Abstract
Solitary insects that feed on floral nectar must use innate knowledge to find their first flower. While innate preferences for flower colours are often described as fixed, species-specific traits, the nature and persistence of these preferences have been debated, particularly in relation to ontogenetic processes such as learning. Here we present evidence for a strong context-dependence of innate colour preferences in the crepuscular hawkmoth Manduca sexta. Contrary to expectations, our results show that innate colour biases shift with changes in the visual environment, namely illuminance and background. This finding reveals that innate responses might emerge from a contextual integration of sensory inputs involved in object class recognition rather than from the deterministic matching of such inputs with a fixed internal representation.
Keywords: innate bias, pollination, Lepidoptera, vision, chromatic
1. Background
For the most part, the mechanisms underlying innate preferences are presently unknown, although they represent a fundamental and ecologically important component of animal behaviour [1]. Animals navigating natural environments are subjected to a torrent of sensory stimuli, and innate sensory biases are crucial, particularly to inexperienced solitary animals, to efficiently locate important resources. This has been well studied in mating systems mediated by acoustic [2], visual [3,4], vibratory [5] and olfactory signals [6], but innate sensory biases also have been explored in pollination systems, with an emphasis on the colour preferences of insects, the main pollinators of angiosperms ([7]; also see below).
Although flowers communicate with pollinators through multiple channels of sensory stimuli, vision is acknowledged to be a key modality for most pollinators to find flowers [7,8]. For newly eclosed anthophilous insects, innate sensory preferences or biases thus provide a ‘search image’ that facilitates flower detection in complex, dynamic environments during their first foraging bouts. Floral colour plays an essential role in this task, and innate colour preferences have been extensively investigated in flower-visiting insects [7–9]. Blue and purple for Hymenoptera and some Lepidoptera [7,10,11] and, to a lesser degree yellow and red for some Lepidoptera and Coleoptera [7], are the innately preferred colour hues among diurnal pollinators. Visual detection is physiologically more challenging for nocturnal pollinators [12], which tend to exploit large, UV-absorbing white or creamy flowers that are characteristically very fragrant [7,8,13,14]. While strong scents facilitate long-distance detection, light coloration might favour visual recognition via achromatic (intensity-related) signals under low-light conditions [9]. Nevertheless, the hawkmoths (Lepidoptera, Sphingidae) that have been studied have three photoreceptor types sensitive to UV, blue and green light, and have been shown to possess colour vision in dim light [9,15].
The well-studied crepuscular/nocturnal hawkmoth Manduca sexta feeds from large, mostly white, but UV absorbing (hereafter: white-UV, which is perceived chromatically as a ‘bee blue/green colour’ [16]) or yellow, night-blooming flowers throughout the Americas [13,17]. However, physiological and behavioural evidence suggests they have an innate feeding bias towards blue [15], as do many other insect pollinators [7]. Indeed, in a previous study, we found that naive M. sexta chose a dark blue artificial flower over a white-UV one [18]. In these experiments, the flight cage was white-UV and was illuminated with light intensities resembling twilight conditions. In another study we tested innate responses under several nocturnal illuminance conditions in a larger, dark-green flight cage. Interestingly, we found that under simulated starlight and dim moonlight levels, moths responded robustly only under floral scent stimulation, and showed an innate bias towards white-UV artificial flowers [19]. Under brighter moonlight illuminances, moths did not appear to rely on olfactory stimulation, and did not distinguish between white-UV and blue flowers [19]. Innate sensory biases are inherited, and these moths forage during rapidly changing, crepuscular illuminations; could they show plasticity in visual bias depending on the conditions under which visual signals are encountered? To answer this question, we have performed a series of binary choice assays under three ecologically relevant illuminances (equivalent to starlight, moonlight and twilight) and with two backgrounds (white-UV and dark-green) to more closely evaluate the innate colour preference of M. sexta.
2. Material and methods
Experiments were performed during January 2015–April 2016 at The University of Tennessee at Martin, Martin, TN, USA. Manduca sexta larvae (Great Lakes Hornworm, MI, USA) were fed an artificial diet based on cornmeal as an added source of beta-carotenoids [18] and kept in incubators (Percival I-30VL, Perry, IA, USA) under a 16 : 8 light/dark, 26 : 24°C cycle. After pupation, males and females were placed in separate incubators and starved for 2–3 days prior to being tested.
