Abstract
Background:
Biomonitoring data shows that people are exposed to phthalates, phenols and perchlorates. Many of these compounds are endocrine disrupting compounds that affect thyroid hormone levels. Yet the effect of these compounds on thyroid hormone levels are often evaluated individually rather than as a mixture. Our objective was to examine the association between 11 urinary endocrine disrupting compounds and thyroid hormones using structural equation models.
Methods:
Using data from the National Health and Nutrition and Examination Survey 2007-2008, we fit a latent variable utilizing urinary measurements of 9 compounds in females (perchlorate, bisphenol A, benzophenone-3, mono-2ethyl5carboxypentyl phthalate, mono-n-butyl phthalate, mono-(3-carboxypropyl) phthalate, mono(2ethyl5hydroxyhexyl) phthalate, mono-benzyl phthalate, and mono-isobutyl phthalate) and 8 compounds in males (without benzophenone-3). The association of the latent variable with serum thyroid hormones (Total T3, Total T4, and Thyroid Stimulating Hormones) was assessed in females (N=710) and males (N=850) over the age of 12 controlling for age, race, and urinary creatinine.
Results:
In males, urinary endocrine disrupting compound levels were negatively associated with thyroxine (β: −0.19, 95% Confidence Interval (95% CI): −0.31, −0.05). In females, urinary endocrine disrupting compound levels were positively associated with triiodothyronine serum concentrations (β: 0.09, 95% CI: −0.03, 0.21) however this association was not statistically significant.
Conclusions:
This cross-sectional analysis provides additional evidence that environmental exposure to phthalates and phenols is associated with endocrine-related processes. Furthermore, these results suggested sex-specific differences in exposure to endocrine disrupting mixtures, and the exposure-response between endocrine disrupting mixtures and thyroid hormone levels. Specifically, higher exposure to a mixture of endocrine disrupting compounds was associated with lower levels of total T4 in males but not in females. While a structural methodological framework was used to assess these complex relationships, the cross sectional nature of this analysis limits causal inference and further research is needed to determine the clinical significance of these findings.
Keywords: Endocrine disrupting compounds, thyroid hormones, structural equation modeling, EDCs
Introduction
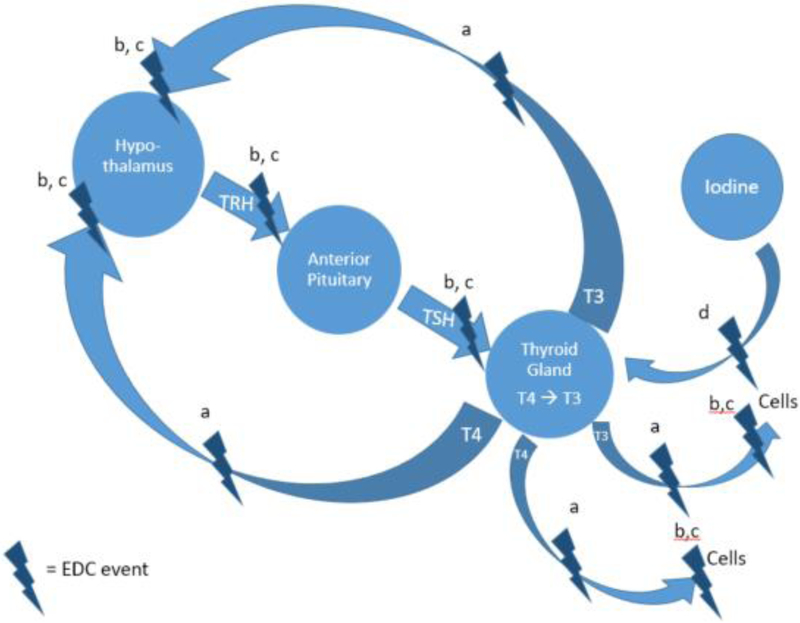
The endocrine system is susceptible to environmental toxicants called endocrine disrupting chemicals (EDCs) [1]. EDCs include many synthetic compounds such as phthalates, bisphenol A (BPA), polyhalogenated compounds, alkylphenols, and perchlorates [2]. These compounds are commonly found in personal care products, food packaging, building materials, and industrial pollution [3-7]. Because phthalates are not chemically bound to the products they are integrated into, they easily off-gas or migrate out of the product leading to human exposure. Some phthalates are also used in medical tubing and devices which can contribute to intravenous exposure [8]. Other routes of exposure to phthalates and EDCs include inhalation of house dust or particulate matter, dermal exposure from personal care products, ingestion from food contaminated with EDCs from contact with food packaging [9-12]. Once absorbed in the body, EDCs can influence the hypothalamic-pituitary-thyroid axis and thyroid homeostasis by binding to transport molecules or receptors [13], mimicking thyroid hormones (THs), or blocking iodine from entering the thyroid [14] (Figure 1). For instance, if an EDC acts as a TH antagonist this effectively lowers the amount of circulating THs which prompts the hypothalamus to secrete thyrotropin releasing hormone (TRH) which then informs the pituitary gland to secrete thyroid stimulating hormone (TSH) [15]. In turn, TSH stimulates follicular cells in the thyroid gland to produce thyroxine (T4) which is converted to triiodothyronine (T3) within cells [16]. In addition to this feedback loop there are other factors that are known to influence THs. Once THs are produced, they interact with almost every cell in the body by binding to the thyroid hormone receptor (TR) and are important for metabolism, growth, bone and heart health, and the respiratory and nervous systems [16-20].
Figure 1.
Relationship between the hypothalamic-pituitary-thyroid axis and EDCs. EDCs can disrupt the axis and influence thyroid homeostasis by (a) binding to transport molecules, (b) binding to receptors, (c) mimicking THs, and (d) blocking iodine from entering the thyroid.
Several toxicology studies have explored the association between exposure to single EDCs and THs. These studies show that rats exposed to di(2-ethylhexyl) phthalate (DEHP), dibutyl phthalates (DBP), and perchlorate had decreased blood levels of T4 and T3 [21-25]. Whereas, BPA exposure in rats during development resulted in increased blood levels of T4 but resulted in no difference in TSH blood levels [26]. Similarly rats exposed to triclosan had increased blood levels of T4 [27] but decreases in T3 [15]. In human observational studies, higher urinary metabolites of DEHP were related to lower total T4, lower total T3, and increased TSH among adults (n=1,346) [28]. Blount and colleagues [29] found a significant positive association between urinary perchlorate and TSH levels in women (n=1,111) and a negative asociation between urinary perchlorate and total T4 among women with iodine levels <100 ug/L. No assocation between TSH or T4 were observed in men. In contrast, 12 adults who were exposed to triclosan for 14 days via toothpaste had no significant difference in plasma TH concentrations before and after exposure [30]. Similarly, 13 volunteers exposed to perchlorate via spring water exhibited no changes in thyroid hormone concentrations during the duration of the study [31].
Yet individuals are exposed to multiple EDCs at any one point in time. Thus, it is important to examine the combined effect of these compounds on thryroid hormones. Structural equation modelling (SEM) is a technique well-suited to examining multiple highly correlated environmental exposures on complex biological pathways [32]. Subsequently, this study hypothesized a priori that urinary concentrations of 11 EDCs expressed as a latent variable would be negatively associated with T3 and T4 levels and positively associated with TSH levels. By utilizing SEM, we are able to address several suggestions for future research put forth by Endocrine Society, as discussed in the Society’s Second Scientific Statement on EDCs [33]. Specifically, we were able to model a mixture of low-dose exposure to EDCs and investigate the mixture’s response on thyroid hormones for each sex. For instance, given that prior epidemiological studies have observed sex-specific relationships between EDCs and these outcomes and important covariates such as menopausal status were present only for females, we examined these hypotheses separately in males and females. It should be noted that in SEM the term “effect” refers to statistical and not causal effect and we use the former meaning throughout the rest of the manuscript.
Methods
Study design and population
We used data from the 2007-2008 NHANES cycle. While thyroid hormone profiles are also analyzed in NHANES 2009-2010, only free T4 and total T3 were available in this cycle. Thus we limited our analytic sample to data from the 2007-2008 NHANES. This cross-sectional survey collected data on nutrition and health measures from a U.S. non-institutionalized population by utilizing physical examinations, specimen collection and surveys [34]. The survey is conducted by the National Center for Health Statistics (NCHS) of the Center for Disease Control (CDC) and the data are publicly available. All protocols were approved by NCHS Research Ethics Review Board (ERB) and documented consent was obtained from all participants [35].
