Abstract
Non-thermal atmospheric pressure plasmas are investigated as augmenting therapy to combat bacterial infections. The strong antibacterial effects of plasmas are attributed to the complex mixture of reactive species, (V)UV radiation and electric fields. The experience with antibiotics is that upon their introduction as medicines, resistance occurs in pathogens and spreads. To assess the possibility of bacterial resistance developing against plasma, we investigated intrinsic protective mechanisms that allow Escherichia coli to survive plasma stress. We performed a genome-wide screening of single-gene knockout mutants of E. coli and identified 87 mutants that are hypersensitive to the effluent of a microscale atmospheric pressure plasma jet. For selected genes (cysB, mntH, rep and iscS) we showed in complementation studies that plasma resistance can be restored and increased above wild-type levels upon over-expression. To identify plasma-derived components that the 87 genes confer resistance against, mutants were tested for hypersensitivity against individual stressors (hydrogen peroxide, superoxide, hydroxyl radicals, ozone, HOCl, peroxynitrite, NO•, nitrite, nitrate, HNO3, acid stress, diamide, heat stress and detergents). k-means++ clustering revealed that most genes protect from hydrogen peroxide, superoxide and/or nitric oxide. In conclusion, individual bacterial genes confer resistance against plasma providing insights into the antibacterial mechanisms of plasma.
Keywords: disinfection, atmospheric pressure plasma, iron–sulfur cluster, antibacterial mechanism, stress response, non-thermal plasma
1. Introduction
The steady discovery of multi-drug-resistant bacteria and cancers is a serious threat in modern medicine [1]. Therefore, development of new strategies, which can act alongside antibiotics and anti-cancer agents, is of growing interest. One of those therapies is the application of cold atmospheric pressure plasmas [2–4]. Several devices for plasma generation are already in clinical use for treatment of wounds or cancer, such as the kINPen MED plasma jet of neoplas (Greifswald, Germany) [5], the PlasmaDerm dielectric barrier discharge of Cinogy (Duderstadt, Germany) [6] or the MicroPlaSter or SteriPlas devices of Adtec Healthcare (Hounslow, UK) for treatment with a plasma effluent [7]. Plasma jets and plasma effluent devices use a defined gas mixture, which is ionized by application of an electric field forming the gas plasma. The plasma effluent is then blown out of a jet nozzle directly onto the patient's wound [8]. The effluents consist of a variety of reactive species, mostly oxygen and nitrogen-based such as atomic oxygen (O•), superoxide , ozone (O3), excited oxygen and nitrogen, and/or nitric oxides (NxOy). Upon interaction with an aqueous sample even more reactive species are formed (•OH, H2O2 and peroxynitrite) [9]. Several mechanisms have been described for bacterial inactivation by plasma including the oxidation or irreversible modification of essential enzymes [10] and the perturbation of the cell envelope, e.g. by lipid peroxidation [11]. Further, due to UV radiation and DNA-damaging species (e.g. hydroxyl radicals) plasma also introduces modifications to DNA causing strand breaks and mutations [12,13].
Several transcriptomic [14–16], proteomic [17,18] and metabolomic studies [19] on prokaryotic as well as eukaryotic cells were performed to gain system-wide insights into the effects of plasma on cells, the cellular response to plasma treatment and cellular defences against plasma-derived reactive species. Inspired by these studies that demonstrated upregulation of cellular stress responses as a means of physiological adaptation, we performed a genome-wide screening in Escherichia coli for plasma-protective genes that confer plasma resistance to the E. coli wild-type. Although different definitions of the term ‘resistance’ can be found in the antibiotic research literature depending on the field of research (e.g. epidemiology or clinical perspective [20]) all definitions have in common that resistance is based on genetic alterations caused by mutations or horizontal gene transfer, and thus a heritable trait [21]. In contrast to resistance, the term ‘tolerance’ refers to the transient state of a subpopulation of genetically identical bacteria that allows this subpopulation to survive antibiotic treatment better than their growing peers. Tolerance is thus not based on a genetic difference, but the result of a different cellular state. One example is the dormancy of cells that refers to a state of reduced metabolism that makes the dormant subpopulation less vulnerable to antibiotics than the fast-growing part of the population [20,21]. In the present study, we address the phenomenon of plasma resistance, since E. coli strains with different genetics were analysed (deletion mutants or strains over-expressing certain genes). Typically, vegetatively growing bacteria survive only short plasma exposures. We show here, that mutants lacking certain protective genes are hypersensitive and survive even shorter plasma exposures. This difference in plasma sensitivity indicates that there are genes that, by means of the encoded protein products, confer a basic level of plasma resistance to wild-type cells. The identification of plasma-protective genes helps identify targets of plasma in cells contributing to our understanding of plasma-mediated bacterial inactivation. The characterization of inherent protection mechanisms may also provide insights into genetic changes that lead to bacteria surviving longer plasma exposures, giving rise to plasma-resistant strains in the future.
2. Results
2.1. Screening for plasma-sensitive mutants
A collection of E. coli single-gene knockout mutants (KEIO collection) [22] was screened to identify genes and proteins that protect wild-type cells when exposed to non-thermal plasma. This strain collection of 3985 strains each lacking a single non-essential gene is comprehensive. It covers the entire E. coli genome. The screening was performed with a microscale atmospheric pressure plasma jet (µAPPJ) operated with He/O2 as feed gas [8]. Similar devices are already used as medical devices to treat patients [21]. Screening was performed by growing the mutant strains in 96-well microtitre plates in LB broth overnight and then spotting 2 µl of culture onto LB agar plates with a replicator. Spotted cells were exposed to the effluent of the plasma jet for 100 s and incubated at 37°C for 16 h. When the wild-type was spotted and exposed, colonies were observed after the 16 h incubation. To identify knockout strains with increased plasma sensitivity, each strain was spotted and exposed three times in independent experiments and the results were scored. Mutants accrued one point for each experiment in which no colonies were detected after the 16 h incubation. Score values of 2 indicated that the knockout mutant did not survive plasma treatment in two out of three biological replicates (electronic supplementary material, figure S1). Mutants with a score of 2 or higher were termed ‘plasma sensitive’ and analysed further.
Of the 3985 mutants tested, 87 (equalling 2.2%) exhibited an increased plasma sensitivity (table 1). Information on the encoded proteins was obtained from the NCBI and UniProt databases [23]. Protein properties of the set of 87 were compared to all E. coli proteins with regard to subcellular protein localization, metal cofactors, amino acid representation and functional category to identify potential patterns in the data.
Table 1.
Plasma-protective genes. Strains of the KEIO collection were spotted onto LB agar and exposed to the effluent of the µAPPJ for 100 s. The jet was driven with He and a 0.6% O2 admixture. Genes were listed as protective when the respective knockout mutant did not form colonies after plasma exposure and a 16 h incubation in at least two out of three experiments. Annotations are based on NCBI database.
| # | gene | protein | NCBI ID | # | gene | protein | NCBI ID |
|---|---|---|---|---|---|---|---|
| 1 | abgT | p-aminobenzoyl glutamate:H+ symporter | 945912 | 29 | holC | DNA polymerase III, χ subunit | 948787 |
| 2 | ada | DNA-binding transcriptional regulator | 946710 | 30 | holD | DNA polymerase III, ψ subunit | 948890 |
