Abstract
Sensing environmental cues requires well-built neuronal circuits linked to the body surface. Sensory neurons generate dendrites to innervate surface epithelium, thereby making it the largest sensory organ in the body. Previous studies have illustrated that neuronal type, physiological function and branching patterns are determined by intrinsic factors. Perhaps for effective sensation or protection, sensory dendrites bind to or are surrounded by the substrate epidermis. Recent studies have shed light on the mechanisms by which dendrites interact with their substrates. These interactions suggest that substrates can regulate dendrite guidance, arborization and degeneration. In this review, we focus on recent studies of Drosophila and Caenorhabditis elegans that demonstrate how epidermal cells can regulate dendrites in several aspects.
Keywords: dendrite, da neuron, PVD, epidermis, pruning, L1CAM
1. Introduction
Sensing environmental information, such as mechanical, chemical or thermal stimulation, is essential for animal survival and fitness. Over the course of evolution, metazoans have developed extremely specialized sensory organs to accomplish vision, hearing, taste, touch and smell. Noxious stimuli of high-temperature or mechanical type are detected via the skin, a sensory organ comprising epidermal cells that is conserved from nematodes to human. Sensory neurons that detect nociceptive stimulations extend highly branched dendrites to fully innervate the epidermis, making the skin the largest sensory organ [1,2]. Although the skin has been known as a sensory organ for a long time, systematic studies of how sensory neurons generate dendrites only started in invertebrate systems in the 1990s [3]. In a little over a decade, researchers had uncovered molecular and cellular mechanisms intrinsic to dendritic arborization and began to reveal direct interactions between sensory dendrites and epidermal cells. To date, mainly through studies of Drosophila, Caenorhabditis elegans and the vertebrate zebrafish, it has been demonstrated that the epidermis is not just passively innervated by dendrites, but in fact it actively instructs dendritic branching. In this review, we summarize and discuss recent progress in dendrite–epidermis interactions in the fruit fly and nematode systems. For recent findings in zebrafish, refer to the outstanding review [4].
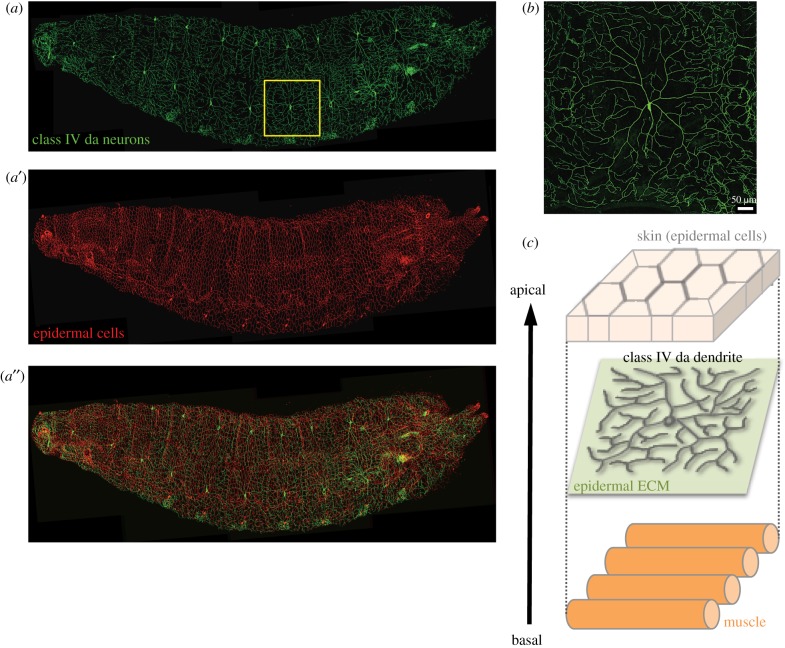
The dendritic arborization (da) neurons of the peripheral sensory system in Drosophila larvae are a model system used to study dendritic arborization. The diversity and complexity of Drosophila da neurons are ideal for studying neuronal fate determination, dendrite–dendrite interactions and dendrite–substrate interplay [2,5]. These da neurons are classified into four classes (I–IV) based on increasing complexity of their dendrites [5]. The diversity of dendritic patterning often reflects distinct functions and requirements for receptive field sizes. Class I da neurons function as proprioceptors, mainly projecting primary branches and only a few side branches [6,7]. The dendritic branches of class I da neurons are enriched with microtubules [8], rendering them suitable for detecting the contraction wave during larval crawling. Class III da neurons extend several long primary or secondary branches that protrude numerous spike-like branches, tiling 70–80% of the innervating field [5]. A recent study indicates that these spiked neurons function in sensing gentle touch via low-threshold mechano-transduction NOMPC channels located on the spikes [9]. The dendritic spikes are thin and built primarily of actin without microtubules, possibly making them more flexible and facilitating easier detection of weak mechanical force. Notably, animal body surfaces are filled with nociceptive sensors to instantly detect strong thermal stress and intense light from all directions so that they can escape these harmful stimuli. In Drosophila larvae, the class IV da neurons are capable of sensing multiple noxious stimuli [5,10]. Only three class IV da neurons innervate the epidermis of each hemi-segment, forming space-filling patterns and tiling each other (figures 1a and 2b) [5]. These complex dendritic patterns necessitate generating seventh- or eighth-order branches to reach up to 800 terminal ends for each neuron and to cover almost 100% of each hemi-segment [5]. These comprehensive dendritic branches express the mechano-transducers Painless [11], Pickpocket [12] and Piezo [13] for nociception and express the light sensor Gr28b [10] to detect strong light. Although class IV dendrites exhibit much more extensive branching and cover a larger area than class III dendrites, these arbors are not responsive to gentle touch [9]. This functional limitation is primarily due to the lack of NOMPC expression. The mixed contents of actin and microtubules [14] could also possibly make class IV dendrites less flexible than class III dendritic spikes but suitable for high-threshold mechano-sensation. Therefore, elaboration of appropriate dendritic arbors and expression of correct sensory molecules are important for neuronal function. Based on genetic studies in Drosophila over the past two decades, key intrinsic regulators in dendritic patterning have been characterized [2]. Transcription factors function in concert to determine neuronal fate [8,15–18], cytoskeletal regulators and motor proteins shape dendritic patterns [19–23], secretory and endocytic pathways promote dendrite growth [24,25] and cell adhesion molecules (CAMs) limit dendrite outgrowth into the surroundings [26–29] and mediate dendritic self-avoidance [30–32].
Figure 1.
Class IV da neurons and epidermal cells in Drosophila larvae. A Drosophila larva co-expressing membrane-tethered GFP (mCD8-GFP) in class IV da neurons and membrane-tethered Tomato (CD4-tdTom) in epidermal cells. (a) GFP, (a′) tdTom and (a″) merged image. The yellow box indicates a single class IV da neuron and its receptive field. (b) A class IV da neuron located in the dorsal region of a larva, showing the complex but non-overlapping dendritic pattern. (c) Schematic of the epidermis–dendrite–muscle spatial arrangement. Dendrites are attached to the ECM (green plane) secreted by epidermal cells and are separated from muscles.
Figure 2.