Subjects were included in this analysis if data were available on: i) urinary concentrations of the 11 phenols, perchlorate, and phthalates, and ii) serum thyroid hormones. These measurements were assessed only in 1/3 of participants aged 12 and above. Of the 2,278 individuals who had their urinary EDCs and blood TH levels measured, we excluded individuals taking medication for a thyroid problem (n=114) and those with self-reported thyroid problems (n=72) resulting in 2,092 participants who were not at risk for thyroid dysfunction. Out of those participants, we excluded participants who did not complete data on urinary perchlorate (n=113), thyroid hormones (n=126), urinary iodine (n=63), serum cotinine (n=3), BMI (n=18), and poverty-income ratio (n=135), leaving 1,634 participants. Ten women were excluded because they indicated they were breastfeeding which can alter thyroid hormone levels [36]. Finally, 64 women were missing data on menopausal status leaving 710 females and 850 males (total 1,560) with complete data.
Measurement of chemical biomarkers in urine
A total of nineteen different phthalates, phenols (BPA, triclosan, and benzophenone-3) and perchlorate were measured in the urine of the same participants and used in this analysis. NCHS recommends using chemical data that are above 60% frequency detection [37]. Therefore, only 7 of the 15 urinary phthalate metabolites were retained for use in this study (mono-2ethyl5carboxypentyl phthalate [MECPP], mono-n-butyl phthalate [MnBP], mono-(3-carboxypropyl) phthalate [MCPP], mono-ethyl phthalate [MEP], mono(2ethyl5hydroxyhexyl) phthalate [MEHHP], mono-benzyl phthalate [MBzP], mono-isobutyl phthalate [MiBP]) for a total of 11 EDCs used in this analysis. Additional suspected endocrine disrupting compounds such as polychlorinated biphenyls (PCBs) were assessed in NHANES 2007-2008, however since NHANES does not measured every chemical in the same person we were unable to included additional endocrine disruptors because they were not measured in the same subgroup used in this analysis. Phenol analytes were measured using gas chromatograph-mass spectrometry (GC-MS) [38], phthalates were measured using high performance liquid chromatograph (HPLC) with electrospray ionization (ESI) combined with MS/MS [39], and perchlorate was analyzed with ion chromatography (IC) coupled with electrospray tandem MS [40]. Samples that were below the limit of detection (LOD) for a selected analyte were assigned the analyte LOD divided by the by NCHS.
Thyroid Hormones
Total T3 (pg/mL), total T4 (ng/dL) and TSH (mIU/L) were measured in serum using immunoenzymatic assays described elsewhere [41].
Covariates
Several variables were examined as potential confounders including: poverty index ratio (PIR, at or below 0.99 and at or above 1.00), race (Mexican American [MA], Other Hispanic [OH] including multi-racial, non-Hispanic White [NHW], non-Hispanic Black [NHB]), and age (continuous). Smoking status was determined using cotinine values (≤ 10 ng/L cotinine was classified as a non-smoker, >10ng/L cotinine was classified as a smoker) [42]. Urinary iodine concentrations were investigated as a covariate because thyroxine production is dependent on iodine concentrations [43]. For women, menopausal status (pre or post-menopausal) was assessed. BMI was calculated by dividing the weight of the participant in kilograms by the height squared (meters) of the individual [44] and was used as a continuous variable in this analysis. For all questions, responses of “don’t know” or refused responses were coded as missing. Finally, urinary creatinine was included as a continuous variable.
Statistical Modeling
The concentration of all urinary chemicals, iodine, urinary creatinine, BMI, and THs were natural log transformed to address skewness. Descriptive statistics were calculated using sampling weights for the subsample who had their biospecimens analyzed for phenols and phthalates using Stata for Windows (version 14, StataCorp LP, www.stata.com). To account for the complex sampling design of the NHANES, we used survey variables including stratification, clustering (PSU), and sample weights that corresponded to the weights of the subsample [37]. SEMs were used to evaluate our a priori hypothesis that EDC exposure, defined as a latent variable, would have a direct effect on THs (Figure 2). We modeled a single latent variable to represent the body burden of the available EDCs as a mixture. The SEM models were stratified by sex. Model building and testing of the SEM was performed using Mplus (version 7.4, Muthen and Muthen; www.StatModel.com), although Stata was used to construct the SEM diagram.
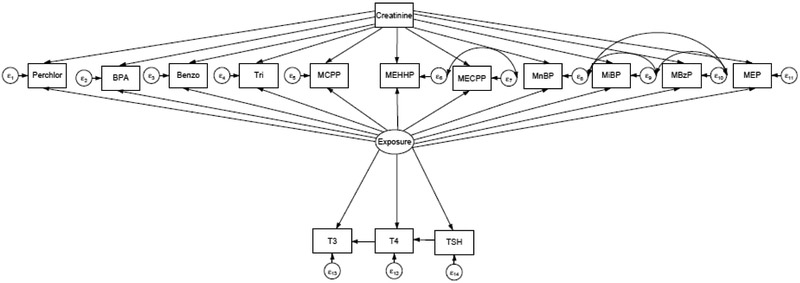
Figure 2.
A priori model of relationship between 11 EDC compounds and THs after adjustment for creatinine. The latent Exposure variable represent exposure to a mixture of EDCs. Perchlorate is represented by “Perchlor”, benzophenone-3 is represented by “Benzo”, and triclosan is represented by “Tri”, mono-2ethyl5carboxypentyl phthalate is represented by “MECPP”, mono-n-butyl phthalate is represented by “MnBP”, mono-(3-carboxypropyl) phthalate is represented by “MCPP”, mono-ethyl phthalate is represented by “MEP”, mono(2ethyl5hydroxyhexyl) phthalate is represented by “MEHHP”, mono-benzyl phthalate is represented by “MBzP”, and mono-isobutyl phthalate is represented by “MiBP”.
In SEM diagrams the latent, or unmeasured, variables are represented by ovals. Measured variables are represented by rectangles. Single headed arrows represent regressions between variables (called factor loadings when an observed variable is regressed on the latent factor it indicates). The small circles designated as εx are residuals, or unexplained variance. A double-headed arrow between the residuals indicates that the residuals are correlated. In this analysis, the Exposure latent variable is indicated by the available measured EDCs variables but it has no inherent metric because latent variables are unmeasured. In order to set the measurement scale, a measured variable’s unstandardized loading can be set to 1. We arbitrarily chose perchlorate’s unstandardized loading to be set as 1. Thus allowing us 1) to determine the association of the EDCs with the latent variable despite the EDCs being measured on different scales and 2) determine the association of the latent variable with the outcome variables despite the latent variable having no inherent metric. Subsequently, the standardize parameter estimates can be used to compare the relative influence among variables and are similar to beta coefficients computed in regression analysis. Maximum likelihood estimation with robust standard errors was used as our estimator.
Initially, we created an SEM based on our a priori hypothesis where all available urinary EDCs regressed by urinary creatinine to control for dilution differences in urine samples [45]. Additionally, we correlated the residuals εx of phthalate metabolites that shared the same parent compound because they were highly correlated and likely shared a similar source (e.g. MEHHP with MECPP, MnBP with MBzP, MiBP with MBzP) [46, 47]. After these initial models were fit, all EDCs were associated with the latent Exposure. Subsequently, we removed EDCs that did not significantly load (i.e., a p value ≥ 0.05) to the latent variable. We then incorporated thyroid hormones into the model following the a prior hypothesis shown in Figure 2. If an association between T4, T3, or THS and the latent variable Exposure were not statistically significant (p value ≥0.05) they were removed. Next, selected covariates were added to the model to adjust latent and endogenous variables because they were perceived to be biological relevant with regards to EDCs and thyroid hormones. After the addition of covariates into the model, some of the relationships between Exposure and THs were attenuated (p value ≥ 0.05). These relationships were left in the model to control for potential confounding. Subsequently, the latent variable Exposure was adjusted for age, race, and PIR, and thyroid hormones were adjusted for age, smoking status, iodine, and menopausal status (females only). Since BMI is related to both thyroid hormones and EDCs, BMI was allowed to correlate with THs and all the individual EDCs (except perchlorate) [48-52]. Neither age nor PIR were significantly related to the latent variable Exposure (p value ≥ 0.05). Additionally iodine and menopausal status were not significant related to thyroid hormones, nor was BMI correlated with thyroid hormones and most individual EDCs.
