ABSTRACT
Canonical Wnts promote myoblast differentiation; however, the role of β-catenin in adult myogenesis has been contentious, and its mechanism(s) unclear. Using CRISPR-generated β-catenin-null primary adult mouse myoblasts, we found that β-catenin was essential for morphological differentiation and timely deployment of the myogenic gene program. Alignment, elongation and fusion were grossly impaired in null cells, and myogenic gene expression was not coordinated with cytoskeletal and membrane remodeling events. Rescue studies and genome-wide analyses extended previous findings that a β-catenin-TCF/LEF interaction is not required for differentiation, and that β-catenin enhances MyoD binding to myogenic loci. We mapped cellular pathways controlled by β-catenin and defined novel targets in myoblasts, including the fusogenic genes myomaker and myomixer. We also showed that interaction of β-catenin with α-catenin was important for efficient differentiation. Overall the study suggests dual roles for β-catenin: a TCF/LEF-independent nuclear function that coordinates an extensive network of myogenic genes in cooperation with MyoD; and an α-catenin-dependent membrane function that helps control cell-cell interactions. β-Catenin-TCF/LEF complexes may function primarily in feedback regulation to control levels of β-catenin and thus prevent precocious/excessive myoblast fusion.
KEY WORDS: Homeobox genes, Muscle stem cells, Myogenesis, Skeletal muscle, Transcription factors, Epigenetics, Cell signaling, Differentiation
Summary: Differentiation of adult myoblasts requires the interaction of β-catenin with MyoD but not with TCF/LEF; future selective targeting of different β-catenin complexes might allow inhibition of TCF/LEF-dependent fibrosis whilst sparing myoblast differentiation.
INTRODUCTION
Skeletal muscle owes its considerable regenerative capacity to a population of resident muscle stem cells called satellite cells (Lepper et al., 2011; McCarthy et al., 2011; Murphy et al., 2011; Sambasivan et al., 2011). These cells, which are situated between the basal lamina and myofiber, are largely quiescent in uninjured adult muscle (Mauro, 1961; Rudnicki et al., 2008; Buckingham et al., 2003). Soluble factors produced after muscle injury trigger satellite cells to re-enter the cell cycle, giving rise to a transit-amplifying population called myoblasts. Myoblasts express a hierarchy of myogenic regulatory factors (MRFs), such as MyoD, which is involved in both proliferation and differentiation, and myogenin, which drives and modulates the differentiation program (Schultz and Jaryszak, 1985; Wozniak et al., 2005). Differentiation involves multiple highly coordinated processes, including exit from the cell cycle, migration, adhesion, fusion and formation of the contractile apparatus that defines functional myofibers.
Several studies have defined crucial functions for the canonical Wnt/β-catenin signaling pathway in muscle development and also in adult muscle regeneration. Wnt ligands bind to membrane receptor co-complexes involving the Frizzled and low-density lipoprotein receptor protein (LRP) families that associate with the so-called destruction complex (Stamos and Weis, 2013). Wnt binding elicits a change in the destruction complex that suppresses ubiquitylation and degradation of β-catenin (Li et al., 2012) As the core transcriptional effector of the Wnt pathway, β-catenin does not bind DNA directly but instead interacts with partner proteins to regulate target genes. The classical transcriptional partners for β-catenin are the TCF/LEF family factors. In the absence of Wnt signals, TCF/LEF proteins bind and repress Wnt target genes via interaction with Groucho/TLE co-repressors (Arce et al., 2009; Daniels and Weis, 2005) and HDAC1 (Arce et al., 2009; Billin et al., 2000). Upon binding of Wnt ligand, stabilized β-catenin binds to TCF/LEF proteins and recruits histone acetyltransferases (HATs) (Ogryzko et al., 1996; Parker et al., 2008; Takemaru and Moon, 2000; Hecht et al., 2000) to activate these targets (Hecht et al., 2000; Wodarz and Nusse, 1998; Posokhova et al., 2015).
Canonical Wnt signals have been shown to play crucial roles in muscle development; in particular, canonical Wnts induce expression of Myf5 and MyoD in the somite/myotome and thus embryonic myogenesis (see von Maltzahn et al., 2012 for a review). Less well understood is the role of canonical Wnt signals in adult (regenerative) myogenesis, although insights in this regard come from recent studies both in vitro and in vivo. Canonical Wnt ligands have been found to promote myoblast differentiation in vitro (Otto et al., 2008; Bernardi et al., 2011; Zhuang et al., 2014; Pansters et al., 2011; Tanaka et al., 2011; Brack et al., 2008), and can increase expression or activity of MyoD and/or myogenin (Ridgeway et al., 2000; Petropoulos and Skerjanc, 2002; Jones et al., 2015). Moreover, studies by Kim et al. (2008) using C2C12 cells showed that β-catenin can interact with MyoD and enhance the ability of MyoD to bind and activate target genes. This study also found that β-catenin knockdown impaired C2C12 differentiation; however, dominant-negative (DN) forms of TCF/LEF did not. This finding led to the suggestion that β-catenin function in myogenesis is TCF/LEF independent (Kim et al., 2008). However, a more recent study reported that expression of DN-TCF4 in human adult primary myoblasts did impair differentiation, and concluded that TCF4 is required for this process (Agley et al., 2017). The reason for these discrepant results remains unclear, but may relate to the use of different cell models and assays. We have recently reported that Wnt treatment of adult primary myoblasts leads to acquisition of active chromatin marks at myogenic loci that are enriched for E-boxes but not for TCF/LEF elements (Hulin et al., 2016). These data suggest that Wnt activates a gene expression program that is TCF/LEF independent in adult myoblasts; this conclusion is consistent with the findings of Kim et al. (2008), but not those of Agley et al. (2017).
Conditional deletion studies in mice have raised uncertainty about whether β-catenin is actually required for adult myogenesis in vivo, regardless of mechanism. In particular, Murphy et al. (2014) performed inducible Cre-mediated ablation of β-catenin in satellite cells and reported no defect in adult muscle regeneration (Murphy et al., 2014). They suggested that, in contrast to the embryonic state, β-catenin may be redundant in adult myoblasts. Subsequently however, Rudolf et al. (2016) performed very similar conditional deletion experiments using a different Cre-driver line, and found β-catenin to be essential for efficient adult muscle repair. They also reported that explanted myoblasts lacking β-catenin showed delayed differentiation in vitro. The reason for the discrepancy between the two in vivo studies remains unresolved, but may relate to the presence of undetected ‘recombination-escaper’ cells, which are known to have confounded previous conditional ablation studies in satellite cells (Brack, 2014).
To address the ongoing uncertainty about the cell-autonomous requirement for β-catenin and its mechanism(s) of action, we used CRISPR to generate adult primary mouse myoblast-derived β-catenin-null models. We then investigated the functions of β-catenin via rescue studies with mutant forms of the protein. We confirmed that β-catenin is essential for timely activation of the myogenic gene expression program by Wnt in primary myoblasts, and that β-catenin is required for Wnt to enhance genome-wide MyoD binding. Interestingly, even though key myogenic genes such as myogenin were expressed in null cells in a delayed fashion, the cells still displayed grossly impaired morphological differentiation, suggesting that these molecular and morphological events are uncoupled by loss of β-catenin. Our rescue studies supported the previous suggestion that β-catenin-TCF/LEF interactions are not required for differentiation. However, in contrast to a previous report, we also found that interactions of β-catenin with α-catenin at membrane junction complexes are important for efficient differentiation.
RESULTS
Phenotypic characterization of β-catenin-null primary adult myoblasts
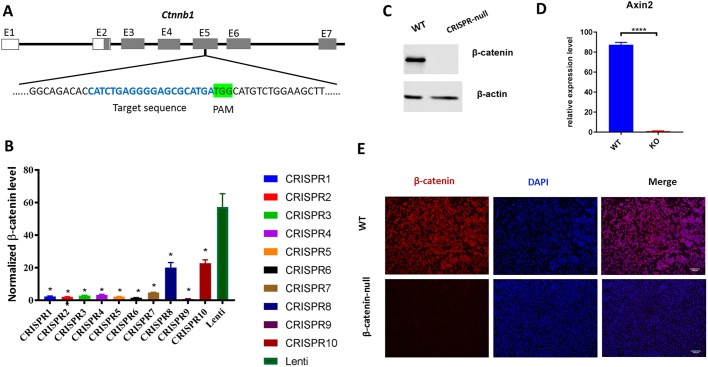
Low passage primary myoblasts derived from adult mice were transduced with a viral vector targeting exon 5 of β-catenin (Fig. 1A). Loss of β-catenin mRNA was observed in several clones (<5% of parental cell levels), likely as a result of nonsense-mediated decay (NMD) (Fig. 1B). All null clones had a similar phenotype (Fig. S1A), which included a rounded morphology and impaired differentiation, as discussed further below. Loss of β-catenin protein was confirmed at the protein level by immunostaining and immunoblotting for selected lines (e.g. Fig. 1C,E). Consistent with the loss of β-catenin, Axin2 failed to be induced in null cells after Wnt3a treatment (Fig. 1D). Sequencing analysis of selected clones revealed frameshift deletions generating premature stop codons in both alleles; the resulting predicted proteins would be truncated to fewer than 40 amino acids (see Fig. S2A,B), although these are unlikely to be produced because of NMD-mediated loss of the transcript.
