ABSTRACT
STIM1- and Orai1-mediated store-operated Ca2+ entry (SOCE) constitutes the major Ca2+ influx in almost all electrically non-excitable cells. However, little is known about the spatiotemporal organization at the elementary level. Here, we developed Orai1-tethered or palmitoylated biosensor GCaMP6f to report subplasmalemmal Ca2+ signals. We visualized spontaneous discrete and long-lasting transients (‘Ca2+ glows’) arising from STIM1-Orai1 in invading melanoma cells. Ca2+ glows occurred preferentially in single invadopodia and at sites near the cell periphery under resting conditions. Re-addition of external Ca2+ after store depletion elicited spatially synchronous Ca2+ glows, followed by high-rate discharge of asynchronous local events. Knockout of STIM1 or expression of the dominant-negative Orai1-E106A mutant markedly decreased Ca2+ glow frequency, diminished global SOCE and attenuated invadopodial formation. Functionally, invadopodial Ca2+ glows provided high Ca2+ microdomains to locally activate Ca2+/calmodulin-dependent Pyk2 (also known as PTK2B), which initiates the SOCE–Pyk2–Src signaling cascade required for invasion. Overall, the discovery of elemental Ca2+ signals of SOCE not only unveils a previously unappreciated gating mode of STIM1-Orai1 channels in situ, but also underscores a critical role of the spatiotemporal dynamics of SOCE in orchestrating complex cell behaviors such as invasion.
This article has an associated First Person interview with the first author of the paper.
KEY WORDS: Ca2+ biosensor, Store-operated Ca2+ entry, Ca2+ glows, Pyk2, Invasion
Summary: Elemental Ca2+ signals of store-operated Ca2+ entry, visualized in native cancer and endothelial cells by newly developed plasmalemmal targeted sensors, are critical for invasion via regulation of the Pyk2–Src pathway.
INTRODUCTION
Ca2+ is a remarkably versatile second messenger that controls a wide range of physiological and pathophysiological processes (Berridge et al., 2003). Cytosolic Ca2+ is tightly controlled by an elaborate system consisting of channels, pumps and exchangers as well as buffers. The spatial and temporal organization of cytosolic and organellar Ca2+ is also crucial for the speed, specificity and robustness of Ca2+ signaling (Berridge et al., 2003; Cheng and Lederer, 2008). Among a plethora of Ca2+ entry pathways, store-operated Ca2+ channels (SOCs) provide the predominant mechanism responsible for replenishing the endoplasmic reticulum (ER) Ca2+ store (Parekh and Putney, 2005) in electrically non-excitable cells. Stromal interacting molecule 1 (STIM1)- and Orai1-mediated store-operated Ca2+ entry (SOCE) has been described in some excitable cells (Chen and Sanderson, 2017; Feldman et al., 2017; Wei-LaPierre et al., 2013). The ER-resident Ca2+ sensor, STIM1, and the plasma membrane (PM) pore-forming subunit Orai1, are the two critical components of SOCE. Upon depletion of the ER Ca2+ store, STIM1 oligomerizes and translocates to the ER-PM junctions to cluster and activate Orai1 channels and Ca2+ influx.
To date, little is known about the spatiotemporal organization of SOCE at the elementary level, in particular, it is not clear whether SOCE comprises elementary Ca2+ signals that are similar to Ca2+ sparks (Cheng et al., 1993) and Ca2+ puffs (Yao and Parker, 1994) for stored Ca2+ release mediated by ryanodine receptors and inositol 1,4,5-triphosphate receptors. It is believed that Ca2+ concentration microdomains are confined to tens of nanometers within the open mouth of activated SOCs (Hogan, 2015), and the clustering of Orai1 proteins significantly enhances the signaling downstream of SOCE (Samanta et al., 2015). Therefore, detection of such STIM1-Orai1 puncta-mediated Ca2+ microdomains could provide insightful information into how the spatiotemporal dynamics of SOCE orchestrates downstream signaling, as well as check the veracity of models for STIM1-Orai1 gating in the cluster.
STIM1 and Orai1 have been increasingly implicated in cancer and are reported to be essential for cancer cell migration and invasion (Sun et al., 2014). Invadopodia, the invasion machinery, are proteolytic membrane protrusions used by malignant cells to remodel the extracellular matrix and to facilitate metastatic dissemination (Murphy and Courtneidge, 2011). The invadopodium consists of an actin-rich core surrounded by a ring of adhesive protein complexes (Gimona et al., 2008; Linder, 2007). The assembly of invadopodia is stimulated in response to pro-invasion cues in the tumor microenvironment (Murphy and Courtneidge, 2011), which activate the non-receptor tyrosine kinase Src to initiate the nucleation of the invadopodial F-actin core (Gimona et al., 2008). Here, we mainly used a cancer cell model to investigate the spatiotemporal Ca2+ dynamics of SOCE in intact cells, to illustrate its interplay with subcellular morphological structures, and to explore the cellular and molecular mechanisms by which SOCE controls downstream signaling.
By fusing the genetically encoded Ca2+ indicator GCaMP6f to the N-terminus of the Orai1 channel or attaching the palmitoylation sequence of the human GAP43 protein to GCaMP6f, we developed plasmalemma-targeted biosensors to preferentially report Ca2+ influx. We discovered spontaneous, long-lasting Ca2+ transients confined to single invadopodia as well as discrete sites at the cell periphery of invading melanoma cells. Such local Ca2+ transients, dubbed ‘Ca2+ glows’, constitute elementary events of SOCE mediated by STIM1 and Orai1 clusters. Similar Ca2+ glows of STIM1 and Orai1 origin were found in human umbilical vein endothelial cells (HUVECs). We further demonstrated that, in the cancer cell model, invadopodial Ca2+ glows play an important role in orchestrating local Pyk2 (also known as PTK2B) and Src activation to signal cancer cell invasion.
RESULTS
Targeting GCaMP6f to the plasma membrane to report subplasmalemmal Ca2+
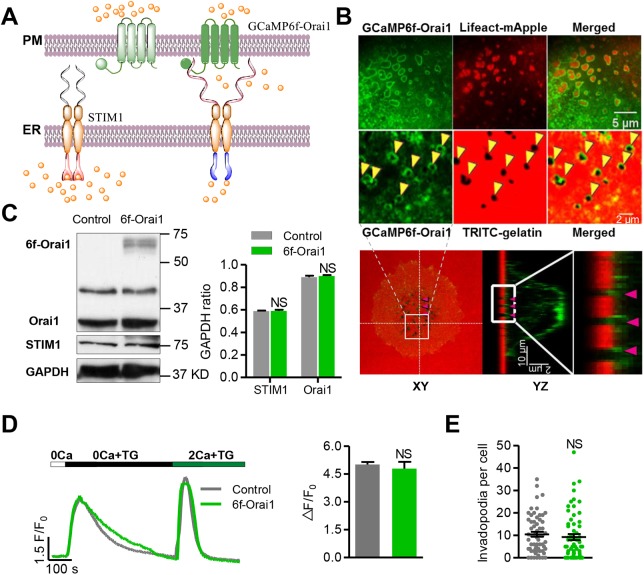
Upon depletion of the ER Ca2+ store, STIM1 translocates and accumulates in regions of the ER-PM junction, where it clusters Orai1 through physical contact to form structures commonly referred to as ‘puncta’ (Liou et al., 2005; Wu et al., 2006). STIM1 also provides the signal that activates the apposed Orai1, a highly Ca2+-selective channel with extremely small single-channel conductance (Prakriya and Lewis, 2015). We reckoned that targeting a Ca2+ sensor to the microdomain of the Orai1 channel mouth would enable us to investigate how the STIM-Orai1 puncta operate in live cells. We therefore fused the genetically encoded Ca2+ probe GCaMP6f to the N-terminus of Orai1 adjacent to the transmembrane domain (Fig. 1A). It has been reported that protein fusion in this region does not alter the assembly, trafficking and activity of SOCs (Li et al., 2007; Park et al., 2009; Zhou et al., 2016). The Ca2+ biosensor GCaMP6f was chosen because of its demonstrated capabilities in resolving ryanodine receptor-mediated Ca2+ sparks in heart cells (Shang et al., 2014) and Ca2+ transients elicited by single action potentials in neurons (Chen et al., 2013). As an alternative strategy to probe subsurface Ca2+ without altering the stoichiometry of STIM1 and Orai1, we generated another targeted Ca2+ sensor, PM-GCaMP6f, by attaching the palmitoylation sequence of the human GAP43 protein to the N-terminus of GCaMP6f (Varnai et al., 2006).