Using the a prior SEM model with α=0.05 and 1-β=0.08, the minimum sample size was determined to be 210, indicating that both male and female models are well powered [53]. Global model fit was evaluated using the following indices: Comparative Fit Index (CFI), Tucker-Lewis Index (TLI), Standardized Root Mean Square Residual (SRMR), Root Mean Square Error of Approximation (RMSEA), and the RMSEA 90% confidence interval (RMSEA 90% CI). Due to the concern that BPA concentrations might be a result of external contamination [54], models were run with and without BPA. However, no substantial differences were observed between these two models and we opted to retain BPA in the EDC mixture because it contributed to the latent variable Exposure. Sensitivity analysis showed that a model which included all covariates in the males and female models yielded estimates that were of similar magnitude, direction, and significance. Although the inclusion of the additional, non-statistically significant covariates in each model decreased the values for goodness of fit.
Results
Descriptive Statistics
The characteristics of the sample population are described in Table 1. The mean concentration of T3, T4, and TSH in males was 114.69 ng/dL (112.32, 117.11 ng/dL), 7.35 μg/dL (7.23, 7.47 μg/L), 1.63 mIU/mL (1.54, 1.72 mIU/mL), respectively. In females, the mean concentration of T3, T4 and TSH was 111.79 ng/dL (108.19, 114.70 ng/dL), 7.58 μg/L (7.40, 7.76 μg/L), and 1.57 mIU/mL (1.41, 1.75 mIU/mL), respectively. To assess the impact of missing data on the representativeness of the subpopulation used in this analysis, we compared the characteristics of this subsample with the larger population that had measured EDCs and THs. Males included in this analysis were more likely to be Non-Hispanic White, have a PIR ≥ 1, be classified as obese and be a non-smoker than males who were excluded due to missing data. Females included in this analysis were more likely to be older, Non-Hispanic White, have a PIR ≥ 1, overweight, smokers, and pre-menopausal than females who were excluded due to missing data.
Table 1.
Descriptive characteristics for males and females who had their serum analyzed for multiple EDCs that were included in this analysis compared to those that were excluded due to missing data (NHANES 1999-2002)
| | ||||||
|---|---|---|---|---|---|---|
| Males who had complete data (n=850 ) |
Males who did not have complete data (n=231) |
Chi- square (p- value) |
Females who had complete data (n=710 ) |
Females who did not have complete data (n=291) |
Chi- square (p- value) |
|
| Variable | N (%a) | N (%a) | N (%a) | N (%a) | ||
| Age (years) | 0.29 (0.82) |
4.00 (0.03) |
||||
| Geometric Meana | 37.06 (35.32, 38.89) | 35.78 (32.16, 39.82) | 37.65 (35.27, 40.19) | 36.46 (33.82, 39.31) | ||
| (95% CI) | ||||||
| 12-19 | 162 (14.52) | 50 (14.96) | 131 (13.97) | 65 (17.93) | ||
| 20-39 | 224 (33.44) | 68 (37.78) | 195 (31.87) | 83 (35.66) | ||
| 40-59 | 218 (34.17) | 52 (30.26) | 216 (36.69) | 61 (20.43) | ||
| ≥60 | 246 (17.86) | 61 (17.00) | 168 (17.47) | 85 (25.97) | ||
| Race | 5.85 (0.004) |
3.83 (0.03) |
||||
| MA | 151 (9.46) | 40 (11.35) | 153 (9.05) | 53 (8.14) | ||
| OH | 118 (9.83) | 37 (11.27) | 109 (10.39) | 62 (14.35) | ||
| NHW | 417 (70.74) | 88 (60.27) | 287 (68.17) | 92 (58.84) | ||
| NHB | 164 (9.96) | 66 (17.11) | 161 (12.38) | 84 (18.66) | ||
| PIR | 158.18 (<0.001) |
67.25 (<0.001) |
||||
| Geometric Meana | 2.46 (2.27, 2.67) | 2.15 (1.75, 2.63) | 2.25 (2.02, 2.49) | 1.95 (1.73, 2.20) | ||
| (95% CI) | ||||||
| ≤0.99 | 174 (12.56) | 30 (18.44) | 164 (15.47) | 57 (20.27) | ||
| ≥1.00 | 676 (87.44) | 105 (81.56) | 546 (84.593 | 140 (79.73) | ||
| Missing (N) | NA | 96 | NA | 94 | ||
| BMI (kg/m2)b | 19.77 (<0.001) |
6.89 (<0.001) |
||||
| Geometric Meana | 27.58 (27.07, 28.11) | 26.49 (25.50, 27.53) | 26.78 (26.22, 27.34) | 26.59 (25.51, 27.71) | ||
| (95% CI) | ||||||
| Underweight | 7 (0.50) | 2 (0.24) | 10 (1.32) | 8 (3.26) | ||
| Normal | 267 (30.66) | 72 (35.54) | 252 (41.08) | 100 (42.05) | ||
| Overweight | 287 (34.81) | 82 (38.82) | 213 (27.74) | 65 (20.79) | ||
| Obese | 289 (34.03) | 61 (25.40) | 235 (29.85) | 102 (33.90) | ||
| Missing (N) | NA | 14 | NA | 16 | ||
| Smoking Status | 89.02 (<0.001) |
34.89 (<0.001) |
||||
| Smoker | 235 (29.45) | 54 (38.91) | 147 (21.16) | 33 (14.63) | ||
| Non-smoker | 615 (70.54) | 106 (61.09) | 563 (78.84) | 197 (85.37) | ||
| Missing (N) | NA | 71 | NA | 61 | ||
| Menopausal Status | NA | 116.54 (<0.001) |
||||
| Pre-menopause | NA | NA | 425 (63.72) | 102 (62.70) | ||
| Post-menopause | NA | NA | 285 (36.28) | 81 (37.30) | ||
| Missing (N) | NA | NA | NA | 108 | ||
Weighted using survey variables including stratification, clustering (PSU), and laboratory sample weights
BMI categories for males and females aged 20 and above were determined using the CDC’s definition. BMI < 18.5 is underweight, BMI ≥ 18.5 and ≤ 24.9 is normal, BMI ≥ 25.0 and ≤ 29.9 is overweight, and BMI ≥ 30.0 is obese [55]. BMI definition for males and females aged 12-19 are determined using percentiles calculated from the CDC growth charts [56].
We initially created a latent variable based on 11 urinary EDCs which met our criteria of being measured in the same individual at a detection frequency ≥ 60%. However, not all EDCs were significantly associated with the latent variable. Additionally, the number of EDCs that were significantly associated with the latent variable differed by sex. Thus, the latent variable was comprised of 9 EDCs for females and 8 EDCs for males. The mean concentrations and 95% confidence intervals for the urinary analytes that were significantly associated with the latent variable are described in Table 2. The geometric mean for perchlorate was 4.37 ng/L (95% CI: 3.97, 4.82 ng/L) and 3.48 ng/L (95% CI: 3.08, 3.94 ng/L) for males and females, respectively. The highest geometric mean of the phenols for men and women was benzophenone-3 with a geometric mean of 11.73 ng/mL (95% CI: 8.99, 15.30 ng/mL) and 27.83 ng/mL (95% CI: 19.62, 39.45 ng/mL), respectively. Mono-(2-ethyl-5-carboxypentyl) phthalate had the highest geometric mean of the phthalates with geometric mean concentrations of 34.35 ng/mL (95% CI: 28.75, 41.05 ng/mL) and 31.77 ng/mL (95% CI: 26.43, 38.18 ng/mL) for males and females, respectively.
Table 2.
