Abstract
Background
The essence of osteoporosis is mainly the imbalance of bone formation and absorption. Previous studies indicated that SIRT1 is closely related to bone metabolism and bone mass as a regulator of bone mass. The literature reports that microRNAs are significant regulators of osteoblast proliferation and differentiation.
Material/Methods
In this study, SIRT1 protein and mRNA levels were examined by Western blot and RT-PCR. Osteogenic proliferation was examined by CCK8 assay and osteogenic markers, including ALP, OCN, and RUNX2, were examined by ELISA. The target of miR-132-3p was identified by luciferase reporter assay.
Results
LPS downregulated the SIRT1 protein level and β-glycerophosphate upregulated the SIRT1 protein level. The results demonstrated that SIRT1 overexpression promoted the proliferation and differentiation in MC3T3-E1 cells, and SIRT1 interference had the opposite effect. Luciferase reporter assay revealed that miR-132-3p inhibited the reporter gene activity of SIRT1. LPS upregulated the mRNA level of miR-132-3p, and β-glycerophosphate downregulated the mRNA level of miR-132-3p.
Conclusions
miR-132-3p is a pivotal regulator in osteogenic proliferation and differentiation by targeting SIRT1.
MeSH Keywords: Cell Differentiation, Cell Proliferation, MicroRNAs, Osteoblasts, Sirtuin 1
Background
Osteoporosis is a common bone metabolic disease and has become a public problem that seriously threatens health [1–4]. Prior studies have noted that the essence of osteoporosis is mainly the imbalance of bone tissue formation and absorption. Osteoporosis shows significant reduction of osteoblast activity and increasing osteoclast activity. Osteoblasts (OB) and osteoclasts (OC) are the 2 main cell types that maintain bone mass during bone remodeling [5–8]. In recent years, many researchers have focused on the phenomenon that abnormalities of osteoblast proliferation, differentiation, or apoptosis lead to decreased bone mass. Therefore, in-depth study on the molecular mechanisms of regulating bone formation of osteoblasts will provide new strategies and methods for the treatment of osteoporosis.
SIRT1 was first discovered in humans in 1999 [9] and is the first member of the Sirtuin protein family to be discovered [10]. SIRT1, a deacetylase, interacts with a variety of proteins and is involved in the regulation of cell apoptosis and senescence under stress conditions, enhancing cell activity and self-healing and survival ability [11–14]. The literature reports that SIRT1 is closely related to bone metabolism and bone mass as a regulator of bone mass [15–17]. It has been reported in the literature that conditional knockout of SIRT1 has a great influence on bone densitometry and bone mass. In comparison to wild-type (WT) mice, the body weight and size, skeletal size, bone volume, osteoblast numbers, alkaline phosphatase- and type I collagen-positive areas, and osteogenic-related gene expression levels are all significantly increased in SIRT1TG mice. [18] Further study the strong relationship between SIRT1 and osteoporosis to clarify the molecular mechanism of SIRT1 acting in the pathogenesis and prevention of osteoporosis. It will also provide new research targets on the treatment of osteoporosis.
MicroRNAs (miRNAs), a class of small non-coding RNAs with 21–23 nucleotide molecules, influence almost every genetic pathway (e.g., cell cycle checkpoint, cell proliferation, differentiation, and apoptosis), with a wide range of target genes. MicroRNAs inhibit the translation of the target gene or directly promote degradation of target gene mRNA by binding to the specific sequence of the target gene in the 3′UTR and the coding region in a base-complementary pairing manner [19–21]. The literature reports miRNAs are important regulators in proliferation and differentiation of osteoblasts. miR-150-3p directly binds to the 3′ non-coding region of β-catenin mRNA, reducing the expression level of β-catenin and inhibiting osteoblast differentiation [22]. miR-33-5p acts on the 3′ non-coding region of Hmga2, reducing Hmga2 protein levels to promote osteoblast differentiation [23].
miR-132-3p is dysregulated in some human cancers. For instance, evidence has proved that miR-132-3p in CRC cells influence cell proliferation, migration, and metastasis [24], but the exact role of miR-132-3p in osteoblasts remains unclear. The aim of the present study was to investigate the role of miR-132-3p in targeting the SIRT1 gene in osteoblasts. Given the roles of SIRT 1 and miRNAs in regulating bone formation, we hypothesized that SIRT1 and miR-132-3p are involved in both osteoblast proliferation and differentiation. In this study, the role of SIRT1 and miR-132-3p in MC3T3-E1 were investigated, which included cell proliferation and differentiation.
