Abstract
Background
Increasing albumin to creatinine ratio (ACR) within the normal range is a risk factor for cardiovascular disease in the general population. Clinical and epidemiological studies often store urine samples for long durations prior to ACR assessment. The stability of ACR at the lowest urinary albumin concentrations during prolonged storage has not been previously studied because routine clinical assays can’t quantify very low concentrations of albumin.
Aim
To determine the stability of urinary albumin and creatinine over 12 months in samples stored at −20 °C and −80 °C using an assay which enables assessment of previously undetectable levels of albumin and to investigate if additives can be used to prevent urinary albumin degradation.
Method
ACR was measured in 30 urine samples from healthy subjects on the day of collection. Each sample was divided into 5 portions, each receiving a different treatment; alkalisation, protease inhibiter, boric acid, low protein binding tubes and no treatment (control). Samples were stored at −20 °C and −80 °C and ACR was analysed again after 12 months.
Results
Mean (95% CI) percent change in ACR was −34.3% (−47.2 to −21.4; p < 0.0001) and −1.8% (−9.4 to 5.8; p = 0.91) in samples stored at −20 °C and −80 °C respectively. Treating samples did not prevent the reduction in albumin at −20 °C (p < 0.001).
Conclusion
The loss in urinary albumin concentration which occurs during storage at −20 °C for 12 months is not prevented by pre-treating samples prior to storage. For accurate determination of albumin concentration or ACR, samples should be stored at −80 °C on day of collection.
Keywords: Albumin, Creatinine, ACR, Storage, Stability, Albuminuria
Highlights
-
•
Urine samples should be stored at −80 °C on day of collection to prevent loss of albumin.
-
•
ACR is reduced by over 30% in samples stored at −20 °C for 12 months.
-
•
Treating samples prior to storage at −20 °C does not prevent loss in urinary albumin.
1. Introduction
Moderately increased urinary albumin excretion, formerly known as microalbuminuria, has been established as a risk factor for diabetic kidney disease since the 1980’s [1]. Since then a wealth of studies have demonstrated that increasing urinary albumin excretion, even within the normal range, is a risk factor for cardiovascular disease (CVD) in the general population [2,3]. Urinary albumin to creatinine ratio (ACR) measured in spot samples is clinically preferred as it is more convenient than assessing albumin excretion in timed collections [4].
Clinical and epidemiological studies investigating the continuous relationship between albumin excretion and the risk of CVD often store urine samples for long durations before measuring the ACR. It is therefore important to understand the stability of ACR during prolonged frozen storage in samples with low levels of albumin, to ensure the reported ACR is valid.
Urinary creatinine appears to be stable during prolonged frozen storage [5]. The strongest agreement amongst studies investigating albumin stability is that urinary albumin, at all levels, is more stable when stored at −80 °C when compared to −20 °C, and the loss in albumin is variable between samples [[6], [7], [8], [9]]. The effect of frozen storage on albumin at levels undetectable by standard methods is currently unknown. At higher levels of albumin, Brinkman and colleagues reported a ∼30% decrease in urinary albumin concentration in samples stored for 12 months at −20 °C [10] and later reported this loss in albumin decreases the predictive properties of urine albumin for mortality [11].
Lambers Heerspink et al. demonstrated that urinary albumin degradation at −20 °C (assessed by immunonephelometry) can be prevented by alkalising samples [12]. Kania and colleagues conducted in vitro experiments and reported that pepstatin, a protease inhibitor, prevents urinary albumin degradation [13]. The cause of urinary albumin degradation is not fully understood and some authors speculate that it may result from bacterial contamination [13]. Thus, collecting samples in boric acid, a bacteriostatic agent, might prevent the loss of urinary albumin in stored samples. Investigators have reported that the influence of albumin adsorption to storage containers is <1% in urine samples with an albumin concentration of 4–5 mg/L [14]. This influence might be greater at lower concentrations, of interest in this paper, as the amount adsorbed becomes a larger fraction of the total albumin concentration. Commercially available sample tubes designed to reduce protein binding may decrease albumin adsorption during storage, reducing this effect.
The aim of this investigation is to examine the stability of urinary albumin and creatinine over 12 months in samples stored at −80 °C using an assay which enables assessment of previously undetectable levels of albumin and to investigate if additives and low protein binding tubes can be used to prevent the loss of urinary albumin in samples stored at −20 °C.