Fig. 1.
Characterization of β-catenin-null primary myoblasts. (A) Strategy for Cas9/gRNA-mediated mutation of β-catenin. The blue nucleotides represent the gRNA target sequence; the highlighted nucleotides represent the protospacer adjacent motif (PAM). (B) β-Catenin mRNA expression was measured by RT-PCR in multiple independent CRISPR-generated myoblast lines and compared with that in wild-type myoblasts transfected with the empty vector (LentiCRISPRv2). Data are normalized to the housekeeping gene RPS26 and show the average of two experiments performed in duplicate. Statistical analysis: one-way ANOVA (*P<0.05). (C) Western blot analysis using β-catenin antibody shows that β-catenin-null primary myoblasts are completely devoid of β-catenin protein expression. β-Actin was used as a loading control. (D) β-Catenin-null myoblasts show no induction of Axin2 mRNA after treatment with Wnt3a for 24 h. Statistical analysis: t-test (****P<0.05). (E) Immunofluorescence labeling of β-catenin (red) in cultured wild-type and β-catenin-null primary myoblasts; nuclei are stained with DAPI (blue). Scale bars: 25 µm.
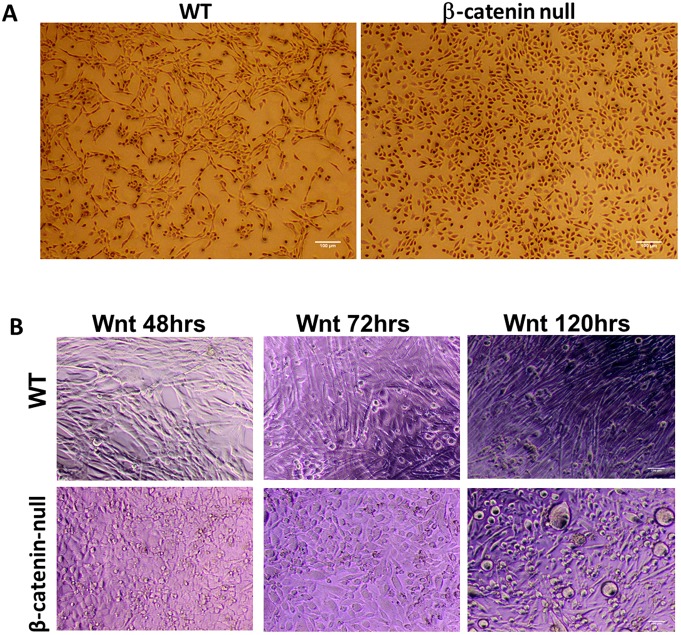
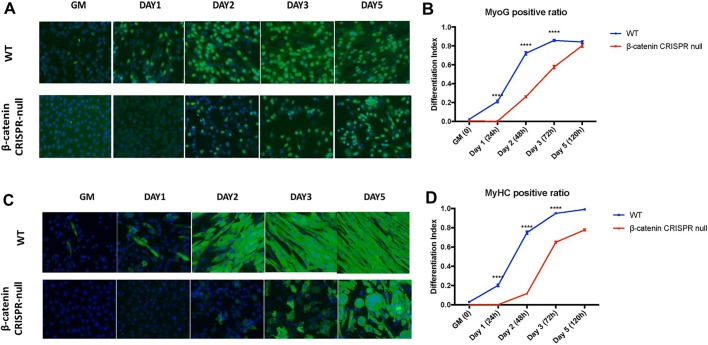
A representative null line is shown in Fig. 2 in comparison with control cells that were transduced with empty lentiCRISPRv2 vector and passaged a similar number of times. When cultured in growth media, the β-catenin-null cells appeared rounded relative to wild-type cells, and failed to undergo spontaneous differentiation upon reaching confluence (Fig. 2A). Treatment with Wnt3a induced differentiation of wild-type cells, leading to formation of aligned fibers within 24 h. After 5 days in culture, cells began to beat, indicating spontaneous contractility (Fig. 2B and Movies 1-3). In contrast, β-catenin-null cells continued to proliferate after Wnt3a treatment (Fig. S1B) and fused slowly and infrequently, giving rise to only very short fibers that were poorly aligned and did not beat, even after 5 days in culture (Fig. 2B and Movies 4-6). Upon long-term culture, some β-catenin-null cells fused into syncytia that became detached from the substrate and formed spherical balls (Fig. 2B), indicating that loss of β-catenin impaired substrate attachment. Cultures were immunostained with myogenin and MyHC antibodies at different time points after treating cells with Wnt3a. In wild-type myoblasts, myogenin and MyHC were first detected on day 1, whereas in β-catenin-null myoblasts, these markers were first detected on days 2-3 after induction of differentiation (Fig. 3A-D). Interestingly, MyHC was highly expressed in unfused cells in both wild-type and null cultures, and in syncytia that did not elongate in the latter. Despite the eventual acquisition of these myogenic markers in β-catenin-null cells (Fig. 3A-D), morphological myogenesis (formation of multinucleated elongated myofibers) was essentially prevented.
Fig. 2.
Differentiation capacity of wild-type and β-catenin-null primary myoblasts. (A) Phenotype of wild-type and β-catenin-null primary myoblasts cultured in growth media (GM) showing lack of spontaneous differentiation. (B) Phenotype of wild-type and β-catenin-null primary myoblasts after 5 days of culture in Wnt3a-containing media showing greatly impaired differentiation. Scale bars: 100 µm.
Fig. 3.
Immunostaining analysis of myogenic markers in wild-type and β-catenin-null primary myoblasts after Wnt3a treatment. (A,C) Immunofluorescence staining for myogenin (A) or MyHC (C), both green. Nuclei were stained with DAPI (blue). Images were captured at 0, 24, 48, 72 and 120 h post-treatment. (B,D) Quantification of myogenin- (B) or MyHC- (D) positive cells at each time point. Data are mean±s.e.m. Statistical analysis: one-way ANOVA (****P<0.0001).
To confirm that the phenotype of the null cells was due to loss of β-catenin, we transfected them with a constitutively active β-catenin expression plasmid together with a GFP-expression plasmid to assess efficiency. Notably, the null myoblasts transfected much more efficiently than did wild-type myoblasts (∼80% of null cells were GFP-positive relative to 50-60% of wild-type myoblasts), possibly owing to their rounded/poorly adherent phenotype. Null cells transfected with stable β-catenin spontaneously elongated and fused, and increased expression of myogenic markers (Fig. S3). Overall, these data suggest that β-catenin is important for coupling of fusion and cytoskeletal remodeling with acquisition of molecular markers of differentiation.
