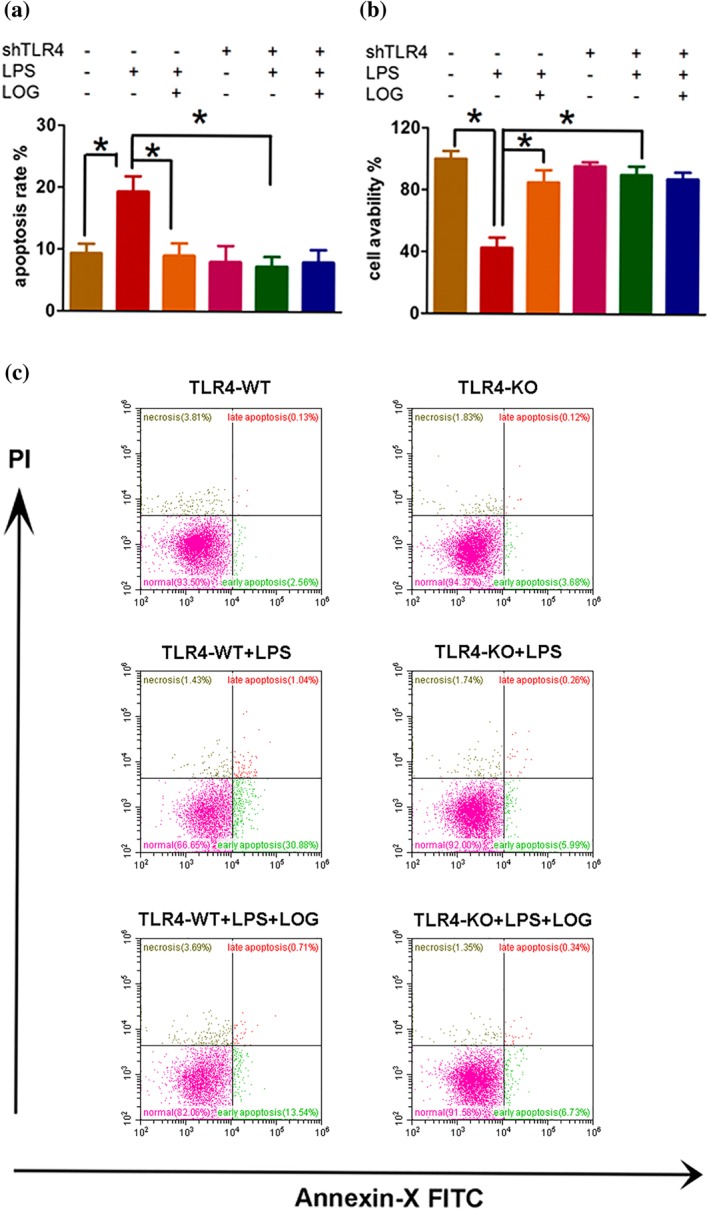
Deletion of TLR4 ameliorated renal dysfunction, renal injury, apoptosis, and cell death in vivo and in vitro. The anti‐apoptotic effects of Loganetin on HK‐2 cells were compared with or without shTLR4 plasmid transfection; apoptosis rate (a) and cell viability (b) were determined. To explore the role of Loganetin in LPS‐induced cell death, cells were collected and analysed by flow cytometry based on Annexin V/PI staining (c). Experiments were performed in triplicate for each group. Data are expressed as the means ± SD. *P < 0.05
3.6. Deletion of TLR4 ameliorated renal dysfunction, renal injury, apoptosis, and cell death in RIAKI mice
To further clarify whether Loganetin exerts its protective effects on renal damage via TLR4, C57BL/10ScSn mice were treated with glycerol (50%, 10 ml·kg−1, i.m. injection) alone or combined with LPS (1 mg·kg−1, i.p. injection) with or without Loganetin (18 mg·kg−1, i.p. injection). After 72 hr, all mice were killed, and the kidney function and morphology including ATN score, brush border, and TUNEL‐positive cells were evaluated. The histological results demonstrated that glycerol‐induced renal damage was enhanced when glycerol was combined with LPS injection but significantly ameliorated by Loganetin treatment. In addition, compared with glycerol only group, the protective effect of Loganetin on renal damage in the glycerol + LPS group was weaker but not significant (Supporting Information Figure S9a). In addition, PAS and TUNEL staining were performed, and relative parameters such as ATN score, brush border, and TUNEL‐positive cells (Supporting Information Figure S9b) were in accordance with the H&E staining.
