Abstract
Dispersal can strongly influence ecological and evolutionary dynamics. Besides the direct contribution of dispersal to population dynamics, dispersers often differ in their phenotypic attributes from non-dispersers, which leads to dispersal syndromes. The consequences of such dispersal syndromes have been widely explored at the population and community level; however, to date, ecosystem-level effects remain unclear. Here, we examine whether dispersing and resident individuals of two different aquatic keystone invertebrate species have different contributions to detrital processing, a key function in freshwater ecosystems. Using experimental two-patch systems, we found no difference in leaf consumption rates with dispersal status of the common native species Gammarus fossarum. In Dikerogammarus villosus, however, a Ponto-Caspian species now expanding throughout Europe, dispersers consumed leaf litter at roughly three times the rate of non-dispersers. Furthermore, this put the contribution of dispersing D. villosus to leaf litter processing on par with native G. fossarum, after adjusting for differences in organismal size. Given that leaf litter decomposition is a key function in aquatic ecosystems, and the rapid species turnover in freshwater habitats with range expansions of non-native species, this finding suggests that dispersal syndromes may have important consequences for ecosystem functioning.
Keywords: amphipods, decomposition, leaf litter, metapopulation, meta-ecosystem, non-random dispersal
1. Introduction
Dispersal, the movement from a natal site to another site or habitat patch with potential consequences for gene flow, is an essential process in ecology and evolution [1,2]. Dispersal connects local populations and allows colonization of new patches, and thus governs the spatial distribution of biodiversity. Although it is often treated as a stochastic event, dispersal between patches is neutral with respect neither to species [3] nor to individuals within species [4]. Within species, individuals may disperse depending on their own phenotype (dispersal syndrome) [5–7]. Across the animal kingdom, dispersing and non-dispersing individuals have identifiable differences in a broad range of phenotypic characteristics [2,4,8,9].
To date, the consequences of dispersal syndromes have primarily been considered at the population and community levels. For example, in Glanville fritillary butterflies, polymorphism in an isomerase gene is such that heterozygotes disperse 70% more often than homozygotes, and because this gene is also associated with differences in clutch size, lifespan and other traits, this contributes to colonization–extinction dynamics [2]. An example of community-level effects is found in western bluebirds, where the increased aggressiveness of dispersers enables them to out-compete mountain bluebirds in patches they colonize [10].
While such correlations are interesting in the context of population and community dynamics, ecosystems could also be impacted by dispersal syndromes, via resource flux, a measure of ecosystem functioning [11]. In fact, some work has demonstrated that dispersers consume resources differently from non-dispersers; for example, mosquitofish that had dispersed between pools in an experimental stream were four times as efficient at reducing prey abundance after arriving in a new location as are non-dispersers, though this effect attenuated over time [12]. However, this finding was framed in a behavioural context only, ignoring potential ecosystem-level effects. Thus, resource dynamics, and resource consumption in particular, are a potentially unexplored consequence of dispersal syndromes on ecosystems [13].
Detritus consumption by detritivores is a strong determinant of decomposition rate, one of the key fluxes in ecosystems [14,15]. Decomposition of organic matter is especially important in freshwater ecosystems, because it enables terrestrial detritus to subsidize the aquatic food web [16], and shredding of leaf litter by invertebrate detritivores is a key step in the decomposition process [17,18]. Here, we used shredding freshwater detritivores to test whether dispersers differ in their leaf litter consumption rate and thus their contribution to ecosystem function. We used one native and one non-native species of amphipod (Crustacea: Amphipoda), a guild of dominant shredding invertebrates in European streams [19]. Amphipod abundance can drive total terrestrial leaf litter shredding [20,21]; however, these two species are functionally non-equivalent in their shredding activity [22–24]. After an initial experiment in which we simulated dispersal by allowing individuals to move between two patches in experimental landscapes, we examined whether dispersers and non-dispersers (henceforth ‘residents’) differed in leaf consumption rates.