Molecular characterization of β-catenin-null primary adult myoblasts
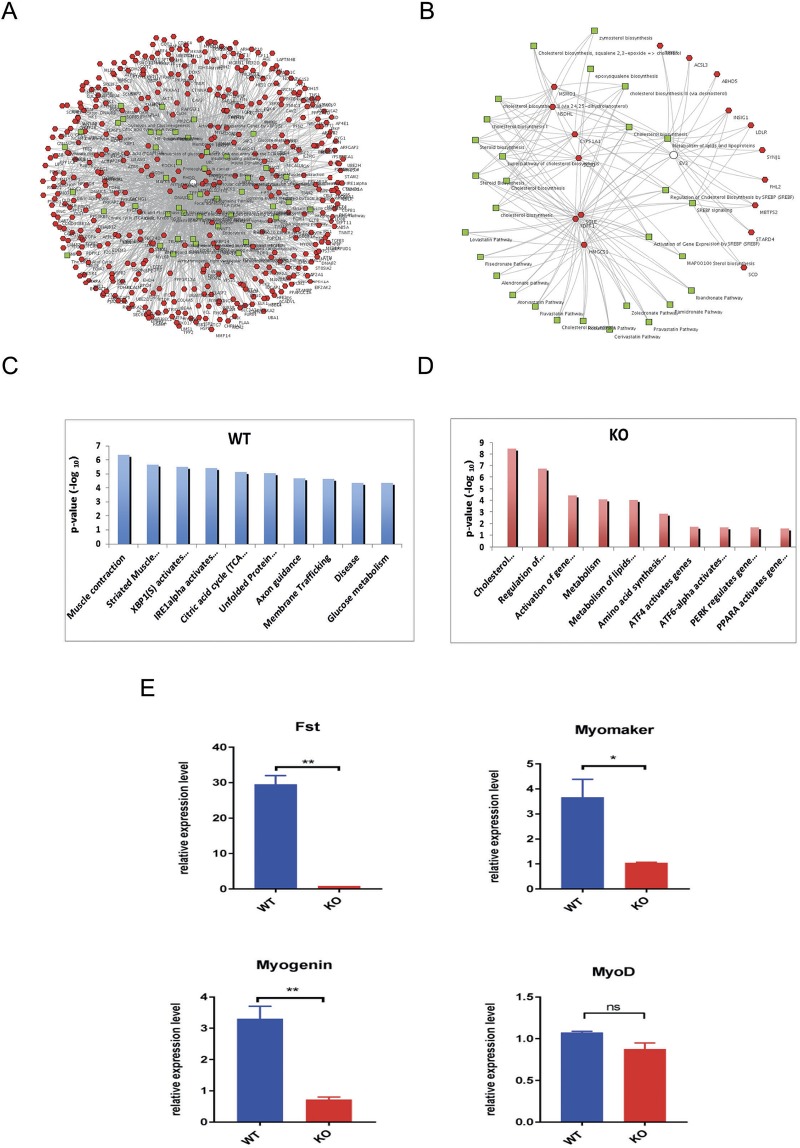
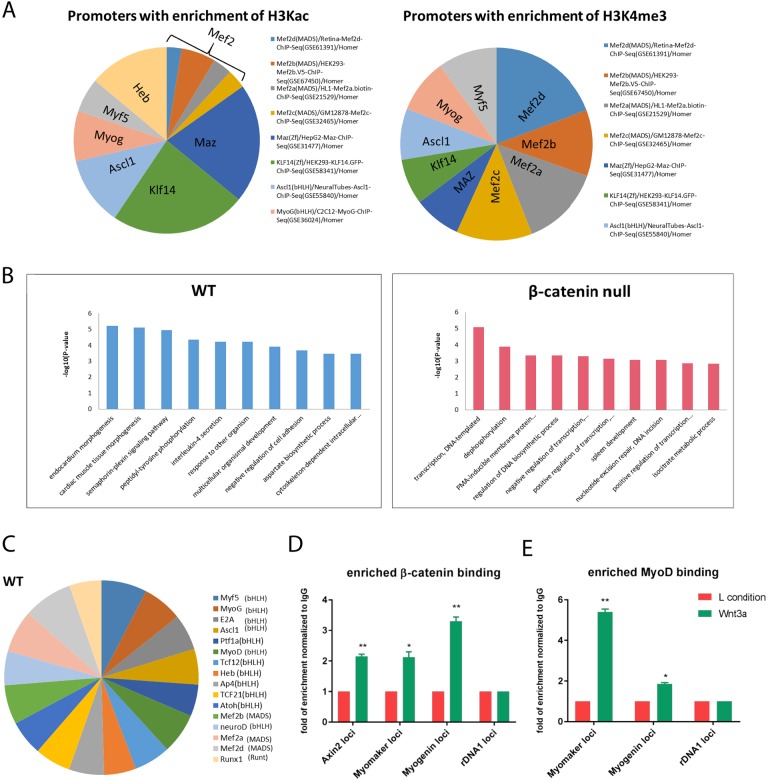
To understand the molecular changes underlying the phenotypic effects of loss of β-catenin, we performed transcriptomic analysis of wild-type myoblasts and two of the β-catenin-null lines after 24 h of Wnt3a treatment. Comparative enrichment clustering of wild-type myoblast RNAseq data using Toppcluster (Kaimal et al., 2010) identified the most significantly enriched Gene Ontology (GO) terms associated with the Wnt-induced transcriptome as muscle contraction and striated muscle formation (Fig. 4A,C). Equivalent analysis of β-catenin-null cells found no muscle-associated terms among the top 10 enriched terms GO categories (Fig. 4B,D). Moreover, comparison of Fig. 4A with B shows that wild-type cells (A) activated a very large number of genes (red nodes) that were tightly linked within a small number of functional pathways (green nodes), which is indicative of a highly coordinated response to Wnt, whereas null cells (B) activated very few genes within a large number of pathways, suggesting a nonspecific response. The top 25 pathways associated with the Wnt-induced transcriptome in wild-type cells were further assessed using REACTOME: muscle contraction (general, striated and smooth) were the top 3 enriched pathways, followed by ECM proteoglycans/ECM organization, Cdo, Cdkn1a/p21, calcitonin receptors, circadian clock, Igf-binding proteins, leptin, Tfap2, growth hormone signaling, Runx factors and NrCAM (see Table S2). Interestingly, of the top 25 enriched pathways associated with Wnt-downregulated genes in wild-type cells, 12 relate to Notch signaling and eight involve DNA repair (see Table S3). REACTOME analysis of the Wnt-induced transcriptome in null cells suggested a weak enrichment of lipid-associated pathways, although significance was low and no pathway was represented by more than two gene members (see Table S4).
Fig. 4.
Transcriptomic analysis of wild-type and β-catenin-null cells after Wnt3a treatment identifies the Wnt-induced gene set. (A,B) RNA-seq was performed on wild-type (A) and β-catenin-null (B) myoblasts treated with Wnt3a or control (L-cell) medium for 24 h. Genes upregulated by Wnt3a treatment (Wnt3a/L cell≥twofold, red nodes) were analyzed using Toppcluster to define associated pathways (green nodes). Network analysis of global gene expression changes in wild type shows many genes upregulated by Wnt3a in wild type but not in β-catenin-null myoblasts. (C,D) Gene Ontology (GO) terms significantly enriched in the differentially expressed gene set (Wnt3a/L cell≥twofold) identified in wild-type (C) and β-catenin-null (D) myoblasts. (E) Wnt3a induces expression of myogenic genes myomaker (Tmem8c), Fst and myogenin in a β-catenin-dependent manner. Quantitative RT-PCR was performed on wild-type and β-catenin-null myoblasts treated with Wnt3a-containing or control (L-cell) medium for 24 h. Data are normalized to the housekeeping gene RPS26 and are the average of three experiments performed in duplicate. Data are mean±s.e.m. Statistical analysis: t-test (*P<0.05, **P<0.01).
To validate the RNA-seq data, the β-catenin-dependence of selected targets was tested by RT-PCR (Fig. 4E). Myogenin, follistatin, and myomaker (see further details below) were induced by Wnt treatment of wild-type but not null cells, consistent with RNA-seq analysis. MyoD was not induced by Wnt3a in either cell type, again consistent with RNA-seq.
Essential fusion pore components myomaker and myomixer are targets of Wnt/β-catenin signaling in primary myoblasts
Among the novel Wnt/β-catenin-induced targets revealed by RNA-seq were two genes essential for formation of the myoblast fusion pore: myomaker (Mymk; previously called Tmem8c) and myomixer (Mymx; previously called myomerger, minion and GM7325) (Millay et al., 2014, 2016; Quinn et al., 2017; Takei et al., 2017). Both genes were induced fivefold by Wnt3a in wild-type cells but not in null cells based on RNA-seq data, and were induced by β-catenin transfection, as assessed by RT-PCR (see Fig. S4). In addition, RNA-seq showed that four other factors recently identified within the myomaker-myomixer complex (Takei et al., 2017) were induced at least twofold by Wnt3a in wild-type cells but not null cells. These genes were dysferlin (Dysf), synaptopodin-2-like (Synpo2l), junctional sarcoplasmic reticulum protein 1 (Jsrp1) and tripartite motif 72 (Trim72). Overall, these data suggest that Wnt might function in coordinating fusion pore assembly.
Wnt induces MyoD binding to myogenic loci in a β-catenin-dependent manner
Previous work using C2C12 cells showed that β-catenin could enhance the ability of MyoD to bind and activate specific target genes (Kim et al., 2008). Here, we had the opportunity to examine whether loss of β-catenin altered the global landscape of MyoD binding and gene activation in primary myoblasts. To achieve this, we performed two genome-wide analyses: the first was of activating-histone modifications in wild-type and β-catenin-null cells, the second was of MyoD binding. In wild-type cells, activating modifications (H3KAc and H3K4me3) were enriched at myogenic gene promoters 24 h after Wnt3a treatment. These activated promoters were enriched in MRF- and MEF-binding motifs, but not in TCF/LEF-binding motifs (Fig. 5A), consistent with our earlier study (Hulin et al., 2016). In contrast, β-catenin-null cells showed no enrichment of activating histone modifications at myogenic promoters, and there were no significantly enriched motifs. MyoD-ChIP-seq analysis in wild-type cells 24 h after Wnt3a treatment revealed increased binding of endogenous MyoD at genes associated with myogenic differentiation (Fig. 5B, left), and these loci were enriched in MRF- and MEF-binding motifs as expected (Fig. 5C). In contrast, in null cells, there was very little increase in MyoD binding after Wnt3a treatment, and the loci showing enhanced binding were not associated with myogenic genes (Fig. 5B, right), and contained no statistically enriched motifs, suggesting that the enrichment was non-specific.
Fig. 5.
Epigenomic analyses indicate that β-catenin promotes MyoD association with chromatin. (A) H3Kac and H3K4me3 ChIP-seq was performed using chromatin from wild-type myoblasts treated with control or Wnt3a-containing media for 24 h. Homer analysis was used to identify the top 10 motifs that are enriched in promoter regions that show increased levels of H3Kac and H3K4me3 (Wnt/L cell≥threefold) after Wnt3a induction (represented as target sequences with motif/total sequences with motif). (B) MyoD ChIP-seq analyses of wild-type and β-catenin-null primary myoblasts treated with Wnt3a medium for 24 h. Gene ontology (GO) analysis was performed on the set of genes showing increased MyoD binding after Wnt3a treatment (Wnt/L cell≥twofold) in wild-type (left) and β-catenin CRISPR-null (right) myoblasts. Wild-type myoblasts show significant enrichment of muscle-associated GO categories; β-catenin-null myoblasts do not show enrichment in any muscle-specific categories. (C) The top 15 motifs (Homer analysis) seen in promoter regions showing increased MyoD binding (Wnt/L cell≥twofold) after Wnt3a treatment of wild-type myoblasts (target sequences with motif/total sequences with motifs). Statistical analysis: multiple t-tests with P<0.05 considered significant. (D,E) Wnt3a increases binding of β-catenin and MyoD to the proximal promoters of the myomaker and myogenin genes, as shown by ChIP-qPCR analysis. Chromatin from wild-type primary myoblasts treated with control or Wnt3a-containing medium for 48 h was used for ChIP with β-catenin (D) or MyoD (E) antibodies. qPCR was used to assess enrichment of the myomaker promoter in both β-catenin- and MyoD-ChIP samples; the myogenin promoter was used as a control for MyoD-ChIP and the Axin2 enhancer was used as a control for β-catenin-ChIP. Data were first normalized to a control non-target locus (rDNA1) and then to the mock ChIP with preimmune IgG, set to a value of 1. n=3. Data are mean±s.e.m. *P<0.05, **P<0.001.