Fig. 1.
Targeting the Ca2+ biosensor GCaMP6f to the plasma membrane. (A) Schematic showing the design strategy of the GCaMP6f-Orai1 Ca2+ biosensor. GCaMP6f was fused to the N-terminus of Orai1 (GCaMP6f-Orai1) to target it to the plasma membrane with preferential accessibility to Orai1-mediated Ca2+ entry. This vector was expressed in WM793 cells by adenoviral infection. (B) Localization of GCaMP6f-Orai1. Upper panel: confocal images of GCaMP6f-Orai1 (top left), invadopodia visualized by Lifeact-mApple fluorescence (top middle) or degradation holes (arrowheads) left after TRITC-labeled gelatin was digested (bottom middle, an enlarged view of the cell area is indicated in the lower panel), and their merged images. Note that the GCaMP6f-Orai1 fluorescence encircles invadopodia. Lower panel: orthogonal views of confocal z stacks, showing the localization of the targeted sensor: XY view (left), YZ view (middle) and an enlarged view of the boxed area indicated in the YZ view (right). (C) Western blots (left) showing the expression of GCaMP6f-Orai1 and its effect on endogenous STIM1 and Orai1 levels, and quantification (right). (D) Effects of biosensor expression on thapsigargin (TG)-induced SOCE. Left: after store Ca2+ release induced by 5 μM TG in 0 Ca2+ solution, reintroducing Ca2+ into the extracellular solution elicited SOCE. Note that Rhod4 indicated similar release and SOCE-induced cytosolic Ca2+ responses in control and GCaMP6f-Orai1-expressing cells. Right: statistics of the magnitude of SOCE, indexed by ΔF/F0 of Rhod4 after Ca2+ restoration following store depletion. n=172 and 49 cells from 7 independent experiments for control and GCaMP6f-Orai1 groups, respectively. (E) The number of invadopodia per cell was unaltered by biosensor expression. n=62 cells from 4 independent experiments for both groups. Data represent mean±s.e.m.; NS, not statistically significant.
To investigate the spatiotemporal properties of SOCE, we used the highly invasive WM793 human melanoma cell as a model system of non-excitable cells. WM793 cells plated on gelatin-coated coverslips were first infected with GCaMP6f-Orai1 adenovirus for 36 h. After stimulation with 10% fetal bovine serum (FBS), serum-starved WM793 cells start to assemble actin-rich protrusion structures and form invadopodia arrays, usually beneath the nucleus region. Functional invadopodia secrete proteases to degrade the extracellular matrix, producing pits and holes in the thin tetramethylrhodamine (TRITC)-gelatin coating, which were visualized by the loss of TRITC fluorescence (Fig. 1B). Confocal imaging revealed that the invadopodia were intensely stained with GCaMP6f-Orai1, giving rise to a ring-like structure wrapping around the actin-rich invadopodial core labeled by Lifeact-mApple (Fig. 1B, upper panel, top row) or surrounding the TRITC-free pits and holes in the gelatin coat as seen from both 2D and 3D z-stack images (Fig. 1B, upper panel, bottom row and lower panel). Intensely fluorescent puncta were also evident at sites near the cell periphery. Because a similar pattern of distribution was evident with PM-GCaMP6f (Fig. S1A), the appearance of inhomogeneity was likely due to unevenness of the plasma membrane seen in the confocal optical section (e.g. the ring structure at invadopodia could be explained by the vertical dimension of the plasma membrane at these loci). Thus, both of the membrane-targeted Ca2+ biosensors, GCaMP6f-Orai1 and PM-GCaMP6f, would enable the investigation of SOCE activity at single-invadopodium resolution.
To determine whether the expression of GCaMP6f-Orai1 altered SOCE due to a change in the stoichiometry of STIM1 and Orai1, we measured the expression level of GCaM6f-Orai1 and found that it was ∼30% of that of endogenous Orai1 in WM793 cells, without an appreciable compensatory change in STIM1 expression (Fig. 1C). Functionally, GCaM6f-Orai1 expression did not alter the magnitude of SOCE induced by Ca2+ re-addition after ER depletion (Fig. 1D), and did not affect the number of invadopodia in WM793 cells (Fig. 1E), indicating that perturbation of SOCE by GCaMP6f-Orai1 expression, if any, is negligible. Further, to appraise the relative sensitivity of the membrane-targeted biosensors to Ca2+ influx versus Ca2+ release, we made dual-indicator measurements using GCaMP6f-Orai1 and Rhod4, the latter of which is diffusible in the cytosol. We found that, whereas global SOCE led to similar fold increases in fluorescence of the two indicators, thapsigargin (TG)-induced ER Ca2+ release elicited a fold change in GCaMP6f-Orai1 fluorescence that was merely 50% of that of Rhod4 (Fig. S2A). This result suggests that the membrane-targeted Ca2+ biosensor preferentially reports subplasmalemmal Ca2+ and is relatively insensitive to Ca2+ release from the ER, which is in agreement with a previous report (Dynes et al., 2016). In the rest of the study, we opted to use GCaMP6f-Orai1 as the Ca2+ biosensor in most experiments, and PM-GCaMP6f in a subset of experiments mainly for the purpose of validation.
Spontaneous Ca2+ glows in invading melanoma cells
In WM793 cells expressing GCaMP6f-Orai1, we used time-lapse confocal imaging to acquire 400 consecutive images at 5-s intervals. Discrete, sudden and transient GCaMP6f-Orai1 fluorescence increases arose spontaneously from the biosensor-labeled invadopodia (Fig. 2A; Movie 1). Individual transients were confined to single invadopodia, with no sign of propagation into adjacent invadopodia or extra-invadopodial areas (Fig. 2A, top left). Their full duration at half maximum (FDHM) lasted up to tens of seconds with an average value of 11.5 s (Fig. 2A,D). Transient increases in GCaMP6f-Orai1 fluorescence were also found at discrete sites at the cell periphery (Fig. S3A). We named them Ca2+ glows because of their exceptionally long duration. On average, the rate of occurrence was 0.5 events/min/cell for invadopodial Ca2+ glows and 0.24 events/min/cell for peripheral Ca2+ glows. The spatial size of invadopodial Ca2+ glows ranged from 0.5 µm2 to 16.5 µm2, with an average of 3.4 µm2 (Fig. 2B). The histogram of invadopodial glow amplitude displayed a broad distribution ranging from 0.1 to 4.36 (ΔF/F0), with an average 0.63-fold increase in GCaMP6f-Orai1 fluorescence (Fig. 2C). Invadopodial Ca2+ glows exhibited highly variable kinetics: often a rapid rise was followed by either a spiky peak or a prolonged plateau; stepwise fluctuations were sometimes evident in the rise or the plateau phase; likewise, their termination also manifested different patterns, with an abrupt fall or a gradual decline, suggestive of the complex gating behavior of the underlying Ca2+ channels. To resolve possible faster Ca2+ dynamics, we applied linescan imaging at 3.78-ms resolution and were able to identify substructures lasting <5 s among long-lasting Ca2+ glows, but failed to detect any subsecond events (Fig. 2E,F). Therefore, Ca2+ glows are kinetically distinctive compared with known elementary Ca2+ signaling events, such as Ca2+ sparks originating from ryanodine receptor Ca2+ release channels (∼20 ms) in muscles (Cheng and Lederer, 2008), Ca2+ puffs from the inositol 1,4,5-trisphosphate receptor (IP3R) Ca2+ release channels (∼300 ms) in non-excitable cells (Smith et al., 2009), and Ca2+ flickers from TRPM7 and coupled activation of IP3Rs (∼500 ms) in WI-38 cells undergoing directional movement (Wei et al., 2012). Notably, peripheral Ca2+ glows, such as those in filopodia, exhibited comparable, although not identical, amplitude (ΔF/F0, 1.26) (Fig. S3B), duration (FDHM, 8.44 s) (Fig. S3C) and spatial size (12.3 µm2) (Fig. S3D). Taken together, invadopodial and peripheral Ca2+ glows reflect previously unappreciated local Ca2+ signaling events in invading melanoma cells.