(a). Experimental set-up
Experiments were performed in a flight cage (47 cm × 61 cm × 47 cm) whose floor was made of brown cardboard, and whose walls were made of cotton cloth, with the exception of one wall, which was made of transparent plastic to allow the experimenter to record the behaviour of the moths (electronic supplementary material, S1). The cloth was either white-UV or dark-green (see figure 1a for relative spectral reflectance). The cage was illuminated with an LED panel with diffusor (LEDtronics, Torrance, CA, USA; see figure 1a for the spectral distribution of the lights), whose output was adjusted with a variable DC voltage power supply (Elenco-XP-752A, Wheeling, IL, USA). Three different illuminances were used: 0.0011 ± 0.003 lx (hereafter: starlight), 0.1556 ± 0.03 lx (hereafter: moonlight) and 9.13 ± 0.16 lx (hereafter: twilight), as measured with a ILT1700 radiometer using a SED100 detector and Y filter to measure illuminance (International Light Technologies, Peabody, MA, USA). Changes in illuminance do not affect the spectral distribution of LED-emitted light. The combination of the three illuminances and two backgrounds resulted in six experimental treatments: starlight/white-UV, starlight/dark-green, moonlight/white-UV, moonlight/dark-green, twilight/white-UV, and twilight/dark-green.
Figure 1.
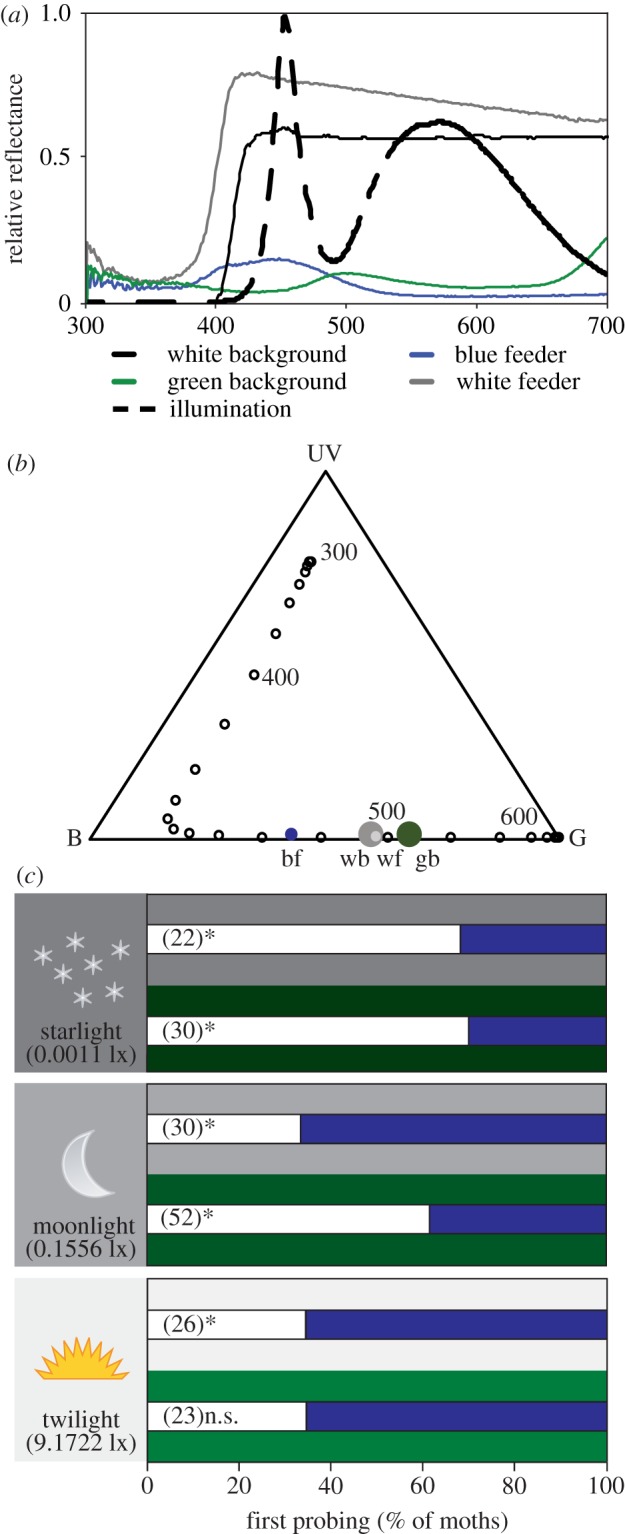
(a) Spectral distribution of the lights used in the experiment and relative spectral reflectance of experimental backgrounds and feeders. (b) Maxwell colour triangle depicting the colour space of stimuli and background colours. UV, B and G denote the colours exciting only the UV, blue and green receptors, respectively. We used the spectral absorption curves of the photoreceptors of the hawkmoth Macroglossum stellatarum, assuming they are similar to those of the closely related Manduca sexta; bf: blue feeder, wf: white-UV feeder, wb: white-UV background, gb: green background. (c) Colour choices of naive M. sexta hawkmoths between the white-UV feeder (white stacked bars) and blue feeder (blue stacked bars) offered against a light, white-UV background (white/grey backgrounds behind stacked bars) or a dark, green background (green backgrounds behind the stacked bars) under three different illuminances resembling starlight (upper panel), moonlight (middle panel) and twilight (lower panel). Numbers in parentheses represent the number of independent replicates for each treatment. *p < 0.05; n.s: p = 0.0585. See text for details.
