Abstract
Introduction
Early supported discharge (‘step-down’) and prevention of admission (‘step-up’) services require safe medicines reconciliation. Medication discrepancies at transfer of care are a potential cause of patient harm. There is currently no published work examining level of medication discrepancies and associated risk in this setting.
Objectives
Working within a ‘step-up’ and ‘step-down’ integrated service based in the community, caring for patients in their home to:
▸ quantify the number of medicines discrepancies and allergy status discrepancies
▸ ascertain the type of discrepancies
▸ assess the potential for harm caused by these discrepancies.
Methods
Medicines reconciliation was performed by two pharmacists for patients within the ‘step-up’ and ‘step-down’ service as well as patients recently discharged from hospital to a care home. The resulting medication history was compared with the original medication history documented in the service's notes. Allergy status and medication discrepancies were identified and the type of discrepancy was recorded. National Patient Safety Agency (NPSA) risk assessment was used to categorise the level of risk of each discrepancy.
Results
20 out of the 54 patients (37%) did not have an allergy status recorded at baseline. Of the 573 medications listed for patients reviewed following medicines reconciliation, 317 (55%) had a medication discrepancy. There was an average of 5.87 (95% CI 4.53 to 7.18) medication discrepancies per patient of which 49% were classified (NPSA) as moderate, high or extreme risk.
Conclusions
There was a high level of medication discrepancies in this service with implications for patient safety and cost. Such services would benefit from pharmacist-led medicines reconciliation.
Keywords: Medication errors, Pharmacists, CLINICAL PHARMACY, Review Drug-use, Community health services
EAHP Statement 4: Clinical Pharmacy Services.
EAHP Statement 5: Patient Safety and Quality Assurance
Introduction
It is known that there is lack of accurate and complete information about patients' medicines when their care is transferred between healthcare settings. Estimates suggest that between 30% and 70% of patients have either an error or an unintentional change to their medicines when care is transferred.1 Hence, a significant number of medicines-related incidents occur when patients transfer between care settings.2 The literature suggests that 6%–10% of admissions have a medicines-related contribution due to adverse drug events.3 4 Hospitals in the UK are expected to comply with relevant standards5 and guidance.2 National Institute for Health and Care Excellence (NICE) guidance states that medicines reconciliation in an acute setting should be carried out within 24 hours, or sooner if clinically necessary, when the person moves from one care setting to another.6
Recent years have seen the rise of early supported discharge (‘step-down’) and prevention of admission (‘step-up’) integrated services based in the community, which aim to reduce inappropriate hospital admission and enable early hospital discharge. The service in this pilot is one such example where the service accepts referrals from general practitioners (GPs) and specialist healthcare professionals through a single point of access. The multidisciplinary team of approximately 150 staff offers a range of healthcare, rehabilitation and re-enablement services for patients across a locality including:
Rapid response service: a multidisciplinary, holistic assessment for patients in urgent need of care and at risk of admission into hospital. Patients are seen in their home or in the accident and emergency department (A&E), within 2 hours of referral by two members of the clinical team. They are then followed up in their home over the required number of days.
Early supported discharge: hospital-at-home services in the community are provided by suitably skilled team members facilitating early discharge for patients in hospital.
Short-term rehabilitation: neurological and general rehabilitation at home is available, together with a falls prevention service.
There is currently minimal medicines reconciliation performed by the members of the multidisciplinary team that visit the patients (includes nurses, paramedics, occupational therapists, physiotherapists and dieticians; none of whom are prescribers) and without pharmacist input.7
Traditionally, services addressing medication errors at transfer of care have focused on the model of moving from primary care (general practice) to secondary care (hospital) and vice versa. To the best of the authors' knowledge, there is no published work that looks at medication errors during transfer to ‘step-up’ and ‘step-down’ integrated community services, where patients are cared for in their home. Given the expected rise in use of these types of services,8 it is important to specifically evaluate the level of errors in patients' medication when their care is transferred to such services.
Aim
To explore the role of a pharmacist in undertaking medicines reconciliation within a ‘step-up’ and ‘step-down’ integrated service based in the community.