Fig. 2.
Spontaneous invadopodial Ca2+ glows detected by GCaMP6f-Orai1. (A) Spontaneous invadopodial Ca2+ glows from a WM793 cell expressing GCaMP6f-Orai1. WM793 cells were infected with GCaMP6f-Orai1 adenovirus and plated on gelatin-coated coverslips. After starvation overnight, they were exposed to 10% FBS for 20 min to initiate the formation of invadopodia and then subjected to imaging. Individual invadopodia are outlined and numerically labeled. Top left: time-lapse F/F0 images at 5-s intervals of a representative invadopodial Ca2+ glow (first row) and local staining of GCaMP6f-Orai1 and Lifeact-mApple as well as the merged image (second row from left to right, respectively). Bottom left: mask map of the cell periphery and individual invadopodia. The invadopodia are numerically labeled. Right: time courses of invadopodial Ca2+ glows. Note that the occurrence of Ca2+ glows appears to be independently regulated, even among adjacent invadopodia. See also Movie 1. (B–D) Distributions of the area (B), amplitude (C) and duration (FDHM) (D) of invadopodial Ca2+ glows (n=566 events from 36 cells in 30 independent experiments). (E) High-resolution imaging of invadopodial Ca2+ signals. Inset: dashed line across three adjacent invadopodia denotes the selected line for imaging at the 3.78 ms/line. (F) Time courses of GCaMP6f-Orai1 fluorescence corresponding to the image part marked by the green bar (shown above the scale bar) in E are shown in parallel with the image, and an expanded view of the boxed time window is shown below.
Invadopodial and peripheral glows of similar amplitude and kinetics were also reliably detected with the other biosensor, PM-GCaMP6f (Fig. S1B,C), suggesting that low-level Orai1 overexpression caused by the expression of the biosensor GCaMP6f-Orail did not alter the properties of Ca2+ glows. More importantly, Ca2+ glows were better detected by the membrane-targeting biosensor than the diffusible cytosolic indicator Rhod4: in dual-indicator imaging experiments, Ca2+ glows reported by GCaMP6f-Orail were significantly brighter and had a higher signal-to-noise ratio (Fig. S2B), and small Ca2+ glows clearly visible with GCaMP6f-Orai1 were indiscernible with Rhod4 (Fig. S2C). Together with the in situ comparison of indicator properties shown in Fig. S2A, this result is consistent with the idea that invadopodial and peripheral glows originate mainly from Ca2+ influx rather than Ca2+ release.
Ca2+ glows represent elemental Ca2+ signals of SOCE
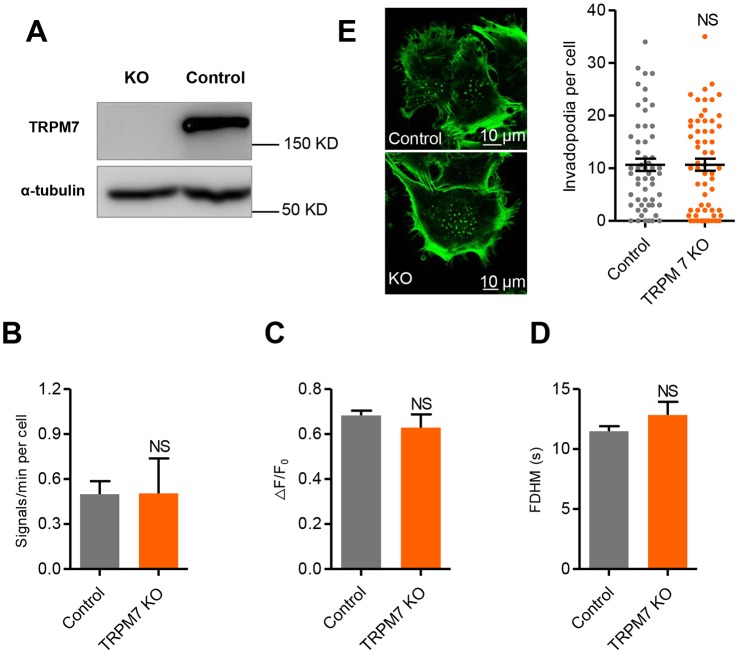
Next, we sought to determine the specific link between specific Ca2+ sources and invadopodial or peripheral Ca2+ glows. We first demonstrated that removal of external Ca2+ led to a complete inhibition of Ca2+ glow activity (Movie 2), indicating that Ca2+ influx is a prerequisite of Ca2+ glow production. As we have previously shown that TRPM7 (a stretch-activated Ca2+-permeant channel of the transient receptor potential superfamily) was able to generate discrete, although much briefer, local Ca2+ transients (Ca2+ flickers) in the leading lamellipodium of migrating fibroblasts (Wei et al., 2009), we investigated the role of TRPM7 in the generation of Ca2+ glows in WM793 cells. We showed that ablation of TRPM7 using CRISPR-Cas9 (Fig. 3A) had little effect on the frequency (Fig. 3B; Fig. S3E), amplitude (Fig. 3C) and kinetics of glows (Fig. 3D), and did not significantly change the formation of invadopodia (Fig. 3E). Taken together, our data indicate that TRPM7 is unlikely to be the primary Ca2+ entry pathway in generating Ca2+ glows or in regulating invadopodial formation in WM793 cells.
Fig. 3.
TRPM7 is not responsible for the genesis of Ca2+ glows. (A) Western blots showing knockout of TRPM7. (B–D) Negligible effects of TRPM7 KO on the frequency (B), amplitude (ΔF/F0) (C) and duration (FDHM) (D) of invadopodial Ca2+ glows (n=54 and 15 cells from 30 and 12 independent experiments for the control and TRPM7 KO groups, respectively). (E) Left: fluorescence micrographs showing invadopodial formation in control (top) and TRPM7 KO (bottom) cells. Right: quantification of invadopodial formation (n=56 and 61 cells from 4 independent experiments for the control and TRPM7 KO groups). Data are presented as means±s.e.m.; NS, not statistically significant.