Material and Methods
Cell culture
Mouse MC3T3-E1 cell lines, purchased from the Type Culture Collection of the Chinese Academy of Sciences, Shanghai, China, were cultured in Dulbecco’s modified Eagle’s medium/nutrient mixture F12 (DMEM/FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), supplemented with antibiotic and antimycotic agents (100 units/ml penicillin, 100 μg/ml streptomycin; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) in an incubator with standard culture conditions (37°C, 5% CO2, and 95% humidity).
CCK8 assay
The cells were digested, counted, and prepared into a cell suspension at a concentration of 5×104 cells/ml in 96-well plates. After adding 100 uL of cell suspension per well, the 96-well cell culture plates were incubated in an incubator with standard culture conditions (37°C, 5% CO2, and 95% humidity) for 24 h. We then discarded the medium and washed cells twice with PBS. We added 200 μl medium to the untreated group, and the vector control group and the overexpression group were transfected with the empty vector and the SIRT1 overexpression vector, respectively. The medium was changed after 6 h, and 96-well cell culture plates were incubated at 37°C in a 5% CO2 incubator for 24 h. Then, 96-well plates were stained with CCK-8, λ=450 nm, and the OD value was measured: (1) 10 μL of CCK-8 was added to each well, and incubation was continued for 3 h in the incubator; (2) Shaking gently for 10 min; and (3) λ=450 nm, and the OD value of each well was read by a microplate reader.
ELISA
Levels of alkaline phosphatase (ALP), Osteocalcin (OCN), and RUNX2 in the supernatant of the MC3T3-E1 cell culture, obtained by centrifugation (15 min; 1000×g; 2–8°C) were measured using alkaline phosphatase, a Bone ELISA Kit, (ABIN368017, CUSABIO), Osteocalcin ELISA Kit, Bone gamma-Carboxyglutamate (Gla) Protein, (ABIN415574, CLOUD-CLONE CORP) and RUNX2 ELISA Kit, Runt-Related Transcription Factor 2, (ABIN424555, CLOUD-CLONE CORP) in accordance with the manufacturer’s protocol. MC3T3-E1cells were seeded in 96-well cell culture plates followed by 24-h transfection with either SIRT1, SIRT1 inhibitor, or NC at 37°C.
Alkaline phosphatase (ALP) staining
MC3T3-E1 cells were either untreated or treated by transfecting with vector or NCshRNA, overexpression plasmid of SIRT1 or shRNA of SIRT1. Then, the cells were stained with the ALP staining kit (D0001-1; Nanjing Jiancheng Bioengineering Institute, China), according to the manufacturer’s instructions.
Western blot analysis
Western blot analysis was used to detect SIRT 1. Cells were harvested and lysed with 0.5 ml radioimmunoprecipitation assay buffer (Beyotime Institute of Biotechnology, Hai men, China). The concentration of protein was determined using an Enhanced BCA Protein Assay kit (P0010; Beyotime Institute of Biotechnology) according to the manufacturer’s protocol. Protein samples (20 μg per lane) from the lysates were separated by 8% SDS-PAGE and blotted onto polyvinylidene fluoride (PVDF) membranes. After blocking with 5% skimmed milk for 1–1.5 h at room temperature, the PVDF membranes were incubated with primary antibody against Anti- SIRT1 antibody (1: 1000; S5196; Sigma-Aldrich; Merck KGaA) and Monoclonal Anti-GAPDH (1: 20000; G8795; Sigma-Aldrich; Merck KGaA) at 4°C overnight. The PVDF membranes were treated with horseradish peroxidase (HRP)-conjugated secondary antibody (Anti-Mouse IgG; 1: 80 000; A9917; Sigma-Aldrich; Merck KGaA) at room temperature for 1 h. Protein bands were visualized by an enhanced chemiluminescence kit (sc-2048, Sigma-Aldrich, Merck KGaA). Data were analyzed with Image Pro Plus v.6.0 software (Media Cybernetics, Inc., Rockville, MD, USA) for densitometry. GAPDH was used as an endogenous control.
