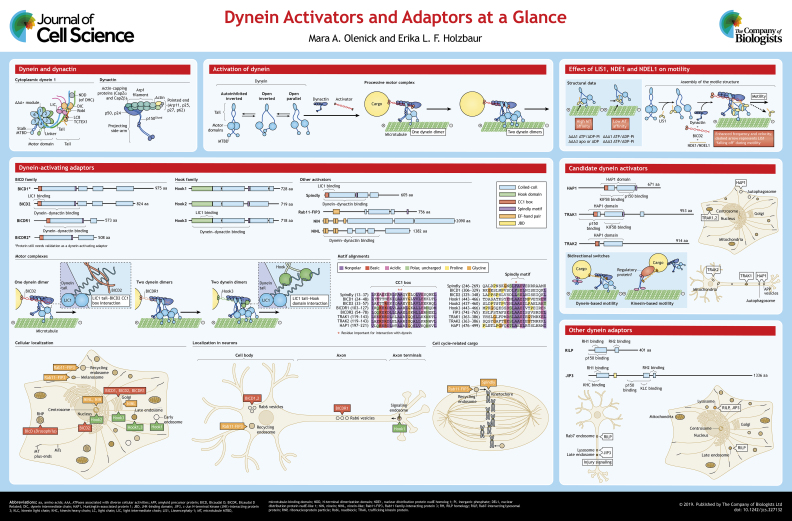
ABSTRACT
Cytoplasmic dynein-1 (hereafter dynein) is an essential cellular motor that drives the movement of diverse cargos along the microtubule cytoskeleton, including organelles, vesicles and RNAs. A long-standing question is how a single form of dynein can be adapted to a wide range of cellular functions in both interphase and mitosis. Recent progress has provided new insights – dynein interacts with a group of activating adaptors that provide cargo-specific and/or function-specific regulation of the motor complex. Activating adaptors such as BICD2 and Hook1 enhance the stability of the complex that dynein forms with its required activator dynactin, leading to highly processive motility toward the microtubule minus end. Furthermore, activating adaptors mediate specific interactions of the motor complex with cargos such as Rab6-positive vesicles or ribonucleoprotein particles for BICD2, and signaling endosomes for Hook1. In this Cell Science at a Glance article and accompanying poster, we highlight the conserved structural features found in dynein activators, the effects of these activators on biophysical parameters, such as motor velocity and stall force, and the specific intracellular functions they mediate.
KEY WORDS: BICD2, Cytoplasmic dynein, Dynactin, Hook1, Microtubule motors, Trafficking
Summary: We review recent progress identifying and characterizing activating adaptors for the microtubule motor protein cytoplasmic dynein that enhance motor processivity and couple dynein to specific cargos within the cell.
Introduction
Microtubule-based transport is vital to cellular development and survival. Microtubules provide a polarized highway to facilitate active transport by the molecular motors dynein and kinesin. While many types of kinesins drive transport toward microtubule plus-ends, there is only one major form of dynein, cytoplasmic dynein-1, which drives the trafficking of a wide array of minus-end-directed cargo within the cell. Recent work has brought new insight into the spatial and temporal regulation of cytoplasmic dynein by adaptor proteins, which link dynein to cargo (Fu and Holzbaur, 2014; Kardon and Vale, 2009; Reck-Peterson et al., 2018). Interestingly, many of these adaptors modulate the motile properties of dynein, either enhancing or inhibiting movement, while some act as motility switches by co-ordinately regulating dynein and kinesin. Here, we use ‘adaptor’ as a more general term to discuss proteins that can link dynein to cargo and use ‘activating adaptor’ or ‘activator’ to identify a subclass of adaptor proteins that have been shown to enhance the processivity of dynein. Below, and in the accompanying poster, we summarize our current understanding of both the structure and function of dynein adaptor proteins.
Dynein and dynactin
Cytoplasmic dynein 1 (henceforth referred to as dynein) is a 1.4 MDa motor complex consisting of dimerized heavy chains (DHCs; symbol DYNC1H1), each with an N-terminal tail and a C-terminal motor domain (see poster). The N-terminal tail mediates homodimerization of the heavy chains, along with binding sites for non-catalytic subunits, including two intermediate chains (DICs; DYNC1I1 and DYNC1I2) and two light intermediate chains (LIC1 and LIC2, also known as DYNC1LI1 and DYNC1LI2). Additional light chains (LCs) are also bound to the DICs. These non-catalytic subunits of dynein are thought to stabilize the complex and may contribute to the regulation of specific dynein functions (recently reviewed by Reck-Peterson et al., 2018). The motor domain of dynein is composed of six concatenated AAA+ domains that form a motor ring with a protruding flexible 15-nm-long stalk with the microtubule-binding domain localized to the end (Gee et al., 1997; Burgess et al., 2003; Kon et al., 2011). AAA1 is the primary site of ATP hydrolysis, while the nucleotide state of AAA3 has an allosteric effect on the motile properties of dynein (Takahide Kon et al., 2004; DeWitt et al., 2015; Nicholas et al., 2015).
Dynein requires the co-factor dynactin, a 1 MDa, 23-subunit complex, the first identified activator for dynein and essential for most cellular functions of the motor (see poster). The core of dynactin is comprised of an ∼37-nm-long actin-like filament called the Arp1 filament (Schroer, 2004), which is composed of eight Arp1 subunits (also known as ACTR1A), one β-actin molecule and one Arp11 molecule (also known as ACTR10). Arp11 interacts with p25, p27 and p62 (also known as DCTN5, DCTN6 and DCTN4, respectively) to form the pointed-end complex of dynactin. The barbed end of the Arp1 filament within dynactin is capped by actin capping protein, a heterodimer of a CapZα and CapZβ family protein. A shoulder complex sits on the barbed-end, comprised of two copies of p24 (DCTN3), four copies of p50 (dynamtin or DCTN2) and two copies of p150Glued (DCTN1) (Chowdhury et al., 2015; Urnavicius et al., 2015). The pointed-end of dynactin has been suggested to play a role in facilitating cargo interaction (Zhang et al., 2011; Yeh et al., 2012; Qiu et al., 2018) while the p150Glued subunit has an independent and ATP-insensitive microtubule-binding domain (Waterman-Storer et al., 1995).
Activation of dynein
Dynein is responsible for the long-distance transport of many cargos, some of which display highly processive motility. In vitro, isolated or recombinant mammalian dynein is poorly processive unless bound to a bead or other surface. Electron microscopy (EM) studies have indicated that in the absence of binding partners, mammalian dynein is found in an auto-inhibited or phi state, which has a low affinity for microtubules (Torisawa et al., 2014; Zhang et al., 2017). In the presence of dynactin and a coiled-coil activating adaptor, such as Bicaudal D (BICD) protein 2 (BICD2), the motor heads of the dynein dimer are reoriented to facilitate motility (Zhang et al., 2017); single-molecule studies have shown that the complex between dynein–dynactin and an activating adaptor is superprocessive compared to the motility of dynein alone (McKenney et al., 2014; Schlager et al., 2014a), moving at velocities ranging from ∼0.4 to 1.4 µm/s over run-lengths of up to 40 µm. Furthermore, some activating adaptors such as Hook3 can recruit two dynein dimers to one dynactin complex, enhancing both the velocity and the force produced by the motor complex (Urnavicius et al., 2018; Grotjahn et al., 2018). While it is difficult to compare velocity and run length data across multiple studies due to differing experimental conditions such as buffer composition and ionic strength, studies that have directly compared dynein activators have found differences in velocities and force production that suggest that these cofactors can fine-tune dynein motor function (McKenney et al., 2014; Olenick et al., 2016; Redwine et al., 2017; Urnavicius et al., 2018).