2. Material and methods
We used one native amphipod species, Gammarus fossarum (Koch), and one non-native amphipod species, Dikerogammarus villosus (Sowinsky), in our experiments. Gammarus fossarum is very common in headwater streams throughout Switzerland and central Europe [25–27], but also known to co-occur with D. villosus in lakes [27]. We collected adult G. fossarum from the Sagentobelbach stream in Dübendorf, Switzerland (47.39° N, 8.59° E) in November 2016. In the laboratory, amphipods were placed in holding containers of approximately 500 individuals and acclimated to 18°C laboratory conditions for 60 h, and were provided ad libitum alder (Alnus glutinosa (Gaertner)) leaves as food. This was repeated in January 2017 with D. villosus, a Ponto-Caspian species that has expanded into central Europe in the last three decades [28], with individuals collected from Lake Constance at Kesswil, Switzerland (47.60° N, 9.32° E), where the species is known to co-occur with G. fossarum in the lake, and where G. fossarum is also found in stream populations [27]. For each species, the experiment was conducted in two steps: a dispersal experiment followed by a leaf consumption experiment. Experimental protocols, including length of dispersal phase and length of consumption experiment, were adapted depending on the species' activity levels and consumption rates, based on pilot experiments. Gammarus fossarum used in the experiment had a mean dry weight of 3.30 mg (s.d. ± 1.33), and D. villosus had a mean dry weight of 8.59 mg (s.d. ± 2.60).
(a). Dispersal experiment
A common method for examining the causes and consequences of dispersal is to allow organisms to disperse through linked experimental patches ranging from two-patch pairings [29,30] to larger grids or networks [31,32]. The dispersal experiments were run according to the Dispersal Network (DispNet) distributed experiment protocol, detailed in [29]. Briefly, we set up 40 replicates of a two-patch mesocosm system in order to address rates of amphipod dispersal from one to the other patch. The experiment had a factorial design of resource availability (alder leaves versus no food) and predator cues (fish kairomones versus no kairomones) in the patch of origin, with each experimental context replicated 10 times. Because we found no effect of the resource or predator cue context on dispersal rates in amphipods [29]—perhaps in part because some gammarid amphipods have a hiding response to predator cues [33,34]—we here pooled all data from the different treatments together and only considered the effect of dispersal status (dispersed versus resident individuals) on subsequent leaf consumption. Residuals from the models (described below) confirmed that no additional variation in leaf consumption rates was explained by experimental context/treatment (electronic supplementary material, figure S1).
Each patch was a 3 l (198 × 198 mm) polypropylene box, and each pair of patches (one ‘origin’ and one ‘target’ patch, with their relative positions randomized) was connected by 30 cm of silicone tubing with 20 mm diameter. There are few published estimates of dispersal distances by gammarid amphipods. Dispersal can occur by drift or upstream movement. Humphries & Ruxton [35] used a modelling approach based on data from Elliott [36] to estimate that Gammarus pulex, a related taxon, drifted an average of 2.25 m per drift event, which lasted on average 8 s; across a wider range of taxa, distances of drift events are often estimated between 2 and 10 m [37]. Hughes [38] used a laboratory experiment, estimating upstream movement by G. pulex from 4 to 21 cm h−1. Thus, our 30 cm connection tube is shorter than many dispersal events, but within the same order of magnitude of movements that might be expected over a time frame of a few hours. Furthermore, we focused on emigration decisions as the component of dispersal to measure, rather than travel or settlement decisions. We thus designed the experimental landscape such that the tubing represented a hostile matrix different from the mesocosms themselves, to try to ensure that the decision to swim between patches did not represent only routine foraging movement. To accomplish this, patches were covered with a black lid to reduce light permeability, while the connection tube was left uncovered; this light difference between patches and matrix rendered the connection tube a hostile matrix, because amphipods are photophobic [39]. We thus assume that movement between patches in our experimental landscape will correlate with dispersal.
Twenty amphipods were placed in each origin patch and allowed to habituate for 30 min. We then opened a clamp that had been used to close the connection and amphipods could disperse for a period of 4.5 h (G. fossarum) or 7 h (D. villosus) before the connection tube was closed again. To confirm that relocation from the origin to target patch was not simply due to routine movement in the course of foraging, but represented dispersal decisions, we also measured movement (gross swimming speed, extracted from videos of the animals using the ‘BEMOVI’ package [40] in R) of residents and dispersers, and found that speed was not correlated with dispersal status (electronic supplementary material, figure S2).