2. Methods
Ethical permission was obtained to collect thirty urine samples (20 from random spot collections and 10 from overnight collections) from 17 participants, all without any known disease (Cornwall & Plymouth REC 14/SW/0049). Only subjects with normoalbuminuria were included in the study to ensure low levels of urinary albumin were investigated. Each sample was divided into five portions, each undergoing different treatments:
-
1.
No treatment; 400 μl aliquots were stored in standard 2 ml polypropylene micro tubes (Sarstedt, Nümbrecht, Germany, P72.609.001). These tubes were also used to store 400 μl aliquots from samples which had received the other treatments.
-
2.
Alkalisation; 15 μl of NaOH 1 M (Sigma Aldrich, Missouri, USA) was added to 10 ml of sample. After gentle mixing by four hand inversions, pH was measured by a portable pH tester (Hanna instruments, Rhode Island, USA) to ensure pH > 8.
-
3.
Boric acid; 20 ml of each sample was added to containers precoated with boric acid (Sarstedt, Nümbrecht, Germany, P51.9923.820).
-
4.
Protease inhibitor; 1 mg pepstatin (Sigma Aldrich, Missouri, USA, P5318) was dissolved in 1 ml of a 10% acetic acid: 90% methanol solution (Sigma Aldrich, Missouri, USA). A 1:10 dilution was made using deionised water to make a working solution of 0.1 mg/ml. The manufacturer states the effective concentration of pepstatin is 1 μg/ml. Therefore 100 μl of the working solution was added to 9,900 μl of urine sample.
-
5.
Low protein binding tubes; 400 μl aliquots of untreated samples were stored in 1.5 ml low protein binding micro tubes (Sarstedt, Germany, P72.706.600).
Urinary albumin and creatinine were measured on the day of collection. After the samples had undergone the different treatments, 400 μl aliquots were stored at −20 °C or −80 °C. After storage for 12 months, aliquots from all 300 samples were thawed to room temperature and mixed by four hand inversions prior to measuring urinary albumin and creatinine again. Corresponding samples which were stored at different temperatures were analysed in the same batch.
Urinary albumin was measured using an enzyme linked immunosorbent assay (ELISA) (Orgentec, Mainz, Deutschland) on an automated platform (DS2, Dynex technologies, VA, USA). Samples were measured in duplicate with four quality controls tested on each microplate (two containing albumin in a serum/buffer matrix and two from pooled urine samples). In-house validation of the ELISA demonstrated a strong agreement with the routine immunoturbidmetric assay (COBAS, Roche Diagnostics Ltd., Burgess Hill, UK) (y = 0.98 (0.90–1.08) x + 3.66 (2.69–4.50) for samples measurable by the COBAS method). The Orgentec ELISA has a limit of quantification of 0.74 mg/L, the limit of detection was 0.24 mg/L and within batch coefficient of variation (CV) is <12%. Urinary creatinine was measured by the Jaffe method on the P8000 COBAS analyser (Roche Diagnostics Ltd., Burgess Hill, UK), which demonstrated a within batch CV <4.2%.
The difference in urinary albumin, creatinine and ACR after 12 months storage is expressed as the percent change relative to the baseline concentration when measured fresh, and compared using the Wilcoxon sign-rank test. Normality of data was tested by Shapiro Wilks test. The mean and standard deviation (SD), and median and interquartile range (IQR) are reported for data with normal and non-normal distribution respectively.
3. Results
All urines had measurable urinary albumin using the validated ELISA. Median age [IQR] was 34 [22 to 47] years, 47% were men and all participants were Caucasian and without any known disease (Table 1). Median ACR [IQR] was 0.52 [0.45 to 0.79] mg/mmol (measured fresh), with all urine samples in the normoalbuminuria range.
Table 1.
Participant characteristics.
Data presented as mean (SD) or median [IQR] depending on normality of data. eGFR calculated using CKD-EPI equation. 30 samples, 20 from random spot collections and 10 from overnight collections were stored from the 17 participants.
