Abstract
Stress exposure can leave long-term footprints within the organism, like in telomeres (TLs), protective chromosome caps that shorten during cell replication and following exposure to stressors. Short TLs are considered to indicate lower fitness prospects, but why TLs shorten under stressful conditions is not understood. Glucocorticoid hormones (GCs) increase upon stress exposure and are thought to promote TL shortening by increasing oxidative damage. However, evidence that GCs are pro-oxidants and oxidative stress is causally linked to TL attrition is mixed. Based on new biochemical findings, we propose the metabolic telomere attrition hypothesis: during times of substantially increased energy demands, TLs are shortened as part of the transition into an organismal ‘emergency state’, which prioritizes immediate survival functions over processes with longer-term benefits. TL attrition during energy shortages could serve multiple roles including amplified signalling of cellular energy debt to re-direct critical resources to immediately important processes. This new view of TL shortening as a strategy to resolve major energetic trade-offs can improve our understanding of TL dynamics. We suggest that TLs are master regulators of cell homeostasis and propose future research avenues to understand the interactions between energy homeostasis, metabolic regulators and TL.
Keywords: glucocorticoid, telomere, energetic stress, life-history trade-off, metabolism, TOR
1. Introduction
All living beings, from unicellular organisms to humans, have to endure challenges in their daily lives. To overcome the strains of nutrient shortage, competition, intense work load or psychological pressure, organisms have evolved a complex array of physiological and behavioural solutions. Nevertheless, major challenges can leave long-term footprints in the organisms' functioning, affecting life-history decisions such as the relative investment in growth, fecundity, somatic protection and longevity. One biomarker that has been used to assess the cumulative effects of conditions experienced in early and late life is the length of telomeres (TLs) [1–5].
TLs are complexes comprising DNA repeats of (T2AG3)n, proteins and RNA, which protect chromosomes from degradation caused by an incomplete DNA replication. This occurs because during replication, the ends of linear chromosomes cannot fully be copied, leading to a continuous shortening of these DNA repeats with each round of replication [6]. A critical lower threshold of erosion can result in severely compromised cells, including DNA damage responses, cellular senescence, arrest of cell replication and apoptosis [7–9]. TL maintenance mechanisms exist, but often are unable to prevent their attrition [10–13]. Short TL lengths or high TL attrition rates in early life and adulthood are associated with lower life expectancy and higher disease risk in various species including humans [14–22]. However, recent reviews found the evidence for a causal role of TLs in organismal deterioration including fitness to be mixed in humans [23] and non-model animal species [24]. It has been recently proposed that for fitness not only TL length matters, but also the state of the protein and RNA structures surrounding TLs [25–29].
Findings of age-related gradual declines in TL length in proliferative (e.g. germinal cells, stem cells) and quiescent tissues (e.g. cardiac muscle, liver and pancreas) indicate that the rate of cell division cannot entirely explain TL dynamics [30]. One mechanism currently considered to be a second major cause of TL attrition is cellular oxidative stress [31–34], which can arise when the concentration of pro-oxidant molecules in the organism is high relative to that of antioxidant substances [35]. This condition can result primarily from increased metabolic rate but also from other processes including inflammation or exposure to exogenous noxious compounds [36–39]. TLs are thought to be particularly sensitive to oxidative insult because they contain many guanine triplet sequences, which are more vulnerable to oxidation compared to other bases [31]. However, although biochemical evidence for a negative association between oxidative stress and TL length exists in vitro [31], many field studies in intact organisms have not been able to find such patterns [40] (but see [32,34]). A caveat of the hypothesis that oxidative stress causes TL attrition is that the consequences of oxidative damage should primarily become evident mostly during cell division, when the damaged DNA has to be replicated [32,41], irrespective of whether this damage happened before or during the replication. This mechanism can therefore not fully explain stress-induced TL attrition in post-mitotic tissues where cell replication is rare or absent [42]. The high correlation in TL lengths among tissues with different rates of cell division also supports the notion that TL dynamics are not caused by cell replication alone [43].
Recent reports have highlighted interactions of TLs with key metabolic pathways of cells [30,44–46] (figure 1; further details below). Based on this evidence, we propose the metabolic telomere attrition hypothesis, which suggests that energetic limitations will be an additional cause of TL attrition. Biochemical evidence has also suggested a signalling function of TLs within metabolic pathways [7,30,46,47], providing a scope for TLs to convey critical information on cellular states for life-history decisions. Such TL functions would be in line with the recently advanced hypotheses that (i) TL attrition can be adaptive because TLs are costly to maintain (costly maintenance hypothesis), and (ii) because the signalling function of TL attrition can regulate life-history decisions according to cell integrity (functional telomere attrition hypothesis) [48]. The metabolic telomere attrition hypothesis goes beyond these proposed explanations by introducing specific metabolic processes as potential regulators of TL dynamics (table 1).
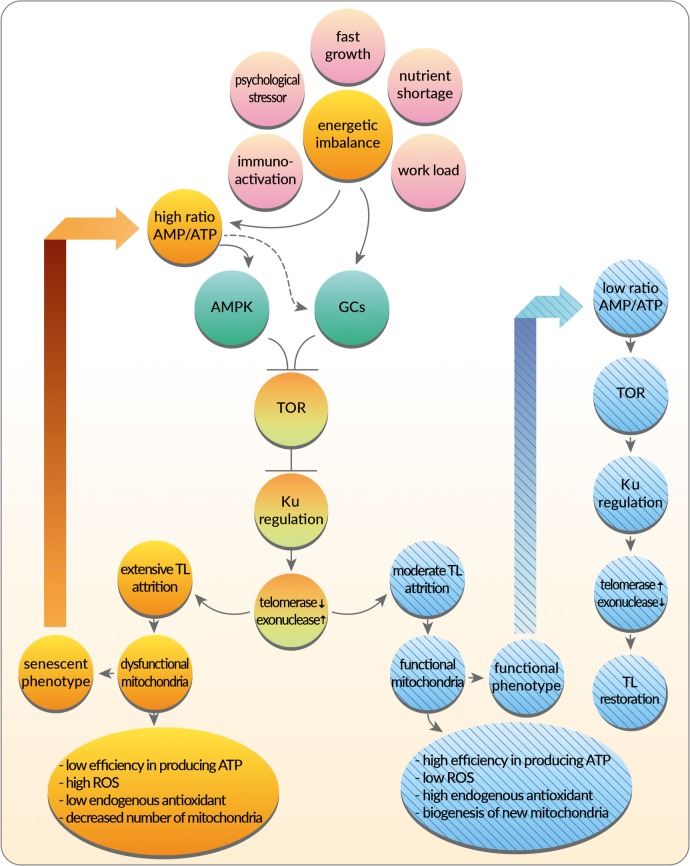
Figure 1.
Simplified illustration of the main biochemical pathways for a metabolic control of TL length as proposed by the metabolic telomere attrition hypothesis. Immune activation, psychological stressors, growth, nutrient shortage and work load can negatively affect the cellular energy balance, leading to increased concentrations of metabolic regulators like glucocorticoids (GCs) and AMP-activated kinase (AMPK), partly through low ATP levels (i.e. at high AMP/ATP ratios, which activate AMPK). AMPK can inhibit the kinase TOR (target of rapamycin), which downregulates enzymes and proteins that maintain TL length. Likewise, GCs may also be able to inhibit TOR via REDD1 (‘Regulated in development and DNA Damage’ response proteins). TOR inhibition in turn suppresses Ku-proteins, which inhibit the TL maintenance enzyme telomerase while increasing exonuclease concentrations and thus the excision of nucleotides from TLs. Extensive TL shortening can cause ageing processes (dysfunctional mitochondria and cellular senescence pathway—left non-barred/yellow loop), while moderate TL attrition can promote immediate survival functions (functional phenotype pathway—right barred-blue loop). For example, when low levels of protein p53 are secreted, they promote moderate TL attrition, leading to mitochondrial functionality that includes increased concentrations of enzymatic antioxidants, improved oxidative phosphorylation and the generation of new mitochondria. By contrast, at high concentrations p53 disrupt mitochondria function, thereby decreasing cellular ATP levels while increasing the concentrations of oxidative molecules by depleting and/or downregulating antioxidant enzymes. The latter pathway is part of the ageing circuitry. Solid arrows, known activating processes; dashed arrows, proposed activating processes; black ‘Ts’, inhibiting processes. (Online version in colour.)