(b). Consumption experiment
After the dispersal experiment, amphipods were transferred to new single-patch mesocosms (2 l plastic containers with 0.4 m2 of substrate area, placed on racks, with a constant trickling inflow/outflow of water) to measure leaf litter consumption. The density of amphipods used in the leaf consumption experiment was standardized between dispersers and residents to account for possible effects of density on leaf consumption rates [41]. Thus, from each two-patch system, all dispersers were moved to one new mesocosm (G. fossarum: mean 3.6 ± s.d. 2.0 dispersers; D. villosus: 1.1 ± 0.3 dispersers), and an identical number of haphazardly chosen residents was moved to a separate new mesocosm. Densities remained highly correlated at the replicate block level throughout the experiment (G. fossarum: r = 0.89, p < 0.001; D. villosus: r = 0.53, p = 0.05). Mesocosms were provisioned with 1.5 g (dry weight) of conditioned alder leaves. All leaves used in the experiment originated from the same batch (see electronic supplementary material). The leaf consumption experiments were run for 19 (G. fossarum) and 12 (D. villosus) days, respectively—when a visual estimate suggested that 50% of leaf litter was remaining in the most quickly consumed mesocosms—at which point leaves from the mesocosms were collected and dried for 48 h at 60°C, then weighed to calculate mass loss from the beginning of the experiment. Microbial and fungal decomposition was not explicitly considered because previous experiments showed it to be negligible over these time periods (see electronic supplementary material; [24]). Amphipods were counted every 2–3 days throughout the experiments to track mortality; overall, survival was 76.3% for G. fossarum and 95.4% for D. villosus. These mortality estimates were used to calculate an average daily amphipod density for each mesocosm over the length of the experiment (see electronic supplementary material). At the end of the experiment, amphipods were sacrificed and dried for 48 h at 60°C. The average daily biomass in a mesocosm (mg m–2) was then calculated as the average daily density (above) multiplied by the average weight of individuals in the mesocosm. Leaf consumption rates were calculated as the dry weight of leaf litter consumed per milligram of amphipod dry weight per day.
(c). Analysis
Consumption rates were compared between residents and dispersers of each species separately using linear mixed-effects models with the ‘lme4’ package, v. 1.1-18-1 [42], in R v. 3.5.0 (R Core Team, Vienna, Austria, 2018; https://cran.r-project.org/). Distributions of consumption rates were positively skewed. To meet assumptions regarding error structure, the data were square-root transformed. For both species, the response was modelled with dispersal status (disperser versus resident) and density as fixed factors, and replicate block (the two-patch experimental metapopulation from which dispersers and residents originated) as a random intercept. The replicate block accounted for all potential differences associated with the experimental metapopulation of origin. After building the mixed-effect models, marginal R2 (attributable for fixed effects only) and conditional R2 values (accounting for both random and fixed effects) were calculated using the ‘MuMIn’ package, v. 1.42.1 [43]. Differences in consumption rates between dispersers and residents were tested using the Tukey HSD test using the ‘multcomp’ package, v. 1.4-8 [44].