| Characteristic | |
|---|---|
| N (%male) | 17 (47%) |
| Age (years) | 34 [22 to 47] |
| Ethnicity (% white British) | 100 |
| Weight (kg) | 75.1 (15.9) |
| BMI (kg/m2) | 24.3 [23.0 to 25.3] |
| Systolic BP (mmHg) | 116 (12) |
| Diastolic BP (mmHg) | 72 (9) |
| Blood Creatinine (μmol/L) | 77.0 (16.5) |
| eGFR (ml/min/1.73m2) | 101.3 (15.6) |
| Urinary Albumin (mg/L) | 2.7 [1.7 to 6.3] |
| Urinary Creatinine (mmol/L) | 5.9 [1.9 to 9.9] |
| ACR (mg/mmol) | 0.52 [0.45 to 0.79] |
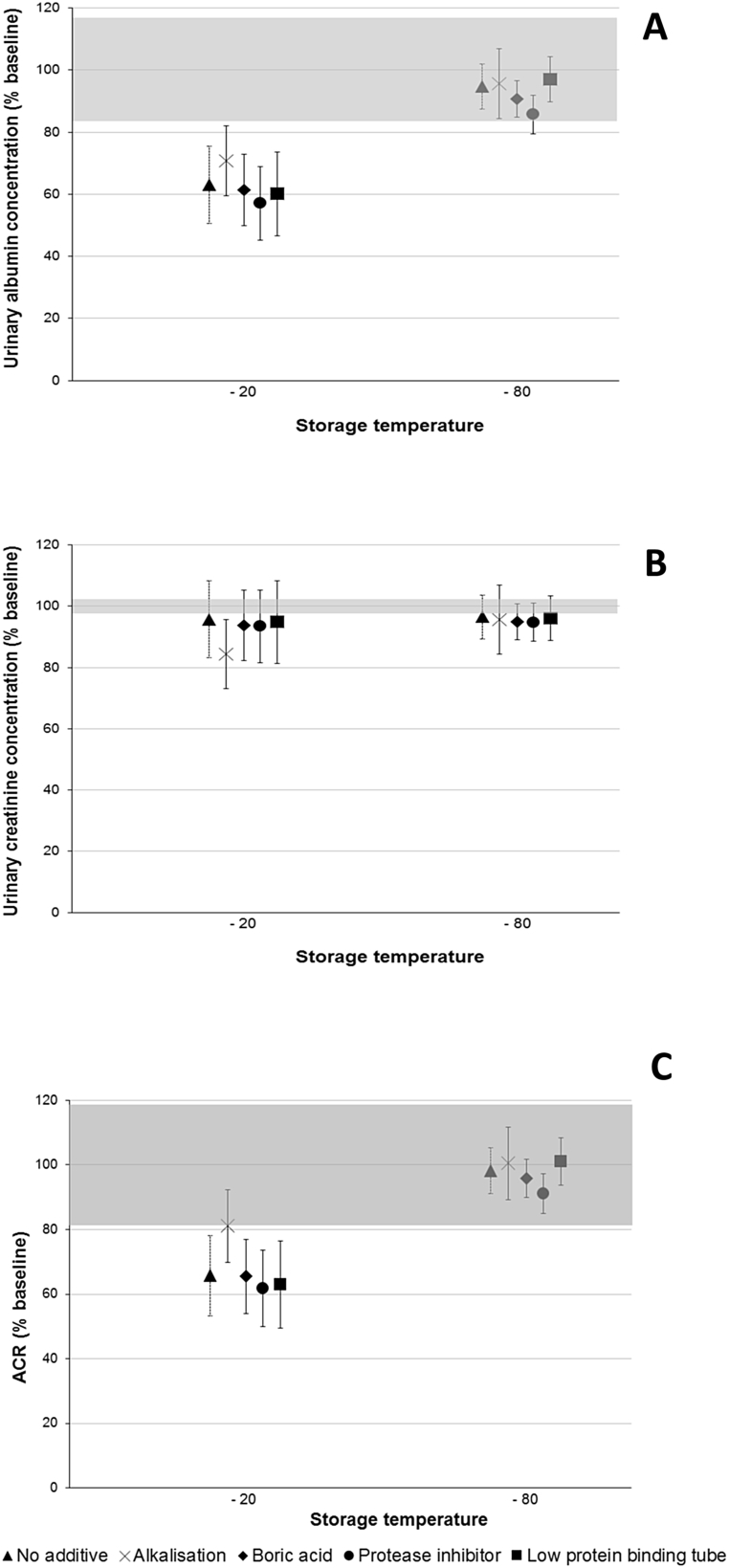
The mean (95% CI) percent change in albumin concentration was −5.3% (−12.5 to 2.0; p = 0.06) in untreated samples stored for 12 months at −80 °C, compared to not frozen samples (Fig. 1). Urinary albumin concentration was significantly reduced in untreated samples stored at −20 °C, with a mean (95% CI) change of −36.9% (−49.4 to −24.4; p < 0.0001). Treating samples and using low protein binding tubes did not prevent the loss of urinary albumin at −20 °C (p < 0.001).
Fig. 1.