| 3 | apaH | diadenosine tetraphosphatase | 944770 | 31 | ihfA | integration host factor, α subunit | 945472 |
| 4 | asnB | asparagine synthetase B | 945281 | 32 | ihfB | integration host factor, β subunit | 945533 |
| 5 | atoA | β subunit, acetate CoA transferase | 946719 | 33 | iscS | cysteine desulfurase | 947004 |
| 6 | atoE | short chain fatty acid transporter | 946721 | 34 | iscU | scaffold protein for iron–sulfur cluster assembly | 947002 |
| 7 | bamB | outer membrane protein assembly complex | 946982 | 35 | katE | catalase II | 946234 |
| 8 | bcsC | cellulose biosynthesis protein | 948047 | 36 | lpd | lipoamide dehydrogenase | 944854 |
| 9 | carA | carbamoyl phosphate synthetase | 949025 | 37 | lsrD | autoinducer-2 ABC transporter | 946264 |
| 10 | cpxA | sensory histidine kinase | 948405 | 38 | menC | O-succinylbenzoate synthase | 946734 |
| 11 | csgG | curli secretion channel | 945619 | 39 | mglB | d-galactose/d-galactoside ABC transporter | 949041 |
| 12 | cysB | transcriptional regulator | 945771 | 40 | mngA | 2-O-α-mannosyl-d-glycerate PTS permease | 945355 |
| 13 | degP | serine protease Do | 947139 | 41 | mntH | Mn2+/Fe2+:H+ symporter | 946899 |
| 14 | dksA | RNA polymerase-binding transcriptional factor | 944850 | 42 | moaC | cyclic pyranopterin monophosphate synthase | 945397 |
| 15 | dnaT | primosomal protein | 948813 | 43 | nagC | DNA-binding transcriptional dual regulator | 945285 |
| 16 | ecnB | bacteriolytic entericidin B lipoprotein | 2847737 | 44 | napC | periplasmic nitrate reductase | 946706 |
| 17 | essQ | Qin prophage, lysis protein | 946093 | 45 | nlpE | outer membrane lipoprotein involved in surface sensing | 946782 |
| 18 | fimC | periplasmic chaperone for type 1 fimbriae | 948843 | 46 | oppD | murein tripeptide ABC transporter | 945802 |
| 19 | fimD | export and assembly of type 1 fimbriae | 948844 | 47 | oxyR | OxyR DNA-binding transcriptional dual regulator | 948462 |
| 20 | flgB | flagellar basal-body rod protein FlgB | 945678 | 48 | pepQ | XAA-Pro dipeptidase | 948335 |
| 21 | flgG | flagellar basal-body rod protein FlgG | 945647 | 49 | pgm | phosphoglucomutase | 945271 |
| 22 | ftn | ferritin iron storage protein | 946410 | 50 | pnp | polynucleotide phosphorylase | 947672 |
| 23 | fucA | l-fuculose-phosphate aldolase | 947282 | 51 | poxA | EF-P-lysine lysyltransferase | 7156434 |
| 24 | gspL | putative protein secretion protein for export | 947842 | 52 | priA | primosome factor N' | 948426 |
| 25 | guaA | GMP synthetase | 947334 | 53 | purH | AICAR transformylase/IMP cyclohydrolase | 948503 |
| 26 | hdeB | acid stress chaperone | 948026 | 54 | rcsC | sensory histidine kinase | 948993 |
| 27 | hicA | toxin of the HicA–HicB toxin–antitoxin system | 945989 | 55 | recA | DNA strand exchange and recombination protein | 947170 |
| 28 | hlpA | chaperone protein | 944861 | 56 | rep | DNA helicase | 948292 |
| 57 | rimM | ribosome maturation protein | 947101 | 73 | ydhQ | conserved protein | 944851 |
| 58 | rnt | RNase T | 946159 | 74 | ydhX | predicted 4Fe-4S ferredoxin-type protein | 947308 |
| 59 | rpmJ | 50S ribosomal subunit protein L36 | 947805 | 75 | ydiL | conserved protein | 946181 |
| 60 | rsxA | integral membrane protein of SoxR-reducing complex | 946148 | 76 | ydjA | predicted oxidoreductase | 945964 |
| 61 | rsxC | member of SoxR-reducing complex | 946137 | 77 | yeiA | NADH-dependent dihydropyrimidine dehydrogenase subunit | 949037 |
| 62 | sufB | SufBCD iron–sulfur cluster scaffold protein | 945753 | 78 | yeiG | S-formylglutathione hydrolase/S-lactoylglutathione hydrolase | 949045 |
| 63 | thyA | thymidylate synthase | 949035 | 79 | yfaU | 2-keto-3deoxy-l-rhamnoate aldolase | 948054 |
| 64 | trpB | tryptophan synthase, β subunit | 945768 | 80 | ygjR | predicted NAD(P)-binding dehydrogenase | 947600 |
| 65 | tufA | elongation factor Tu | 947838 | 81 | yifL | predicted lipoprotein | 1450304 |
| 66 | wzxE | lipid III flippase | 948294 | 82 | yigA | conserved protein | 948359 |
| 67 | yaiT | putative protein | 4056040 | 83 | ymgG | putative protein | 945728 |
| 68 | ybcS | DLP12 prophage, lysozyme | 7156271 | 84 | yncK | predicted transposase | 4056028 |
| 69 | ybdO | predicted DNA-binding transcriptional regulator | 945216 | 85 | yojL | flavin transferase | 946711 |
| 70 | ycjU | β-phosphoglucomutase | 945891 | 86 | yqfA | predicted oxidoreductase | 947381 |
| 71 | ydeS | predicted fimbrial-like adhesin protein | 946047 | 87 | zapA | cell division factor | 947404 |
| 72 | ydfH | DNA-binding transcriptional regulator | 7158646 |
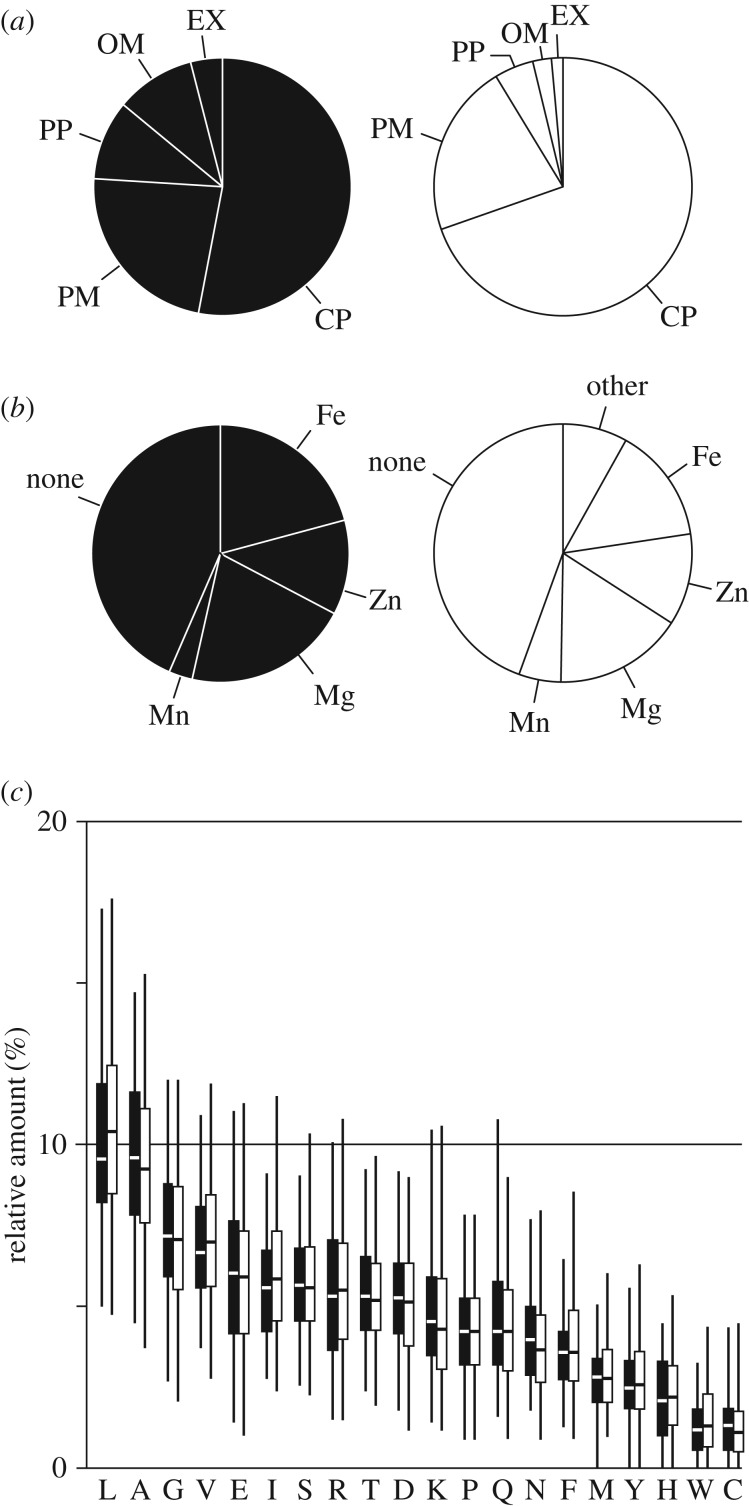
The 87 proteins were analysed with regard to their subcellular localization to see if e.g. extracellular proteins were over-represented in the screening (figure 1a). While the majority of the 87 proteins are located in the cytoplasm, compared to all proteins of E. coli, the set of 87 is enriched in proteins with extracellular localization, i.e. proteins located in the outer membrane or in the periplasm. For instance, in this screening FlgB and FlgG (flagellar basal-body rod proteins) [24] as well as FimC and FimD (type 1 fimbriae formation) [25] came up. Based on our current knowledge of the proteins, their role in protecting the cells against plasma is not obvious. Possibly, extracellular proteins act as unspecific scavengers of reactive species at the cell surface.
Figure 1.
Properties of the 87 identified proteins compared to the entirety of E. coli proteins. (a) Subcellular localization of the set of 87 plasma-protective proteins (black) compared to all proteins (white). CP, cytoplasm; EX, extracellular; OM, outer membrane; PM, plasma membrane; PP, periplasm. (b) Metal cofactors of the 87 proteins (black) compared to all proteins (white). (c) Relative content of each amino acid per protein averaged over the set of 87 proteins (black bars) or over all E. coli proteins (white bars). Horizontal lines mark the median. The whiskers cover 95% of all data points. For all comparisons (a–c), the UniProt database was used as reference [23].
Transition metals are redox-active and many enzymes, such as catalases or superoxide dismutases contain metal cofactors that are needed to complete the catalytic cycle [26,27]. We therefore compared the set of 87 proteins to all E. coli proteins with regard to metal cofactor content (figure 1b). We found that iron-containing proteins were over-represented (37% in our dataset compared to 26% in all proteins encoded in the E. coli genome), but no over-representation was observed for other metal ions or the overall presence of metal cofactors.
It has been observed previously that some amino acids are more susceptible to plasma-induced modifications than others [28]. Cysteine for instance is one of the most vulnerable amino acids as it is easily oxidized to sulfenic or sulfonic acid [10,27–29]. We investigated the amino acid composition of the proteins in the set of 87 proteins and compared it to the average for E. coli proteins. However, on the whole, no amino acid was over-represented significantly, neither those with comparably reactive groups like cysteine, nor those with aromatic rings like tryptophan or histidine (figure 1c). The average cysteine content of E. coli proteins is 1.3% (figure 1c). And while most of the 87 plasma-protective proteins have a similar cysteine content (on average 1.4%), YdhX contains 7.2% cysteines (16 cysteine residues of 222 amino acids total). It ranks among the top 30 E. coli proteins with regard to relative cysteine content, making YdhX a very cysteine-rich protein. YdhX is a predicted [4Fe-4S]-ferredoxin-type protein with yet unknown function located in the outer membrane facing the periplasm [29]. Interestingly, in the screening proteins directly involved in asparagine (AsnB), cysteine (CysB) and tryptophan (TrpB) biosynthesis were identified as protective, suggesting that these amino acids might be of special importance as free amino acids and/or as sulfur donors for repair mechanisms after plasma treatment.
To identify pathways and functional categories that might be over-represented, the 87 genes were grouped based on GO terminology (electronic supplementary material, figure S2) [30]. Protein-related pathways were found to be most strongly represented. This includes protein synthesis, translocation, protection and degradation.