As visual targets we used a dark-blue and a white-UV feeder [18] offering a 20% (w/w) sucrose solution at a height of 30 cm, ensuring moths perceived the targets against the vertical background. The spectral reflectance of the feeders and green background was measured with a spectroradiometer (RSP900-R; International Light, Peabody, MA, USA) using an integrating sphere with illumination from a Xenon lamp (HPX-2000-HP-DUV, Ocean Optics, Dunedin, FL, USA). For the white-UV background we used the illumination from a halogen lamp (DH-2000-BAL; Ocean Optics) to avoid measuring the blue fluorescence it showed under UV illumination. This was necessary as the LED illumination in the experimental cage did not provide UV. The colour loci are plotted in a Maxwell colour triangle depicting the moth colour space (figure 1b). The feeders were positioned 15 cm apart from each other, equidistant from the centre of the cage and the plastic window. The blue and white-UV feeders were pseudo-randomly swapped to control for any position bias. Olfactory stimulation increases feeding responsiveness, thus we concealed a cotton swab soaked in bergamot oil at the base of the feeders.
(b). Experimental procedure
We transferred starved, flower-naive moths of both sexes to the experimental room at least one hour prior to the onset of their scotophase (beginning of moth activity) and ran the assays for 2–3 h. Moths were tested individually and only once. After a moth was pseudo-randomly assigned a treatment, it was placed inside the cage and allowed up to 10 min to take off. After take-off, it was allowed up to 10 min to probe the feeders. A probing response consisted of the moth hovering in front of the feeder and contacting it with its proboscis. The time elapsed between take-off and the first response was recorded as the latency time (s). Moths that flew for less than 30 s were discarded. If a moth flew for between 30 s and 10 min without responding to the feeders, it was recorded as unresponsive.
(c). Statistical analysis
Feeder choices were analysed using binomial tests against the null hypothesis of random choice (probability of 0.5).
3. Results and discussion
Under starlight, naive M. sexta moths showed a bias towards the bright white-UV feeder irrespective of the background (binomial tests; starlight and white-UV background: p = 0.0407; N = 22; starlight and green background: p = 0.0133; N = 30; figure 1c, upper panel). Conversely, under twilight, the brightest illuminance that M. sexta moths encounter in the wild, naive moths preferred the dark blue feeder, again, without an effect of background (binomial tests; twilight and white-UV background: p = 0.0466; N = 26; twilight and green background: p = 0.0585, N = 23; figure 1c, lower panel). However, under the intermediate moonlight illuminance, we saw a clear background effect on choices: moths showed a bias for the blue feeder when the background was white-UV but preferred the white-UV feeder when the background was dark-green (binomial tests; moonlight and white-UV background: p = 0.028; N = 30; moonlight and green background: p = 0.028; N = 52; figure 1c, middle panel). Innate sensory biases are inherited, yet our results show that they can be modified by the context in which signals are perceived, resulting in different biases as a function of illuminance and background.