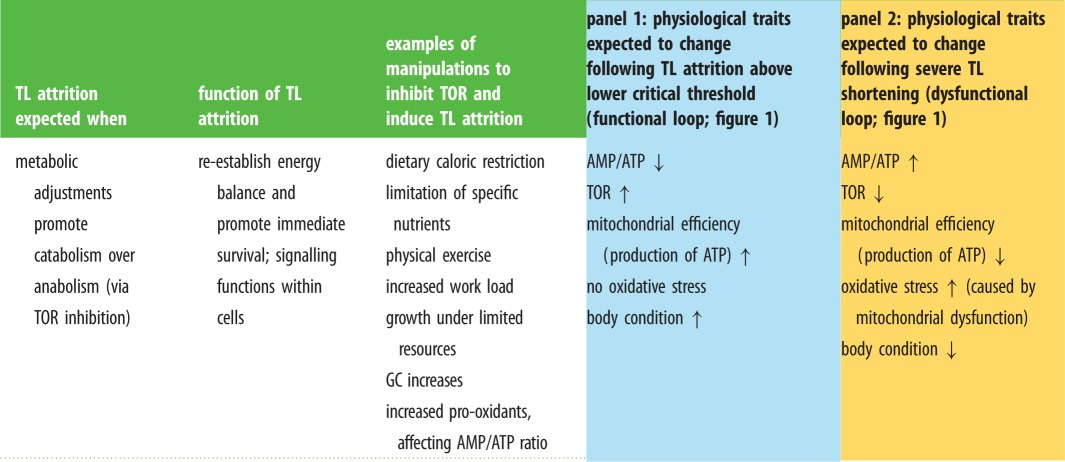
Table 1.
Assumptions, predictions and experimental tests of the metabolic telomere attrition hypothesis. Note that when TL attrition is moderate (pale blue panel 1), no oxidative stress is expected (in contrast to predictions of the oxidative stress telomere attrition hypothesis). When TL attrition is severe (yellow panel 2), oxidative damage is expected to increase (similarly to the oxidative stress telomere attrition hypothesis). However, the mechanisms envisioned for the association between oxidative stress and TL attrition differ between the two hypotheses, with the metabolic telomere attrition hypothesis viewing oxidative damage more as a consequence of TL attrition than a cause. TOR, target of rapamycin; GC, glucocorticoid; AMP/ATP: adenosine monophosphate/adenosine triphosphate.
 |
(a). Glucocorticoid-induced telomere attrition to cope with energy imbalances?
TL loss can be promoted by stressors like energy limitation, work load, disease, oxidative imbalance or noxious stimuli [4,49–51], which often result in an upregulation of glucocorticoid hormones (GCs [52–56]). GCs have fundamental metabolic functions by stimulating gluconeogenesis, lipolysis and proteolysis, by modulating the immune system, and by regulating the synthesis and action of a number of hormones, kinases and other enzymes [52,57,58]. GCs have extensive pleiotropic actions on metabolic processes, and during times of increased energy demands, activate specific pathways to release endogenous energy stores while inhibiting processes that are not essential to cope with major challenges [52]. Elevated GC levels can cause TL shortening in vertebrates including humans (reviewed in [54,59]). The effects of GCs on TLs are particularly evident during development, an energetically highly demanding phase of life. For example, endogenously or exogenously elevated GC levels reduced TL lengths in nestlings from different avian species [60,61]. Similar effects were found when GC exposure occurred before birth, either when bird eggs were injected with GCs [62] or when maternal GC concentrations were experimentally elevated [63]. The accepted explanation for this phenomenon is that elevated GCs increase cellular oxidative stress [54,59] by downregulating endogenous antioxidant defences and/or increasing the production of reactive oxygen molecules [54,59,64]. However, recent experimental studies that investigated the potential of GCs to promote oxidative stress were not able to find such an effect [65–68]. GCs can also impair TL lengths by downregulating the expression of telomerase, the enzyme that can counteract the loss of telomeric nucleotides. However, to our knowledge, only one study thus far has examined a GC-induced inhibition of telomerase transcription, but without assessing consequences for TL length [69]. Since telomerase also has functions that are unrelated to TL maintenance, e.g. to protect mitochondria from oxidative stress [70,71], at present there is no conclusive evidence that GCs shorten TLs by suppressing telomerase. However, as outlined above, GCs interact intimately with metabolic processes and are typically upregulated during times of high energy need to mobilize internal resources, often at the deficit of other processes that also require these resources as fuel.
2. The metabolic telomere attrition hypothesis
Biochemists recently proposed the existence of active TL shortening processes that are activated when the cell is in energetic debt and needs to reduce its anabolic metabolism through a key regulatory kinase called ‘target of rapamycin’ (TOR; figure 1) in yeast, mice and humans [44,47,72–74]. In this proposed pathway, various factors that affect an individual's energy balance cause the upregulation of metabolic mediators like GCs and AMP-activated protein kinase (AMPK), which in animals typically increase when energy reserves are low [75–77]. This upregulation of GCs and AMPK could either be direct or mediated by low cellular adenosine-triphospate (ATP) levels ([47]; figure 1). There is also evidence that GCs and AMPK can interact with each other at different levels to regulate cellular homeostasis [78,79]. GCs can inhibit TOR via REDD1 (‘Regulated in development and DNA Damage’ response proteins [76]), while AMPK can do it directly (figure 1). TOR in turn plays a crucial role in physiological trade-offs by regulating cell proliferation, differentiation, growth and anabolic processes [80–82]. The inhibition of TOR causes a downregulation of enzymes and proteins that maintain TL length in yeast [44,72,83,84] and humans [74,85]. The view that TL length is a regulated process is supported by the recent discovery that Ku-proteins, which are regulated by TOR (figure 1), can affect TL length in both directions and through two pathways: first, when Ku-proteins are present, they can directly interact with telomeric repeats to increase telomerase expression, thus promoting TL maintenance [86]. Conversely, TOR inhibition leads to a suppression of Ku-protein expression, resulting in an inhibition of telomerase expression and TL attrition (figure 1). Second, Ku-proteins appear able to bind to telomeric RNA repeats called TERRA (TElomeric Repeats containing RNA with repetitive UUAGGG sequences [87]). When bound to TERRA, Ku-proteins can induce TL shortening by promoting the expression of the enzyme exonuclease 1, which cleaves nucleotides from telomeric DNA ([44,86]; figure 1). The interactions between Ku-proteins and TERRA are not yet fully understood and their importance in regulating TL dynamics is only starting to emerge. However, in humans, TERRA may be regulated by the energetic state of an individual, and possibly within relatively short time frames. For example, only 45 min of endurance exercise induced TERRA expression in muscle cells of athletes [45]. The authors speculated that the role of the TERRA activation was to protect TLs from the oxidative insult generated by the exercise (though oxidative stress was not measured in this study). According to the metabolic telomere attrition hypothesis, TERRA could have instead been activated to promote TL shortening in skeletal muscle cells in response to the energetic imbalance caused by the exercise [86]. In trained mice, 30 min of running performed at 65% of their peak speed induced a reduction in the TL-sheltering protein TRF1 [88], which could explain the shortening of TLs observed in mice exposed to chronic exercise [89]. Together with the previous example, this study suggests that when facing energetic challenges, organisms can trigger a rather swift reorganization of TL-associated complexes like TERRA and sheltering proteins, which are involved in TL dynamics. We are still far from understanding these processes but studying the gene expression of components involved in TL regulation will constitute important first steps towards fully elucidating these pathways.
Within the framework of the metabolic telomere attrition hypothesis, we could envision several functional reasons for an active TL length regulation: first, TL attrition could serve an important signalling function in the metabolic regulation of the cell (which would also provide a mechanism for the ‘functional attrition hypothesis' [48]). There is biochemical evidence in mice [30,47] that TL shortening can either cause dysfunctional mitochondria and thus cellular senescence or maintain functional mitochondria and healthy cells depending on the level of proteins like p53 that are secreted following TL attrition ([47,90]; figure 1). At low concentrations, p53 increases levels of enzymatic antioxidants and boosts the production of mitochondrial ATP as well as the generation of new mitochondria ([47,91,92]; figure 1, ‘functional phenotype’ loop). By contrast, at high concentrations, p53 disrupts mitochondria function, thereby decreasing ATP production and cellular energy levels, while increasing concentrations of pro-oxidative molecules through the depletion and/or downregulation of antioxidant enzymes (figure 1). This TL–mitochondria–oxidative stress axis pathway is part of the ageing circuitry [30,90].
Second, TLs could be regulated because they are costly to maintain (see also ‘costly maintenance hypothesis' [48]). On theoretical grounds, costs are plausible because the TL structure is rather complex, consisting of DNA–protein and DNA–RNA complexes, and because an elaborate TL maintenance machinery exists (figure 1). However, it is methodologically difficult to quantify such costs. Nevertheless, tantalizing evidence in yeast suggests that alterations in the ratio of the four dNTPs (nucleoside triphosphates like ATP, GTP, CTP and TTP, which comprise the nucleotide building blocks of DNA) can dramatically affect TL length, both positively and negatively [93]. Nucleoside concentrations can vary with nutritional state, biosynthesis potential, rate of cell replication and other conditions [94], and directly affect cell replication [95,96]. Whenever nucleotide shortages occur, cells preferentially activate salvage pathways to obtain nucleotides from existing sources like DNA before resorting to de novo biosynthesis because the former is less expensive in terms of ATP-requirements [94]. If a cell cannot obtain enough nucleotides, it will stop proliferating and trigger the replicative stress response [95,97,98]. These observations raise the intriguing possibility that during energy shortages, cells may induce these salvage pathways to gain ready-to-use nucleotides through TL shortening, as a beneficial short-term strategy to avoid the costs of the replicative stress response. However, it is currently unclear whether the gain in nucleotides (or other resources) achieved via TL attrition would be sufficient to prevent the cell from entering such a detrimental state. The central role of nucleotide metabolism in affecting cell replication is only beginning to be understood, but it seems to be a central feature for regulating cell proliferation [95]. Further studies in this field are needed to obtain a more complete picture of these processes.
