ABSTRACT
Differentiation of germline stem cells (GSCs) in the Drosophila ovary is induced by somatic escort cells (ECs), which extend membrane protrusions encapsulating the germline cells (GCs). Germline encapsulation requires activated epidermal growth factor receptor (Egfr) signaling within the ECs, following secretion of its ligands from the GCs. We show that the conserved family of irre cell recognition module (IRM) proteins is essential for GC encapsulation by ECs, with a requirement for roughest (rst) and kin of irre (kirre) in the germline and for sticks and stones (sns) and hibris (hbs) in ECs. In the absence of IRM components in their respective cell types, EC extensions are reduced concomitantly with a decrease in Egfr signaling in these cells. Reintroducing either activated Egfr in the ECs, or overexpressing its ligand Spitz (Spi) from the germline, rescued the requirement for IRM proteins in both cell types. These experiments introduce novel essential components, the IRM proteins, into the process of inductive interactions between GCs and ECs, and imply that IRM-mediated activity is required upstream of the Egfr signaling.
KEY WORDS: Germline stem cells, Escort cells, Drosophila, Egfr, Germarium
Statement: IRM proteins mediate adhesion between escort cells and the germline cells, promoting short range signaling required for escort cells’ encapsulation of germline cells.
INTRODUCTION
Stem cell function depends on proper input from their environment. Anchoring of the germline stem cells (GSCs) to the maintenance niche is essential for them to preserve a stem cell state (Song and Xie, 2002; Xie and Spradling, 2000), and the encapsulation of the differentiating germline cells (GCs) by escort cells (ECs) is a prerequisite for their differentiation (Kirilly et al., 2011; Maimon et al., 2014; Su et al., 2018). While the anchoring process and its contribution to GSCs maintenance have been well studied, much less is known about the nature of the encapsulation process and its contribution to GCs differentiation.
Each adult Drosophila ovary is composed of 16–20 ovarioles, which serve as egg production lines. Continuous egg production depends on GSCs, which are housed at the anterior tip of each ovariole, in the germarium (Kirilly and Xie, 2007; Xie and Spradling, 2000). The cap cells of the maintenance niche and the GSCs express the DE-cadherin shotgun, which mediates their attachment to each other, and lack of DE-cadherin results in stem cell loss (Song and Xie, 2002). Close proximity between the stem cells and the maintenance niche is vital for the GSCs to receive the BMP2/4 homologue decapentaplegic (Dpp) from the cap cells (Kai and Spradling, 2003; Xie and Spradling, 2000). Dpp signaling in the GSCs results in phosphorylation of Mad (Mothers against Dpp, a SMAD2/3 homologue) and repression of bam (bag-of-marbles) expression, a key germline differentiation effector (Chen and McKearin, 2003; Ohlstein and McKearin, 1997; Song et al., 2004).
The GSCs undergo asymmetric division, with one daughter cell remaining in the niche and replenishing the stem cell reservoir. The other daughter cell, the cystoblast, exits the maintenance niche and begins to differentiate (Xie and Spradling, 2000). During differentiation, GCs undergo incomplete cell divisions and remain connected by cytoplasmic bridges, thereby forming cysts (de Cuevas et al., 1997). The fusome, an intracellular organelle connecting GCs, changes shape throughout GCs’ differentiation from a round structure in GSCs and in cystoblasts, into a branched profile in differentiated cysts (de Cuevas et al., 1997; Lin et al., 1994).
Exiting the maintenance niche is a necessary but not sufficient step towards GSC differentiation, and the latter is further supported by ECs, which provide essential signals for differentiation. ECs encapsulate the GCs with membrane extensions, shown to promote GC differentiation. In the absence of these protrusions, differentiation does not progress (Kirilly et al., 2011; Maimon et al., 2014; Su et al., 2018). JAK/STAT (Maimon et al., 2014; Rozario and DeSimone, 2010) and Egfr (Gilboa and Lehmann, 2006; Liu et al., 2010; Schulz et al., 2002) activation are both required to induce the ECs’ specialized membrane processes (Liu et al., 2010; Maimon et al., 2014; Schulz et al., 2002). The germline supports the EC extensions through the secretion of Egfr ligands (Liu et al., 2010), which in turn activates the Egfr signaling in the ECs (Gilboa and Lehmann, 2006; Liu et al., 2010; Schulz et al., 2002). Recent studies demonstrated that EC membrane extensions behave dynamically, with a subset of them continuously retracting and extending, whereas others become stabilized, allowing firm encapsulation of the germline (Banisch et al., 2017). Such dynamic behavior might provide essential signals for GC differentiation, but still allows physical progression of the differentiated GCs posteriorly. Although some of the factors that act from the soma are known, little is known about the role of GCs in the encapsulation.
In an attempt to identify novel components mediating the cross talk between GCs and ECs, we performed an RNAi-based screen with a group of candidate genes. One hit from this screen was Kirre, a member of the irre cell recognition module (IRM) family. IRM proteins belong to the Ig superfamily of proteins, with conserved structure and function from C. elegans and flies, to humans (Fischbach et al., 2009). Drosophila IRM family includes four membrane proteins: Rst, Kirre, SNS and Hbs, which are expressed by multiple cell types, where they mediate various heterotypic adhesion processes. These include myoblast fusion to myotubes (Artero et al., 2001; Dworak et al., 2001; Paululat et al., 1995; Ruiz-Gómez et al., 2000; Strunkelnberg et al., 2001), cell sorting during ommatidia formation (Bao and Cagan, 2005; Bao et al., 2010), cell spacing in the olfactory sensory organs, the formation of the wing margins (Venugopala Reddy et al., 1999), axon pathfinding in the optic lobe (Boschert et al., 1990; Schneider et al., 1995), programmed cell death in the eye (Reiter et al., 1996; Wolff and Ready, 1991) and the establishment of a slit diaphragm-like structure in garland and in pericardial nephrocytes (Weavers et al., 2009; Zhuang et al., 2009). IRM proteins also function in the peritoneal and epithelial muscle sheaths of larval ovaries during egg chamber oogenesis (Valer et al., 2018).
The IRM-mediated attachment is characterized by heterotypic interactions between two paralog sets; Rst and Kirre bind with either Sns or Hbs. In some systems one binding pair is dominant while other systems utilize them redundantly. Additionally, the expression pattern of the different IRM components is not necessarily limited to specific cell types involved in the interaction. In eye development the only active IRM proteins are Rst and Hibris (Bao and Cagan, 2005), with interommatidial precursor cells expressing Rst and primary pigment cells expressing Hibris (Bao and Cagan, 2005). In myoblast fusion, Kirre acts redundantly with Rst (Ruiz-Gómez et al., 2000; Strunkelnberg et al., 2001) and Hbs can partially substitute for Sns (Shelton et al., 2009), with expression of Rst on both fusion competent myoblasts and founder cells (Galletta et al., 2004; Strunkelnberg et al., 2001). During myoblast fusion, IRM proteins promote attachment between the myotube and the myoblast through direct binding of their extracellular domains (Galletta et al., 2004). Subsequently, the cytoplasmic domains of both Kirre and SNS recruit the actin polymerization machinery through verprolin/WASP interacting protein (WIP), a process suggested to support the membrane fusion process (Kaipa et al., 2013; Kim et al., 2007; Massarwa et al., 2007; Mukherjee et al., 2011).