Dynein activators
BICD proteins were initially identified in Drosophila where mutations cause abnormal development of the abdomen resulting in a bicaudal (‘two-tailed’) phenotype (Mohler and Wieschaus, 1986). Drosophila BICD was found to be vital for mRNA transport in ribonucleoprotein (RNP) complexes and nuclear positioning (Wharton and Struhl, 1989; Suter and Steward, 1991; Swan and Suter, 1996; Swan et al., 1999; Mach and Lehmann, 1997; Bullock and Ish-Horowicz, 2001). In mammals, there are two BICD orthologs, BICD1 and BICD2, as well as two related proteins, BICDR1 and BICDR2, which are slightly shorter. BICD proteins form dimers characterized by long coiled-coil domains. Cryo-EM analysis of the N-terminus of BICD2 shows an extended coiled-coil of ∼250 amino acid residues that extends for ∼30 nm and docks onto the Arp1 filament of dynactin (Chowdhury et al., 2015; Urnavicius et al., 2015). BICD2 also interacts with the N-terminal tail of the DHC (Chowdhury et al., 2015; Urnavicius et al., 2015) and the dynein LIC1, via coiled-coil interactions (Schroeder et al., 2014; Lee et al., 2018). Together, these interactions enhance the affinity of the dynein–dynactin interaction (Splinter et al., 2012; McKenney et al., 2014; Schlager et al., 2014a). While BICD2 is mainly found in a complex with one dynein and one dynactin, BICDR1 can recruit two dynein dimers to a single dynactin, which further enhances the force and velocity of the motor complex (Urnavicius et al., 2018; Grotjahn et al., 2018; Schlager et al., 2014b). In mammalian cells, BICD proteins have been implicated in Golgi vesicle transport via a C-terminal interaction with the small GTPase Rab6 proteins (Hoogenraad et al., 2001; Matanis et al., 2002; Schlager et al., 2010; Short et al., 2002). BICD2 has also been implicated in nuclear positioning (Splinter et al., 2012; Hu et al., 2013). Furthermore, the C-terminal region of BICD proteins can bend back on itself to produce an autoinhibited state (Terawaki et al., 2015; Liu et al., 2013; Wharton and Struhl, 1989; Urnavicius et al., 2015), which can be relieved by cargo binding (Liu et al., 2013; Huynh and Vale, 2017; McClintock et al., 2018; Sladewski et al., 2018), suggesting an efficient mechanism to regulate dynein motility.
Members of the Hook protein family activate dynein in a similar manner. There are three Hook proteins expressed in mammalian cells, characterized by three conserved regions: a globular N-terminal Hook domain, a central coiled-coil domain that drives dimerization and forms a 31-nm helix that aligns along the Arp1 filament, and a divergent, predicted unstructured C-terminal domain thought to mediate cargo binding (Walenta et al., 2001; Lee et al., 2018; Urnavicius et al., 2018) (see poster). Hook1 and Hook3 enhance the binding of dynein and dynactin to effectively activate dynein motility, inducing longer run lengths and higher velocities than BICD2 (Olenick et al., 2016; Schroeder and Vale, 2016; Urnavicius et al., 2018). Complex formation requires the N-terminal Hook domain, which directly interacts with a helix of the dynein subunit LIC1; this interaction is important for Hook-induced processive motility of dynein in vitro and in cells (Lee et al., 2018; Olenick et al., 2016; Schroeder and Vale, 2016). Like BICDR1, Hook3 can interact with two dimeric dynein motors per dynactin (Urnavicius et al., 2018; Grotjahn et al., 2018). In mammalian cells, Hook2 is thought to function at the centrosome and during mitotic progression (Szebenyi et al., 2007; Moynihan et al., 2009; Guthrie et al., 2009; Dwivedi et al., 2019), while Hook1 and Hook3 have been implicated in a variety of endosomal trafficking pathways (Luiro et al., 2004; Maldonado-Báez et al., 2013; Xu et al., 2008; Walenta et al., 2001; Guo et al., 2016), similar to the role of fungal Hook proteins (Zhang et al., 2014; Bielska et al., 2014). Most recently, Hook1 has been shown to be required for the transport of TrkB–BDNF-containing signaling endosomes in neurons, a role specific for Hook1 but not Hook3 (Olenick et al., 2019) (see poster).
Spindly is another dynein activator which plays a role in mitosis by silencing a mitotic checkpoint after proper spindle assembly (McKenney et al., 2014; Barisic et al., 2010; Griffis et al., 2007; Gassmann et al., 2010). Spindly recruits dynein to kinetochores, which induces the movement of chromosomes to the poles (Griffis et al., 2007; Gassmann et al., 2008; Chan et al., 2009). Comparisons of Spindly with other dynein activators has identified two conserved features, the CC1 box and the Spindly motif (Gama et al., 2017) (see poster). The CC1 box is found in both Spindly and BICD proteins, and is a segment of coiled-coil that mediates an interaction with LIC1, analogous to the role of the Hook domain in Hook proteins (Lee et al., 2018). In the CC1 box, mutations of two conserved alanine residues to valine residues within BICD proteins causes a loss of interaction with dynein–dynactin in vitro (Schlager et al., 2014b) and a loss-of-function phenotype in Drosophila (Oh et al., 2000). Similar alanine-to-valine mutations in Spindly also resulted in loss of dynein interaction (Gama et al., 2017).
The Spindly motif has been identified in most but not all known dynein activators. The sequence L(F/A)xE is located just after the extended coiled-coil domain characteristic of validated dynein activators (Gama et al., 2017). This region of Spindly was found to interact with the pointed end of dynactin. Mutation of the phenylalanine residue to an alanine residue caused a loss of the Spindly–dynactin interaction with dynein. However, the phenylalanine residue is not conserved in other dynein adaptors and is actually an alanine residue in most cases (Gama et al., 2017). Thus, further work is required to fully define this motif and its function within other dynein adaptors.
There are three shared elements found in experimentally validated dynein activators: an extended ∼30 nm coiled-coil domain, flanked at its N-terminus by a CC1 box or Hook domain, and at its C-terminus by a Spindly motif. These elements have also been identified in additional proteins thought to interact with dynein. For example, Rab11-FIP3 shares these elements and was also found to activate the motility of dynein (McKenney et al., 2014). In addition, Rab11-FIP3 contains N-terminal EF-hand domains, which might act as regulatory modules. Rab11-FIP proteins are mainly known to regulate the trafficking of recycling endosomes via a conserved Rab11 GTPase-binding domain (reviewed in Horgan and McCaffrey, 2009; Jing and Prekeris, 2009). Rab11-FIP3 plays an important role in the cell-cycle-dependent trafficking of recycling endosomes (Horgan et al., 2010; Inoue et al., 2008; Wilson et al., 2005; Simon et al., 2008) and has been implicated in dendrite formation through trafficking of Rab11- and Arf6-dependent endosomal transport in neurons (Yazaki et al., 2014; Song et al., 2015). So far, only Rab11-FIP3 has been shown to interact with dynein, despite the high similarities between Rab11-FIP3 and Rab11-FIP4 (Horgan et al., 2010; McKenney et al., 2014).
Ninein and ninein-like proteins have been identified as activating adaptors for dynein through a BioID mass spectrometry screen for novel dynein–dynactin interactors (Redwine et al., 2017). These proteins contain a long coiled-coil stretch similar to other activators, but have EF-hand domains similar to Rab11-FIP3 (see poster). Functionally, ninein proteins have been previously described as centrosomal proteins and as microtubule-anchoring factors (Delgehyr et al., 2005; Casenghi et al., 2003; Mogensen et al., 2000; Wang et al., 2015; Moss et al., 2007). Ninein proteins have been linked to trafficking, as overexpression leads to dispersion of the Golgi and lysosomes (Casenghi et al., 2005). In zebrafish, loss of ninein leads to defects in brain and skull development (Dauber et al., 2012), while loss of ninein-like causes mislocalized trafficking of cilia-directed cargo marked by Rab8a and impaired melanosome transport (Bachmann-Gagescu et al., 2015; Dona et al., 2015).
Candidate activators
A number of proteins have been identified as candidate activators due to shared structural elements found in bona fide activating adaptors, but have not yet been shown to enhance processive motility of dynein in vitro. One of these candidate activators is huntingtin-associated protein 1 (HAP1). HAP1 interacts with huntingtin, known for its causative role in Huntington's disease. Huntingtin is an extended scaffolding protein with many known interactors, one of which is the intermediate chain of dynein (Caviston et al., 2007) (see poster). HAP1 also interacts with the p150Glued subunit of dynactin (Li et al., 1998; Engelender et al., 1997), as well as the kinesin heavy chain and light chain (Twelvetrees et al., 2010; McGuire et al., 2006), implicating the two proteins as having a role in intracellular transport (Block-Galarza et al., 1997). While huntingtin has been linked to the axonal transport of several vesicle populations (Wong and Holzbaur, 2014; Gunawardena et al., 2003; Weiss and Littleton, 2016; Colin et al., 2008; Her and Goldstein, 2008), HAP1 is required for the huntingtin-mediated transport of autophagosomes (Wong and Holzbaur, 2014), as well as for APP trafficking (Yang et al., 2012). It has been suggested that huntingtin and HAP1 together act as a platform for both dynein and kinesin attachment to vesicles (Box 1), although the large size of the complex has made it difficult to dissect the underlying mechanisms through in vitro assays.
Box 1. Bidirectional adaptors.