RNA isolation and qRT-PCR
Total RNA was isolated MC3T3-E1 cell with TRIZOL reagent (Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s instructions. For the evaluation of mRNA, synthesis of cDNA was performed using an RNA PCR kit (Takara, Japan) and quantitative real-time PCR was carried out with the SYBR premix Ex TaqII kit (Takara) according to the manufacturer’s instructions. All reactions were performed in triplicate. The 2–ΔΔCt method was used to calculate the relative quantities of each gene. GAPDH was used as an endogenous control.
Luciferase reporter assay
The SIRT 1 wild-type (WT) and mutant (MUT) 3′UTR firefly luciferase construct were generated. MC3T3-E1 cells (80% confluence) were cotransfected with SIRT 1 -WT-3′UTR or SIRT 1-MUT-3′UTR luciferase reporter and Renilla luciferase reporter (pRL), and miRNA NC or miR-132-3p-mimic. Forty-eight hours after the incubation, cells were lysed and the supernatants were used for the detection of luciferase activity using a dual luciferase assay system (Promega, Madison, WI, USA). We calculated the relative fluorescence intensity and compared it with the no-load control to determine whether the miR-132-3p inhibited the reporter gene activity of SIRT 1.
Statistical analysis
All the experiments were performed at least 3 times. Data are presented as the mean ± standard deviation. Data were analyzed by one-way analysis of variance followed by the Scheffe post hoc test to evaluate the effects of different treatments. Analyses were conducted using SPSS 12.0 software (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
SIRT1 were significantly increased in osteogenic differentiation
To analyze whether SIRT1 was associated with osteogenic differentiation, we used a well-accepted model to investigate the osteogenic differentiation. SIRT1 protein levels were significantly decreased exposed to lipopolysaccharides (LPS) and increased exposed to β-glycerophosphate in MC3T3-E1 (Figure 1).
Figure 1.
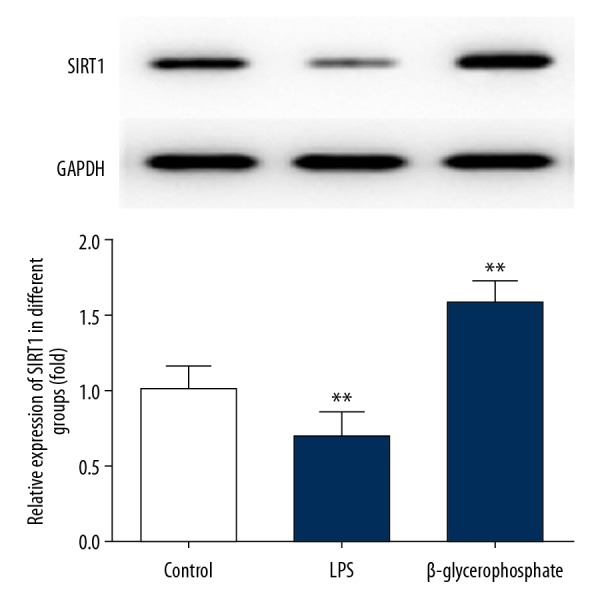
SIRT1 was significantly increased in osteogenic differentiation. MC3T3-E1 cells were either untreated or treated with 2 ug LPS or 5 Mm β-glycerophosphate. GAPDH was used as the endogenous control. SIRT1 protein levels were measured by Western blotting, then quantified and statistically analyzed. ** P<0.01 vs. control group.
