Highlights
-
•
Thiazole Yellow G dye was completely mineralised by Aspergillus niger LAG.
-
•
A degradation pathway was proposed showing initial asymmetric cleavage action.
-
•
Laccase was implicated in the desulfonation and demethylation of the dye.
-
•
The increase in germination percentage further affirmed the dye’s detoxified status.
Keywords: Biodegradation, Aspergillus niger, Thiazole yellow G dye, Detoxification
Abstract
Filamentous fungi perform tremendously in adsorption of dyes from polluted environment. In this study, Aspergillus niger LAG decolorized thiazole yellow G dye within 5 days. Scale up studies done revealed that maximum decolorization (98%) was achieved at a concentration (10 mg L−1), temperature (35 °C) and pH 6. The fungus exhibited significant inductions in laccase (71%) and lignin peroxidase (48%) respectively. Spectrometric analysis (UV–vis, HPLC and gas chromatography-mass spectrometry) was used in analyzing the degraded products of the dye. The GCMS analysis revealed the production of two metabolites; sodium 6-methyl-2-phenyl-1,3-benzothiazole-7-sulfonate and 2-phenyl-4,5-dihydro-1,3-thiazole after degradation of thiazole yellow G dye. A metabolic pathway of thiazole yellow G dye degradation by Aspergillus niger was proposed. Significant growth in plumule and radicle couple with an attendant increase in germination further confirmed the detoxified status of the dye after degradation.
1. Introduction
The brunt (toxigenic and carcinogenic hazards) of indiscriminate disposal of dyeing effluents are borne by the aquatic systems and humans [1]. These adverse effects are made worse due to the complex aromatic nature of most dyes which makes the treatment of dyeing effluent extremely difficult [2]. Mishra and Tripathy [3] reported that synthetic dyes (direct, acid and reactive dyes) are ionic, basic are cationic and disperse are non-ionic in nature. Different reactive groups (vinyl sulfone, chlorotriazine, trichloropyrimidine and difluorochloropyrimidine) makes up the chromophores of most azo dyes [4]. Excellent light fastness, water fastness, wash fastness, colour fastness, and perspiration fastness qualities endeared most dye practitioners to the use of reactive dyes [4]. Little success has been recorded on dye effluent treatment through convention means [5]. Thiazole yellow G is a cationic basic thiazine dye extensively used in dyeing cotton [6]. Thiazole yellow G dye can cause health problems in humans after inhalation [7]. It is important to treat dyeing wastewaters containing reactive dyes and thiazole yellow G dye because of their harmful effects.
Ayed et al. [8] reported that dye effluent management could be relatively expensive using known physical (biosorption and adsorption) and chemical methods (oxidation, ozonation, and flocculation). The non- functionality and non- feasibility of these methods were further corroborated by Dönmez [9]. Biotreatment of dye wastewater is regarded and accorded the most economical and eco-friendly alternative [10]. Several microbial dye treatment systems have been employed which includes the use of growing culture in medium and biosorption (living or dead). Fu and Viraraghavan [11] reported that bacteria, fungi, algae and yeasts have been found very potent in the treatment of virtually all classes of dyes.
The polymers obtainable in the cells of some filamentous fungi like Aspergillus niger gave them the edge in the biotechnological treatment of textile dye wastewater through adsorption process [12]. This was demonstrated when Aksu and Karabayır [13] reported effective dye removal properties of Rhizopus arrhizus dead biomass. Several enzymes (lignin peroxidase, laccase and manganese peroxidase) have been implicated in the dye decolorization potency of some filamentous fungi [14].
This study aims to investigate the decolorization and detoxification efficiency of Aspergillus niger on thiazole yellow G dye. The physico-chemical parameters were further scaled up during the experiment to study their effect of decolorization process. The toxic status were determined using toxicity analyses after degradation.
2. Materials and methods
2.1. Dye collection
Thiazole yellow G dye was kindly donated by Sunflag Nigeria Limited, textile manufacturing industry in Surulere, Lagos State, Nigeria. The dye collected was kept in sterile airtight plastic can. It was of best analytical grade and high purity (97%).