To further confirm previous results and our hypothesis, TLR4 knockout (TLR4‐KO) mice were used to duplicate an RIAKI mouse model. Based on the immunoblot and qRT‐PCR analysis, TLR4 was completely abolished in TLR4‐KO mice compared with WT mice, even after glycerol treatment (Supporting Information Figure S7). To assess the role of TLR4 in the pathogenesis of RIAKI, wild‐type and TLR4‐KO littermate mice were treated with or without Loganetin (18 mg·kg−1) for 72 hr after glycerol injection. The survival status of all treatment groups was recorded and calculated (Figure 9b). Compared with the control group (none died), mortality was higher both in the WT (five died between the 36th and 59th hour after glycerol injection) and KO mice (two died between the 40th and 69th hour after glycerol injection) treated with glycerol. In contrast, Loganetin treatment resulted in dramatically lower mortality in both TLR4‐WT‐RIAKI mice (three died) and TLR4‐KO‐RIAKI mice (two died). In addition to the elevated survival percentage, Loganetin prolonged lifespan regardless of whether TLR4 was knocked out. In the non‐Loganetin treatment group, levels of BUN and serum CREA were similarly low. At 72 hr after glycerol injection, wild‐type mice developed moderate renal failure, which was significantly lower with Loganetin treatment or in TLR4‐KO mice without Loganetin treatment. However, the levels of BUN and CREA were not different between WT or KO mice after Loganetin treatment (Figure 9b). Histological analysis confirmed that the glycerol‐induced kidney tissue damage occurred in TLR4‐WT mice, but was significantly ameliorated in TLR4‐KO mice (Figure 9a, upper). Consistent with H&E staining, PAS and TUNEL assays showed that in wild‐type mice, the ATN score, brush border, and TUNEL‐positive cell were 3.82 ± 0.6, 3.5 ± 0.5, and 352 ± 34 in RIAKI mice respectively. In contrast, these parameters were 1.13 ± 0.2, 7.6 ± 0.8, and 138 ± 24 in Loganetin‐treated RIAKI mice respectively. Additionally, Loganetin treatment did not affect these parameters when TLR4 was knocked out (Figure 9a, lower, c). However, the specific agonist of TLR4, LPS, partially inhibited this effect of Loganetin. Overall, the above results clearly demonstrated that TLR4 mediated the pathological process of RIAKI induced by glycerol injection. TLR4 depletion significantly ameliorated kidney dysfunction, renal injury, and cell death. And Loganetin can ameliorate RIAKI by inhibiting TLR4 activity.
Figure 9.
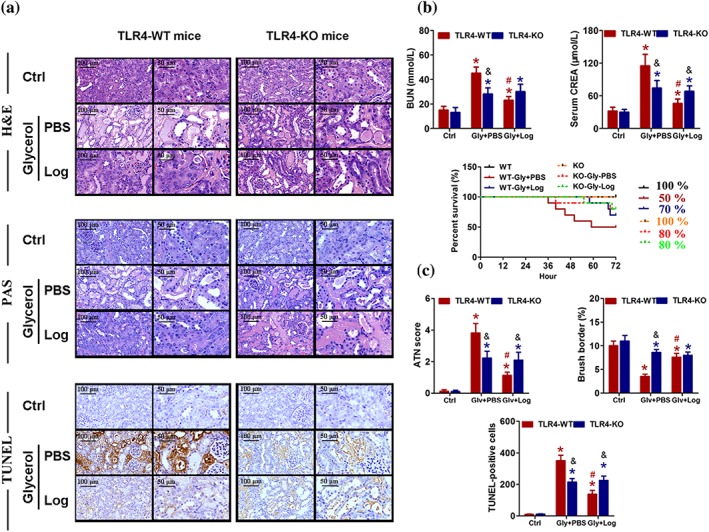
Loganetin improved rhabdomyolysis‐induced acute kidney injury in mice by acting on the TLR4. TLR‐WT and TLR‐KO mice subjected to different treatments were killed at 72 hr after glycerol injection. Representative haematoxylin and eosin (upper), periodic acid‐Schiff (middle), and TUNEL (lower) staining images of kidney (a), blood urea nitrogen (BUN, left), serum levels of (right) creatinine (CREA, right), and survival curves of each groups (lower) were detected and performed (b). (c) Representative images of acute tubular necrosis score, brush borders, and TUNEL‐positive cells were showed. Data are expressed as means + SD, n = 10. *P < 0.05 versus control group of the same genotype; #P < 0.05 versus PBS group of the same genotype; &P < 0.05 versus the same treated group of different genotype
4. DISCUSSION AND CONCLUSIONS
Although there have been few studies on Loganetin’s bioactivity, the research on Loganin in general is much more widely available. It has been reported that Loganin can affect lipid metabolism, oxidative stress, and inflammation. Loganin can inhibit NF‐κB activation (Kim et al., 2015). In addition, Loganin exhibits neuroprotection (Kwon et al., 2011), anti‐amnesic activity (Lee et al., 2009), protective effects against neurodegenerative diseases (Kwon, Kim, Lee, & Jang, 2009), hepatic injury, and other diabetic complications (Yamabe et al., 2010). In Schwann SW10 cells, Loganin blocks the effects of TNF‐α (Chao et al., 2017). Loganetin is produced by the deglucosylation of Loganin (Miettinen et al., 2014). Therefore, we speculated that the two may have similar biological functions.