Description of the concentration of EDCs in males and females including geometric mean, 95% confidence interval (95% CI), percent above LOD, and percent missing (NHANES 2007-2008).
| Males (N=850) | Females (N=710) | ||||||
|---|---|---|---|---|---|---|---|
| Biomarkers | Limit of Detection (LOD) |
Geometric Meana (95 % CI) |
Above LOD (%) |
Percent Missing (%) |
Geometric Meana (95% CI) |
Above LOD (%) |
Percent Missing (%) |
| Perchlorate (ng/L) | 0.13 | 4.37 (3.97, 4.82) | 100 | 8.51 | 3.48 (3.08, 3.94) | 100 | 6.59 |
| Bisphenol A (ng/mL) (BPA) | 0.39 | 2.19 (1.94, 2.47) | 94 | 2.52 | 2.00 (1.80, 2.23) | 92 | 3.50 |
| Benzophenone-3 (ng/mL) | 0.39 | 11.73 (8.99, 15.30) | 95 | 2.52 | 27.83 (19.62, 39.45) | 97 | 3.50 |
| Mono-2ethyl5carboxypentyl phthalate (ng/mL) (MECPP) | 0.49 | 34.35 (28.75, 41.05) | 99 | 2.52 | 31.77 (26.43, 38.18) | 99 | 3.50 |
| Mono-n-butyl phthalate (ng/mL)(MnBP) | 0.59 | 17.65 (16.19, 19.26) | 99 | 2.52 | 20.01 (17.16, 23.34) | 99 | 3.50 |
| Mono-(3-carboxypropyl) phthalate(ng/mL) (MCPP) | 0.20 | 2.88 (2.56, 3.24) | 98 | 2.52 | 2.43 (2.11, 2.80) | 96 | 3.50 |
| Mono(2ethyl5hydroxyhexyl)phthalate (ng/mL) (MEHHP) | 0.69 | 23.32 (19.33, 28.14) | 99 | 2.52 | 21.28 (17.65, 25.67) | 99 | 3.50 |
| Mono-benzyl phthalate (ng/mL)(MBzP) | 0.22 | 7.29 (6.46, 8.26) | 65 | 2.52 | 6.87 (5.80, 8.14) | 97 | 3.50 |
| Mono-isobutyl phthalate (ng/mL)(MiBP) | 0.30 | 7.30 (6.59, 8.25) | 98 | 2.52 | 6.75 (6.10, 7.46) | 98 | 3.50 |
| Total T3 (ng/dL) | NA | 114.69 (112.32, 117.11) | NA | 9.57 | 111.79 (108.19, 114.70) | NA | 8.09 |
| Total T4 (ug/L) | NA | 7.35 (7.23, 7.47) | NA | 9.57 | 7.58 (7.40, 7.76) | NA | 8.09 |
| TSH (mIU/mL) | NA | 1.63 (1.54, 1.72) | NA | 9.57 | 1.57 (1.41, 1.75) | NA | 8.09 |
| Iodine (ug/L) | NA | 170.98 (158.41, 184.54) | NA | 8.50 | 145.92 (135.34, 157.33) | NA | 9.79 |
| eUrinary Creatinineb | NA | 122.76 (116.30, 129.57) | NA | 2.19 | 86.34 (79.28, 94.02) | NA | 2.60 |
Weighted using survey variables including stratification, clustering (PSU), and laboratory sample weights.
Creatinine used to adjust urinary EDCs for concentration and/or dilution differences in urine samples
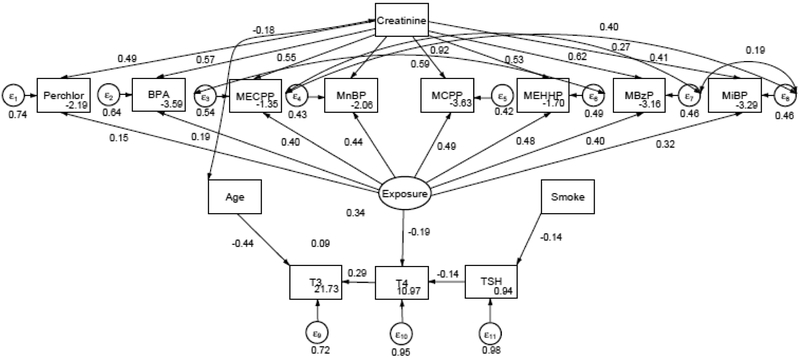
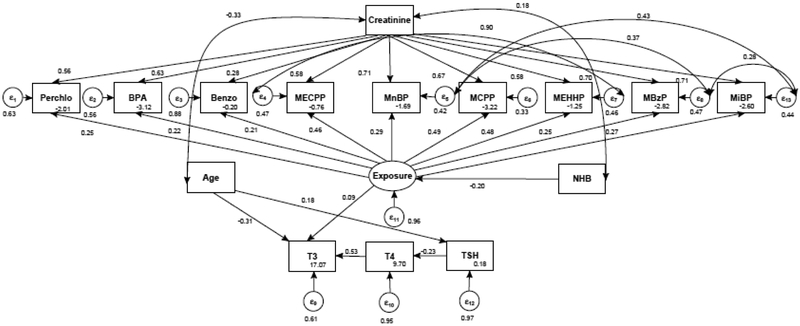
Figures 3 and 4 show the fitted SEM for the associations between Exposure and THs for males and females 12-85 years of age in NHANES 2007-2008, respectively. In SEM, coefficients can be interpreted in a similar manner to multiple regression. For males, MCPP had the highest loading of 0.49 (95% CI: 0.31, 0.63) onto Exposure. The SEM models showed that Exposure was negatively associated with T4 (β= −0.19, 95% CI: −0.31, −0.05) in males (Table 3). Whereas, in females MCPP had the highest loading on the latent Exposure variable of 0.49 (95% CI: 0.41, 0.58) and Exposure was positively associated with T3 (0.09, 95% CI. −0.03, 0.21) among women after adjusting T3 for age (Table 4). Fit indices showed that the all models were well above acceptable criteria (Table 5).
Figure 3.
SEM of relationship between EDC and THs in males after adjusted EDCs for creatinine, T3 for age, and TSH for Smoking Status. Not pictured: correlation between TSH and BMI.
Figure 4.
SEM of relationship between EDC and THs in females after adjusting EDCs for being Non-Hispanic Black (NHB) and T3 and TSH for age. Not pictured: correlation between T4 and BMI and correlation between BMI and benzophenone-3.
Table 3.
Standardized estimates SEM depicting the relationship between 8 EDCs, THs, and covariates in males 12-85 years of age, NHANES 2007-2008. These values are depicted in Figure 2.
| Loadings/effects | Standard Estimates (95% CI) | Standard Errors | p-value |
|---|---|---|---|
| Exposure → Perchlorate | 0.15 (0.06, 0.23) | 0.05 | 0.001 |
| Exposure → BPA | 0.19 (0.09, 0.29) | 0.05 | <0.001 |
| Exposure → MECPP | 0.40 (0.30, 0.51) | 0.05 | <0.001 |
| Exposure → MnBP | 0.44 (0.36, 0.52) | 0.04 | <0.001 |
| Exposure → MCPP | 0.49 (0.31, 0.63) | 0.06 | <0.001 |
| Exposure → MEHHP | 0.48 (0.35, 0.60) | 0.06 | <0.001 |
| Exposure → MBzP | 0.40 (0.33, 0.47) | 0.04 | <0.001 |
| Exposure → MiBP | 0.32 (0.23, 0.43) | 0.06 | <0.001 |
| Exposure → T4 | −0.19 (−0.31, −0.05) | 0.07 | 0.008 |
| Creatinine → Perchlorate | 0.49 (0.42, 0.56) | 0.03 | <0.001 |
| Creatinine → BPA | 0.57 (0.51, 0.63) | 0.03 | <0.001 |
| Creatinine → MECPP | 0.55 (0.49, 0.63) | 0.03 | <0.001 |
| Creatinine → MnBP | 0.62 (0.53, 0.71) | 0.04 | <0.001 |
| Creatinine → MCPP | 0.59 (0.51, 0.67) | 0.04 | <0.001 |
| Creatinine → MEHHP | 0.53 (0.48, 0.61) | 0.03 | <0.001 |
| Creatinine →MBzP | 0.62 (0.56, 0.67) | 0.03 | <0.001 |
| Creatinine → MiBP | 0.66 (0.60, 0.72) | 0.03 | <0.001 |
| TSH → T4 | −0.14 (−0.21, −0.06) | 0.03 | <0.001 |
| T4 → T3 | 0.29 (0.23, 0.35) | 0.03 | <0.001 |
| Age → T3 | −0.44 (−0.48, −0.41) | 0.02 | <0.001 |
| Smoke → TSH | −0.14 (0.01, 0.17) | 0.03 | <0.001 |
| Residual variances | |||
| e.Perchlorate | 0.74 (0.66, 0.82) | 0.04 | <0.001 |
| e. BPA | 0.64 (0.55, 0.73) | 0.04 | <0.001 |
| e. MECPP | 0.52 (0.40, 0.65) | 0.06 | <0.001 |
| e. MnBP | 0.43 (0.35, 0.50) | 0.04 | <0.001 |
| e. MCPP | 0.40 (0.33, 0.48) | 0.04 | <0.001 |
| e. MEHHP | 0.48 (0.34, 0.62) | 0.07 | <0.001 |
| e. MBzP | 0.46 (0.39, 0.54) | 0.04 | <0.001 |
| e. MiBP | 0.45 (0.35, 0.56) | 0.05 | <0.001 |
| e.T3 | 0.72 (0.68, 0.76) | 0.02 | <0.001 |
| e.T4 | 0.95 (0.90, 1.00) | 0.03 | <0.001 |
| e.TSH | 0.99 (0.98, 1.01) | 0.01 | <0.001 |
| e.Iodine | 0.71 (0.61, 0.81) | 0.05 | <0.001 |
| Covariance | |||
| MEHPP ↔ MECPP | 0.92 (0.89, 0.94) | 0.01 | <0.001 |
| MnBP ↔ MiBP | 0.40 (0.26, 0.57) | 0.08 | <0.001 |
| MnBP ↔ MBzP | 0.27 (0.17, 0.36) | 0.05 | <0.001 |
| MiBP ↔ MBzP | 0.19 (−0.02, 0.39) | 0.10 | 0.07 |
| BMI ↔ TSH | 0.14 | 0.04 | 0.001 |
| Creatinine ↔ Age | −0.18 (−0.23, −0.13) | 0.03 | <0.001 |
Table 4.