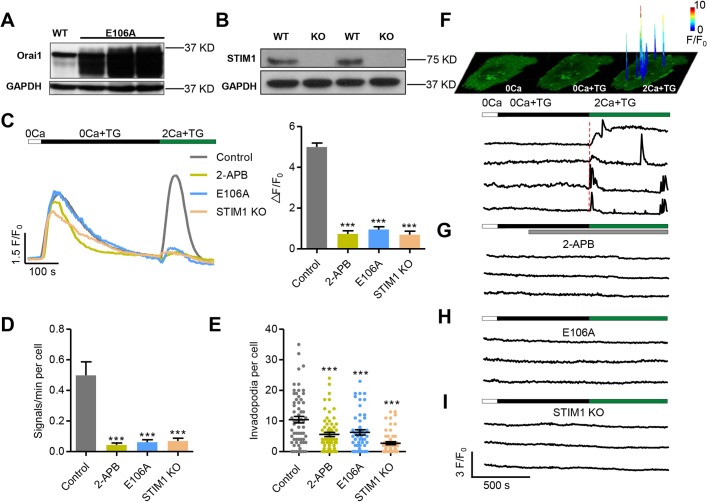
In non-excitable cells, SOCE is activated by the depletion of the ER store and constitutes the major Ca2+ entry mechanism for refilling the internal store and sustaining cytoplasmic Ca2+ signaling. Recent reports have shown that STIM1-Orai1-mediated SOCE occurs at discrete sites in which STIM1 anchored in the ER and Orai1 in the plasma membrane form closely apposed clusters in transfected cells. In testing whether Ca2+ glows reflect local, intermittent SOCE activity in native cells, we found that addition of 100 μM 2-aminoethoxydiphenyl borate (2-APB), a commonly used SOCE inhibitor, significantly suppressed the glow activity (Fig. 4C,D), consistent with the notion that glows depend on SOCE-related activity. Because 2-APB is not a very specific inhibitor, to directly test the SOCE hypothesis for the genesis of Ca2+ glows, we either expressed the dominant-negative Orai1 mutant, Orai1-E106A (Fig. 4A), or used CRISPR-Cas9 to knock out STIM1 in WM793 cells (Fig. 4B). Measurement of the cytosolic Ca2+ response using the Ca2+ indicator Fluo4 confirmed that, upon restoration of external Ca2+ after ER depletion, global SOCE was largely abolished by 2-APB, Orai1 E106A or STIM1 knockout (KO) in WM793 cells (Fig. 4C). In these SOCE-abrogated cells, the occurrence of invadopodial and peripheral glows was dramatically suppressed by 87.7% and 67.4% in Orai1-E106A cells, and by 86.1% and 63.9% in STIM1 KO cells (Fig. 4D; Fig. S3E), strongly suggesting that Ca2+ glows originate from STIM1-Orai1-mediated local SOCE. Meanwhile, the formation of invadopodia was also significantly attenuated under the above conditions (Fig. 4E), confirming an important role for SOCE in invadopodial formation.
Fig. 4.
Invadopodial Ca2+ glows originate from SOCE mediated by Orai1 and STIM1. (A,B) Manipulating SOCE in WM793 cells. Western blots showing the expression of the dominant-negative mutant Orai1-E106A (E106A) (A) and knockout of STIM1 (STIM1 KO) (B). (C) Representative traces (left) and average amplitude (right) of SOCE recorded by Fluo4. 100 μM 2-APB was added to the extracellular solution when the fluorescence returned to baseline after TG treatment. Note that ER Ca2+ release was largely comparable in these groups (n=69, 32, 61 and 83 cells from 3, 3, 9 and 3 independent experiments for control, 2-APB, E106A and STIM1 KO groups, respectively). (D) Statistics of the frequencies of spontaneous invadopodial Ca2+ glows (n=54, 18, 21 and 36 cells from 30, 8, 16 and 25 independent experiments for control, 2-APB, E106A mutant and STIM1 KO groups, respectively). (E) Depression of invadopodial formation by treatment with 2-APB (n=66), expression of the E106A mutant (n=52) and STIM1 KO (n=57) compared with control (n=62) from 6 independent experiments. (F) Invadopodial Ca2+ glows resolved during global SOCE. Top: space-time plots of local Ca2+ events reported by GCaMP6f-Orai1 in response to 0 Ca, TG and subsequent 2 mM Ca2+ in a WM793 cell. The colored peaks are overlays of surface plots of discrete Ca2+ glows at their peaks, indicating both the locations and the amplitudes (coded by colors) of these local signals. Bottom: corresponding traces of representative invadopodial Ca2+ glows during global SOCE. Note the initial near-synchronous activation at all invadopodia followed by delayed, asynchronous activity at individual invadopodia. (G–I) Invadopodial Ca2+ glows were abolished by 2-APB treatment (G), expression of the E106A mutant (H) and STIM1 KO (I). Data are presented as mean±s.e.m.; ***P<0.001 compared with control.
More direct evidence that local, intermittent SOCE underlies invadopodial and peripheral Ca2+ glows was obtained in experiments with the standard protocol of Ca2+ re-addition after store depletion. When ER depletion was induced by TG in the absence of external Ca2+, Ca2+ glow activity was completely quiescent, as was the case with removal of external Ca2+ alone (Movie 2). Strikingly, upon re-addition of extracellular Ca2+ (2 mM), bright Ca2+ glows discharged immediately and synchronously in all invadopodia, as well as at discrete peripheral sites (Fig. 4F; Movie 2). In the continued presence of TG to prevent ER refilling, delayed Ca2+ glows occurred at a high rate, in an asynchronous manner, with latencies ranging from 10 s to 490 s. Compared with spontaneous Ca2+ glows in resting cells, these delayed evoked invadopodial glows had a much higher amplitude (ΔF/F0=2.27, N=46 events), suggesting a higher STIM1-Orai1 channel open probability and/or the involvement of greater numbers of channels. The overall duration of the cluster activation, however, was unchanged because the FDHM of evoked Ca2+ glows (∼9.44 s) was comparable to that of spontaneous glows. Repetitive activity of Ca2+ glows was also evident in the same invadopodia, indicative of STIM1-Orai1 clusters operating in a discrete and intermittent fashion. These evoked Ca2+ glows were effectively abrogated by the SOCE blocker 2-APB (Fig. 4G), the expression of Orai1 E106A (Fig. 4H) and the KO of STIM1 (Fig. 4I). Taken together, we conclude that spontaneous and evoked Ca2+ glows originate from local, transient SOCE at the single-invadopodium (and peripheral punctum) level. In other words, SOCE does not operate in a continuous mode; rather, it comprises spatiotemporally discrete elementary events in the form of Ca2+ glows in WM793 cells.
Similar SOCE Ca2+ glows were also found in HUVECs (Fig. S3F), in which SOCE has been shown to actively participate in the regulation of proliferation and migration (Abdullaev et al., 2008; Li et al., 2011). These Ca2+ glows were significantly inhibited by the SOCE blocker BTP-2 and the Orai1 dominant-negative mutant E106A (Fig. S3G). Thus, the conclusion that SOCE comprises discrete Ca2+ glows, akin to Ca2+ sparks and puffs for store Ca2+ release, might be generalizable to other non-excitable cells.
Ca2+/calmodulin-dependent Pyk2 activation by STIM1 and Orai1 in invadopodial formation
High-Ca2+ microdomains created by SOCE in the assembling invadopodium or invadopodial SOCE glows may spatially and temporally activate a multitude of local Ca2+-dependent signaling cascades critical to cytoskeletal dynamics, such as actin remodeling, focal adhesion detachment and relocation, and actin-myosin contraction (Wei et al., 2012). SOCE can regulate invadopodial initiation by activating non-receptor tyrosine kinase Src (Sun et al., 2014). Interestingly, the activation of Src by STIM1 was found in adherent cells but not in suspended cells (Fig. S4A), suggesting a role for cell adhesion in SOCE-mediated Src activation. Pyk2 is a Ca2+/calmodulin-dependent cell adhesion kinase (Riggs et al., 2011). The binding of Ca2+/calmodulin to the autoinhibitory FERM domain leads to the formation of Pyk2 dimers (Kohno et al., 2008), and promotes the autophosphorylation and activation of Pyk2. To determine whether Pyk2 plays roles in SOCE-mediated invadopodial formation, we first investigated the effects of STIM1 and Orai1 on Pyk2 activity in WM793 cells. Depletion of STIM1 and Orai1 with short hairpin RNA (shRNA) reduced the levels of pY402-Pyk2 in WM793 cells by 50% and 80%, respectively (Fig. 5A). When ectopically expressed, STIM1 or STIM1 plus Orai1 increased phospho-Pyk2 by more than 3-fold (Fig. 5B). Blockade of SOCE with 2-APB decreased phospho-Pyk2 by >80% (Fig. 5C), suggesting that SOCE is critical for Pyk2 activation.
Fig. 5.