Loganin has anti‐inflammatory and immune‐regulatory activities (Yamabe et al., 2010). In this study, we evaluated the effects of Loganetin on a glycerol‐induced AKI mouse model. Loganetin efficiently improved the symptoms of AKI. Treatment with Loganetin resulted in decreased levels of BUN and serum CREA in AKI mice. Both high and low doses of Loganetin prolonged the lifespan of AKI mice. Loganetin prevented the apoptosis of renal tubular epithelial cells. It has been reported that Loganin effectively inhibits H2O2‐induced SH‐SY5Y apoptosis, and the protective mechanism has been attributed to decrease apoptosis as a result of p38, caspase‐3, and cytochrome c regulation (Kao et al., 2017). We found that Loganetin suppressed apoptosis in the corticomedullary junction of kidney tissue in the AKI model. Our investigation of the mechanism found that 50% glycerol resulted in an elevation of p‐p65, p‐JNK, p‐p38, p21, and c‐Jun protein levels, whereas Loganetin reduced these protein levels. In the mouse model, Loganetin treatment decreased the expression of inflammatory factors, including TNF‐α, IFN‐γ, and IL‐17A. And Loganetin also reduced the infiltration of CD3+ T cells, CD45+ immune cells, and CD11b + F4/80 macrophages into the kidney (Huang et al., 2017). Glycerol‐induced AKI increased the expression of TNF‐α, IFN‐γ, IL‐6, IL‐17, and IL‐1β in both peripheral blood and kidney tissue. These results are in agreement with the those obtained previously (Wu et al., 2017). TNF‐α and IL‐6 could trigger the release of other pro‐inflammatory cytokines and inflammatory mediators. IFN‐γ, IL‐17, and IL‐1β are all involved in kidney injury (Li et al., 2010). These inflammatory factors aggravate kidney injury. Therefore, we determined the levels of these inflammatory cytokines and found that Loganetin inhibited their expression and ameliorated kidney injury.
In HK‐2 cells, we showed that TLR4 plays a central role in AKI development. To imitate the pathological process of AKI in vitro, HK‐2 cells were stimulated with LPS. Loganetin pretreatment markedly inhibited LPS‐induced expression of p‐p65, p‐JNK, p‐p38, p21, and c‐Jun proteins. When TLR4 was knocked down by specific shRNA in the presence of LPS, Loganetin treatment did not alter the phosphorylation of p65, JNK, and p38 and did not affect the expression of p21, c‐Jun, Bax, or caspase 3, which suggests that TLR4 plays an important role in the effects of Loganetin on apoptosis induced by LPS in HK‐2 cells. Also Loganetin, pre‐ or post‐treatment, protected HK‐2 cells from apoptosis. In TLR4 knockout (TLR4‐KO) mice, Loganetin did not display a protective effect on glycerol‐induced AKI. The levels of BUN and serum CREA were not restored by Loganetin treatment, and the ATN score, brush border, and apoptosis in kidneys of glycerol‐stimulated TLR4‐KO mice were not ameliorated by Loganetin treatment compared with TLR4‐WT mice. Loganetin increased the survival of glycerol‐treated mice with AKI but not of the glycerol‐treated TLR4‐KO mice.
Novel inhibitors of TLR4 have been designed and synthesized according to the structure of the molecule, including TAK‐242, which disrupts the interactions of TLR4 with its adaptor molecules (Matsunaga, Tsuchimori, Matsumoto, & Ii, 2011). Here, we report, for the first time, a natural small molecular product, Loganetin, which can significantly inhibit NF‐κB activity by acting on TLR4. However, the exact binding site is still not clear and needs further study.
AUTHOR CONTRIBUTIONS
Y.R., G.Z., and J.Y. contributed to the conception and design of the study and approved the final version to be submitted. J.L. contributed to drafting the article and data analysis and interpretation. Y.T., M.W., and G.L. performed samples collection from experimental animals. Y.T., and J.L. performed H&E, PAS, TUNEL, immunohistochemistry, and immunofluorescence staining. Q.W., Y.S., Z.L., and J.Y. performed elisa and immunoblot analysis.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for Design & Analysis, Immunoblotting and Immunochemistry, and Animal Experimentation, and as recommended by funding agencies, publishers and other organisations engaged with supporting research.
Supporting information
Figure S1 The effects of Loganetin on mice renal function. Mice subjected to Loganetin injection and killed after 72 hr. Representative H&E, PAS, and TUNEL staining images of kidney (A) and the ATN scores (B), blood urea nitrogen (BUN, C) creatinine (CREA, D), brush border (E), TUNEL‐positive cells (F) at 72 hr were calculated. (G) Representative survival curves of each group, sold line: Loganetin pretreatment; dotted line: Loganetin post‐treatment. Data are expressed as the means ± S.D., n = 10.
Figure S2 Loganetin regulated the expressions of TLR4/Jnk/p38 pathway in RIKAI. Quantitative analysis of p65, Jnk, p38, and phospho‐p65, −Jnk, −p38, p21, c‐Jun, Bax, Caspase‐9 and cleaved caspase‐3 expression by QRT‐PCR of kidney tissues. Data are expressed as means + SD, n = 10. *P < 0.05, versus Gly + PBS group.
Figure S3 The protection effects of Loganetin on myoglobin stimulated mouse peritoneal macrophages and HK2 cells. Time‐ and concentration‐dependence of the inhibitory effect of Loganetin on the production of TNF‐α and IL‐6 in mouse peritoneal macrophages stimulated with myoglobin were determined. Cells were incubated with TAK‐242 (100 nM) or Loganetin (100 nM) at the indicated times, washed, and stimulated with myoglobin. The concentrations of TNF‐α and IL‐6 (A) in the culture supernatants were determined using specific elisa kits. (B) HK2 cells pretreated with Loganetin (100 nM) for 24 hr, washed, and stimulated with myoglobin for 6 hr were lysed using cell lysis buffer for immunoblots. Experiments were performed in triplicate for each group.