Some cargo in cells, such as mitochondria, display highly bidirectional motility characterized by movement toward both microtubule plus- and minus-ends, along with directional switching. Both dynein and kinesins are bound to some cargos, leading to the question of how overall motility is regulated. Some dynein adaptors have been suggested to act as bidirectional adaptors since they can interact directly with both dynein and kinesin. In many cases, the dynein interaction region overlaps with the kinesin interaction region, suggesting that the adaptor might be able to act as a bidirectional switch. For example, HAP1 and TRAKs have been reported to interact with dynein and kinesin via the HAP coiled-coil domain (see poster) (McGuire et al., 2006; Twelvetrees et al., 2010; Engelender et al., 1997; Li et al., 1998; van Spronsen et al., 2013). Furthermore, some motor adaptors have regulatory signals such as binding partners or post-translational modifications that may mediate a controlled switch between dynein or kinesin-based motility. For example, TRAK proteins interact with Miro, a Ca2+ sensor which alters the association of TRAK and kinesin upon Ca2+ binding to reduce motility of mitochondria (MacAskill et al., 2009; Wang and Schwarz, 2009). In addition, JIP1 has a phosphorylation site that regulates the switch from kinesin- to dynein-based motility of APP-positive vesicles (Fu and Holzbaur, 2013). These bidirectional adaptors can help improve the spatial and temporal targeting of cargo by regulating which motors are active in response to cellular cues and demands.
Milton (in Drosophila) and TRAK1 and TRAK2 (in mammals) belong to a family of proteins that act as motor adaptors for mitochondria. The Milton/TRAK family have an N-terminal coiled-coil region with a high degree of similarity to the HAP1 domain and a C-terminal region that interacts with mitochondrial Rho GTPase (Miro) proteins. In Drosophila, Milton in complex with Miro interacts with kinesin-1 to deliver mitochondria to neuronal synapses (Stowers et al., 2002; Glater et al., 2006). The mammalian homologs of Milton, TRAK1 and TRAK2, have been linked to dynein and kinesin motility (Box 1) and are required for mitochondria distribution in a variety of cell types including neurons (reviewed in Melkov and Abdu, 2018). TRAK1 binds dynein–dynactin and kinesin-1, while TRAK2 predominately interacts with dynein–dynactin (van Spronsen et al., 2013). In neurons, TRAK1 is mainly localized in the axon, while TRAK2 is localized to the dendrites (van Spronsen et al., 2013; Loss and Stephenson, 2015), which could reflect the dependence of each compartment on distinct mechanisms of mitochondrial transport (see poster).
Other adaptors
Rab7-interacting lysosomal protein (RILP) has been suggested to link dynein to Rab7-marked vesicles, including late endosomes and lysosomes (Cantalupo et al., 2001; Jordens et al., 2001). Biochemical studies support a stepwise process of dynein recruitment by RILP, where RILP and oxysterol-binding protein-related protein 1L (ORP1L, also known as OSBPL1A) form a complex with the small GTPase Rab7 and then RILP can interact with the p150Glued subunit of dynactin, which in turn recruits dynein to the vesicle (Johansson et al., 2007). This stepwise recruitment suggests that the association of dynein with vesicles can be regulated by cholesterol levels, which are sensed by ORP1L. In addition, RILP has been shown to self-interact, likely as a homodimer, similar to other dynein-activating adaptors like BICD2 (Wu et al., 2005; Colucci et al., 2005). However, RILP has not yet been shown to specifically activate dynein-driven motility in vitro or in cells.
c-Jun N-terminal kinase (JNK)-interacting proteins (JIPs) have also been identified as motor adaptors (see poster). There are four mammalian JIPs, JIP1 to JIP4 (also known as MAPK8IP1–MAPK8IP3 and SPAG9, respectively) which are highly expressed in the brain (Dickens et al., 1997; Yasuda et al., 1999; Kelkar et al., 2005, 2000). Each JIP protein contains a JNK-binding domain near the N-terminus, which can interact with kinases of the JNK pathway and p38 MAPK pathway to signal for growth, differentiation and apoptosis (Whitmarsh, 2006). In addition to signaling factors, JIPs interact with microtubule motors. All of the JIPs have been found to interact with kinesin-1 (Verhey et al., 2001; Bowman et al., 2000; Montagnac et al., 2009; Fu and Holzbaur, 2013). JIP3 and JIP4, which each contain N-terminal coiled-coil regions (Kelkar et al., 2005, 2000), have also been implicated in minus-end motility via interactions with dynein and/or dynactin (Box 1). In Drosophila, JIP3 (also known as Sunday Driver) associates with dynein–dynactin during the transport of axonal JNK-injury signals via an endosomal pathway (Cavalli et al., 2005; Abe et al., 2009). Lysosomal accumulation and maturation defects have been observed in JIP3-knockout mice, consistent with previous observations in zebrafish and C. elegans (Gowrishankar et al., 2017; Drerup and Nechiporuk, 2013; Edwards et al., 2013). JIP4 was shown to transport recycling endosomes during cytokinesis via its interaction with kinesin-1 and dynactin, with ARF6 binding acting as the regulatory switch for JIP4 interaction with motor proteins (Montagnac et al., 2009). However, none of the JIPs have yet to be shown to activate dynein motility in vitro or in vivo.
LIS1, NDE1 and NDEL1
LIS1 (also known as PAFAH1B1) is an important regulator of dynein, first described as the gene mutated in the neurodevelopmental disease Type 1 lissencephaly (Reiner et al., 1993). LIS1 has been linked to the motility of vesicles, mitochondria, nuclei and centrosomes in a variety of organisms and cell types, so it is likely to be more of a global regulator of dynein motility rather than a cargo-specific activator (Shao et al., 2013; Moughamian et al., 2013; Lenz et al., 2006; Zhang et al., 2010; Yi et al., 2011; Egan et al., 2012; Tsai et al., 2007; Xiang et al., 1995; Gambello et al., 2003). LIS1 has an N-terminal dimerization domain, a coiled-coil region, a disordered loop and a β-propeller domain with seven WD repeats (Kim et al., 2004; Tarricone et al., 2004) (see poster). LIS1 interacts with the homologs NDE1 and NDEL1, which are dimeric, coiled-coil proteins that also interact with dynein (Wang and Zheng, 2011; Zyłkiewicz et al., 2011; Stehman et al., 2007). NDE1 and NDEL1 have both been suggested to tether LIS1 to dynein, forming a regulatory module that is controlled by Cdk5-dependent phosphorylation (Hebbar et al., 2008; Pandey and Smith, 2011; Klinman and Holzbaur, 2015). The β-propeller domain of LIS1 interacts with the dynein motor domain, at the AAA3 and AAA4 modules (Huang et al., 2012; Toropova et al., 2014). Initial experiments suggested that LIS1 increases the affinity of dynein for microtubules and slows the velocity of dynein in vitro (Huang et al., 2012; McKenney et al., 2010; Toropova et al., 2014; Yamada et al., 2008), but recent work has challenged this idea. For yeast dynein, LIS1 is now proposed to induce either a weak or tight microtubule-binding state of dynein, depending on the nucleotide bound to AAA3 and the number of LIS1 β-propeller domains (one or two) interacting with the motor domain (DeSantis et al., 2017). In recent single-molecule studies using mammalian dynein in complex with dynactin and BICD2, LIS1 was found to increase the frequency and velocity of dynein motility in a concentration-dependent manner (Baumbach et al., 2017; Gutierrez et al., 2017). Current models suggest that the binding of LIS1 favors or stabilizes the ‘open’ state of dynein and enhances the formation of a motile dynein–dynactin-activating adaptor complex, but is not required for motility once the complex is fully assembled (see poster). Further studies are required to fully elucidate the mechanisms of LIS1-dependent regulation of dynein.
Conclusions
Great strides have been made to uncover the structure and function of individual dynein activators and adaptors but there is still much to learn about the regulation of dynein. Significant further work is needed to understand the specific regulatory mechanisms involved, how they may be co-ordinated to mediate dynein function in vivo and how these proteins might play a role in disease states.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
Our work in this area is supported by the National Institutes of Health (NIH) (R35 GM126950) to E.L.F.H. Deposited in PMC for release after 12 months.