SOCE regulates invadopodial formation through the Ca2+/calmodulin–Pyk2–Src pathway. (A) STIM1 shRNA and Orai1 shRNA decreased active Pyk2 (pY402-Pyk2) levels in WM793 cells. (B) Ectopic expression of STIM1- and Orai1-activated Pyk2 in WM793 cells. (C) SOCE blockade with 2-APB (100 µM) decreased pY402-Pyk2 levels. (D) Depletion of Pyk2 with shRNA abrogated the STIM1-mediated activation of pY416-Src. (E) Immunofluorescence staining of pY402-Pyk2, showing that Pyk2 was activated in invadopodia and focal adhesions in WM793 cells. (F) Immunofluorescence staining showing that calmodulin was recruited to invadopodia in WM793 cells. (G) Pyk2 knockdown abolished STIM1-mediated invadopodial formation (n=120, 123, 125 and 127 for control shRNA, control shRNA+STIM1, Pyk2 shRNA, and Pyk2 shRNA+STIM1, respectively). (H) STIM1-promoted WM793 cell invasion was abrogated after Pyk2 knockdown. Cell numbers were collected from triplicate independent invasion chamber assays. (I) Ectopic overexpression of STIM1 stimulated the interaction of Src with wild-type Pyk2, but not the calmodulin-binding motif mutant of Pyk2 (L176Q/Q177A). (J) A model for the Ca2+/calmodulin–Pyk2–Src pathway downstream of SOCE. Local high Ca2+ influx mediated by SOCE promotes the binding of Ca2+/calmodulin to the FERM domain of Pyk2, which releases the intramolecular auto-inhibition. Ca2+/calmodulin-bound Pyk2 autophosphorylate each other on tyrosine 402. pY402-Pyk2 interacts with the SH2 domain of Src to activate Src. Data are representative results from at least 3 independent experiments. Horizontal bars represent mean±s.e.m; ***P<0.001, **P<0.01; NS, not statistically significant.
To further test the hypothesis that SOCE activates Src through Pyk2, we used shRNA to knock down Pyk2, which resulted in a 70% reduction in Pyk2 protein levels (Fig. 5D). Overexpression of STIM1 in control shRNA-treated cells more than doubled the pY416-Src level; strikingly, the activation of Src by ectopic STIM1 was almost abrogated when Pyk2 was knocked down in WM793 cells (Fig. 5D), suggesting that Pyk2 is required for the SOCE-mediated activation of Src. Furthermore, the invadopodial formation and melanoma cell invasion promoted by SOCE were abolished after Pyk2 knockdown (Fig. 5G,H).
To determine the mechanism by which SOCE activates Pyk2, we used W7 to inhibit calmodulin in WM793 cells expressing ectopic STIM1. W7 almost abolished the STIM1-induced Pyk2 autophosphorylation, suggesting that calmodulin is required for SOCE-mediated Pyk2 activation (Fig. S4B). It has been reported that 8–12 µM free Ca2+ is required for the initial Pyk2 activation, although the activity of activated Pyk2 can be maintained in 500 nM free Ca2+ (Kohno et al., 2008). These results suggested that Pyk2 might serve as a downstream effector mediating the regulation of invadopodial formation by glows. Indeed, we noted that the active form of Pyk2 (pY402) and calmodulin were mostly localized to invadopodia and focal adhesions, implying that calmodulin and Pyk2 might be involved in modulating invadopodia and Src (Fig. 5E,F). Moreover, Pyk2 was co-immunoprecipitated with calmodulin after 10% FBS induction initiated the growth of invadopodia, whereas Pyk2 and calmodulin remained without interaction when cells were starved and quiescent (Fig. S4C). To further critically test this hypothesis, we generated a L176A/Q177A Pyk2 IQ motif mutant, which prevents the binding of calmodulin to the Pyk2 FERM domain (Kohno et al., 2008). Stimulation of Ca2+ influx with STIM1 overexpression remarkably increased the amount of Src co-immunoprecipitated by Pyk2, suggesting that SOCE promotes the physical interaction between Pyk2 and Src (Fig. 5I). However, when the Ca2+/calmodulin-binding motif in the FERM domain of Pyk2 was mutated, this Pyk2-Src interaction was inhibited (Fig. 5I), indicating that the binding of calmodulin to the Pyk2 FERM domain is required for SOCE to regulate Src activity. Therefore, our data suggested that SOCE promotes invadopodial formation and melanoma invasion via the Ca2+/calmodulin–Pyk2–Src pathway (Fig. 5J).
DISCUSSION
In order to directly visualize the Ca2+ dynamics mediated by Orai1-STIM1 at high spatiotemporal resolution, we developed two types of Ca2+ biosensors, GCaMP6f-Orai1 and PM-GCaMP6f, and both were successfully targeted to the plasma membrane. This approach enabled us to visualize subsurface Ca2+ microdomains with high fidelity (as compared with the diffusible Ca2+ indicator Rhod4), while retaining sensitivity to Ca2+ changes in the bulk of the cytosol (through diffusion of Ca2+ into the subsurface). Our imaging data showed that Orai1 was locally and intermittently activated to mediate invadopodial and peripheral Ca2+ glows in invading WM793 cells, as well as spatiotemporally discrete Ca2+ glows in HUVECs. We provided strong evidence that the Ca2+ glows in WM793 cells are elementary SOCE events, presumably mediated by the STIM1-Orai1 channel clusters. Unlike Ca2+ sparks of ryanodine receptor origin or Ca2+ puffs of IP3R origin (Yao and Parker, 1994), the Ca2+ glows of SOCE origin were exceptionally long lasting, and were blocked by removal of external Ca2+ and persisted under conditions that do not support any ER Ca2+ release (i.e. re-addition of external Ca2+ after store depletion in the continued presence of TG). SOCE Ca2+ glows are not of the same nature as ‘Ca2+ flickers’ (Wei et al., 2009) as their rate of occurrence was unaffected by TRPM7 KO, and because of their distinctive kinetics. More importantly, inhibition of SOCE using pharmacological inhibitors, Orai1 dominant-negative mutant, or STIM1 KO, effectively abolished Ca2+ glow activity. Upon restoration of external Ca2+ to cells with a depleted ER and disabled ER Ca2+ recycling, evoked STIM1-Orai1 Ca2+ glows occurred abruptly, followed by persistent discharge of high-rate, asynchronous Ca2+ glows. Using the plasma membrane-targeted biosensors G-GECO1-Orai1 and G-GECO1-Orai3 in conjunction with total internal reflection fluorescence microscopy, Dynes et al. have previously visualized single Orai1 channel Ca2+ influx, artificially activated by co-expressing CAD or STIM1, or by 2-APB acting as an agonist of Orai3, in transfected cells (Dynes et al., 2016). However, the individual SOCE Ca2+ glows in the present study must each involve numerous Orai1 channels, given their spatial extent (up to 16 µm2), large amplitude (ΔF/F0 up to 4-fold) and the small conductance of a single Orai1 channel. Thus, we have provided the first demonstration of elementary SOCE events originating from STIM1-Orai1 clusters in native cells under physiologically and pathologically relevant conditions.
The characterization of SOCE Ca2+ glows has uncovered surprising gating properties of STIM1 and Orai1 channel clusters in situ. First, the presence of discrete transient SOCE events indicates that SOCE operates in a digital, intermittent fashion, whereby store depletion markedly increases the rate of occurrence of Ca2+ glows with only modest changes in their unitary properties (i.e. amplitude and duration). Second, the presence of Ca2+ glow activity in resting cells shows that spontaneous SOCE activity is under tight spatial control such that adjacent invadopodia can exhibit distinctly different temporal patterns of activity. The high-Ca2+ microdomains so created by Ca2+ glows, coupled with local enrichment of Ca2+ effectors including calmodulin and Pyk2, might play a critical role in shaping the invadopodial dynamics as well as secretory functions for the initiation of invadopodial formation. Indeed, our data show that the number of invadopodia in different experimental settings is closely related to the level of STIM1-Orai1 Ca2+ glow activity.
Based on the kinetics of Ca2+ glows, the gating of Orai1 channels within single invadopodia appear to be temporally synchronized and spatially coordinated. The rapid on- and off-kinetics of individual Ca2+ glows strongly argue against the hypothesis that individual Orai1 channels gate stochastically and independently. Possible mechanisms for the spatiotemporal coordination of Orai1 channels at the invadopodial level could be 2-fold. If the ER store fluctuates locally, then the Orai1 channels in the same invadopodium may share a common modulatory signal so that they operate in concert. However, invadopodial and peripheral Ca2+ glows occur in ER-depleted cells with disabled Ca2+ recycling, indicating that this could not be the sole explanation. Alternatively, a single STIM1-Orai1 channel may act as an intrinsic oscillator and manifest dampened oscillatory behavior under certain experimental conditions (Dynes et al., 2016). In this scenario, an entrained oscillation of many STIM1-Orai1 channels in the same invadopodium (punctum) would afford a possible explanation for the invadopodium (punctum)-level synchrony.
