Abstract
Objectives
To analyse the risk factors of gastropathy caused by using non-steroidal anti-inflammatory drugs (NSAIDs) in detected hospital admissions and to analyse the use of gastroprotective treatment concerning these risk factors.
Methods
A retrospective observational study was carried out in the framework of an integral risk management plan of drugs and proactive pharmacovigilance of hospital admissions for NSAID-induced gastropathy occurring between 2011 and 2015. Cases were identified after reviewing the ICD-9 codes related to NSAID-induced gastropathy in hospital discharge reports. Various biometric, clinical and pharmacotherapeutic variables of each patient were registered. The gastroprotective criteria set out in the therapeutic decision algorithm of the Valencian Health System were followed.
Results
62 hospital admissions for NSAID-induced gastropathy were detected. The mean length of stay was 5.3±3.8 days. Ibuprofen was the most prevalent NSAID (28 cases, 45.2%). 24 cases (38.7%) took NSAIDs in the week before hospitalisation. The prevalence of relevant risk factors for gastropathy were age >60 years (37 cases, 59.7%), concomitant medication (24 cases, 38.7%) and a history of peptic ulcer (9 cases, 14.5%). 41 patients (66.1%) met gastroprotective major criteria, 18 of whom (43.9%) were using a proton pump inhibitor following a prevention plan.
Conclusions
In this study all relevant gastroprotective criteria were associated with the use of gastroprotection in detected hospital admissions for NSAID-induced gastropathy. However, a lack of gastroprotection was observed in a large number of detected cases with the criteria to use it. The feedback of our results to health area agents can serve to reinforce the safe use of NSAIDs.
Keywords: upper gastrointestinal bleeding, nonsteroidal anti-inflammatory agents, acetylsalicylic acid, hospitalization, risk factors, anti-ulcer agents, pharmacovigilance, safety, metamizole
EAHP Statement 5: Patient Safety and Quality Assurance
Introduction
Non-steroidal anti-inflammatory drugs (NSAIDs) are agents with broad chemical heterogeneity, but they share as a mechanism of action the inhibition of the cyclo-oxygenase enzyme (COX). Consequently, the synthesis of prostaglandins, prostacyclins and thromboxanes is blocked, and the effects mediated by them are inhibited. Within this group are considered traditional NSAIDs (such as ibuprofen, diclofenac or naproxen), selective COX-2 inhibitors (coxibs) and acetylsalicylic acid (ASA).1 In Spain NSAIDs are currently among the most prescribed therapeutic groups,2 but it is also considered a therapeutic group closely linked to self-medication.3
Gastropathy is one of the most important health problems caused by these drugs. It presents a wide clinical expression, ranging from dyspeptic symptoms to serious complications causing hospitalisation such as upper gastrointestinal bleeding (UGIB).4–7
The medical literature provides evidence of an increased risk of gastropathy by patient-related factors (age, history of peptic ulcer, smoking, alcohol consumption, Helicobacter pylori infection and severe systemic disease) and treatment-related factors (dose, duration and type of NSAID used and concomitant use with other drugs including systemic corticosteroids, oral anticoagulants, platelet aggregation inhibitors or other NSAIDs).1 8–13 Identification and assessment of these risk factors is necessary to determine the need for prophylaxis with gastroprotective medications to reduce the prevalence of this type of injury. In this regard, some studies have shown an inappropriate use of anti-ulcer drugs, especially as prophylaxis for gastrointestinal (GI) complications associated with drugs.14 15 However, a gradual increase in anti-ulcer drug consumption in Spain has been observed since completion of these studies, especially proton pump inhibitors (PPIs). This increase may be related to their use for the prophylaxis of NSAID-induced gastropathy.16
This study, developed in the context of an integral risk management plan of drugs, aims to describe the most common risk factors associated with NSAID-induced gastropathy in detected hospital admissions and to measure the relationship between the use of gastroprotection and these relevant risk factors.
Methods
Study design
A cross-sectional analysis of a retrospective case series of hospital admissions for gastropathy by treatment with NSAIDs occurring between 2011 and 2015 was performed. Cases were selected from hospital admissions included in the integral risk management plan of drugs and proactive pharmacovigilance of Francesc de Borja Hospital (Gandia, Valencia, Spain) pertaining to ‘Conselleria Valenciana de Sanitat Universal i Salut Pública’.