Cell science at a glance
A high-resolution version of the poster and individual poster panels are available for downloading at http://jcs.biologists.org/lookup/doi/10.1242/jcs.227132.supplemental
References
- Abe N., Almenar-Queralt A., Lillo C., Shen Z., Lozach J., Briggs S. P., Williams D. S., Goldstein L. S. B. and Cavalli V. (2009). Sunday driver interacts with two distinct classes of axonal organelles. J. Biol. Chem. 284, 34628-34639. 10.1074/jbc.M109.035022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann-Gagescu R., Dona M., Hetterschijt L., Tonnaer E., Peters T., de Vrieze E., Mans D. A., van Beersum S. E. C., Phelps I. G., Arts H. H. et al. (2015). The ciliopathy protein CC2D2A associates with NINL and functions in RAB8-MICAL3-regulated vesicle trafficking. PLoS Genet. 11, e1005575 10.1371/journal.pgen.1005575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barisic M., Sohm B., Mikolcevic P., Wandke C., Rauch V., Ringer T., Hess M., Bonn G. and Geley S. (2010). Spindly/CCDC99 is required for efficient chromosome congression and mitotic checkpoint regulation. Mol. Biol. Cell 21, 1968-1981. 10.1091/mbc.e09-04-0356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumbach J., Murthy A., McClintock M. A., Dix C. I., Zalyte R., Hoang H. T. and Bullock S. L. (2017). Lissencephaly-1 is a context-dependent regulator of the human dynein complex. Elife 6, e21768 10.7554/eLife.21768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielska E., Schuster M., Roger Y., Berepiki A., Soanes D. M., Talbot N. J. and Steinberg G. (2014). Hook is an adapter that coordinates kinesin-3 and dynein cargo attachment on early endosomes. J. Cell Biol. 204, 989-1007. 10.1083/jcb.201309022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block-Galarza J., Chase K. O., Sapp E., Vaughn K. T., Vallee R. B., DiFiglia M. and Aronin N. (1997). Fast transport and retrograde movement of huntingtin and HAP 1 in axons. Neuroreport 8, 2247-2251. 10.1097/00001756-199707070-00031 [DOI] [PubMed] [Google Scholar]
- Bowman A. B., Kamal A., Ritchings B. W., Philp A. V., McGrail M., Gindhart J. G. and Goldstein L. S. (2000). Kinesin-dependent axonal transport is mediated by the sunday driver (SYD) protein. Cell 103, 583-594. 10.1016/S0092-8674(00)00162-8 [DOI] [PubMed] [Google Scholar]
- Bullock S. L. and Ish-Horowicz D. (2001). Conserved signals and machinery for RNA transport in Drosophila oogenesis and embryogenesis. Nature 414, 611-616. 10.1038/414611a [DOI] [PubMed] [Google Scholar]
- Burgess S. A., Walker M. L., Sakakibara H., Knight P. J. and Oiwa K. (2003). Dynein structure and power stroke. Nature 421, 715-718. 10.1038/nature01377 [DOI] [PubMed] [Google Scholar]
- Cantalupo G., Alifano P., Roberti V., Bruni C. B. and Bucci C. (2001). Rab-interacting lysosomal protein (RILP): the Rab7 effector required for transport to lysosomes. EMBO J. 20, 683–693. 10.1093/emboj/20.4.683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casenghi M., Meraldi P., Weinhart U., Duncan P. I., Körner R. and Nigg E. A. (2003). Polo-like Kinase 1 Regulates Nlp, a centrosome protein involved in microtubule nucleation. Dev. Cell 5, 113-125. 10.1016/S1534-5807(03)00193-X [DOI] [PubMed] [Google Scholar]
- Casenghi M., Barr F. A. and Nigg E. A. (2005). Phosphorylation of Nlp by Plk1 negatively regulates its dynein-dynactin-dependent targeting to the centrosome. J. Cell Sci. 118, 5101-5108. 10.1242/jcs.02622 [DOI] [PubMed] [Google Scholar]
- Cavalli V., Kujala P., Klumperman J. and Goldstein L. S. B. (2005). Sunday driver links axonal transport to damage signaling. J. Cell Biol. 168, 775-787. 10.1083/jcb.200410136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviston J. P., Ross J. L., Antony S. M., Tokito M. and Holzbaur E. L. F. (2007). Huntingtin facilitates dynein/dynactin-mediated vesicle transport. Proc. Natl. Acad. Sci. USA 104, 10045-10050. 10.1073/pnas.0610628104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan Y. W., Fava L. L., Uldschmid A., Schmitz M. H. A., Gerlich D. W., Nigg E. A. and Santamaria A. (2009). Mitotic control of kinetochore-associated dynein and spindle orientation by human Spindly. J. Cell Biol. 185, 859-874. 10.1083/jcb.200812167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury S., Ketcham S. A., Schroer T. A. and Lander G. C. (2015). Structural organization of the dynein-dynactin complex bound to microtubules. Nat. Struct. Mol. Biol. 22:345-347. 10.1038/nsmb.2996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colin E., Zala D., Liot G., Rangone H., Borrell-Pagès M., Li X.-J., Saudou F. and Humbert S. (2008). Huntingtin phosphorylation acts as a molecular switch for anterograde/retrograde transport in neurons. EMBO J. 27, 2124-2134. 10.1038/emboj.2008.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colucci A. M. R., Campana M. C., Bellopede M. and Bucci C. (2005). The Rab-interacting lysosomal protein, a Rab7 and Rab34 effector, is capable of self-interaction. Biochem. Biophys. Res. Commun. 334, 128-133. 10.1016/j.bbrc.2005.06.067 [DOI] [PubMed] [Google Scholar]
- Dauber A., LaFranchi S. H., Maliga Z., Lui J. C., Moon J. E., McDeed C., Henke K., Zonana J., Kingman G. A., Pers T. H. et al. (2012). Novel microcephalic primordial dwarfism disorder associated with variants in the centrosomal protein ninein. J. Clin. Endocrinol. Metab. 97, E2140-E2151. 10.1210/jc.2012-2150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgehyr N., Sillibourne J., Bornens M. and Bornens M. (2005). Microtubule nucleation and anchoring at the centrosome are independent processes linked by ninein function. J. Cell Sci. 118, 1565-1575. 10.1242/jcs.02302 [DOI] [PubMed] [Google Scholar]
- DeSantis M. E., Cianfrocco M. A., Htet Z. M., Tran P. T., Reck-Peterson S. L. and Leschziner A. E. (2017). Lis1 has two opposing modes of regulating cytoplasmic dynein. Cell 170, 1197-1208.e12. 10.1016/j.cell.2017.08.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWitt M. A., Cypranowska C. A., Cleary F. B., Belyy V. and Yildiz A. (2015). The AAA3 domain of cytoplasmic dynein acts as a switch to facilitate microtubule release. Nat. Struct. Mol. Biol. 22, 73-80. 10.1038/nsmb.2930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickens M., Rogers J. S., Cavanagh J., Raitano A., Xia Z., Halpern J. R., Greenberg M. E., Sawyers C. L. and Davis R. J. (1997). A cytoplasmic inhibitor of the JNK signal transduction pathway. Science 277, 693-696. 10.1126/science.277.5326.693 [DOI] [PubMed] [Google Scholar]
- Dona M., Bachmann-Gagescu R., Texier Y., Toedt G., Hetterschijt L., Tonnaer E. L., Peters T. A., van Beersum S. E. C., Bergboer J. G. M., Horn N. et al. (2015). NINL and DZANK1 co-function in vesicle transport and are essential for photoreceptor development in zebrafish. PLoS Genet. 11, e1005574 10.1371/journal.pgen.1005574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drerup C. M. and Nechiporuk A. V. (2013). JNK-interacting protein 3 mediates the retrograde transport of activated c-Jun N-terminal kinase and lysosomes. PLoS Genet. 9, e1003303 10.1371/journal.pgen.1003303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwivedi D., Kumari A., Rathi S., Mylavarapu S. V. S. and Sharma M. (2019). The dynein adaptor Hook2 plays essential roles in mitotic progression and cytokinesis. J. Cell Biol. 218, 871-894. 10.1083/JCB.201804183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S. L., Yu S., Hoover C. M., Phillips B. C., Richmond J. E. and Miller K. G. (2013). An organelle gatekeeper function for Caenorhabditis elegans UNC-16 (JIP3) at the axon initial segment. Genetics 194, 143-161. 10.1534/genetics.112.147348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan M. J., Tan K. and Reck-Peterson S. L. (2012). Lis1 is an initiation factor for dynein-driven organelle transport. J. Cell Biol. 197, 971-982. 10.1083/jcb.201112101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelender S., Sharp A. H., Colomer V., Tokito M. K., Lanahan A., Worley P., Holzbaur E. L. F. and Ross C. A. (1997). Huntingtin-associated protein 1 (HAP1) interacts with the p150Glued subunit of dynactin. Hum. Mol. Genet. 6, 2205-2212. 10.1093/hmg/6.13.2205 [DOI] [PubMed] [Google Scholar]