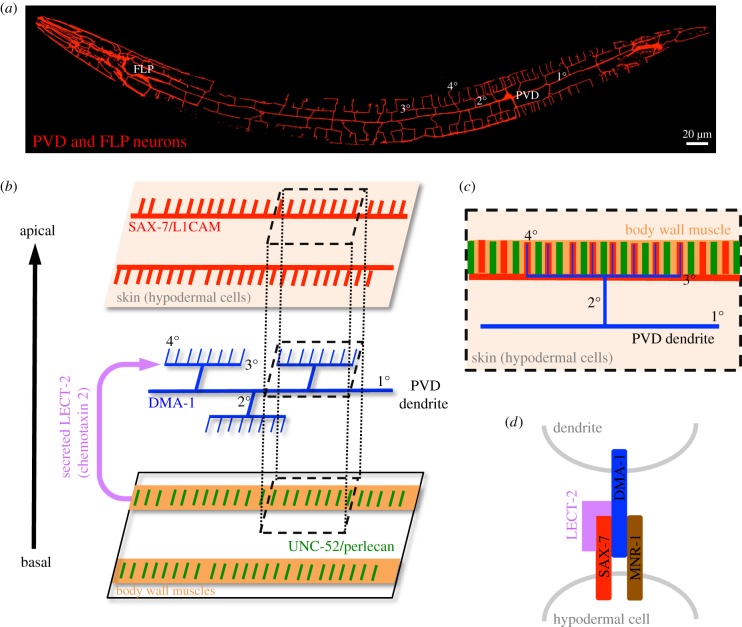
PVD neuron in C. elegans. (a) Somas and dendritic patterns of PVD and FLP neurons in a C. elegans larva are labelled by expressing membrane-tethered mCherry (myr::mCherry) in both neurons. Primary, secondary, tertiary and quaternary branches are indicated as 1°, 2°, 3° and 4° on PVD dendrites, respectively. FLP neuron and its dendrites are located in the anterior part, which are not overlapped to PVD dendrites. (b) Expression of guiding ligands and receptors in the interphase of skin, dendrites and muscle cells. SAX-7 (red) is expressed as horizontal stripes (corresponding to the 3° dendritic branches) and vertical stripes (corresponding to the 4° dendritic branches) on skin. DMA-1 (blue) is expressed on PVD dendrites. UNC-52 (green) is expressed as spaced stripes on the body wall muscles (orange). LECT-2 (magenta) is secreted from the body wall muscles to the dendrite–SAX-7 interface. (c) Dorsal view of menorah-like dendrites in the skin–dendrite–muscle arrangement represented by a grey dashed box in (b). Note that the 4° dendritic branches overlap with body wall muscle fibres (orange). (d) Receptor–ligand interactions between dendrites and skin.
The model organism C. elegans has emerged as an elegant system for studying dendrite–environment interactions, given the availability of powerful genetic tools. The two PVD neurons, each located on the left or right sides of this nematode, generate stereotypic menorah-like dendrites (figure 2a) [33]. Dendrite growth of PVD neurons is initiated by extending two first-order (1°) branches in the anterior and posterior directions at larval stage 2 (L2). Then, at late L2/early L3, 2° branches sprout vertically from the 1° branches, extending to reach the lateral boundaries of outer body wall muscles. At the muscle boundary, most 2° branches bifurcate and extend horizontally to form 3° branches. Finally, at early L4, an array of short 4° branches extend vertically from the horizontal 3° branches to form menorah-like structures. To construct these highly branched dendrites, PVD neurons have been reported to use some intrinsic branching mechanisms that are analogous to those of Drosophila da neurons. For example, different levels of the transcription factor Cut confer class-specific da neuronal types in Drosophila [15], whereas low and high levels of the transcription factor MEC-3 confer, respectively, highly branching PVD neurons and restricted-branching AVM neurons in C. elegans [34,35]. Both systems are also analogous in generating distal branches. The dynein motor that functions in transporting cargo along microtubules is selectively required to promote distal branch formation in class IV da neurons [21]. Another study in C. elegans also identified mutations in the dynein accessory factor that selectively disrupted distal branching of PVD neurons [36]. Thus, both invertebrate systems exhibit shared characteristics for some of the intrinsic mechanisms that promote dendrite branching.
Although it might be expected that both systems would also share some common mechanisms in terms of dendrite–epidermis interaction, which is the focus of this review, significant differences might also be anticipated due to differences in their dendritic morphologies and stereotypies, as well as their interacting environments. Functioning as nociceptors, dendrites of both class IV da and PVD neurons innervate a confined two-dimensional space between the epidermis and muscle cells (figures 1c and 2b). Whereas Drosophila class IV da neurons co-innervate the same field as other classes of da neurons, C. elegans PVD neurons exclusively target most body parts, leaving only the anterior segment to be innervated by the multi-dendritic FLP neuron (figure 2a). In addition, dendrites of Drosophila class IV da neurons are pruned completely in the pupal stage during metamorphosis and regrow new arbors for adulthood, which is not the case for C. elegans PVD neurons and thus prevents studies of remodelling in the nematode system. However, the major difference between these two neuron types is the morphological complexity of their dendrites. Class IV da neurons elaborate numerous branches in a space-filling pattern to fully cover the target field (figure 1a,b), in a pattern similar to zebrafish Rohon–Beard (RB) sensory neurons [37] and retinal ganglion cells in mice [38]. These radial and extensively branching patterns are indicative of a space-filling mechanism whereby branches sprout and extend into uncovered areas. This mechanism has to be combined with a self-avoidance mechanism by which growth of branches is repulsed by iso-neuronal branches, preventing crossovers between branches (figure 1b) [39]. By contrast, C. elegans PVD dendrites are highly stereotypic, branching orthogonally from previous orders (figure 2a), so it would not be surprising if guidance cues are identified as being located along the growth pathways to direct this dendritic pattern. Thus, studies on these two systems provide distinct and complementary insights into how dendrites interact with epidermis during growth and patterning. Below, we first discuss the molecular and cellular mechanisms responsible for guiding dendritic branching of PVD neurons. We then focus on how epidermis regulates dendritic patterning of class IV da neurons.