(a). Telomere attrition acting as a signal for life-history decisions?
A third functional role for TL shortening during energy shortages could be to act as a cellular signal to support life-history decisions (see also ‘life-history regulation hypothesis' [48]). Energetic limitations necessitate trade-offs in resource allocation to processes that compete for access to the same reserves [99,100]. A common vertebrate trade-off is the one between investment in current versus future reproductive events (the latter requiring investment in self-maintenance processes to increase the probability of surviving and reproducing again). GCs are thought to mediate this trade-off, with baseline concentrations supporting traits that increase reproductive success (e.g. parental provisioning [101–105]), and high stress-induced concentrations inhibiting reproductive behaviour and promoting the ‘emergency life-history state’ to support immediate survival processes [106].
Further, it is known that long TLs can silence certain genes located in the subtelomeric region (called telomere position effect—TPE). This gene silencing appears to be an evolutionarily conserved strategy found in several species from yeast to humans that allows metabolic adjustment to changes to environmental conditions [107,108]. These genes are activated by decreasing the TPE when TLs become shorter (but much before a DNA damage response is induced [107,109]), providing a mechanism by which organismal performance can be programmed according to TL length [110,111]. Overall, these functions suggest that TLs are regulators of cell function, sensors of energetic stress and mediators of life-history trade-offs. During periods of energy limitation, moderate TL attrition could support GC-induced metabolic changes associated with prioritizing processes that increase immediate survival (via the functional phenotype pathway; figure 1). In this way, TL attrition would help to re-direct resource investment in processes that promote survival and future reproduction instead of investment in current reproduction. Once energy balance has been restored, cellular processes could return to their former state, TL length could be maintained again and life-history decisions reversed. Evidence of TL restoration after attrition has also been reported [4,18,112,113], but thus far, it is unclear whether TL-lengthening represents a methodological artefact or has a biological significance [114–116]. Within the concept of the metabolic telomere attrition hypothesis, TL restoration is conceivable, and in the following, we will summarize some tentative biomedical evidence that might support its existence. For example, telomerase is active in all tissues during development, while in adults, it is present only in mitotic tissues and stem cells (at least in humans), but its activity can vary greatly with life-history stage, tissue type and across species [117–119]. Telomerase is quite conserved and present in almost all organisms ranging from yeast to humans [10,70,120]. TL-lengthening via mechanisms other than telomerase have also been described in somatic cells [121]. Further, androgens are used to treat TL-related human diseases, because they are able to restore TL loss by activating the expression of telomerase reverse transcriptase (TERT, one of the subunits of telomerase [122,123]). Interestingly, in these experiments, androgenic TERT regulation was achieved through the oestrogen receptor, i.e. either following the enzymatic conversion of androgens into oestradiol or through direct effects of oestradiol [123]. This would explain why TL elongation is also observed after oestrogen treatment [124]. Similar results were obtained in mice suffering from aplastic anaemia induced by short TLs, where testosterone implants caused TL elongation by upregulating telomerase activity [125]. The latter study also showed that the effect of testosterone on TLs was independent of that on cell proliferation, which can be promoted by androgens [125]. Thus far, studies on the effects of sex steroids on TLs have been conducted on individuals afflicted by diseases and treated with pharmacological doses of sex steroids. Hence, the actions of naturalistic sex steroid concentrations on TL dynamics have not yet been tested, but it is likely that similar pathways would be activated. Since the metabolic telomere attrition hypothesis predicts that TL shortening should occur when cells have to switch to a catabolic state, the converse is also expected in that TL restoration should occur when cells are in an anabolic state. Sex steroids typically promote anabolic processes, in contrast with the catabolic effects of GCs [126,127]. Indeed, androgens are known to activate TOR-mediated anabolic processes by inhibiting both REDD1 and AMPK [128], which in turn activate TOR (figure 1).
(b). Telomere attrition solves trade-offs?
The metabolic telomere attrition hypothesis assumes that TL attrition is strongest during times of energy limitation, and that TL shortening has a functional role as a signal for life-history decisions. These proposed functions raise questions about the evolutionary processes that could have shaped such pathways. In particular, could TL attrition be a solution of energetic trade-offs that has been shaped by evolutionary forces (see also [48])? Several recent reports indeed indicate that TL shortening might be an optimal strategy under certain circumstances. TL attrition typically is very high during the early growth phase, which is a highly energy-demanding phase [18,34,129]. Individuals' growth rates can determine their health and fitness prospects [130]. Evidence supporting the existence of trade-offs between TL length and growth is accumulating (reviewed in [34]). For example, king penguin (Aptenodytes patagonicus) nestlings with a higher TL attrition were in better body condition [131]. Similarly, male and female common terns (Sterna hirundo) with shorter age-corrected TLs were in better body condition, arrived earlier on the breeding grounds and produced more offspring [132]. Furthermore, male common terns with higher baseline GC levels achieved higher reproductive success and had shorter TLs, showing that short TLs can be associated with higher fitness [133]. In line with the latter finding, Atlantic salmon (Salmo salar) individuals with the shortest TLs at the time of migration had the greatest probability of surviving [134]. A caveat of the studies reviewed above is that they are correlative and did not determine causal relationships. Furthermore, they did not offer thorough mechanistic explanations for their findings. Nevertheless, they fit with the predictions of the metabolic telomere attrition hypothesis that during times of energy limitations, a diversion of resources away from TL maintenance to other processes could be beneficial by improving body condition, performance and fitness. There are also reports of negative relationships between TL length and fitness proxies [17,18,21,135–137], which would not be in contrast with the metabolic telomere attrition. For example, after reaching a certain lower threshold, short TLs are expected to negatively impact body condition by promoting dysfunctional mitochondria and cell senescence (figure 1).
3. Testing the metabolic telomere attrition hypothesis
Several testable predictions arise from the metabolic telomere attrition hypothesis (table 1):
-
(1)
TL shortening is expected whenever organisms face major energetic challenges that are perceived by the cell (via TOR) and induce specific metabolic adjustments like a promotion of catabolic over anabolic processes to re-establish energy balance. Thus, TL attrition is likely to occur in life-history stages characterized by high metabolic demands, like growth [138], migration and reproduction [137], during challenging environmental conditions [135], and in the wake of energetically costly processes like immune activation [50], high work load [133], antagonistic challenges [139] or poor availability of resources [140].
-
(2)
During times of energy shortages, organismal changes should occur (and will have to be studied in detail) at two levels: (i) in the physiological mechanisms that link energetic state, cell metabolism and TL dynamics like mitochondrial functioning, phosphorylation status of target kinases or gene expression; (ii) in the relationship between TL attrition and the resulting condition of the organism. Repeated assessments of TL lengths and metabolic processes in individuals of different body condition experiencing demanding conditions are needed to test this prediction [4]. Ideally, such tests should be carried out in natural populations, which are exposed more frequently to settings that require resource trade-offs [133,135,137–140] than populations living in sheltered laboratory conditions.
-
(3)
TL dynamics will be influenced by a range of metabolic regulators, including GCs, thyroid and sex hormones, insulin, energy-sensing kinases and proteins.
-
(4)
Shortened TLs have a cell signalling function in triggering metabolic adjustments that boost mitochondrial activity to increase ATP production. This prediction could be tested by evaluating the metabolic efficiency of cells with short versus long TLs, paying specific attention to the expression of TOR regulators and TOR actions to disentangle the causes from the consequences of TL dynamics. This could also improve our understanding of the role of metabolically induced oxidative stress in determining TL length.
-
(5)
Oxidative stress could be indirectly related to TL length through specific cell signals between TLs and mitochondria ([47]; figure 1), without being the cause of attrition. For example, severe oxidative stress can impair mitochondrial ATP production, thus increasing the AMP/ATP ratio, which in turn inhibits TOR and shortens TL lengths ([141]; figure 1). TL attrition to a dysfunctional degree causes mitochondrial dysregulation, which in turn can lead to a high production of radical oxidative molecules and low antioxidant concentrations. In this case, oxidative stress would cause TL shortening primarily through metabolic pathways and not, or not only, by damaging TL integrity through oxidation. Similarly, administration of antioxidants could benefit TL maintenance because it has positive effects on mitochondrial metabolism [30], thus improving the energetic state of the cell, and not because it mitigates oxidative damage (table 1).