Objectives
For two cohorts of patients seen by the service:
To quantify the number of medicines discrepancies and drug allergy status discrepancies
To ascertain the type of discrepancies
To assess the potential for harm caused by these discrepancies.
Method
This pilot study was undertaken by two pharmacists with postgraduate clinical diplomas. The pharmacists had strong clinical skills and experience in working in both primary and secondary care settings. Both had a background of working in care homes and with GPs and other healthcare professionals to ensure safe and effective prescribing, implementing good medicines management practice and reducing inappropriate polypharmacy.
The pilot was carried out over 8 days (Monday, Wednesday or Thursday) in the 4 weeks between 18 May 2015 and 12 June 2015. This time period was chosen due to staff availability. The initial plan was to review two discrete groups of patients to investigate medicines discrepancies at step-up and step-down as follows:
Group 1 medicines reconciliation on step-up: A&E referrals and GP referrals of patients in the community, to the service
Group 2 medicines reconciliation on step-down: complex discharges from hospital to care homes supported by the service
For group 1, on each day of the pilot, all patients admitted to the service within the previous 24 hours were included and where time allowed, this was expanded to include patients admitted in the last 48 hours. For group 2, complex discharge cases were obtained from the hospital discharge coordinator. These patients were selected on the basis of location (discharged to a care home), complexity in terms of multiple medicines, comorbid conditions and presenting complaint for admission to hospital. These patients were visited by the pharmacist undertaking the pilot at their care home. Four patients who had been recently discharged from hospital who could also benefit from medicines reconciliation support, although not directly referred to the service, were identified at the care homes visited and included in group 2 of the study.
Data collection was undertaken using a specifically designed medicines reconciliation form. This was piloted in five patients, modified and then used and completed for each patient reviewed by the pharmacist. Data collected included reason for referral to the service, drug allergy status including adverse drug reactions, medications and type of discrepancy. All patients included in the pilot received medicines reconciliation according to the process recommended by NICE.6 The outcome of medicines reconciliation resulted in a ‘best possible’ medication history, which was compared with the baseline medication history as documented on the generic clinical information system (GCIS) in group 1 which is the medication list most commonly used by all clinicians in the service. For group 2, the baseline medication history was taken to be that recorded on the medication administration record (MAR) chart, which is used in care homes to administer medication. Temporary MAR charts are normally written by care homes against the hospital discharge letters until a new and updated copy is produced and dispensed by the community pharmacy. Any discrepancies were highlighted to the clinical team and where appropriate recommendations were made. In addition to NICE recommendation, the pharmacists also undertook medication review and medicines optimisation for all patients to provide appropriate pharmaceutical care. This included recommendations to optimise therapy and patient consultation where appropriate.
Outcomes from medicines reconciliation on admission were documented by the pharmacist making an entry of medication history and reconciliation notes on GCIS, highlighting changes from the original, if any.
Each discrepancy identified was then categorised for risk of harm to the patient using the National Patient Safety Agency (NPSA) risk assessment tool.9 A review of 25 discrepancies from five randomly selected patients was undertaken by the two pharmacists and doctor separately and results were compared. The clinicians categorised the risk, taking into account the complete clinical picture which included nature of discrepancy, medicine involved, other medications, presenting complaint, the clinical setting and how these could influence the severity of the potential outcome of the discrepancy and the likelihood of the outcome occurring. Where categorisation varied, consensus was achieved through case discussion. Having agreed the process for categorisation and tested on a sample, a single clinician categorised the rest of the discrepancies.
Results were analysed using Microsoft Excel 2010.
Results
Two hundred and ten patients were seen in the ‘step-up’, rapid response, care pathway (group 1) during the 4 weeks of the data collection period. Pharmacist-led medicines reconciliation was completed for 39 patients in the 8 days over which the pilot was conducted. Pharmacist-led medicines reconciliation was completed for 15 patients (group 2) from the ‘step-down’ pathway (ie, complex discharge and recently discharged to care home). The results below summarise the activities for groups 1 and 2:
Drug allergy status (including adverse drug reactions) documentation
In total, there were 31 discrepancies in allergy status documentation between medication history following medicines reconciliation and the medication history documented on GCIS or the MAR chart. Twenty out of the 54 patients (37%) did not have an allergy status recorded at baseline.