2.2. Description of the dye
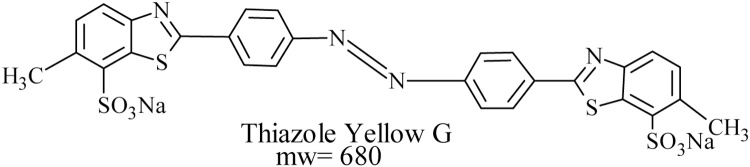
Thiazole yellow G dye is a complex di-azo dye with the chemical formula C52H26Cl2N14Na8O26S8, Molecular weight 680 g mol−1, and chemical structure (Fig. 1).
Fig. 1.
Chemical structure of thiazole yellow G dye.
2.3. Micro-organisms and culture conditions
The method used by Bankole et al [15].was adopted in the polluted soil collection procedure. Isolation of the fungus was then carried out on potato dextrose agar (PDA) slants and maintained at 4 °C. The fungus was later grown in 250 ml flask (Erlenmeyer) containing 100 ml potato dextrose broth (PDB) at 37 °C for 24 h. The 24 h culture was kept at 4 °C.
2.4. Decolorization experiments
The maximum wavelength (λmax-503 nm) of thiazole yellow G dye solution was determined and recorded using UV–vis spectrophotometer. Spore suspension (5 ml) was later transferred into an Erlenmeyer flask (already containing 100 ml of PDB and 100 mg L−1 of the dye). The set up was done in triplicates with respective biocontrols (non-inoculated) and monitored for 15 days at room temperature. The supernatant obtained after centrifugation (10,000 x g for 10 min) of aliquots taken after 0, 3, 6, 9, 12 and 15 days were later diluted (at 1:15 ratio) with sterile distilled water. Spectrophotometer-UV/Vis was used in monitoring change in absorbance as described by Bankole et al [15].
2.4.1. GCMS analysis
Helium was used as carrier gas. Shimadzu QP 2010 GCMS Engine (Shimadzu Corporation, Japan) was used following the earlier procedure reported by Bankole et al [14]. The pattern of spectral peaks and retention times were used in identifying the metabolites.
2.4.2. HPLC analysis
The HPLC machine has a dual wavelength UV detector and C18 column (symmetry, 4.6 × 250 mm) (Waters, USA 2690 system). The flow rate was set at 0.80 mL min−1 with methanol (being used as the mobile phase). The analysis was left to run for 10 min.
2.5. Optimization of physicochemical parameters during decolorization studies
The pH values were varied from 4 to 9 for the experiments in static cultures in a water bath with the temperature preset and maintained at 32 °C. The studies were equally repeated by varying the temperature between 30 and 40 °C at pH of 7.0. Effect of initial concentrations (10–60 mg L−1) on decolorization of thiazole yellow G dye by Aspergillus niger was further done Bankole et al. [15].
2.6. Enzymatic analyses
Time course laccase and lignin peroxidase enzyme analyses were conducted using the method used by Bankole et al. [16].
2.7. Phytotoxicity studies
The toxic effects on plants of the dye and dye metabolites after decolorization by the fungi were determined using the method of Bankole et al. [17] with slight modifications. Ethyl acetate extract of Thiazole Yellow G dye metabolic products was dried and dissolved in sterile distilled water to a final concentration of 1000 ppm. The phytotoxicity studies were performed on seeds of Sorghum biocolor and Vigna unguiculata, two plants commonly found in Nigeria. Sixty seeds (60 each) were wetted (20 ml per day) with dye metabolites and dye (1000 ppm) in separate petri plates. Seeds wet with tap water were included as controls. Germination percentage (%), Length of plumule (cm), and radicle (cm) and was recorded after 12 days.
3. Results and discussion
3.1. UV–vis spectroscopy
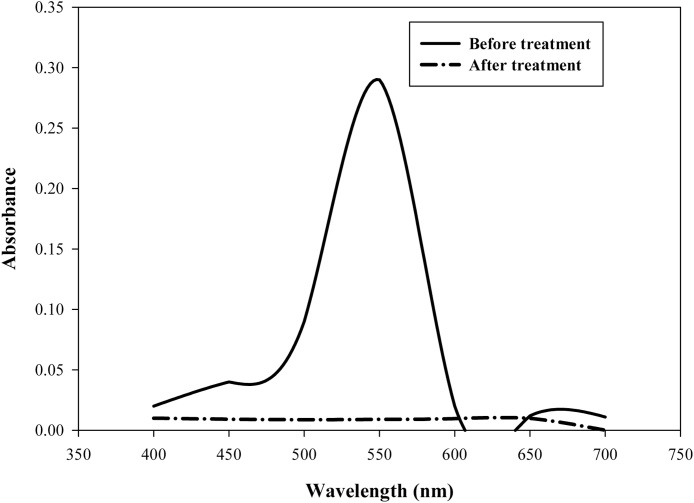
There was relative disappearance (at day 5) of the peak observed at 503 nm (control-day 0) in the UV/Vis absorbance spectra of thiazole yellow G dye (Fig. 2). Significant disintegration of peaks was observed which suggested a reduction of the dye components thus decolorization. The visual changes observed in the Erlenmeyer flasks corroborated the near disappearance of the peak at day 5.