The mammalian homologs of rst and of kirre are KIRREL (Neph1), KIRREL2 (Neph3) and KIRREL3 (Neph2, mkirre), while both sns and hbs have one known homolog, Nephrin (Fischbach et al., 2009). These are required for processes that are akin to the Drosophila ones: muscle fusion (Sohn et al., 2009; Wagner et al., 2011), axon pathfinding and synapse formation of proprioceptive neurons on muscle spindles (Komori et al., 2008), sensory organ formation (Morikawa et al., 2007), and the establishment of filtration barrier of the kidney podocytes (Tryggvason et al., 2006). In humans, mutations in Neph1 or Nephrin associate with congenital nephrotic syndrome of the Finnish type (NPHS1), in which the glomerular filtration barrier breaks down (Kestilä et al., 1998).
Here we show that the IRM proteins are required for the formation of EC membrane extensions which encapsulate the GCs, as well as for activation of the Egfr signaling pathway within the ECs. Moreover, we show that hyper activation of the Egfr signaling in the ECs bypasses the requirement for IRM proteins-dependent adhesion, supporting a model in which IRM-dependent adhesion acts upstream of the Egfr signaling, to stabilize ECs membrane extensions.
RESULTS
Kirre and Rst are required within germline cells for their encapsulation by ECs
In order to identify novel components required for germline encapsulation by ECs, an RNAi-based screen with candidate genes coding for putative membrane or ECM proteins was performed. Two drivers were used, nanos-Gal4 (nos-Gal4) driving expression in the germline, and traffic jam-Gal4 (tj-Gal4), driving expression in the somatic ECs (Bolívar et al., 2006; Li et al., 2003; Van Doren et al., 1998). Encapsulation defects manifest in aberrant ECs extensions, or in abnormal GC differentiation. The extensions were visualized by anti-Coracle (Cora), a membrane protein that is highly expressed in ECs membrane protrusions (Fairchild et al., 2015; Maimon et al., 2014). Differentiation was detected by round fusome amount, visualized by anti-hu-li tai shao (Hts). Summary of the genes identified in this screen is described in Table S1.
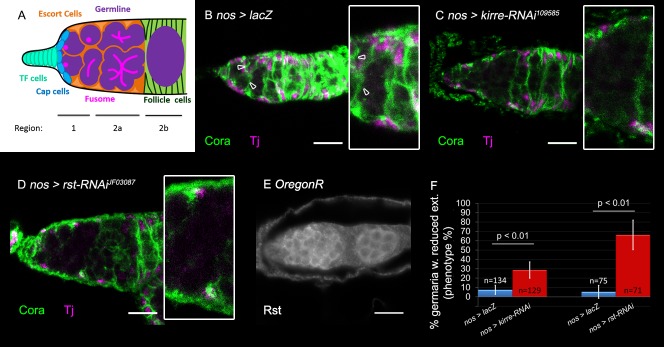
An interesting hit of this screen was kirre, coding for one of four IRM family members in the Drosophila genome. kirre knockdown in the germline resulted in a non-autonomous effect on the ECs. In region 2a of the germarium the differentiating germline cells associates with ECs (Fig. 1A). When kirre was knocked-down using kirre-RNAi109585, EC membrane extensions were reduced in this region (Fig. 1, compare B to C). Quantification of this phenotype was achieved by scoring germaria into one of two groups: those lacking extensions in region 2a in at least three consecutive focal planes (z=6 µm) and those with normal extensions in this region in all focal planes. kirre knockdown in the germline resulted in 29% germaria with a decreased number of extensions (germaria, n=129 P<0.01) (Fig. 1F). Notably, this reduction was not due to an absence of ECs in the affected area, as they were still visible when labeled with anti-Traffic jam (Tj) (Fig. 1C). Knockdown of kirre using a second RNAi line (kirre-RNAi27227) caused partial lethality of the animals, and hence was not quantified.
Fig. 1.
Germline knockdown of kirre or rst inhibits ECs membrane extensions. (A) Schematic representation of the germarium. In region 1 GSCs are attached to the maintenance niche and differentiation begins with their cystoblast daughter cells, all of which contain round fusomes. In region 2a the differentiating germline cells, characterized by branched fusomes, are encapsulated by EC extensions. In region 2b the 16-cell cyst is surrounded by follicle cells. (B–D) Anti-Coracle (Cora, green) marks somatic cell membranes, Anti-Tj marks ECs (magenta) arrowheads point to existing extensions, insets show region 2a magnified twofold. (B) Control germline nos-GAL4>lacZ germaria exhibits normal EC extensions (green). (C) Representative image of germline knockdown of kirre (using kirre RNAi) which reduces EC extensions. (D) Representative image of germline knockdown of rst (using rst RNAi) which reduces EC extensions. (E) Rst staining is present in the germline, as revealed by Anti-Rst. (F) Quantification of the percentage of germaria with reduced EC extensions in germline knockdown of kirre (n=129, eight independent experiments) or rst (n=71, six independent experiments). P-values were calculated using binomial proportions z-test, bars represent s.d. Scale bars: 10 µm.
Similarly to kirre, roughest (rst) knockdown in the germline led to a reduction in EC extensions (Fig. 1, compare B to D). Decreased extensions were detected in 66% of analyzed germaria (n=71 P<0.01) (Fig. 1F), whereas no effect on the presence of the ECs was observed (Fig. 1D). Anti-Rst staining revealed Rst localization along the GC boundaries (Fig. 1E). It was therefore concluded that Kirre and Rst are both required in the germline for a non-autonomous induction of EC membrane extensions.
Knockdown of kirre and rst in the ECs using tj-Gal4, did not lead to a visible phenotype (Fig. S1A-C, quantification in G), confirming the non-autonomous activity of both Kirre and Rst in the GCs. Taken together, these experiments support a non-autonomous function of Kirre and Rst in the germline, essential for inducing membrane extensions of the ECs.
Hbs and Sns are required in ECs for germ cell encapsulation
Next, we knocked down hbs and sns, the binding partners of kirre and rst, either in the germline or in the soma, driving their corresponding RNAi lines with either soma (tj) or germline (nos) GAL4 drivers. hbs knockdown in the soma resulted in 30% of analyzed germaria presenting reduced extension in region 2a (n=80 P<0.01) (Fig. 2, compare A to B, quantification in D), without affecting EC number. Similarly, knockdown of sns in the ECs led to a phenotype of reduced EC membrane extensions in region 2a in 37% of the analyzed germaria (n=57 P<0.01) (Fig. 2, compare A to C, quantification in D), with no effect on EC number. In contrast, knockdown of hbs or sns in the germline did not impact the formation of EC extensions (Fig. S1D-F, quantification in H). It was therefore concluded that whereas Kirre and Rst promote EC extensions non-autonomously acting within the GCs, Sns and Hbs activities are required autonomously in the ECs.
Fig. 2.
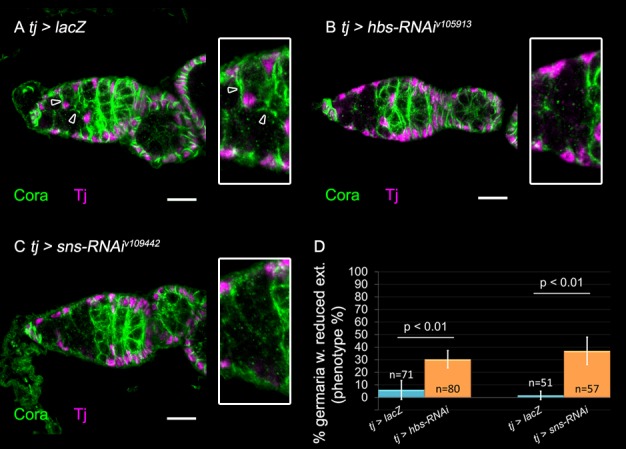
Somatic knockdown of hbs or sns inhibits EC membrane extension. In all panels: Anti-Coracle (Cora, green) marks somatic cell membranes, Anti-Tj marks ECs (magenta). Arrowheads point to existing extensions, insets show region 2a magnified twofold. (A) Control tj-GAL4>lacZ exhibits normal EC extensions. (B) Somatic knockdown of hbs using RNAi exhibits reduced EC extensions. (C) Somatic knockdown of sns using RNAi exhibits reduced EC extensions. (D) Quantification of the percentage of germaria with reduced extensions observed in somatic knockdown of hbs (n=80, six independent experiments) or sns (n=57, four independent experiments). P-values were calculated using binomial proportions z-test, bars represent s.d. Scale bars: 10 µm.