Risk assessment of the allergy status documentation revealed that 15 discrepancies (48%) had a potential for moderate, high or extreme risk of harm to the patient; some examples are highlighted in table 1.
Table 1.
Number of allergy discrepancies, by risk category, with examples
| Risk assessment for discrepancies in allergy records | Number of discrepancies (percentage of total) | Examples |
|---|---|---|
| Low risk | 16 (52) | No entry: NKDA not recorded, intolerances not recorded |
| Moderate risk | 8 (26) | Patient referred with high INR, rash when takes Diltiazem not documented on GCIS |
| High risk | 4 (13) | Asthmatic with previous worsening of symptoms while on atenolol, not documented on GCIS |
| Extreme risk | 3 (10) | Patient with raised inflammatory markers allergic to erythromycin but stated ‘no known drug allergies’ noted on GCIS |
GCIS, generic clinical information system; INR, international normalised ratio; NKDA, no known drug allergies.
Medicines discrepancies
Twelve patients (30.77%) in group 1 had no medication history documented on GCIS, which required clinicians to make decisions without access to a medication list.
In both groups 1 and 2, a total number of 317 discrepancies were identified, that is, an average number of 5.87 (95% CI 4.53 to 7.18) discrepancies per patient. When those patients that had no medication history recorded on GCIS are excluded, the average number of discrepancies per patient is 5.15. A detailed breakdown of patients seen and discrepancies is given in table 2.
Table 2.
Levels of medication discrepancies overall, in group 1 (step-up care) and in group 2 (step-down care and recent hospital discharges to care home)
| Group 1: referred by A&E | Group 1: referred by GP | Group 1 total | Group 2 | Total | |
|---|---|---|---|---|---|
| Number of patients | 14 | 25 | 39 | 15 | 54 |
| Number of patients with a medication history recorded on GCIS for group 1/MAR chart for group 2 | 5 | 22 | 27 | 15 | 42 |
| Number of medications (as per best possible medication history) | 161 | 256 | 417 | 156 | 573 |
| Mean number of medications per patient | 11.5 (range 6 to 21) | 10.24 (range 5 to 21) | 10.69 (range 5 to 21) | 10.4 (range 3 to 22) | 10.61 (range 3 to 22) |
| Total number of medication discrepancies | 160 | 115 | 275 | 42 | 317 |
| Mean discrepancies per patient | 11.43 (range 4 to 22) | 4.6 (range 0 to 13) | 7.05 (range 0 to 22) | 2.8 (range 0 to 8) | 5.87 (range 0 to 22) |
| Mean discrepancies per medication | 0.99 | 0.45 | 0.66 | 0.27 | 0.55 |
A&E, accident and emergency department; GCIS, generic clinical information system; GP, general practitioner; MAR, medication administration record.
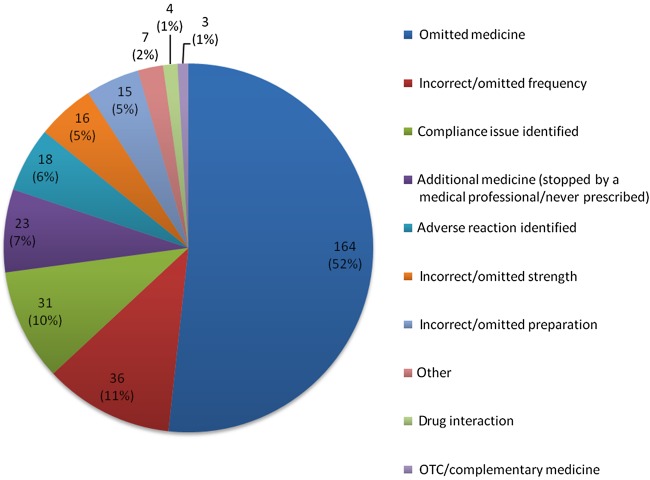
The most common discrepancy found was omitted medicines (164 discrepancies, 52%) which included records which did not have any medication history documented. Incorrect/omitted frequency, compliance issues, additional medicines (stopped by a medical professional/never prescribed) and adverse reaction identified accounted for a total of 28% of discrepancies (figure 1).