2.2. Quantitative analyses
The screening of the KEIO collection was based on a qualitative assay that only distinguishes between growth and no growth after plasma exposure. To determine the influence of a general growth defect that the knockout mutants might have, the 87 mutants were tested for plasma sensitivity in a quantitative assay that takes into account plasma-independent growth defects. Cell suspensions containing approximately 500 colony forming units (CFU) in 30 µl LB broth were exposed for 30 s to the µAPPJ operated as described above or left untreated. The cells were then plated onto agar and CFU counted after overnight incubation. With this quantitative approach, the plasma sensitivity of about 20 mutants was confirmed to be significantly higher compared to the wild-type, which means that when cell suspensions were treated with plasma the mutants did not survive as well as the wild-type (electronic supplementary material, figure S3). The strain ΔiscS lacking the cysteine desulfurase of one of the two iron–sulfur cluster biosynthesis operons [31] exhibited the lowest survival rate after plasma treatment (22%) compared with the wild-type (60%). The second most sensitive strain was Δrep with 29% survival rate. Rep is an ATP-dependent DNA helicase and a component of the replisome [32]. It has been shown to be crucial for the resumption of replication after UV radiation-induced replication arrest [33]. Since the plasma jet emits UV radiation [8], the role of Rep in plasma resistance seems evident.
The knockout mutants of the KEIO collection were made by substituting the open reading frames with a kanamycin resistance cassette [22]. To ensure that the observed plasma sensitivity was indeed a consequence of the gene replacement, exemplarily four deletion mutants with significantly increased plasma sensitivity (ΔcysB, ΔiscS, ΔmntH and Δrep) were complemented with the deleted gene encoded episomally on the plasmid pCA24N (figure 2). The plasmids were taken from the ASKA collection, a comprehensive plasmid library for over-expression of E. coli genes [34]. Controls with the empty vector (not expressing an E. coli gene) were performed as well to control for effects of plasmid replication and maintenance (selection pressure by chloramphenicol). Induction was performed with IPTG concentrations ranging from 0.01 to 1000 µM. The production of the plasmid-encoded proteins was analysed by western blot analysis (figure 2a). Furthermore, growth of the strains at each IPTG concentration was recorded (electronic supplementary material, figure S4). For each complemented strain, an IPTG concentration was chosen for plasma sensitivity assays based on protein expression levels and growth rates of the mutants (electronic supplementary material, figure S4). The survival rates of all complemented strains were significantly higher compared with the corresponding deletion strains harbouring the empty vector as control (figure 2b). For ΔcysB, ΔiscS and Δrep complementation caused the survival rate to increase above that of the wild-type harbouring pCA24N, which indicates that over-production of these proteins can increase the plasma resistance of bacteria. For ΔmntH the effect of complementation was less pronounced.
Figure 2.
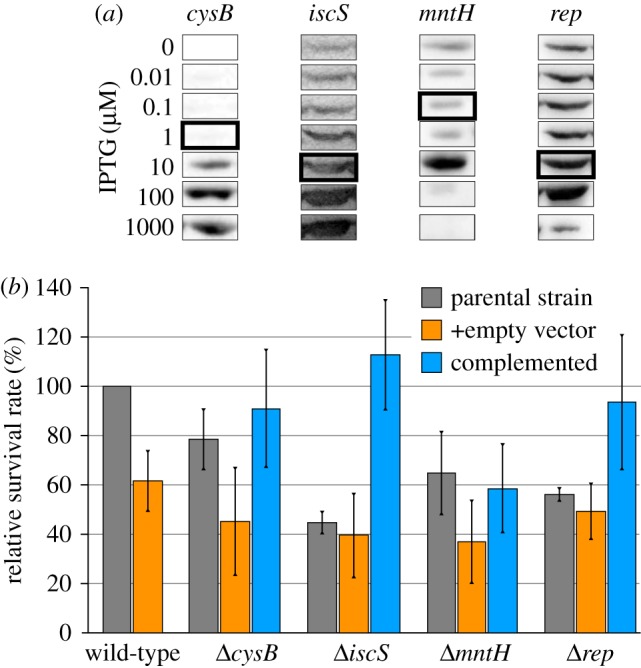
Protein levels and plasma sensitivity of complemented deletion strains. (a) Western blot analysis with anti-His5 antibody was performed to measure protein levels at different IPTG concentrations. The IPTG concentrations of the highlighted expression levels were chosen for the plasma sensitivity assay. (b) Complementation was performed by inducing with 0.1 µM IPTG (mntH), 1 µM (cysB) or 10 µM (iscS, rep). Strains harbouring the empty vector (pCA24N) served as control. Relative survival rates were calculated by dividing the CFU of plasma-treated samples by the CFU of the gas-treated control and by subsequently normalizing to the wild-type (set to 100%). Experiments were performed three times independently. Averages and standard deviations are shown. (Online version in colour.)
2.3. Stressors mimicking individual plasma-derived stress factors
Plasma is a cocktail mostly consisting of short-lived reactive species, like excited states of helium, oxygen, and nitrogen, atomic oxygen, or superoxide. When in contact with water (in air or in aqueous samples), these species recombine giving rise to a different set of reactive molecules, e.g. peroxynitrite or hydroxyl radicals. Formation of hypochlorous acid (HOCl) is also possible, when atomic oxygen reacts with chloride ions in the liquid [9].
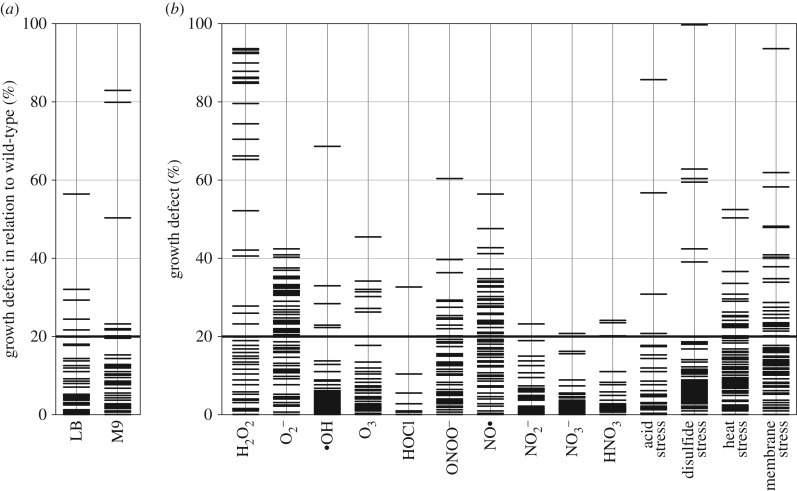
For some of the identified proteins, the participation in plasma resistance can be explained, like for the helicase Rep described above, the proteolytic enzyme DegP (periplasmic endoprotease), which participates in degrading damaged proteins [35], AtoA/E, which engage in short chain fatty acid degradation [36] and could thus play a role in protecting cells from lipid oxidation, or the hydrogen peroxide detoxifying KatE (catalase E) [26]. For many others, like the above-mentioned FlgB/G, the roles remain to be elucidated. To gain first insights into which plasma components the 87 genes protect the cells from, the mutants were tested for their sensitivity to conditions mimicking single species generated by plasma, plasma air and plasma–liquid interaction. Some molecules like H2O2, nitrite and nitrate were added to the medium directly, while stressors like paraquat, nitroprusside and copper ions combined with hydrogen peroxide (promoting Fenton reaction) were used to generate , NO• and •OH, respectively. Further, acid stress (generated by HCl), heat stress or membrane stress (induced by SDS, CTAB, saponine or Triton X-100) were tested. With the exception of nitrate, the mutants were incubated with each stressor at a defined concentration, at which the growth of the wild-type was reduced to about 60% compared with an untreated control. Since none of the tested nitrate concentrations (0.1–45 mM) inhibited the growth of the wild-type, 10 mM nitrate were used in the assay (higher concentrations are not expected to be generated during treatment with the µAPPJ [37]). The growth of the mutants was measured after a 16 h incubation with each stressor, and normalized first to an untreated culture and subsequently to the wild-type (figure 3). The greater the growth defect, the higher the sensitivity of the mutant to that particular stress condition. Already without addition of external stressors, five strains showed a growth defect in LB medium (ΔcysB, ΔfimC, ΔguaA, ΔiscS and ΔpriA), and eight other mutants exhibited reduced growth in M9 minimal medium (ΔapaH, ΔdksA, ΔnlpE, Δpnp, ΔtrpB, ΔtufA, ΔydiL and ΔyigA) (figure 3a). These results confirm that general growth restrictions are indeed occurring and need to be considered when comparing plasma resistance of the knockout strains.
Figure 3.
Effects of stressors on the growth of 87 plasma-sensitive strains. (a) To determine growth defects of the mutants in LB and M9 medium at 37°C, the OD after a 16 h incubation in the respective medium was set in relation to that of the wild-type, for which the growth defect was set to 0%. (b) To determine growth defects of the strains in the presence of stressors, cells were incubated in LB medium containing the indicated plasma-mimicking components. Acid stress was caused by HCl, disulfide stress by diamide, heat stress by overnight incubation at 40.5°C, and membrane stress by addition of different detergents. The OD of the deletion strains after 16 h incubation was set in relation to the corresponding unstressed control and subsequently in relation to the wild-type (set to 0% growth defect). A growth defect greater than 20% was regarded significant. All data represent three independent biological replicates.