SIRT1 overexpression significantly increased osteogenic proliferation
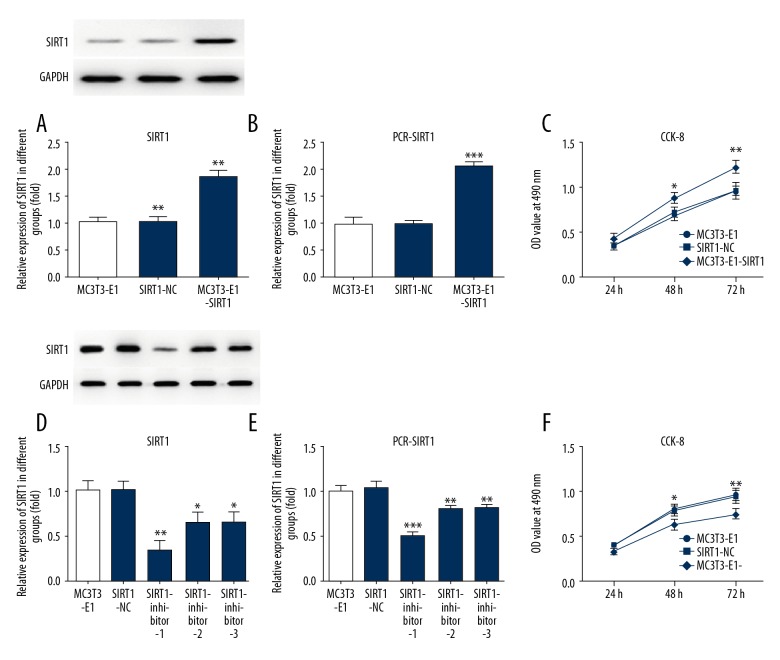
To analyze whether SIRT1 was involved in osteogenic proliferation, we detected the protein and mRNA levels of SIRT1 by western blot and RT-qPCR following treatment with SIRT1 overexpression or interference. The cell viability of MC3T3-E1 cells was assessed by CCK8 assay. SIRT1 overexpression significantly elevated the protein level (Figure 2A) and mRNA level (Figure 2B) compared with the MC3T3-E1 group. SIRT1 overexpression significantly increased cell viability for 48 h and 72 h (Figure 2C) compared with the control group. Three kinds of SIRT1 interference decreased the protein level (Figure 2D) and decreased the mRNA level (Figure 2E) compared with the MC3T3-E1 group. Consequently, SIRT1-inhibitor 1 was selected for subsequent experiments. SIRT1 interference significantly decreased cell viability for 48 h and 72 h (Figure 2F) compared with the control group.
Figure 2.
SIRT1 overexpression markedly upregulates osteogenic proliferation. SIRT1 overexpression upregulated the protein (A) and mRNA (B) levels and cell proliferation (C) in MC3T3-E1. Cells were either untreated or transfected with empty plasmid transfected with overexpression plasmid of SIRT1. The SIRT1 interference downregulated the protein (D) and mRNA (E) levels and cell proliferation (F) in MC3T3-E1 cells. Cells were either untreated or transfected with empty plasmid transfected with SIRT1 interference plasmid. SIRT1 protein and mRNA levels were measured by Western blotting and PCR, GAPDH was used as the endogenous control, and the cell viability of MC3T3-E1 cells was measured with CCK8 assay, then quantified and statistically analyzed. * P<0.05, ** P<0.01, *** P<0.001 vs. MC3T3-E1 group.