Study population
All cases of drug-related gastropathy resulting in admission to hospital deemed to be associated with the use of NSAIDs were selected. Patients with gastropathy associated with other drugs or without relevant information for the analysis were excluded.
Detection system, analysis and verification of cases
The identification of cases was carried out systematically and retrospectively by the Clinical Documentation and Admission Department of the hospital through the central computing application for hospital management (IRIS) of the Valencian Health System. International Classification of Diseases, Ninth revision, Clinical Modification (ICD-9 CM) was used for coding diagnoses of the hospital discharge reports. The medical criteria, set out explicitly in the same discharge reports, were considered as sufficient for acceptance of accountability. However, by using the Naranjo adverse drug reaction probability,17 it was confirmed that the detected cases had at least a probable causal relationship.
The cases were analysed, validated and recorded retrospectively by the pharmacist team participating in the integral risk management plan of drugs and proactive pharmacovigilance of the hospital. Information regarding sex, age, main pathology, relevant medical history, laboratory and diagnostic tests, evolution during hospitalisation and ongoing medication (generic name, dosage schedules and number of days of treatment) was collected. The following corporate electronic applications were used: the pharmacotherapeutic history (Farmasyst, MDIS), the medical history (MIZAR, Abucasis, Orion Clinic) and the laboratory results application (GestLab). A record of the cases was made in a database designed for this purpose using Microsoft Access 2007. This file is subject to safety requirements established by Law 15/1999 and Royal Decree 1720/2007 of personal data protection.
The gastroprotective criteria set out in the recommendations of 2003 from the Spanish Association of Gastroenterology (AEG) and the Spanish Society of Rheumatology (SER)12 and in the therapeutic decision algorithm of the Valencian Health System for the management and pharmacological prescription in the prevention of NSAID-induced gastropathy were used.18 Patients were categorised as users of gastroprotective treatment if they had used PPIs or misoprostol during NSAID intake and before the onset of symptoms of gastropathy. Electronic dispensing records from the pharmacotherapeutic history were checked to evaluate adherence to medication.
Statistical analyses
Statistical analyses were performed using Microsoft Excel 2007 and the statistical package SPSS V.19.0 for Windows. Data were reported as mean and SD or as percentage. The χ2 test was used to compare proportions and the Student t-test was used to compare means. Comparisons were analysed using a two-tailed test and p<0.05 was considered statistically significant.
Ethical issues
This healthcare quality programme was approved by the Teaching, Investigation and Ethics Commission of Gandia Health Department. Also, it is integrated in research projects managed by the Foundation for the Promotion of Health and Biomedical Research of Valencia (ref. FISABIO 2015/31).
Results
Sixty-two hospital admissions for NSAID-induced gastropathy were detected from 2011 to 2015 (32 men, 30 women). Table 1 shows the demographic and clinical characteristics according to sex. Other than anaemia, there were no differences in the distribution of these characteristics between men and women.
Table 1.
Demographic and clinical characteristics of detected cases at hospital admission
| Variable | Men | Women | Total sample |
|---|---|---|---|
| Cases, n (%) | 32 (51.6) | 30 (48.4) | 62 (100) |
| Age, mean (SD), years | 60.6 (21.2) | 65.1 (23.3) | 62.8 (22.1) |
| Age, n (%) | |||
| ≥75 years | 10 (31.2) | 15 (50.0) | 25 (40.3) |
| 65–74 years | 7 (21.9) | 3 (10.0) | 10 (16.1) |
| 45–64 years | 8 (25.0) | 6 (20.0) | 14 (22.6) |
| 11–44 years | 7 (21.9) | 6 (20.0) | 13 (21.0) |
| Mode of presentation, n (%) | |||
| Gastric ulcer bleeding | 9 (28.1) | 8 (26.7) | 17 (27.5) |
| Non-bleeding peptic lesion events* | 7 (21.8) | 8 (26.7) | 15 (24.2) |
| Gastroduodenal ulcer bleeding | 6 (18.8) | 4 (13.3) | 10 (16.1) |
| Unspecified GI bleeding† | 4 (12.5) | 6 (20.0) | 10 (16.1) |
| Duodenal ulcer bleeding | 6 (18.8) | 3 (10.0) | 9 (14.5) |
| Oesophageal ulcer bleeding | – | 1 (3.3) | 1 (1.6) |
| Clinical manifestations, n (%) | |||
| Anaemia‡ | 29 (90.6) | 17 (56.7)§ | 46 (74.2) |
| Melaena | 24 (75.0) | 16 (53.3) | 40 (64.5) |
| Peptic pain | 11 (34.4) | 15 (50.0) | 26 (41.9) |
| Coffee ground vomitus/haematemesis | 5 (15.6) | 8 (26.7) | 13 (21.0) |
| Haemoglobin, mean (SD), g/dL | 9.7 (2.6) | 10.9 (2.6) | 10.3 (2.6) |
| Red blood cell count, mean (SD), ×109/L | 3.4 (0.9) | 3.9 (0.7) | 3.6 (0.8) |
*Cases without symptoms of melaena and/or coffee ground vomitus/haematemesis but with evidence of ulcer injury on gastroscopy.