Fig. 2.
UV/Vis spectra of thiazole yellow G dye before and after treatment with Aspergillus niger.
3.2. Decolorization at different pH
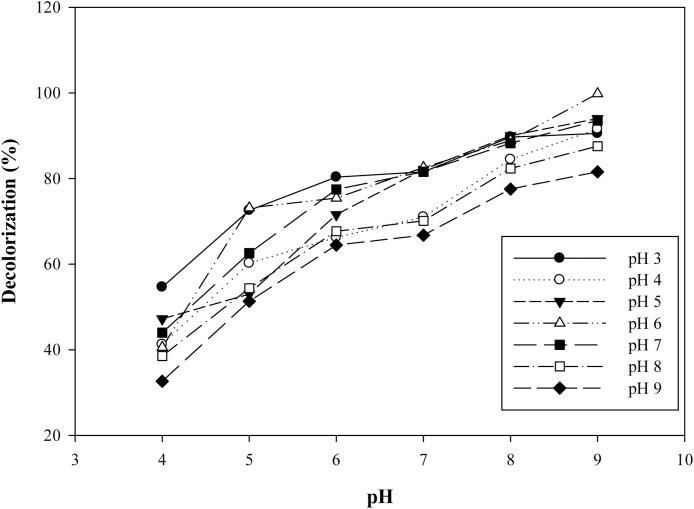
Optimum decolorization efficiency, 97.85 and 99.97% was observed at pH 5 and 6 respectively while a steady decline was recorded from pH 4 to pH 9 (Fig. 3). Previous works by Kaushik and Malik [18] and Tian et al [19] reported that at pH 2–6, optimum dye adsorption is greatly enhanced between the adsorbent and adsorbate. The result of this study further corroborated Wu et al [20] suggestion that fungal cell surface is highly charged and active in acidic medium. Dye decolorization is largely due to the van der waal forces between the negatively charged thiazole yellow G dye anions and positively charged Aspergillus niger cells. Iscen et al [21] reported that alkaline pH brings about depletion in adsorption process between the dye and the adsorbent due to repulsion on the active sites of the fungal biomass surface.
Fig. 3.
Effect of pH on decolorization of thiazole yellow G dye by Aspergillus niger.
3.3. Decolorization at varying concentrations
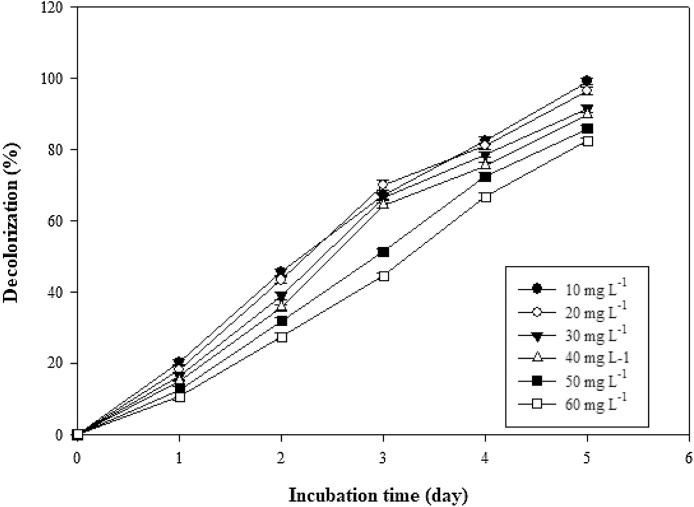
An upsurge in concentration (10, 20, 30, 40, 50 and 60 mg L−1) of thiazole yellow G dye led to depletion in decolorization efficiency (99.1, 96.54, 91.44, 89.67, 85.76 and 82.44%) (Fig. 4). Higher concentrations of the dye exhibited a negative effect on adsorption process. This result was in concordance with previous work done by Solís et al [22]. Yargic et al [23] also corroborated the fact that lower concentrations of thiazole yellow G dye propel mass transfer resistance between adsorbent and adsorbate. Increasing dye concentration usually leads to surface saturation of the fungal cell surface which in turn depletes decolorization efficiency [21].