2. Pre-patterning growth pathways
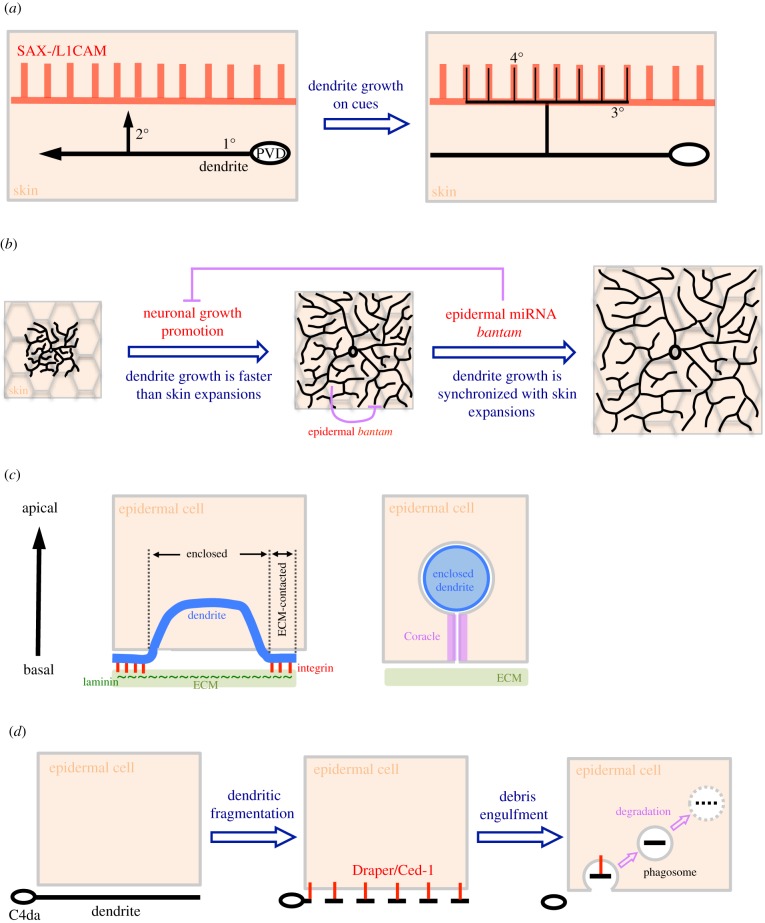
A novel transmembrane leucine-rich repeat (LRR) protein, DMA-1, is expressed in PVD neurons, and mutations in dma-1 disrupt higher-order dendrite branching after normal growth of 1° branches [40]. The LRR domain is located in the extracellular region of DMA-1 and mediates protein–protein interactions [41], suggesting the existence of unidentified extracellular binding partners to direct dendrite growth. Through forward genetic screens, two independent research groups found that PVD neurons in sax-7 mutants exhibit higher-order dendrite patterning defects; whereas 1° branches extend normally and some 2° branches successfully reach the sublateral muscle boundary, 3° branches fail to branch horizontally [42,43]. The sax-7 gene encodes an L1-type CAM of the evolutionarily conserved immunoglobulin CAM protein superfamily (IgSF CAM) [44]. As a transmembrane protein, L1CAM contains immunoglobulin domains linked to fibronectin III domains in the extracellular region, a single-pass transmembrane domain and a conserved cytoplasmic tail. In Drosophila and vertebrates, L1CAMs have critical functions in neurite outgrowth and guidance, and are expressed at high levels in neural tissues [25,45,46]. Interestingly, SAX-7 is expressed in non-neuronal hypodermal cells as sublateral longitudinal stripes that match the horizontal 3° branching pattern [42] (figure 2b,c). These results suggest that skin (hypodermal cells) directs pre-patterned growth of 3° branches. Indeed, ectopic expression of SAX-7 in lateral hypodermal seam cells of the sax-7 mutant, which are located along 1° branches, induced dendritic growth into the seam cells. Like other IgSF CAMs, skin-derived SAX-7 might interact with proteins presented on the dendritic surface via their extracellular domains. Through an additional genetic screen, mnr-1 mutations were identified as producing branching defects in PVD 3° branches similar to those displayed by dma-1 and sax-7 mutants [40,43]. The mnr-1 gene encodes an uncharacterized type I transmembrane protein [43]. Analyses of gene expression and mutant rescue suggest that DMA-1 on dendritic surfaces interacts with SAX-7 and MNR-1 on hypodermal cell membranes. Drosophila S2 cells transfected with DMA-1 form strong aggregations with cells expressing both SAX-7 and MNR-1, but not with cells expressing only one or the other [42]. Accordingly, dendritic DMA-1 forms a tripartite complex with hypodermal SAX-7 and MNR-1 (figure 2d). Thus, skin cells appear to play a pre-patterning role by guiding dendrite branching via expression of SAX-7 in narrow stripes [47] (figure 3a). An immediate and obvious question is how SAX-7 expression is restricted to continuous stripes through skin. Recent forward genetic screens have shown that mutations in the dynein light intermediate chain DLI-1 and the cell–cell fusion protein EFF-1 caused disruption of PVD dendritic branching patterns similar to those observed in the sax-7 mutant [48]. Importantly, orderly SAX-7 stripes are scrambled into numerous ectopic patches between lateral and sublateral lines in both dli-1 and eff-1 mutants. Moreover, hypodermal but not neuronal expression of DLI-1 or EFF-1 rescues the respective mutant phenotypes in terms of SAX-7 stripe formation and PVD dendrite organization. Therefore, the microtubule-based transport system might be important for establishing or maintaining striped expression of SAX-7 in fused hypodermal cells.
Figure 3.
Examples of how epidermis shapes dendritic patterns. (a) Pre-patterning growth pathways. SAX-7 is expressed in a striped pattern in skin that guides future dendritic patterning before PVD 3° and 4° branch formation. Then, the upcoming 2° branch turns and branches out new branches according to the SAX-7 stripes. (b) Synchronizing dendrite growth with body expansion. Initial dendrite growth is rapid until the epidermal field is fully covered at mid-larval stages. This rapid growth of dendrites is decelerated by epidermal bantam microRNA to synchronize the growth rate with epidermis for later stages. (c) ECM-attached and epidermis-enclosed branches. Dendritic branches are attached on the epidermal ECM by interactions between dendritic integrin (red) and epidermal laminin (green) on the ECM. A portion of an enclosed branch inside an epidermal cell is also shown. The enclosed dendrite is encircled by epidermal membranes (grey) that are invaginated from the basal surface of the epidermal cell. The septate junction protein Coracle (magenta) is expressed in the two closely aligned invaginated membrane layers at the basal side of the enclosed dendrite. (d) Cleaning up dendritic debris. During pruning of class IV da (C4da) neurons, dendrites are first fragmented and recognized by the apoptotic cell clearance receptor Draper. Dendritic debris is then engulfed by epidermal cells and further fused to phagosomes for degradation.
The orderly array of PVD 4° branches hints at a pre-patterning event responsible for their parallel growth. Highly sensitive spinning-disk confocal microscopy revealed a stripe-like expression pattern of SAX-7 in skin arrays matching PVD 4° branching patterns, albeit weaker than that observed for 3° branches [49] (figure 2b,c). These SAX-7 stripes are expressed in hypodermal cells at the L3 stage, prior to the appearance of 4° branches, suggesting that terminal 4° branches might adopt the same strategy as 3° branches to grow along pre-patterned SAX-7 stripes. However, it is not possible to assay 4° dendrite branching defects in sax-7 mutants as their 3° branches are disrupted. Consequently, a genetic screen was undertaken for mutants in which only 4° branches are affected. This analysis identified mutations in unc-52, which encodes the extracellular heparan sulfate proteoglycan (HSPG) Perlecan, as specifically disrupting 4° branches, while 1°, 2° and 3° branches remained intact. This branch specificity largely depends on regulation of SAX-7 expression, as SAX-7 expression is normal in the 3° branching pathways but is absent from the 4° branching pathways in unc-52 mutants. Immunostaining revealed that UNC-52 is expressed in muscles, forming interdigitating stripes to SAX-7 stripes in the epidermis (figure 2b,c). Together with genetic studies, these findings resulted in a proposed model whereby localized UNC-52 stripes link muscle sarcomeres to hypodermal hemidesmosomes prior to SAX-7 stripe formation [49]. Thus, to make an array of well-spaced terminal branches, hypodermal substrates are first divided into equal units through local interactions at muscle contact sites, which then restricts SAX-7 stripe formation within these units (figure 3a). Interestingly, according to different orders of branches and local environments, extracellular matrix (ECM) proteins may shape dendrites in different ways. In the unc-52 mutant, apart from the effect on 4° branches, 2° branches are also reduced, although not with complete penetrance [50]. Away from the epidermis, the ECM protein NID-1/Nidogen interacts with four specific immunoglobulin domains of UNC-52 to pattern 2° branches [51,52]. However, these UNC-52-NID-1 interactions are dispensable for skin–muscle attachments during 4° branch formation. Therefore, a single ECM molecule can differentially direct dendrite growth in different environments.