3. Results
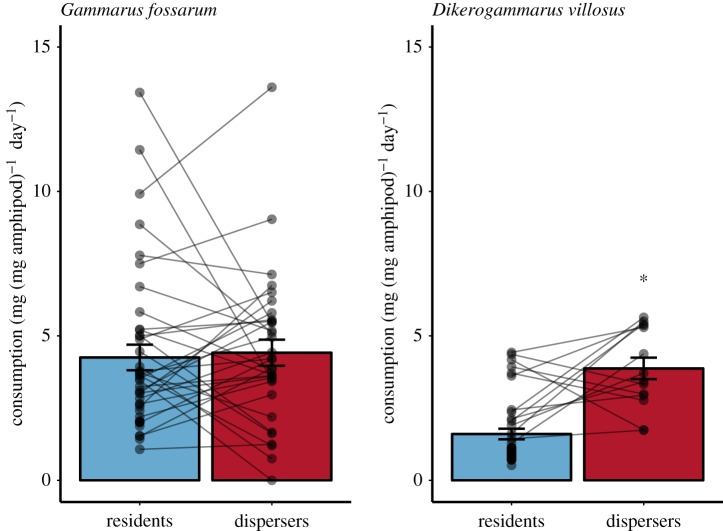
For both species, higher density of amphipods had a negative influence on biomass-adjusted leaf consumption rates (table 1). For G. fossarum, the estimated difference between square-root transformed daily consumption rates of residents and dispersers was not significantly different from zero (figure 1 and table 1). For D. villosus, dispersal status was associated with differences in leaf consumption rate (table 1). Post hoc testing indicated that dispersing D. villosus had significantly higher consumption rates than residents (Tukey's HSD: mean difference in square-root transformed daily consumption = 0.231, z = −2.52, p = 0.01; figure 1). Dispersing D. villosus had biomass-adjusted consumption rates similar to G. fossarum, and approximately three times higher than non-dispersing D. villosus (figure 1).
Table 1.
Results from the linear mixed-effects models of biomass-adjusted consumption rates as a function of dispersal status and density, for G. fossarum (n = 73 mesocosms) and D. villosus (n = 53). Estimates and their standard errors are drawn from linear mixed-effects models. Variance associated with the random factor of replicate blocks, and its standard deviation, are reported in italics. Marginal R2 (associated with fixed factors only) and conditional R2 (associated with fixed and random factors) are reported for each model.
| coefficient | std error/std dev. | |
|---|---|---|
| G. fossarum (marginal R2 = 0.18, conditional R2 = 0.46) | ||
| intercept (residents) | 2.494 | 0.171 |
| dispersers | −0.002 | 0.081 |
| density | −0.009 | 0.002 |
| variance due to replicates | 0.119 | 0.345 |
| D. villosus (marginal R2 = 0.78, conditional R2 = 0.78) | ||
| intercept (residents) | 2.102 | 0.068 |
| dispersers | 0.163 | 0.065 |
| density | −0.022 | 0.002 |
| variance due to replicates | 0 | 0 |
Figure 1.
Daily average leaf litter consumption by dispersing and non-dispersing (resident) amphipods of G. fossarum (n = 73 mesocosms) and D. villosus (n = 53), adjusted for biomass of the individuals in each experimental replicate. Error bars show standard error of the mean, and grey dots show raw data points from experimental mesocosms; lines connect observations from resident and disperser populations from the same experimental patch pair of the original dispersal experiment. Asterisk shows a significant difference (p < 0.05) between consumption rates of dispersers and residents according to a linear mixed-effect model. (Online version in colour.)
4. Discussion
We identified a dispersal syndrome with consequences for ecosystem functioning in a non-native but not in a native amphipod species: D. villosus dispersers consumed leaf litter at roughly three times the rate of residents, while there was no difference in leaf consumption rate with dispersal status in G. fossarum. To date, most research addressing consumption rates in relation to dispersal status or range fronts has been in a behavioural context, addressing personality and aggression as contributions to predator–prey interactions [12,45,46], for example. To our knowledge, there has been little research into consumption of basal resources as a component of non-random dispersal. This is despite the importance of such traits to energy flows through food webs and ecosystems. Furthermore, differences in traits that may depend on resource consumption—such as size, metabolism and growth rates [2,8]—with dispersal propensity render resource consumption a logical component of a dispersal phenotype, and thus one that could have consequences for energy fluxes through food webs and ecosystems.
Our study species are omnivorous aquatic invertebrates, which despite a wide diet breadth contribute the bulk of leaf litter processing in central European headwater streams [20]. Our results show that in D. villosus, dispersers make a greater contribution to the detritus-based pathway integrating terrestrial energy into the food web than do residents. This species also has lower overall contributions to leaf litter processing than G. fossarum [22–24], but we suggest that both species identity and dispersal status of individuals within a species could jointly determine their contribution to ecosystem function. Interestingly, leaf consumption rates declined with density for both species, consistent with previous experimental work [41], but the association between dispersal status and leaf consumption rate in D. villosus was independent of the effect of density. Given that dispersers are likely to be in low densities in their new habitats, this could create interesting synergistic effects.