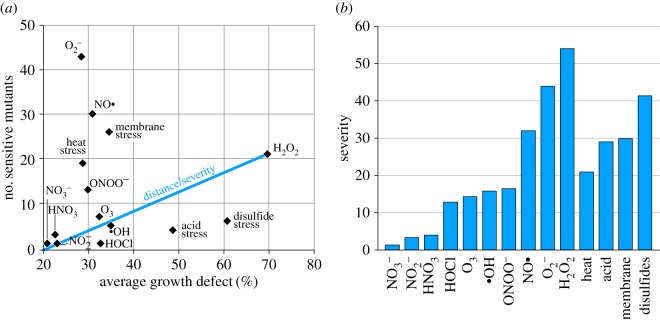
Strains exhibiting a growth defect of 20% or higher when exposed to a stressor were termed ‘sensitive’ towards that stressor (figure 3b). To compare the importance of the stressors, for each stressor the number of sensitive mutants was plotted against the average growth defect of these mutants (figure 4a). We used a ‘severity score’ based on the Euclidean distance (0, no impact; 118 maximal impact on all mutants; figure 4b). According to the severity score, hydrogen peroxide had the highest impact causing a growth defect of 70% in 21 mutants (severity score of 54). Superoxide was another important stressor, affecting the growth of 43 mutants, with a 29% inhibition on average (severity score 44). These results indicate that wild-type bacteria have a number of genes that protect them from two of the main plasma-derived reactive oxygen species.
Figure 4.
Comparison of the importance of individual stressors. (a) The number of knockout mutants with a growth defect greater 20% were plotted against the average growth defect of those strains. To illustrate the calculation of the severity score, the distance of the stressor H2O2 to the point of origin is shown as an example. (b) Severity score based on the Euclidean distance of each stressor from the point of origin. (Online version in colour.)
The tested nitrogen salts impacted only weakly on the growth of few of the E. coli mutants resulting in severity scores below 5 and indicating that they are probably not important in bacterial resistance against plasma. The addition of 4 mM HNO3 decreased the pH of the medium to 5.5 by dissociation into H+ and , but even at that low pH, nitrate had little effect on the mutants. Peroxynitrite, a strong oxygen- and nitrogen-based oxidant causing e.g. lipid peroxidation, had a severity score of 16 (figure 4b). Of all reactive nitrogen species tested, nitric oxide generated by the decay of sodium nitroprusside affected the highest number of mutants (30 mutants, severity score 32).
The four strains that were selected for complementation studies (ΔcysB, ΔiscS, ΔmntH and Δrep) and tested for curing the plasma-sensitive phenotype (figure 2), were also tested for curing stressor sensitivity (figure 5a). The strain ΔiscS pCA24N (empty vector) exhibited an increased H2O2 sensitivity. No growth was detectable at 0.6 mM H2O2, whereas the wild-type harbouring the empty vector still showed growth at 1 mM H2O2. Complementation of ΔiscS partially restored the wild-type phenotype and the complemented mutant grew at 0.8 mM. The complementation further cured the sensitivity against superoxide, SDS, and an acidic milieu (electronic supplementary material, figure S5A). The complementation of the ΔcysB mutant did not restore H2O2 (figure 5a) or peroxynitrite resistance to wild-type levels (electronic supplementary material, figure S5B). The complementation of Δrep increased the H2O2 resistance beyond wild-type levels such that growth was detected at 2 mM H2O2 (figure 5a). Similarly, the complementation of ΔmntH enabled the cells to grow at elevated superoxide levels (electronic supplementary material, figure S5C).
Figure 5.
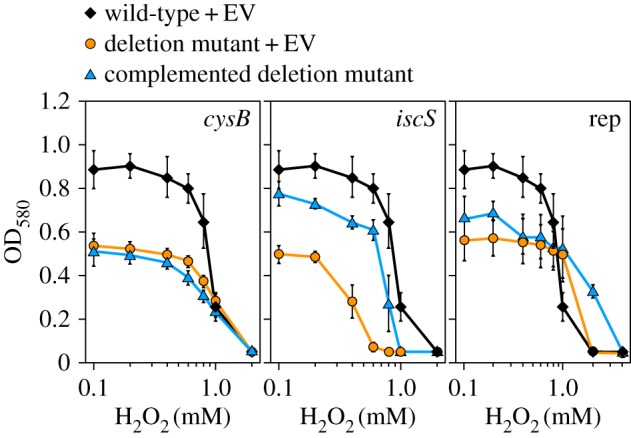
Stressor sensitivity of complemented strains. (a) The OD after 16 h incubation at varying concentrations of H2O2 is shown for wild-type and deletion strains harbouring the empty vector (EV) pCA24N and for the corresponding complemented strains. Complementation was performed by induction with 1 µM IPTG (cysB) or 10 µM (iscS, rep), respectively. The data represent three independent experiments. Averages and standard deviations are shown. (Online version in colour.)
2.4. Clustering
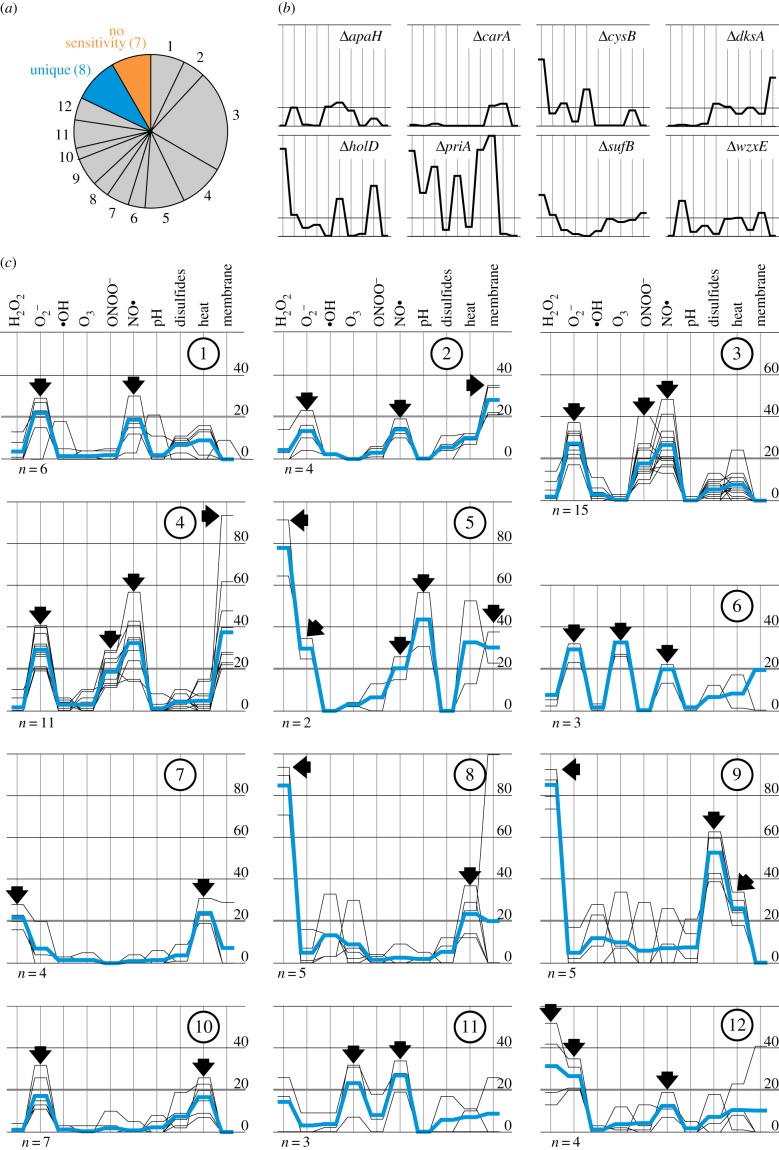
In the first screening, several genes organized in operons or belonging to the same metabolic pathways were identified, like atoA/E, fimC/D, flgB/G, holC/D, ihfA/B, iscS/U/sufB and rsxA/C. A clustering based on the k-mean++ algorithm was performed to reveal whether mutants belonging to the same pathways exhibit similar profiles with regard to stressor sensitivity as determined in the second screening approach (figure 6). The stressors with the lowest severity scores (nitrite, nitrate, nitric acid and HOCl) were excluded from this calculation to lower the number of dimensions and to thereby increase the reliability of the clustering. The strains sensitive to all stressors (ΔholC, ΔlpdA and ΔrimM) or none of the stressors (ΔasnB, ΔcsgG, ΔhicA, ΔnapC, ΔoppD, ΔyfaU and ΔzapA) were excluded (figure 6a). Eight mutants exhibited a unique stressor profile and were not grouped (figure 6b). The remaining 72 mutants were sorted into 12 clusters (figure 6c).
Figure 6.
The mutants were clustered based on their sensitivity against plasma-relevant stressors. (a) Mutants are grouped into 12 clusters, strains with unique profiles and strains not sensitive to any of the tested stressors. (b) Stressor profiles of the strains with unique stressor profiles. The order of the stressors is the same as in (c). (c) The 12 clusters (numbered 1–12) were generated using the k-mean++ clustering algorithm. Arrows mark the stressors characteristic for each cluster; n indicates the number of strains in the cluster and blue lines represent the average stressor profile of the cluster members. (Online version in colour.)
As expected, some mutants impaired in the same operon or pathway grouped together in the same cluster. The two strains ΔiscS and ΔiscU forming cluster #5 are defective in one of the two iron–sulfur cluster biosynthesis pathways and have very similar stressor profiles (sensitive against H2O2, , NO•, acidic pH and membrane stress). ΔsufB, another strain lacking a gene of the iron–sulfur cluster biosynthesis network, had a unique stressor profile. Two strains ΔihfA and ΔihfB missing genes encoding different subunits of histone-like proteins grouped to cluster #2 and #1, respectively. Both are sensitive to superoxide and nitric oxide, but the ΔihfA strain, like the other strains in cluster #2, was also susceptible to general membrane stress. Both the genes cpxA and degP are part of the heat shock response [38] and the knockout strains both cluster in cluster #8.
The hallmark of cluster #10 is a significant sensitivity against superoxide. The strain ΔmntH previously used in complementation experiments (figures 2 and 4c) is part of this cluster. We investigated whether superoxide is indeed the plasma component causing the ΔmntH strain to be plasma-sensitive. To counteract superoxide stress, sodA (encoding a cytoplasmic superoxide dismutase) was over-expressed from the plasmid pCA24N::sodA in the ΔmntH background (figure 7). Induction of sodA with 10 µM IPTG completely restored the plasma resistance of the ΔmntH mutant (compare WT with empty vector, ΔmntH with empty vector and ΔmntH with pSodA) emphasizing that indeed superoxide is the plasma-generated species responsible for the sensitivity of the ΔmntH mutant.