Figure 1.
Types of medication discrepancies, number of discrepancies (%).
Risk assessment of medicines discrepancies
A total of 156 (49%) of the discrepancies had a risk of harm that was classified as moderate, high or extreme risk as shown in table 3.
Table 3.
Number of medication discrepancies, by risk category, with examples
| Risk assessment for medication discrepancies | Number of discrepancies (percentage of total) | Example |
|---|---|---|
| Low risk | 161 (51) | Bimatoprost eye-drops omitted from GCIS medicines list |
| Moderate risk | 68 (21) | Syrup formulation omitted for antitubercular drugs on MAR chart |
| High risk | 82 (26) | Prednisolone on GCIS medicines list for a patient presenting with an exacerbation of COPD, when in fact had not been prescribed |
| Extreme risk | 6 (2) | 161 units of Lantus written on MAR chart |
COPD, chronic obstructive pulmonary disease; GCIS, generic clinical information system; MAR, medication administration record.
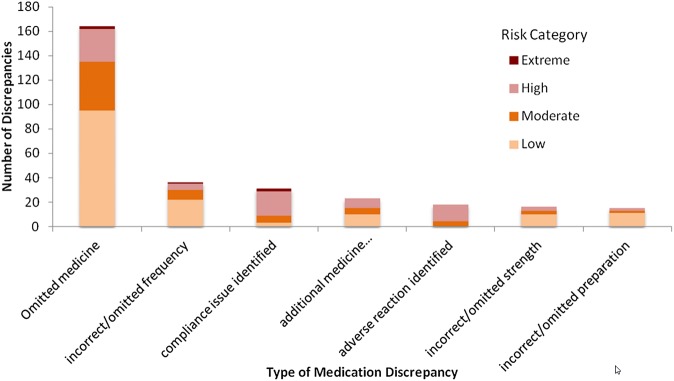
It is worth noting that of these moderate, high and extreme risk discrepancies, 44% (69 discrepancies) were accounted for by omitted medicines, 18% (28 discrepancies) by compliance issues, 11% (17 discrepancies) by adverse reactions and 11% (17 discrepancies) by adverse reaction identified. The breakdown of risk level for the top seven discrepancy areas is displayed in figure 2.
Figure 2.
Numbers of medication discrepancies, by risk category, for the top seven discrepancy areas.
Discussion
Summary of main findings
This study found a high level of both drug allergy status and medication discrepancies. Thirty-seven per cent of patients did not have an allergy status recorded and of those that did there was a discrepancy in 24% of cases. Of the 573 medications listed for patients reviewed following medicines reconciliation, 317 (55%) were subject to a medication discrepancy. In other words, for approximately every two drugs there was one discrepancy identified. There was an average of 5.87 medication discrepancies per patient of which 49% were of moderate, high or extreme risk. The mean medication discrepancies per patient varied greatly between the different groups, being lowest in those recently discharged to a care home. This could be due to these patients being more likely to have recently undergone medicines reconciliation while admitted to hospital. However, even in this population there were notable discrepancies with the mean of 2.8 medication discrepancies per patient. In those patients who were seen by the ‘step-up’ element of the service, group 1, those referred by A&E had the highest level of mean medication discrepancies per patient at 11.43, compared with 4.6 for those referred by their GP. This could be as a result of the referral paperwork from GPs including a copy of currently prescribed medication by the GP, whereas the A&E referrals included the medication history obtained by the referring A&E doctor. Despite these variations in levels of medication discrepancies, in all groups there were high levels of medication discrepancies. Furthermore, it was found in this pilot that 31% of patients had no documented medication history on the electronic medical record used by the service which is detrimental to decision making and as such poses a high risk to patients.