- Fu M. and Holzbaur E. L. F. (2013). JIP1 regulates the directionality of APP axonal transport by coordinating kinesin and dynein motors. J. Cell Biol. 202, 495-508. 10.1083/jcb.201302078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu M. and Holzbaur E. L. F. (2014). Integrated regulation of motor-driven organelle transport by scaffolding proteins. Trends Cell Biol. 24, 564-574. 10.1016/j.tcb.2014.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gama J. B., Pereira C., Simões P. A., Celestino R., Reis R. M., Barbosa D. J., Pires H. R., Carvalho C., Amorim J., Carvalho A. X. et al. (2017). Molecular mechanism of dynein recruitment to kinetochores by the Rod-Zw10-Zwilch complex and Spindly. J. Cell Biol. 216, 943-960. 10.1083/jcb.201610108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambello M. J., Darling D. L., Yingling J., Tanaka T., Gleeson J. G., Wynshaw-Boris A., Marchionni M. and Dubois-Dalcq M. (2003). Multiple dose-dependent effects of Lis1 on cerebral cortical development. J. Neurosci. 23, 1719-1729. 10.1523/JNEUROSCI.23-05-01719.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassmann R., Essex A., Hu J.-S., Maddox P. S., Motegi F., Sugimoto A., O'Rourke S. M., Bowerman B., McLeod I., Yates J. R. et al. (2008). A new mechanism controlling kinetochore-microtubule interactions revealed by comparison of two dynein-targeting components: SPDL-1 and the Rod/Zwilch/Zw10 complex. Genes Dev. 22, 2385-2399. 10.1101/gad.1687508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassmann R., Holland A. J., Varma D., Wan X., Civril F., Cleveland D. W., Oegema K., Salmon E. D. and Desai A. (2010). Removal of Spindly from microtubule-attached kinetochores controls spindle checkpoint silencing in human cells. Genes Dev. 24, 957-971. 10.1101/gad.1886810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee M. A., Heuser J. E. and Vallee R. B. (1997). An extended microtubule-binding structure within the dynein motor domain. Nature 390, 636-639. 10.1038/37663 [DOI] [PubMed] [Google Scholar]
- Glater E. E., Megeath L. J., Stowers R. S. and Schwarz T. L. (2006). Axonal transport of mitochondria requires milton to recruit kinesin heavy chain and is light chain independent. J. Cell Biol. 173, 545-557. 10.1083/jcb.200601067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowrishankar S., Wu Y. and Ferguson S. M. (2017). Impaired JIP3-dependent axonal lysosome transport promotes amyloid plaque pathology. J. Cell Biol. 216, 3291-3305. 10.1083/jcb.201612148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffis E. R., Stuurman N. and Vale R. D. (2007). Spindly, a novel protein essential for silencing the spindle assembly checkpoint, recruits dynein to the kinetochore. J. Cell Biol. 177, 1005-1015. 10.1083/jcb.200702062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotjahn D. A., Chowdhury S., Xu Y., McKenney R. J., Schroer T. A. and Lander G. C. (2018). Cryo-electron tomography reveals that dynactin recruits a team of dyneins for processive motility. Nat. Struct. Mol. Biol. 25, 203-207. 10.1038/s41594-018-0027-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunawardena S., Her L.-S., Brusch R. G., Laymon R. A., Niesman I. R., Gordesky-Gold B., Sintasath L., Bonini N. M. and Goldstein L. S. B. (2003). Disruption of axonal transport by loss of huntingtin or expression of pathogenic polyQ proteins in Drosophila. Neuron 40, 25-40. 10.1016/S0896-6273(03)00594-4 [DOI] [PubMed] [Google Scholar]
- Guo X., Farías G. G., Mattera R. and Bonifacino J. S. (2016). Rab5 and its effector FHF contribute to neuronal polarity through dynein-dependent retrieval of somatodendritic proteins from the axon. Proc. Natl. Acad. Sci. USA 113, E5318-E5327. 10.1073/pnas.1601844113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie C. R., Schellenberg G. D. and Kraemer B. C. (2009). SUT-2 potentiates tau-induced neurotoxicity in Caenorhabditis elegans. Hum. Mol. Genet. 18, 1825-1838. 10.1093/hmg/ddp099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez P. A., Ackermann B. E., Vershinin M. and McKenney R. J. (2017). Differential effects of the dynein-regulatory factor Lissencephaly-1 on processive dynein-dynactin motility. J. Biol. Chem. 292, 12245-12255. 10.1074/jbc.M117.790048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebbar S., Mesngon M. T., Guillotte A. M., Desai B., Ayala R. and Smith D. S. (2008). Lis1 and Ndel1 influence the timing of nuclear envelope breakdown in neural stem cells. J. Cell Biol. 182, 1063-1071. 10.1083/jcb.200803071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Her L.-S. and Goldstein L. S. B. (2008). Enhanced sensitivity of striatal neurons to axonal transport defects induced by mutant huntingtin. J. Neurosci. 28, 13662-13672. 10.1523/JNEUROSCI.4144-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogenraad C. C., Akhmanova A., Howell S. A., Dortland B. R., De Zeeuw C. I., Willemsen R., Visser P., Grosveld F. and Galjart N. (2001). Mammalian Golgi-associated Bicaudal-D2 functions in the dynein-dynactin pathway by interacting with these complexes. EMBO J. 20, 4041-4054. 10.1093/emboj/20.15.4041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horgan C. P. and McCaffrey M. W. (2009). The dynamic Rab11-FIPs. Biochem. Soc. Trans. 37, 1032-1036. 10.1042/BST0371032 [DOI] [PubMed] [Google Scholar]
- Horgan C. P., Hanscom S. R., Jolly R. S., Futter C. E. and McCaffrey M. W. (2010). Rab11-FIP3 links the Rab11 GTPase and cytoplasmic dynein to mediate transport to the endosomal-recycling compartment. J. Cell Sci. 123, 181-191. 10.1242/jcs.052670 [DOI] [PubMed] [Google Scholar]
- Hu D. J.-K., Baffet A. D., Nayak T., Akhmanova A., Doye V. and Vallee R. B. (2013). Dynein recruitment to nuclear pores activates apical nuclear migration and mitotic entry in brain progenitor cells. Cell 154, 1300-1313. 10.1016/j.cell.2013.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Roberts A. J., Leschziner A. E. and Reck-Peterson S. L. (2012). Lis1 acts as a “clutch” between the ATPase and microtubule-binding domains of the dynein motor. Cell 150, 975-986. 10.1016/j.cell.2012.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh W. and Vale R. D. (2017). Disease-associated mutations in human BICD2 hyperactivate motility of dynein-dynactin. J. Cell Biol. 216, 3051-3060. 10.1083/jcb.201703201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H., Ha V. L., Prekeris R. and Randazzo P. A. (2008). Arf GTPase-activating protein ASAP1 Interacts with Rab11 effector FIP3 and regulates pericentrosomal localization of transferrin receptor–positive recycling endosome. Mol. Biol. Cell 19, 4224-4237. 10.1091/mbc.e08-03-0290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing J. and Prekeris R. (2009). Polarized endocytic transport: The roles of Rab11 and Rab11-FIPs in regulating cell polarity. Histol. Histopathol. 24, 1171-1180. 10.14670/HH-24.1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson M., Rocha N., Zwart W., Jordens I., Janssen L., Kuijl C., Olkkonen V. M. and Neefjes J. (2007). Activation of endosomal dynein motors by stepwise assembly of Rab7–RILP–p150Glued, ORP1L, and the receptor βlll spectrin. J. Cell Biol. 176, 459-471. 10.1083/jcb.200606077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordens I., Fernandez-Borja M., Marsman M., Dusseljee S., Janssen L., Calafat J., Janssen H., Wubbolts R. and Neefjes J. (2001). The Rab7 effector protein RILP controls lysosomal transport by inducing the recruitment of dynein-dynactin motors. Curr. Biol. 11, 1680-1685. 10.1016/S0960-9822(01)00531-0 [DOI] [PubMed] [Google Scholar]