SIRT1 overexpression significantly increased osteogenic differentiation
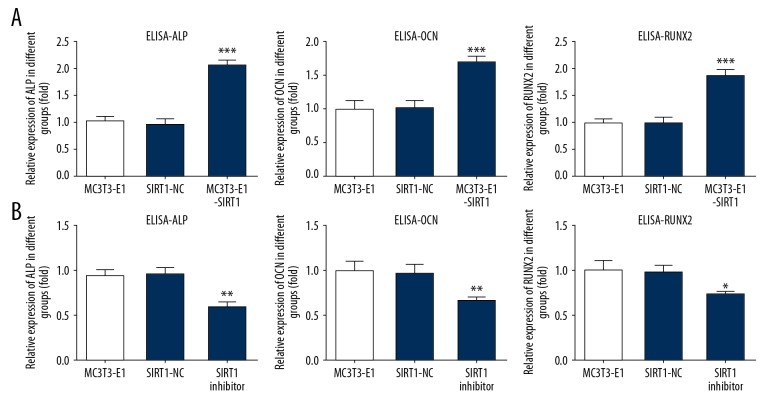
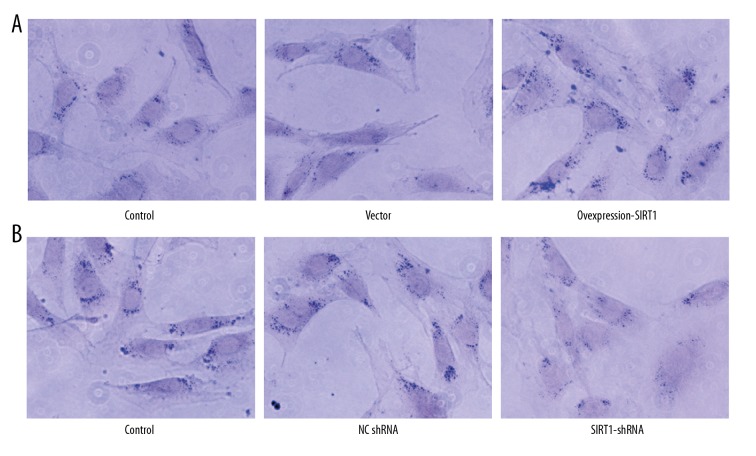
To determine the effect of SIRT1 overexpression or interference in osteogenic differentiation, we detected protein level of osteogenic markers including ALP, OCN, and RUNX2 by ELISA. We found that SIRT1 overexpression obviously increased osteogenic differentiation, as indicated by the upregulated levels of ALP, OCN, and RUNX2 (Figure 3A). However, SIRT1 interference suppressed osteogenic differentiation with decreased levels of ALP, OCN, and RUNX2 (Figure 3B). The morphology of osteogenic differentiation was shown by staining with alkaline phosphatase. We found that SIRT1 overexpression promoted osteogenic differentiation (Figure 4A) and SIRT1 interference inhibited osteogenic differentiation (Figure 4B)
Figure 3.
SIRT1 overexpression markedly upregulated osteogenic differentiation. The SIRT1 overexpression or interference influenced ALP, OCN, and Runx2 in MC3T3-E1. ALP, OCN, and Runx2 protein levels in MC3T3-E1 were assessed with ELISA kits. MC3T3-E1 cells were either untreated, or treated by transfecting with control plasmid, overexpression plasmid of SIRT1 (A), and interference plasmid of SIRT1 (B). * P<0.05, ** P<0.01, *** P<0.001 vs. control group.
Figure 4.
SIRT1 overexpression promoted osteogenic differentiation and SIRT1 interference inhibited osteogenic differentiation. The morphology of osteogenic differentiation was shown by staining with alkaline phosphatase. MC3T3-E1 cells were either untreated, or treated by transfecting with vector or NCshRNA, overexpression plasmid of SIRT1 (A), shRNA of SIRT1 (B).
SIRT1 was negatively regulated by miR-132-3p involved in osteogenic differentiation
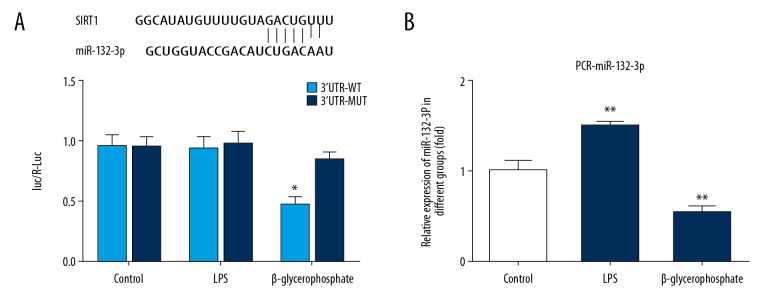
To verify that SIRT1 is a direct target of miR-132-3p, we constructed luciferase reporter plasmids containing either wild-type or mutant 3′UTR segments of SIRT1. The wild-type or mutant reporter plasmid was transfected into MC3T3-E1 cells. Reporter assays showed that miR-132-3p significantly inhibited the luciferase reporter activity of miR-132-3p-mimic-3′UTR-WT (Figure 5A) compared with miR-NC-3′UTR-WT. In contrast, transfection of miR-132-3p had no obvious effect on reporter activity of miR-132-3p-mimic-3′UTR-MUT.