Immunoblotting analysis of myoblasts showed no obvious difference in MyoD or ID1 protein levels after Wnt treatment (Fig. S5). Thus, increased MyoD binding to target genes likely involves interaction of MyoD with β-catenin, as previously suggested (Kim et al., 2008). To confirm this, recruitment of β-catenin and MyoD was tested using ChIP-qPCR with the myogenin and myomaker promoters as exemplars. Wnt3a treatment of wild-type myoblasts increased binding of endogenous β-catenin and MyoD, at the same proximal promoter location, for both genes (Fig. 5D,E). The myogenin findings are consistent with previous work showing that β-catenin knockdown reduces MyoD binding to the myogenin promoter (Kim et al., 2008). However, the binding of β-catenin and MyoD to the mouse myomaker promoter is a novel finding. We also further confirmed the binding of MyoD to this promoter using heterologous MyoD expression (Fig. S6).
TCF/LEF-β-catenin interaction is not required for Wnt-mediated differentiation of primary myoblasts
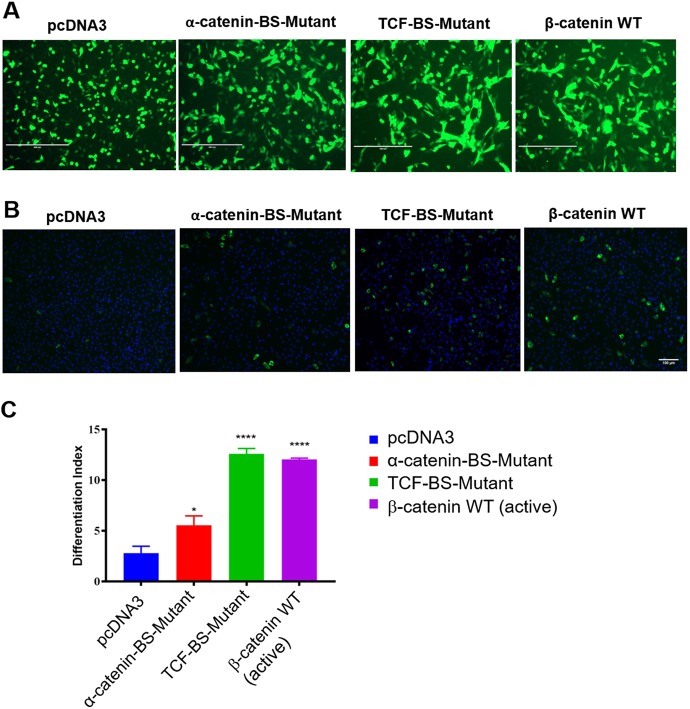
Although a previous study indicated that β-catenin controls C2C12 differentiation in a TCF/LEF-independent manner (Kim et al., 2008), a more recent report concluded that adult primary myoblasts require TCF/LEF for differentiation (Agley et al., 2017). To clarify the role of TCF/LEF family members in differentiation, we attempted to rescue the phenotype of the β-catenin-null cells using a variant of β-catenin that does not interact with TCF/LEF. We mutated two positively charged residues (‘charged buttons’) in β-catenin that are known to be essential for interaction with TCF/LEF proteins (Graham et al., 2000). The mutant form of stable β-catenin (referred to here as the TCF-binding site mutant) was unable to induce Axin2 expression or TOPflash activity (Fig. S7), confirming functional ablation of TCF/LEF interaction. When this TCF-binding site mutant was transfected into β-catenin null myoblasts, morphological differentiation was indistinguishable from that of cells transfected with wild-type β-catenin (Fig. 6A). The full rescue of differentiation by the TCF-binding site mutant was confirmed by quantifying the number of nuclei in MyHC-positive fibers (Fig. 6B,C). Overall, these data support the model (Kim et al., 2008) that β-catenin-mediated differentiation does not require TCF/LEF.
Fig. 6.
Comparison of the ability of wild-type and mutant forms of β-catenin to rescue differentiation of β-catenin-null myoblasts. (A) β-Catenin-null myoblasts were co-transfected with GFP and one of the following expression plasmids: empty pcDNA3, an α-catenin-binding site (BS) mutant form of β-catenin (which cannot interact with α-catenin), a TCF-binding site (BS) mutant form of β-catenin (which cannot interact with TCF/LEF) and wild-type β-catenin (constitutively active/stable). Scale bars: 50 µm. (B,C) Quantitation of differentiation was achieved by immunostaining with MyHC (B) and counting the percentage of nuclei (blue, DAPI) in MyHC-positive myofibers (C). Data are mean±s.e.m. Statistical analysis: one-way ANOVA (*P<0.05, ****P<0.0001).
Interaction of β-catenin and α-catenin is required for efficient differentiation of primary myoblasts
Although these studies have focused on nuclear functions, β-catenin is also crucial at adherens junctions where cadherin/β-catenin/α-catenin complexes link to the cytoskeleton (Huveneers and de Rooij, 2013) and control local filament assembly (Yamada et al., 2005; Xu and Kimelman, 2007). We examined whether expression of a mutant form of β-catenin that cannot interact with α-catenin could induce differentiation of β-catenin-null myoblasts. The residues that control interaction of β-catenin with α-catenin have been previously defined (Aberle et al., 1996; Nieset et al., 1997); we mutated these residues (threonine 120 and valine 122) to alanine, generating a variant of stable β-catenin that does not bind to α-catenin. This mutant (referred to here as the α-catenin-binding site mutant) was able to induce Axin2 expression and TOPflash activity as effectively as wild-type β-catenin (Fig. S7). The α-catenin-binding site mutant only partially rescued differentiation (Fig. 6A-C), suggesting that, in addition to the nuclear functions of β-catenin, interactions between β-catenin and α-catenin at membrane junction complexes are also required for efficient differentiation.
DISCUSSION
The overall goal of this study was to understand the cell-autonomous requirement for β-catenin in myoblast differentiation. This was prompted in part by conflicting findings from previous studies of β-catenin disruption both in vitro and in vivo. Our morphological and gene expression data using the β-catenin-null primary myoblast model are consistent with, but extend beyond, those of Kim et al. (2008) and Rudolf et al. (2016). Pathway analysis of transcriptomic data indicates that Wnt/β-catenin Wnt targets multiple myogenic pathways: many involved in forming contractile apparatus, but others related to cell cycle control (e.g. Cdkn1a/p21 that was previously implicated in myoblast cell cycle exit; Zhang et al., 1999), ECM modulation, adhesion and fusion, and Igf signaling. Wnt-mediated regulation of clock-related genes is intriguing and broadly consistent with previous studies showing that circadian regulators control differentiation in muscle (Chatterjee and Ma, 2016). The most highly Wnt-induced of the circadian genes, Bhlhe40 (SHARP-2, Dec1, Stra13), is a bHLH factor that functions in negative-feedback loop with CLOCK/BMAL (Honma et al., 2002). Interestingly, Bhlhe40 was also reported to antagonize Notch in satellite cells (Sun et al., 2007), preventing Notch-imposed inhibition of differentiation. Very recently, Wnt was reported to mediate intercellular coupling of the circadian clock to the cell cycle in organoids (Matsu-Ura et al., 2016); thus, it is possible that Wnt-mediated regulation of Bhlhe40 helps to coordinate the synchronous cell cycle exit of neighboring myoblasts.
Among the novel targets of Wnt/β-catenin identified were the central fusion pore factors myomaker and myomixer. Myomaker was recently found to be essential for C2C12 cell fusion. Moreover, its ectopic expression in fibroblasts could induce them to fuse with C2C12 cells, but not with each other (Millay et al., 2014, 2016), suggesting the existence of a myoblast-specific fusion partner. Myomixer was very recently identified as this protein (Leikina et al., 2018). Interestingly, we found that transfection of myomaker into β-catenin-null myoblasts did not rescue their fusion and instead caused toxicity (not shown). This may have been due to insufficient levels of myomixer and suggests that the levels of these two proteins must be carefully titrated. A set of potential fusion pore components were recently identified via a proteomic analysis of myomixer-interacting proteins (Takei et al., 2017). Of these, four were Wnt/β-catenin induced based on RNA-seq data; this finding prompts further analysis of the role of Wnt in controlling fusion pore assembly.
Overall, the dysregulation of multiple classes of myogenic regulators and effectors is consistent with the observation that longer-term null myoblast cultures ultimately expressed myogenin and MyHC, yet still failed to align, and any fusion that occurred appeared uncoupled from the assembly of the myofibrils that maintain myofiber architecture (particularly evident in the formation of ball-shaped syncytia). Overall, we conclude that β-catenin coordinates multiple processes, including cell cycle exit, matrix remodeling, fusion and cytoskeletal rearrangement/myofibril assembly.