Figure S4 Loganetin regulated the mRNA levels of pro‐inflammatory factors in RIAKI mice. Renal mRNA levels of IFN‐γ, TNF‐α, IL‐17A, IL‐6 and IL‐1β were analysed by qPCR. Data are expressed as means + SD, n = 10. *P < 0.05, versus Gly + PBS group; #P < 0.05, versus Loganetin treated at 2 mg/kg.
Figure S5 Loganetin regulated the mRNA levels of TLR4‐related factors in HK‐2 cells. HK2 cells pretreated by Loganetin (100 nM) at the indicated times, washed, and stimulated with LPS (1 ng/ml) for 6 hr, mRNA levels of TLR4‐related factors were detected by QRT‐PCR. Data are expressed as means + SD, n = 6.
Figure S6 The protection effects of Loganetin in HK‐2 cells. HK2 cells pretreated with LPS (1 ng/ml) for 6 hr, washed, and stimulated with Loganetin (100 nM) for 6, 12, and 24 hr, the apoptosis rate (A) and cell viability (B) were determined. Experiments were performed in triplicate for each group. Data are expressed as the means ± S.D. *P < 0.05.
Figure S7 The protective mechanisms of Loganetin on HK‐2 cells were mediated by TLR4. shTLR4 plasmid transfected into HK‐2 cells with or without shTLR4 plasmid transfection were pretreated by Loganetin (100 nM) for 12 hr, then washed and incubated with LPS (1 ng/ml) for 6 hr, semi‐quantitative analysis of immunofluorescence assay was performed to detected mRNA levels of TLR4‐related genes. Data are expressed as means + SD, n = 6. *P < 0.05, versus Loganetin pretreated in TLR4‐WT + LPS group.
Figure S8 Deletion of TLR4 ameliorated renal dysfunction, renal injury, apoptosis, and cell death in vivo and in vitro. (A) Semi‐quantitative analysis of Annexin V/PI staining in HK‐2 cells with or without shTLR4 plasmid by flow cytometry assay. Data are expressed as means + SD, n = 6. *P < 0.05, versus LPS‐treated group of the same genotype group (TLR4‐WT or ‐KO). (B) Immunoblot assay of TLR4 expressions in TLR4‐WT or TLR4‐KO mice kidney tissues at 72 hr after glycerol‐induced RIAKI. (n = 10).
Figure S9 Loganetin improved RIAKI in mice by TLR4. TLR‐WT mice subjected to different treatments were killed at 72 hr after glycerol alone or combined with LPS injection. Representative H&E (upper), PAS (middle), and TUNEL (lower) staining images of kidney (A), ATN score (B, left), blood urea nitrogen (BUN, left), serum levels of (right) creatinine (CREA, right), brush borders (middle), and TUNEL‐positive cells (left) were detected and performed (B). Data are expressed as means + SD, n = 10. *P < 0.05 versus glycerol‐treated group; #P < 0.05, versus glycerol + LPS treated group.
ACKNOWLEDGEMENTS
This study was supported by the National Natural Science Foundation of China (Grants 81400752 to C.Y.; 81370852 to M.X.; 81270832 to R.R.; and 81270833 and 81570674 to T.Z.).
Li J, Tan Y, Wang M, et al. Loganetin protects against rhabdomyolysis‐induced acute kidney injury by modulating the toll‐like receptor 4 signalling pathway. Br J Pharmacol. 2019;176:1106–1121. 10.1111/bph.14595
Contributor Information
Gui‐min Zhang, Email: lnzhangguimin@lunan.cn.
Yu‐shan Ren, Email: newtimes2015@yeah.net.
REFERENCES
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … Collaborators, C. G. T. P. (2017a). 2017/18: Catalytic receptors. British Journal of Pharmacology, 174(S1), S225–S271. 10.1111/bph.13876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … Collaborators, C. G. T. P. (2017b). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. British Journal of Pharmacology, 174(S1), S272–S359. 10.1111/bph.13877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , Harding, S. D. , … CGTP Collaborators (2017). The Concise Guide to PHARMACOLOGY 2017/18: Overview. British Journal of Pharmacology, 174(Suppl 1), S1–S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora, M. K. , & Singh, U. K. (2014). Oxidative stress: Meeting multiple targets in pathogenesis of diabetic nephropathy. Current Drug Targets, 15(5), 531–538. 10.2174/1389450115666140321120635 [DOI] [PubMed] [Google Scholar]
- Ashall, V. , & Millar, K. (2014). Endpoint matrix: A conceptual tool to promote consideration of the multiple dimensions of humane endpoints. ALTEX, 31(2), 209–213. 10.14573/altex.1307261 [DOI] [PubMed] [Google Scholar]
- Bellomo, R. , Kellum, J. A. , & Ronco, C. (2012). Acute kidney injury. Lancet, 380(9843), 756–766. 10.1016/S0140-6736(11)61454-2 [DOI] [PubMed] [Google Scholar]