Figure 5.
SIRT1 was negatively regulated by miR-132-3p in osteogenic differentiation. Luciferase reporter assay was used to test whether the miR-132-3p was bound to the 3′UTR of SIRT1 mRNA in MC3T3-E1. MC3T3-E1 cells were either untreated, or treated by transfecting with control plasmid, constructed luciferase reporter plasmid WT, or MUT (A). * P<0.05 vs. 3′UTR-MUT. The mRNA level of miR-132-3p was significantly increased by exposure to LPS and decreased by exposure to β-glycerophosphate in MC3T3-E1 cells (B). MC3T3-E1 cells were either untreated, or treated with 2 ug LPS or 5 Mm β-glycerophosphate for 24 h before transfecting miR-132-3p. The miR-132-3p levels were measured by PCR, then quantified and statistically analyzed. ** P<0.01 vs. control group.
mRNA levels of miR-132-3p were detected by RT-qPCR following treatment with LPS or β-glycerophosphate. miR-132-3p mRNA levels were significantly increased in cells exposed to LPS and decreased in cells exposed to β-glycerophosphate (Figure 5B) in MC3T3-E1.
Discussion
We found that SIRT1 was obviously upregulated during osteogenic proliferation and differentiation. Furthermore, we demonstrated that SIRT1 is a positive regulator of osteogenic proliferation and differentiation because its overexpression increased osteogenic proliferation and differentiation, whereas its silencing led to the opposite effect. Finally, we demonstrated that SIRT1 influences osteogenic proliferation and differentiation of MC3T3-E1 cells through the regulation of miR-132-3p.
It has been well documented that exposure to lipopolysaccharides (LPS) causes inhibition of osteoblast differentiation [25], while exposure to β-glycerophosphate causes induction of osteoblast differentiation [26,27]. In the present study, we found that SIRT1 protein levels were significantly decreased in MC3T3-E1 cells exposed to LPS and were significantly increased in cells exposed to β-glycerophosphate. It has been reported in the literature that SIRT1 promotes osteogenic differentiation through downregulation of peroxisome proliferator-activated receptor γ in MC3T3-E1 cells [28]. Results revealed that SIRT1 overexpression markedly increased osteogenic proliferation and differentiation, while SIRT1 interference considerably decreased osteogenic proliferation and differentiation in MC3T3-E1 cells, further supporting the hypothesis that SIRT1 acts as an important regulator in osteogenic proliferation and differentiation. Previous studies demonstrated that miR-132-3p inhibits the differentiation and functional activity of osteoblast by reducing the stability and acetylation levels of Runx2 [29]. Our results confirmed that the miR-132-3p bound to the 3′UTR of SIRT1 mRNA in MC3T3-E1 cells and miR-132-3p negatively regulated the expression of SIRT1, thereby suppressing osteogenic proliferation and differentiation.
Conclusions
Our study explored the function and mechanisms of SIRT1 in osteogenic proliferation and differentiation, and miR-132-3p was identified as a novel regulator of osteogenic proliferation and differentiation by negatively regulating SIRT1. This study provides new insights into the potential of SIRT1 and miR-132-3p as therapeutic targets in osteoporosis.
Abbreviations
- SIRT1
silent mating type information regulation 2 homolog
- CCK8
cell counting Kit-8
- ELISA
enzyme-linked immunosorbent assay
- qRT-PCR
quantitative real-time polymerase chain reaction
- NC
negative control
- LPS
lipopolysaccharides
- ALP
alkaline phosphatase
- OCN
osteocalcin
- RUNX2
runt-related transcription factor 2
Footnotes
Source of support: Departmental sources
Conflict of interests
None.