Figure 7.
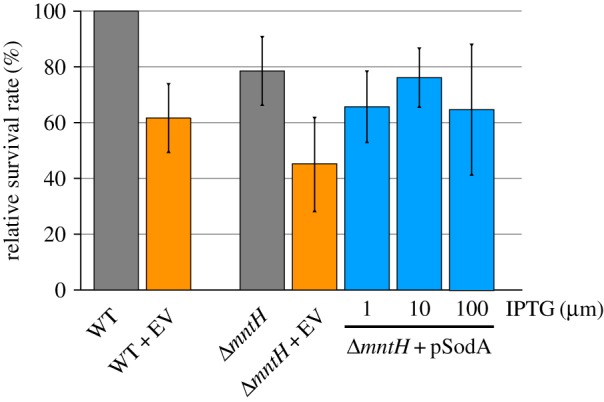
Plasma sensitivity of the wild-type and a ΔmntH mutant harbouring either the empty vector (EV) or the plasmid pCA24N::sodA (pSodA) was investigated by determining survival rates relative to the wild-type after 30 s plasma treatment. sodA was induced with increasing amounts of IPTG. The data represent three independent experiments. Averages and standard deviations are shown. (Online version in colour.)
3. Discussion
The reaction of different eukaryotic and prokaryotic cells to plasma treatment was already investigated on different levels using system-wide approaches like transcriptomic [14–16], proteomic [17,18] and metabolomic analyses [19]. Here, we focused on the genetic level. Using a genome-wide screening approach, we identified genes that contribute to the capability of E. coli to survive short exposures to the effluent of a plasma jet operated with a He/O2 mixture as feed gas.
3.1. What do the plasma-sensitive mutants reveal about plasma targets?
The first screening of the KEIO collection of approximately 4000 E. coli single-gene knockout mutants [20] for sensitivity to treatment with the µAPPJ identified 87 genes that mediate protection against plasma. Among these are genes indicative of DNA damage such as ada encoding a transcriptional regulator with protective function against DNA-alkylating agents [39], or recA [40] with a number of functions related to DNA repair and recombination, most prominently the SOS response allowing error-prone DNA repair when high fidelity repair systems are overwhelmed. Two other genes do not encode proteins considered classical stress proteins but are also connected functionally to DNA. The phosphoglucomutase Pgm is responsible for the breakdown of glycogen to maltose and galactose [41]. Besides this role in supplying energy, Pgm was found to indirectly modulate DNA topology and favour supercoiled structures in a high-throughput screening for DNA topology-manipulating proteins in E. coli [42]. A similar role was attributed to DksA [42], an RNA polymerase-binding transcription factor, which in addition participates in the repair of double-strand DNA breaks [43]. While DNA-damaging effects of plasma have been observed and are actively being studied by different groups, the influence of the degree of supercoiling on plasma sensitivity has not yet been considered in great depth.
Several genes encoding components of cellular appendices were identified as protective: proteins of the flagellar apparatus (FlgB, FlgG), fimbriae formation (BamB, FimC and FimD) and curli assembly (CsgG). These structures are responsible for motility [24], adhesion to surfaces and biofilm formation [44], respectively, and are thus needed under different growth conditions. Rather than by functional commonality, the different cell appendices might mediate protection from plasma components in an unspecific manner, e.g. by acting as scavengers of reactive species on the cell surface preventing their entering the cell.
Iron-containing proteins were over-represented among the 87 proteins. In particular, mntH is a manganese and iron transporter induced upon iron limitation [45] and at least seven of the identified genes are directly or indirectly involved in the biosynthesis (iscS, iscU and sufB), repair (ftn), or regulation (ihfA, ihfB and oxyR) of iron–sulfur clusters [45]. Iron–sulfur clusters are important cofactors in many cellular pathways and processes, i.e. in the respiratory electron transport chain and the TCA cycle [46,47]. However, iron–sulfur clusters are known to be highly sensitive to oxidation by several ROS, especially superoxide [48]. Taken together, the over-representation of iron-containing metalloproteins, the identification of the iron–sulfur cluster-related genes, the high severity score of superoxide and the protective effects of sodA over-expression in a ΔmntA mutant indicate that the disruption of iron–sulfur clusters poses a severe challenge to cells exposed to plasma. As the oxidative inactivation of those clusters has been described to be very fast (rate constants in the order of 108–109 M−1 s−1) [49–51], targeting of these structures might be among the first survival-limiting challenges occurring during plasma treatment.
3.2. What do the plasma-sensitive mutants reveal about plasma components?
The effluent of the µAPPJ is composed of a versatile mixture of reactive oxygen species. At the relevant distance of the jet from the sample, atomic oxygen (approx. 1014 cm−3), ozone (approx. 1015 cm−3) and superoxide (1011 cm−3) have been identified as dominant species [8]. Atomic oxygen has recently been shown to enter the liquid phase [49]. For the generation of H2O2 or NO•, humidity or impurities from ambient air are necessary, respectively [50]. All those species target a variety of molecules in cells. In the presence of water, superoxide and nitric oxide readily recombine to peroxynitrite [51], which then reacts directly with biomolecules like metalloproteins [52] and thiols [53]. Depending on the pH and CO2 availability, peroxynitrite can decay to •NO2 and or •NO2 and •OH, causing nitrosylations and oxygenations of proteins, DNA and lipids [54]. Owing to this diverse range of reactions, peroxynitrite is often described as more harmful to cells than either superoxide or nitric oxide alone [51,55]. In recent studies, the antibacterial properties of liquids exposed to plasma were even attributed mainly to peroxynitrite [56,57].
In the presented stressor profiling, H2O2, and NO• were the stressors with the highest severity scores (figure 4b), indicating that when the respective protective genes are missing, the cells become highly sensitive to plasma exposure. Among the plasma-protective proteins were for instance the stress-inducible catalase KatE [26] and the hydrogen peroxide-sensing transcriptional regulator OxyR [58], which regulates a number of genes protecting from hydrogen peroxide stress. The clustering of the mutants based on stressor profiles resulted in seven clusters (#1–6, #12) with sensitivity towards superoxide and nitric oxide, encompassing 45 of the 72 strains. Both reactive species target similar molecules in cells, like metallo- and iron–sulfur cluster proteins [52] or DNA [48,59–61] that are discussed as plasma targets above. Somewhat surprisingly, the stressor profiling indicated that reactive nitrogen species like NO• play an important role in bacterial inactivation. Given that nitrogen was not admixed in the process gas, reactive nitrogen species can stem from impurities of the feed gases or secondary reactions with ambient air-derived nitrogen. Nevertheless, both sources of nitrogen have been shown to play only minor roles regarding the device and distance to the sample used here [62,63]. Further reactive nitrogen species might form by interaction of plasma-generated species with the liquid phase, like the medium or cellular components. When using plasmas with higher levels of reactive nitrogen species, we would expect to observe an increased importance of reactive nitrogen protective mechanisms, i.e. more mutants lacking genes encoding reactive nitrogen detoxifying genes. Such a plasma could be generated, for example, by increasing the distance of the µAPPJ to the sample, as the admixture of ambient air increases from almost zero at 4 mm to about 20% with 2 cm distance [62].
In our screening, more genes protecting against and NO• were identified than genes protecting from peroxynitrite. This could reflect that the bacterial defence is focused on detoxifying the two precursors rather than the recombination product peroxynitrite. It could further reflect an adaptation of E. coli to interacting with the mammalian immune system, which exposes E. coli to superoxide and nitric oxide, which act as precursors of other reactive oxygen and nitrogen species including peroxynitrite [51]. However, it might also simply be a reflection of the number of genes that E. coli harbours that protect against individual stressors and the type of stressors that were available for profiling. Many plasma-generated species and components as well as recombination products were not tested as individual stressors, like atomic oxygen, singlet oxygen, or UV radiation, although cellular targets of those species are described manifold [64,65]. Furthermore, plasma jets like the one applied here use noble gases for ionization. Winter et al. observed a significant impact of argon gas treatment (without plasma ignition) on the proteome of Bacillus subtilis [17]. The effect of the helium/oxygen gas used in this study was not investigated. Nonetheless, our screening revealed that for inherent plasma resistance, E. coli relies heavily on mechanisms of detoxification of species stemming from the effluent, namely H2O2, and NO•.
3.3. Implications of inherent plasma resistance for medical applications
Plasmas are complex and dynamic mixtures exerting antibacterial properties through reactive species, (V)UV emission, and some by means of electric fields. The joint action of multiple stressors acting on multiple molecular targets simultaneously has led to the possibility of emergence of plasma resistance being broadly discounted [66,67]. Yet, plasma resistance has already been described at least once [68]. In the present study, we identified a set of genes that when missing leave the bacterial cell less well protected from plasma, thus conferring a certain level of resistance to the wild-type. The complementation and over-expression studies, e.g. of the strains ΔiscS pCA24N::iscS and ΔcysB pCA24N::cysB indicate that some of the genes can increase the level of plasma resistance beyond the levels of the wild-type with empty vector simply through elevated levels of a single protein (figure 2). Such an elevation in gene expression can easily occur in natural mutants e.g. by point mutations in the promoter region [69,70]. Additionally, plasmas have been shown to be mutagenic [10,13,71], thus they may increase the frequency at which such mutants occur. It is therefore conceivable that strains over-producing protective proteins evolve under non-laboratory conditions and that their evolution is expedited by plasma exposure both through increased mutation rates and selective pressure. The duration of plasma exposure tolerated by prokaryotic and eukaryotic cells are within the same order of magnitude ranging from seconds to a few minutes depending on the device and treatment conditions [72,73]. An increase in plasma resistance of bacteria by a factor of 3–10 may therefore already limit the clinical application of plasmas as antibacterial strategy.