The role of muscle-derived UNC-52/Perlecan in PVD branch formation implies that other proximal yet non-contact tissues may modulate dendrite–substrate interactions. Forward genetic screens revealed lect-2 mutants as exhibiting disorganized dendrite phenotypes [53,54]. Interestingly, these lect-2 mutant phenotypes could be rescued by ectopic lect-2 expression in several different tissues, even though the lect-2 transcript is localized in muscle cells. Consistent with the idea that LECT-2 is secreted to exert its function in dendritic arborization (figure 2b), lect-2 encodes a homologue of chemotaxin 2 (LECT2)/Chondromodulin II, a chemotactic factor secreted from leucocytes [55,56]. The secreted LECT-2 protein decorates PVD dendritic branches and hypodermal cells in a pattern analogous to SAX-7 expression. As the SAX-7/MNR-1/DMA-1 tripartite complex directs 3° branching, genetic analyses suggest that LECT-2 acts in this pathway by stabilizing ligand–receptor complex formation [57] (figure 2d). Thus, the stereotypic menorah-like pattern of PVD dendrites is pre-determined by regulated SAX-7 localization in the hypodermal substrate, thereby guiding dendritic branch growth (figures 2 and 3a).
3. Stabilizing new branches
Dendritic branch formation necessitates initiation and stabilization of new branches. In C. elegans, SAX-7 is pre-patterned in the epidermis, establishing the pathways that guide dendrite growth. As a CAM, it is possible that skin-derived SAX-7 also functions in stabilizing new dendrites. Time-lapse recordings reveal that newly formed PVD 3° branches exhibit dynamic extension and retraction [42]. Whereas more than half of new branches are ultimately stabilized to form T-shape dendrites in wild-type C. elegans, nearly all 3° branches in sax-7 mutants are retracted after branch initiation. Thus, in addition to being a guiding factor, SAX-7 also appears to function as a stabilizer of branches during dendritic growth.
Apart from SAX-7, another skin-derived component, the receptor tyrosine phosphatase CLR-1 was also identified in a C. elegans genetic screen [58]. Expression of clr-1::GFP was detected in hypodermal and muscle cells but not in PVD neurons. PVD 4° branches are largely disrupted in clr-1 mutants, but SAX-7 stripes are normal, suggesting an alternative pathway for regulating terminal branch growth. Domain deletion assays on CLR-1 indicate that only the intracellular phosphatase domain, but not the extracellular adhesion domain, is required for branch growth. Genetic analysis also supports that CLR-1 functions parallel to the SAX-7/DMA-1 pathway. Interestingly, unlike SAX-7 that is required both for branch initiation and stabilization, new 4° branches grow onto the SAX-7 stripes of clr-1 mutants but are ultimately retracted. Hence, hypodermal cells first instruct PVD 4° branch growth through the pre-patterned SAX-7 stripes, and then stabilize those branches through CLR-1 intracellular phosphatase activity.
In Drosophila class IV da neurons, numerous dendritic branches that sprout out to innervate the skin (epidermal cells) also require stabilization throughout development. One recent study has shown that mutations in core proteins of HSPG or its biogenesis in epidermal cells strongly impede dendritic growth of class IV da neurons [59]. Time-lapse recordings showed that branches initially grew into HSPG-deficient areas but were not retained there during dynamic growth, suggesting epidermal HSPGs are required for branch stabilization. Consistently, stabilized microtubules were found to be largely depleted from branches located at the borders between wild-type and HSPG-deficient areas. These data suggest that epidermal HSPGs support dendrite growth by stabilizing and bundling dendritic microtubules. Similar epidermal HSPG-based regulation of dendrite growth has been reported for zebrafish, in which the HSPGs are highly enriched in the basal membranes of the skin [60]. Upon disrupting HSPG synthesis, sensory axons of zebrafish RB neurons failed to reach the skin. Time-lapse examination revealed that epidermal HSPGs in zebrafish guide sensory branches to innervate the skin [60], unlike their role in maintaining branch stability, as is the case for Drosophila class IV da neurons.
4. Synchronizing dendrite growth with body expansion
During development, dendrites actively extend and branch to cover their target fields; in the case of class IV da neurons, this is the layer of epidermis covering the larval body. As the body size of larvae continuously increases through developmental stages, how is the expansion of the epidermal layer coordinated with dendrite growth to maintain full coverage of the target field? Imaging of early-stage class IV da neurons has revealed that dendrite outgrowth starts around 16 h after egg laying (AEL), after the epidermal sheet has formed [61]. Dendrites rapidly grow and branch to catch up with epidermal sheet expansion until they fully cover the body wall by 48 h AEL (figure 3b). Immediately after this rapid growth phase, dendrite growth decelerates to a rate similar to that of epidermal cell expansion (in a process called scaling growth) until pupa formation [61] (figure 3b). Interestingly, dendrites were found to greatly interact with epidermal cells at 48 h AEL, coinciding with the growth phase transition [62]. This coincidence implies that epidermal cells may send inhibitory signals to slow down dendrite growth. A genetic screen targeting scaling growth identified the microRNA mutant bantam, which exhibits small body size and over-sized dendritic arbors [61]. Although bantam is expressed in neurons, epidermal cells and muscle cells, only epidermal expression could rescue the scaling growth defect, suggesting that bantam functions non-cell-autonomously in epidermis to inhibit dendrite growth (figure 3b). In photo-ablation experiments, da neurons regenerate more dendrites in bantam mutants than in wild-type flies, suggesting that bantam is involved in generating growth-inhibitory signals in scaling growth [63]. The microRNA bantam may restrict dendrite regeneration by downregulating Akt activity that facilitates dendrite regeneration in neurons [63]. In epidermis, bantam regulates pathways involved in cell cycle regulation, cell growth and cell adhesion [62]. During development, epidermal cells complete proliferation at embryonic stages and then enter endoreplication around 48 h AEL to scale up cell size and enhance protein synthesis, thereby supporting body expansion [62]. Epithelial endoreplication in larvae also autonomously increases β-integrin expression to enhance interactions with the ECM. In bantam mutants, epidermal endoreplication is arrested and results in diverse abnormalities including disrupted dendrite–ECM interactions when dendrites are starting to contact the epidermis extensively. These findings suggest that epidermal cells play active roles in synchronizing dendrite growth when they undergo developmental transitions, thereby matching dendritic arborization to body expansion (figure 3b).