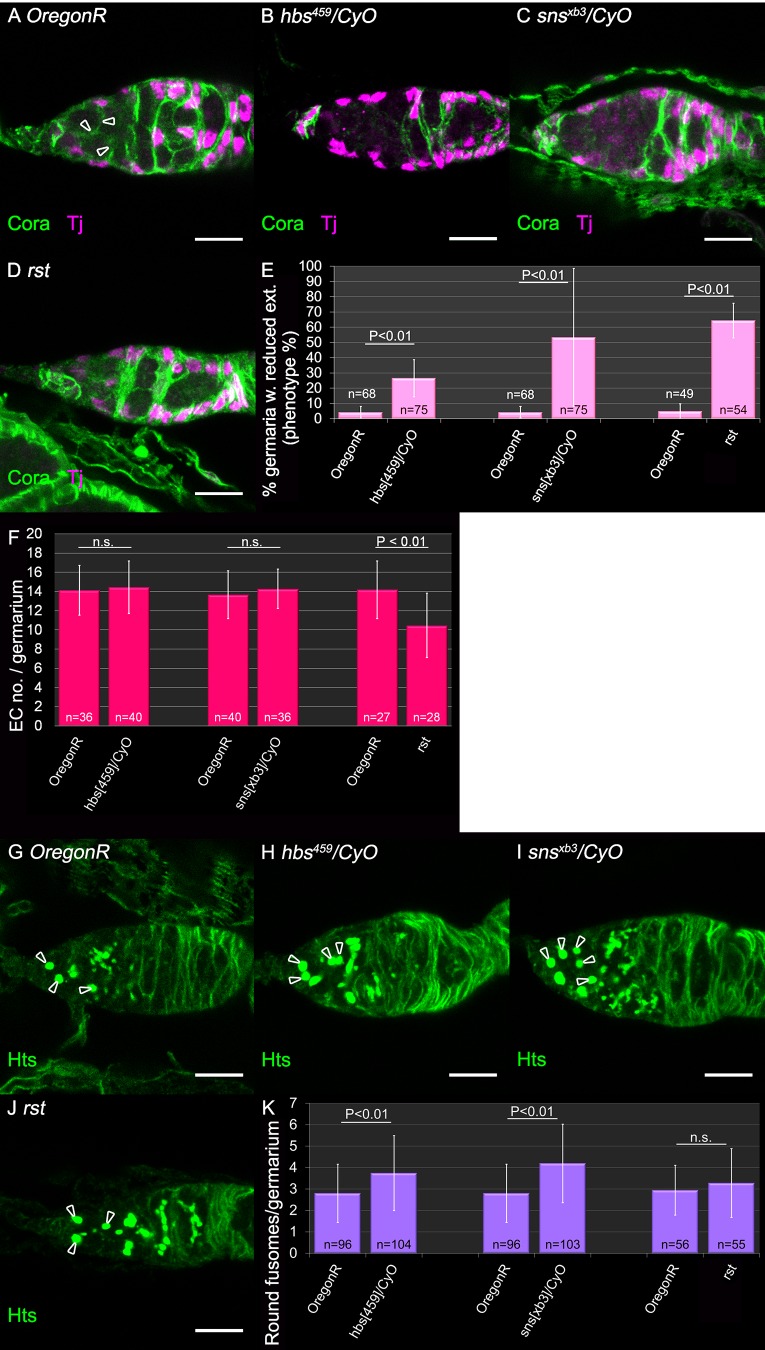
The requirement for IRM components is haploinsufficient
Homozygous null mutant embryos for each of the IRM genes do not survive to adulthood due to their requirements in other tissues. Interestingly, ovaries heterozygous for either hbs459, snsxb3, as well as homozygous rst hypomorphic allele (rstMI04842-GFSTF1) did show a phenotype of loss of ECs extensions (Fig. 3A–D, and quantification in E). A significant reduction of EC membrane extensions was observed in 27% of hbs459/CyO germaria (n=75 P<0.01) (Fig. 3B,E), as well as in 53% germaria of snsxb3/CyO (n=75 P<0.01) (Fig. 3C,E) and in 42% of snss660/CyO germaria (n=36 P<0.01) (Fig. S2A,B). Germaria of rst homozygous hypomorphic allele had fewer extensions in 64% of the germaria (n=54 P<0.01) (Fig. 3D,E). The number of ECs remained constant in all of the heterozygous background, while rst homozygous hypomorphic germaria had less ECs and were smaller (n=28 P<0.01) (Fig. 3F, Fig. S2C). Interestingly, kirre,rst heterozygous females carrying a deficiency that deletes both genes did not exhibit a phenotype of lack of ECs extensions, possibly pointing to an unequal functional contribution of the IRM proteins to GC–EC adhesion (Fig. S3).
Fig. 3.
Haplo insufficient requirements for IRM genes in inducing EC membrane extensions and in differentiation. (A) WT germarium, each germline cell is surrounded by EC extensions (arrowheads). Anti-Coracle (Cora, green) marks somatic cell membranes, Anti-Tj marks ECs (magenta). (B,C) Representative images of heterozygous mutants of hbs (B) or sns (C) exhibit reduced extensions. (D) Homozygous rst mutant has fewer extensions. (E) Quantification of the percentage of germaria with reduced extensions in heterozygous females of hbs (n=75), sns (n=75), rst (n=54) mutants. For each mutation, five independent experiments are shown. P-values were calculated using binomial proportions z-test, bars represent s.d. (F) Quantification of ECs present in heterozygous germaria of hbs (n=40, three independent experiments), sns (n=36, three independent experiments), rst (n=28, two independent experiments) mutants. P-values were calculated using two-sample t-test, bars represent s.d. (G) WT germline contains about three germline cells with round fusomes (arrowheads). Anti-Hts (green) stains fusomes and membranes. (H,I) Representative heterozygous mutant germarium of hbs (H) or sns (I) contain more GCs with round fusomes, indicating undifferentiated germlines (arrowheads). (J) Homozygous rst mutant germarium contains about three round fusomes (arrowheads). (K) Quantification of the number of round fusomes per germarium in heterozygous females of hbs (n=104, seven independent experiments), sns (n=103, seven independent experiments), and rst (n=55, four independent experiments). P-values were calculated using two-sample t-test, bars represent s.d. Scale bars: 10 µm.
These results corroborate the conclusions obtained by the RNAi experiments, and support the direct involvement of the IRM proteins in mediating communication between the germline and ECs, required for germline encapsulation by the ECs. We also produced mutant clones for the IRM alleles in the soma or in the germline, to evaluate the phenotype of EC extensions in a complete absence of either of the IRM components. Unfortunately, EC mutant clones were less frequent then control EC clones and were often comprised of a single cell, making it difficult to distinguish between its own extensions and extensions from neighboring wild-type (WT) EC. Germline clones were of equal frequency as in the control. However, completely mutant germline was not achieved, so that each EC always came into contact with WT germline and we were unable to infer the outcome of full absence of the relevant IRM (Fig. S4 shows that the mutant cell is still in contact with one WT cell).