4. Experimental procedure
4.1. Plasma sources
Plasma was generated by a well-described microscale atmospheric pressure plasma jet (µAPPJ) [8]. Helium (1.4 slm, 5.0 purity) with an admixture of oxygen (0.6%, 0.0084 slm, 4.8 purity) was used as the feed gas. Plasma was driven with 13.56 MHz and 230 VRMS. Samples were placed at 4 mm distance to the nozzle of the jet.
To generate ozone as a stressor (see below), a dielectric barrier discharge plasma device (Cinogy, Duderstadt, Germany) was used [6,74]. The copper electrode had a diameter of 20 mm and was driven with a pulsing frequency of 300 Hz with 13.5 kV peak amplitude.
4.2. Strains, media and chemicals
The KEIO collection of single-gene knockout mutants was used [22]. All strains were cultivated in LB medium at 37°C under aerobic conditions. Knockout mutants were maintained in the presence of 50 µg ml−1 kanamycin. The strains were stored in 96-well microtitre plates filled with Hogness modified freezing medium at −80°C [75]. Before usage for experiments, cells were freshly stamped into new microtitre plates filled with LB medium and incubated overnight.
All chemicals used for media, as additives, stressors or for synthesis were reagent grade and purchased from Sigma Aldrich.
4.3. Screening of the KEIO collection
From each well of a microtitre plate containing different strains 2 µl were dropped onto an LB agar plate using a 96-well replicator. After a 2 h incubation at 37°C, the spots were treated with the effluent of the μAPPJ for 100 s. Afterwards, the plates were incubated at 37°C overnight. The experiment was repeated three times independently. For each mutant, the number of experiments in which no cells grew after overnight incubation was summed up to give a score. Mutants with a score of 2 or higher were classified as plasma sensitive.
4.4. Plasma sensitivity
To quantify the plasma sensitivity of the 87 mutants, the optical density of overnight cultures was determined, adjusted to OD580 = 0.03, and then further diluted 1 : 500 in LB medium to give approximately 16 000 CFU ml−1. To prevent the cell suspension from spreading on the support due to the gas flow coming from the jet, 30 µl (containing approx. 500 CFU) of this low-density bacterial suspension were soaked into filter paper (1 cm2, Whatman 3 MM) which was then exposed to the effluent of the µAPPJ for 30 s with or without (gas control) igniting the plasma. Directly after treatment, the filter paper was immersed in 1 ml NaCl (0.9% (w/v)) and incubated at 37°C for 20 min to detach the cells from the filter and bring them into suspension. Afterwards, the filter was removed and the suspension plated and incubated overnight to determine CFU counts. The CFU counts of the gas controls were set to 100% to calculate plasma survival rates for each strain. This experiment was performed at least three times independently for each mutant.
To study the effects of complementation, the survival rate of the mutants was normalized to that of the wild-type (set to 100%) for each of the three replicates prior to calculating the average.
4.5. Complementation
The mutants of the KEIO collection were transformed with the plasmid of the ASKA collection [34] that harbours the respective gene under the control of an IPTG-inducible promoter for complementation of the knockout. Chloramphenicol (50 µg ml−1) was added to the culture medium to maintain the plasmid. For any of the following experiments, the strains were inoculated 1 : 100 (v/v) from an overnight culture and incubated with IPTG ranging from 0.01 to 1000 µM for 120 min. Afterwards, the OD580 was determined and the cultures used as described.
4.6. SDS-PAGE and western blot
For detection and semi-quantitative analysis of the episomally encoded proteins, SDS-PAGE and subsequent western blot analysis according to standard protocols were performed [76]. Like for the plasma sensitivity assay, 1 ml of the bacterial culture was harvested and resuspended in 75 µl denaturing loading buffer. After incubation at 95°C for 10 min and removal of cell debris by centrifugation (10 min, 12 000g), 5 µl of each sample were applied to SDS-PAGE. Detection of the His6-taged proteins was performed with an anti-His5 antibody (Qiagen, Hilden, Germany).
4.7. Synthesis of peroxynitrite
Peroxynitrite was synthesized freshly from isopentyl nitrite and hydrogen peroxide as described by Uppu & Pryor [77]. The concentration of peroxynitrite was determined photometrically by Beer–Lambert Law using ɛ302 = 1670 M−1 cm−1 and adjusted to 100 mM with potassium phosphate buffer (100 mM, pH 7) directly prior to use.
4.8. Screening against defined stressors
The plasma-sensitive mutants were treated with the following stressors: hydrogen peroxide (2 mM), paraquat (0.5 mM), products of the Fenton reaction (0.5 mM CuCl2 and 0.8 mM H2O2 in LB buffered with 100 mM potassium phosphate, pH 6), hypochlorous acid (3 mM), peroxynitrite (5 mM in LB buffered with 100 mM potassium phosphate, pH 7), nitric acid (4 mM), sodium nitrate (10 mM), sodium nitrite (4 mM), sodium nitroprusside (4 mM), heat shock (40.5°C) and acidic medium (LB pH 4.5 adjusted with HCl). Membrane stress was induced by incubation with sodium dodecyl sulfate (SDS, 1 mM), cetrimonium bromide (CTAB, 0.02 mM), Triton X-100 (3% (v/v)) or saponin (2% (w/v)). For each mutant, the results for the highest-impact membrane stressor were used to describe the sensitivity to membrane stress. Except for ozone, treatment with the stressors took place in microtitre plates. To this end, LB medium containing the stressor was inoculated by stamping from overnight pre-cultures. For treatment with ozone, a 96-well replicator was briefly immersed in an overnight pre-culture. Droplets were then allowed to dry on the stamp for 15 min. Afterwards, the stamp was placed in a glass beaker with approximately 2 mm distance to the bottom. Ozone was generated by the DBD device described above [6] positioned next to the stamp. After operating the DBD for 30 min, the cells were suspended in fresh LB medium. Concentrations/doses of all the stressors were determined to be non-lethal for the wild-type but reducing its growth to about 60%. Growth of the cultures was recorded photometrically at 580 nm with a plate reader (EnSpire, PerkinElmer) after 16 h of incubation at 37°C, or 40.5°C (heat stress). The sensitivity was calculated according to formula 1. A mutant was classified as stressor sensitive, when the relative growth defect was greater than 20% compared with the parental strain.
4.9. Clustering of stressor profiles
The stressor profiles of the mutants were grouped by performing the k-mean++ algorithm (MatLab, R2016a) with the cosine distance for calculating the similarity between two profiles. To avoid local minima, the algorithm was repeated 10 000 times searching for the lowest sum of distances. As the k-mean++ algorithm needs a predefined number of clusters, this procedure was performed assuming 2–20 clusters using the silhouette coefficient for identification of the best fitting number of clusters.
Supplementary Material
Data accessibility
The data have been uploaded as electronic supplementary material.
Authors' contributions
M.K. carried out experiments, data analysis, designed the study and wrote the manuscript; F.J., T.D. and B.S. carried out experiments; J.B. provided the plasma source and its characterization; J.W.L. designed the study; J.E.B. designed the study and wrote the manuscript. All authors gave final approval for publication.
Competing interests
The authors declare no competing interests.
Funding
This study was funded by the German Research Foundation (DFG): BA 4193/7-1 and PAK728 (Plasmadecon).