-
(6)
Within early-life stages, individuals that still undergo growth, i.e. high cell replication, should be more likely to show significant TL attrition during metabolically challenging conditions than individuals that already have completed growth.
-
(7)
Individuals with longer TLs are more likely to lose telomeric nucleotides, as has been observed in some passerines [20,142], because these are actively adjusted in relation to metabolic needs. Cells with longer TLs can tolerate more extensive erosion without entering cellular senescence than cells with short TLs. Consequently, TL loss relative to starting length is expected to predict fitness outcomes for individuals [18,20]. However, once the lower threshold of TL loss has been surpassed, afflicted cells would become senescent, with negative fitness consequences for the organism. Note that it has been difficult to assess the lower threshold at which short TLs become dysfunctional. In humans suffering from certain diseases, a lower threshold of 2.6 kb was assumed to indicate dysfunctionality [143], but much shorter TL lengths have been measured in humans and generalizations to other taxa have proven difficult [144]. Moreover, most biomedical and ecological studies use methods that provide average and relative estimates of TLs rather than chromosome-specific and absolute ones [145]. It will be crucial that future studies attempt to delineate lower TL length thresholds at the level of individuals, populations and species to improve our understanding of the cellular and organismal consequences of TL attrition [144,146].
-
(8)
Species with higher metabolic rates and smaller body sizes are expected to suffer more readily from energy shortages and should, therefore, be more prone to TL shortening than large species with low metabolic rates. A meta-analysis on rodent species supports this prediction by showing a negative correlation between telomerase activity in several tissues and body size (which in turn is negatively correlated with metabolic rate [118]). These results were interpreted as being an evolved tumour protection strategy by turning off telomerase in large, long-lived species [118,119]. However, another, not mutually exclusive explanation is that smaller species need to maintain high telomerase activity to restore their larger TL losses.
-
(9)
Hibernating species offer an ideal model to test the metabolic telomere attrition hypothesis as the increased longevity of hibernating species relative to that of non-hibernating species with a comparable life-history strategy seems to be related to TL dynamics [147,148]. Dormice (Glis glis) that show high torpor levels and low arousal frequencies limit their TL attrition, while post-torpor feeding can elongate TLs [147–149]. This fits the predictions of the metabolic telomere attrition hypothesis: TLs are finely regulated according to an individual's energetic state, with TL attrition being almost absent during torpor, when hardly any energy is needed owing to the substantially reduced metabolic rate. TLs can even be elongated following torpor when individuals become active again, increase their metabolic rate and have plenty of access to food resources [149].
-
(10)
Other models suitable to test the metabolic telomere attrition hypothesis are species that have to fast for a long time. For example, king penguin chicks show an extended growth phase during which they have to endure long periods of food shortages during winter [131]. The growth of smaller chicks during this time is more strongly reduced than that of larger chicks, but they later exhibit catch-up growth to compensate for their reduced size. As a consequence of the accelerated compensatory growth rate, these smaller chicks incur a greater oxidative damage and an accelerated TL loss compared to chicks that had a size advantage from the outset [131]. However, it is hard to disentangle the costs of catch-up growth and lack of nutrients in this experiment. The authors also found a negative association between TL loss and oxidative damage, supporting the hypothesis that a fast growth rate causes oxidative damage and in turn eroded TLs. However, this study also found that ‘independently of the growth trajectory, ending with a good body condition was also associated with a higher loss of telomere sequences' [131]. These puzzling results are actually in line with our hypothesis of an adaptive solution to a metabolic trade-off between TL and growth. In harsh environmental conditions, allocating resources to functions other than TL maintenance may allow king penguin chicks to achieve a better body condition and consequently to increase their survival probabilities [150].
4. Conclusion
While a shortening of TLs with age is a matter of fact, the mechanisms underlying TL attrition and their organismal consequences are still unresolved [23,48]. New insights into TL biology are gradually but dramatically changing our view of TL dynamics. Recent studies in different species have shown that TLs are transcribed by RNA polymerase into long RNAs repeats (TERRA, see also above [27,151]). The main functions of TERRA still have to be established, but thus far, it is clear that they have an essential role in the dynamics and functioning of TLs [28,152].
Our understanding of the relationship between TLs and oxidative stress is also changing. Although it is generally assumed that oxidative stress accelerates TL erosion in vitro [31], it is now becoming evident from in vivo studies that the picture is much more complex, with TL length and metabolism being more tightly linked than initially thought and not consistently explained by the production of reactive oxygen species [44,45,47]. The relationship between TL attrition and metabolism as proposed by the metabolic telomere attrition hypothesis may in fact explain some contradictory results concerning the pathways leading to TL shortening as well as resulting fitness consequences (table 1). This review proposes that one pathway of TL erosion is linked to energetic trade-offs, with shortened TLs also fulfilling an important signalling function for life-history trade-offs. We suggest viewing TL length not only as a biomarker for cumulative damage but also as a determinant of organismal condition, integrating the impacts of both past and current lifetime experiences.
Acknowledgements
The authors thank Simon Verhulst and several anonymous reviewers for valuable comments on previous versions of the manuscript.
Data accessibility
This article has no additional data.
Authors' contributions
S.C. developed the concept and S.C. and M.H. refined the hypothesis and wrote the manuscript.
Competing interests
We declare we have no competing interests.
Funding
We are grateful to the Max Planck Society and the German Science Foundation (DFG grant CA 1789/1-1-2017) for supporting our work.
References
- 1.Monaghan P, Haussmann MF. 2006. Do telomere dynamics link lifestyle and lifespan? Trends Ecol. Evol. 21, 47–53. ( 10.1016/j.tree.2005.11.007) [DOI] [PubMed] [Google Scholar]
- 2.Monaghan P. 2010. Telomeres and life histories: the long and the short of it. Ann. NY Acad. Sci. 1206, 130–142. ( 10.1111/j.1749-6632.2010.05705.x) [DOI] [PubMed] [Google Scholar]
- 3.Bateson M, Nettle D. 2018. Why are there associations between telomere length and behaviour? Phil. Trans. R. Soc. B 373, 20160438 ( 10.1098/rstb.2016.0438) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hau M, Greives TJ, Haussmann MF, Matlack C, Costantini D, Quetting M, Adelman JS, Partecke J. 2015. Repeated stressors in adulthood increase the rate of biological ageing. Front. Zool. 12, 4 ( 10.1186/s12983-015-0095-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mathur MB, Epel E, Kind S, Desai M, Parks CG, Sandler DP, Khazeni N. 2016. Perceived stress and telomere length: a systematic review, meta-analysis, and methodologic considerations for advancing the field. Brain Behav. Immun. 54, 158–169. ( 10.1016/j.bbi.2016.02.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blackburn EH. 1991. Structure and function of telomeres. Nature 350, 569–573. ( 10.1038/350569a0) [DOI] [PubMed] [Google Scholar]
- 7.Passos JF, Saretzki G, Von Zglinicki T. 2007. DNA damage in telomeres and mitochondria during cellular senescence: is there a connection? Nucleic Acids Res. 35, 7505–7513. ( 10.1093/nar/gkm893) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blackburn EH, Epel ES, Lin J. 2015. Human telomere biology: a contributory and interactive factor in aging, disease risks, and protection. Science 350, 1193–1198. ( 10.1126/science.aab3389) [DOI] [PubMed] [Google Scholar]