Fig. 4.
Effect of initial concentrations (mg L−1) on decolorization of thiazole yellow G dye by Aspergillus niger.
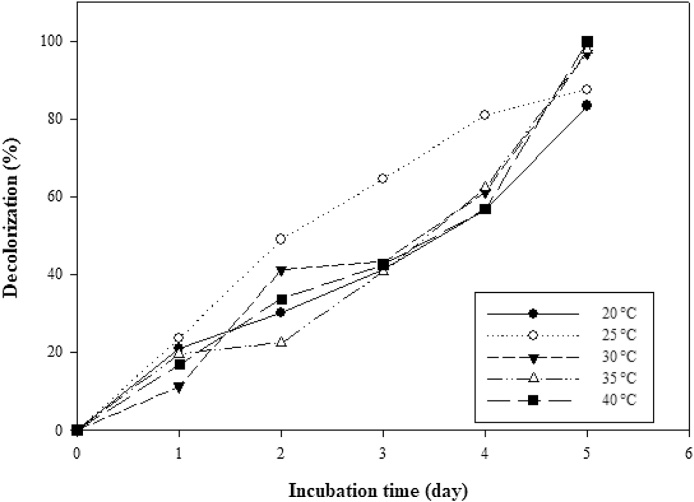
3.4. Decolorization at different temperatures
At lower temperatures (20 and 25 °C), significant decolorization was recorded while optimum decolorization (99.95%) of thiazole yellow G dye was observed at almost ambient temperature (40 °C) (Fig. 5). Decolorization by biosorption is actively aided through an increasing temperature of the reaction medium of the biomass and thiazole yellow G dye [19]. Our study characteristically showed that myco-removal of colour usually decreases with attendant increase in temperature.
Fig. 5.
Effect of temperature (°C) on decolorization of thiazole yellow G dye by Aspergillus niger.
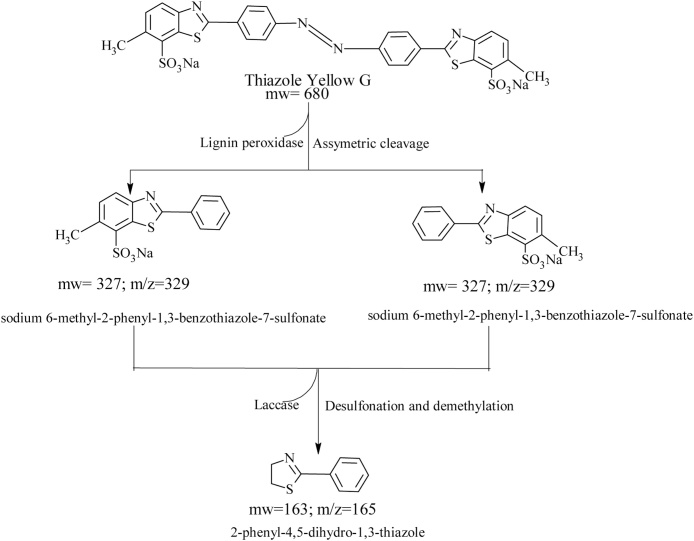
3.5. GCMS analysis
The GCMS analyses results (Table 1) revealed the formation of two major intermediate products with a molecular weight of 327 and 163 g mol−1 representing sodium 6-methyl-2-phenyl-1,3-benzothiazole-7-sulfonate and 2-phenyl-4,5-dihydro-1,3-thiazole (Fig. 6) respectively. Laccase was however responsible for the demethylation and desulfonation of the intermediate metabolites to 2-phenyl-4,5-dihydoxy-1,3-thiazole. Laccase is known for its azo dye desulfonation dexterity during biodegradation process [16]. The GCMS data obtained was used to propose a schematic pathway of degradation of thiazole yellow G dye by Aspergillus niger (Fig. 6).
Table 1.