Standardized estimates from the SEM depicting the relationship between 9 EDCs, THs, and covariates in females 12-85 years of age, NHANES 2007-2008. These values are depicted in Figure 3.
| Loadings/effects | Standard Estimates (95% CI) | Standard Errors | p-value |
|---|---|---|---|
| Exposure → Perchlorate | 0.25 (0.13, 0.35) | 0.06 | <0.001 |
| Exposure → BPA | 0.22 (0.13, 0.31) | 0.05 | <0.001 |
| Exposure → Benzophenone-3 | 0.21 (0.08, 0.34) | 0.05 | 0.001 |
| Exposure → MECPP | 0.46 (0.33, 0.59) | 0.07 | <0.001 |
| Exposure → MnBP | 0.29 (0.20, 0.37) | 0.04 | <0.001 |
| Exposure → MCPP | 0.49 (0.41, 0.58) | 0.04 | <0.001 |
| Exposure → MEHHP | 0.48 (0.37, 0.59) | 0.06 | <0.001 |
| Exposure → MBzP | 0.25 (0.16, 0.33) | 0.04 | <0.001 |
| Exposure → MiBP | 0.27 (0.15, 0.40) | 0.06 | <0.001 |
| NHB → Exposure | −0.20 (−0.31, −0.10) | 0.05 | <0.001 |
| Exposure → T3 | 0.09 (−0.03, 0.21) | 0.06 | 0.13 |
| Creatinine → Perchlorate | 0.56 (0.48, 0.64) | 0.05 | <0.001 |
| Creatinine → BPA | 0.63 (0.52, 0.74) | 0.05 | <0.001 |
| Creatinine → Benzo | 0.28 (0.20, 0.36) | 0.05 | <0.001 |
| Creatinine → MECPP | 0.58 (0.51, 0.66) | 0.03 | <0.001 |
| Creatinine → MnBP | 0.71 (0.66, 0.77) | 0.03 | <0.001 |
| Creatinine → MCPP | 0.67 (0.59, 0.75) | 0.04 | <0.001 |
| Creatinine → MEHHP | 0.58 (0.50, 0.66) | 0.04 | <0.001 |
| Creatinine →MBzP | 0.70 (0.64, 0.75) | 0.03 | <0.001 |
| Creatinine → MiBP | 0.71 (0.66, 0.76) | 0.04 | <0.001 |
| TSH → T4 | −0.23 (−0.35, −0.11) | 0.06 | <0.001 |
| T4 → T3 | 0.53 (0.43, 0.62) | 0.05 | <0.001 |
| Age → T3 | −0.31 (−0.42, −0.20) | 0.06 | <0.001 |
| Age → TSH | 0.18 (0.10, 0.25) | 0.04 | <0.001 |
| Residual variances | |||
| e.Perchlorate | 0.63 (0.54, 0.72) | 0.05 | <0.001 |
| e. BPA | 0.56 (0.45, 0.68) | 0.06 | <0.001 |
| e. Benzophenone-3 | 0.88 (0.81, 0.96) | 0.04 | <0.001 |
| e. MECPP | 0.47 (0.31, 0.60) | 0.08 | <0.001 |
| e. MnBP | 0.42 (0.35, 0.50) | 0.04 | <0.001 |
| e. MCPP | 0.33 (0.21, 0.42) | 0.05 | <0.001 |
| e. MEHHP | 0.46 (0.31, 0.57) | 0.06 | <0.001 |
| e. MBzP | 0.47 (0.39, 0.56) | 0.04 | <0.001 |
| e. MiBP | 0.44 (0.37, 0.52) | 0.04 | <0.001 |
| e.TSH | 0.97 (0.92, 0.97) | 0.01 | <0.001 |
| e.T3 | 0.61 (0.57, 0.69) | 0.03 | <0.001 |
| e.T4 | 0.95 (0.93, 1.00) | 0.03 | <0.001 |
| e. Exposure | 0.96 (0.91, 1.00) | 0.02 | <0.001 |
| Covariance | |||
| MEHPP ↔ MECPP | 0.90 (0.87, 0.93) | 0.01 | <0.001 |
| MnBP ↔ MiBP | 0.43 (0.35, 0.51) | 0.04 | <0.001 |
| MnBP ↔ MBzP | 0.37 (0.28, 0.46) | 0.05 | <0.001 |
| MBzP ↔ MiBP | 0.28 (0.19, 0.37) | 0.05 | <0.001 |
| BMI ↔ T4 | 0.15 (0.08, 0.22) | 0.04 | <0.001 |
| BMI ↔ Benzophenone-3 | −0.08 (−0.16, −0.01) | 0.04 | 0.03 |
| Creatinine ↔ Age | −0.33 (−0.40. −0.27) | 0.03 | <0.001 |
| Creatinine ↔ NHB | 0.18 (0.12, 0.24) | 0.03 | <0.001 |
Table 5.
Comparative Fit Index (CFI), Tucker Lewis Index (TLI), Standardize Root Mean Square Residual (SRMR), Root Mean Square Error of Approximation (RMSEA), and 90% Confidence Interval (90% CI) SRMR for Figures 2 and 3.
Discussion
In this cross-sectional study of the U.S. population, we observed that exposure to multiple EDCs was associated with modest differences in THs in both men and women aged 12 years and older. Specifically, exposure to multiple EDCs was signifcantly negatively associated with Total T4 in males but not in females. Multiple EDCs were associated with higher T3 levels in females although there is a possibility that this observation is due to chance. These observations support the hypothesis that exposure to multiple EDCs may alter thyroid hormone levels in the general population, but in a sex-specific manner. However, the clinical relevance of these findings to thyroid disease requires further study.
It is difficult to compare the magnitudes of the estimates of EDCs on thyroid hormone levels observed in our study with previous studies because we used a latent variable to capture the body burden of available EDCs which yields a mixture. This approach is unlike previous studies that describe EDCs body burden using geometric means or medians of individual compounds. In our analysis, the magnitidue of an EDC in SEM is relative to the magnitude of the other compound loading onto the latent variable. Conclusions can be made, however, on the presence or absence of EDCs between models and strengths of loadings between individual EDCs within each model. For instance, triclosan and MEP did not significantly load onto the latent variable in either the male or female model. DEP (parent compound of MEP) is found in personal care products like scents or shampoos, medications, and industrial solvent [8]. Triclosan is an antimicrobial agent used in personal care products, such as toothpaste, or in kitchenware and furniture [55]. Failure to signficantly load on the latent variable could represent lack of exposure to triclosan and DEP because these compounds have different sources than the other EDCs which did signficanlty load onto the latent variable. It is also possible that these compounds were excreted quickly in the urine and exposure was not accurately captured in the spot urine sample collected by NHANES [56, 57]. Benzophenone-3 loaded on the latent variable in females but not in males. This is not suprising given that benzophenone-3 is a sunscreen agent and females have higher exposure to benzophenone-3 compared to males [58] and research indicates that the body burden of enviornmental chemicals differ by sex [59-61]. It is worth mentioning that our analysis provides no information on the association between thyroid hormone levels and the EDCs that did not load on the latent variable Exposure.