A: Albumin; B: Creatinine; C: ACR stability after 12 months storage at -20oC and -80oC. Thirty samples had five aliquots taken, each undergoing different treatments. The shaded region shows the percent difference of each analyte which may be attributed to inter-assay variation. The mean percent of the baseline values with 95% confidence intervals is reported.
Urinary creatinine concentration was reduced (compared to not frozen samples) in untreated samples stored for 12 months at −20 °C and −80 °C, with a mean (95% CI) percent change of −4.3% (−6.0 to −2.5) and −3.5% (−5.1 to −1.9) (p = 0.0001) respectively. However, this is not a clinically significant change (<10%) [[15], [16], [17]]. This is not true for alkalised samples stored at −20 °C, as the change in creatinine was much greater at −15.6% (−25.0 to −6.1) (p < 0.0001). This magnitude of change was not observed in alkalised samples stored at −80 °C (−4.4% (−5.9 to −2.8); p < 0.0001).
Mean (95% CI) percent change in ACR was −34.3% (−47.2 to −21.4; p < 0.0001) and −1.8% (−9.4 to 5.8; p = 0.91) in untreated samples stored for 12 months at −20 °C and −80 °C, respectively, compared to not frozen samples. Treating samples and using low protein binding tubes did not prevent the loss of ACR at −20 °C (p < 0.001). Observed decreases in ACR reflect the change in albumin concentration, however the increase in ACR observed in alkalised samples stored at −20 °C reflects decreased creatinine concentration.
4. Discussion
This work demonstrates that, even when urinary albumin is quantified below the levels usually detectable by standard methods, urinary albumin and ACR are stable and can be accurately assessed following storage for 12 months at −80 °C but not at −20 °C. This confirms the findings from previous studies carried out at higher albumin concentrations [7]. Our findings demonstrate that treating samples or using low protein binding tubes does not prevent the loss of low levels of urinary albumin in samples stored at −20 °C.
Our findings do not support previous research which suggests alkalising samples [12] and adding pepstatin [13] prevents urinary albumin degradation. Different cohorts and methodological approaches may contribute to the discordance in findings, but we demonstrate that these are not suitable methods of preserving the lowest concentrations of urinary albumin in samples stored at −20 °C for 12 months. In our study, storing samples in boric acid did not prevent urinary albumin loss at −20 °C, this has also been reported at higher albumin concentrations in a recent study by Herrington et al. [9].
We identified a small loss in urinary creatinine concentration in untreated samples after storage for 12 months at both −20 °C and −80 °C. This small difference is not clinically significant [[15], [16], [17]] and did not influence the ACR, it may reflect assay imprecision. Creatinine stability during prolonged frozen storage has been reported in other studies [5,12].
In conclusion, clinical and epidemiological studies investigating the relationship between increasing ACR in the normal range and risk of CVD in the general population should store samples at −80 °C if ACR measurement is not carried out on the day of collection. Collecting urine samples in boric acid, storing in low protein binding tubes, pre-treating by alkalisation or by adding pepstatin, does not prevent the decline in urinary albumin concentration over 12 months storage at −20 °C.
Declarations of interest
None.
Funding
This study was supported by the National Institute for Health Research (NIHR) Exeter Clinical Research Facility (UK) and funded by The University of Exeter Medical School, UK.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.plabm.2019.e00120.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Mogensen C.E. Microalbuminuria as a predictor of clinical diabetic nephropathy. Kidney Int. 1987;31(2):673–689. doi: 10.1038/ki.1987.50. [DOI] [PubMed] [Google Scholar]
- 2.Hillege H.L., Fidler V., Diercks G.F., van Gilst W.H., de Zeeuw D., van Veldhuisen D.J., Gans R.O., Janssen W.M., Grobbee D.E., de Jong P.E. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation. 2002;106(14):1777–1782. doi: 10.1161/01.cir.0000031732.78052.81. [DOI] [PubMed] [Google Scholar]