GC–MS data of obtained metabolite after degradation of thiazole yellow G dye by Aspergillus niger.
| Peak | RT (min) |
m/z | Mol. weight | Description | Mass spectrum |
|---|---|---|---|---|---|
| 1 | 19.689 | 329 | 327 | sodium 6-methyl-2-phenyl-1,3-benzothiazole-7-sulfonate |  |
| 2 | 11.421 | 165 | 163 | 2-phenyl-4,5-dihydro-1,3-thiazole | 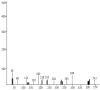 |
Fig. 6.
Proposed schematic pathway for degradation of thiazole yellow G dye by Aspergillus niger.
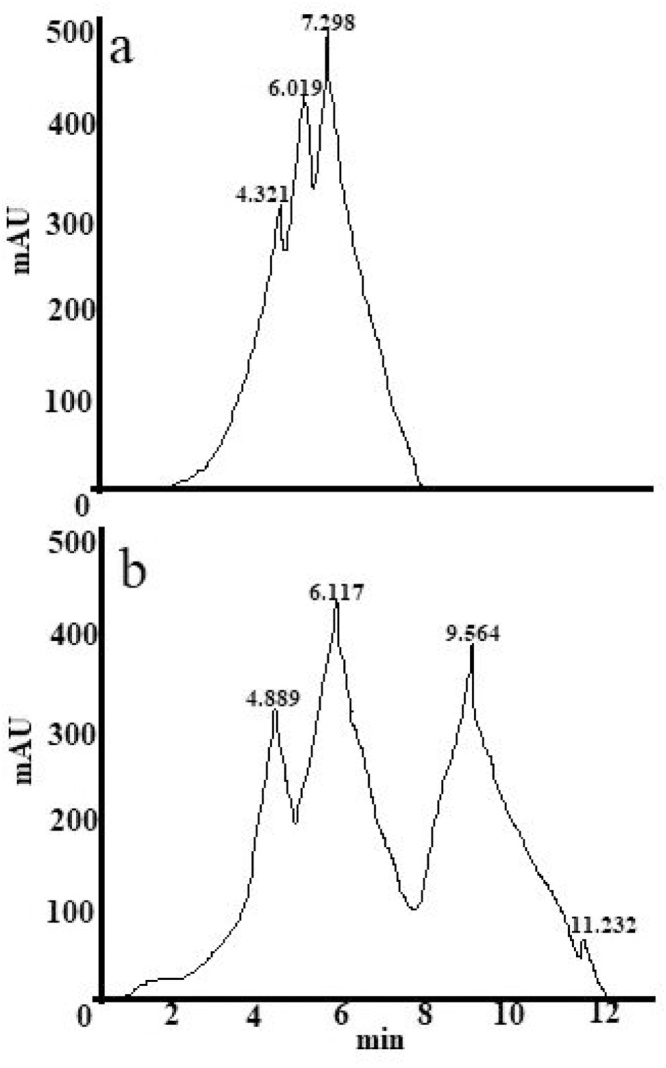
3.6. HPLC analysis
HPLC spectra of thiazole yellow G dye showed the peaks at retention time 4.321, 6.019 and 7.298 min (Fig. 7a) and the metabolites obtained after its degradation by Aspergillus niger showed the peaks at retention time 4.889, 6.117, 9.564, and 11.232 min (Fig. 7b). The abundance of enzymes usually dictates the cleavage of a typical azo dye [14].
Fig. 7.
HPLC pattern of (a) thiazole yellow G dye before degradation, (b) metabolites obtained after degradation of thiazole yellow G dye by Aspergillus niger.
3.7. Enzymatic analyses
Fungi elicit extracellular non-stereotypic enzymes which play vital roles in the biodegradation process. There were 71% and 48% inductions of laccase and lignin peroxidase respectively during the decolorization process (Table 2).
Table 2.
Enzyme profile in control Aspergillus niger cells (0 h) and the induced cells obtained after complete decolorization (96 h) of thiazole yellow G dye.
| Enzymes | Control cells (0 h) | Cells obtained after complete decolorization (96 h) |
|---|---|---|
| Lignin peroxidasea | 2.74 ± 0.002 | 4.75 ± 0.02* |
| Laccasea | 0.635 ± 0.007 | 0.899 ± 0.022* |
Values are mean of three experiments ± SEM. Significantly different from the control cells at.