Local control of Ca2+ signaling is essential for this vital intracellular messenger to control diverse physiological and pathophysiological processes (Berridge et al., 2000). The activation of low-affinity Ca2+-binding proteins requires tens to hundreds of micromoles of Ca2+, which is orders of magnitude higher than the physiological Ca2+ concentration in the bulk of the cytosol. With a 104-fold Ca2+ gradient built up across the plasma membrane, SOCE, which is prevalent in most cell types, comprises the major Ca2+ entry pathway. It has been estimated that in the microdomain within the opening mouth of activated SOCs, the Ca2+ concentration can reach tens of micromoles (Hogan, 2015; Kar et al., 2014), a concentration sufficient to activate many Ca2+ effectors with low Ca2+-binding affinity. In this study, we found that calmodulin and Pyk2 are enriched in invadopodia and co-exist with intermittent high-Ca2+ signals in the form of Ca2+ glows. In this regard, previous studies have provided evidence that the NFAT1 (also known as NFATC1) transcription factor and adenylyl cyclase 8 are selectively activated by SOC-mediated subplasmalemmal Ca2+ microdomains, but not by a global increase in cytosolic Ca2+ (Kar et al., 2011; Willoughby et al., 2012). In this case, invadopodia and podosomes are proteolytic membrane protrusions used by invasive cells to remodel the extracellular matrix. It has been demonstrated that Ca2+ channels and effectors such as Orai1, STIM1, myosin and calcineurin are enriched in invadopodia and podosomes (Alexander et al., 2008; Baldassarre et al., 2003; Chen et al., 2017; Siddiqui et al., 2012). Interestingly, calmodulin and the Ca2+-activated adhesion kinase Pyk2 are also locally recruited to invadopodia. By recruiting SOCs and Ca2+ effectors, invadopodia may serve as signaling hubs (Mo and Yang, 2018), coordinating invadopodial initiation and extracellular matrix degradation through a Ca2+/calmodulin–Pyk2–Src pathway.
In summary, we have investigated the spatiotemporal dynamics of SOCE using Orai1-tethered or palmitoylated Ca2+ biosensors in conjunction with confocal microscopy. We discovered elementary SOCE events, namely Ca2+ glows, in invading cancer cells as well as primary cultured endothelial cells. Using Ca2+ glows as optical recordings of the activation and Ca2+ permeation of STIM1-Orai1 channel clusters, we revealed unexpected gating properties and their regulation of these SOCs in situ. Functionally, intermittent invadopodial Ca2+ glows may play an essential role in the activation of locally enriched, high-threshold Ca2+ effectors required for invadopodial formation and cancer metastasis. Future investigations are warranted to determine to what extent the current findings can be generalized to other types of non-excitable cells and how the spatiotemporal organization of elemental SOCE events can orchestrate complex cell behaviors in physiology and pathology.
MATERIALS AND METHODS
Vector construction and recombinant adenovirus production
The GCaMP6f and human ORAI1 genes were amplified from pGP-CMV-GCaMP6f (Addgene #40755) and Orai1-EGFP (Yang et al., 2009), respectively, and then inserted into pEGFP-C1 via an in-fusion cloning kit (Clontech). The Orai1 dominant-negative mutant Orai1-E106A was amplified from myc-Orai1 E106A pHAGE (Addgene #22752). For PM tethering, the GCaMP6f sequence was inserted into the BamHI and NotI restriction sites of PM-FRB-CFP (Addgene #67517) through ligation. The fusion gene GCaMP6f-Orai1, the Orai1-E106A mutant and PM-GCaMP6f were then amplified and inserted into the pENTR/TEV/D-TOPO vector (Invitrogen). The adenovirus was produced in HEK293A cells using an adenoviral expression system (Invitrogen).
Cell culture
The culture media used for WM793 melanoma cells were RPMI (Gibco) and Dulbecco's modified Eagle medium (Gibco) for HEK293A and HEK293T cells. All culture media were supplemented with 10% FBS and penicillin/streptomycin.
RNA interference
RNA interference of STIM1, ORAI1 and Pyk2 was performed using the pSUPER.Retro.puro vector (Oligoengine) encoding shRNA. The target sequences were as following: AGAAGGAGCTAGAATCTCAC (STIM1sh), TCGGCCTGATCTTTATCGT (Orai1sh1), CCAGCATTGAGTGTGTACA (Orai1sh2), TGCACTTGACAAGAAGTCC (Pyk2sh1) and ACCCAGAAACTGCTCAACA (Pyk2sh2). To efficiently knock down ORAI1 and Pyk2, two shRNAs targeting two different regions of the same gene were used simultaneously. Cells with stable knockdown were selected using 2 μg/ml puromycin.
CRISPR-Cas9 gene KO system
The targeting single-guide RNAs were cloned into the BsmBI restriction site of the pLentiCRISPRv2 backbone vector (Addgene #52961). The sequences were GGCGACAGGAACCAGCTCGG for STIM1 and TGACTCCATAAGCATCCGTT for TRPM7.
Stable cell line construction
Stable cell lines were generated via the pLPCX and pLNCX2 retroviral systems. The pLNCX2 retroviral vectors encoding human STIM1 have been previously described (Yang et al., 2012). pLPCX-Flag-Pyk2 was generated by cloning Flag-Pyk2 into pLPCX between the BglII and XhoI restriction sites. The Flag-Pyk2 FERM domain mutation (Pyk2 L176A/Q177A) was generated by direct mutagenesis using pLPCX-Flag-Pyk2 as template, with the forward primer, 5′-CTGGGCCACTACGCGGAGCGGAACAAGAACTCCGCGAAGGCGCTCACCCTCGCGCTGTACTCACTG-3′, and the reverse primer, 5′-CAGTGAGTACAGCGCGAGGGTGAGCGCCTTCGCGGAGTTCTTGTTCCGCTCCGCGTAGTGGCCCAG-3′. The vectors were then transfected into HEK293T cells along with VSV-G and gag-pol to generate mature retrovirus. Positive stable cells were selected using G418 (400 µg/ml) for pLNCX2 and puromycin (2 µg/ml) for pLPCX.
Western blot analysis
Cell lysates were separated by 4–12% SDS-PAGE and transferred to nitrocellulose membranes (Millipore). The membranes were blocked with 5% non-fat dry milk and incubated with primary antibody overnight at 4°C, followed by incubation with secondary antibodies for 1 h at room temperature. Antibodies against STIM1 (Cell Signaling Technology, #5668S), Orai1 (Sigma-Aldrich, #O8264), GAPDH (Sigma-Aldrich, #G8795), Src (Millipore, clone GD11, #05-184), p-Src (Tyr416) (Cell Signaling Technology, #2101), Pyk2 (Cell Signaling Technology, #3480), p-Pyk2 (Tyr402) (Cell Signaling Technology, #3291), tubulin (Sigma-Aldrich, #T6199), calmodulin (Santa Cruz Biotechnology, #sc-137079) and TRPM7 (Abcam, #ab109438) were used. For the western blot analysis, all primary antibodies were used at 1:1000 dilution and all secondary antibodies were diluted at 1:2500. Unprocessed images of western blots can be seen in Fig. S5.