Encapsulation of GCs by ECs had been shown previously to contribute to differentiation of the GCs, detected by a transformation of the round fusomes into elongated ones (Kirilly et al., 2011; Lim and Fuller, 2012; Maimon et al., 2014; Schulz et al., 2002; Su et al., 2018). We further analyzed the degree of GCs differentiation by quantifying the number of round fusomes (not including dividing GSCs) in the germaria of either hbs, or sns heterozygous, as well as in rst hypomorphic homozygous females, all of which exhibited a phenotype of loss of EC membrane extensions. hbs459/CyO germaria did exhibit higher number of rounded fusomes (n=104 P<0.01) (Fig. 3H, quantification in K). Similarly, snsxb3/CyO germaria were found to contain a higher number of rounded fusomes (n=103 P<0.01) (Fig. 3I, quantification in K). However, heterozygotes snss660/CyO (Fig. S2D-E), or rst hypomorphic homozygous (Fig. 3J, quantification in K) did not exhibit a significant increase in the number of round fusomes. These results support the notion that a primary role of IRM proteins is mediating EC extensions rather than directly affecting differentiation of the GCs. RNAi mediated knockdown for rst, hbs, sns, but not for kirre similarly exhibited increased number of round fusomes (Fig. S5A,B).
IRM proteins are required for Egfr signaling in ECs
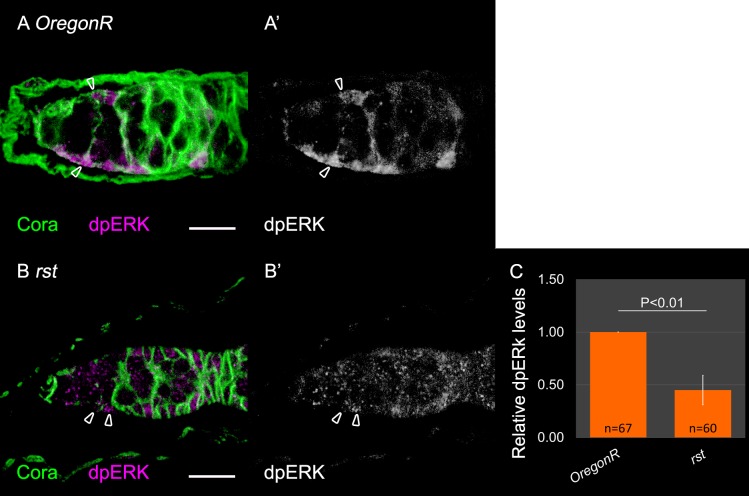
Two signaling pathways, Egfr, as well as JAK/STAT had been previously shown to promote the formation of EC membrane extensions. To assess whether activation of these pathways is downstream of IRM-dependent communication between ECs and GCs, we compared the levels of their respective targets in rst homozygous mutant germaria. Namely, double phosphorylated ERK (dpERK) for Egfr (Kirilly et al., 2011; Schulz et al., 2002), and the transcriptional target ZFh1 for JAK/STAT (Leatherman and Dinardo, 2010; Maimon et al., 2014; Singh et al., 2010). Zfh1 levels in the ECs were unaffected in rst homozygous mutant germaria (Fig. S6), suggesting no connection between IRM and JAK/STAT signaling. In contrast, dpERK levels were significantly reduced in rst homozygous mutant ECs (fluorescence quantification indicated that the intensity of dpERK was 0.45-fold less than that of control, n=60 P<0.01) (Fig. 4), suggesting that IRM-dependent EC–GC adhesion is upstream of Egfr activation in the ECs. Analysis of heterozygous mutant germaria did not show a statistically significant change in dpERK signaling, unlike the analysis of the rst homozygous mutant germaria, possibly indicating a different sensitivity of the dpERK intensity relative to the phenotype of EC membrane extension.
Fig. 4.
rst is required for Egfr activation in ECs. In all panels: Anti-Coracle (Cora, green) marks somatic cell membranes, Anti-dpERK (magenta) marks somatic cell bodies. (A,A′) Control ECs express dpERK. (B,B′) rst homozygous mutant ECs have reduced dpERK levels. Arrowheads indicate ECs. (C) Quantification of dpERK fluorescent levels in ECs of control (OregonR, n=67) or rst (n=60) mutant germaria. Six independent experiments are shown. P-values were calculated using two-sample t-test, bars represent s.d. Scale bars: 10 µm.
Next, a constitutively active Egfr (Egfr-CA) transgene was expressed in the ECs, in combination with an RNAi against sns to address its ability to rescue the extensions of the ECs in which sns was knocked down. We used UAS-lacZ to compensate for the addition of a UAS responsive site. Egfr-CA was indeed able to partially restore the formation of membrane extensions by the ECs. Whereas sns-RNAi caused decreased ECs extensions in 61% of the germaria (n=40), its co-expression with Egfr-CA decreased the reduction of EC extension to 38% of the germaria (n=42, P<0.05, Fig. 5, compare C to D, quantification in E). These results indicate that Egfr signaling in ECs can compensate for the loss of sns, and together they support the notion that IRM proteins act upstream of Egfr signaling in the ECs.
Fig. 5.
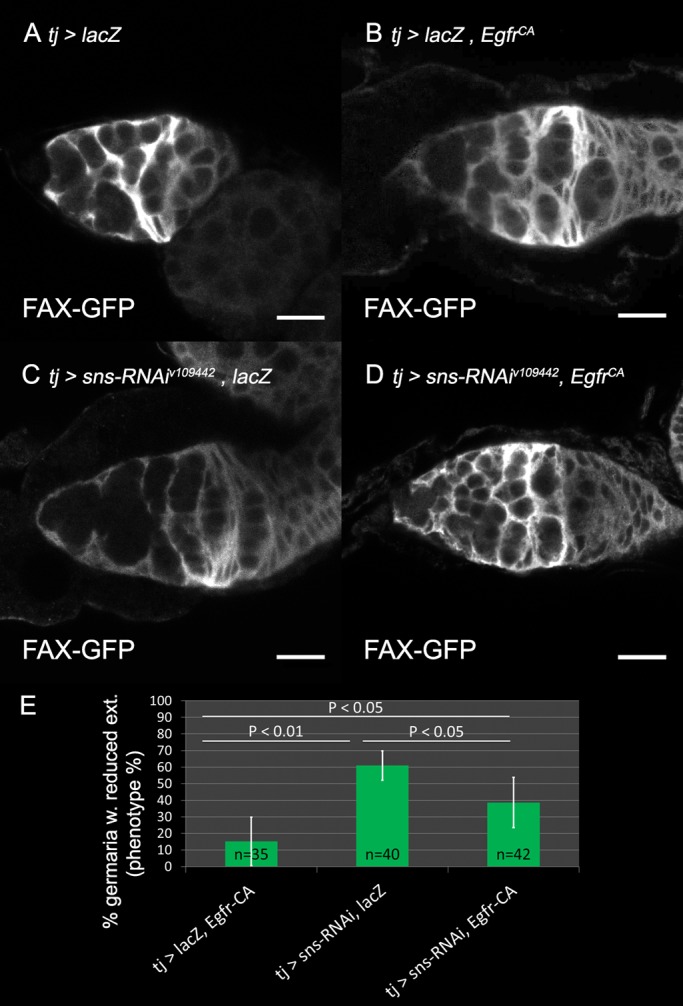
Constitutive active Efgr in ECs partially rescues extension formation. In all panels: FAX-GFP (white) marks somatic cell membranes. (A) FAX-GFP labels EC extensions. (B) Constitutively active Egfr (EGFRCA) in control germaria labeled with FAX-GFP and also carrying UAS-lacZ. (C) sns knockdown in the soma labeled with FAX-GFP and carrying UAS-lacZ. (D) sns knockdown in the soma combined with EGFRCA in the soma labeled with FAX-GFP restores EC extensions. (E) Quantification of the percentage of germaria with reduced extensions in control (n=35), sns knockdown (n=40), or sns knockdown combined with constitutive active Egfr (n=42). Four independent experiments are shown. P-values were calculated using binomial proportions z-test, bars represent s.d. Scale bars: 10 µm.