- 9.Blackburn EH. 2001. Switching and signaling at the telomere. Cell 106, 661–673. ( 10.1016/S0092-8674(01)00492-5) [DOI] [PubMed] [Google Scholar]
- 10.Hornsby PJ. 2007. Telomerase and the aging process. Exp. Gerontol. 42, 575–581. ( 10.1016/j.exger.2007.03.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang M, Arneric M, Lingner J. 2007. Telomerase repeat addition processivity is increased at critically short telomeres in a Tel1-dependent manner in Saccharomyces cerevisiae. Genes Dev. 21, 2485–2494. ( 10.1101/gad.1588807) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu RA, Upton HE, Vogan JM, Collins K. 2017. Telomerase mechanism of telomere synthesis. Annu. Rev. Biochem. 86, 439–460. ( 10.1146/annurev-biochem-061516-045019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hartmann N, et al. 2009. Telomeres shorten while Tert expression increases during ageing of the short-lived fish Nothobranchius furzeri. Mech. Ageing Dev. 130, 290–296. ( 10.1016/j.mad.2009.01.003) [DOI] [PubMed] [Google Scholar]
- 14.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. 2013. The hallmarks of aging. Cell 153, 1194–1217. ( 10.1016/j.cell.2013.05.039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cawthon R, Smith K, O'Brien E, Sivatchenko A, Kerber R. 2003. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet 361, 393–395. ( 10.1016/S0140-6736(03)12384-7) [DOI] [PubMed] [Google Scholar]
- 16.Haussmann MF, Vleck CM, Nisbet ICT. 2003. Calibrating the telomere clock in common terns, Sterna hirundo. Exp. Gerontol. 38, 787–789. ( 10.1016/S0531-5565(03)00109-8) [DOI] [PubMed] [Google Scholar]
- 17.Bize P, Criscuolo F, Metcalfe NB, Nasir L, Monaghan P. 2009. Telomere dynamics rather than age predict life expectancy in the wild. Proc. R. Soc. B 276, 1679–1683. ( 10.1098/rspb.2008.1817) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heidinger BJ, Blount JD, Boner W, Griffiths K, Metcalfe NB, Monaghan P. 2012. Telomere length in early life predicts lifespan. Proc. Natl Acad. Sci. USA 109, 1743–1748. ( 10.1073/pnas.1113306109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boonekamp JJ, Simons MJP, Hemerik L, Verhulst S. 2013. Telomere length behaves as biomarker of somatic redundancy rather than biological age. Aging Cell 12, 330–332. ( 10.1111/acel.12050) [DOI] [PubMed] [Google Scholar]
- 20.Boonekamp JJ, Mulder G, Salomons HM, Dijkstra C, Verhulst S. 2014. Nestling telomere shortening, but not telomere length, reflects developmental stress and predicts survival in wild birds. Proc. R. Soc. B 281, 20133287 ( 10.1098/rspb.2013.3287) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barrett ELB, Burke TA, Hammers M, Komdeur J, Richardson DS. 2013. Telomere length and dynamics predict mortality in a wild longitudinal study. Mol. Ecol. 22, 249–259. ( 10.1111/mec.12110) [DOI] [PubMed] [Google Scholar]
- 22.Fairlie J, Holland R, Pilkington JG, Pemberton JM, Harrington L, Nussey DH. 2016. Lifelong leukocyte telomere dynamics and survival in a free-living mammal. Aging Cell 15, 140–148. ( 10.1111/acel.12417) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simons MJP. 2015. Questioning causal involvement of telomeres in aging. Ageing Res. Rev. 24, 191–196. ( 10.1016/j.arr.2015.08.002) [DOI] [PubMed] [Google Scholar]
- 24.Wilbourn RV, Moatt JP, Froy H, Walling CA, Nussey DH, Boonekamp JJ. 2018. The relationship between telomere length and mortality risk in non-model vertebrate systems: a meta-analysis. Phil. Trans. R. Soc. B 373, 20160447 ( 10.1098/rstb.2016.0447) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bandaria JN, Qin P, Berk V, Chu S, Yildiz A. 2016. Shelterin protects chromosome ends by compacting telomeric chromatin. Cell 164, 735–746. ( 10.1016/j.cell.2016.01.036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Lange T. 2005. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 19, 2100–2110. ( 10.1101/gad.1346005) [DOI] [PubMed] [Google Scholar]
- 27.Azzalin CM, Reichenbach P, Khoriauli L, Giulotto E, Lingner J. 2007. Telomeric repeat-containing RNA and RNA surveillance factors at mammalian chromosome ends. Science 318, 798–801. ( 10.1126/science.1147182) [DOI] [PubMed] [Google Scholar]
- 28.Cusanelli E, Perez Romero CA, Chartrand P. 2013. Telomeric noncoding RNA TERRA is induced by telomere shortening to nucleate telomerase molecules at short telomeres. Mol. Cell 51, 780–791. ( 10.1016/j.molcel.2013.08.029) [DOI] [PubMed] [Google Scholar]
- 29.Karlseder J, Smogorzewska A, de Lange T. 2002. Senescence induced by altered telomere state, not telomere loss. Science 295, 2446–2449. ( 10.1126/science.1069523) [DOI] [PubMed] [Google Scholar]
- 30.Sahin E, et al. 2011. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature 470, 359–365. ( 10.1038/nature09787) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Von Zglinicki T. 2002. Oxidative stress shortens telomeres. Trends Biochem. Sci. 27, 339–344. ( 10.1016/S0968-0004(02)02110-2) [DOI] [PubMed] [Google Scholar]
- 32.Reichert S, Stier A. 2017. Does oxidative stress shorten telomeres in vivo ? A review. Biol. Lett. 13, 20170463 ( 10.1098/rsbl.2017.0463) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haussmann MF, Marchetto NM. 2010. Telomeres: linking stress and survival, ecology and evolution. Curr. Zool. 56, 714–727. [Google Scholar]
- 34.Monaghan P, Ozanne SE. 2018. Somatic growth and telomere dynamics in vertebrates: relationships, mechanisms and consequences. Phil. Trans. R. Soc. B 373, 20160446 ( 10.1098/rstb.2016.0446) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Halliwell B, Gutteridge JMC. 2007. Free radicals in biology and medicine. Oxford, UK: Oxford University Press. [Google Scholar]
- 36.Monaghan P, Metcalfe NB, Torres R. 2009. Oxidative stress as a mediator of life history trade-offs: mechanisms, measurements and interpretation. Ecol. Lett. 12, 75–92. ( 10.1111/j.1461-0248.2008.01258.x) [DOI] [PubMed] [Google Scholar]
- 37.Metcalfe NB, Alonso-Alvarez C. 2010. Oxidative stress as a life-history constraint: the role of reactive oxygen species in shaping phenotypes from conception to death. Funct. Ecol. 24, 984–996. ( 10.1111/j.1365-2435.2010.01750.x) [DOI] [Google Scholar]
- 38.Isaksson C. 2015. Urbanization, oxidative stress and inflammation: a question of evolving, acclimatizing or coping with urban environmental stress. Funct. Ecol. 29, 913–923. ( 10.1111/1365-2435.12477) [DOI] [Google Scholar]
- 39.Costantini D. 2008. Oxidative stress in ecology and evolution: lessons from avian studies. Ecol. Lett. 11, 1238–1251. ( 10.1111/j.1461-0248.2008.01246.x) [DOI] [PubMed] [Google Scholar]
- 40.Boonekamp JJ, Bauch C, Mulder E, Verhulst S. 2017. Does oxidative stress shorten telomeres? Biol. Lett. 13, 20170164 ( 10.1098/rsbl.2017.0164) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Von Zglinicki T, Pilger R, Sitte N. 2000. Accumulation of single-strand breaks is the major cause of telomere shortening in human fibroblasts. Free Radic. Biol. Med. 28, 64–74. ( 10.1016/S0891-5849(99)00207-5) [DOI] [PubMed] [Google Scholar]
- 42.Reichert S, Criscuolo F, Verinaud E, Zahn S, Massemin S. 2013. Telomere length correlations among somatic tissues in adult zebra finches. PLoS One 8, e81496 ( 10.1371/journal.pone.0081496) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Daniali L, Benetos A, Susser E, Kark JD, Labat C, Kimura M, Desai K, Granick M, Aviv A. 2013. Telomeres shorten at equivalent rates in somatic tissues of adults. Nat. Commun. 4, 1597 ( 10.1038/ncomms2602) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ungar L, Harari Y, Toren A, Kupiec M. 2011. Tor complex1 controls telomere length by affecting the level of Ku. Curr. Biol. 21, 2115–2120. ( 10.1016/j.cub.2011.11.024) [DOI] [PubMed] [Google Scholar]
- 45.Diman A, et al. 2016. Nuclear respiratory factor 1 and endurance exercise promote human telomere transcription. Sci. Adv. 2, e1600031 ( 10.1126/sciadv.1600031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ye J, Renault VM, Jamet K, Gilson E. 2014. Transcriptional outcome of telomere signalling. Nat. Rev. Genet. 15, 491–503. ( 10.1038/nrg3743) [DOI] [PubMed] [Google Scholar]