Immunofluorescence assay
WM793 cells were permeabilized with 0.1% Triton X-100 and blocked with 10% normal goat serum. The sections were incubated with rabbit anti-p-Pyk2 (Cell Signaling Technology, #3291) or mouse anti-calmodulin (Santa Cruz Biotechnology, #sc-137079), both at 1:100 dilution, for 2 h at room temperature, washed with PBS, and then incubated with Alexa Fluor 488-conjugated anti-mouse IgG (1:300, Invitrogen) or anti-rabbit IgG (1:300, Invitrogen) and TRITC-labeled phalloidin (5 µg/ml, Sigma-Aldrich) for 1 h at room temperature. The immunofluorescence staining was visualized using a Zeiss LSM710 confocal microscope at 488 nm and 543 nm excitation and 490–540 nm and >560 nm emission, respectively.
Co-immunoprecipitation assay
WM793 cells expressing pLPCX control and pLPCX-Flag-Pyk2 grown to 90% confluence were washed three times with ice-cold PBS and lysed in 0.5 ml lysis buffer (50 mM Tris, 150 mM NaCl, 0.5% sodium deoxycholate, 1% Triton X-100, pH 8.0). The cell lysate was briefly sonicated (5 s) and centrifuged at 12,000 g on a 4°C benchtop centrifuge. The supernatant was incubated with M2 beads for 4 h at 4°C, then the beads were washed three times with wash buffer (50 mM Tris, 150 mM NaCl, 1 mM PMSF and 1% NP-40, pH 8.0) and the bound proteins were eluted with protein sample buffer, resolved on SDS-PAGE and detected with anti-calmodulin or anti-Src antibody.
Fluorescent gelatin labeling
Fluorescent gelatin was synthesized by incubating bovine skin gelatin with amine-reactive fluorescent dyes. TRITC isothiocyanate (Thermo Fisher Scientific) was dissolved in dimethyl sulfoxide at 10 mg/ml. Dye solution (50 µl) was added slowly to 1 ml bovine skin gelatin solution (Sigma-Aldrich) (40 mg/ml, dissolved in 0.1 M sodium bicarbonate buffer, pH 8.3) while vortexing. After incubation at room temperature for 1 h, the free dyes in the mixture were removed with a HiTrap desalting column (GE Life Sciences). The desalted fluorescent gelatin solution can be stored at 4°C for short-term storage or at −80°C with 10% glycerol as cryoprotectant for long-term storage.
Coverslip coating
Acid-washed coverslips (or glass-bottomed dishes) were incubated with 0.4% bovine gelatin for 30–60 min and washed with PBS. The gelatin was fixed with 0.5% glutaraldehyde for 10 min and then washed extensively with PBS for cell culture and imaging.
Live cell imaging
WM793 cells (in RPMI growth medium with 10% FBS) were plated onto gelatin-coated coverslips or glass-bottomed dishes to attach and spread for at least 3 h and then starved overnight (>12 h) in RPMI with 1% FBS. Invadopodial formation was initiated by adding 10% FBS to the starvation medium.
Confocal imaging was carried out with a Zeiss LSM710 microscope with a 40×, 1.3 NA oil-immersion objective; the pinhole was set for a nominal 1-μm optical section. Cells were kept in a Heating Insert P S1 incubator (Pecon) at a constant temperature of 37°C under 5% CO2 (vol/vol). For simultaneous measurement of GCaMP6f-Orai1 or Fluo4 and Lifeact-mApple or Rhod4, excitation was at 488 nm and 543 nm and their fluorescence emission was collected at 490–520 nm (with Rhod4) or 490–540 nm (with Lifeact-mApple) and >560 nm, respectively. For chemical Ca2+ indicator loading, WM793 cells were incubated with 5 µmol/l Rhod4 AM (AAT Bioquest) or Fluo4 AM (Invitrogen) for 10 min at 37°C. Linescan imaging was performed at 3.78 ms/line and x-y time-lapse images were acquired at 0.78 s/frame every 5 s.
Image processing and data analysis
Digital images were processed using customer-devised routines written in IDL (ITT, New York, NY). Data are reported as the mean±s.e.m. Two-tailed P-values were determined by Student's t-test or the Mann–Whitney test as indicated, with P<0.05 defined as statistically significant.
Supplementary Material
Acknowledgements
We thank Dr. Iain C. Bruce for manuscript editing; Dr. Shiqiang Wang, Chaoliang Wei and Kunfu Ouyang for valuable comments; and Dr. Yujie Sun, Dong Li and Liangyi Chen for assistance with imaging experiments.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: F.L.; Methodology: F.L., J.S., Q.Z., Y.H., P.Y., H.H.; Software: F.L., J.S., J.L., P.Y.; Validation: F.L., J.S.; Formal analysis: F.L., J.S., P.Y., Y.Z.; Investigation: F.L., J.S.; Resources: F.L., J.S.; Data curation: F.L., J.S., J.L., Y.H., H.H.; Writing - original draft: F.L.; Writing - review & editing: H.C., S.Y.; Visualization: H.C.; Supervision: H.C., S.Y., X.W.; Project administration: F.L., X.W.; Funding acquisition: H.C., S.Y., J.S.
Funding
This work was supported by the National Key R&D Program of China [2017YFA0504000 and 2016YFA0500403 to H.C.], the National Natural Science Foundation of China [31470811, 31670039 and 31821091 to H.C.; 31671448 and 81572618 to J.S.] and the National Institutes of Health [R01CA175741 to S.Y.]. Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://jcs.biologists.org/lookup/doi/10.1242/jcs.224923.supplemental
References
- Abdullaev I. F., Bisaillon Jonathan M., Potier M., Gonzalez Jose C., Motiani Rajender K. and Trebak M. (2008). Stim1 and orai1 mediate CRAC currents and store-operated calcium entry important for endothelial cell proliferation. Circ. Res. 103, 1289-1299. 10.1161/01.RES.0000338496.95579.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander N. R., Branch K. M., Parekh A., Clark E. S., Iwueke I. C., Guelcher S. A. and Weaver A. M. (2008). Extracellular matrix rigidity promotes invadopodia activity. Curr. Biol. 18, 1295-1299. 10.1016/j.cub.2008.07.090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldassarre M., Pompeo A., Beznoussenko G., Castaldi C., Cortellino S., McNiven M. A., Luini A. and Buccione R. (2003). Dynamin participates in focal extracellular matrix degradation by invasive cells. Mol. Biol. Cell. 14, 1074-1084. 10.1091/mbc.e02-05-0308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Lipp P. and Bootman M. D. (2000). The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 1, 11-21. 10.1038/35036035 [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Bootman M. D. and Roderick H. L. (2003). Calcium signalling: dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 4, 517-529. 10.1038/nrm1155 [DOI] [PubMed] [Google Scholar]
- Chen J. and Sanderson M. J. (2017). Store-operated calcium entry is required for sustained contraction and Ca2+ oscillations of airway smooth muscle. J. Physiol. 595, 3203-3218. 10.1113/JP272694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T.-W., Wardill T. J., Sun Y., Pulver S. R., Renninger S. L., Baohan A., Schreiter E. R., Kerr R. A., Orger M. B., Jayaraman V. et al. (2013). Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295-300. 10.1038/nature12354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.-W., Chen Y.-F., Chiu W.-T., Chen H.-C. and Shen M.-R. (2017). STIM1-dependent Ca(2+) signaling regulates podosome formation to facilitate cancer cell invasion. Sci. Rep. 7, 11523 10.1038/s41598-017-11273-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H. and Lederer W. J. (2008). Calcium sparks. Physiol. Rev. 88, 1491-1545. 10.1152/physrev.00030.2007 [DOI] [PubMed] [Google Scholar]
- Cheng H., Lederer W. and Cannell M. (1993). Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science 262, 740-744. 10.1126/science.8235594 [DOI] [PubMed] [Google Scholar]