- 3.Dwyer J.P., Parving H.H., Hunsicker L.G., Ravid M., Remuzzi G., Lewis J.B. Renal dysfunction in the presence of normoalbuminuria in type 2 diabetes: results from the DEMAND study. Cardiorenal Med. 2012;2(1):1–10. doi: 10.1159/000333249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Kidney Foundation KDOQI, K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am. J. Kidney Dis. :Off. J. Natl. Kidney Found. 2002;39(2 Suppl 1):S1–S266. [PubMed] [Google Scholar]
- 5.Remer T., Montenegro-Bethancourt G., Shi L. 2014. Long-term Urine Biobanking: Storage Stability of Clinical Chemical Parameters under Moderate Freezing Conditions without Use of Preservatives. [DOI] [PubMed] [Google Scholar]
- 6.Elving L.D., Bakkeren J.A., Jansen M.J., de Kat Angelino C.M., de Nobel E., van Munster P.J. Screening for microalbuminuria in patients with diabetes mellitus: frozen storage of urine samples decreases their albumin content. Clin. Chem. 1989;35(2):308–310. [PubMed] [Google Scholar]
- 7.Brinkman J.W., de Zeeuw D., Lambers Heerspink H.J., Gansevoort R.T., Kema I.P., de Jong P.E., Bakker S.J. Apparent loss of urinary albumin during long-term frozen storage: HPLC vs immunonephelometry. Clin. Chem. 2007;53(8):1520–1526. doi: 10.1373/clinchem.2007.088823. [DOI] [PubMed] [Google Scholar]
- 8.Hara F., Nakazato K., Shiba K., Shimoda J., Kojima T., Fukumura Y., Kobayashi I. Studies of diabetic nephropathy. I. Effects of storage time and temperature on microalbuminuria. Biol. Pharm. Bull. 1994;17(9):1241–1245. doi: 10.1248/bpb.17.1241. [DOI] [PubMed] [Google Scholar]
- 9.Herrington W., Illingworth N., Staplin N., Kumar A., Storey B., Hrusecka R., Judge P., Mahmood M., Parish S., Landray M., Haynes R., Baigent C., Hill M., Clark S. Effect of processing delay and storage conditions on urine albumin-to-creatinine ratio. Clin. J. Am. Soc. Nephrol. 2016;11(10):1794–1801. doi: 10.2215/CJN.13341215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brinkman J.W., de Zeeuw D., Duker J.J., Gansevoort R.T., Kema I.P., Hillege H.L., de Jong P.E., Bakker S.J.L. Falsely low urinary albumin concentrations after prolonged frozen storage of urine samples. Clin. Chem. 2005;51(11):2181–2183. doi: 10.1373/clinchem.2005.053777. [DOI] [PubMed] [Google Scholar]
- 11.Brinkman J.W., de Zeeuw D., Gansevoort R.T., Duker J.J., Kema I.P., de Jong P.E., Bakker S.J.L. Prolonged frozen storage of urine reduces the value of albuminuria for mortality prediction. Clin. Chem. 2007;53(1):153–154. doi: 10.1373/clinchem.2006.081471. [DOI] [PubMed] [Google Scholar]
- 12.Lambers Heerspink H.J., Nauta F.L., van der Zee C.P., Brinkman J.W., Gansevoort R.T., de Zeeuw D., Bakker S.J. Alkalinization of urine samples preserves albumin concentrations during prolonged frozen storage in patients with diabetes mellitus. Diabet. Med. 2009;26(5):556–559. doi: 10.1111/j.1464-5491.2009.02721.x. [DOI] [PubMed] [Google Scholar]
- 13.Kania K., Byrnes E.A., Beilby J.P., Webb S.A.R., Strong K.J. Urinary proteases degrade albumin: implications for measurement of albuminuria in stored samples. Ann. Clin. Biochem. 2010;47(2):151–157. doi: 10.1258/acb.2009.009247. [DOI] [PubMed] [Google Scholar]
- 14.Robinson M.K., Caudill S., Koch D.D., Ritchie J., Hortin G., H Eckfeldt J., Sandberg S., Williams D., Myers G., Greg Miller W. 2014. Albumin Adsorption onto Surfaces of Urine Collection and Analysis Containers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDonald T.J., Knight B.A., Shields B.M., Bowman P., Salzmann M.B., Hattersley A.T. Stability and reproducibility of a single-sample urinary C-Peptide/Creatinine ratio and its correlation with 24-h urinary C-peptide. Clin. Chem. 2009;55(11):2035–2039. doi: 10.1373/clinchem.2009.129312. [DOI] [PubMed] [Google Scholar]
- 16.Chakera A.J., McDonald T.J., Knight B.A., Vaidya B., Jones A.G. Current laboratory requirements for adrenocorticotropic hormone and renin/aldosterone sample handling are unnecessarily restrictive. Clin. Med. 2017;17(1):18–21. doi: 10.7861/clinmedicine.17-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDonald T.J., Perry M.H., Peake R.W., Pullan N.J., O'Connor J., Shields B.M., Knight B.A., Hattersley A.T. EDTA improves stability of whole blood C-peptide and insulin to over 24 hours at room temperature. PLoS One. 2012;7(7) doi: 10.1371/journal.pone.0042084. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.