References
- 1.Dimitriou R, Jones E, McGonagle D, Giannoudis PV. Bone regeneration: Current concepts and future directions. BMC Med. 2011;9:66. doi: 10.1186/1741-7015-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gu SX, Li X, Hamilton JL, et al. MicroRNA-146a reduces IL-1 dependent inflammatory responses in the intervertebral disc. Gene. 2015;555(2):80–87. doi: 10.1016/j.gene.2014.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Y, Tao Y, Hyman ME, et al. Osteoporosis in china. Osteoporos Int. 2009;20(10):1651–62. doi: 10.1007/s00198-009-0925-y. [DOI] [PubMed] [Google Scholar]
- 4.Li Y, Xuan M, Wang B, et al. Comparison of parathyroid hormone (1–34) and elcatonin in postmenopausal women with osteoporosis: An 18-month randomized, multicenter controlled trial in China. Chin Med J. 2013;126(3):457–63. [PubMed] [Google Scholar]
- 5.Qi H, Aguiar DJ, Williams SM, et al. Identification of genes responsible for osteoblast differentiation from human mesodermal progenitor cells. Proc Natl Acad Sci USA. 2003;100(6):3305–10. doi: 10.1073/pnas.0532693100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weinstein RS, Manolagas SC. Apoptosis and osteoporosis. Am J Med. 2000;108:153–64. doi: 10.1016/s0002-9343(99)00420-9. [DOI] [PubMed] [Google Scholar]
- 7.Gunaratnam K, Vidal C, Boadle R. Mechanisms of palmitate-induced cell death in human osteoblasts. Biol Open. 2013;2(12):1382–89. doi: 10.1242/bio.20136700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yagi M, King AB, Boachie-Adjei O. Characterization of osteopenia/osteoporosis in adult scoliosis: Does bone density affect surgical outcome? Spine. 2011;36(20):1652–57. doi: 10.1097/BRS.0b013e31820110b4. [DOI] [PubMed] [Google Scholar]
- 9.Frye RA. Characterization of five human cDNAs with homology to the yeast SIR2 gene: Sir2-like proteins (sirtuins) metabolize NAD and may have protein ADP-ribosyltransferase activity. Biochem Biophys Res Commun. 1999;260(1):273–79. doi: 10.1006/bbrc.1999.0897. [DOI] [PubMed] [Google Scholar]
- 10.Sherman JM, Stone EM, Freeman-Cook LL, et al. The conserved core of a human SIR2 homologue functions in yeast silencing. Mol Biol Cell. 1999;10(9):3045–59. doi: 10.1091/mbc.10.9.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang F, Kume S, Koya D. SIRT1 and insulin resistance. Nat Rev Endocrinol. 2009;5(7):367–73. doi: 10.1038/nrendo.2009.101. [DOI] [PubMed] [Google Scholar]
- 12.Chua KF, Mostoslavsky R, Lombard DB, et al. Mammalian SIRT1 limits replicative life span in response to chronic genotoxic stress. Cell Metab. 2005;2(1):67–76. doi: 10.1016/j.cmet.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 13.Haigis MC, Guarente LP. Mammalian sirtuins – emerging roles in physiology, aging, and calorie restriction. Genes Dev. 2006;20(21):2913–21. doi: 10.1101/gad.1467506. [DOI] [PubMed] [Google Scholar]
- 14.Leibiger IB, Berggren PO. SIRT1: A metabolic master switch that modulates lifespan. Nat Med. 2006;12(1):34–36. doi: 10.1038/nm0106-34. [DOI] [PubMed] [Google Scholar]
- 15.Lee HW, Suh JH, Kim AY, et al. Histone deacetylase 1-mediated histone modification regulates osteoblast differentiation. Mol Endocrinol. 2006;20(10):2432–43. doi: 10.1210/me.2006-0061. [DOI] [PubMed] [Google Scholar]
- 16.Cohen-Kfir E, Artsi H, Levin A, et al. SIRT1 is a regulator of bone mass and a repressor of Sost encoding for sclerostin, a bone formation inhibitor. Endocrinology. 2011;152(12):4514–24. doi: 10.1210/en.2011-1128. [DOI] [PubMed] [Google Scholar]