References
- 1.Adegoke AA, Faleye AC, Singh G, Stenstroem TA. 2017. Antibiotic resistant superbugs: assessment of the interrelationship of occurrence in clinical settings and environmental niches. Molecules 22, 29 ( 10.3390/molecules22010029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haertel B, von Woedtke T, Weltmann K-D, Lindequist U. 2014. Non-thermal atmospheric-pressure plasma possible application in wound healing. Biomol. Ther. 22, 477–490. ( 10.4062/biomolther.2014.105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morfill GE, Kong MG, Zimmermann JL. 2009. Focus on plasma medicine. New J. Phys. 11, 115011 ( 10.1088/1367-2630/11/11/115011) [DOI] [Google Scholar]
- 4.Metelmann HR, et al. 2015. Head and neck cancer treatment and physical plasma. Clin. Plasma Med. 3, 17–23. ( 10.1016/j.cpme.2015.02.001) [DOI] [Google Scholar]
- 5.Bekeschus S, Schmidt A, Weltmann K-D, von Woedtke T. 2016. The plasma jet kINPen—a powerful tool for wound healing. Clin. Plasma Med. 4, 19–28. ( 10.1016/j.cpme.2016.01.001) [DOI] [Google Scholar]
- 6.Kuchenbecker M, Bibinov N, Kaemlimg A, Wandke D, Awakowicz P, Viöl W. 2009. Characterization of DBD plasma source for biomedical applications. J. Phys. D: Appl. Phys. 42, 045212 ( 10.1088/0022-3727/42/4/045212) [DOI] [Google Scholar]
- 7.Isbary G, Shimizu T, Li Y-F, Stolz W, Thomas HM, Morfill GE, Zimmermann JL. 2013. Cold atmospheric plasma devices for medical issues. Expert Rev. Med. Devices 10, 367–377. ( 10.1586/ERD.13.4) [DOI] [PubMed] [Google Scholar]
- 8.Ellerweg D, Benedikt J, von Keudell A, Knake N, Schulz-von der Gathen V. 2010. Characterization of the effluent of a He/O2 microscale atmospheric pressure plasma jet by quantitative molecular beam mass spectrometry. New J. Phys. 12, 013021 ( 10.1088/1367-2630/12/1/013021) [DOI] [Google Scholar]
- 9.Bruggeman PJ, et al. 2016. Plasma–liquid interactions: a review and roadmap. Plasma Sources Sci. Technol. 25, 053002 ( 10.1088/0963-0252/25/5/053002) [DOI] [Google Scholar]
- 10.Lackmann J-W, Schneider S, Edengeiser E, Jarzina F, Brinckmann S, Steinborn E, Havenith M, Benedikt J, Bandow JE. 2013. Photons and particles emitted from cold atmospheric-pressure plasma inactivate bacteria and biomolecules independently and synergistically. J. R. Soc. Interface 10, 20130591 ( 10.1098/rsif.2013.0591) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joshi SG, Cooper M, Yost A, Paff M, Ercan UK, Fridman G, Friedman G, Fridman A, Brooks AD. 2011. Nonthermal dielectric-barrier discharge plasma-induced inactivation involves oxidative DNA damage and membrane lipid peroxidation in Escherichia coli. Antimicrob. Agents Chemother. 55, 1053–1062. ( 10.1128/AAC.01002-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edengeiser E, Lackmann J-W, Bründermann E, Schneider S, Benedikt J, Bandow JE, Havenith M. 2015. Synergistic effects of atmospheric pressure plasma-emitted components on DNA oligomers: a Raman spectroscopic study. J. Biophotonics 8, 918–924. ( 10.1002/jbio.201400123) [DOI] [PubMed] [Google Scholar]
- 13.Zhang X, Zhang C, Zhou Q-Q, Zhang X-F, Wang L-Y, Chang H-B, Li H-P, Oda Y, Xing X-H. 2015. Quantitative evaluation of DNA damage and mutation rate by atmospheric and room-temperature plasma (ARTP) and conventional mutagenesis. Appl. Microbiol. Biotechnol. 99, 5639–5646. ( 10.1007/s00253-015-6678-y) [DOI] [PubMed] [Google Scholar]
- 14.Joshi SG, Yost A, Joshi SS, Addya S, Ehrlich G, Brooks A. 2015. Microarray analysis of transcriptomic response of Escherichia coli to nonthermal plasma-treated PBS solution. Adv. Biosci. Biotechnol. 06, 49–62. ( 10.4236/abb.2015.62006) [DOI] [Google Scholar]
- 15.Sharma A, Collins G, Pruden A. 2009. Differential gene expression in Escherichia coli following exposure to nonthermal atmospheric pressure plasma. J. Appl. Microbiol. 107, 1440–1449. ( 10.1111/j.1365-2672.2009.04323.x) [DOI] [PubMed] [Google Scholar]
- 16.Mols M, Mastwijk H, Nierop Groot M, Abee T. 2013. Physiological and transcriptional response of Bacillus cereus treated with low-temperature nitrogen gas plasma. J. Appl. Microbiol. 115, 689–702. ( 10.1111/jam.12278) [DOI] [PubMed] [Google Scholar]
- 17.Winter T, Bernhardt J, Winter J, Mäder U, Schlüter R, Weltmann K-D, Hecker M, Kusch H. 2013. Common versus noble Bacillus subtilis differentially responds to air and argon gas plasma. Proteomics 13, 2608–2621. ( 10.1002/pmic.201200343) [DOI] [PubMed] [Google Scholar]
- 18.Winter T, et al. 2011. Characterization of the global impact of low temperature gas plasma on vegetative microorganisms. Proteomics 11, 3518–3530. ( 10.1002/pmic.201000637) [DOI] [PubMed] [Google Scholar]
- 19.Xu D, Xu Y, Ning N, Cui Q, Liu Z, Wang X, Liu D, Chen H, Kong MG. 2018. Alteration of metabolite profiling by cold atmospheric plasma treatment in human myeloma cells. Cancer Cell Int. 18, 42 ( 10.1186/s12935-018-0541-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davison HC, Low JC, Woolhouse ME. 2000. What is antibiotic resistance and how can we measure it? Trends Microbiol. 8, 554–559. ( 10.1016/S0966-842X(00)01873-4) [DOI] [PubMed] [Google Scholar]
- 21.Brauner A, Fridman O, Gefen O, Balaban NQ. 2016. Distinguishing between resistance, tolerance and persistence to antibiotic treatment. Nat. Rev. Microbiol. 14, 320–330. ( 10.1038/nrmicro.2016.34) [DOI] [PubMed] [Google Scholar]
- 22.Baba T, et al. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the KEIO collection. Mol. Syst. Biol. 2, 2006.0008 ( 10.1038/msb4100050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bateman A, et al. 2017. UniProt: the universal protein knowledgebase. Nucleic Acids Res. 45, D158–D169. ( 10.1093/nar/gkw1099) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saijo-Hamano Y, Uchida N, Namba K, Oosawa K. 2004. In vitro characterization of FlgB, FlgC, FlgF, FlgG, and FliE, flagellar basal body proteins of Salmonella. J. Mol. Biol. 339, 423–435. ( 10.1016/j.jmb.2004.03.070) [DOI] [PubMed] [Google Scholar]
- 25.Remaut H, Tang C, Henderson NS, Pinkner JS, Wang T, Hultgren SJ, Thanassi DG, Waksman G, Li H. 2008. Fiber formation across the bacterial outer membrane by the chaperone/usher pathway. Cell 133, 640–652. ( 10.1016/j.cell.2008.03.033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bravo J, Verdaguer N, Tormo J, Betzel C, Switala J, Loewen PC, Fita I. 1995. Crystal structure of catalase HPII from Escherichia coli. Structure 3, 491–502. ( 10.1016/S0969-2126(01)00182-4) [DOI] [PubMed] [Google Scholar]
- 27.Miller A-F. 2012. Superoxide dismutases: ancient enzymes and new insights. FEBS Lett. 586, 585–595. ( 10.1016/j.febslet.2011.10.048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takai E, Kitamura T, Kuwabara J, Ikawa S, Yoshizawa S, Shiraki K, Kawasaki H, Arakawa R, Kitano K. 2014. Chemical modification of amino acids by atmospheric-pressure cold plasma in aqueous solution. J. Phys. D: Appl. Phys. 47, 285403 ( 10.1088/0022-3727/47/28/285403) [DOI] [Google Scholar]
- 29.Tullman-Ercek D, DeLisa MP, Kawarasaki Y, Iranpour P, Ribnicky B, Palmer T, Georgiou G. 2007. Export pathway selectivity of Escherichia coli twin arginine translocation signal peptides. J. Biol. Chem. 282, 8309–8316. ( 10.1074/jbc.M610507200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huerta-Cepas J, et al. 2016. eggNOG 4.5: a hierarchical orthology framework with improved functional annotations for eukaryotic, prokaryotic and viral sequences. Nucleic Acids Res. 44, D286–D293. ( 10.1093/nar/gkv1248) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tokumoto U, Takahashi Y. 2001. Genetic analysis of the isc operon in Escherichia coli involved in the biogenesis of cellular iron–sulfur proteins. J. Biochem. 130, 63–71. ( 10.1093/oxfordjournals.jbchem.a002963) [DOI] [PubMed] [Google Scholar]
- 32.Lane HE, Denhardt DT. 1975. The rep mutation. IV. Slower movement of replication forks in Escherichia coli rep strains. J. Mol. Biol. 97, 99–112. ( 10.1016/S0022-2836(75)80025-8) [DOI] [PubMed] [Google Scholar]
- 33.Courcelle CT, Landstrom AJ, Anderson B, Courcelle J. 2012. Cellular characterization of the primosome and Rep helicase in processing and restoration of replication following arrest by UV-induced DNA damage in Escherichia coli . J. Bacteriol. 194, 3977–3986. ( 10.1128/JB.00290-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kitagawa M, Ara T, Arifuzzaman M, Ioka-Nakamichi T, Inamoto E, Toyonaga H, Mori H. 2005. Complete set of ORF clones of Escherichia coli ASKA library (a complete set of E. coli K-12 ORF archive): unique resources for biological research. DNA Res. 12, 291–299. ( 10.1093/dnares/dsi012) [DOI] [PubMed] [Google Scholar]
- 35.Isaac DD, Pinkner JS, Hultgren SJ, Silhavy TJ. 2005. The extracytoplasmic adaptor protein CpxP is degraded with substrate by DegP. Proc. Natl Acad. Sci. USA 102, 17 775–17 779. ( 10.1073/pnas.0508936102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jenkins LS, Nunn WD. 1987. Regulation of the ato operon by the atoC gene in Escherichia coli. J. Bacteriol. 169, 2096–2102. ( 10.1128/jb.169.5.2096-2102.1987) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wende K, et al. 2015. Identification of the biologically active liquid chemistry induced by a nonthermal atmospheric pressure plasma jet. Biointerphases 10, 029518 ( 10.1116/1.4919710) [DOI] [PubMed] [Google Scholar]
- 38.Danese PN, Snyder WB, Cosma CL, Davis L, Silhavy TJ. 1995. The Cpx two-component signal-transduction pathway of Escherichia coli regulates transcription of the gene specifying the stress-inducible periplasmic protease, DegP. Genes Dev. 9, 387–398. ( 10.1101/gad.9.4.387) [DOI] [PubMed] [Google Scholar]