5. Restricting dendritic branching within a thin two-dimensional space
Maximizing sensory coverage of the innervating field is a fundamental process during polymodal nociceptor morphogenesis. Space-filling neurons such as class IV da neurons may use self-avoidance mechanisms to ensure new branch innervation in regions that are not occupied by other iso-neuronal branches, thereby attaining complete field coverage (figure 1b). Establishing the non-overlapping dendritic pattern of class IV da neurons also requires restricting branch growth in the same plane, which prevents crossovers caused by non-contact passing in three-dimensional space. In fact, these sensory dendrites attach to the epidermis to sense externally derived stimuli. Imaging analyses have revealed that dendritic branches are restricted between the basal surface and ECM of epidermal cells [64,65] (figures 1c and 3c). Dendrite attachment to the ECM is mediated by interactions between integrins in dendrites and epidermis-secreted laminins (figure 3c). In mutants for integrin subunits, many dendritic branches escape ECM attachment and are enclosed by epidermis (figure 3c). Although the functional significance (or defects) associated with dendritic enclosure is not clear, it would allow non-contact crossover of iso-neuronal branches at the basal side of enclosed dendrites. Furthermore, a semaphorin ligand and its receptor are required for dendrite–ECM interactions to avoid crossovers [66]. The epidermis-secreted Sema-2b ligand interacts with the neuronal Plexin B receptor on dendritic surfaces, with this latter also physically associating with integrin subunits. Genetic analyses have indicated that this Sema-2b ligand–receptor complex promotes dendrite positioning at the ECM by activating the downstream Trc kinase. Thus, multiple mechanisms are involved in mediating the ECM–dendrite interaction, ensuring that dendritic branches are positioned in a thin two-dimensional space for executing self-avoidance and maximizing non-redundant dendrite coverage.
Although dendrites are mainly ECM-contacting, some dendrites are enclosed inside epidermal cells in wild-type larvae. A previous study identified that Coracle (Cora), a septate junction protein, is expressed along enclosed dendrites [65], and a follow-up study revealed that Cora is expressed at the basal side of epidermal cells to these enclosed dendrites (figure 3c) [67]. When dendrite positioning was examined, epidermal cora knockdown resulted in less dendritic enclosure, suggesting it is required to enclose dendritic branches [67]. Transmission electron microscopy revealed that enclosed dendrites are encircled by epidermal membranes that are invaginated from the basal surface [64,65], and the two layers of invaginated membranes closely align with each other until they reach the enclosed branch (figure 3c). Cora is a component of septate junction protein complexes, but it is not clear whether other components are also localized and function in dendrite enclosure. Overall, then, epidermal cells actively regulate dendrite positioning at the ECM or their enclosure to establish the non-crossover space-filling pattern.
6. Engulfment of fragmented dendrites during pruning
Clearance of degenerating axons and dendrites is an essential process for tissue homeostasis and to avoid inflammation. Studies of programmed axon pruning in mushroom bodies during metamorphosis and axon degeneration after injury in olfactory receptor neurons indicate that glia, the axon-supporting and -ensheathing cells, mediate clearance of degenerating axons [68,69]. Which cells are involved in dendrite clearance? During Drosophila metamorphosis from larva to adult, dendrites of larval class IV da neurons are pruned completely at the early pupal stage. In these surviving neurons, dendrites are then regenerated from the remaining soma at later pupal stages but with a different pattern in adults. Although early pruning steps, such as severing of dendrites, are largely controlled cell-autonomously, subsequent clearance is conducted on-site by epidermal cells [70]. Similar to how glial cells engulf axonal debris, epidermal cells express the transmembrane protein Draper/Ced-1 to recognize fragmented dendrites (figure 3d). Engulfed dendrite debris is then transported and fused to phagosomes for further degradation [70]. Furthermore, breakdown of long dendrites into short fragments also seems to be regulated by the epidermis, as delayed dendrite fragmentation has frequently been observed upon epidermal knockdown of Draper, Vps16A or WASP to inhibit engulfment or phagosome maturation [71–73]. Time-lapse recordings have revealed that epidermal cells wrap around dendrite-thinning and -beading sites with actin-rich membranes. This actin accumulation was not detected at dendrite–epidermis contact sites when the receptor Draper was depleted by RNAi knockdown [70]. Thus, epidermal cells not only phagocytose dendrite debris, but they also actively promote dendrite fragmentation. The interaction between dendrites and epidermis also extends to lesion-induced dendrite degeneration, which follows the same pathway as the developmentally regulated pruning process [70]. A similar skin-mediated clearance mechanism has also been reported for zebrafish. After laser axotomy of somatosensory peripheral axons in zebrafish, neuronal debris was phagocytosed by epidermal cells rather than by blood-derived phagocytes [74]. Thus, epidermal cells not only act as dendrite supporting and ensheathing cells, but also serve as phagocytes to clean up neuronal debris during programmed or injury-induced pruning.
7. Reshaping dendrites
Interestingly, the regenerated dendrites of class IV da neurons further undergo remodelling in early adulthood. At 24 h after eclosion, ventral class IV da neurons rapidly reshape their radially patterned dendrites into a lattice-like pattern that aligns well with lateral tergosternal muscles (LTMs) [75]. This reshaping of dendrites does not involve dendritic pruning and regrowth. Instead, this process is achieved by relocating the existing branches to the grooves between LTM fibres [75]. By a genetic screen, mutations in matrix metalloproteinase 2 (Mmp2) were found to cause obvious defects in dendrite reshaping [75]. Mmp is known to modify the basement membrane by degrading ECM components [76]. Consistent with its molecular functions, Mmp2 expression was found to be transiently elevated in epidermal cells when the ECM between the epidermis and the LTMs is being selectively degraded [75]. These findings suggest that epidermis actively modifies the ECM microenvironment to allow relocation of dendritic branches.
8. Concluding remarks and future prospects
Using powerful genetic tools to manipulate gene expression, accumulating evidence from studies of C. elegans and Drosophila has shed light on the active roles of epidermis in regulating dendrite patterning and degeneration. Epidermal cells, the substrate of sensory dendrites, instruct and stabilize dendritic growth by forming SAX-7/L1CAM stripes in C. elegans. L1CAM family proteins are conserved and required for neural development in other organisms like Drosophila and mice. It is possible that substrates in higher organisms may also express L1CAM homologues to regulate dendritic growth. Although only one isoform of sax-7 is essential for dendritic growth in nematodes [43], both fly and mouse express alternatively spliced isoforms in neurons and non-neuronal tissues [44]. Thus, substrates could employ these alternatively spliced isoforms to regulate dendrite arborization. Although dynein components and fusogen are required for SAX-7 expression into stripes, it is unclear how these SAX-7 stripes are localized along the muscle–skin interface. It is possible that muscles express unidentified cues that recruit and stabilize hypodermal SAX-7 expression.
In Drosophila, the dendrites of da neurons are restricted to a thin space between the basal surface of epidermis and the ECM. Through self-avoidance, dendritic arbors do not overlap. However, some dendrites are enclosed by epidermal cells [64,65]. The percentage of enclosed dendrites progressively increases from second instar to the final larval stages [62], suggesting an increasing requirement in later stages. Although dendritic ensheathment could allow other branches to crossover without direct contact, some enclosed dendrites are solitary without crossover, suggesting unidentified functions for enclosed dendrites [66]. Interestingly, damaged epidermal cells are reported to secrete an inflammatory cytokine Eiger, a homologue of tumour necrosis factor, to induce thermal hyperalgesia and allodynia through da neurons, suggesting that epidermal cells could also play a role in transmitting signals to neurons [77].
Even though epidermal cells support dendritic arborization during larval stages, they promote dendrite degeneration during pupal stages, suggesting an interesting transition from protective to attacking roles. Larval epidermal cells are also degenerated during pupal stages to be replaced by adult epidermal cells. During larval stages, lesion-induced dendritic pruning also requires epidermal cells to exert an attacking role. Epidermal cells seem to be able to interchange these two conflicting roles at any time, depending on the status of dendrites. The lessons learned from invertebrate studies could potentially be extrapolated, with some variations, to mammalian systems, with considerable implications for sensory regeneration in patients suffering burns or physical lesions.