Predicting these populations' contributions to ecosystem function is important because D. villosus has been deemed one of the 100 worst invaders in European freshwater ecosystems [47]. Because the non-native species is currently undergoing a range expansion, the signature of either trade-offs for increased dispersal ability or selection for success in new habitats is likely more prominent than in populations that are in their range core (such as the G. fossarum populations used in our experiment), consistent with spatial selection theory [48]. Identifying whether this is true or whether the dispersal syndrome is consistent across the range of D. villosus would require performing experiments with D. villosus from its range core in the Ponto-Caspian region. This would also address whether it is appropriate to make interspecific comparisons of this and other phenotypic traits using populations with different recent dispersal/range expansion histories, depending on the research question.
Regardless, how non-native species will affect ecosystem function is a central question in an era of global change and increased connectivity [49]. As the distribution of suitable habitat is altered and human activity continues to contribute to global organismal dispersal, the potential effects of phenotype-dependent dispersal should be considered when attempting to predict impacts on ecosystem function. This may be challenging, because it means that predictions made based on species contributions to ecosystem function in their range core may not be valid at the edges of their range expansions [49]. However, considering the increasing evidence of how dispersal phenotypes can alter system dynamics, it is crucial to extend this understanding into the realm of ecosystem function.
Supplementary Material
Acknowledgements
The authors thank Samuel Hürlemann, Remo Wüthrich, Georg Flückiger and Sascha Brunner for help in the laboratory and field. We thank Felix Moerman, Heidi Kaech, Corinne Hertäg and two anonymous reviewers for comments on the manuscript. We thank the members of DispNet for the collaboration that led to this experiment. This is publication ISEM-2019-002 of the Institut des Sciences de l'Evolution—Montpellier.
Data accessibility
Data are available via the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.8b9j040 [50]. Code used to clean data, run analyses and produce figures is available at https://github.com/chelseajlittle/DispNetConsumption.
Authors' contributions
E.A.F. conceived the dispersal experiment and all authors together designed the consumption experiment. C.J.L. and E.A.F. ran the experiments. C.J.L. analysed the data and drafted the manuscript. All authors contributed to revisions, gave final approval for publication and agreed to be held accountable for the work within the article.
Competing interests
We have no competing interests.
Funding
Funding is from the Swiss National Science Foundation Grant no. PP00P3_179089 and the University of Zurich Research Priority Programme URPP Global Change and Biodiversity (to F.A.).
References
- 1.Ronce O. 2007. How does it feel to be like a rolling stone? Ten questions about dispersal evolution. Annu. Rev. Ecol. Evol. Syst. 38, 231–253. ( 10.1146/annurev.ecolsys.38.091206.095611) [DOI] [Google Scholar]
- 2.Hanski IA. 2011. Eco-evolutionary spatial dynamics in the Glanville fritillary butterfly. Proc. Natl Acad. Sci. USA 108, 14 397–14 404. ( 10.1073/pnas.1110020108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lowe WH, McPeek MA. 2014. Is dispersal neutral? Trends Ecol. Evol. 29, 444–450. ( 10.1016/j.tree.2014.05.009) [DOI] [PubMed] [Google Scholar]