Nocturnal hawkmoths have scotopic colour vision, and show colour constancy, but they can also use achromatic contrast, or brightness differences between visual stimuli [9]. A possible explanation for our results would be a switch from using chromatic to achromatic cues depending on illuminance: under twilight, moths preferred blue because of an innate preference based on chromaticity, but under starlight, they displayed an innate preference for the brighter (white-UV) target regardless of target contrast with the background. Finally, under the intermediate moonlight illuminance, naive moths preferred the feeder presenting the highest contrast with the background, using achromatic vision. Considering the variability of flower types, landscapes and light conditions that M. sexta experiences in nature, it is intriguing but not surprising that innate biases are not towards a fixed, specific signal.
Goal-directed behaviours—such as nectar-foraging—are selected according to their efficiency [20]. In this type of purposeful system, sensory information is evaluated according to its functionality, which emerges from selective processes acting on the relationship between the animal and its environment (see [20–22]). For example, it has recently been shown that naive Papilio xuthus butterflies change their innate colour preferences in the presence of some relevant odours [8]. These butterflies, which preferred blue when there were no odours, increased their landing frequency on green discs when stimulated with host plant odours. Females were unmated and did not show oviposition behaviours. Yet, in this study the host plant scent appeared to modulate the behavioural state from a foraging one, with a blue innate preference, towards a reproductive one, with a green innate preference, with the putative function of keeping virgin females close to their host plant, as the authors of the study suggest.
Here, we show an unprecedented effect of the visual environment on innate colour preferences. As we and others have argued, blue objects in nature are almost exclusively flowers and colour vision can mediate their recognition [18]. However, under dimly lit conditions, colour vision is physiologically challenging and moths flying in starlight could forage more efficiently by responding to bright objects. It seems that innate preferences may not be fixed, specific signals, but the result of a contextual integration of sensory information, acting as object class recognition systems that may use cross-modal integration [8,19], and, as shown here, the visual context of potential nectar sources.
Supplementary Material
Supplementary Material
Acknowledgements
We would like to thank William Thornton, Somsak Sukittanon and Dan Comer for helping with the construction of the experimental set-up.
Ethics
Experiments were performed in compliance with current U.S.A. laws and international ethical standards.
Data accessibility
The behavioural data and analysis, and experimental set-up photos, can be found in electronic supplementary material.
Authors' contributions
J.G. and A.K. designed the experiment. W.K., J.W. and J.T. collected data. A.K. performed colour measurements and elaborated the figures. A.K., R.A.R. and J.G. analysed and interpreted the data. W.K., A.K., J.W., J.T., R.A.R. and J.G. wrote the manuscript. All authors approved the final version of the manuscript and agree to be held accountable for the content therein.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by the College of Engineering and Natural Sciences of the University of Tennessee at Martin through the Bill and Roberta Blankenship Endowment for Undergraduate Research.