Supplementary Material
Acknowledgements
We thank Chun-Liang Pan at National Taiwan University for comments on the manuscript, as well as Chan-Yen Ou and Ying-Chun Chen at National Taiwan University for providing the image for figure 2a.
Data accessibility
This article has no additional data.
Competing interests
We declare we have no competing interests.
Funding
We received no funding for this study.
References
- 1.Albeg A, Smith CJ, Chatzigeorgiou M, Feitelson DG, Hall DH, Schafer WR, Miller DM III, Treinin M. 2011. C. elegans multi-dendritic sensory neurons: morphology and function . Mol. Cell. Neurosci. 46, 308–317. ( 10.1016/j.mcn.2010.10.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jan YN, Jan LY. 2010. Branching out: mechanisms of dendritic arborization. Nat. Rev. Neurosci. 11, 316–328. ( 10.1038/nrn2836) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao FB, Brenman JE, Jan LY, Jan YN. 1999. Genes regulating dendritic outgrowth, branching, and routing in Drosophila. Genes Dev. 13, 2549–2561. ( 10.1101/gad.13.19.2549) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang F, Julien DP, Sagasti A. 2013. Journey to the skin: somatosensory peripheral axon guidance and morphogenesis. Cell Adh. Migr. 7, 388–394. ( 10.4161/cam.25000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grueber WB, Jan LY, Jan YN. 2002. Tiling of the Drosophila epidermis by multidendritic sensory neurons. Development 129, 2867–2878. [DOI] [PubMed] [Google Scholar]
- 6.Hughes CL, Thomas JB. 2007. A sensory feedback circuit coordinates muscle activity in Drosophila. Mol. Cell. Neurosci. 35, 383–396. ( 10.1016/j.mcn.2007.04.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hwang RY, Zhong L, Xu Y, Johnson T, Zhang F, Deisseroth K, Tracey WD. 2007. Nociceptive neurons protect Drosophila larvae from parasitoid wasps. Curr. Biol. 17, 2105–2116. ( 10.1016/j.cub.2007.11.029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jinushi-Nakao S, Arvind R, Amikura R, Kinameri E, Liu AW, Moore AW. 2007. Knot/Collier and cut control different aspects of dendrite cytoskeleton and synergize to define final arbor shape. Neuron 56, 963–978. ( 10.1016/j.neuron.2007.10.031) [DOI] [PubMed] [Google Scholar]
- 9.Yan Z, Zhang W, He Y, Gorczyca D, Xiang Y, Cheng LE, Meltzer S, Jan LY, Jan YN. 2013. Drosophila NOMPC is a mechanotransduction channel subunit for gentle-touch sensation. Nature 493, 221–225. ( 10.1038/nature11685) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiang Y, Yuan Q, Vogt N, Looger LL, Jan LY, Jan YN. 2010. Light-avoidance-mediating photoreceptors tile the Drosophila larval body wall. Nature 468, 921–926. ( 10.1038/nature09576) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tracey WD Jr, Wilson RI, Laurent G, Benzer S. 2003. Painless, a Drosophila gene essential for nociception. Cell 113, 261–273. ( 10.1016/S0092-8674(03)00272-1) [DOI] [PubMed] [Google Scholar]
- 12.Zhong L, Hwang RY, Tracey WD. 2010. Pickpocket is a DEG/ENaC protein required for mechanical nociception in Drosophila larvae. Curr. Biol. 20, 429–434. ( 10.1016/j.cub.2009.12.057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim SE, Coste B, Chadha A, Cook B, Patapoutian A. 2012. The role of Drosophila Piezo in mechanical nociception. Nature 483, 209–212. ( 10.1038/nature10801) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stone MC, Roegiers F, Rolls MM. 2008. Microtubules have opposite orientation in axons and dendrites of Drosophila neurons. Mol. Biol. Cell. 19, 4122–4129. ( 10.1091/mbc.E07-10-1079) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grueber WB, Jan LY, Jan YN. 2003. Different levels of the homeodomain protein cut regulate distinct dendrite branching patterns of Drosophila multidendritic neurons. Cell 112, 805–818. ( 10.1016/S0092-8674(03)00160-0) [DOI] [PubMed] [Google Scholar]
- 16.Kim MD, Jan LY, Jan YN. 2006. The bHLH-PAS protein spineless is necessary for the diversification of dendrite morphology of Drosophila dendritic arborization neurons. Genes Dev. 20, 2806–2819. ( 10.1101/gad.1459706) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li W, Wang F, Menut L, Gao FB. 2004. BTB/POZ-zinc finger protein abrupt suppresses dendritic branching in a neuronal subtype-specific and dosage-dependent manner. Neuron 43, 823–834. ( 10.1016/j.neuron.2004.08.040) [DOI] [PubMed] [Google Scholar]
- 18.Sugimura K, Satoh D, Estes P, Crews S, Uemura T. 2004. Development of morphological diversity of dendrites in Drosophila by the BTB-zinc finger protein abrupt. Neuron 43, 809–822. ( 10.1016/j.neuron.2004.08.016) [DOI] [PubMed] [Google Scholar]
- 19.Lee A, Li W, Xu K, Bogert BA, Su K, Gao FB. 2003. Control of dendritic development by the Drosophila fragile X-related gene involves the small GTPase Rac1. Development 130, 5543–5552. ( 10.1242/dev.00792) [DOI] [PubMed] [Google Scholar]
- 20.Scott EK, Reuter JE, Luo L. 2003. Small GTPase Cdc42 is required for multiple aspects of dendritic morphogenesis. J. Neurosci. 23, 3118–3123. ( 10.1523/JNEUROSCI.23-08-03118.2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Satoh D, Sato D, Tsuyama T, Saito M, Ohkura H, Rolls MM, Ishikawa F, Uemura T. 2008. Spatial control of branching within dendritic arbors by dynein-dependent transport of Rab5-endosomes. Nat. Cell Biol. 10, 1164–1171. ( 10.1038/ncb1776) [DOI] [PubMed] [Google Scholar]
- 22.Zheng Y, Wildonger J, Ye B, Zhang Y, Kita A, Younger SH, Zimmerman S, Jan LY, Jan YN. 2008. Dynein is required for polarized dendritic transport and uniform microtubule orientation in axons. Nat. Cell Biol. 10, 1172–1180. ( 10.1038/ncb1777) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nithianandam V, Chien CT. 2018. Actin blobs prefigure dendrite branching sites. J. Cell Biol. 217, 3731–3746. ( 10.1083/jcb.201711136) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ye B, Zhang Y, Song W, Younger SH, Jan LY, Jan YN. 2007. Growing dendrites and axons differ in their reliance on the secretory pathway. Cell 130, 717–729. ( 10.1016/j.cell.2007.06.032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang WK, et al. 2011. Nak regulates localization of clathrin sites in higher-order dendrites to promote local dendrite growth. Neuron 72, 285–299. ( 10.1016/j.neuron.2011.08.028) [DOI] [PubMed] [Google Scholar]
- 26.Gao FB, Kohwi M, Brenman JE, Jan LY, Jan YN. 2000. Control of dendritic field formation in Drosophila: the roles of flamingo and competition between homologous neurons. Neuron 28, 91–101. ( 10.1016/S0896-6273(00)00088-X) [DOI] [PubMed] [Google Scholar]