- 4.Clobert J, Le Galliard JF, Cote J, Meylan S, Massot M.. 2009. Informed dispersal, heterogeneity in animal dispersal syndromes and the dynamics of spatially structured populations. Ecol. Lett. 12, 197–209. ( 10.1111/j.1461-0248.2008.01267.x) [DOI] [PubMed] [Google Scholar]
- 5.Bowler DE, Benton TG. 2005. Causes and consequences of animal dispersal strategies: relating individual behaviour to spatial dynamics. Biol. Rev. 80, 205–225. ( 10.1017/S1464793104006645) [DOI] [PubMed] [Google Scholar]
- 6.O'Riain MJ, Jarvis JUM, Faulkes CG. 1996. A dispersive morph in the naked mole-rat. Nature 380, 619–621. ( 10.1038/380619a0) [DOI] [PubMed] [Google Scholar]
- 7.Sih A, Cote J, Evans M, Fogarty S, Pruitt J. 2012. Ecological implications of behavioural syndromes. Ecol. Lett. 15, 278–289. ( 10.1111/j.1461-0248.2011.01731.x) [DOI] [PubMed] [Google Scholar]
- 8.Stevens VM, et al. 2014. A comparative analysis of dispersal syndromes in terrestrial and semi-terrestrial animals. Ecol. Lett. 17, 1039–1052. ( 10.1111/ele.12303) [DOI] [PubMed] [Google Scholar]
- 9.Fronhofer EA, Altermatt F. 2015. Eco-evolutionary feedbacks during experimental range expansions. Nat. Commun. 6, 1–9. ( 10.1038/ncomms7844) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duckworth A, Badyaev AV. 2007. Coupling of dispersal and aggression facilitates the rapid range expansion of a passerine bird. Proc. Natl Acad. Sci. USA 104, 15 017–15 022. ( 10.1073/pnas.0706174104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barnes AD, Jochum M, Lefcheck JS, Eisenhauer N, Scherber C, O'Connor MI, de Ruiter P, Brose U. 2018. Energy flux: the link between multitrophic biodiversity and ecosystem functioning. Trends Ecol. Evol. 33, 186–197. ( 10.1016/j.tree.2017.12.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cote J, Brodin T, Fogarty S, Sih A. 2017. Non-random dispersal mediates invader impacts on the invertebrate community. J. Anim. Ecol. 86, 1298–1307. ( 10.1111/1365-2656.12734) [DOI] [PubMed] [Google Scholar]
- 13.Massol F, Altermatt F, Gounand I, Gravel D, Leibold MA, Mouquet N. 2017. How life-history traits affect ecosystem properties: effects of dispersal in meta-ecosystems. Oikos 126, 532–546. ( 10.1111/oik.03893) [DOI] [Google Scholar]
- 14.Cebrian J. 1999. Patterns in the fate of production in plant communities. Am. Nat. 154, 449–468. ( 10.1086/303244) [DOI] [PubMed] [Google Scholar]
- 15.Krishna MP, Mohan M. 2017. Litter decomposition in forest ecosystems: a review. Energy Ecol. Environ. 2, 236–249. ( 10.1007/s40974-017-0064-9) [DOI] [Google Scholar]
- 16.Webster JR, Benfield EF. 1986. Vascular plant breakdown in freshwater ecosystems. Annu. Rev. Ecol. Syst. 17, 567–594. ( 10.1146/annurev.es.17.110186.003031) [DOI] [Google Scholar]
- 17.Graça MAS. 2001. The role of invertebrates on leaf litter decomposition in streams—a review. Int. Rev. Hydrobiol. 86, 383–393. () [DOI] [Google Scholar]
- 18.Tonin AM, Pozo J, Monroy S, Basaguren A, Pérez J, Gonçalves JF, Pearson R, Cardinale BJ, Boyero L. 2018. Interactions between large and small detritivores influence how biodiversity impacts litter decomposition. J. Anim. Ecol. 87, 1465–1474. ( 10.1111/1365-2656.12876) [DOI] [PubMed] [Google Scholar]
- 19.Pöckl M, Webb BW, Sutcliffe DW. 2003. Life history and reproductive capacity of Gammarus fossarum and G. roeseli (Crustacea: Amphipoda) under naturally fluctuating water temperatures: a simulation study. Freshw. Biol. 48, 53–66. ( 10.1046/j.1365-2427.2003.00967.x) [DOI] [Google Scholar]
- 20.Macneil C, Dick JTA, Elwood RW. 1997. The trophic ecology of freshwater Gammarus spp. (Crustacea: Amphipoda): problems and perspectives concerning the functional feeding group concept. Biol. Rev. 72, 349–364. ( 10.1111/j.1469-185X.1997.tb00017.x) [DOI] [Google Scholar]