- 47.Sahin E, DePinho RA. 2012. Axis of ageing: telomeres, p53 and mitochondria. Nat. Rev. Mol. Cell Biol. 13, 397–404. ( 10.1038/nrm3352) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Young AJ. 2018. The role of telomeres in the mechanisms and evolution of life-history trade-offs and ageing. Phil. Trans. R. Soc. B 373, 20160452 ( 10.1098/RSTB.2016.0452) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zalli A, Carvalho LA, Lin J, Hamer M, Erusalimsky JD, Blackburn EH, Steptoe A. 2014. Shorter telomeres with high telomerase activity are associated with raised allostatic load and impoverished psychosocial resources. Proc. Natl Acad. Sci. USA 111, 4519–4524. ( 10.1073/pnas.1322145111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Asghar M, Hasselquist D, Hansson B, Zehtindjiev P, Westerdahl H, Bensch S. 2015. Hidden costs of infection: chronic malaria accelerates telomere degradation and senescence in wild birds. Science 347, 436–438. ( 10.1126/science.1261121) [DOI] [PubMed] [Google Scholar]
- 51.Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. 2004. Accelerated telomere shortening in response to life stress. Proc. Natl Acad. Sci. USA 101, 17 312–17 315. ( 10.1073/pnas.0407162101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sapolsky RM, Romero LM, Munck AU. 2000. How do glucocorticoids influence stress responses ? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 21, 55–89. ( 10.1210/er.21.1.55) [DOI] [PubMed] [Google Scholar]
- 53.Romero LM. 2004. Physiological stress in ecology: lessons from biomedical research. Trends Ecol. Evol. 19, 249–255. ( 10.1016/j.tree.2004.03.008) [DOI] [PubMed] [Google Scholar]
- 54.Haussmann MF, Heidinger BJ. 2015. Telomere dynamics may link stress exposure and ageing across generations. Biol. Lett. 11, 20150396 ( 10.1098/rsbl.2015.0396) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Romero LM, Platts SH, Schoech SJ, Wada H, Crespi E, Martin LB, Buck CL. 2015. Understanding stress in the healthy animal—potential paths for progress. Stress 18, 491–497. ( 10.3109/10253890.2015.1073255) [DOI] [PubMed] [Google Scholar]
- 56.Hau M, Casagrande S, Ouyang JQ, Baugh AT. 2016. Glucocorticoid-mediated phenotypes in vertebrates: multilevel variation and evolution. In Advances in the study of behavior (eds Naguib M, Mitani JC, Simmons LW, Barrett L, Healy S, Zuk M), pp. 41–115. New York, NY: Academic Press. [Google Scholar]
- 57.Picard M, Juster R-P, McEwen BS. 2014. Mitochondrial allostatic load puts the ‘gluc’ back in glucocorticoids. Nat. Rev. Endocrinol. 10, 303–310. ( 10.1038/nrendo.2014.22) [DOI] [PubMed] [Google Scholar]
- 58.Opata AA, Cheesman KC, Geer EB. 2017. Glucocorticoid regulation of body composition and metabolism. In The hypothalamic–pituitary–adrenal axis in health and disease (ed. Geer E.), pp. 3–26. Cham, Switzerland: Springer; ( 10.1007/978-3-319-45950-9) [DOI] [Google Scholar]
- 59.Angelier F, Costantini D, Blévin P, Chastel O. 2018. Do glucocorticoids mediate the link between environmental conditions and telomere dynamics in wild vertebrates? A review. Gen. Comp. Endocrinol. 256, 99–111. ( 10.1016/j.ygcen.2017.07.007) [DOI] [PubMed] [Google Scholar]
- 60.Herborn KA, Heidinger BJ, Boner W, Noguera JC, Adam A, Daunt F, Monaghan P. 2014. Stress exposure in early post-natal life reduces telomere length: an experimental demonstration in a long-lived seabird. Proc. R. Soc. B 281, 20133151 ( 10.1098/rspb.2013.3151) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stier A, et al. 2014. Starting with a handicap: phenotypic differences between early- and late-born king penguin chicks and their survival correlates. Funct. Ecol. 28, 601–611. ( 10.1111/1365-2435.12204) [DOI] [Google Scholar]
- 62.Haussmann MF, Longenecker AS, Marchetto NM, Juliano SA, Bowden RM. 2012. Embryonic exposure to corticosterone modifies the juvenile stress response, oxidative stress and telomere length. Proc. R. Soc. B 279, 1447–1456. ( 10.1098/rspb.2011.1913) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tissier ML, Williams TD, Criscuolo F. 2014. Maternal effects underlie ageing costs of growth in the zebra finch (Taeniopygia guttata). PLoS ONE 9, e97705 ( 10.1371/journal.pone.0097705) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Costantini D, Marasco V, Møller AP. 2011. A meta-analysis of glucocorticoids as modulators of oxidative stress in vertebrates. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 181, 447–456. ( 10.1007/s00360-011-0566-2) [DOI] [PubMed] [Google Scholar]
- 65.Ouyang JQ, Lendvai ÁZ, Moore IT, Bonier F, Haussmann MF. 2016. Do hormones, telomere lengths, and oxidative stress form an integrated phenotype? A case study in free-living tree swallows. Integr. Comp. Biol. 56, 138–145. ( 10.1093/icb/icw044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lendvai AZ, Ouyang JQ, Schoenle LA, Fasanello V, Haussmann MF, Bonier F, Moore IT. 2014. Experimental food restriction reveals individual differences in corticosterone reaction norms with no oxidative costs. PLoS ONE 9, e110564 ( 10.1371/journal.pone.0110564) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vitousek MN, Taff CC, Ardia DR, Stedman JM, Zimmer C, Salzman TC, Winkler DW. 2018. The lingering impact of stress: brief acute glucocorticoid exposure has sustained, dose-dependent effects on reproduction. Proc. R. Soc. B 285, 20180722 ( 10.1098/rspb.2018.0722) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Casagrande S, Hau M. 2018. Enzymatic antioxidants but not baseline glucocorticoids mediate the reproduction–survival trade-off in a wild bird. Proc. R. Soc. B 285, 20182141 ( 10.1098/rspb.2018.2141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Choi J, Fauce SR, Effros RB. 2008. Reduced telomerase activity in human T lymphocytes exposed to cortisol. Brain. Behav. Immun. 22, 600–605. ( 10.1016/j.bbi.2007.12.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rubtsova MP, Vasilkova DP, Malyavko AN, Naraikina YV, Zvereva MI, Dontsova OA. 2012. Telomere lengthening and other functions of telomerase. Acta Naturae 4, 44–61. [PMC free article] [PubMed] [Google Scholar]
- 71.Ahmed S, Passos JF, Birket MJ, Beckmann T, Brings S, Peters H, Birch-Machin MA, von Zglinicki T, Saretzki G. 2008. Telomerase does not counteract telomere shortening but protects mitochondrial function under oxidative stress. J. Cell Sci. 121, 1046–1053. ( 10.1242/jcs.019372) [DOI] [PubMed] [Google Scholar]
- 72.Harari Y, Romano GH, Ungar L, Kupiec M. 2013. Nature vs nurture: interplay between the genetic control of telomere length and environmental factors. Cell Cycle 12, 3465–3470. ( 10.4161/cc.26625) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dogan F, Avci CB. 2018. Correlation between telomerase and mTOR pathway in cancer stem cells. Gene 641, 235–239. ( 10.1016/j.gene.2017.09.072) [DOI] [PubMed] [Google Scholar]
- 74.Gopalakrishnan K, Venkatesan S, Su E, Low H, Hande MP. 2018. Effects of rapamycin on the mechanistic target of rapamycin (mTOR) pathway and telomerase in breast cancer cells. Mutat. Res. Gen Toxicol Environ Mutagen 836, 103–113. ( 10.1016/j.mrgentox.2018.03.008) [DOI] [PubMed] [Google Scholar]
- 75.Hardie DG, Ross FA, Hawley SA. 2012. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 13, 251–262. ( 10.1038/nrm3311) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang H, Kubica N, Ellisen LW, Jefferson LS, Kimball SR. 2006. Dexamethasone represses signaling through the mammalian target of rapamycin in muscle cells by enhancing expression of REDD1. J. Biol. Chem. 281, 39 128–39 134. ( 10.1074/jbc.M610023200) [DOI] [PubMed] [Google Scholar]
- 77.Martin DE, Hall MN. 2005. The expanding TOR signaling network. Curr. Opin. Cell Biol. 17, 158–166. ( 10.1016/j.ceb.2005.02.008) [DOI] [PubMed] [Google Scholar]
- 78.Lim CT, Kola B, Korbonits M. 2010. AMPK as a mediator of hormonal signalling. J. Mol. Endocrinol. 44, 87–97. ( 10.1677/JME-09-0063) [DOI] [PubMed] [Google Scholar]
- 79.Nader N, Ng SSM, Lambrou GI, Pervanidou P, Wang Y, Chrousos GP, Kino T. 2010. Adenosine 5′-monophosphate-activated protein kinase regulates metabolic actions of glucocorticoids by phosphorylating the glucocorticoid receptor through p38 mitogen-activated protein kinase. Mol. Endocrinol. 24, 1748–1764. ( 10.1210/me.2010-0192) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Avruch J, Long X, Ortiz-Vega S, Rapley J, Papageorgiou A, Dai N. 2009. Amino acid regulation of TOR complex1. Am. J. Physiol. Endocrinol. Metab. 296, E592–E602. ( 10.1152/ajpendo.90645.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Betz C, Hall MN. 2013. Where is mTOR and what is it doing there? J. Cell Biol. 203, 563–574. ( 10.1083/jcb.201306041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wei Y, Zhang YJ, Cai Y. 2013. Growth or longevity: the TOR's decision on lifespan regulation. Biogerontology 14, 353–363. ( 10.1007/s10522-013-9435-6) [DOI] [PubMed] [Google Scholar]