- 27.Kimura H, Usui T, Tsubouchi A, Uemura T. 2006. Potential dual molecular interaction of the Drosophila 7-pass transmembrane cadherin Flamingo in dendritic morphogenesis. J. Cell Sci. 119, 1118–1129. ( 10.1242/jcs.02832) [DOI] [PubMed] [Google Scholar]
- 28.Dimitrova S, Reissaus A, Tavosanis G. 2008. Slit and Robo regulate dendrite branching and elongation of space-filling neurons in Drosophila. Dev. Biol. 324, 18–30. ( 10.1016/j.ydbio.2008.08.028) [DOI] [PubMed] [Google Scholar]
- 29.Matsubara D, Horiuchi SY, Shimono K, Usui T, Uemura T. 2011. The seven-pass transmembrane cadherin Flamingo controls dendritic self-avoidance via its binding to a LIM domain protein, Espinas, in Drosophila sensory neurons. Genes Dev. 25, 1982–1996. ( 10.1101/gad.16531611) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hughes ME, Bortnick R, Tsubouchi A, Baumer P, Kondo M, Uemura T, Schmucker D. 2007. Homophilic Dscam interactions control complex dendrite morphogenesis. Neuron 54, 417–427. ( 10.1016/j.neuron.2007.04.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matthews BJ, Kim ME, Flanagan JJ, Hattori D, Clemens JC, Zipursky SL, Grueber WB. 2007. Dendrite self-avoidance is controlled by Dscam. Cell 129, 593–604. ( 10.1016/j.cell.2007.04.013) [DOI] [PubMed] [Google Scholar]
- 32.Soba P, Zhu S, Emoto K, Younger S, Yang SJ, Yu HH, Lee T, Jan LY, Jan YN. 2007. Drosophila sensory neurons require Dscam for dendritic self-avoidance and proper dendritic field organization. Neuron 54, 403–416. ( 10.1016/j.neuron.2007.03.029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith CJ, Watson JD, Spencer WC, O'Brien T, Cha B, Albeg A, Treinin M, Miller DM III. 2010. Time-lapse imaging and cell-specific expression profiling reveal dynamic branching and molecular determinants of a multi-dendritic nociceptor in C. elegans. Dev. Biol. 345, 18–33. ( 10.1016/j.ydbio.2010.05.502) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Way JC, Chalfie M. 1989. The mec-3 gene of Caenorhabditis elegans requires its own product for maintained expression and is expressed in three neuronal cell types. Genes Dev. 3, 1823–1833. ( 10.1101/gad.3.12a.1823) [DOI] [PubMed] [Google Scholar]
- 35.Smith CJ, et al. 2013. Sensory neuron fates are distinguished by a transcriptional switch that regulates dendrite branch stabilization. Neuron 79, 266–280. ( 10.1016/j.neuron.2013.05.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aguirre-Chen C, Bulow HE, Kaprielian Z. 2011. C. elegans bicd-1, homolog of the Drosophila dynein accessory factor Bicaudal D, regulates the branching of PVD sensory neuron dendrites. Development 138, 507–518. ( 10.1242/dev.060939) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palanca AM, et al. 2013. New transgenic reporters identify somatosensory neuron subtypes in larval zebrafish. Dev. Neurobiol. 73, 152–167. ( 10.1002/dneu.22049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lefebvre JL, Kostadinov D, Chen WV, Maniatis T, Sanes JR. 2012. Protocadherins mediate dendritic self-avoidance in the mammalian nervous system. Nature 488, 517–521. ( 10.1038/nature11305) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zipursky SL, Grueber WB. 2013. The molecular basis of self-avoidance. Annu. Rev. Neurosci. 36, 547–568. ( 10.1146/annurev-neuro-062111-150414) [DOI] [PubMed] [Google Scholar]
- 40.Liu OW, Shen K. 2011. The transmembrane LRR protein DMA-1 promotes dendrite branching and growth in C. elegans. Nat. Neurosci. 15, 57–63. ( 10.1038/nn.2978) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bella J, Hindle KL, McEwan PA, Lovell SC. 2008. The leucine-rich repeat structure. Cell. Mol. Life Sci. 65, 2307–2333. ( 10.1007/s00018-008-8019-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dong X, Liu OW, Howell AS, Shen K. 2013. An extracellular adhesion molecule complex patterns dendritic branching and morphogenesis. Cell 155, 296–307. ( 10.1016/j.cell.2013.08.059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salzberg Y, Diaz-Balzac CA, Ramirez-Suarez NJ, Attreed M, Tecle E, Desbois M, Kaprielian Z, Bulow HE. 2013. Skin-derived cues control arborization of sensory dendrites in Caenorhabditis elegans. Cell 155, 308–320. ( 10.1016/j.cell.2013.08.058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mualla R, Nagaraj K, Hortsch M. 2013. A phylogenetic analysis of the L1 family of neural cell adhesion molecules. Neurochem. Res. 38, 1196–1207. ( 10.1007/s11064-012-0892-0) [DOI] [PubMed] [Google Scholar]
- 45.Kamiguchi H. 2003. The mechanism of axon growth: what we have learned from the cell adhesion molecule L1. Mol. Neurobiol. 28, 219–228. ( 10.1385/MN:28:3:219) [DOI] [PubMed] [Google Scholar]
- 46.Siegenthaler D, Enneking EM, Moreno E, Pielage J. 2015. L1CAM/Neuroglian controls the axon–axon interactions establishing layered and lobular mushroom body architecture. J. Cell Biol. 208, 1003–1018. ( 10.1083/jcb.201407131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ziegenfuss JS, Grueber WB. 2013. SAX-7 and menorin light the path for dendrite morphogenesis. Cell 155, 269–271. ( 10.1016/j.cell.2013.09.029) [DOI] [PubMed] [Google Scholar]
- 48.Zhu T, Liang X, Wang XM, Shen K. 2017. Dynein and EFF-1 control dendrite morphology by regulating the localization pattern of SAX-7 in epidermal cells. J. Cell Sci. 130, 4063–4071. ( 10.1242/jcs.201699) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liang X, Dong X, Moerman DG, Shen K, Wang X. 2015. Sarcomeres pattern proprioceptive sensory dendritic endings through UNC-52/Perlecan in C. elegans. Dev. Cell. 33, 388–400. ( 10.1016/j.devcel.2015.03.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Celestrin K, Diaz-Balzac CA, Tang LTH, Ackley BD, Bulow HE. 2018. Four specific immunoglobulin domains in UNC-52/Perlecan function with NID-1/Nidogen during dendrite morphogenesis in Caenorhabditis elegans. Development 145, dev158881 ( 10.1242/dev.158881) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim S, Wadsworth WG. 2000. Positioning of longitudinal nerves in C. elegans by nidogen. Science 288, 150–154. ( 10.1126/science.288.5463.150) [DOI] [PubMed] [Google Scholar]