- Campbell, M. T. , Hile, K. L. , Zhang, H. , Asanuma, H. , Vanderbrink, B. A. , Rink, R. R. , & Meldrum, K. K. (2011). Toll‐like receptor 4: A novel signaling pathway during renal fibrogenesis. The Journal of Surgical Research, 168(1), e61–e69. 10.1016/j.jss.2009.09.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao, G. , Tian, X. , Zhang, W. , Ou, X. , Cong, F. , & Song, T. (2017). Blocking Smad2 signalling with loganin attenuates SW10 cell cycle arrest induced by TNF‐alpha. PLoS ONE, 12(5), e0176965 10.1371/journal.pone.0176965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J. , John, R. , Richardson, J. A. , Shelton, J. M. , Zhou, X. J. , Wang, Y. , … Lu, C. Y. (2011). Toll‐like receptor 4 regulates early endothelial activation during ischemic acute kidney injury. Kidney International, 79(3), 288–299. 10.1038/ki.2010.381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis, M. J. , Alexander, S. , Cirino, G. , Docherty, J. R. , George, C. H. , Giembycz, M. A. , … Ahluwalia, A. (2018). Experimental design and analysis and their reporting II: Updated and simplified guidance for authors and peer reviewers. British Journal of Pharmacology, 175(7), 987–993. 10.1111/bph.14153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El‐Achkar, T. M. , Huang, X. , Plotkin, Z. , Sandoval, R. M. , Rhodes, G. J. , & Dagher, P. C. (2006). Sepsis induces changes in the expression and distribution of Toll‐like receptor 4 in the rat kidney. American Journal of Physiology. Renal Physiology, 290(5), F1034–F1043. 10.1152/ajprenal.00414.2005 [DOI] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Research, 46(D1), D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, R. , Shi, M. , Guo, F. , Feng, Y. , Liu, J. , Li, L. , … Fu, P. (2018). Pharmacological Inhibition of fatty acid‐binding protein 4 (FABP4) protects against rhabdomyolysis‐induced acute kidney injury. Frontiers in Pharmacology, 9, 917 10.3389/fphar.2018.00917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, R. S. , Zhou, J. J. , Feng, Y. Y. , Shi, M. , Guo, F. , Gou, S. J. , … Fu, P. (2017). Pharmacological inhibition of macrophage Toll‐like receptor 4/nuclear factor‐kappa B alleviates rhabdomyolysis‐induced acute kidney injury. Chinese Medical Journal, 130(18), 2163–2169. 10.4103/0366-6999.213406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang, H. R. , Ko, G. J. , Wasowska, B. A. , & Rabb, H. (2009). The interaction between ischemia‐reperfusion and immune responses in the kidney. Journal of Molecular Medicine (Berl), 87(9), 859–864. 10.1007/s00109-009-0491-y [DOI] [PubMed] [Google Scholar]
- Jesinkey, S. R. , Funk, J. A. , Stallons, L. J. , Wills, L. P. , Megyesi, J. K. , Beeson, C. C. , & Schnellmann, R. G. (2014). Formoterol restores mitochondrial and renal function after ischemia‐reperfusion injury. Journal of the American Society of Nephrology: JASN, 25(6), 1157–1162. 10.1681/ASN.2013090952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao, C. J. , Chen, W. F. , Guo, B. L. , Feng, C. W. , Hung, H. C. , Yang, W. Y. , … Wen, Z. H. (2017). The 1‐tosylpentan‐3‐one protects against 6‐hydroxydopamine‐induced neurotoxicity. International Journal of Molecular Sciences, 18(5). 10.3390/ijms18051096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny, C. , Browne, W. , Cuthill, I. C. , Emerson, M. , & Altman, D. G. (2010). Animal research: Reporting in vivo experiments: The ARRIVE guidelines. British Journal of Pharmacology, 160, 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, M. G. , Koo, T. Y. , Yan, J. J. , Lee, E. , Han, K. H. , Jeong, J. C. , … Yang, J. (2013). IL‐2/anti‐IL‐2 complex attenuates renal ischemia‐reperfusion injury through expansion of regulatory T cells. Journal of the American Society of Nephrology: JASN, 24(10), 1529–1536. 10.1681/ASN.2012080784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, M. J. , Bae, G. S. , Jo, I. J. , Choi, S. B. , Kim, D. G. , Shin, J. Y. , … Park, S. J. (2015). Loganin protects against pancreatitis by inhibiting NF‐kappaB activation. European Journal of Pharmacology, 765, 541–550. 10.1016/j.ejphar.2015.09.019 [DOI] [PubMed] [Google Scholar]
- Kwon, S. H. , Kim, H. C. , Lee, S. Y. , & Jang, C. G. (2009). Loganin improves learning and memory impairments induced by scopolamine in mice. European Journal of Pharmacology, 619(1–3), 44–49. 10.1016/j.ejphar.2009.06.062 [DOI] [PubMed] [Google Scholar]
- Kwon, S. H. , Kim, J. A. , Hong, S. I. , Jung, Y. H. , Kim, H. C. , Lee, S. Y. , & Jang, C. G. (2011). Loganin protects against hydrogen peroxide‐induced apoptosis by inhibiting phosphorylation of JNK, p38, and ERK 1/2 MAPKs in SH‐SY5Y cells. Neurochemistry International, 58(4), 533–541. 10.1016/j.neuint.2011.01.012 [DOI] [PubMed] [Google Scholar]
- Lee, K. Y. , Sung, S. H. , Kim, S. H. , Jang, Y. P. , Oh, T. H. , & Kim, Y. C. (2009). Cognitive‐enhancing activity of loganin isolated from Cornus officinalis in scopolamine‐induced amnesic mice. Archives of Pharmacal Research, 32(5), 677–683. 10.1007/s12272-009-1505-6 [DOI] [PubMed] [Google Scholar]
- Li, J. , Wan, Y. , Na, S. , Liu, X. , Dong, G. , Yang, Z. , … Yue, J. (2015). Sex‐dependent regulation of hepatic CYP3A by growth hormone: Roles of HNF6, C/EBPalpha, and RXRalpha. Biochemical Pharmacology, 93(1), 92–103. 10.1016/j.bcp.2014.10.010 [DOI] [PubMed] [Google Scholar]