Comparison with existing literature
The importance of conducting accurate and timely medicines reconciliation has been emphasised by NICE guidance6 and NPSA alerts.1 However, the published literature tends to focus on medication reconciliation at the point of admission or discharge to hospital. One such study shows that unintentional variances of 30%–70% occur between the medications charted at a patient's admission and their medication prior to admission.1 This is the first study to examine the level of medication discrepancies in an early supported discharge (‘step-down’) and prevention of admission (‘step-up’) integrated community service, where patients are treated in their home and have not necessarily had a recent hospital admission. The overall average of 5.87 medication discrepancies per patient, which reduces to an average of 5.15 discrepancies per patient, when those without a documented medication history are excluded, is greater than the average of 1.32 found by Dodds in an inpatient setting.10 A possible explanation for the higher level of discrepancy is that the medication history was documented by nurses and other allied healthcare professionals, all of whom were non-prescribers, rather than doctors. The drug history was formed from information obtained from their visit to the patient and where available from the GP referral letter. Staff did not have access to Summary Care Records. Another study examining medication discrepancies on transfer from hospital to a skilled nursing facility for subacute care found that 21.3% of medications reviewed had a discrepancy,11 again this is lower than the level of discrepancy found in this pilot where 55% of medications from the best possible medication history had a discrepancy.
Evidence is lacking from the community setting in which this pilot was conducted. However, studies in inpatient settings demonstrate that 10% of patients are admitted to hospital due to adverse drug events and approximately 5% of these are preventable medicines-related issues.4 Furthermore, medication errors are one of the leading causes of injury to hospital patients, and medication chart reviews reveal that over half of all hospital medication errors occur at the interfaces of care.12 More than 40% of medication errors are believed to result from inadequate reconciliation in handovers during admission, transfer and discharge of patients of which approximately 20% are believed to result in patient harm.13 14
The potential outcomes from medication discrepancies identified through medicines reconciliation have important clinical, economic and humanistic consequences. Errors in drug allergy documentation and medicines reconciliation may result in serious harm and while this is rare, the consequences can be fatal. The potential economic impact includes reduced clinical benefit to patients, leading to increased healthcare utilisation as well as risk to trusts from litigation following errors. Further research is needed to explore these areas.
Strengths and limitations
Limitations to this study include the sample size being small and that patients were not selected randomly. This is because pharmacists were limited to the number of patients that could be reviewed and the number of certain days the study could be carried out over as this was a pilot. Furthermore, in the ‘step-down’ arm of the study, patients not seen by the service but recently discharged to a care home were also included. The pilot was carried out in the summer the effect of the higher number of referrals over the winter months could not be taken into account. The risk assessment was performed by clinicians involved in the pilot. Ideally independent clinicians, not involved in the data collection or the care of the patients, would have performed the risk assessment.
Strengths include that the two main referral sources for patients to the ‘step-up’ arm of the study were well represented in the sample. Referrals from the ambulance service were not represented but these form a small minority of the patients seen by the service.
A further strength of the study is that two pharmacists and a doctor were involved in the risk assessment of the identified discrepancies. They initially performed the risk assessment separately on a smaller sample and then checked for agreement, before proceeding to complete the risk assessment for the rest of the study, therefore making the judgement used for risk assessment more robust. However, limitations to this are that the pharmacists performed the data collection, the doctor was involved in the care of the patients and there are no figures available as to how many of the risk assessments there was initial disagreement upon.
The results of this pilot suggest the need for further studies to confirm the findings.
Implications
The high level of medication discrepancies found in this study has patient safety and cost implications especially considering that such services tend to be used by patients who due to age, comorbidities and polypharmacy are already at high risk of medication adverse effects.
The current study examined medication discrepancies at the point of admission to a ‘step-up’ and ‘step-down’ service based in the community. Future work could include exploring medication changes made by the service and how these are communicated to the GP at discharge and subsequently the level of medication discrepancies after discharge from such services.
Currently, the service that is the subject of this study does not include a pharmacist and there are no staff dedicated to optimising medicines reconciliation. This pilot study suggests that inclusion of pharmacists in such services to support medicines reconciliation can provide an effective service, which improves safety for patients. In addition to medicines reconciliation, a pharmacist could also bring other medicines-related skills into the service. These include development of medicines-related procedures and policies, contributing medicines-related knowledge to support evidence-based decision making and education and training of various healthcare professionals that form part of the multidisciplinary team.