References
- 1.Lorenz KZ. 1981. The foundations of ethology. New York, NY: Springer-Verlag New York Inc. [Google Scholar]
- 2.Ryan MJ, Fox JH, Wilczynski W, Rand AS. 1990. Sexual selection for sensory exploitation in the frog Physalaemus pustulosus. Nature 343, 66 ( 10.1038/343066a0) [DOI] [PubMed] [Google Scholar]
- 3.Basolo AL. 1990. Female preference predates the evolution of the sword in swordtail fish. Science 250, 808–810. ( 10.1126/science.250.4982.808) [DOI] [PubMed] [Google Scholar]
- 4.Smith C, Barber I, Wootton RJ, Chittka L. 2004. A receiver bias in the origin of three-spined stickleback mate choice. Proc. R. Soc. Lond. B 271, 949–955. ( 10.1098/rspb.2004.2690) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Proctor HC. 1991. Courtship in the water mite Neumania papillator: males capitalize on female adaptations for predation. Anim. Behav. 42, 589–598. ( 10.1016/S0003-3472(05)80242-8) [DOI] [Google Scholar]
- 6.Stökl J, Steiger S. 2017. Evolutionary origin of insect pheromones. Curr. Opin. Insect Sci. 24, 36–42. ( 10.1016/j.cois.2017.09.004) [DOI] [PubMed] [Google Scholar]
- 7.Lunau K, Maier EJ. 1995. Innate color preferences of flower visitors. J. Comp. Physiol. A 177, 1–19. ( 10.1007/BF00243394) [DOI] [Google Scholar]
- 8.Kinoshita M, Stewart FJ, Ômura H. 2017. Multisensory integration in Lepidoptera: insights into flower–visitor interactions. Bioessays 39, 1600086 ( 10.1002/bies.201600086) [DOI] [PubMed] [Google Scholar]
- 9.Kelber A, Balkenius A, Warrant EJ. 2003. Colour vision in diurnal and nocturnal hawkmoths. Integr. Comp. Biol. 43, 571–579. ( 10.1093/icb/43.4.571) [DOI] [PubMed] [Google Scholar]
- 10.Kelber A. 1997. Innate preferences for flower features in the hawkmoth Macroglossum stellatarum. J. Exp. Biol. 200, 827–836. [DOI] [PubMed] [Google Scholar]
- 11.Frisch KV. 1914. Der Farbensinn und Formensinn der Biene. Zool Jb Abt Allg Zool Physiol 35, 1–188. (doi:0.5962/bhl.title.11736) [Google Scholar]
- 12.Borges RM, Somanathan H, Kelber A. 2016. Patterns and processes in nocturnal and crepuscular pollination services. Q. Rev. Biol. 91, 389–418. ( 10.1086/689481) [DOI] [PubMed] [Google Scholar]
- 13.White RH, Stevenson RD, Bennett RR, Cutler DE, Haber WA. 1994. Wavelength discrimination and the role of ultraviolet vision in the feeding-behavior of hawkmoths. Biotropica 26, 427–435. ( 10.2307/2389237) [DOI] [Google Scholar]
- 14.Haber WA, Frankie GW. 1989. A tropical hawkmoth community—Costa Rican dry forest Sphingidae. Biotropica 21, 155–172. ( 10.2307/2388706) [DOI] [Google Scholar]
- 15.Cutler DE, Bennett RR, Stevenson RD, White RH. 1995. Feeding-behavior in the nocturnal moth Manduca sexta is mediated mainly by blue receptors, but where are they located in the retina? J. Exp. Biol. 198, 1909–1917. [DOI] [PubMed] [Google Scholar]
- 16.Kevan PG, Giurfa M, Chittka L. 1996. Why are there so many and so few white flowers? Trends Plant Sci. 1, 280–284. ( 10.1016/1360-1385(96)20008-1) [DOI] [Google Scholar]
- 17.Haber WA, Frankie GW. 1982. Pollination of Luehea (Tiliaceae) in Costa Rican deciduous forest. Ecology 63, 1740–1750. ( 10.2307/1940116) [DOI] [Google Scholar]
- 18.Goyret J, Pfaff M, Raguso RA, Kelber A. 2008. Why do Manduca sexta feed from white flowers? Innate and learnt colour preferences in a hawkmoth. Naturwissenschaften 95, 569–576. ( 10.1007/s00114-008-0350-7) [DOI] [PubMed] [Google Scholar]
- 19.Goyret J, Yuan ML. 2015. Influence of ambient illumination on the use of olfactory and visual signals by a nocturnal hawkmoth during close-range foraging. Integr. Comp. Biol. 55, 486–494. ( 10.1093/icb/icv009) [DOI] [PubMed] [Google Scholar]
- 20.Mayr E. 1988. Toward a new philosophy of biology: observations of an evolutionist. Cambridge, MA: Harvard University Press. [Google Scholar]
- 21.Corning PA, Kline SJ. 1998. Thermodynamics, information and life revisited, Part II: ‘Thermoeconomics’ and ‘Control information’. Syst. Res. Behav. Sci. 15, 453–482. () [DOI] [Google Scholar]
- 22.Corning PA. 2001. ‘Control information’: the missing element in Norbert Wiener's cybernetic paradigm? Kybernetes 30, 1272–1288. ( 10.1108/EUM0000000006552) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The behavioural data and analysis, and experimental set-up photos, can be found in electronic supplementary material.