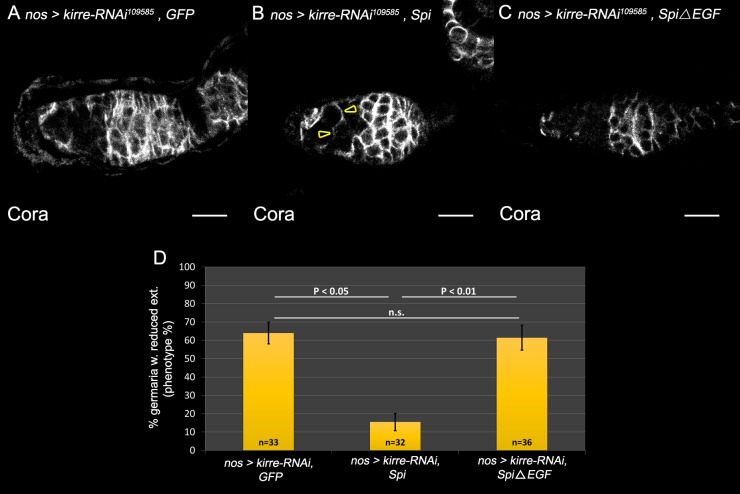
Previous reports support a model in which the Egfr ligand Spitz is provided by the GCs where it is cleaved and activated by the Rhomboid protease Stet (Gilboa and Lehmann, 2006; Liu et al., 2010; Schulz et al., 2002). Processing of Spi by Stet and its secretion from the GCs is necessary to activate the Egfr pathway non-autonomously in the ECs, leading to the formation of membrane extensions. In an attempt to further verify the hypothesis that IRM activity takes place upstream of Egfr signaling, we tested the ability of secreted active Spi driven in the GCs to rescue the phenotype of kirre knockdown in these cells. We used UAS-GFP to compensate for the addition of another UAS site. Significantly, membrane extensions of the ECs were restored in region 2a of the germaria in which kirre was knocked down by RNAi, relative to control in which kirre knockdown was combined with either UAS-GFP, or with UAS-Spi lacking the EGF domain. Quantification of these results showed that whereas 64% of the germaria exhibited reduced EC extensions following kirre knocked down alone (n=33), or in combination with inactive Spi (n=36), only 15% of kirre knocked down germaria which was combined with Spi showed reduced EC extensions (n=32, P<0.05, Fig. 6, compare B to A and C, quantification in D). We therefore conclude that Egfr signaling is acting downstream of the IRM cassette.
Fig. 6.
Germline expression of Spi rescues EC extensions in kirre knockdown germaria. In all panels: Anti-Coracle (Cora, white) marks EC membranes. (A) kirre knockdown in the germline results in reduced extensions. (B) Expression of Spi in the germline combined with kirre knockdown partially restores EC extensions (arrowheads). (C) Spi lacking the EGF domain does not restore extensions in the kirre knockdown background. (D) Quantification of EC extensions in kirre knockdown alone, in kirre knockdown expressed together with Spi (n=32) or kirre knockdown expressed together with inactive Spi (n=36), indicating a significant rescue by Spi. Three independent experiments are shown. P-values were calculated using binomial proportions z-test, bars represent s.d. Scale bars: 10 µm.
DISCUSSION
A positive feedback loop between ECs and GCs ensures germline differentiation in the germarium. The ECs encapsulate the GCs with elongated membrane extensions, isolating them from signals originating from the maintenance niche, and promoting their differentiation, whereas the GCs further provide signals that promote the formation of these membrane projections by the ECs (Mottier-Pavie et al., 2016). Here we identified additional elements required for the reinforcement of EC-GC interaction, namely the four Drosophila members of the IRM protein family. These proteins function differentially in each of the cell types and presumably stabilize and shorten the distance between the two opposing plasma membranes. Our findings imply that kirre and rst are required exclusively in the germline, while hbs and sns function in the ECs. We suggest that stabilization of ECs membrane extensions and shortening the distance between the membranes of the two cell types are essential for efficient Egfr activation by the short range ligand Spi (Fig. 7).
Fig. 7.
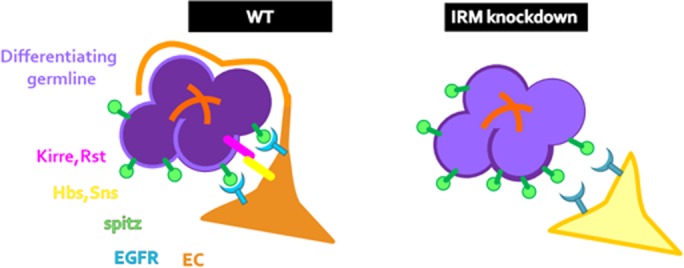
Model: IRM maintains ECs and germline within signaling distance enabling EC extension formation. In IRM knockdown, attachment of ECs and germline is hindered, increasing the distance between these two cell types. As a result, the soluble ligand Spi does not activate Egfr signaling in ECs to the same extent and less membrane protrusions form and encapsulate the germline.
Other systems in which IRM proteins function to maintain heterotypic interactions between cells had been described. In the Drosophila eye, similar pairs of IRM proteins are differentially expressed by ommatidial (Hbs and Sns) or inter-ommatidial (Kirre and Rst) cells and promote their preferential adhesion (Bao et al., 2010). Similarly, IRM proteins function to mediate proper spacing between bristles in the anterior D–V border of the wing imaginal disc (Linneweber et al., 2015). Whereas in these systems IRM proteins were not described to promote better signaling between the heterotypic cell types, such function cannot be excluded.
Full penetrance of the phenotype of the IRM could not be achieved since homozygous mutant suffer from defects in other tissues, and do not survive to adulthood. Furthermore, clonal analysis led to isolated mutant cells (either ECs or GCs) surrounded by WT cells, and since the IRM function non-autonomously the mutant cells were often rescued by their neighboring WT cells. When comparing the phenotype of EC membrane extensions between the heterozygous females and the knockdown cells, we noticed that the heterozygous mutant phenotype was higher relative to the tissue specific knockdown. This was unexpected and could possibly support a broader functional contribution of a given IRM, in both GCs as well as in ECs.
ECs must balance between two contradictory demands – the need to extend membrane protrusions between the GCs maintaining their tight encapsulation, and the need to release the germ cells allowing their progression through the germarium. IRM proteins mediate attachment, therefore must permit dissociation and re-attachment of the GCs to the ECs. Low levels of IRM constituents may control this grip-loosening behavior through a comparable number of attachment sites between the two cell types. Such low levels are consistent with the haploinsufficiency of the phenotype of EC membrane extensions in the IRM mutants. In such a model, maximal grip would depend on the number of EC membrane extensions, which express relatively low levels of IRM proteins. Loosening might take place when the number of extensions decreases by lowering Egfr signaling.