- 52.Ackley BD, Kang SH, Crew JR, Suh C, Jin Y, Kramer JM. 2003. The basement membrane components nidogen and type XVIII collagen regulate organization of neuromuscular junctions in Caenorhabditis elegans. J. Neurosci. 23, 3577–3587. ( 10.1523/JNEUROSCI.23-09-03577.2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Diaz-Balzac CA, Rahman M, Lazaro-Pena MI, Martin Hernandez LA, Salzberg Y, Aguirre-Chen C, Kaprielian Z, Bulow HE. 2016. Muscle- and skin-derived cues jointly orchestrate patterning of somatosensory dendrites. Curr. Biol. 26, 2379–2387. ( 10.1016/j.cub.2016.07.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zou W, Shen A, Dong X, Tugizova M, Xiang YK, Shen K. 2016. A multi-protein receptor-ligand complex underlies combinatorial dendrite guidance choices in C. elegans. Elife 5, e18345 ( 10.7554/eLife.18345) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hiraki Y, Inoue H, Kondo J, Kamizono A, Yoshitake Y, Shukunami C, Suzuki F. 1996. A novel growth-promoting factor derived from fetal bovine cartilage, chondromodulin II: purification and amino acid sequence. J. Biol. Chem. 271, 22 657–22 662. ( 10.1074/jbc.271.37.22657) [DOI] [PubMed] [Google Scholar]
- 56.Yamagoe S, Yamakawa Y, Matsuo Y, Minowada J, Mizuno S, Suzuki K. 1996. Purification and primary amino acid sequence of a novel neutrophil chemotactic factor LECT2. Immunol. Lett. 52, 9–13. ( 10.1016/0165-2478(96)02572-2) [DOI] [PubMed] [Google Scholar]
- 57.O'Brien B, Miller DM III. 2016. Neurodevelopment: three's a crowd, four is a receptor complex. Curr. Biol. 26, R799–R801. ( 10.1016/j.cub.2016.07.055) [DOI] [PubMed] [Google Scholar]
- 58.Liu X, Wang X, Shen K. 2016. Receptor tyrosine phosphatase CLR-1 acts in skin cells to promote sensory dendrite outgrowth. Dev. Biol. 413, 60–69. ( 10.1016/j.ydbio.2016.03.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Poe AR, Tang L, Wang B, Li Y, Sapar ML, Han C. 2017. Dendritic space-filling requires a neuronal type-specific extracellular permissive signal in Drosophila. Proc. Natl Acad. Sci. USA 114, E8062–E8071. ( 10.1073/pnas.1707467114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang F, Wolfson SN, Gharib A, Sagasti A. 2012. LAR receptor tyrosine phosphatases and HSPGs guide peripheral sensory axons to the skin. Curr. Biol. 22, 373–382. ( 10.1016/j.cub.2012.01.040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Parrish JZ, Xu P, Kim CC, Jan LY, Jan YN. 2009. The microRNA bantam functions in epithelial cells to regulate scaling growth of dendrite arbors in Drosophila sensory neurons. Neuron 63, 788–802. ( 10.1016/j.neuron.2009.08.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jiang N, Soba P, Parker E, Kim CC, Parrish JZ. 2014. The microRNA bantam regulates a developmental transition in epithelial cells that restricts sensory dendrite growth. Development 141, 2657–2668. ( 10.1242/dev.107573) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Song Y, Ori-McKenney KM, Zheng Y, Han C, Jan LY, Jan YN. 2012. Regeneration of Drosophila sensory neuron axons and dendrites is regulated by the Akt pathway involving Pten and microRNA bantam. Genes Dev. 26, 1612–1625. ( 10.1101/gad.193243.112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Han C, Wang D, Soba P, Zhu S, Lin X, Jan LY, Jan YN. 2012. Integrins regulate repulsion-mediated dendritic patterning of Drosophila sensory neurons by restricting dendrites in a 2D space. Neuron 73, 64–78. ( 10.1016/j.neuron.2011.10.036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim ME, Shrestha BR, Blazeski R, Mason CA, Grueber WB. 2012. Integrins establish dendrite–substrate relationships that promote dendritic self-avoidance and patterning in Drosophila sensory neurons. Neuron 73, 79–91. ( 10.1016/j.neuron.2011.10.033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Meltzer S, et al. 2016. Epidermis-derived semaphorin promotes dendrite self-avoidance by regulating dendrite-substrate adhesion in Drosophila sensory neurons. Neuron 89, 741–755. ( 10.1016/j.neuron.2016.01.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tenenbaum CM, Misra M, Alizzi RA, Gavis ER. 2017. Enclosure of dendrites by epidermal cells restricts branching and permits coordinated development of spatially overlapping sensory neurons. Cell Rep. 20, 3043–3056. ( 10.1016/j.celrep.2017.09.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Awasaki T, Ito K. 2004. Engulfing action of glial cells is required for programmed axon pruning during Drosophila metamorphosis. Curr. Biol. 14, 668–677. ( 10.1016/j.cub.2004.04.001) [DOI] [PubMed] [Google Scholar]
- 69.MacDonald JM, Beach MG, Porpiglia E, Sheehan AE, Watts RJ, Freeman MR. 2006. The Drosophila cell corpse engulfment receptor Draper mediates glial clearance of severed axons. Neuron 50, 869–881. ( 10.1016/j.neuron.2006.04.028) [DOI] [PubMed] [Google Scholar]
- 70.Han C, Song Y, Xiao H, Wang D, Franc NC, Jan LY, Jan YN. 2014. Epidermal cells are the primary phagocytes in the fragmentation and clearance of degenerating dendrites in Drosophila. Neuron 81, 544–560. ( 10.1016/j.neuron.2013.11.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lorenzi R, Brickell PM, Katz DR, Kinnon C, Thrasher AJ. 2000. Wiskott-Aldrich syndrome protein is necessary for efficient IgG-mediated phagocytosis. Blood 95, 2943–2946. [PubMed] [Google Scholar]
- 72.Pulipparacharuvil S, Akbar MA, Ray S, Sevrioukov EA, Haberman AS, Rohrer J, Kramer H. 2005. Drosophila Vps16A is required for trafficking to lysosomes and biogenesis of pigment granules. J. Cell Sci. 118, 3663–3673. ( 10.1242/jcs.02502) [DOI] [PubMed] [Google Scholar]
- 73.Awasaki T, Tatsumi R, Takahashi K, Arai K, Nakanishi Y, Ueda R, Ito K. 2006. Essential role of the apoptotic cell engulfment genes draper and ced-6 in programmed axon pruning during Drosophila metamorphosis. Neuron 50, 855–867. ( 10.1016/j.neuron.2006.04.027) [DOI] [PubMed] [Google Scholar]
- 74.Rasmussen JP, Sack GS, Martin SM, Sagasti A. 2015. Vertebrate epidermal cells are broad-specificity phagocytes that clear sensory axon debris. J. Neurosci. 35, 559–570. ( 10.1523/JNEUROSCI.3613-14.2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yasunaga K, Kanamori T, Morikawa R, Suzuki E, Emoto K. 2010. Dendrite reshaping of adult Drosophila sensory neurons requires matrix metalloproteinase-mediated modification of the basement membranes. Dev. Cell. 18, 621–632. ( 10.1016/j.devcel.2010.02.010) [DOI] [PubMed] [Google Scholar]
- 76.Srivastava A, Pastor-Pareja JC, Igaki T, Pagliarini R, Xu T. 2007. Basement membrane remodeling is essential for Drosophila disc eversion and tumor invasion. Proc. Natl Acad. Sci. USA 104, 2721–2726. ( 10.1073/pnas.0611666104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Babcock DT, Landry C, Galko MJ. 2009. Cytokine signaling mediates UV-induced nociceptive sensitization in Drosophila larvae. Curr. Biol. 19, 799–806. ( 10.1016/j.cub.2009.03.062) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article has no additional data.