P < 0.05 by one-way ANOVA with Tukey-Kramer multiple comparisons test.
U min−l mg protein−l.
3.8. Toxicity study
The phytotoxicity experiment results showed a significant effect on the % germination and length of the plumule and radicle of the thiazole G dye solution (1000 ppm) wetted seeds. The germination percentage of Sorghum bicolor and Vigna unguiculata seeds was higher (100%) when treated with water than the dye 5 days decolorized metabolites on treatment with A. niger. The results as presented in Table 3 revealed remarkable growth with attendant changes in the length of plumule and radicle when wetted with the dye metabolites. These results further elucidated dye detoxifying potency of A. niger on thiazole yellow G. This may be due to the removal of aromatic amines by the fungus used in this study [17].
Table 3.
Phyto-toxicity study of thiazole yellow G dye and its degraded products after 5 days of treatment using Aspergillus niger.
| Parameters studied |
Water | Thiazole Yellow G dye (Control) |
Thiazole Yellow G dye (metabolites) |
|---|---|---|---|
| Vigna unguiculata (dicot) | |||
| Germination (%) | 100 | 70 | 100 |
| Plumule (cm) | 11.42 ± 0.05 | 5.89 ± 0.01* | 9.15 ± 0.09** |
| Radicle (cm) | 5.76 ± 0.02 | 2.05 ± 0.02* | 4.43 ± 0.01** |
| Sorghum bicolor (monocot) | |||
| Germination (%) | 100 | 80 | 90 |
| Plumule (cm) | 6.47 ± 0.04 | 3.21 ± 0.05* | 5.17 ± 0.03** |
| Radicle (cm) | 5.15 ± 0.01 | 2.57 ± 0.09* | 4.03 ± 0.15** |
Valuesa are presented as mean of three experiments ± S.E.M.
Root and shoot lengths of fifty (50) plants grown in thiazole G dye and its metabolites are significantly different from that of plants grown in sterile distilled water by *P < 0.001, **P < 0.01.
Root and shoot lengths of fifty (50) plants grown in thiazole G dye and its metabolites are significantly different from that of plants grown in sterile distilled water by *P < 0.001, **P < 0.01.
4. Conclusion
The degradation process of thiazole dye by Aspergillus niger proved to be dependent largely on the pH of the solution, temperature and concentration of the dye. The study reported three major intermediate metabolites after degradation of thiazole yellow G dye. Thiazole yellow G dye degraded dye products exhibited less toxic potentials on V. unguiculata and S. bicolor than the control dye. Conclusively, Aspergillus niger has proven to be a cheap, economic, effective, efficient and eco-friendly alternative in the bio-removal of azo dyes from polluted environment.
Funding information
Bankole Paul Olusegun give profound thanks to the Association of Commonwealth Universities (ACU) for the 2018 Early Career Grant Award.
References
- 1.Crini G. Non-conventional low-cost adsorbents for dye removal: a review. Bioresour. Technol. Rep. 2006;97:1061–1085. doi: 10.1016/j.biortech.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Hadibarata T., Kristanti R. Effect of environmental factors in the decolourisation of Remazol Brillant Blue R by Polyporus sp. S133. J. Chilean Chem. Soc. 2012;57:1095–1998. [Google Scholar]
- 3.Mishra G., Tripathy M. A critical review of the treatments for decolourization of textile effluent. Colourage. 1993;40:35–38. [Google Scholar]
- 4.Aksu Z. Application of biosorption for the removal of organic pollutants: a review. Process Biochem. 2005;40:997–1026. [Google Scholar]
- 5.Zhao G., Li M., Hu Z., Hu H. Dissociation and removal of complex chromium ions contained in dye wastewaters. Separat. Purif. Technol. 2005;43:227–232. [Google Scholar]
- 6.Aksu Z., Ertuğrul S., Dönmez G. Methylene Blue biosorption by Rhizophus arrhizus: effect of SDS (sodium dodecylsulfate) surfactant on biosorption properties. Chem. Eng. J. 2010;158:474–481. [Google Scholar]