An interesting outcome from our experiments was that the degree of EC extension phenotype and the extra number of round fusomes did not always correlate. It is therefore suggestive that GC differentiation defects are apparently influenced by additional factors which are independent of EC extensions.
Spi is a diffusible paracrine ligand of the Egfr, which undergoes several phases of processing from a pro-protein inactive form into an active ligand, including, Spi processing, its export to the membrane, and its further palmitoylation at its N-terminal end (Miura et al., 2006). Spi palmitoylation retains its association with the plasma membrane, restricting its range of activity. Spi palmitoylation in the GCs might limits its range of activity only to proximal ECs. It is hypothesized that IRM proteins activity is possibly required in this case to close the gap between the two membranes, allowing efficient Egfr activation in the ECs.
In summary, our results identify novel essential components, the IRM proteins, which act to mediate heterotypic interactions between ECs and GCs, upstream of the Egfr signaling pathway, necessary for encapsulation of the GCs by ECs, and induce their further differentiation.
MATERIALS AND METHODS
Fly stocks
tj-Gal4 is a NP insertion (P{GawB}NP1624) into the traffic jam gene, and was obtained from the Drosophila Genetic Resource Centre. nos-Gal4 was from Dr Ruth Lehmann (NYU, USA). FAX-GFP originated in Yale FlyTrap (Quinones-Coello et al., 2007). UAS-lacZ was provided by Dr Jessica Treisman (NYU School of Medicine, USA). snsxb3 (Bour et al., 2000), snss660 (Shelton et al., 2009) and hbs459 (Artero et al., 2001) were a gift from Dr Susan Abmayr (Stowers Institute for Medical Research). Oregon Red (BL#5), rst[MI04842-GFSTF.1]/FM7j,B[1] (BL#59410), mFRP(nls), hs-flp FRT19A (BL#31418) and FRT19A (BL#1744) were from Bloomington Stock Center. UAS-EGFRCA, UAS-Spi, UAS-SpiΔEGF (Tsruya et al., 2002) and Df67k30(duf, rst)/FM7,GFP (Ruiz-Gómez et al., 2000) were a gift from Dr Benny Shilo. All RNAi lines used are detailed in Table S1 and in Table S2.
Antibody staining
Antibodies were used in the following concentrations: from the Developmental Studies Hybridoma Bank (DSHB), mouse monoclonal anti-Hts (1B1, 1:20) deposited by Lipshitz, H.D. and mouse monoclonal anti-Coracle (1:200) deposited by Fehon, R.; guinea pig anti-traffic-jam (1:700) was from Dr Dorothea Godt (University of Toronto); rabbit anti-Diphosphorylated ERK (1:200, #4370) was from Cell Signaling; rabbit anti-GFP (1:1000, #ab290) was from Invitrogen. Rabbit anti-Zfh1 (1:5000) was a present from Dr Ruth Lehmann (NYU, USA). Mouse anti-Rst (1:25) was a gift and from Dr Ricardo Guelerman P. Ramos (University of São Paulo, Brazil) from Dr Renate Renkawitz-Pohl (Philipps-Universität Marburg). Secondary antibodies were from Jackson Immunoresearch or from Invitrogen. Fixation and immunostaining of young adult ovaries were performed according to standard protocols. Images were acquired on Zeiss LSM 710, on a Zeiss Observer.Z1 or on Zeiss LSM 800 confocal microscope with Zeiss C-Apo-chromat 40×/1.20-W Korr M27 lens.
Quantification of dpERK and Zfh1 staining intensity
Control and experimental animals were dissected and stained on the same day. Images acquisition was on the same day, with the same parameters. The brightest section for each EC was measured with the measure tool in ImageJ software.
Quantification of fusome and EC numbers
Confocal images with plane to plane distance of ∼2.5 μm were analyzed. Round fusomes were counted, dividing (‘exclamation mark’) fusomes were excluded from the count. Distinct ECs were counted manually in all planes excluding the top and bottom planes as to exclude follicle cells.
Statistical analyses
Experiments were repeated as reported in text. Statistical analysis of extension phenotype was by Binomial proportions z-test (error for binary scoring of germaria), statistical analysis of round fusome amount and of EC numbers was by two-sample t-test. Round fusome count did not include elongated fusomes of dividing GSCs. Bars represent s.d.
Supplementary Material
Acknowledgements
This work was initiated in the lab of Dr Lilach Gilboa in the Weizmann Institute. The authors thank Lilach for her support and advice throughout the project. We also thank Lilach Gilboa and Eyal Schejter for insightful comments on the manuscript. We thank the Bloomington Stock Centre for various fly lines, the Developmental Studies Hybridoma Bank (DSHB) for antibodies, and FlyBase for important genomic information. We are grateful to Dorothea Godt, Suzan Abmayr, Renate Renkawitz-Pohl and Ricardo Guelerman P. Ramos for providing valuable primary antibodies.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: D.S.B.-Z., T.V.; Methodology: D.S.B.-Z.; Software: D.S.B.-Z.; Validation: D.S.B.-Z.; Investigation: D.S.B.-Z.; Writing - original draft: D.S.B.-Z.; Writing - review & editing: T.V.; Visualization: D.S.B.-Z.; Supervision: T.V.; Project administration: T.V.; Funding acquisition: T.V.
Funding
This study was supported by a grant from the German Cancer Research Center (DKFZ)-Israel Ministry of Science and Technology (IMOS) Foundation GR2536 (T.V.). FlyBase is supported by a grant from the National Human Genome Research Institute at the U.S. National Institutes of Health #U41 HG000739. Support is also provided by the British Medical Research Council (#MR/N030117/1) and the Indiana Genomics Initiative. The BDSC is supported by a grant from the Office of the Director of the National Institutes of Health under Award Number P40OD018537. NIH ICs OD, NIGMS, NICHD and NINDS contribute to funding of this award.