In agreement with Kim et al. (2008), our studies indicated that β-catenin enhances MyoD binding to target genes. Moreover, using ChIP-seq we were able to generalize this model, showing that Wnt enhances MyoD binding to myogenic loci across the genome. We also confirmed that β-catenin and MyoD co-bind to common loci using analytical ChIP; however, we were unable to gain a genome-wide picture of colocalization because β-catenin ChIP-seq results were inconsistent (not shown). This is possibly because β-catenin associates in large complexes in myoblasts that are inconsistently represented during shearing/library construction; a technical limitation to be addressed in future work. How β-catenin promotes MyoD binding is not yet clear, but might involve post-transcriptional changes. Given that Wnt/β-catenin drives histone modification, it may also increase the stability of MyoD binding within chromatin. β-Catenin can associate with p300 and CBP, and these complexes are known to target different gene programs (Sasaki et al., 2013). MyoD also interacts with p300 and CBP; however, only p300 appears to be required for embryonic myogenesis (Polesskaya et al., 2001; Roth et al., 2003). We found that chemical inhibition of the β-catenin-p300 interaction can impair myoblast differentiation, whereas disruption of β-catenin-CBP interaction does not (Fig. S8). Whether these co-activators are differentially involved in the functions of β-catenin-MyoD and β-catenin-TCF/LEF complexes, is being further examined.
Kim et al. (2008) used dominant-negative (DN)-TCF/LEF expression to conclude that TCF/LEF proteins were redundant for differentiation. We came to the same conclusion via a different approach in which null cells were transfected with a form of β-catenin that cannot interact with TCF/LEF. However, a very recent study by Agley et al. (2017) found that overexpression of dominant-negative (DN)-TCF4 in human myoblasts impaired differentiation. The reasons for these discrepant results may relate to different cell models and assays; however, in the study by Agley et al., it is notable that DN-TCF4 expression dramatically reduced levels of active β-catenin (Agley et al., 2017); this would be expected to impair differentiation regardless of the mechanism of β-catenin action. Overexpression of active β-catenin also led to formation of thinner fibers (Agley et al., 2017), which is presumably due to precocious differentiation of myoblasts at the expense of proliferation (Brack et al., 2008; Murphy et al., 2014; Rudolf et al., 2016). A parsimonious model that agrees with all of these studies is that β-catenin-TCF interactions are not required to activate the differentiation program of committed myoblasts, but are required for appropriate feedback to the Wnt pathway (via targets such as Axin2) to prevent precocious/excessive fusion. There is also evidence that Wnt/β-catenin induces proliferation of uncommitted (e.g. MyoD-negative) satellite cells (Otto et al., 2008), and this is likely to involve TCF/LEF-mediated targeting of genes such as Ccna2 and Cdc25c (Suzuki et al., 2015). Consistent with this, when Murphy et al. (2014), examined Wnt/β-catenin activity during muscle regeneration using a TCF/LEF-GFP reporter, they found that 6% of cells were GFP+ before injury, 23% at 1 day post-injury (dpi) and only 0.6% at 3 dpi. This suggested that β-catenin/TCF/LEF activity is high in activated/proliferating satellite cells, but very low in differentiating myoblasts where we would argue that β-catenin/MyoD activity predominates instead (MyoD+ cells were also highest at 3 dpi in Murphy et al., 2014). Of note, during embryogenesis, TCF/LEF is also involved in Wnt-mediated induction of somitic Myf5 expression in proliferating progenitor cells (Borello et al., 2006). In general, it is clear that Wnt/β-catenin regulates multiple steps of myogenesis (Suzuki et al., 2015) and TCF/LEF-dependent functions of Wnt in progenitor and satellite cells may be distinct from its MyoD-dependent functions in committed myoblasts.
We found that interaction of β-catenin with α-catenin is important for efficient differentiation, which is in contrast to the findings of Kim et al. (2008) in C2C12 cells. However, Kim et al. expressed mutant β-catenin in the background of wild-type β-catenin, and the extent to which the mutant can act in a dominant-negative manner is unclear. Thus, our rescue studies in null cells might appear to be a more-reliable approach, although with the caveat that any overexpression of β-catenin could cause artefacts by altering stoichiometry. It is also possible that primary myoblasts and C2C12 cells have different requirements for these interactions. Adherens junctions might be more important for interaction, alignment and fusion of primary myoblasts than C2C12 cells, which are less migratory and more adhesive. The exact role of β-catenin at the myoblast membrane remains to be determined, although it likely relates to coupling cadherin-mediated adhesion to cytoskeletal remodeling (Wrobel et al., 2007; Ozawa, 2015). It is also interesting to consider the relationship between fusion pores and adherens junctions. In Drosophila muscle, electron-dense fusion plaques overlap with N-cadherin-rich junctions (Hamp et al., 2016). Thus, it is possible that myomaker/myomixer and β-catenin membrane functions overlap; future work should examine their localization and interaction during differentiation. Interestingly, recent work has shown that a β-catenin-α-catenin interaction may also be involved in the pro-proliferative functions of Wnt/β-catenin in satellite cells; in particular, loss of this interaction after deletion of cadherins induced a break in quiescence by increasing nuclear β-catenin/TCF complexes (Goel et al., 2017). Thus, it appears that membrane-linked β-catenin functions (like its nuclear functions) may also be involved in multiple steps of myogenesis.
Although this study focused on in vitro models to define β-catenin function, it is relevant to discuss the recent in vivo studies of satellite cell-specific β-catenin ablation. As mentioned previously, Murphy et al. (2014) found that loss of β-catenin had no effect on regeneration, whereas Rudolf et al. (2016) reported significantly impaired regeneration. The reason for the discrepancy remains unclear, although the possibility that the earlier study was compromised by the presence of unrecognized ‘recombination escapers’ has been put forward (Rudolf et al., 2016). Among the Wnt/β-catenin targets that we have identified are miRNAs that repress Pax7 by binding the 3′UTR (Cui et al., 2019). Murphy et al. (2014) used a Pax7-Cre-driver allele that contained the Pax7 3′UTR (Murphy et al., 2011); it is possible that endogenous β-catenin (via its miRNA targets) inhibited cre expression via this 3′UTR, increasing the likelihood that a subset of cells may escape recombination. Given the apparently pro-proliferative role of β-catenin (via TCF/LEF) in satellite cells (Suzuki et al., 2015), any such escapers might have a selective advantage post-activation. In contrast, Rudolf et al. (2016) used a Pax7-cre driver allele that did not contain this 3′UTR (Lepper et al., 2009) and hence would not have been subject to repression, possibly allowing more efficient recombination. Although this hypothesis obviously requires formal testing, it is reasonable to state that any regulation of a cre driver allele (in this case Pax7) by its intended target (in this case β-catenin) has potential to generate confounding effects.
In summary, we report that differentiation of primary adult myoblasts requires interaction of β-catenin with MyoD, but not TCF/LEF, in agreement with previous findings in C2C12 cells (Kim et al., 2008), but also involves the interaction of β-catenin with α-catenin at adherens junction complexes. We also identify a novel role for Wnt/β-catenin in regulation of fusion pore assembly. Further work will be required to understand exactly how β-catenin coordinates activation of myogenic gene expression with cell alignment and fusion, and the extent to which the membrane functions of β-catenin are important for this. In addition, defining differential co-factor requirements for β-catenin/MyoD and β-catenin/TCF/LEF complexes might ultimately allow differential targeting of desirable pro-myogenic versus other potentially deleterious (e.g. pro-fibrotic) functions of β-catenin in muscle.
MATERIALS AND METHODS
Primary myoblast cultures
Primary myoblasts were isolated from postnatal day 21 TP1-Venus mice by immuno-FACS, as previously described (Zhuang et al., 2014); details are provided in the supplementary Materials and Methods. TP1-Venus are an ICR transgenic strain [RIKEN BioResource Center (RBRC06137)] (Kohyama et al., 2005; Sasaki et al., 2011). Imaging of myoblast cultures was carried out using an Olympus CKX41 inverted microscope and DP22 camera.
Plasmids
LentiCRISPR v2 and TOPflash plasmids were from Addgene (plasmids 52961 and 12456) (Sanjana et al., 2014). The constitutively active β-catenin cDNA was obtained from Dr Victor Korinek and subcloned into pcDNA3 vector. The MyoD1/pcDNA3 vector was generated in the laboratory previously. pMM043 (GFP-expression) plasmid was obtained from Dr Michael Michael (Flinders University, Australia). TOPFlash plasmid was obtained from Addgene (plasmid 12456).
Site-directed mutagenesis
The TCF/LEF interaction sites in the β-catenin/pcDNA3 were mutated by the replacement K312 and K435 with glutamic acid: the β-catenin TCF-mutant (K312E/K435E). The α-catenin interaction sites in the β-catenin/pcDNA3 were mutated by the replacement T120 and V122 with alanines: β-catenin α-catenin-mutant (T120A/ V122A). Site-directed mutagenesis (SDM) was then performed using the QuikChange site directed mutagenesis protocol (Agilent). All mutant plasmids were confirmed by sequencing.