- 21.Piscart C, Genoel R, Doledec S, Chauvet E, Marmonier P. 2009. Effects of intense agricultural practices on heterotrophic processes in streams. Environ. Pollut. 157, 1011–1018. ( 10.1016/j.envpol.2008.10.010) [DOI] [PubMed] [Google Scholar]
- 22.Piscart C, Mermillod-Blondin F, Maazouzi C, Merigoux S, Marmonier P. 2011. Potential impact of invasive amphipods on leaf litter recycling in aquatic ecosystems. Biol. Invasions 13, 2861–2868. ( 10.1007/s10530-011-9969-y) [DOI] [Google Scholar]
- 23.Jourdan J, Westerwald B, Kiechle A, Chen W, Streit B, Klaus S, Oetken M, Plath M. 2016. Pronounced species turnover, but no functional equivalence in leaf consumption of invasive amphipods in the River Rhine. Biol. Invasions 18, 763–774. ( 10.1007/s10530-015-1046-5) [DOI] [Google Scholar]
- 24.Little CJ, Altermatt F. 2018. Species turnover and invasion of dominant freshwater invertebrates alter biodiversity–ecosystem–function relationship. Ecol. Monogr. 88, 461–480. ( 10.1002/ecm.1299) [DOI] [Google Scholar]
- 25.Altermatt F, Alther R, Fišer C, Jokela J, Konec M, Küry D, Mächler E, Stucki P, Westram AM. 2014. Diversity and distribution of freshwater amphipod species in Switzerland (Crustacea: Amphipoda). PLoS One 9, e110328 ( 10.1371/journal.pone.0110328) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Little CJ, Altermatt F. 2018. Do priority effects outweigh environmental filtering in a guild of dominant freshwater macroinvertebrates? Proc. R. Soc. B 285, 20180205 ( 10.1101/216267) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Altermatt F, Alther R, Mächler E. 2016. Spatial patterns of genetic diversity, community composition and occurrence of native and non-native amphipods in naturally replicated tributary streams. BMC Ecol. 16, 23 ( 10.1186/s12898-016-0079-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van den Brink FWB, Van der Velde G, Bij de Vaate A. 1991. Amphipod invasion on the Rhine. Nature 352, 576 ( 10.1038/352576a0) [DOI] [Google Scholar]
- 29.Fronhofer EA, et al. 2018. Bottom-up and top-down control of dispersal across major organismal groups: a coordinated distributed experiment. Nat. Ecol. Evol. 2, 1859–1863. ( 10.1038/s41559-018-0686-0) [DOI] [PubMed] [Google Scholar]
- 30.Fronhofer EA, Klecka J, Melián CJ, Altermatt F. 2015. Condition-dependent movement and dispersal in experimental metacommunities. Ecol. Lett. 18, 954–963. ( 10.1111/ele.12475) [DOI] [PubMed] [Google Scholar]
- 31.Altermatt F, Fronhofer EA. 2018. Dispersal in dendritic networks: ecological consequences on the spatial distribution of population densities. Freshw. Biol. 63, 22–32. ( 10.1111/fwb.12951) [DOI] [Google Scholar]
- 32.Legrand D, et al. 2012. The Metatron: an experimental system to study dispersal and metaecosystems for terrestrial organisms. Nat. Methods 9, 828–833. ( 10.1038/nmeth.2104) [DOI] [PubMed] [Google Scholar]
- 33.Wooster DE. 1998. Amphipod (Gammarus minus) responses to predators and predator impact on amphipod density. Oecologia 115, 253–259. ( 10.1007/s004420050514) [DOI] [PubMed] [Google Scholar]
- 34.Williams KL, Navins KC, Lewis SE. 2016. Behavioral responses to predation risk in brooding female amphipods (Gammarus pseudolimnaeus). J. Freshw. Ecol. 31, 571–581. ( 10.1080/02705060.2016.1196464) [DOI] [Google Scholar]
- 35.Humphries S, Ruxton GD. 2003. Estimation of intergenerational drift dispersal distances and mortality risk for aquatic macroinvertebrates. Limnol. Oceanogr. 48, 2117–2124. ( 10.4319/lo.2003.48.6.2117) [DOI] [Google Scholar]