Supplementary information
Supplementary information available online at http://bio.biologists.org/lookup/doi/10.1242/bio.039842.supplemental
References
- Artero R. D., Castanon I. and Baylies M. K. (2001). The immunoglobulin-like protein Hibris functions as a dose-dependent regulator of myoblast fusion and is differentially controlled by Ras and Notch signaling. Development 128, 4251-4264. [DOI] [PubMed] [Google Scholar]
- Banisch T. U., Maimon I., Dadosh T. and Gilboa L. (2017). Escort cells generate a dynamic compartment for germline stem cell differentiation via combined Stat and Erk signalling. Development 144, 1937-1947. 10.1242/dev.143727 [DOI] [PubMed] [Google Scholar]
- Bao S. and Cagan R. (2005). Preferential adhesion mediated by Hibris and Roughest regulates morphogenesis and patterning in the Drosophila eye. Dev. Cell 8, 925-935. 10.1016/j.devcel.2005.03.011 [DOI] [PubMed] [Google Scholar]
- Bao S., Fischbach K. F., Corbin V. and Cagan R. L. (2010). Preferential adhesion maintains separation of ommatidia in the Drosophila eye. Dev. Biol. 344, 948-956. 10.1016/j.ydbio.2010.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolívar J., Pearson J., López-Onieva L. and González-Reyes A. (2006). Genetic dissection of a stem cell niche: the case of the Drosophila ovary. Dev. Dyn. 235, 2969-2979. 10.1002/dvdy.20967 [DOI] [PubMed] [Google Scholar]
- Boschert U., Ramos R. G., Tix S., Technau G. M. and Fischbach K.-F. (1990). Genetic and developmental analysis of irreC, a genetic function required for optic chiasm formation in Drosophila. J. Neurogenet. 6, 153-171. 10.3109/01677069009107107 [DOI] [PubMed] [Google Scholar]
- Bour B. A., Chakravarti M., West J. M. and Abmayr S. M. (2000). Drosophila SNS, a member of the immunoglobulin superfamily that is essential for myoblast fusion. Genes Dev. 14, 1498-1511. [PMC free article] [PubMed] [Google Scholar]
- Chen D. and McKearin D. (2003). Dpp signaling silences bam transcription directly to establish asymmetric divisions of germline stem cells. Curr. Biol. 13, 1786-1791. 10.1016/j.cub.2003.09.033 [DOI] [PubMed] [Google Scholar]
- de Cuevas M., Lilly M. A. and Spradling A. C. (1997). Germline cyst formation in Drosophila. Annu. Rev. Genet. 31, 405-428. 10.1146/annurev.genet.31.1.405 [DOI] [PubMed] [Google Scholar]
- Dworak H. A., Charles M. A., Pellerano L. B. and Sink H. (2001). Characterization of Drosophila hibris, a gene related to human nephrin. Development 128, 4265-4276. [DOI] [PubMed] [Google Scholar]
- Fairchild M. J., Smendziuk C. M. and Tanentzapf G. (2015). A somatic permeability barrier around the germline is essential for Drosophila spermatogenesis. Development 142, 268-281. 10.1242/dev.114967 [DOI] [PubMed] [Google Scholar]
- Fischbach K.-F., Linneweber G. A., Andlauer T. F., Hertenstein A., Bonengel B. and Chaudhary K. (2009). The irre cell recognition module (IRM) proteins. J. Neurogenet. 23, 48-67. 10.1080/01677060802471668 [DOI] [PubMed] [Google Scholar]
- Galletta B. J., Chakravarti M., Banerjee R. and Abmayr S. M. (2004). SNS: adhesive properties, localization requirements and ectodomain dependence in S2 cells and embryonic myoblasts. Mech. Dev. 121, 1455-1468. 10.1016/j.mod.2004.08.001 [DOI] [PubMed] [Google Scholar]
- Gilboa L. and Lehmann R. (2006). Soma-germline interactions coordinate homeostasis and growth in the Drosophila gonad. Nature 443, 97-100. 10.1038/nature05068 [DOI] [PubMed] [Google Scholar]
- Kai T. and Spradling A. (2003). An empty Drosophila stem cell niche reactivates the proliferation of ectopic cells. Proc. Natl. Acad. Sci. USA 100, 4633-4638. 10.1073/pnas.0830856100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaipa B. R., Shao H., Schäfer G., Trinkewitz T., Groth V., Liu J., Beck L., Bogdan S., Abmayr S. M. and Önel S.-F. (2013). Dock mediates Scar- and WASp-dependent actin polymerization through interaction with cell adhesion molecules in founder cells and fusion-competent myoblasts. J. Cell Sci. 126, 360-372. 10.1242/jcs.113860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kestilä M., Lenkkeri U., Männikkö M., Lamerdin J., McCready P., Putaala H., Ruotsalainen V., Morita T., Nissinen M., Herva R. et al. (1998). Positionally cloned gene for a novel glomerular protein--nephrin--is mutated in congenital nephrotic syndrome. Mol. Cell 1, 575-582. 10.1016/S1097-2765(00)80057-X [DOI] [PubMed] [Google Scholar]
- Kim S., Shilagardi K., Zhang S., Hong S. N., Sens K. L., Bo J., Gonzalez G. A. and Chen E. H. (2007). A critical function for the actin cytoskeleton in targeted exocytosis of prefusion vesicles during myoblast fusion. Dev. Cell 12, 571-586. 10.1016/j.devcel.2007.02.019 [DOI] [PubMed] [Google Scholar]
- Kirilly D. and Xie T. (2007). The Drosophila ovary: an active stem cell community. Cell Res. 17, 15-25. 10.1038/sj.cr.7310123 [DOI] [PubMed] [Google Scholar]
- Kirilly D., Wang S. and Xie T. (2011). Self-maintained escort cells form a germline stem cell differentiation niche. Development 138, 5087-5097. 10.1242/dev.067850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori T., Gyobu H., Ueno H., Kitamura T., Senba E. and Morikawa Y. (2008). Expression of kin of irregular chiasm-like 3/mKirre in proprioceptive neurons of the dorsal root ganglia and its interaction with nephrin in muscle spindles. J. Comp. Neurol. 511, 92-108. 10.1002/cne.21838 [DOI] [PubMed] [Google Scholar]
- Leatherman J. L. and Dinardo S. (2010). Germline self-renewal requires cyst stem cells and stat regulates niche adhesion in Drosophila testes. Nat. Cell Biol. 12, 806-811. 10.1038/ncb2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M. A., Alls J. D., Avancini R. M., Koo K. and Godt D. (2003). The large Maf factor Traffic Jam controls gonad morphogenesis in Drosophila. Nat. Cell Biol. 5, 994-1000. 10.1038/ncb1058 [DOI] [PubMed] [Google Scholar]
- Lim J. G. Y. and Fuller M. T. (2012). Somatic cell lineage is required for differentiation and not maintenance of germline stem cells in Drosophila testes. Proc. Natl. Acad. Sci. USA 109, 18477-18481. 10.1073/pnas.1215516109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H., Yue L. and Spradling A. C. (1994). The Drosophila fusome, a germline-specific organelle, contains membrane skeletal proteins and functions in cyst formation. Development 120, 947-956. [DOI] [PubMed] [Google Scholar]
- Linneweber G. A., Winking M. and Fischbach K.-F. (2015). The cell adhesion molecules roughest, hibris, kin of irre and sticks and stones are required for long range spacing of the drosophila wing disc sensory sensilla. PLoS ONE 10, e0128490 10.1371/journal.pone.0128490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M., Lim T. M. and Cai Y. (2010). The Drosophila female germline stem cell lineage acts to spatially restrict DPP function within the niche. Sci. Signal. 3, ra57 10.1126/scisignal.2000740 [DOI] [PubMed] [Google Scholar]
- Maimon I., Popliker M. and Gilboa L. (2014). Without children is required for Stat-mediated zfh1 transcription and for germline stem cell differentiation. Development 141, 2602-2610. 10.1242/dev.109611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massarwa R., Carmon S., Shilo B.-Z. and Schejter E. D. (2007). WIP/WASp-based actin-polymerization machinery is essential for myoblast fusion in Drosophila. Dev. Cell 12, 557-569. 10.1016/j.devcel.2007.01.016 [DOI] [PubMed] [Google Scholar]