CRISPR
gRNAs were inserted into the LentiCRISPRv2 vector according to the recommended protocols (Sanjana et al., 2014). Briefly, oligonucleotides corresponding to sgRNA targeting exon 5 of β-catenin were annealed and ligated into LentiCRISPRv2. After confirmation by sequencing, the LentiCRISPRv2-β-catenin vector was packaged as described below and used to transduce primary adult mouse myoblasts at low passage. Transduced myoblasts were subjected to puromycin selection and fluorescence-activated cell sorting or limiting dilution plating to obtain clonal lines. Multiple mutant cell lines were expanded and characterized by sequencing and gene expression analysis.
Viral packaging and transduction
HEK293T cells were plated at a density of 4×105 cells/well in a 6-well plate 24 h before transfection. Transfection complexes consisted of plasmid DNA (1.5 µg viral plasmid, 1.5 µg gag-pol plasmid, 1 µg VSV-G plasmid), 8 µl of Lipofectamine 2000 (Thermo Fisher Scientific) and 200 µl of serum-free media per well. Cells were cultured for 24 h before collecting viral supernatant. Viral supernatant was harvested and cleared by centrifugation, and polybrene (Sigma) was added at 4 µg/ml final concentration. Spinfection was carried out by spinning plates for 3 h at 1150 g at 30°C. The viral supernatant was replaced with fresh media and cells were grown for a further 48 h before analysis. Lentiviral methods were approved by the Flinders Institutional Biosafety Committee (IBC2008-14).
Transfections
Primary myoblasts seeded in 12-well plates at 1×105 cells/well were co-transfected with pcDNA3, β-catenin/pcDNA3, MyoD1/pcDNA3, β-Cat K312E/K435E or β-Cat T120A/V122A and GFP expression plasmids pMM043 (0.66 µg each plasmid unless otherwise stated) using Lipofectamine LTX (Thermo Fisher Scientific). Quantitative-RT-PCRs were performed 48 h post-transfection. Transfections were performed in triplicate and repeated two to six times; significance was assessed using Student's t-test. Transfection with TOPflash used Lipofectamine LTX, as well as pRL-Null renilla-luciferase reporter (Promega) as an internal reference. Luciferase assays were performed 48 h post-transfection using a Dual Luciferase assay kit (Promega). TOPFlash luciferase assays were performed in HEK293T cells according to our previous studies (Zhuang et al., 2014).
Chromatin immunoprecipitation
ChIP was performed using a modified MicroChIP protocol (Dahl and Collas, 2008) in primary myoblasts as described previously (Zhuang et al., 2014) using MyoD antibody (M-318, Santa Cruz Biotechnology), rabbit β-catenin antibody (9562, Cell Signaling Technology) or an equivalent amount of appropriate pre-immune IgG (Santa Cruz Biotechnology or Cell Signaling Technology). In some ChIP experiments, wild-type myoblasts and β-catenin-null CRISPR myoblasts were plated at the same density and treated for 24 h with Wnt3a medium or L cell control medium. In other ChIP experiments, wild-type myoblasts were transfected with MyoD1/pcDNA3 using Lipofectamine LTX (Thermo Fisher Scientific). After ChIP, purified genomic DNA was analyzed by quantitative PCR (qPCR) with primers that amplify the target regions or a control non-target locus (rDNA1). Details are provided in the supplementary Materials and Methods, and sequences of primer used for genomic PCR are in Table S1.
RNA isolation and quantitative reverse transcription-PCR
RNA was prepared from cells using TRIzol (Life Technologies); after DNase treatment, cDNA was synthesized using NxGen M-MuLV reverse transcriptase (Lucigen) and random primers (New England Biolabs). Quantitative RT-PCR was performed using a Corbett Rotorgene (Qiagen) and GoTaq SYBR green (Promega). Significance was assessed using Student's t-test. Sequences of primers used for qRT-PCR are shown in Table S1.
Immunofluorescence labeling
Wild-type and β-catenin-null primary myoblasts were plated at 1.5×104 per well in collagen-coated 96-well plates before treatments with Wnt3a or L-cell control medium. Wells were fixed at 0 h, 24 h, 48 h, 72 h and 120 h post-treatment with 3.7% formaldehyde in PBS for 10 min at room temperature, rinsed with PBS and permeabilized with 0.5% Triton-X for 5 min. Primary myoblasts were then blocked with 1% BSA in PBS for 30 min before addition of antibodies. Rabbit anti-β-catenin (9562, Cell Signaling Technology), mouse anti-myogenin (F5D-c, DSHB) or anti-MyHC (MF-20-c, DSHB) antibodies were applied at 4 µg/ml overnight at 4°C; fluorescently labeled secondary antibodies (Dylight-594 or Dylight-488; Vector Labs) were applied at 10 µg/ml for 2 h. Nuclei were counterstained with 1 µg/ml DAPI for 5 min. Cells were imaged with an Olympus IX71 microscope. To quantify the percentage of myogenin- or MyHC-positive cells at each time point, the number of fluorescently labeled cells was divided by the total number of DAPI stained nuclei using ImageJ in 20 fields per condition.
Proliferation assay
A dye-retention assay was used to assess proliferation. Briefly, 1×106 wild-type or β-catenin-null myoblasts were washed and incubated with 1 µl of Vibrant DiI cell-labeling solution (Thermo Fisher Scientific) in 1 ml serum-free RPMI at 37⁰C for 10 min. Labeling efficiency was assessed by flow cytometry using the Accuri C6 (BD Biosciences) and was always more than 95%. The cells were plated in a 24-well plate at 5×104 cells/well. After various times in culture, the cells were harvested using trypsin and the mean DiI fluorescence level of the population was measured by flow cytometry. The change (decrement) in mean DiI fluorescence at each time point was calculated as a fold over the initial (time 0) fluorescence level. The experiment was repeated twice in duplicate with similar results and a representative experiment is shown.
RNA-seq
Wild-type and β-catenin-null CRISPR myoblasts were plated at 3.3×105 cells per well in collagen-coated six-well plates before treatment with Wnt3a or control conditioned L-cell medium. Primary myoblasts were harvested at 24 h post-treatment, and RNA was prepared as previously described. RNA quality was confirmed using the Agilent 2100 Bioanalyzer and RNA-Seq libraries prepared using the TruSeq RNA Sample Preparation Kit v2 according to the Illumina protocol. Multiplexed libraries were validated using the Agilent BioAnalyzer, normalized and pooled for sequencing. High-throughput sequencing was performed on the HiSeq 2500 system (Illumina) with a 100 bp read length. Image analysis and base calling were carried out using Illumina HiSeq Analysis Software. Short read sequences were mapped to a UCSC mm10 reference sequence using the RNA-Seq aligner STAR (Dobin et al., 2013). Known splice junctions from mm10 were supplied to the aligner and de novo junction discovery was also permitted. Differential gene expression analysis, statistical testing and annotation were performed using Cuffdiff 2 (Trapnell et al., 2013). Transcript expression was calculated as gene-level relative abundance in fragments per kilobase of exon model per million mapped fragments and employed correction for transcript abundance bias (Roberts et al., 2011). Results for genes of interest were also explored visually using the UCSC Genome Browser.
Statistical analyses
GraphPad Prism was used for statistical analysis. One-way ANOVA or t-tests were used as specified in the figure legends.
Supplementary Material
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contribution
Conceptualization: H.P.M., R.M.; Methodology: S.C.; Investigation: S.C., L.L., M.D., R.T.Y., J.-A.H., R.M.; Resources: M.D., R.T.Y., R.M.E.; Data curation: M.D., R.T.Y.; Writing - original draft: S.C., H.P.M., R.M.; Writing - review & editing: R.M.; Supervision: M.D., R.T.Y., R.M.; Project administration: R.M.; Funding acquisition: R.M.E., R.M.
Funding
This work was supported by National Institutes of Health (1R01EY026202 and 1R01EY028983 to H.P.M.), by an Australian Research Council Fellowship (FT110100573 to R.M.) and by a Flinders Foundation grant (to R.M.). Deposited in PMC for release after 12 months.