- Kardon J. R. and Vale R. D. (2009). Regulators of the cytoplasmic dynein motor. Nat. Rev. Mol. Cell Biol. 10, 854-865. 10.1038/nrm2804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelkar N., Gupta S., Dickens M. and Davis R. J. (2000). Interaction of a mitogen-activated protein kinase signaling module with the neuronal protein JIP3. Mol. Cell. Biol. 20, 1030-1043. 10.1128/MCB.20.3.1030-1043.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelkar N., Standen C. L. and Davis R. J. (2005). Role of the JIP4 scaffold protein in the regulation of mitogen-activated protein kinase signaling pathways. Mol. Cell. Biol. 25, 2733-2743. 10.1128/MCB.25.7.2733-2743.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M. H., Cooper D. R., Oleksy A., Devedjiev Y., Derewenda U., Reiner O., Otlewski J. and Derewenda Z. S. (2004). The structure of the N-terminal domain of the product of the lissencephaly gene Lis1 and its functional implications. Structure 12, 987-998. 10.1016/j.str.2004.03.024 [DOI] [PubMed] [Google Scholar]
- Klinman E. and Holzbaur E. L. F. (2015). Stress-induced CDK5 activation disrupts axonal transport via Lis1/Ndel1/Dynein. Cell Rep. 12, 462-473. 10.1016/j.celrep.2015.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kon T., Sutoh K. and Kurisu G. (2011). X-ray structure of a functional full-length dynein motor domain. Nat. Struct. Mol. Biol. 18, 638-642. 10.1038/nsmb.2074 [DOI] [PubMed] [Google Scholar]
- Lee I.-G., Olenick M. A., Boczkowska M., Franzini-Armstrong C., Holzbaur E. L. F. and Dominguez R. (2018). A conserved interaction of the dynein light intermediate chain with dynein-dynactin effectors necessary for processivity. Nat. Commun. 9, 986 10.1038/s41467-018-03412-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz J. H., Schuchardt I., Straube A. and Steinberg G. (2006). A dynein loading zone for retrograde endosome motility at microtubule plus-ends. EMBO J. 25:2275-2286. 10.1038/sj.emboj.7601119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S.-H., Gutekunst C.-A., Hersch S. M., Li X.-J., Sheth A., Kim J., Young A., Penney J., Golden J., Aronin N. et al. (1998). Interaction of huntingtin-associated protein with dynactin P150Glued. J. Neurosci. 18, 1261-1269. 10.1523/JNEUROSCI.18-04-01261.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Salter H. K., Holding A. N., Johnson C. M., Stephens E., Lukavsky P. J., Walshaw J. and Bullock S. L. (2013). Bicaudal-D uses a parallel, homodimeric coiled coil with heterotypic registry to coordinate recruitment of cargos to dynein. Genes Dev. 27, 1233-1246. 10.1101/gad.212381.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loss O. and Stephenson F. A. (2015). Localization of the kinesin adaptor proteins trafficking kinesin proteins 1 and 2 in primary cultures of hippocampal pyramidal and cortical neurons. J. Neurosci. Res. 93, 1056-1066. 10.1002/jnr.23549 [DOI] [PubMed] [Google Scholar]
- Luiro K., Yliannala K., Ahtiainen L., Maunu H., Järvelä I., Kyttälä A. and Jalanko A. (2004). Interconnections of CLN3, Hook1 and Rab proteins link Batten disease to defects in the endocytic pathway. Hum. Mol. Genet. 13, 3017-3027. 10.1093/hmg/ddh321 [DOI] [PubMed] [Google Scholar]
- MacAskill A. F., Rinholm J. E., Twelvetrees A. E., Arancibia-Carcamo I. L., Muir J., Fransson A., Aspenstrom P., Attwell D. and Kittler J. T. (2009). Miro1 Is a Calcium Sensor for Glutamate Receptor-Dependent Localization of Mitochondria at Synapses. Neuron 61, 541-555. 10.1016/j.neuron.2009.01.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mach J. M. and Lehmann R. (1997). An Egalitarian-BicaudalD complex is essential for oocyte specification and axis determination in Drosophila. Genes Dev. 11, 423-435. 10.1101/gad.11.4.423 [DOI] [PubMed] [Google Scholar]
- Maldonado-Báez L., Cole N. B., Krämer H. and Donaldson J. G. (2013). Microtubule-dependent endosomal sorting of clathrin-independent cargo by Hook1. J. Cell Biol. 201, 233-247. 10.1083/jcb.201208172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matanis T., Akhmanova A., Wulf P., Del Nery E., Weide T., Stepanova T., Galjart N., Grosveld F., Goud B., De Zeeuw C. I. et al. (2002). Bicaudal-D regulates COPI-independent Golgi–ER transport by recruiting the dynein–dynactin motor complex. Nat. Cell Biol. 4, 986-992. 10.1038/ncb891 [DOI] [PubMed] [Google Scholar]
- McClintock M. A., Dix C. I., Johnson C. M., McLaughlin S. H., Maizels R. J., Hoang H. T. and Bullock S. L. (2018). RNA-directed activation of cytoplasmic dynein-1 in reconstituted transport RNPs. Elife. 7, e36312 10.7554/eLife.36312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire J. R., Rong J., Li S.-H. and Li X.-J. (2006). Interaction of Huntingtin-associated protein-1 with kinesin light chain: implications in intracellular trafficking in neurons. J. Biol. Chem 281, 3552-3559. 10.1074/jbc.M509806200 [DOI] [PubMed] [Google Scholar]
- McKenney R. J., Vershinin M., Kunwar A., Vallee R. B. and Gross S. P. (2010). LIS1 and NudE Induce a persistent dynein force-producing state. Cell 141, 304-314. 10.1016/j.cell.2010.02.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenney R. J., Huynh W., Tanenbaum M. E., Bhabha G. and Vale R. D. (2014). Activation of cytoplasmic dynein motility by dynactin-cargo adapter complexes. Science 345, 337-341. 10.1126/science.1254198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melkov A. and Abdu U. (2018). Regulation of long-distance transport of mitochondria along microtubules. Cell. Mol. Life Sci. 75, 163-176. 10.1007/s00018-017-2590-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen M. M., Malik A., Piel M., Bouckson-Castaing V. and Bornens M. (2000). Microtubule minus-end anchorage at centrosomal and non-centrosomal sites: the role of ninein. J. Cell Sci. 113, 3013-3023. [DOI] [PubMed] [Google Scholar]
- Mohler J. and Wieschaus E. F. (1986). Dominant maternal-effect mutations of Drosophila melanogaster causing the production of double-abdomen embryos. Genetics 112, 803-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagnac G., Sibarita J.-B., Loubéry S., Daviet L., Romao M., Raposo G. and Chavrier P. (2009). ARF6 Interacts with JIP4 to control a motor switch mechanism regulating endosome traffic in cytokinesis. Curr. Biol. 19, 184-195. 10.1016/j.cub.2008.12.043 [DOI] [PubMed] [Google Scholar]
- Moss D. K., Bellett G., Carter J. M., Liovic M., Keynton J., Prescott A. R., Lane E. B. and Mogensen M. M. (2007). Ninein is released from the centrosome and moves bi-directionally along microtubules. J. Cell Sci. 120, 3064-3074. 10.1242/jcs.010322 [DOI] [PubMed] [Google Scholar]
- Moughamian A. J., Osborn G. E., Lazarus J. E., Maday S. and Holzbaur E. L. F. (2013). Ordered recruitment of dynactin to the microtubule plus-end is required for efficient initiation of retrograde axonal transport. J. Neurosci. 33, 13190-13203. 10.1523/JNEUROSCI.0935-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moynihan K. L., Pooley R., Miller P. M., Kaverina I. and Bader D. M. (2009). Murine CENP-F regulates centrosomal microtubule nucleation and interacts with Hook2 at the centrosome. Mol. Biol. Cell 20, 4790-4803. 10.1091/mbc.e09-07-0560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas M. P., Berger F., Rao L., Brenner S., Cho C. and Gennerich A. (2015). Cytoplasmic dynein regulates its attachment to microtubules via nucleotide state-switched mechanosensing at multiple AAA domains. Proc. Natl. Acad. Sci. USA 112, 6371-6376. 10.1073/pnas.1417422112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J., Baksa K. and Steward R. (2000). Functional domains of the Drosophila bicaudal-D protein. Genetics 154, 713-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olenick M. A., Dominguez R. and Holzbaur E. L. F. (2019). Dynein activator Hook1 is required for trafficking of BDNF-signaling endosomes in neurons. J. Cell Biol. 218, 220-233. 10.1083/jcb.201805016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olenick M. A., Tokito M., Boczkowska M., Dominguez R. and Holzbaur E. L. F. (2016). Hook adaptors induce unidirectional processive motility by enhancing the Dynein-Dynactin interaction. J. Biol. Chem. 291, 18239-18251. 