- 17.Iyer S, Han L, Bartell SM, et al. Sirtuin1 (SIRT1) promotes cortical bone formation by preventing β-catenin sequestration by FoxO transcription factors in osteoblast progenitors. J Biol Chem. 2014;89(35):24069–78. doi: 10.1074/jbc.M114.561803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun W, Qiao W, Zhou B, et al. Overexpression of SIRT1 in mesenchymal stem cells protects against bone loss in mice by FOXO3a deacetylation and oxidative stress inhibition. Metabolism. 2018;88:61–71. doi: 10.1016/j.metabol.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Olena AF, Patton JG. Genomic organization of microRNAs. J Cell Physiol. 2010;222(3):540–45. doi: 10.1002/jcp.21993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tilghman SL, Rhodes LV, Bratton MR, et al. Phytoalexins, miRNAs and breast cancer: A review of phytochemical-mediated miRNA regulation in breast cancer. J Health Care Poor Underserved. 2013;24(1 Suppl):36–46. doi: 10.1353/hpu.2013.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abouheif MM, Nakasa T, Shibuya H, et al. Silencing microRNA-34a inhibits chondrocyte apoptosis in a rat osteoarthritis model in vitro. Rheumatology (Oxford) 2010;49(11):2054–60. doi: 10.1093/rheumatology/keq247. [DOI] [PubMed] [Google Scholar]
- 22.Wang N, Zhou Z, Wu T, et al. TNF-α-induced NF-κB activation upregulates microRNA-150-3p and inhibits osteogenesis of mesenchymal stem cells by targeting β-catenin. Open Biol. 2016;6(3):150258. doi: 10.1098/rsob.150258. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Wang H, Sun Z, Wang Y, et al. miR-33-5p, a novel mechano-sensitive microRNA promotes osteoblast differentiation by targeting Hmga2. Sci Rep. 2016;6:23170. doi: 10.1038/srep23170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang M, Li Y, Wang H, et al. LncRNA SNHG5 affects cell proliferation, metastasis and migration of colorectal cancer through regulating miR-132-3p/CREB5. Cancer Biol Ther. 2018;5:1–13. doi: 10.1080/15384047.2018.1537579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma J, Wang Z, Zhao J, et al. Resveratrol attenuates lipopolysaccharides (LPS)-induced inhibition of osteoblast differentiation in MC3T3-E1 Ccells. Med Sci Monit. 2018;24:2045–52. doi: 10.12659/MSM.905703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chaves Neto AH, Machado D, Yano CL, Ferreira CV. Antioxidant defense and apoptotic effectors in ascorbic acid and β-glycerophosphate-induced osteoblastic differentiation. Dev Growth Differ. 2011;53(1):88–96. doi: 10.1111/j.1440-169X.2010.01232.x. [DOI] [PubMed] [Google Scholar]
- 27.Chaves Neto AH, Queiroz KC, Milani R, et al. Profiling the changes in signaling pathways in ascorbic acid/β-glycerophosphate-induced osteoblastic differentiation. J Cell Biochem. 2011;112(1):71–77. doi: 10.1002/jcb.22763. [DOI] [PubMed] [Google Scholar]
- 28.Qu B, Ma Y, Yan M, et al. Sirtuin1 promotes osteogenic differentiation through downregulation of peroxisome proliferator-activated receptor γ in MC3T3-E1 cells. Biochem Biophys Res Commun. 2016;478(1):439–45. doi: 10.1016/j.bbrc.2016.06.154. [DOI] [PubMed] [Google Scholar]
- 29.Hu Z, Wang Y, Sun Z, et al. miRNA-132-3p inhibits osteoblast differentiation by targeting Ep300 in simulated microgravity. Sci Rep. 2015;5:18655. doi: 10.1038/srep18655. [DOI] [PMC free article] [PubMed] [Google Scholar]