- 7.Cengiz S., Cavas L. Removal of methylene blue by invasive marine seaweed: caulerpa racemosa var. Cylindracea. Bioresour. Technol. 2008;99:2357–2363. doi: 10.1016/j.biortech.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 8.Ayed L., Achour S., Bakhrouf A. Application of the mixture design to decolourise effluent textile wastewater using continuous stirred bed reactor. Water Sa. 2011;37(37):21–26. [Google Scholar]
- 9.Dönmez G. Bioaccumulation of the reactive textile dyes by Candida tropicalis growing in molasses medium. Enzy. Microb. Technol. 2002;30:363–366. [Google Scholar]
- 10.Davies L.C., Carias C.C., Novais J.M., Martins-Dias S. Phytoremediation of textile effluents containing azo dye by using Phragmites australis in a vertical flow intermittent feeding constructed wetland. Ecol. Eng. 2005;25:594–605. [Google Scholar]
- 11.Fu Y., Viraraghavan T. Fungal decolourisation of dye wastewaters: a review. Bioresour. Technol. Rep. 2001;79:251–262. doi: 10.1016/s0960-8524(01)00028-1. [DOI] [PubMed] [Google Scholar]
- 12.Aksu Z., Karabayir G. Comparison of biosorption properties of different kinds of fungi for the removal of Gryfalan Black RL metal-complex dye. Bioresour. Technol. Rep. 2008;99:7730–7741. doi: 10.1016/j.biortech.2008.01.056. [DOI] [PubMed] [Google Scholar]
- 13.Aksu Z. Biosorption of reactive dyes by dried activated sludge: equilibrium and kinetic modelling. Biochem. Eng. J. 2001;7:79–84. doi: 10.1016/s1369-703x(00)00098-x. [DOI] [PubMed] [Google Scholar]
- 14.Bankole P.O., Adekunle A.A., Obidi O.F., Chandanshive V.V., Govindwar S.P. Biodegradation and detoxification of Scarlet RR dye by a newly isolated filamentous fungus, Peyronellaea prosopidis. Sustain. Environ. Res. 2018;28:214–222. [Google Scholar]
- 15.Bankole P.O., Adekunle A.A., Obidi O.F., Olukanni O.D., Govindwar S.P. Degradation of indigo dye by a newly isolated yeast, Diutina rugosa from dye wastewater polluted soil. J. Environ. Chem. Eng. 2017;5:4639–4648. [Google Scholar]
- 16.Bankole P.O., Adekunle A.A., Govindwar S.P. Enhanced decolorization and biodegradation of acid red 88 dye by newly isolated fungus, Achaetomium strumarium. J. Environ. Chem. Eng. 2018;6:1589–1600. [Google Scholar]
- 17.Bankole P.O., Adekunle A.A., Govindwar S.P. Biodegradation of a monochlorotriazine dye, cibacron brilliant Red 3B-A in solid state fermentation by wood-rot fungal consortium, Daldinia concentrica and Xylaria polymorpha. Int. J. Biol. Macromol. 2018;120(Part A):19–27. doi: 10.1016/j.ijbiomac.2018.08.068. [DOI] [PubMed] [Google Scholar]
- 18.Kaushik P., Malik A. Fungal dye decolorization: recent advances and future potential review article. Environ. Int. 2009;35:127–141. doi: 10.1016/j.envint.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 19.Tian C., Tian R., Zhou Y., Chen Q., Cheng H. Decolorization of indigo dye and indigo dye-containing textile effluent by Ganoderma weberianum. Afr. J. Microbiol. Res. 2013;7:941–947. [Google Scholar]
- 20.Wu Y., Li T., Yang L. Mechanisms of removing pollutants from aqueous solutions by microorganisms and their aggregates: a review. Bioresour. Technol. Rep. 2012;107:10–18. doi: 10.1016/j.biortech.2011.12.088. [DOI] [PubMed] [Google Scholar]
- 21.Iscen C.F., Kiran I. Biosorption of reactive black 5 dye by Penicillium restrictum: the kinetic study. J. Hazard. Mater. 2007;143:335–340. doi: 10.1016/j.jhazmat.2006.09.028. [DOI] [PubMed] [Google Scholar]
- 22.Solís M., Solís A., Pérez H.I., Manjarrez N., Flores M. Microbial decolouration of azo dyes: a review. Process Biochem. 2012;47:1723–1748. [Google Scholar]
- 23.Yargic A.S., Yarbay Sahin R.Z., Ozbay N., Onal E. The effect of different operating conditions on removal of reactive dye by green carbon adsorption. JOSUNAS. 2013:498–510. [Google Scholar]