- Miura G. I., Buglino J., Alvarado D., Lemmon M. A., Resh M. D. and Treisman J. E. (2006). Palmitoylation of the EGFR ligand Spitz by Rasp increases Spitz activity by restricting its diffusion. Dev. Cell 10, 167-176. 10.1016/j.devcel.2005.11.017 [DOI] [PubMed] [Google Scholar]
- Morikawa Y., Komori T., Hisaoka T., Ueno H., Kitamura T. and Senba E. (2007). Expression of mKirre in the developing sensory pathways: its close apposition to nephrin-expressing cells. Neuroscience 150, 880-886. 10.1016/j.neuroscience.2007.10.013 [DOI] [PubMed] [Google Scholar]
- Mottier-Pavie V. I., Palacios V., Eliazer S., Scoggin S. and Buszczak M. (2016). The Wnt pathway limits BMP signaling outside of the germline stem cell niche in Drosophila ovaries. Dev. Biol. 417, 50-62. 10.1016/j.ydbio.2016.06.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee P., Gildor B., Shilo B.-Z., VijayRaghavan K. and Schejter E. D. (2011). The actin nucleator WASp is required for myoblast fusion during adult Drosophila myogenesis. Development 138, 2347-2357. 10.1242/dev.055012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlstein B. and McKearin D. (1997). Ectopic expression of the Drosophila Bam protein eliminates oogenic germline stem cells. Development 124, 3651-3662. [DOI] [PubMed] [Google Scholar]
- Paululat A., Burchard S. and Renkawitz-Pohl R. (1995). Fusion from myoblasts to myotubes is dependent on the rolling stone gene (rost) of Drosophila. Development 121, 2611-2620. [DOI] [PubMed] [Google Scholar]
- Quinones-Coello A. T., Petrella L. N., Ayers K., Melillo A., Mazzalupo S., Hudson A. M., Wang S., Castiblanco C., Buszczak M., Hoskins R. A. et al. (2007). Exploring strategies for protein trapping in Drosophila. Genetics 175, 1089-1104. 10.1534/genetics.106.065995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter C., Schimansky T., Nie Z. and Fischbach K. F. (1996). Reorganization of membrane contacts prior to apoptosis in the Drosophila retina: the role of the IrreC-rst protein. Development 122, 1931-1940. [DOI] [PubMed] [Google Scholar]
- Rozario T. and DeSimone D. W. (2010). The extracellular matrix in development and morphogenesis: a dynamic view. Dev. Biol. 341, 126-140. 10.1016/j.ydbio.2009.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Gómez M., Coutts N., Price A., Taylor M. V. and Bate M. (2000). Drosophila dumbfounded: a myoblast attractant essential for fusion. Cell 102, 189-198. 10.1016/S0092-8674(00)00024-6 [DOI] [PubMed] [Google Scholar]
- Schneider T., Reiter C., Eule E., Bader B., Lichte B., Nie Z., Schimansky T., Ramos R. G. and Fischbach K. F. (1995). Restricted expression of the irreC-rst protein is required for normal axonal projections of columnar visual neurons. Neuron 15, 259-271. 10.1016/0896-6273(95)90032-2 [DOI] [PubMed] [Google Scholar]
- Schulz C., Wood C. G., Jones D. L., Tazuke S. I. and Fuller M. T. (2002). Signaling from germ cells mediated by the rhomboid homolog stet organizes encapsulation by somatic support cells. Development 129, 4523-4534. [DOI] [PubMed] [Google Scholar]
- Shelton C., Kocherlakota K. S., Zhuang S. and Abmayr S. M. (2009). The immunoglobulin superfamily member Hbs functions redundantly with Sns in interactions between founder and fusion-competent myoblasts. Development 136, 1159-1168. 10.1242/dev.026302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S. R., Zheng Z., Wang H., Oh S. W., Chen X. and Hou S. X. (2010). Competitiveness for the niche and mutual dependence of the germline and somatic stem cells in the Drosophila testis are regulated by the JAK/STAT signaling. J. Cell. Physiol. 223, 500-510. 10.1002/jcp.22073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn R. L., Huang P., Kawahara G., Mitchell M., Guyon J., Kalluri R., Kunkel L. M. and Gussoni E. (2009). A role for nephrin, a renal protein, in vertebrate skeletal muscle cell fusion. Proc. Natl. Acad. Sci. USA 106, 9274-9279. 10.1073/pnas.0904398106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X. and Xie T. (2002). DE-cadherin-mediated cell adhesion is essential for maintaining somatic stem cells in the Drosophila ovary. Proc. Natl. Acad. Sci. USA 99, 14813-14818. 10.1073/pnas.232389399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X., Wong M. D., Kawase E., Xi R., Ding B. C., McCarthy J. J. and Xie T. (2004). Bmp signals from niche cells directly repress transcription of a differentiation-promoting gene, bag of marbles, in germline stem cells in the Drosophila ovary. Development 131, 1353-1364. 10.1242/dev.01026 [DOI] [PubMed] [Google Scholar]
- Strunkelnberg M., Bonengel B., Moda L. M., Hertenstein A., de Couet H. G., Ramos R. G. and Fischbach K. F. (2001). rst and its paralogue kirre act redundantly during embryonic muscle development in Drosophila. Development 128, 4229-4239. [DOI] [PubMed] [Google Scholar]
- Su Y. H., Rastegri E., Kao S. H., Lai C. M., Lin K. Y., Liao H. Y., Wang M. H. and Hsu H. J. (2018). Diet regulates membrane extension and survival of niche escort cells for germline homeostasis via insulin signaling. Development 145, 1-13. 10.1242/dev.159186 [DOI] [PubMed] [Google Scholar]
- Tryggvason K., Pikkarainen T. and Patrakka J. (2006). Nck links nephrin to actin in kidney podocytes. Cell 125, 221-224. 10.1016/j.cell.2006.04.002 [DOI] [PubMed] [Google Scholar]
- Tsruya R., Schlesinger A., Reich A., Gabay L., Sapir A. and Shilo B. Z. (2002). Intracellular trafficking by Star regulates cleavage of the Drosophila EGF receptor ligand Spitz. Genes Dev. 16, 222-234. 10.1101/gad.214202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valer F. B., Machado M. C. R., Silva-Junior R. M. P. and Ramos R. G. P. (2018). Expression of Hbs, Kirre and Rst during Drosophila ovarian development. Genesis 56, e23242 10.1002/dvg.23242 [DOI] [PubMed] [Google Scholar]
- Van Doren M., Williamson A. L. and Lehmann R. (1998). Regulation of zygotic gene expression in Drosophila primordial germ cells. Curr. Biol. 8, 243-246. 10.1016/S0960-9822(98)70091-0 [DOI] [PubMed] [Google Scholar]
- Venugopala Reddy G., Reiter C., Shanbhag S., Fischbach K. F. and Rodrigues V. (1999). Irregular chiasm-C-roughest, a member of the immunoglobulin superfamily, affects sense organ spacing on the Drosophila antenna by influencing the positioning of founder cells on the disc ectoderm. Dev. Genes Evol. 209, 581-591. 10.1007/s004270050292 [DOI] [PubMed] [Google Scholar]
- Wagner N., Morrison H., Pagnotta S., Michiels J. F., Schwab Y., Tryggvason K., Schedl A. and Wagner K. D. (2011). The podocyte protein nephrin is required for cardiac vessel formation. Hum. Mol. Genet. 20, 2182-2194. 10.1093/hmg/ddr106 [DOI] [PubMed] [Google Scholar]
- Weavers H., Prieto-Sánchez S., Grawe F., Garcia-López A., Artero R., Wilsch-Bräuninger M., Ruiz-Gómez M., Skaer H. and Denholm B. (2009). The insect nephrocyte is a podocyte-like cell with a filtration slit diaphragm. Nature 457, 322-326. 10.1038/nature07526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff T. and Ready D. F. (1991). Cell death in normal and rough eye mutants of Drosophila. Development 113, 825-839. [DOI] [PubMed] [Google Scholar]
- Xie T. and Spradling A. C. (2000). A niche maintaining germ line stem cells in the Drosophila ovary. Science 290, 328-330. 10.1126/science.290.5490.328 [DOI] [PubMed] [Google Scholar]
- Zhuang S., Shao H., Guo F., Trimble R., Pearce E. and Abmayr S. M. (2009). Sns and Kirre, the Drosophila orthologs of Nephrin and Neph1, direct adhesion, fusion and formation of a slit diaphragm-like structure in insect nephrocytes. Development 136, 2335-2344. 10.1242/dev.031609 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.