Data availability
RNA-seq and ChIP-seq data reported in this paper have been deposited in the National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA) database under accession number SRP185730.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.167080.supplemental
References
- Aberle H., Schwartz H., Hoschuetzky H. and Kemler R. (1996). Single amino acid substitutions in proteins of the armadillo gene family abolish their binding to -catenin. J. Biol. Chem. 271, 1520-1526. 10.1074/jbc.271.3.1520 [DOI] [PubMed] [Google Scholar]
- Agley C., Lewis F., Jaka O., Lazarus N., Velloso C., Francis-West P., Ellison-Hughes G. M. and Harridge S. D. R. (2017). Active GSK3β and an intact β-catenin TCF complex are essential for the differentiation of human myogenic progenitor cells. Sci. Rep. 7, 13189 10.1038/s41598-017-10731-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arce L., Pate K. T. and Waterman M. L. (2009). Groucho binds two conserved regions of LEF-1 for HDAC-dependent repression. BMC Cancer 9, 159 10.1186/1471-2407-9-159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi H., Gay S., Fedon Y., Vernus B., Bonnieu A. and Bacou F. (2011). Wnt4 activates the canonical β-catenin pathway and regulates negatively myostatin: functional implication in myogenesis. Am. J. Physiol. Cell Physiol. 300, C1122-C1138. 10.1152/ajpcell.00214.2010 [DOI] [PubMed] [Google Scholar]
- Billin A. N., Thirlwell H. and Ayer D. E. (2000). Beta-catenin-histone deacetylase interactions regulate the transition of LEF1 from a transcriptional repressor to an activator. Mol. Cell. Biol. 20, 6882-6890. 10.1128/MCB.20.18.6882-6890.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borello U., Berarducci B., Murphy P., Bajard L., Buffa V., Piccolo S., Buckingham M. and Cossu G. (2006). The Wnt/β-catenin pathway regulates Gli-mediated Myf5 expression during somitogenesis. Development 133, 3723-3732. 10.1242/dev.02517 [DOI] [PubMed] [Google Scholar]
- Brack A. S. (2014). Pax7 is back. Skeletal Muscle 4, 24-24 10.1186/s13395-014-0024-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brack A. S., Conboy I. M., Conboy M. J., Shen J. and Rando T. A. (2008). A temporal switch from notch to Wnt signaling in muscle stem cells is necessary for normal adult myogenesis. Cell Stem Cell 2, 50-59. 10.1016/j.stem.2007.10.006 [DOI] [PubMed] [Google Scholar]
- Buckingham M., Bajard L., Chang T., Daubas P., Hadchouel J., Meilhac S., Montarras D., Rocancourt D. and Relaix F. (2003). The formation of skeletal muscle: from somite to limb. J. Anat. 202, 59-68. 10.1046/j.1469-7580.2003.00139.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S. and Ma K. (2016). Circadian clock regulation of skeletal muscle growth and repair. F1000Research 5, 1549-1549 10.12688/f1000research.9076.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui S., Li L., Mubarokah S. N. and Meech R. (2019). Wnt/β-catenin signalling induces the MyomiRs miR-133b and miR-206 to suppress Pax7 and induce the myogenic differentiation program. J. Cell. Biochem. (in press). [DOI] [PubMed] [Google Scholar]
- Dahl J. A. and Collas P. (2008). A rapid micro chromatin immunoprecipitation assay (ChIP). Nat. Protocols 3, 1032-1045. 10.1038/nprot.2008.68 [DOI] [PubMed] [Google Scholar]
- Daniels D. L. and Weis W. I. (2005). Beta-catenin directly displaces Groucho/TLE repressors from Tcf/Lef in Wnt-mediated transcription activation. Nat. Struct. Mol. Biol. 12, 364-371. 10.1038/nsmb912 [DOI] [PubMed] [Google Scholar]
- Dobin A., Davis C. A., Schlesinger F., Drenkow J., Zaleski C., Jha S., Batut P., Chaisson M. and Gingeras T. R. (2013). STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15-21. 10.1093/bioinformatics/bts635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel A. J., Rieder M. K., Arnold H. H., Radice G. L. and Krauss R. S. (2017). Niche cadherins control the quiescence-to-activation transition in muscle stem cells. Cell Rep 21, 2236-2250. 10.1016/j.celrep.2017.10.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham T. A., Weaver C., Mao F., Kimelman D. and Xu W. (2000). Crystal structure of a β-catenin/Tcf complex. Cell 103, 885-896. 10.1016/S0092-8674(00)00192-6 [DOI] [PubMed] [Google Scholar]
- Hamp J., Löwer A., Dottermusch-Heidel C., Beck L., Moussian B., Flötenmeyer M. and Önel S.-F. (2016). Drosophila Kette coordinates myoblast junction dissolution and the ratio of Scar-to-WASp during myoblast fusion. J. Cell Sci. 129, 3426-3436. 10.1242/jcs.175638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht A., Vleminckx K., Stemmler M. P., van Roy F. and Kemler R. (2000). The p300/CBP acetyltransferases function as transcriptional coactivators of [beta]-catenin in vertebrates. EMBO J. 19, 1839-1850. 10.1093/emboj/19.8.1839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honma S., Kawamoto T., Takagi Y., Fujimoto K., Sato F., Noshiro M., Kato Y. and Honma K. (2002). Dec1 and Dec2 are regulators of the mammalian molecular clock. Nature 419, 841-844. 10.1038/nature01123 [DOI] [PubMed] [Google Scholar]
- Hulin J. A., Nguyen T. D., Cui S., Marri S., Yu R. T., Downes M., Evans R. M., Makarenkova H. and Meech R. (2016). Barx2 and Pax7 regulate Axin2 expression in myoblasts by interaction with beta-catenin and chromatin remodelling. Stem Cells 34, 2169-2182. 10.1002/stem.2396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huveneers S. and de Rooij J. (2013). Mechanosensitive systems at the cadherin–F-actin interface. J. Cell Sci. 126, 403-413. 10.1242/jcs.109447 [DOI] [PubMed] [Google Scholar]
- Jones A. E., Price F. D., Le Grand F., Soleimani V. D., Dick S. A., Megeney L. A. and Rudnicki M. A. (2015). Wnt/beta-catenin controls follistatin signalling to regulate satellite cell myogenic potential. Skelet Muscle 5, 14 10.1186/s13395-015-0038-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaimal V., Bardes E. E., Tabar S. C., Jegga A. G. and Aronow B. J. (2010). ToppCluster: a multiple gene list feature analyzer for comparative enrichment clustering and network-based dissection of biological systems. Nucleic Acids Res. 38, W96-W102. 10.1093/nar/gkq418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C.-H., Neiswender H., Baik E. J., Xiong W. C. and Mei L. (2008). {beta}-Catenin interacts with MyoD and regulates its transcription activity. Mol. Cell. Biol. 28, 2941-2951. 10.1128/MCB.01682-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohyama J., Tokunaga A., Fujita Y., Miyoshi H., Nagai T., Miyawaki A., Nakao K., Matsuzaki Y. and Okano H. (2005). Visualization of spatiotemporal activation of Notch signaling: live monitoring and significance in neural development. Dev. Biol. 286, 311-325. 10.1016/j.ydbio.2005.08.003 [DOI] [PubMed] [Google Scholar]
- Leikina E., Gamage D. G., Prasad V., Goykhberg J., Crowe M., Diao J., Kozlov M. M., Chernomordik L. V. and Millay D. P. (2018). Myomaker and myomerger work independently to control distinct steps of membrane remodeling during myoblast fusion. Dev. Cell 46, 767-780.e7. 10.1016/j.devcel.2018.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepper C., Conway S. J. and Fan C.-M. (2009). Adult satellite cells and embryonic muscle progenitors have distinct genetic requirements. Nature 460, 627-631. 10.1038/nature08209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepper C., Partridge T. A. and Fan C.-M. (2011). An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development 138, 3639-3646. 10.1242/dev.067595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li V. S. W., Ng S. S., Boersema P. J., Low T. Y., Karthaus W. R., Gerlach J. P., Mohammed S., Heck A. J. R., Maurice M. M., Mahmoudi T. et al. (2012). Wnt signaling through Inhibition of β-catenin degradation in an intact Axin1 complex. Cell 149, 1245-1256. 10.1016/j.cell.2012.05.002 [DOI] [PubMed] [Google Scholar]
- Matsu-Ura T., Dovzhenok A., Aihara E., Rood J., Le H., Ren Y., Rosselot A. E., Zhang T., Lee C., Obrietan K. et al. (2016). Intercellular coupling of the cell cycle and circadian clock in adult stem cell culture. Mol. Cell 64, 900-912. 10.1016/j.molcel.2016.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauro A. (1961). Satellite cell of skeletal muscle fibers. J. Cell Biol. 9, 493-495. 10.1083/jcb.9.2.493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy J. J., Mula J., Miyazaki M., Erfani R., Garrison K., Farooqui A. B., Srikuea R., Lawson B. A., Grimes B., Keller C. et al. (2011). Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development 138, 3657-3666. 10.1242/dev.068858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millay D. P., Sutherland L. B., Bassel-Duby R. and Olson E. N. (2014). Myomaker is essential for muscle regeneration. Genes Dev. 28, 1641-1646. 10.1101/gad.247205.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millay D. P., Gamage D. G., Quinn M. E., Min Y.-L., Mitani Y., Bassel-Duby R. and Olson E. N. (2016). Structure-function analysis of myomaker domains required for myoblast fusion. Proc. Natl. Acad. Sci. USA 113, 2116-2121. 10.1073/pnas.1600101113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M. M., Lawson J. A., Mathew S. J., Hutcheson D. A. and Kardon G. (2011). Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development 138, 3625-3637. 10.1242/dev.064162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M. M., Keefe A. C., Lawson J. A., Flygare S. D., Yandell M. and Kardon G. (2014). Transiently active Wnt/beta-catenin signaling is not required but must be silenced for stem cell function during muscle regeneration. Stem Cell Reports 3, 475-488. 10.1016/j.stemcr.2014.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieset J. E., Redfield A. R., Jin F., Knudsen K. A., Johnson K. R. and Wheelock M. J. (1997). Characterization of the interactions of alpha-catenin with alpha-actinin and beta-catenin/plakoglobin. J. Cell Sci. 110, 1013-1022. [DOI] [PubMed] [Google Scholar]