What this paper adds.
What is already known on this subject?
When patients' care is transferred between healthcare settings medication errors or unintentional changes occur.
This has been shown to be the case when patients are admitted to and discharged from hospital.
No study has examined whether this occurs and to what extent when patients care is transferred to step-up and step-down integrated community services, where patients are cared for in their home.
What this study adds?
This study demonstrates that when patients' care is transferred to a ‘step-up’ and ‘step-down’ community service there are a high number of medication discrepancies.
These discrepancies are clinically significant, with approximately half of the medication discrepancies classified as moderate, high or extreme risk of patient harm.
Footnotes
Contributors: HKV and NG designed the pilot study, collected the data, reviewed the initial sample for risk categorisation and drafted the paper. NG performed data analysis. S-JL contributed to study design, performed risk categorisation, data analysis and interpretation. NLB advised on data collection tools and data analysis. S-JL revised the paper. NG, HKV and NLB reviewed drafts of the paper. All authors reviewed and agreed on the final version of the paper.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Technical patient safety solutions for medicines reconciliation on admission of adults to hospital. National Institute for Health and Clinical Excellence (NICE) and the National Patient Safety Agency (NPSA), December 2007.
- 2. Keeping patient safe when they transfer between care providers—getting the medicines right. Royal Pharmaceutical Society June 2012. http://www.rpharms.com/current-campaigns-pdfs/rps-transfer-of-care-final-report.pdf.
- 3.Pirmohamed M, James S, Meakin S, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ 2004; 329:15–19. 10.1136/bmj.329.7456.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kongkaew C, Hann M, Mandal J, et al. Risk factors for hospital admissions associated with adverse drug events. Pharmacotherapy 2013;33:827–37. 10.1002/phar.1287 [DOI] [PubMed] [Google Scholar]
- 5.2014. Professional standards for hospital pharmacy services. Royal Pharmaceutical Society Vs 2 July. http://www.rpharms.com/support-pdfs/rps—professional-standards-for-hospital-pharmacy.pdf.
- 6.Medicines optimisation: the safe and effective use of medicines to enable the best possible outcomes. National Institute for Health and Clinical Excellence (NICE), March 2015.
- 7.Dodds LJ. Optimising pharmacy input to medicines reconciliation at admission to hospital: lessons from a collaborative service evaluation of pharmacy-led medicines reconciliation services in 30 acute hospitals in England. Eur J Hosp Pharm 2014;21:95–10. http://ejhp.bmj.com/content/early/2013/12/10/ejhpharm-2013-000385 [Google Scholar]
- 8. Five year forward view: http://www.england.nhs.uk/ourwork/futurenhs/5yfv-exec-sum/
- 9.A risk matrix for risk managers. NPSA January 2008.
- 10.Dodds L. Results of a Collaborative Audit of Pharmacy-led Medicines Reconciliation (MR) in 56 trusts across E & SE England. Medicines Use and Safety Division, East and South East Specialist Pharmacy Services 2010.
- 11.Tjia J, Bonner A, Briesacher BA, et al. Medication discrepancies upon hospital to skilled nursing facility transitions. J Gen Intern Med 20095:630–5. 10.1007/s11606-009-0948-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Improving the Quality of Medicines Reconciliation—A best practice resource and toolkit. Summary by Chetan Shah, Medicines Use and Safety Division, East and South East England Specialist Pharmacy Services. Date published: 24 June 2015.
- 13.Rozich JD, Howard RJ, Justeson JM, et al. Patient safety standardization as a mechanism to improve safety in health care. J Qual Saf 2004;30:5–14. [DOI] [PubMed] [Google Scholar]
- 14.Gleason KM, Groszek JM, Sullivan C, et al. Reconciliation of discrepancies in medication histories and admission orders of newly hospitalized patients. Am J Health Syst Pharm 2004;61:1689–95. [DOI] [PubMed] [Google Scholar]