It is possible that the loading of each EDC on the latent variable Exposure reflects compounds that come from similar sources and the metabolism of each parent compound. For instance, high molecular weight phthalates, such as DEHP and di-n-octyl phthalate (DOP), are used in vinyl products like toys, furniture or upholstery, building material, food packaging, food production machinary, and tubing [8, 62-65]. In the male SEM model, MCPP and MEHHP have high molecular weight parent compounds of DOP and DEHP, respectively. Both MCPP and MEHHP had almost identifical loadings on the latent variable Exposure. This is not suprising given that their parent compounds potentially have similair sources of exposure. Moreover, MECPP which shares the same parent compound of DEHP with MEHHP, loaded less strongly on the latent variable Exposure than MEHHP. Yet metabolism studies show that DEHP metabolizes quickly to MEHHP which is the most abudant metabolite after an administered dose with MECPP becoming the primary metabolite approximately 12 hours after exposure [66]. This suggests that the observed loadings in our model may represent body burden at the time when the urinary spot sample was collected. Finally, the low molecular weight phthalates (1 to 4 carbons on ester side chains), represented by metabolites MnBP, MBzP, MiBP, are found in personal care products, like cosmetics, adhesives and caulks. Differences in loadings between low molecular weight phthalates may be explained through differences in sources of parent phthalates. Specifically, DBP and diisobutyl phthalate (DiBP) parent compounds of MnBP and MiBP, respectively are found in caulks, cosmetics industrial sovlents and adhesives. While butyl benzyl phthalate, parent compound of MBzP, is found in adhesives, industrial solvents, sealants and vinyl flooring [8, 64, 67]. Perchlorate and BPA loaded to a lesser degree on the latent variable, suggesting that these compounds may not contribute as much as phthalates to the body burden of EDCs in males.We observed a similair pattern for high molecular weight phthalates having greater loadings than low molecular weight phthalates in the female SEM model. Although it was suprising that low molecular weight phthalates did not represent a greater contribution to the body burden of EDCs given that they are frequently found in cosmetics [8].
Based on the known relationships between thyroid hormones, we observed the expected inverse association between TSH and T4 and positive association between T4 and T3 for both females and males. This makes biological sense because TSH signals the production of T4, and T4 is converted to T3. However, the relationship between the latent variable Exposure and THs differed by sex where higher levels of Exposure were associated with lower levels of T4 in males. Whereas, higher levels of Exposure were associated with higher levels of T3 in females although the strength of this association was weaker and may be due to chance. While these associations support the hypothesis that exposure to multiple EDCs influences thyroid hormone levels at a population level, the clinical relevance of these effects is unknown. TSH levels are used to screen for hypothyroidism or hyperthyroidism. This is because subclinical thyroid disease can have elevated or diminished TSH levels and normal T3 and T4 levels [68]. Although our findings are not necessarily informative about the risk of thyroid disease, the observed associations between the latent variable Exposure, T4, and T3 lends biological plausibility to the potential for low level EDCs exposure to disrupt the complex feedback mechanisms between thyroid hormones.
Although limited knowledge exists on how EDCs can disrupt the thyroid feedback loop, it is possible that the synthesis of thryoid hormones after exposure to EDCs is compromised. This would result in the decreased production of T4 which could explain the negative association we observed between Exposure and T4 in males. This theory is based on data from experimental studies which show that perchlorate, phthalates, and phenols can disrupt production by interfering with the sodium-iodide symporter (NIS) and thyroid peroxidase (TPO) enzyme [69-71]. Further ways in which T4 levels can be impacted is through disruption of liver function, since the conversion of T3 to T4 takes place mainly in the liver. For instance, male rats exposed to DBP, the parent compound of MnBP, exhibited increased liver weight, typically associated with liver enzyme induction and decreased T4 blood levels [25], which is consistent with our observed negative association of Exposure and T4 in males.
Since we investigated the effects of EDC mixtures on THs separately for males and females, we were limited in the ability to directly compare the directionality and magnitude of the effect estimates of the latent variable on all THs in each sex. The models suggests a sex-specific response between the latent variable and THs, however the models are not identical and are made from different subpopulations so we cannot be certain that the observed associations between the latent Exposure variable and THs differs by sex. However, there are several possible explainations for sex-specific associations between Exposure and TH levels. First, the differences in loadings of individual EDCs on the latent variable between males and females could result in different EDC mixtures, and subsquently different associations between Exposure and THs. Second, hormonal loads [72] and the binding capacity of thyroid hormones may differ by sex [73], which could affect the relationships between EDCs and THs.
This study has several strengths, including the use of a large nationally representative population which allowed us to control for multiple confounders. By utilizing SEM we were able to extend on previous research by modelling multiple EDCs and examine the associations between environmentally-relevant EDC mixtures and THs. Also, NHANES employs a rigorous quality control program which provides high quality environmental and biological measurements. There were also limitations to this study. While SEM models can be used to support causal inference especially when used in longitudinal analyses with repeated biomarker exposure measurements, the cross-sectional nature of this analysis precludes causal assumption. Although the results from this study support the hypothesis that environmentally-relevant EDC mixtures could affect thyroid homeostasis. Furthermore, the urinary biomarkers used in this analysis are non-persistent and represent relatively recent exposures and that spot urine samples provide only a moderate representation of long term exposure for perchlorates, phthalates, and BPA [3, 74, 75]. Another limitation is due to data constraints because NHANES does not measure all EDCs in the same individual. Thus we could only examine a limited number of EDCs and could not examine interactions with other chemicals which might play a synergistic or antagonistic role in the association between THs. In addition, our approach did not take into consideration interactions between biomarkers or other covariates even though SEM models can be used to model interactions. Finally, these findings may not be representative of the general population because the male subpopulation had a different racial composition, and the female subpopulation was more likely to be older, overweight, had more than one birth, and have a different racial composition compared to population that did not have missing data.
Conclusion
Our study determined that exposure to multiple EDCs was associated with alterations in TH serum concentrations and that these associations supported a sex-specific exposure response. By incorporating exposure to mixtures of environmental chemicals we were able to model a more realistic exposure scenario. This exposure scenario showed a negative exposure-response relationship between multiple endocrine disrupting compounds and T4 among males. Future works should focus on modelling additional EDCs and other environmental chemicals which may have an additive, synergistic, or antagonistic effects on EDC’s disruption of thyroid homeostasis. Furthermore, it is important that future studies integrate dietary or consumer habits that identify sources of EDC exposure and the resultant body burdens, as well as, estimate the effect of EDC mixtures on thyroid homeostasis and risk of thyroid disease to inform risk assessment and policy decisions.
Highlights.
A structural equation model depicting the relationship between several endocrine disrupting compounds and thyroid hormones is proposed.
A more realistic exposure scenario was modeled by incorporating exposure to mixtures of environmental chemicals.
A negative exposure-response relationship between multiple endocrine disrupting compounds and T4 among males was observed.
Acknowledgements
The authors would like to thank Dr. Robert Tanguay for his input on study design. This work was supported by grants from the US National Institute of Environmental Health Sciences T32 ES007060 and P30 ES000210.