- Ogryzko V. V., Schiltz R. L., Russanova V., Howard B. H. and Nakatani Y. (1996). The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell 87, 953-959. 10.1016/S0092-8674(00)82001-2 [DOI] [PubMed] [Google Scholar]
- Otto A., Schmidt C., Luke G., Allen S., Valasek P., Muntoni F., Lawrence-Watt D. and Patel K. (2008). Canonical Wnt signalling induces satellite-cell proliferation during adult skeletal muscle regeneration. J. Cell Sci. 121, 2939-2950. 10.1242/jcs.026534 [DOI] [PubMed] [Google Scholar]
- Ozawa M. (2015). E-cadherin cytoplasmic domain inhibits cell surface localization of endogenous cadherins and fusion of C2C12 myoblasts. Biol. Open 4, 1427-1435. 10.1242/bio.013938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pansters N., van der Velden J., Kelders M., Laeremans H., Schols A. and Langen R. (2011). Segregation of myoblast fusion and muscle-specific gene expression by distinct ligand-dependent inactivation of GSK-3β. Cell. Mol. Life Sci. 68, 523-535. 10.1007/s00018-010-0467-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker D. S., Ni Y. Y., Chang J. L., Li J. and Cadigan K. M. (2008). Wingless signaling induces widespread chromatin remodeling of target loci. Mol. Cell. Biol. 28, 1815-1828. 10.1128/MCB.01230-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petropoulos H. and Skerjanc I. S. (2002). Beta-catenin is essential and sufficient for skeletal myogenesis in P19 cells. J. Biol. Chem. 277, 15393-15399. 10.1074/jbc.M112141200 [DOI] [PubMed] [Google Scholar]
- Polesskaya A., Naguibneva I., Fritsch L., Duquet A., Ait-Si-Ali S., Robin P., Vervisch A., Pritchard L. L., Cole P. and Harel-Bellan A. (2001). CBP/p300 and muscle differentiation: no HAT, no muscle. EMBO J. 20, 6816-6825. 10.1093/emboj/20.23.6816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posokhova E., Shukla A., Seaman S., Volate S., Hilton M. B., Wu B., Morris H., Swing D. A., Zhou M., Zudaire E. et al. (2015). GPR124 functions as a WNT7-specific coactivator of canonical beta-catenin signaling. Cell Rep 10, 123-130. 10.1016/j.celrep.2014.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn M. E., Goh Q., Kurosaka M., Gamage D. G., Petrany M. J., Prasad V. and Millay D. P. (2017). Myomerger induces fusion of non-fusogenic cells and is required for skeletal muscle development. Nat. Commun. 8, 15665 10.1038/ncomms15665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgeway A. G., Petropoulos H., Wilton S. and Skerjanc I. S. (2000). Wnt signaling regulates the function of MyoD and myogenin. J. Biol. Chem. 275, 32398-32405. 10.1074/jbc.M004349200 [DOI] [PubMed] [Google Scholar]
- Roberts A., Pimentel H., Trapnell C. and Pachter L. (2011). Identification of novel transcripts in annotated genomes using RNA-Seq. Bioinformatics 27, 2325-2329. 10.1093/bioinformatics/btr355 [DOI] [PubMed] [Google Scholar]
- Roth J. F., Shikama N., Henzen C., Desbaillets I., Lutz W., Marino S., Wittwer J., Schorle H., Gassmann M. and Eckner R. (2003). Differential role of p300 and CBP acetyltransferase during myogenesis: p300 acts upstream of MyoD and Myf5. EMBO J. 22, 5186-5196. 10.1093/emboj/cdg473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnicki M. A., Le Grand F., McKinnell I. and Kuang S. (2008). The molecular regulation of muscle stem cell function. Cold Spring Harb. Symp. Quant. Biol. 73, 323-331. 10.1101/sqb.2008.73.064 [DOI] [PubMed] [Google Scholar]
- Rudolf A., Schirwis E., Giordani L., Parisi A., Lepper C., Taketo M. M. and Le Grand F. (2016). beta-catenin activation in muscle progenitor cells regulates tissue repair. Cell Reports 15, 1277-1290. 10.1016/j.celrep.2016.04.022 [DOI] [PubMed] [Google Scholar]
- Sambasivan R., Yao R., Kissenpfennig A., Van Wittenberghe L., Paldi A., Gayraud-Morel B., Guenou H., Malissen B., Tajbakhsh S. and Galy A. (2011). Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. Development 138, 3647-3656. 10.1242/dev.067587 [DOI] [PubMed] [Google Scholar]
- Sanjana N. E., Shalem O. and Zhang F. (2014). Improved vectors and genome-wide libraries for CRISPR screening. Nat. Methods 11, 783-784. 10.1038/nmeth.3047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki N., Kiso M., Kitagawa M. and Saga Y. (2011). The repression of Notch signaling occurs via the destabilization of mastermind-like 1 by Mesp2 and is essential for somitogenesis. Development 138, 55-64. 10.1242/dev.055533 [DOI] [PubMed] [Google Scholar]
- Sasaki T., Hwang H., Nguyen C., Kloner R. A. and Kahn M. (2013). The small molecule Wnt signaling modulator ICG-001 improves contractile function in chronically infarcted rat myocardium. PLoS ONE 8, e75010 10.1371/journal.pone.0075010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz E. and Jaryszak D. L. (1985). Effects of skeletal muscle regeneration on the proliferation potential of satellite cells. Mech. Ageing Dev. 30, 63-72. 10.1016/0047-6374(85)90059-4 [DOI] [PubMed] [Google Scholar]
- Stamos J. L. and Weis W. I. (2013). The beta-catenin destruction complex. Cold Spring Harb. Perspect Biol. 5, a007898 10.1101/cshperspect.a007898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H., Li L., Vercherat C., Gulbagci N. T., Acharjee S., Li J., Chung T.-K., Thin T. H. and Taneja R. (2007). Stra13 regulates satellite cell activation by antagonizing Notch signaling. J. Cell Biol. 177, 647-657. 10.1083/jcb.200609007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A., Pelikan R. C. and Iwata J. (2015). WNT/β-catenin signaling regulates multiple steps of myogenesis by regulating step-specific targets. Mol. Cell. Biol. 35, 1763-1776. 10.1128/MCB.01180-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei D., Nishi M., Fukada S., Doi M., Okamura H., Uezumi A., Zhang L., Yoshida M., Miyazato M., Ichimura A. et al. (2017). Gm7325 is MyoD-dependently expressed in activated muscle satellite cells. Biomed. Res. 38, 215-219. 10.2220/biomedres.38.215 [DOI] [PubMed] [Google Scholar]
- Takemaru K. I. and Moon R. T. (2000). The transcriptional coactivator CBP interacts with beta-catenin to activate gene expression. J. Cell Biol. 149, 249-254. 10.1083/jcb.149.2.249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S., Terada K. and Nohno T. (2011). Canonical Wnt signaling is involved in switching from cell proliferation to myogenic differentiation of mouse myoblast cells. J. Mol. Signal. 6, 12 10.1186/1750-2187-6-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C., Hendrickson D. G., Sauvageau M., Goff L., Rinn J. L. and Pachter L. (2013). Differential analysis of gene regulation at transcript resolution with RNA-seq. Nat. Biotechnol. 31, 10.1038/nbt.2450 10.1038/nbt.2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Maltzahn J., Chang N. C., Bentzinger C. F. and Rudnicki M. A. (2012). Wnt signaling in myogenesis. Trends Cell Biol. 22, 602-609. 10.1016/j.tcb.2012.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodarz A. and Nusse R. (1998). Mechanisms of Wnt signaling in development. Annu. Rev. Cell Dev. Biol. 14, 59-88. 10.1146/annurev.cellbio.14.1.59 [DOI] [PubMed] [Google Scholar]
- Wozniak A. C., Kong J., Bock E., Pilipowicz O. and Anderson J. E. (2005). Signaling satellite-cell activation in skeletal muscle: markers, models, stretch, and potential alternate pathways. Muscle Nerve 31, 283-300. 10.1002/mus.20263 [DOI] [PubMed] [Google Scholar]
- Wrobel E., Brzoska E. and Moraczewski J. (2007). M-cadherin and beta-catenin participate in differentiation of rat satellite cells. Eur. J. Cell Biol. 86, 99-109. 10.1016/j.ejcb.2006.11.004 [DOI] [PubMed] [Google Scholar]
- Xu W. and Kimelman D. (2007). Mechanistic insights from structural studies of β-catenin and its binding partners. J. Cell Sci. 120, 3337-3344. 10.1242/jcs.013771 [DOI] [PubMed] [Google Scholar]
- Yamada S., Pokutta S., Drees F., Weis W. I. and Nelson W. J. (2005). Deconstructing the cadherin-catenin-actin complex. Cell 123, 889-901. 10.1016/j.cell.2005.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Wong C., Liu D., Finegold M., Harper J. W. and Elledge S. J. (1999). p21(CIP1) and p57(KIP2) control muscle differentiation at the myogenin step. Genes Dev. 13, 213-224. 10.1101/gad.13.2.213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang L., Hulin J. A., Gromova A., Tran Nguyen T. D., Yu R. T., Liddle C., Downes M., Evans R. M., Makarenkova H. P. and Meech R. (2014). Barx2 and Pax7 have antagonistic functions in regulation of wnt signaling and satellite cell differentiation. Stem Cells 32, 1661-1673. 10.1002/stem.1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.