- 83.Hall M, Tamanoi F. 2010. Structure, function and regulation of TOR complexes from yeasts to mammals. New York, NY: Academic Press. [Google Scholar]
- 84.Kupiec M, Weisman R. 2012. TOR links starvation responses to telomere length maintenance. Cell Cycle 11, 2268–2271. ( 10.4161/cc.20401) [DOI] [PubMed] [Google Scholar]
- 85.Zhou C, Gehrig P. 2003. Rapamycin inhibits telomerase activity by decreasing the hTERT mRNA level in endometrial cancer cells1. Mol. Cancer Ther. 2, 789–795. [PubMed] [Google Scholar]
- 86.Pfeiffer V, Lingner J. 2012. TERRA promotes telomere shortening through exonuclease 1-mediated resection of chromosome ends. PLoS Genet. 8, e1002747 ( 10.1371/journal.pgen.1002747) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Arnoult NVB, Decottignies AA. 2012. Telomere length regulates TERRA levels through increased trimethylation of telomeric H3K9 and HP1a.7. Nat. Struct. Mol. Biol. 19, 948–956. ( 10.1038/nsmb.2364) [DOI] [PubMed] [Google Scholar]
- 88.Ludlow AT, Lima LCJ, Wang J, Hanson ED, Guth LM, Spangenburg EE, Roth SM. 2012. Exercise alters mRNA expression of telomere-repeat binding factor 1 in skeletal muscle via p38 MAPK. J. Appl. Physiol. 113, 1737–1746. ( 10.1152/japplphysiol.00200.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ludlow AT, Witkowski S, Marshall MR, Wang J, Lima LCJ, Guth LM, Spangenburg EE, Roth SM. 2012. Chronic exercise modifies age-related telomere dynamics in a tissue-specific fashion. J. Gerontol. A Biol. Sci. Med. Sci. 67 A, 911–926. ( 10.1093/gerona/gls002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vousden KH, Lane DP. 2007. P53 in health and disease. Nat. Rev. Mol. Cell Biol. 8, 275–283. ( 10.1038/nrm2147) [DOI] [PubMed] [Google Scholar]
- 91.Ventura N, Rea SL, Schiavi A, Torgovnick A, Testi R, Johnson TE. 2009. p53/CEP-1 increases or decreases lifespan, depending on level of mitochondrial bioenergetic stress. Aging Cell 8, 380–393. ( 10.1111/j.1474-9726.2009.00482.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Feng Z, Levine AJ. 2010. The regulation of energy metabolism and the IGF-1/mTOR pathways by the p53 protein. Trends Cell Biol. 20, 427–434. ( 10.1016/j.tcb.2010.03.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gupta A, Sharma S, Reichenbach P, Marjavaara L, Nilsson AK, Lingner J, Chabes A, Rothstein R, Chang M. 2013. Telomere length homeostasis responds to changes in intracellular dNTP pools. Genetics 193, 1095–1105. ( 10.1534/genetics.112.149120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lane AN, Fan TWM. 2015. Regulation of mammalian nucleotide metabolism and biosynthesis. Nucleic Acids Res. 43, 2466–2485. ( 10.1093/nar/gkv047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kollareddy M, et al. 2015. Regulation of nucleotide metabolism by mutant p53 contributes to its gain-of-function activities. Nat. Commun. 6, 7389 ( 10.1038/ncomms8389) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mathews CK. 2015. Deoxyribonucleotide metabolism, mutagenesis and cancer. Nat. Rev. Cancer 15, 528–539. ( 10.1038/nrc3981) [DOI] [PubMed] [Google Scholar]
- 97.Aird KM, et al. 2013. Suppression of nucleotide metabolism underlies the establishment and maintenance of oncogene-induced senescence. Cell Rep. 3, 1252–1265. ( 10.1016/j.celrep.2013.03.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Austin WR, et al. 2012. Nucleoside salvage pathway kinases regulate hematopoiesis by linking nucleotide metabolism with replication stress. J. Exp. Med. 209, 2215–2228. ( 10.1084/jem.20121061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zera AJ, Harshman LG. 2001. The physiology of life history trade-offs in animals. Annu. Rev. Ecol. Syst. 32, 95–126. ( 10.1146/annurev.ecolsys.32.081501.114006) [DOI] [Google Scholar]
- 100.Roff DA, Fairbairn DJ. 2007. The evolution of trade-offs: where are we? J. Evol. Biol. 20, 433–447. ( 10.1111/j.1420-9101.2006.01255.x) [DOI] [PubMed] [Google Scholar]
- 101.Crespi EJ, Williams TD, Jessop TS, Delehanty B. 2013. Life history and the ecology of stress: how do glucocorticoid hormones influence life-history variation in animals? Funct. Ecol. 27, 93–106. ( 10.1111/1365-2435.12009) [DOI] [Google Scholar]
- 102.Vitousek MN, Taff CC, Hallinger KK, Zimmer C, Winkler DW. 2018. Hormones and fitness: evidence for trade-offs in glucocorticoid regulation across contexts. Front. Ecol. Evol. 6, 1–14. ( 10.3389/fevo.2018.00042) [DOI] [Google Scholar]
- 103.Bókony V, Lendvai ÁZ, Liker A, Angelier F, Wingfield JC, Chastel O. 2009. Stress response and the value of reproduction: are birds prudent parents? Am. Nat. 173, 589–598. ( 10.1086/597610) [DOI] [PubMed] [Google Scholar]
- 104.Casagrande S, et al. 2018. Do seasonal glucocorticoid changes depend on reproductive investment? A comparative approach in birds. Integr. Comp. Biol. 58, 739–750. ( 10.1093/icb/icy022) [DOI] [PubMed] [Google Scholar]
- 105.Wingfield JC, Sapolsky RM. 2003. Reproduction and resistance to stress: when and how. J. Neuroendocrinol. 15, 711–724. ( 10.1046/j.1365-2826.2003.01033.x) [DOI] [PubMed] [Google Scholar]
- 106.Wingfield JC, Maney DL, Breuner CW, Jacobs JD, Lynn S, Ramenofsky M, Richardson RD. 1998. Ecological bases of hormone–behavior interactions: the ‘emergency life history stage’. Integr. Comp. Biol. 38, 191–206. ( 10.1093/icb/38.1.191) [DOI] [Google Scholar]
- 107.Ottaviani A, Gilson E, Magdinier F. 2008. Telomeric position effect: from the yeast paradigm to human pathologies? Biochimie 90, 93–107. ( 10.1016/j.biochi.2007.07.022) [DOI] [PubMed] [Google Scholar]
- 108.Tham WH, Zakian VA. 2002. Transcriptional silencing at Saccharomyces telomeres: implications for other organisms. Oncogene 21, 512–521. ( 10.1038/sj/onc/1205078) [DOI] [PubMed] [Google Scholar]
- 109.Robin JD, Ludlow AT, Batten K, Magdinier F, Stadler G, Wagner KR, Shay JW, Wright WE. 2014. Telomere position effect: regulation of gene expression with progressive telomere shortening over long distances. Genes Dev. 28, 2464–2476. ( 10.1101/gad.251041.114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kim W, et al. 2016. Regulation of the human telomerase gene TERT by telomere position effect—over long distances (TPE-OLD): implications for aging and cancer. PLoS Biol. 14, 1–25. ( 10.1371/journal.pbio.2000016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kim W, Shay JW. 2018. Long-range telomere regulation of gene expression: telomere looping and telomere position effect over long distances (TPE-OLD). Differentiation 99, 1–9. ( 10.1016/j.diff.2017.11.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nettle D, Bateson M. 2017. Detecting telomere elongation in longitudinal datasets: analysis of a proposal by Simons, Stulp and Nakagawa. PeerJ 5, e3265 ( 10.7717/peerj.3265) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.McLennan D, Armstrong JD, Stewart DC, Mckelvey S, Boner W, Monaghan P, Metcalfe NB. 2018. Telomere elongation during early development is independent of environmental temperatures in Atlantic salmon. J. Exp. Biol. 221(Pt 11), jeb.178616 ( 10.1242/jeb.178616) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Simons MJP, Stulp G, Nakagawa S. 2014. A statistical approach to distinguish telomere elongation from error in longitudinal datasets. Biogerontology 15, 99–103. ( 10.1007/s10522-013-9471-2) [DOI] [PubMed] [Google Scholar]
- 115.Steenstrup T, Hjelmborg JVB, Kark JD, Christensen K, Aviv A. 2013. The telomere lengthening conundrum—artifact or biology? Nucleic Acids Res. 41, e131 ( 10.1093/nar/gkt370) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Verhulst S, Susser E, Factor-litvak PR, Simons MJP, Benetos A, Steenstrup T, Kark JD, Aviv A, Bank D. 2015. Commentary: the reliability of telomere length measurements. Int. J. Epidemiol. 44, 1683–1686. ( 10.1093/ije/dyv166) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Collins K, Mitchell JR. 2002. Telomerase in the human organism. Oncogene 21, 564–579. ( 10.1038/sj/onc/) [DOI] [PubMed] [Google Scholar]