- 39.Shevell DE, Friedman BM, Walker GC. 1990. Resistance to alkylation damage in Escherichia coli: role of the Ada protein in induction of the adaptive response. Mutat. Res. 233, 53–72. ( 10.1016/0027-5107(90)90151-S) [DOI] [PubMed] [Google Scholar]
- 40.Bell JC, Kowalczykowski SC. 2016. RecA: regulation and mechanism of a molecular search engine. Trends Biochem. Sci. 41, 491–507. ( 10.1016/j.tibs.2016.04.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brautaset T, Petersen S, Valla S. 1998. An experimental study on carbon flow in Escherichia coli as a function of kinetic properties and expression levels of the enzyme phosphoglucomutase. Biotechnol. Bioeng. 58, 299–302. () [DOI] [PubMed] [Google Scholar]
- 42.Hardy CD, Cozzarelli NR. 2005. A genetic selection for supercoiling mutants of Escherichia coli reveals proteins implicated in chromosome structure. Mol. Microbiol. 57, 1636–1652. ( 10.1111/j.1365-2958.2005.04799.x) [DOI] [PubMed] [Google Scholar]
- 43.Meddows TR, Savory AP, Grove JI, Moore T, Lloyd RG. 2005. RecN protein and transcription factor DksA combine to promote faithful recombinational repair of DNA double-strand breaks. Mol. Microbiol. 57, 97–110. ( 10.1111/j.1365-2958.2005.04677.x) [DOI] [PubMed] [Google Scholar]
- 44.McWilliams BD, Torres AG. 2014. Enterohemorrhagic Escherichia coli adhesins. Microbiol Spectr 2, 1–19. ( 10.1128/microbiolspec.EHEC-0003-2013) [DOI] [PubMed] [Google Scholar]
- 45.Mettert EL, Kiley PJ. 2015. How is Fe–S cluster formation regulated? Annu. Rev. Microbiol. 69, 505–526. ( 10.1146/annurev-micro-091014-104457) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Py B, Barras F. 2010. Building Fe–S proteins: bacterial strategies. Nat. Rev. Microbiol. 8, 436–446. ( 10.1038/nrmicro2356) [DOI] [PubMed] [Google Scholar]
- 47.Krebs HA, Johnson WA. 1980. The role of citric acid in intermediate metabolism in animal tissues. FEBS Lett.117(Suppl.), K1–10 ( 10.1016/0014-5793(80)80564-3) [DOI] [Google Scholar]
- 48.Keyer K, Imlay JA. 1996. Superoxide accelerates DNA damage by elevating free-iron levels. Proc. Natl Acad. Sci. USA 93, 13 635–13 640. ( 10.1073/pnas.93.24.13635) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Benedikt J, Mokhtar Hefny M, Shaw A, Buckley BR, Iza F, Schäkermann S, Bandow JE. 2018. The fate of plasma-generated oxygen atoms in aqueous solutions: non-equilibrium atmospheric pressure plasmas as an efficient source of atomic O(aq). Phys. Chem. Chem. Phys. 20, 12 037–12 042. ( 10.1039/C8CP00197A) [DOI] [PubMed] [Google Scholar]
- 50.Murakami T, Niemi K, Gans T, O'Connell D, Graham WG. 2013. Chemical kinetics and reactive species in atmospheric pressure helium–oxygen plasmas with humid-air impurities. Plasma Sources Sci. Technol 22, 015003 ( 10.1088/0963-0252/22/1/015003) [DOI] [Google Scholar]
- 51.Beckman JS, Koppenol WH. 1996. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am. J. Physiol. -Cell Physiol. 271, C1424–C1437. ( 10.1152/ajpcell.1996.271.5.C1424) [DOI] [PubMed] [Google Scholar]
- 52.Jones-Carson J, Laughlin J, Hamad MA, Stewart AL, Voskuil MI, Vazquez-Torres A. 2008. Inactivation of [Fe–S] metalloproteins mediates nitric oxide-dependent killing of Burkholderia mallei. PLoS ONE 3, e1976 ( 10.1371/journal.pone.0001976) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Viner RI, Williams TD, Schöneich C. 1999. Peroxynitrite modification of protein thiols: oxidation, nitrosylation, and S-glutathiolation of functionally important cysteine residue(s) in the sarcoplasmic reticulum Ca-ATPase. Biochemistry 38, 12 408–12 415. ( 10.1021/bi9909445) [DOI] [PubMed] [Google Scholar]
- 54.Luc R, Vergely C. 2008. Forgotten radicals in biology. Int. J. Biomed. Sci. 4, 255–259. [PMC free article] [PubMed] [Google Scholar]
- 55.Radi R. 2018. Oxygen radicals, nitric oxide, and peroxynitrite: redox pathways in molecular medicine. Proc. Natl Acad. Sci. USA 115, 5839–5848. ( 10.1073/pnas.1804932115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lukes P, Dolezalova E, Sisrova I, Clupek M. 2014. Aqueous-phase chemistry and bactericidal effects from an air discharge plasma in contact with water: evidence for the formation of peroxynitrite through a pseudo-second-order post-discharge reaction of H2O2 and HNO2. Plasma Sources Sci. Technol. 23, 015019 ( 10.1088/0963-0252/23/1/015019) [DOI] [Google Scholar]
- 57.Zhou R, Zhou R, Prasad K, Fang Z, Speight R, Bazaka K, Ostrikov KK. 2018. Cold atmospheric plasma activated water as a prospective disinfectant: the crucial role of peroxynitrite. Green Chem. 20, 5284 ( 10.1039/c8gc02800a) [DOI] [Google Scholar]
- 58.Storz G, Imlay JA. 1999. Oxidative stress. Curr. Opin. Microbiol. 2, 188–194. ( 10.1007/SpringerReference_32759) [DOI] [PubMed] [Google Scholar]
- 59.Keyer K, Gort AS, Imlay JA. 1995. Superoxide and the production of oxidative DNA damage. J. Bacteriol. 177, 6782–6790. ( 10.1128/jb.177.23.6782-6790.1995) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Burney S, Caulfield JL, Niles JC, Wishnok JS, Tannenbaum SR. 1999. The chemistry of DNA damage from nitric oxide and peroxynitrite. Mutat. Res. 424, 37–49. ( 10.1016/S0027-5107(99)00006-8) [DOI] [PubMed] [Google Scholar]
- 61.Tamir S, Burney S, Tannenbaum SR. 1996. DNA damage by nitric oxide. Chem. Res. Toxicol. 9, 821–827. ( 10.1021/tx9600311) [DOI] [PubMed] [Google Scholar]
- 62.Ellerweg D, von Keudell A, Benedikt J. 2012. Unexpected O and O3 production in the effluent of He/O2 microplasma jets emanating into ambient air. Plasma Sources Sci. Technol. 21, 034019 ( 10.1088/0963-0252/21/3/034019) [DOI] [Google Scholar]
- 63.Hefny MM, Pattyn C, Lukes P, Benedikt J. 2016. Atmospheric plasma generates oxygen atoms as oxidizing species in aqueous solutions. J. Phys. D: Appl. Phys. 49, 404002 ( 10.1088/0022-3727/49/40/404002) [DOI] [Google Scholar]
- 64.Piette J. 1991. Biological consequences associated with DNA oxidation mediated by singlet oxygen. J. Photochem. Photobiol. B Biol. 11, 241–260. ( 10.1016/1011-1344(91)80030-L) [DOI] [PubMed] [Google Scholar]
- 65.Santos AL, Oliveira V, Baptista I, Henriques I, Gomes NCM, Almeida A, Correia A, Cunha Â. 2013. Wavelength dependence of biological damage induced by UV radiation on bacteria. Arch. Microbiol. 195, 63–74. ( 10.1007/s00203-012-0847-5) [DOI] [PubMed] [Google Scholar]
- 66.Zimmermann JL, Shimizu T, Schmidt HU, Li Y-F, Morfill GE, Isbary G. 2012. Test for bacterial resistance build-up against plasma treatment. New J. Phys. 14, 073037 ( 10.1088/1367-2630/14/7/073037) [DOI] [Google Scholar]
- 67.Matthes R, Assadian O, Kramer A. 2014. Repeated applications of cold atmospheric pressure plasma does not induce resistance in Staphylococcus aureus embedded in biofilms. GMS Hyg. Infect. Control 9, Doc17 ( 10.3205/dgkh000237) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mai-Prochnow A, Bradbury M, Ostrikov K, Murphy AB. 2015. Pseudomonas aeruginosa biofilm response and resistance to cold atmospheric pressure plasma is linked to the redox-active molecule phenazine. PLoS ONE 10, e0130373 ( 10.1371/journal.pone.0130373) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boldrin F, et al. 2018. Promoter mutagenesis for fine-tuning expression of essential genes in Mycobacterium tuberculosis. Microb. Biotechnol. 11, 238–247. ( 10.1111/1751-7915.12875) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Calos MP. 1978. DNA sequence for a low-level promoter of the lac repressor gene and an ‘up’ promoter mutation. Nature 274, 762–765. ( 10.1038/274762a0) [DOI] [PubMed] [Google Scholar]
- 71.Fang M, Jin L, Zhang C, Tan Y, Jiang P, Ge N, Li H, Xing X. 2013. Rapid mutation of Spirulina platensis by a new mutagenesis system of atmospheric and room temperature plasmas (ARTP) and generation of a mutant library with diverse phenotypes. PLoS ONE 8, e77046 ( 10.1371/journal.pone.0077046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bekeschus S, Wende K, Hefny MM, Rödder K, Jablonowski H, Schmidt A, Woedtke TV, Weltmann K-D, Benedikt J. 2017. Oxygen atoms are critical in rendering THP-1 leukaemia cells susceptible to cold physical plasma-induced apoptosis. Sci. Rep. 7, 263001 ( 10.1038/s41598-017-03131-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Alkawareek MY, Gorman SP, Graham WG, Gilmore BF. 2014. Potential cellular targets and antibacterial efficacy of atmospheric pressure non-thermal plasma. Int. J. Antimicrob. Agents 43, 154–160. ( 10.1016/j.ijantimicag.2013.08.022) [DOI] [PubMed] [Google Scholar]
- 74.Baldus S, Schröder D, Bibinov N, Schulz-von der Gathen V, Awakowicz P. 2015. Atomic oxygen dynamics in an air dielectric barrier discharge: a combined diagnostic and modeling approach. J. Phys. D: Appl. Phys. 48, 275203 ( 10.1088/0022-3727/48/27/275203) [DOI] [Google Scholar]
- 75.Hogness DS, Simmons JR. 1964. Breakage of λdg DNA: chemical and genetic characterization of each isolated half-molecule. J. Mol. Biol. 9, 411–438. ( 10.1016/S0022-2836(64)80217-5) [DOI] [PubMed] [Google Scholar]
- 76.Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual, 3rd edn. New York, NY: CSHL Press. [Google Scholar]
- 77.Uppu RM, Pryor WA. 1996. Synthesis of peroxynitrite in a two-phase system using isoamyl nitrite and hydrogen peroxide. Anal. Biochem. 236, 242–249. ( 10.1006/abio.1996.0162) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data have been uploaded as electronic supplementary material.