- Li, J. , Xie, M. , Wang, X. , Ouyang, X. , Wan, Y. , Dong, G. , … Yue, J. (2015). Sex hormones regulate cerebral drug metabolism via brain miRNAs: Down‐regulation of brain CYP2D by androgens reduces the analgesic effects of tramadol. British Journal of Pharmacology, 172(19), 4639–4654. 10.1111/bph.13206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, L. , Huang, L. , Vergis, A. L. , Ye, H. , Bajwa, A. , Narayan, V. , … Okusa, M. D. (2010). IL‐17 produced by neutrophils regulates IFN‐gamma‐mediated neutrophil migration in mouse kidney ischemia‐reperfusion injury. The Journal of Clinical Investigation, 120(1), 331–342. 10.1172/JCI38702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, P. K. , Burdmann, E. A. , & Mehta, R. L. (2013). World Kidney Day 2013: Acute kidney injury‐global health alert. American Journal of Kidney Diseases: The Official Journal of the National Kidney Foundation, 61(3), 359–363. 10.1053/j.ajkd.2013.01.002 [DOI] [PubMed] [Google Scholar]
- Li, Y. , Li, Z. , Shi, L. , Zhao, C. , Shen, B. , Tian, Y. , & Feng, H. (2016). Loganin inhibits the inflammatory response in mouse 3T3L1 adipocytes and mouse model. International Immunopharmacology, 36, 173–179. 10.1016/j.intimp.2016.04.026 [DOI] [PubMed] [Google Scholar]
- Liu, K. , Xu, H. , Lv, G. , Liu, B. , Lee, M. K. , Lu, C. , … Wu, Y. (2015). Loganin attenuates diabetic nephropathy in C57BL/6J mice with diabetes induced by streptozotocin and fed with diets containing high level of advanced glycation end products. Life Sciences, 123, 78–85. 10.1016/j.lfs.2014.12.028 [DOI] [PubMed] [Google Scholar]
- Matsunaga, N. , Tsuchimori, N. , Matsumoto, T. , & Ii, M. (2011). TAK‐242 (resatorvid), a small‐molecule inhibitor of Toll‐like receptor (TLR) 4 signaling, binds selectively to TLR4 and interferes with interactions between TLR4 and its adaptor molecules. Molecular Pharmacology, 79(1), 34–41. 10.1124/mol.110.068064 [DOI] [PubMed] [Google Scholar]
- Mehta, R. L. , Burdmann, E. A. , Cerda, J. , Feehally, J. , Finkelstein, F. , Garcia‐Garcia, G. , … Remuzzi, G. (2016). Recognition and management of acute kidney injury in the International Society of Nephrology 0by25 Global Snapshot: A multinational cross‐sectional study. Lancet, 387(10032), 2017–2025. 10.1016/S0140-6736(16)30240-9 [DOI] [PubMed] [Google Scholar]
- Miettinen, K. , Dong, L. , Navrot, N. , Schneider, T. , Burlat, V. , Pollier, J. , … Werck‐Reichhart, D. (2014). The seco‐iridoid pathway from Catharanthus roseus. Nature Communications, 5, 3606 10.1038/ncomms4606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagatoshi, M. , Terasaka, K. , Nagatsu, A. , & Mizukami, H. (2011). Iridoid‐specific glucosyltransferase from Gardenia jasminoides. The Journal of Biological Chemistry, 286(37), 32866–32874. 10.1074/jbc.M111.242586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura, D. M. , & Pennathur, S. (2015). The balance of powers: Redox regulation of fibrogenic pathways in kidney injury. Redox Biology, 6, 495–504. 10.1016/j.redox.2015.09.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Press, A. T. , Butans, M. J. , Haider, T. P. , Weber, C. , Neugebauer, S. , Kiehntopf, M. , … Kortgen, A. (2017). Fast simultaneous assessment of renal and liver function using polymethine dyes in animal models of chronic and acute organ injury. Scientific Reports, 7(1), 15397 10.1038/s41598-017-14987-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulskens, W. P. , Teske, G. J. , Butter, L. M. , Roelofs, J. J. , van der Poll, T. , Florquin, S. , & Leemans, J. C. (2008). Toll‐like receptor‐4 coordinates the innate immune response of the kidney to renal ischemia/reperfusion injury. PLoS ONE, 3(10), e3596 10.1371/journal.pone.0003596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren, Y. , Min, Y. Q. , Liu, M. , Chi, L. , Zhao, P. , & Zhang, X. L. (2016). N‐Glycosylation‐mutated HCV envelope glycoprotein complex enhances antigen‐presenting activity and cellular and neutralizing antibody responses. Biochimica et Biophysica Acta, 1860(8), 1764–1775. 10.1016/j.bbagen.2015.08.007 [DOI] [PubMed] [Google Scholar]
- Souza, A. C. , Tsuji, T. , Baranova, I. N. , Bocharov, A. V. , Wilkins, K. J. , Street, J. M. , … Star, R. A. (2015). TLR4 mutant mice are protected from renal fibrosis and chronic kidney disease progression. Physiological Reports, 3(9), e12558 10.14814/phy2.12558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchino, S. , Kellum, J. A. , Bellomo, R. , Doig, G. S. , Morimatsu, H. , Morgera, S. , … Beginning and ending supportive therapy for the kidney (BEST Kidney) investigators (2005). Acute renal failure in critically ill patients: A multinational, multicenter study. Jama, 294(7), 813–818. 10.1001/jama.294.7.813 [DOI] [PubMed] [Google Scholar]
- Valko, M. , Jomova, K. , Rhodes, C. J. , Kuca, K. , & Musilek, K. (2016). Redox‐ and non‐redox‐metal‐induced formation of free radicals and their role in human disease. Archives of Toxicology, 90(1), 1–37. 10.1007/s00204-015-1579-5 [DOI] [PubMed] [Google Scholar]