10.1074/jbc.M116.738211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey J. P. and Smith D. S. (2011). A Cdk5-dependent switch regulates Lis1/Ndel1/dynein-driven organelle transport in adult axons. J. Neurosci. 31, 17207-17219. 10.1523/JNEUROSCI.4108-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu R., Zhang J. and Xiang X. (2018). p25 of the dynactin complex plays a dual role in cargo binding and dynactin regulation. J. Biol. Chem. 293, 15606-15619. 10.1074/jbc.RA118.004000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reck-Peterson S. L., Redwine W. B., Vale R. D. and Carter A. P. (2018). The cytoplasmic dynein transport machinery and its many cargoes. Nat. Rev. Mol. Cell Biol. 19, 382-398. 10.1038/s41580-018-0004-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redwine W. B., DeSantis M. E., Hollyer I., Htet Z. M., Tran P. T., Swanson S. K., Florens L., Washburn M. P. and Reck-Peterson S. L. (2017). The human cytoplasmic dynein interactome reveals novel activators of motility. Elife 6, e28257 10.7554/eLife.28257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner O., Carrozzo R., Shen Y., Wehnert M., Faustinella F., Dobyns W. B., Caskey C. T. and Ledbetter D. H. (1993). Isolation of a Miller–Dicker lissencephaly gene containing G protein β-subunit-like repeats. Nature 364, 717-721. 10.1038/364717a0 [DOI] [PubMed] [Google Scholar]
- Schlager M. A., Kapitein L. C., Grigoriev I., Burzynski G. M., Wulf P. S., Keijzer N., de Graaff E., Fukuda M., Shepherd I. T., Akhmanova A. et al. (2010). Pericentrosomal targeting of Rab6 secretory vesicles by Bicaudal-D-related protein 1 (BICDR-1) regulates neuritogenesis. EMBO J. 29, 1637-1651. 10.1038/emboj.2010.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlager M. A., Hoang H. T., Urnavicius L., Bullock S. L. and Carter A. P. (2014a). In vitro reconstitution of a highly processive recombinant human dynein complex. EMBO J. 33, 1855-1868. 10.15252/embj.201488792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlager M. A., Serra-Marques A., Grigoriev I., Gumy L. F., Esteves da Silva M., Wulf P. S., Akhmanova A. and Hoogenraad C. C. (2014b). Bicaudal d family adaptor proteins control the velocity of Dynein-based movements. Cell Rep. 8, 1248-1256. 10.1016/j.celrep.2014.07.052 [DOI] [PubMed] [Google Scholar]
- Schroeder C. M. and Vale R. D. (2016). Assembly and activation of dynein-dynactin by the cargo adaptor protein Hook3. J. Cell Biol. 214, 309-318. 10.1083/jcb.201604002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder C. M., Ostrem J. M., Hertz N. T. and Vale R. D. (2014). A Ras-like domain in the light intermediate chain bridges the dynein motor to a cargo-binding region. Elife 3, e03351 10.7554/eLife.03351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroer T. A. (2004). Dynactin. Annu. Rev. Cell Dev. Biol. 20, 759-779. 10.1146/annurev.cellbio.20.012103.094623 [DOI] [PubMed] [Google Scholar]
- Shao C.-Y., Zhu J., Xie Y.-J., Wang Z., Wang Y.-N., Wang Y., Su L.-D., Zhou L., Zhou T.-H. and Shen Y. (2013). Distinct functions of nuclear distribution proteins LIS1, Ndel1 and NudCL in regulating axonal mitochondrial transport. Traffic 14, 785-797. 10.1111/tra.12070 [DOI] [PubMed] [Google Scholar]
- Short B., Preisinger C., Schaletzky J., Kopajtich R. and Barr F. A. (2002). The Rab6 GTPase regulates recruitment of the dynactin complex to Golgi membranes. Curr. Biol. 12, 1792-1795. 10.1016/S0960-9822(02)01221-6 [DOI] [PubMed] [Google Scholar]
- Simon G. C., Schonteich E., Wu C. C., Piekny A., Ekiert D., Yu X., Gould G. W., Glotzer M. and Prekeris R. (2008). Sequential Cyk-4 binding to ECT2 and FIP3 regulates cleavage furrow ingression and abscission during cytokinesis. EMBO J. 27, 1791-1803. 10.1038/emboj.2008.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladewski T. E., Billington N., Ali M. Y., Bookwalter C. S., Lu H., Krementsova E. B., Schroer T. A. and Trybus K. M (2018). Recruitment of two dyneins to an mRNA-dependent Bicaudal D transport complex. Elife. 7, e36306 10.7554/eLife.36306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M., Giza J., Proenca C. C., Jing D., Elliott M., Dincheva I., Shmelkov S. V., Kim J., Schreiner R., Huang S.-H. et al. (2015). Slitrk5 Mediates BDNF-Dependent TrkB receptor trafficking and signaling. Dev. Cell 33, 690-702. 10.1016/j.devcel.2015.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Splinter D., Razafsky D. S., Schlager M. A., Serra-Marques A., Grigoriev I., Demmers J., Keijzer N., Jiang K., Poser I., Hyman A. A. et al. (2012). BICD2, dynactin, and LIS1 cooperate in regulating dynein recruitment to cellular structures. Mol. Biol. Cell 23, 4226-4241. 10.1091/mbc.e12-03-0210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehman S. A., Chen Y., McKenney R. J. and Vallee R. B. (2007). NudE and NudEL are required for mitotic progression and are involved in dynein recruitment to kinetochores. J. Cell Biol. 178, 583-594. 10.1083/jcb.200610112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowers R. S., Megeath L. J., Górska-Andrzejak J., Meinertzhagen I. A. and Schwarz T. L. (2002). Axonal transport of mitochondria to synapses depends on milton, a novel Drosophila protein. Neuron 36, 1063-1077. 10.1016/S0896-6273(02)01094-2 [DOI] [PubMed] [Google Scholar]
- Suter B. and Steward R. (1991). Requirement for phosphorylation and localization of the Bicaudal-D protein in Drosophila oocyte differentiation. Cell 67, 917-926. 10.1016/0092-8674(91)90365-6 [DOI] [PubMed] [Google Scholar]
- Swan A. and Suter B. (1996). Role of Bicaudal-D in patterning the Drosophila egg chamber in mid-oogenesis. Development 122, 3577-3586. [DOI] [PubMed] [Google Scholar]
- Swan A., Nguyen T. and Suter B. (1999). Drosophila Lissencephaly-1 functions with Bic-D and dynein in oocyte determination and nuclear positioning. Nat. Cell Biol. 1, 444-449. 10.1038/15680 [DOI] [PubMed] [Google Scholar]
- Szebenyi G., Hall B., Yu R., Hashim A. I. and Krämer H. (2007). Hook2 Localizes to the centrosome, binds directly to centriolin/CEP110 and contributes to centrosomal function. Traffic 8, 32-46. 10.1111/j.1600-0854.2006.00511.x [DOI] [PubMed] [Google Scholar]
- Takahide Kon, Nishiura M., Ohkura R. and Toyoshima Y. Y. and Sutoh K (2004). Distinct functions of nucleotide-binding/hydrolysis sites in the four AAA modules of cytoplasmic dynein. Biochemistry 43, 11266-11274. 10.1021/BI048985A [DOI] [PubMed] [Google Scholar]
- Tarricone C., Perrina F., Monzani S., Massimiliano L., Kim M.-H., Derewenda Z. S., Knapp S., Tsai L.-H. and Musacchio A. (2004). Coupling PAF signaling to dynein regulation: structure of LIS1 in complex with PAF-acetylhydrolase. Neuron 44, 809-821. 10.1016/j.neuron.2004.11.019 [DOI] [PubMed] [Google Scholar]
- Terawaki S., Yoshikane A., Higuchi Y. and Wakamatsu K. (2015). Structural basis for cargo binding and autoinhibition of Bicaudal-D1 by a parallel coiled-coil with homotypic registry. Biochem. Biophys. Res. Commun. 460, 451-456. 10.1016/j.bbrc.2015.03.054 [DOI] [PubMed] [Google Scholar]
- Torisawa T., Ichikawa M., Furuta A., Saito K., Oiwa K., Kojima H., Toyoshima Y. Y. and Furuta K. (2014). Autoinhibition and cooperative activation mechanisms of cytoplasmic dynein. Nat. Cell Biol. 16, 1118-1124. 10.1038/ncb3048 [DOI] [PubMed] [Google Scholar]
- Toropova K., Zou S., Roberts A. J., Redwine W. B., Goodman B. S., Reck-Peterson S. L. and Leschziner A. E. (2014). Lis1 regulates dynein by sterically blocking its mechanochemical cycle. Elife. 3, e03372 10.7554/eLife.03372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai J.-W., Bremner K. H. and Vallee R. B. (2007). Dual subcellular roles for LIS1 and dynein in radial neuronal migration in live brain tissue. Nat. Neurosci. 10, 970-979. 10.1038/nn1934 [DOI] [PubMed] [Google Scholar]