- 118.Gorbunova V, Bozzella MJ. 2008. Rodents for comparative aging studies: from mice to beavers. Age (Omaha) 30, 111–119. ( 10.1007/s11357-008-9053-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gomes NMV, Shay JW, Wright WE. 2010. Telomere biology in Metazoa. FEBS Lett. 584, 3741–3751. ( 10.1016/j.febslet.2010.07.031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Haussmann MF, Winkler DW, Huntington CE, Nisbet ICT, Vleck CM. 2007. Telomerase activity is maintained throughout the lifespan of long-lived birds. Exp. Gerontol. 42, 610–618. ( 10.1016/j.exger.2007.03.004) [DOI] [PubMed] [Google Scholar]
- 121.Neumann AA, Watson CM, Noble JR, Pickett HA, Tam PPL, Reddel RR. 2013. Alternative lengthening of telomeres in normal mammalian somatic cells. Genes Dev. 27, 18–23. ( 10.1101/gad.205062.112.Freely) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Calado RT, Yewdell WT, Wilkerson KL, Regal JA, Kajigaya S, Stratakis CA, Young NS. 2009. Sex hormones, acting on the TERT gene, increase telomerase activity in human primary hematopoietic cells. Blood 114, 2236–2243. ( 10.1182/blood-2008-09-178871) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Townsley DM, et al. 2016. Danazol treatment for telomere diseases. N. Engl. J. Med. 374, 1922–1931. ( 10.1056/NEJMoa1515319) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lin J, Kroenke CH, Epel E, Kenna HA, Wolkowitz OM, Blackburn E, Rasgon NL. 2011. Greater endogenous estrogen exposure is associated with longer telomeres in postmenopausal women at risk for cognitive decline. Brain Res. 1379, 224–231. ( 10.1016/j.brainres.2010.10.033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bär C, Huber N, Beier F, Blasco MA. 2015. Therapeutic effect of androgen therapy in a mouse model of aplastic anemia produced by short telomeres. Haematologica 100, 1267–1274. ( 10.3324/haematol.2015.129239) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mooradian AD, Morley JE, Korenman SG. 1987. Biological actions of androgens. Endocr. Rev. 8, 1–28. ( 10.1210/edrv-8-1-1) [DOI] [PubMed] [Google Scholar]
- 127.Kelly DM, Jones TH. 2013. Testosterone: a metabolic hormone in health and disease. J. Endocrinol. 217, R25–R45. ( 10.1530/JOE-12-0455) [DOI] [PubMed] [Google Scholar]
- 128.White JP, Gao S, Puppa MJ, Sato S, Welle SL, Carson JA. 2013. Testosterone regulation of Akt/mTORC1/FoxO3a signaling in skeletal muscle. Mol. Cell. Endocrinol. 365, 174–186. ( 10.1016/j.mce.2012.10.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hall ME, Nasir L, Daunt F, Gault EA, Croxall JP, Wanless S, Monaghan P. 2004. Telomere loss in relation to age and early environment in long-lived birds. Proc. R. Soc. B 271, 1571–1576. ( 10.1098/rspb.2004.2768) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Metcalfe NB, Monaghan P. 2003. Growth versus lifespan: perspectives from evolutionary ecology. Exp. Gerontol. 38, 935–940. ( 10.1016/S0531-5565(03)00159-1) [DOI] [PubMed] [Google Scholar]
- 131.Geiger S, Le Vaillant M, Lebard T, Reichert S, Stier A, Le Maho Y, Criscuolo F. 2012. Catching-up but telomere loss: half-opening the black box of growth and ageing trade-off in wild king penguin chicks. Mol. Ecol. 21, 1500–1510. ( 10.1111/j.1365-294X.2011.05331.x) [DOI] [PubMed] [Google Scholar]
- 132.Bauch C, Becker PH, Verhulst S. 2012. Telomere length reflects phenotypic quality and costs of reproduction in a long-lived seabird. Proc. R. Soc. B 280, 20122540 ( 10.1098/rspb.2012.2540) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bauch C, Riechert J, Verhulst S, Becker PH. 2016. Telomere length reflects reproductive effort indicated by corticosterone levels in a long-lived seabird. Mol. Ecol. 25, 5785–5794. ( 10.1111/mec.13874) [DOI] [PubMed] [Google Scholar]
- 134.McLennan D, Armstrong JD, Stewart DC, Mckelvey S, Boner W, Monaghan P, Metcalfe NB. 2017. Shorter juvenile telomere length is associated with higher survival to spawning in migratory Atlantic salmon. Funct. Ecol. 31, 2070–2079. ( 10.1111/1365-2435.12939) [DOI] [Google Scholar]
- 135.Salmón P, Nilsson JF, Watson H, Bensch S, Isaksson C. 2017. Selective disappearance of great tits with short telomeres in urban areas. Proc. R. Soc. B 284, 20171349 ( 10.1098/rspb.2017.1349) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bateson M, Brilot BO, Gillespie R, Monaghan P, Nettle D. 2015. Developmental telomere attrition predicts impulsive decision-making in adult starlings. Proc. R. Soc. B 282, 20142140 ( 10.1098/rspb.2014.2140) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Schultner J, Moe B, Chastel O, Bech C, Kitaysky AS. 2014. Migration and stress during reproduction govern telomere dynamics in a seabird. Biol. Lett. 10, 20130889 ( 10.1098/rsbl.2013.0889) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Vedder O, Verhulst S, Bauch C, Bouwhuis S. 2017. Telomere attrition and growth: a life-history framework and case study in common terns. J. Evol. Biol. 30, 1409–1419. ( 10.1111/jeb.13119) [DOI] [PubMed] [Google Scholar]
- 139.Bebbington K, Kingma SA, Fairfield EA, Dugdale HL, Komdeur J, Spurgin LG, Richardson DS. 2017. Kinship and familiarity mitigate costs of social conflict between Seychelles warbler neighbors. Proc. Natl Acad. Sci. USA 114, E9036–E9045. ( 10.1073/pnas.1704350114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Spurgin LG, Bebbington K, Fairfield EA, Hammers M, Komdeur J, Burke T, Dugdale HL, Richardson DS. 2018. Spatio-temporal variation in lifelong telomere dynamics in a long-term ecological study. J. Anim. Ecol. 87, 187–198. ( 10.1111/1365-2656.12741) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Choi S-L, Kim S-J, Lee K-T, Kim J, Mu J, Birnbaum MJ, Soo Kim S, Ha J. 2001. The regulation of AMP-activated protein kinase by H2O2. Biochem. Biophys. Res. Commun. 287, 92–97. ( 10.1006/bbrc.2001.5544) [DOI] [PubMed] [Google Scholar]
- 142.Nettle D, Andrews C, Reichert S, Bedford T, Kolenda C, Parker C, Martin-Ruiz C, Monaghan P, Bateson M. 2017. Early-life adversity accelerates biological ageing: experimental evidence from the European starling. Sci. Rep. 7, 40794 ( 10.1017/CBO9781107415324.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Simpson K, Jones RE, Grimstead JW, Hills R, Pepper C, Baird DM. 2015. Telomere fusion threshold identifies a poor prognostic subset of breast cancer patients. Mol. Oncol. 9, 1186–1193. ( 10.1016/j.molonc.2015.02.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Shay JW, Lai T-P, Wright WE. 2018. Comparison of telomere length measurement methods. Phil. Trans. R Soc. B 373, 20160451 ( 10.1098/rstb.2016.0451) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nussey DH, et al. 2014. Measuring telomere length and telomere dynamics in evolutionary biology and ecology. Methods Ecol. Evol. 5, 299–310. ( 10.1111/2041-210X.12161) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Atema E, Mulder EGA, Noordwijk AV, Verhulst S.. 2019. Ultra-long telomeres shorten with age in nestling great tits but are static in adults and mask attrition of short telomeres. Mol. Ecol. Resour. ( 10.1111/1755-0998.12996) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Turbill C, Ruf T, Smith S, Bieber C. 2013. Seasonal variation in telomere length of a hibernating rodent. Biol. Lett. 9, 20121095 ( 10.1098/rsbl.2012.1095) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hoelzl F, Cornils JS, Smith S, Moodley Y, Ruf T. 2016. Telomere dynamics in free-living edible dormice (Glis glis): the impact of hibernation and food supply. J. Exp. Biol. 219, 2469–2474. ( 10.1242/jeb.140871) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hoelzl F, Smith S, Cornils JS, Aydinonat D, Bieber C, Ruf T. 2016. Telomeres are elongated in older individuals in a hibernating rodent, the edible dormouse (Glis glis). Sci. Rep. 6, 36856 ( 10.1038/srep36856) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Saraux C, Viblanc VA, Hanuise N, Le Maho Y, Bohec C. 2011. Effects of individual pre-fledging traits and environmental conditions on return patterns in juvenile king penguins. PLoS ONE 6, e20407 ( 10.1371/journal.pone.0020407) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cusanelli E, Chartrand P. 2015. Telomeric repeat-containing RNA TERRA: a noncoding RNA connecting telomere biology to genome integrity. Front. Genet. 6, 1–9. ( 10.3389/fgene.2015.00143) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.de Silanes IL, Grana O, De Bonis ML, Dominguez O, Pisano DG, Blasco MA. 2014. Identification of TERRA locus unveils a telomere protection role through association to nearly all chromosomes. Nat. Commun. 5, 4723 ( 10.1038/ncomms5723) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.