- Wang, J. , Li, H. , Qiu, S. , Dong, Z. , Xiang, X. , & Zhang, D. (2017). MBD2 upregulates miR‐301a‐5p to induce kidney cell apoptosis during vancomycin‐induced AKI. Cell Death & Disease, 8(10), e3120 10.1038/cddis.2017.509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, S. , Zhang, C. , Li, J. , Niyazi, S. , Zheng, L. , Xu, M. , … Zhu, T. (2017). Erythropoietin protects against rhabdomyolysis‐induced acute kidney injury by modulating macrophage polarization. Cell Death & Disease, 8(4), e2725 10.1038/cddis.2017.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, H. , Chen, G. , Wyburn, K. R. , Yin, J. , Bertolino, P. , Eris, J. M. , … Chadban, S. J. (2007). TLR4 activation mediates kidney ischemia/reperfusion injury. The Journal of Clinical Investigation, 117(10), 2847–2859. 10.1172/JCI31008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, H. , Ma, J. , Wang, P. , Corpuz, T. M. , Panchapakesan, U. , Wyburn, K. R. , & Chadban, S. J. (2010). HMGB1 contributes to kidney ischemia reperfusion injury. Journal of the American Society of Nephrology: JASN, 21(11), 1878–1890. 10.1681/ASN.2009101048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, J. , Pan, X. , Fu, H. , Zheng, Y. , Dai, Y. , Yin, Y. , … Hou, D. (2017). Effect of curcumin on glycerol‐induced acute kidney injury in rats. Scientific Reports, 7(1), 10114 10.1038/s41598-017-10693-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, C. , Chang, A. , Hack, B. K. , Eadon, M. T. , Alper, S. L. , & Cunningham, P. N. (2014). TNF‐mediated damage to glomerular endothelium is an important determinant of acute kidney injury in sepsis. Kidney International, 85(1), 72–81. 10.1038/ki.2013.286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamabe, N. , Noh, J. S. , Park, C. H. , Kang, K. S. , Shibahara, N. , Tanaka, T. , & Yokozawa, T. (2010). Evaluation of loganin, iridoid glycoside from Corni Fructus, on hepatic and renal glucolipotoxicity and inflammation in type 2 diabetic db/db mice. European Journal of Pharmacology, 648(1–3), 179–187. 10.1016/j.ejphar.2010.08.044 [DOI] [PubMed] [Google Scholar]
- Yao, L. , Peng, S. X. , Xu, Y. D. , Lin, S. L. , Li, Y. H. , Liu, C. J. , … Shen, Y. Q. (2017). Unexpected neuroprotective effects of loganin on 1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine‐induced neurotoxicity and cell death in zebrafish. Journal of Cellular Biochemistry, 118(3), 615–628. 10.1002/jcb.25749 [DOI] [PubMed] [Google Scholar]
- You, H. , Gao, T. , Raup‐Konsavage, W. M. , Cooper, T. K. , Bronson, S. K. , Reeves, W. B. , & Awad, A. S. (2017). Podocyte‐specific chemokine (C‐C motif) receptor 2 overexpression mediates diabetic renal injury in mice. Kidney International, 91(3), 671–682. 10.1016/j.kint.2016.09.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, M. Z. , Wang, X. , Wang, Y. , Niu, A. , Wang, S. , Zou, C. , & Harris, R. C. (2017). IL‐4/IL‐13‐mediated polarization of renal macrophages/dendritic cells to an M2a phenotype is essential for recovery from acute kidney injury. Kidney International, 91(2), 375–386. 10.1016/j.kint.2016.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Q. , Liu, L. , Lin, W. , Yin, S. , Duan, A. , Liu, Z. , & Cao, W. (2017). Rhein reverses Klotho repression via promoter demethylation and protects against kidney and bone injuries in mice with chronic kidney disease. Kidney International, 91(1), 144–156. 10.1016/j.kint.2016.07.040 [DOI] [PubMed] [Google Scholar]
- Zhao, M. , Tao, J. , Qian, D. , Liu, P. , Shang, E. X. , Jiang, S. , … Du, L. (2016). Simultaneous determination of loganin, morroniside, catalpol and acteoside in normal and chronic kidney disease rat plasma by UPLC‐MS for investigating the pharmacokinetics of Rehmannia glutinosa and Cornus officinalis Sieb drug pair extract. Journal of chromatography. B, Analytical Technologies in the Biomedical and Life Sciences, 1009‐1010, 122–129. 10.1016/j.jchromb.2015.12.020 [DOI] [PubMed] [Google Scholar]
- Zhao, Y. , Ren, Y. , Zhang, X. , Zhao, P. , Tao, W. , Zhong, J. , … Zhang, X. L. (2014). Ficolin‐2 inhibits hepatitis C virus infection, whereas apolipoprotein E3 mediates viral immune escape. Journal of Immunology, 193(2), 783–796. 10.4049/jimmunol.1302563 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 The effects of Loganetin on mice renal function. Mice subjected to Loganetin injection and killed after 72 hr. Representative H&E, PAS, and TUNEL staining images of kidney (A) and the ATN scores (B), blood urea nitrogen (BUN, C) creatinine (CREA, D), brush border (E), TUNEL‐positive cells (F) at 72 hr were calculated. (G) Representative survival curves of each group, sold line: Loganetin pretreatment; dotted line: Loganetin post‐treatment. Data are expressed as the means ± S.D., n = 10.