- Dynes J. L., Amcheslavsky A. and Cahalan M. D. (2016). Genetically targeted single-channel optical recording reveals multiple Orai1 gating states and oscillations in calcium influx. Proc. Natl. Acad. Sci. USA 113, 440-445. 10.1073/pnas.1523410113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman C. H., Grotegut C. A. and Rosenberg P. B. (2017). The role of STIM1 and SOCE in smooth muscle contractility. Cell Calcium 63, 60-65. 10.1016/j.ceca.2017.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimona M., Buccione R., Courtneidge S. A. and Linder S. (2008). Assembly and biological role of podosomes and invadopodia. Curr. Opin. Cell Biol. 20, 235-241. 10.1016/j.ceb.2008.01.005 [DOI] [PubMed] [Google Scholar]
- Hogan P. G. (2015). The STIM1-ORAI1 microdomain. Cell Calcium 58, 357-367. 10.1016/j.ceca.2015.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kar P., Nelson C. and Parekh A. B. (2011). Selective activation of the transcription factor NFAT1 by calcium microdomains near Ca(2+) release-activated Ca(2+) (CRAC) channels. J. Biol. Chem. 286, 14795-14803. 10.1074/jbc.M111.220582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kar P., Samanta K., Kramer H., Morris O., Bakowski D. and Parekh A. B. (2014). Dynamic assembly of a membrane signaling complex enables selective activation of NFAT by Orai1. Curr. Biol. 24, 1361-1368. 10.1016/j.cub.2014.04.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno T., Matsuda E., Sasaki H. and Sasaki T. (2008). Protein-tyrosine kinase CAKbeta/PYK2 is activated by binding Ca(2+)/calmodulin to FERM F2 alpha2 helix and thus forming its dimer. Biochem. J. 410, 513-523. 10.1042/BJ20070665 [DOI] [PubMed] [Google Scholar]
- Li Z., Lu J., Xu P., Xie X., Chen L. and Xu T. (2007). Mapping the interacting domains of STIM1 and Orai1 in Ca(2+) release-activated Ca(2+) channel activation. J. Biol. Chem. 282, 29448-29456. 10.1074/jbc.M703573200 [DOI] [PubMed] [Google Scholar]
- Li J., Cubbon R. M., Wilson L. A., Amer M. S., McKeown L., Hou B., Majeed Y., Tumova S., Seymour V. A. L., Taylor H. et al. (2011). Orai1 and CRAC channel dependence of VEGF-activated Ca2+ entry and endothelial tube formation. Circ. Res. 108, 1190-1198. 10.1161/CIRCRESAHA.111.243352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder S. (2007). The matrix corroded: podosomes and invadopodia in extracellular matrix degradation. Trends Cell Biol. 17, 107-117. 10.1016/j.tcb.2007.01.002 [DOI] [PubMed] [Google Scholar]
- Liou J., Kim M. L., Do Heo W., Jones J. T., Myers J. W., Ferrell J. E. Jr. and Meyer T. (2005). STIM is a Ca(2+) sensor essential for Ca(2+)-store-depletion-triggered Ca(2+) influx. Curr. Biol. 15, 1235-1241. 10.1016/j.cub.2005.05.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo P. and Yang S. (2018). The store-operated calcium channels in cancer metastasis: from cell migration, invasion to metastatic colonization. Front. Biosci. 23, 1241-1256. 10.2741/4641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy D. A. and Courtneidge S. A. (2011). The ‘ins’ and ‘outs’ of podosomes and invadopodia: characteristics, formation and function. Nat. Rev. Mol. Cell Biol. 12, 413-426. 10.1038/nrm3141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh A. B. and Putney J. W. Jr. (2005). Store-operated calcium channels. Physiol. Rev. 85, 757-810. 10.1152/physrev.00057.2003 [DOI] [PubMed] [Google Scholar]
- Park C. Y., Hoover P. J., Mullins F. M., Bachhawat P., Covington E. D., Raunser S., Walz T., Garcia K. C., Dolmetsch R. E. and Lewis R. S. (2009). STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell 136, 876-890. 10.1016/j.cell.2009.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakriya M. and Lewis R. S. (2015). Store-operated calcium channels. Physiol. Rev. 95, 1383-1436. 10.1152/physrev.00020.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs D., Yang Z., Kloss J. and Loftus J. C. (2011). The Pyk2 FERM regulates Pyk2 complex formation and phosphorylation. Cell. Signal. 23, 288-296. 10.1016/j.cellsig.2010.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanta K., Kar P., Mirams G. R. and Parekh A. B. (2015). Ca(2+) channel re-localization to plasma-membrane microdomains strengthens activation of Ca(2+)-dependent nuclear gene expression. Cell Rep. 12, 203-216. 10.1016/j.celrep.2015.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang W., Lu F., Sun T., Xu J., Li L.-L., Wang Y., Wang G., Chen L., Wang X., Cannell M. B. et al. (2014). Imaging Ca(2+) nanosparks in heart with a new targeted biosensor. Circ. Res. 114, 412-420. 10.1161/CIRCRESAHA.114.302938 [DOI] [PubMed] [Google Scholar]
- Siddiqui T. A., Lively S., Vincent C. and Schlichter L. C. (2012). Regulation of podosome formation, microglial migration and invasion by Ca(2+)-signaling molecules expressed in podosomes. J. Neuroinflammation 9, 250 10.1186/1742-2094-9-250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith I. F., Wiltgen S. M. and Parker I. (2009). Localization of puff sites adjacent to the plasma membrane: Functional and spatial characterization of Ca(2+) signaling in SH-SY5Y cells utilizing membrane-permeant caged IP3. Cell Calcium 45, 65-76. 10.1016/j.ceca.2008.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Lu F., He H., Shen J., Messina J., Mathew R., Wang D., Sarnaik A. A., Chang W. C., Kim M. et al. (2014). STIM1- and Orai1-mediated Ca(2+) oscillation orchestrates invadopodium formation and melanoma invasion. J. Cell Biol. 207, 535-548. 10.1083/jcb.201407082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnai P., Thyagarajan B., Rohacs T. and Balla T. (2006). Rapidly inducible changes in phosphatidylinositol 4,5-bisphosphate levels influence multiple regulatory functions of the lipid in intact living cells. J. Cell Biol. 175, 377-382. 10.1083/jcb.200607116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C., Wang X., Chen M., Ouyang K., Song L.-S. and Cheng H. (2009). Calcium flickers steer cell migration. Nature 457, 901-905. 10.1038/nature07577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C., Wang X., Zheng M. and Cheng H. (2012). Calcium gradients underlying cell migration. Curr. Opin. Cell Biol. 24, 254-261. 10.1016/j.ceb.2011.12.002 [DOI] [PubMed] [Google Scholar]
- Wei-LaPierre L., Carrell E. M., Boncompagni S., Protasi F. and Dirksen R. T. (2013). Orai1-dependent calcium entry promotes skeletal muscle growth and limits fatigue. Nat. Commun. 4, 2805 10.1038/ncomms3805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby D., Everett K. L., Halls M. L., Pacheco J., Skroblin P., Vaca L., Klussmann E. and Cooper D. M. (2012). Direct binding between Orai1 and AC8 mediates dynamic interplay between Ca(2+) and cAMP signaling. Sci. Signal. 5, ra29 10.1126/scisignal.2002299 [DOI] [PubMed] [Google Scholar]
- Wu M. M., Buchanan J., Luik R. M. and Lewis R. S. (2006). Ca(2+) store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J. Cell Biol. 174, 803-813. 10.1083/jcb.200604014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S., Zhang J. J. and Huang X. Y. (2009). Orai1 and STIM1 are critical for breast tumor cell migration and metastasis. Cancer Cell 15, 124-134. 10.1016/j.ccr.2008.12.019 [DOI] [PubMed] [Google Scholar]
- Yang S., Zhang J. J. and Huang X. Y. (2012). Mouse models for tumor metastasis. Methods Mol. Biol. 928, 221-228. 10.1007/978-1-62703-008-3_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y. and Parker I. (1994). Ca(2+) influx modulation of temporal and spatial patterns of inositol trisphosphate-mediated Ca(2+) liberation in Xenopus oocytes. J. Physiol. 476, 17-28. 10.1113/jphysiol.1994.sp020157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Cai X., Loktionova N. A., Wang X., Nwokonko R. M., Wang X., Wang Y., Rothberg B. S., Trebak M. and Gill D. L. (2016). The STIM1-binding site nexus remotely controls Orai1 channel gating. Nat. Commun. 7, 13725 10.1038/ncomms13725 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.