- 36.Elliott JM. 2002. The drift distances and time spent in the drift by freshwater shrimps, Gammarus pulex, in a small stony stream, and their implications for the interpretation of downstream dispersal. Freshw. Biol. 47, 1403–1417. ( 10.1046/j.1365-2427.2002.00874.x) [DOI] [Google Scholar]
- 37.Naman SM, Rosenfeld JS, Richardson JS. 2016. Causes and consequences of invertebrate drift in running waters: from individuals to populations and trophic fluxes. Can. J. Fish. Aquat. Sci. 73, 1292–1305. ( 10.1139/cjfas-2015-0363) [DOI] [Google Scholar]
- 38.Hughes DA. 1970. Some factors affecting drift and upstream movements of Gammarus pulex. Ecology 51, 301–305. ( 10.2307/1933668) [DOI] [Google Scholar]
- 39.David M, Salignon M, Perrot-Minnot MJ. 2014. Shaping the antipredator strategy: flexibility, consistency, and behavioral correlations under varying predation threat. Behav. Ecol. 25, 1148–1156. ( 10.1093/beheco/aru101) [DOI] [Google Scholar]
- 40.Pennekamp F, Schtickzelle N, Petchey OL. 2015. BEMOVI, software for extracting behavior and morphology from videos, illustrated with analyses of microbes. Ecol. Evol. 5, 2584–2595. ( 10.1002/ece3.1529) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Little CJ, Fronhofer EA, Altermatt F. 2018. Nonlinear effects of intraspecific competition alter landscape-wide upscaling of ecosystem function. bioRXiv ( 10.1101/470591) [DOI] [PubMed] [Google Scholar]
- 42.Bates D, Maechler M, Bolker B. 2015. lme4: linear mixed-effects models using S4 classes. R package version 0.999999-0. See http://CRAN.R-project.org/package=lme4.
- 43.Barton K. 2013. MuMIn: multi-model inference. Vienna, Austria: R Foundation for Statistical Computing. See http://CRAN.R-project.org/package=MuMIn, 2013.
- 44.Hothorn T, Bretz F, Westfall P. 2008. Simultaneous inference in general parametric models. Biometrical J. 50, 346–363. ( 10.1002/bimj.200810425) [DOI] [PubMed] [Google Scholar]
- 45.Pintor LM, Sih A, Bauer ML. 2008. Activity and boldness between native and introduced populations of an invasive crayfish. Oikos 117, 1629–1636. ( 10.1016/j.medengphy.2008.01.010) [DOI] [Google Scholar]
- 46.Juette T, Cucherousset J, Cote J. 2014. Animal personality and the ecological impacts of freshwater non-native species. Curr. Zool. 60, 417–427. ( 10.1093/czoolo/60.3.417) [DOI] [Google Scholar]
- 47.Rewicz T, Grabowski M, MacNeil C, Bacela-Spychalska K. 2014. The profile of a ‘perfect’ invader—the case of killer shrimp, Dikerogammarus villosus. Aquat. Invasions 9, 267–288. ( 10.3391/ai.2014.9.3.04) [DOI] [Google Scholar]
- 48.Phillips BL, Brown GP, Shine R. 2010. Life-history evolution in range-shifting populations. Ecology 91, 1617–1627. ( 10.1890/09-0910.1) [DOI] [PubMed] [Google Scholar]
- 49.Strayer DL. 2012. Eight questions about invasions and ecosystem functioning. Ecol. Lett. 15, 1199–1210. ( 10.1111/j.1461-0248.2012.01817.x) [DOI] [PubMed] [Google Scholar]
- 50.Little CJ, Fronhofer EA, Altermatt F. 2019. Data from: Dispersal syndromes can impact ecosystem functioning in spatially structured freshwater populations Dryad Digital Repository. ( 10.5061/dryad.8b9j040) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Little CJ, Fronhofer EA, Altermatt F. 2019. Data from: Dispersal syndromes can impact ecosystem functioning in spatially structured freshwater populations Dryad Digital Repository. ( 10.5061/dryad.8b9j040) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data are available via the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.8b9j040 [50]. Code used to clean data, run analyses and produce figures is available at https://github.com/chelseajlittle/DispNetConsumption.