Figure S2 Loganetin regulated the expressions of TLR4/Jnk/p38 pathway in RIKAI. Quantitative analysis of p65, Jnk, p38, and phospho‐p65, −Jnk, −p38, p21, c‐Jun, Bax, Caspase‐9 and cleaved caspase‐3 expression by QRT‐PCR of kidney tissues. Data are expressed as means + SD, n = 10. *P < 0.05, versus Gly + PBS group.
Figure S3 The protection effects of Loganetin on myoglobin stimulated mouse peritoneal macrophages and HK2 cells. Time‐ and concentration‐dependence of the inhibitory effect of Loganetin on the production of TNF‐α and IL‐6 in mouse peritoneal macrophages stimulated with myoglobin were determined. Cells were incubated with TAK‐242 (100 nM) or Loganetin (100 nM) at the indicated times, washed, and stimulated with myoglobin. The concentrations of TNF‐α and IL‐6 (A) in the culture supernatants were determined using specific elisa kits. (B) HK2 cells pretreated with Loganetin (100 nM) for 24 hr, washed, and stimulated with myoglobin for 6 hr were lysed using cell lysis buffer for immunoblots. Experiments were performed in triplicate for each group.
Figure S4 Loganetin regulated the mRNA levels of pro‐inflammatory factors in RIAKI mice. Renal mRNA levels of IFN‐γ, TNF‐α, IL‐17A, IL‐6 and IL‐1β were analysed by qPCR. Data are expressed as means + SD, n = 10. *P < 0.05, versus Gly + PBS group; #P < 0.05, versus Loganetin treated at 2 mg/kg.
Figure S5 Loganetin regulated the mRNA levels of TLR4‐related factors in HK‐2 cells. HK2 cells pretreated by Loganetin (100 nM) at the indicated times, washed, and stimulated with LPS (1 ng/ml) for 6 hr, mRNA levels of TLR4‐related factors were detected by QRT‐PCR. Data are expressed as means + SD, n = 6.
Figure S6 The protection effects of Loganetin in HK‐2 cells. HK2 cells pretreated with LPS (1 ng/ml) for 6 hr, washed, and stimulated with Loganetin (100 nM) for 6, 12, and 24 hr, the apoptosis rate (A) and cell viability (B) were determined. Experiments were performed in triplicate for each group. Data are expressed as the means ± S.D. *P < 0.05.
Figure S7 The protective mechanisms of Loganetin on HK‐2 cells were mediated by TLR4. shTLR4 plasmid transfected into HK‐2 cells with or without shTLR4 plasmid transfection were pretreated by Loganetin (100 nM) for 12 hr, then washed and incubated with LPS (1 ng/ml) for 6 hr, semi‐quantitative analysis of immunofluorescence assay was performed to detected mRNA levels of TLR4‐related genes. Data are expressed as means + SD, n = 6. *P < 0.05, versus Loganetin pretreated in TLR4‐WT + LPS group.
Figure S8 Deletion of TLR4 ameliorated renal dysfunction, renal injury, apoptosis, and cell death in vivo and in vitro. (A) Semi‐quantitative analysis of Annexin V/PI staining in HK‐2 cells with or without shTLR4 plasmid by flow cytometry assay. Data are expressed as means + SD, n = 6. *P < 0.05, versus LPS‐treated group of the same genotype group (TLR4‐WT or ‐KO). (B) Immunoblot assay of TLR4 expressions in TLR4‐WT or TLR4‐KO mice kidney tissues at 72 hr after glycerol‐induced RIAKI. (n = 10).
Figure S9 Loganetin improved RIAKI in mice by TLR4. TLR‐WT mice subjected to different treatments were killed at 72 hr after glycerol alone or combined with LPS injection. Representative H&E (upper), PAS (middle), and TUNEL (lower) staining images of kidney (A), ATN score (B, left), blood urea nitrogen (BUN, left), serum levels of (right) creatinine (CREA, right), brush borders (middle), and TUNEL‐positive cells (left) were detected and performed (B). Data are expressed as means + SD, n = 10. *P < 0.05 versus glycerol‐treated group; #P < 0.05, versus glycerol + LPS treated group.