List of abbreviations
- BMI
body mass index
- BPA
bisphenol A
- CDC
Center for Disease Control
- CFI
Comparative Fit Index
- CI
Confidence intervals
- DBP
dibutyl phthalates
- DEHP
di(2-ethylhexyl) phthalate
- ESI
electrospray ionization
- EDCs
endocrine disrupting chemicals
- GC-MS
gas chromatograph-mass spectrometry
- HPLC
high performance liquid chromatograph
- HMW
High-molecular weight
- IC
ion chromatography
- LOD
limit of detection
- LMW
Low-molecular weight
- MA
Mexican American
- MBzP
mono-benzyl phthalate
- MCMC
Markov Chain Monte Carlo
- MCPP
mono-(3-carboxypropyl) phthalate
- MECPP
mono-2ethyl5carboxypentyl phthalate
- MEHHP
mono(2ethyl5hydroxyhexyl) phthalate
- MEHP
mono(2-ethylhexyl)phthalate
- MEP
mono-ethyl phthalate
- MiBP
mono-isobutyl phthalate
- MnBP
mono-n-butyl phthalate
- NCHS
National Center for Health Statistics
- NHW
non-Hispanic White
- NHB
non-Hispanic Black
- NIS
Sodium-iodide symporter
- OH
Other Hispanic
- PIR
poverty index ratio
- RMSEA
Root Mean Square Error of Approximation
- SEM
Structural equation modelling
- SRMR
Standardized Root Mean Square Residual
- THs
Thyroid hormones
- TLI
Tucker-Lewis Index
- TPO
Thyroid peroxidase
- TR
Thyroid hormone receptor
- TRH
Thyrotropin releasing hormone
- TSH
Thyroid stimulating hormone
- T3
Triiodothyronine
- T4
Thyroxine
- TTR
Transthyretin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zoeller TR: Environmental chemicals targeting thyroid. Hormones (Athens). 2010, 9:28–40. [DOI] [PubMed] [Google Scholar]
- 2.Schug TT, Janesick A, Blumberg B, Heindel JJ: Endocrine disrupting chemicals and disease susceptibility. The Journal of steroid biochemistry and molecular biology. 2011, 127:204–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hauser R, Calafat AM: PHTHALATES AND HUMAN HEALTH. Occupational and Environmental Medicine. 2005, 62:806–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koniecki D, Wang R, Moody RP, Zhu J: Phthalates in cosmetic and personal care products: concentrations and possible dermal exposure. Environmental research. 2011, 111:329–336. [DOI] [PubMed] [Google Scholar]
- 5.Sandborgh-Englund G, Adolfsson-Erici M, Odham G, Ekstrand J: Pharmacokinetics of triclosan following oral ingestion in humans. Journal of Toxicology and Environmental Health, Part A. 2006, 69:1861–1873. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez H, Farbrot A, Larkö O, Wennberg AM: Percutaneous absorption of the sunscreen benzophenone-3 after repeated whole-body applications, with and without ultraviolet irradiation. British Journal of Dermatology. 2006, 154:337–340. [DOI] [PubMed] [Google Scholar]
- 7.Gustavsson Gonzalez H, Farbrot A, Larkö O: Percutaneous absorption of benzophenone-3, a common component of topical sunscreens. Clinical and experimental dermatology. 2002, 27:691–694. [DOI] [PubMed] [Google Scholar]
- 8.NRC: Phthalates and Cumulative Risk Assessment: The Tasks Ahead. Washington (DC): National Academies Press; 2008. [PubMed] [Google Scholar]
- 9.Sáiz J, Gómara B: Evaluation of endocrine disrupting compounds migration in household food containers under domestic use conditions. Journal of agricultural and food chemistry. 2017, 65:6692–6700. [DOI] [PubMed] [Google Scholar]
- 10.Lampel HP, Jacob SE: Phthalates in baby skin care products. Dermatitis. 2011, 22:272–276. [DOI] [PubMed] [Google Scholar]
- 11.Wang C, Zhai Y, Zhu Y, Li X, Li C, Zeng G: Concentration and Exposure Evaluation of Perchlorate in Size-Segregated Airborne Particulate Matter from Changsha, China. Water, Air, & Soil Pollution. 2017, 228:369. [Google Scholar]
- 12.Liao C, Kannan K: A survey of alkylphenols, bisphenols, and triclosan in personal care products from China and the United States. Archives of environmental contamination and toxicology. 2014, 67:50–59. [DOI] [PubMed] [Google Scholar]
- 13.Boas M, Feldt-Rasmussen U, Skakkebæk NE, Main KM: Environmental chemicals and thyroid function. European Journal of Endocrinology. 2006, 154:599–611. [DOI] [PubMed] [Google Scholar]
- 14.Wolff J: Perchlorate and the thyroid gland. Pharmacological reviews. 1998, 50:89–106. [PubMed] [Google Scholar]
- 15.Zorrilla LM, Gibson EK, Jeffay SC, Crofton KM, Setzer WR, Cooper RL, Stoker TE: The Effects of Triclosan on Puberty and Thyroid Hormones in Male Wistar Rats. Toxicological Sciences. 2009, 107:56–64. [DOI] [PubMed] [Google Scholar]
- 16.Thyroid Hormone Toxicity [http://emedicine.medscape.com/article/819692-overview#showall]
- 17.Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, Braverman LE: Serum TSH, T4, and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). The Journal of Clinical Endocrinology & Metabolism. 2002, 87:489–499. [DOI] [PubMed] [Google Scholar]
- 18.Chan S, Kilby MD: Thyroid hormone and central nervous system development. Journal of Endocrinology. 2000, 165:1–8. [DOI] [PubMed] [Google Scholar]
- 19.Koibuchi N, Chin WW: Thyroid Hormone Action and Brain Development. Trends in Endocrinology & Metabolism. 2000, 11:123–128. [DOI] [PubMed] [Google Scholar]
- 20.Boelaert K, Franklyn J: Thyroid hormone in health and disease. Journal of Endocrinology. 2005, 187:1–15. [DOI] [PubMed] [Google Scholar]
- 21.Hinton RH, Mitchell FE, Mann A, Chescoe D, Price SC, Nunn A, Grasso P, Bridges JW: Effects of phthalic acid esters on the liver and thyroid. Environmental health perspectives. 1986, 70:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Howarth JA, Price SC, Dobrota M, Kentish PA, Hinton RH: Effects on male rats of di-(2-ethylhexyl) phthalate and di-n-hexylphthalate administered alone or in combination. Toxicology letters. 2001, 121:35–43. [DOI] [PubMed] [Google Scholar]
- 23.Mitchell FE, Price SC, Hinton RH, Grasso P, Bridges JW: TIME AND DOSE-RESPONSE STUDY OF THE EFFECTS ON RATS OF THE PLASTICIZER DI(2-ETHYLHEXYL) PHTHALATE. Toxicology and Applied Pharmacology. 1985, 81:371–392. [DOI] [PubMed] [Google Scholar]
- 24.Price SC, Chescoe D, Grasso P, Wright M, Hinton RH: Alterations in the thyroids of rats treated for long periods with di-(2-ethylhexyl) phthalate or with hypolipidaemic agents. Toxicology letters. 1988, 40:37–46. [DOI] [PubMed] [Google Scholar]
- 25.O'Connor JC, Frame SR, Ladics GS: Evaluation of a 15-day screening assay using intact male rats for identifying antiandrogens. Toxicological sciences. 2002, 69:92–108. [DOI] [PubMed] [Google Scholar]
- 26.Zoeller RT, Bansal R, Parris C: Bisphenol-A, an Environmental Contaminant that Acts as a Thyroid Hormone Receptor Antagonist in Vitro, Increases Serum Thyroxine, and Alters RC3/Neurogranin Expression in the Developing Rat Brain. Endocrinology. 2005, 146:607–612. [DOI] [PubMed] [Google Scholar]
- 27.Crofton KM, Paul KB, DeVito MJ, Hedge JM: Short-term in vivo exposure to the water contaminant triclosan: Evidence for disruption of thyroxine. Environmental Toxicology and Pharmacology. 2007, 24:194–197. [DOI] [PubMed] [Google Scholar]
- 28.Meeker JD, Ferguson KK: Relationship between Urinary Phthalate and Bisphenol A Concentrations and Serum Thyroid Measures in US Adults and Adolescents from the National Health and Nutrition Examination Survey (NHANES) 2007-2008. Environmental Health Perspectives. 2011, 119:1396–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blount BC, Pirkle JL, Osterloh JD, Valentin-Blasini L, Caldwell KL: Urinary perchlorate and thyroid hormone levels in adolescent and adult men and women living in the United States. Environmental health perspectives. 2006:1865–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allmyr M, Panagiotidis G, Sparve E, Diczfalusy U, Sandborgh-Englund G: Human Exposure to Triclosan via Toothpaste does not change CYP3A4 Activity or Plasma Concentrations of Thyroid Hormones. Basic & Clinical Pharmacology & Toxicology. 2009, 105:339–344. [DOI] [PubMed] [Google Scholar]
- 31.Braverman LE, Pearce EN, He X, Pino S, Seeley M, Beck B, Magnani B, Blount BC, Firek A: Effects of six months of daily low-dose perchlorate exposure on thyroid function in healthy volunteers. The Journal of Clinical Endocrinology & Metabolism. 2006, 91:2721–2724. [DOI] [PubMed] [Google Scholar]
- 32.Trzeciakowski JP, Gardiner L, Parrish AR: Effects of environmental levels of cadmium, lead and mercury on human renal function evaluated by structural equation modeling. Toxicology letters. 2014, 228:34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gore AC, Chappell V, Fenton S, Flaws JA, Nadal A, Prins GS, Toppari J, Zoeller R: EDC-2: the Endocrine Society's second scientific statement on endocrine-disrupting chemicals. Endocrine reviews. 2015, 36:E1–E150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.NHANES 2003-2004 Dietary Data [http://wwwn.cdc.gov/nchs/nhanes/search/datapage.aspx?Component=Dietary&CycleBeginYear=2003]