- Twelvetrees A. E., Yuen E. Y., Arancibia-Carcamo I. L., MacAskill A. F., Rostaing P., Lumb M. J., Humbert S., Triller A., Saudou F., Yan Z. et al. (2010). Delivery of GABAARs to Synapses Is Mediated by HAP1-KIF5 and Disrupted by Mutant Huntingtin. Neuron 65, 53-65. 10.1016/j.neuron.2009.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urnavicius L., Zhang K., Diamant A. G., Motz C., Schlager M. A., Yu M., Patel N. A., Robinson C. V. and Carter A. P. (2015). The structure of the dynactin complex and its interaction with dynein. Science 347, 1441-1446. 10.1126/science.aaa4080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urnavicius L., Lau C. K., Elshenawy M. M., Morales-Rios E., Motz C., Yildiz A. and Carter A. P. (2018). Cryo-EM shows how dynactin recruits two dyneins for faster movement. Nature 554, 202-206. 10.1038/nature25462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Spronsen M., Mikhaylova M., Lipka J., Schlager M. A., van den Heuvel D. J., Kuijpers M., Wulf P. S., Keijzer N., Demmers J., Kapitein L. C. et al. (2013). TRAK/Milton motor-adaptor proteins steer mitochondrial trafficking to axons and dendrites. Neuron 77, 485-502. 10.1016/j.neuron.2012.11.027 [DOI] [PubMed] [Google Scholar]
- Verhey K. J., Meyer D., Deehan R., Blenis J., Schnapp B. J., Rapoport T. A. and Margolis B. (2001). Cargo of kinesin identified as JIP scaffolding proteins and associated signaling molecules. J. Cell Biol. 152, 959-970. 10.1083/jcb.152.5.959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walenta J. H., Didier A. J., Liu X. and Krämer H. (2001). The Golgi-associated hook3 protein is a member of a novel family of microtubule-binding proteins. J. Cell Biol. 152, 923-934. 10.1083/jcb.152.5.923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. and Schwarz T. L. (2009). The mechanism of Ca2+-dependent regulation of kinesin-mediated mitochondrial motility. Cell 136, 163-174. 10.1016/j.cell.2008.11.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. and Zheng Y. (2011). Identification of a novel dynein binding domain in nudel essential for spindle pole organization in Xenopus egg extract. J. Biol. Chem 286, 587-593. 10.1074/jbc.M110.181578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Wu D., Quintin S., Green R. A., Cheerambathur D. K., Ochoa S. D., Desai A. and Oegema K. (2015). NOCA-1 functions with γ-tubulin and in parallel to Patronin to assemble non-centrosomal microtubule arrays in C. elegans. Elife. 4 10.7554/eLife.08649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman-Storer C., Karki S. and Holzbaur E. (1995). The p150Glued component of the dynactin complex binds to both microtubules and the actin-related protein centractin (Arp1). Proc. Natl. Acad. Sci. USA 92, 1634-1638. 10.1073/pnas.92.5.1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss K. R. and Littleton J. T. (2016). Characterization of axonal transport defects in Drosophila Huntingtin mutants. J. Neurogenet 30, 212-221. 10.1080/01677063.2016.1202950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton R. P. and Struhl G. (1989). Structure of the Drosophila BicaudalD protein and its role in localizing the the posterior determinant nanos. Cell 59, 881-892. 10.1016/0092-8674(89)90611-9 [DOI] [PubMed] [Google Scholar]
- Whitmarsh A. J. (2006). The JIP family of MAPK scaffold proteins. Biochem. Soc. Trans. 34, 828-832. 10.1042/BST0340828 [DOI] [PubMed] [Google Scholar]
- Wilson G. M., Fielding A. B., Simon G. C., Yu X., Andrews P. D., Hames R. S., Frey A. M., Peden A. A., Gould G. W. and Prekeris R. (2005). The FIP3-Rab11 protein complex regulates recycling endosome targeting to the cleavage furrow during late cytokinesis. Mol. Biol. Cell 16, 849-860. 10.1091/mbc.e04-10-0927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong Y. C. and Holzbaur E. L. F. (2014). The regulation of autophagosome dynamics by huntingtin and HAP1 is disrupted by expression of mutant huntingtin, leading to defective cargo degradation. J. Neurosci. 34, 1293-1305. 10.1523/JNEUROSCI.1870-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M., Wang T., Loh E., Hong W., and Song H (2005). Structural basis for recruitment of RILP by small GTPase Rab7. EMBO J. 24, 1491-1501. 10.1038/sj.emboj.7600643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang X., Osmani A. H., Osmani S. A., Xin M. and Morris N. R. (1995). NudF, a nuclear migration gene in Aspergillus nidulans, is similar to the human LIS-1 gene required for neuronal migration. Mol. Biol. Cell 6, 297-310. 10.1091/mbc.6.3.297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L., Sowa M. E., Chen J., Li X., Gygi S. P. and Harper J. W. (2008). An FTS/Hook/p107(FHIP) complex interacts with and promotes endosomal clustering by the homotypic vacuolar protein sorting complex. Mol. Biol. Cell 19, 5059-5071. 10.1091/mbc.e08-05-0473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M., Toba S., Yoshida Y., Haratani K., Mori D., Yano Y., Mimori-Kiyosue Y., Nakamura T., Itoh K., Fushiki S. et al. (2008). LIS1 and NDEL1 coordinate the plus-end-directed transport of cytoplasmic dynein. EMBO J. 27, 2471-2483. 10.1038/emboj.2008.182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G.-Z., Yang M., Lim Y., Lu J.-J., Wang T.-H., Qi J.-G., Zhong J.-H. and Zhou X.-F. (2012). Huntingtin associated protein 1 regulates trafficking of the amyloid precursor protein and modulates amyloid beta levels in neurons. J. Neurochem. 122, 1010-1022. 10.1111/j.1471-4159.2012.07845.x [DOI] [PubMed] [Google Scholar]
- Yasuda J., Whitmarsh A. J., Cavanagh J., Sharma M. and Davis R. J. (1999). The JIP group of mitogen-activated protein kinase scaffold proteins. Mol. Cell. Biol. 19, 7245-7254. 10.1128/MCB.19.10.7245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazaki Y., Hara Y., Tamaki H., Fukaya M. and Sakagami H. (2014). Endosomal localization of FIP3/Arfophilin-1 and its involvement in dendritic formation of mouse hippocampal neurons. Brain Res. 1557, 55-65. 10.1016/j.brainres.2014.02.018 [DOI] [PubMed] [Google Scholar]
- Yeh T.-Y., Quintyne N. J., Scipioni B. R., Eckley D. M. and Schroer T. A. (2012). Dynactin's pointed-end complex is a cargo-targeting module. Mol. Biol. Cell 23, 3827-3837. 10.1091/mbc.e12-07-0496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi J. Y., Ori-McKenney K. M., McKenney R. J., Vershinin M., Gross S. P. and Vallee R. B. (2011). High-resolution imaging reveals indirect coordination of opposite motors and a role for LIS1 in high-load axonal transport. J. Cell Biol. 195, 193-201. 10.1083/jcb.201104076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Zhuang L., Lee Y., Abenza J. F., Peñalva M. A. and Xiang X. (2010). The microtubule plus-end localization of Aspergillus dynein is important for dynein-early-endosome interaction but not for dynein ATPase activation. J. Cell Sci. 123, 3596-3604. 10.1242/jcs.075259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K., Foster H. E., Rondelet A., Lacey S. E., Bahi-Buisson N., Bird A. W. and Carter A. P. (2017). Cryo-EM Reveals How Human Cytoplasmic Dynein Is Auto-inhibited and Activated. Cell 169, 1303-1314.e18. 10.1016/j.cell.2017.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Yao X., Fischer L., Abenza J. F., Peñalva M. A. and Xiang X. (2011). The p25 subunit of the dynactin complex is required for dynein-early endosome interaction. J. Cell Biol. 193, 1245-1255. 10.1083/jcb.201011022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Qiu R., Arst H. N., Peñalva M. A. and Xiang X. (2014). HookA is a novel dynein-early endosome linker critical for cargo movement in vivo. J. Cell Biol. 204, 1009-1026. 10.1083/jcb.201308009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zyłkiewicz E., Kijańska M., Choi W.-C., Derewenda U., Derewenda Z. S. and Stukenberg P. T. (2011). The N-terminal coiled-coil of Ndel1 is a regulated scaffold that recruits LIS1 to dynein. J. Cell Biol. 192, 433-445. 10.1083/jcb.201011142 [DOI] [PMC free article] [PubMed] [Google Scholar]