- 35.National Health and Nutrition Examination Survey Questionnaires, Datasets and Related Docmentation [http://www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm]
- 36.Strbak V, Macho L, Uhercik D, Kliment V: The effect of lactation on thyroid activity of women. Endokrinologie. 1978, 72:183–187. [PubMed] [Google Scholar]
- 37.Johnson C, Paulose-Ram R, Ogden C: National Health and Nutrition Examination Survey: Analytic guidelines, 1999-2010 In Vital Health Stat (Statistics NCfH ed., vol. 2; 2013. [PubMed] [Google Scholar]
- 38.Laboratory Procedure Manual Analyte: Benzophenone-3, bisphenol A, 2,4-dichlorophenol, 2,5-dichlorophenol, ortho-phenylphenol, methyl-, ethyl-, propyl-, and butyl parabens, 4-tertoctylphenol, 2,4,5-trichlorophenol, 2,4,6-trichlorophenol, triclosan [http://www.cdc.gov/nchs/data/nhanes/nhanes_05_06/eph_d_met_phenols_parabens.pdf]
- 39.CDC.: Laboratory Procedure Manual: Phthalate Metabolites. 2010; 2010. [Google Scholar]
- 40.Perchlorate, Nitrate & Thiocyanate - Urine (PERNT_E) [https://wwwn.cdc.gov/Nchs/Nhanes/2007-2008/PERNT_E.htm]
- 41.2007-2008 Documentation, Codebook, and Frequences for Thyroid Profile [http://wwwn.cdc.gov/nchs/nhanes/2007-2008/THYROD_E.htm]
- 42.Benowitz NL: Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiologic reviews. 1996, 18:188–204. [DOI] [PubMed] [Google Scholar]
- 43.Pedrelli M, Pramfalk C, Parini P: Thyroid hormones and thyroid hormone receptors: effects of thyromimetics on reverse cholesterol transport. World journal of gastroenterology: WJG. 2010, 16:5958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anthropometry Procedures Manual [http://www.cdc.gov/nchs/data/nhanes/nhanes_07_08/manual_an.pdf]
- 45.Boeniger MF, Lowry LK, Rosenberg J: Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: a review. The American Industrial Hygiene Association Journal. 1993, 54:615–627. [DOI] [PubMed] [Google Scholar]
- 46.PubChem Compound Database; CID=3026 [https://pubchem.ncbi.nlm.nih.gov/compound/3026]
- 47.CDC: Fourth National Report on Human Exposure to Environmental Chemicals. Department of Health and Human Services: Center for Disease Control and Prevention; 2009. [Google Scholar]
- 48.Carwile JL, Michels KB: Urinary bisphenol A and obesity: NHANES 2003-2006. Environmental Research. 2011, 111:825–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Elobeid MA, Padilla MA, Brock DW, Ruden DM, Allison DB: Endocrine disruptors and obesity: an examination of selected persistent organic pollutants in the NHANES 1999-2002 data. International journal of environmental research and public health. 2010, 7:2988–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hatch EE, Nelson JW, Qureshi MM, Weinberg J, Moore LL, Singer M, Webster TF: Association of urinary phthalate metabolite concentrations with body mass index and waist circumference: a cross-sectional study of NHANES data, 1999-2002. Environ Health. 2008, 7:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Knudsen N, Laurberg P, Rasmussen LB, Bülow I, Perrild H, Ovesen L, Jørgensen T: Small differences in thyroid function may be important for body mass index and the occurrence of obesity in the population. The Journal of Clinical Endocrinology & Metabolism. 2005, 90:4019–4024. [DOI] [PubMed] [Google Scholar]
- 52.Milionis A, Milionis C: Correlation between body mass index and thyroid function in euthyroid individuals in Greece. ISRN biomarkers. 2013, 2013. [Google Scholar]
- 53.MacCallum RC, Browne MW, Sugawara HM: Power analysis and determination of sample size for covariance structure modeling. Psychological methods. 1996, 1:130. [Google Scholar]
- 54.Ye X, Zhou X, Hennings R, Kramer J, Calafat AM: Potential external contamination with bisphenol A and other ubiquitous organic environmental chemicals during biomonitoring analysis: an elusive laboratory challenge. Environmental Health Perspectives (Online). 2013, 121:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Calafat AM, Ye X, Wong L, Reidy JA, Needham LL: Urinary concentrations of triclosan in the US population: 2003-2004. Environmental health perspectives. 2008, 116:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kanetoshi A, Ogawa H, Katsura E, Okui T, Kaneshima H: Disposition and excretion of Irgasan® DP300 and its chlorinated derivatives in mice. Archives of environmental contamination and toxicology. 1988, 17:637–644. [DOI] [PubMed] [Google Scholar]
- 57.Concise International Chemical Assessment Document (CICADS) 52: Diethyl Phthalate [http://www.inchem.org/documents/cicads/cicads/cicad52.htm]
- 58.Calafat AM, Wong L-Y, Ye X, Reidy JA, Needham LL: Concentrations of the sunscreen agent benzophenone-3 in residents of the United States: National Health and Nutrition Examination Survey 2003-2004. Environmental health perspectives. 2008, 116:893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gochfeld M: Gender in toxicology and risk assessment. Environmental Research. 2007, 104:1. [Google Scholar]
- 60.Vahter M, Åkesson A, Lidén C, Ceccatelli S, Berglund M: Gender differences in the disposition and toxicity of metals. Environmental Research. 2007, 104:85–95. [DOI] [PubMed] [Google Scholar]
- 61.Vahter M, Gochfeld M, Casati B, Thiruchelvam M, Falk-Filippson A, Kavlock R, Marafante E, Cory-Slechta D: Implications of gender differences for human health risk assessment and toxicology. Environmental research. 2007, 104:70–84. [DOI] [PubMed] [Google Scholar]
- 62.Public Health Statement: Lead [http://www.atsdr.cdc.gov/ToxProfiles/tp13-c1-b.pdf]
- 63.Toxicological Profile for Di-n-Octylphthalate. [ http://www.atsdr.cdc.gov/toxprofiles/tp95.pdf]
- 64.Toxicological Profile for Di(2-ethylhexyl)phthalate. [ http://www.atsdr.cdc.gov/toxprofiles/tp9.pdf] [PubMed]
- 65.NICNAC: Dimethyl Phthalate. Existing Chemical Hazard Assessment Report. . (Ageing DoHa ed.; 2008. [Google Scholar]
- 66.Calafat AM, McKee RH: Integrating Biomonitoring Exposure Data into the Risk Assessment Process: Phthalates [Diethyl Phthalate and Di(2-ethylhexyl) Phthalate] as a Case Study. Environmental Health Perspectives. 2006, 114:1783–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Toxicological Profile for Diethyl Phthalate. [ http://www.atsdr.cdc.gov/toxprofiles/tp73.pdf]
- 68.Hypothyroidism and Hyperthyroidism [http://www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/endocrinology/hypothyroidism-and-hyperthyroidism/]
- 69.Breous E, Wenzel A, Loos U: The promoter of the human sodium/iodide symporter responds to certain phthalate plasticisers. Molecular and cellular endocrinology. 2005, 244:75–78. [DOI] [PubMed] [Google Scholar]
- 70.Schmutzler C, Bacinski A, Gotthardt I, Huhne K, Ambrugger P, Klammer H, Schlecht C, Hoang-Vu C, Grüters A, Wuttke W: The ultraviolet filter benzophenone 2 interferes with the thyroid hormone axis in rats and is a potent in vitro inhibitor of human recombinant thyroid peroxidase. Endocrinology. 2007, 148:2835–2844. [DOI] [PubMed] [Google Scholar]
- 71.Tonacchera M, Pinchera A, Dimida A, Ferrarini E, Agretti P, Vitti P, Santini F, Crump K, Gibbs J: Relative potencies and additivity of perchlorate, thiocyanate, nitrate, and iodide on the inhibition of radioactive iodide uptake by the human sodium iodide symporter. Thyroid. 2004, 14:1012–1019. [DOI] [PubMed] [Google Scholar]
- 72.Ahmed Z, Khan MA, Haq A, Rehman S: Effect of race, gender and age on thyroid and thyroid stimulating hormone levels in north west frontier province Pakistan. J Ayub Med Coll. 2009, 21. [PubMed] [Google Scholar]
- 73.Braverman LE, Foster AE, Ingbar IH: Sex-Related Differences in the Binding in Serum of Thyroid Hormones. The Journal of Clinical Endocrinology & Metabolism. 1967, 27:227–232. [DOI] [PubMed] [Google Scholar]
- 74.Mahalingaiah S, Meeker JD, Pearson KR, Calafat AM, Ye X, Petrozza J, Hauser R: Temporal variability and predictors of urinary bisphenol A concentrations in men and women. Environmental Health Perspectives. 2008, 116:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mervish N, Blount B, Valentin-Blasini L, Brenner B, Galvez MP, Wolff MS, Teitelbaum SL: Temporal variability in urinary concentrations of perchlorate, nitrate, thiocyanate and iodide among children. Journal of Exposure Science and Environmental Epidemiology. 2012, 22:212–218. [DOI] [PMC free article] [PubMed] [Google Scholar]