†Cases that did not undergo gastroscopy during hospitalisation or no evidence of ulcer injury detected but with symptoms of melaena and/or coffee ground vomitus/haematemesis.
‡Defined as a haemoglobin level <13 g/dL in men and <12 g/dL in women.
§Statistically significant differences (p<0.05) between men and women.
GI, gastrointestinal.
NSAID used, dose and duration of use
Table 2 shows the detected cases in relation to the type of NSAID used, daily dose, GI risk profile and associated use of gastroprotection. The doses used did not exceed the daily maximum dosage established by the Spanish Agency for Medicines and Health Products (AEMPS). In 19 cases (30.6%), NSAID use related to hospitalisation was not in the pharmacotherapeutic history of the patient (self-medication). Of these cases, 16 were drugs that required a medical prescription and three were over-the-counter (OTC) medicines.
Table 2.
Distribution of hospital admissions for non-steroidal anti-inflammatory drug (NSAID)-induced gastropathy according to the type of NSAID, daily dose, gastrointestinal risk profile and associated use of gastroprotection
| Risk profile, n (n with gastroprotection) |
||||||
|---|---|---|---|---|---|---|
| Drug (mg/day) |
Cases, n (%) |
Mean (SD) age, years | Self-medication, n (%) | High* | Medium† | Low‡ |
| Ibuprofen (600–1800) |
28 (45.2) | 52.1 (24.0) | 13 (21.0) | 7 (5) | 5 (0) | 16 (1) |
| Celecoxib (200) |
7 (11.3) | 79.9 (10.3) | – | 4 (2) | 2 (2) | 1 (0) |
| Dexketoprofen (25–75) |
5 (8.1) | 63.4 (24.1) | 1 (1.6) | 4 (1) | – | 1 (1) |
| Diclofenac (50–150) |
5 (8.1) | 71.8 (15.9) | 1 (1.6) | 4 (3) | 1 (0) | – |
| ASA (100) |
4 (6.4) | 79.0 (7.7) | – | 2 (2) | 2 (0) | – |
| ASA (500) |
4 (6.4) | 62.5 (21.0) | 3 (4.8) | 1 (1) | 1 (1) | 2 (0) |
| Metamizole (475–2000) |
3 (4.8) | 74.3 (3.5) | 1 (1.6) | 3 (1) | – | – |
| Aceclofenac (200) |
2 (3.2) | 76.5 (2.1) | – | 2 (0) | – | – |
| Meloxicam (15) |
2 (3.2) | 72.0 (2.8) | – | – | 2 (0) | – |
| Naproxen (1000–1100) |
2 (3.2) | 56.5 (33.2) | – | 1 (0) | – | 1 (0) |
| Total sample | 62 (100) | 62.8 (22.1) | 19 (30.6) | 28 (15) | 13 (3) | 21 (2) |
*High-risk: patients with a history of complicated ulcer, anticoagulated or with two or more other risk factors (age >60 years, severe comorbidity, Helicobacter pylori infection, history of uncomplicated ulcer and/or concomitant use with other NSAIDs, antiplatelet drugs or systemic corticosteroids).
†Medium risk: patients not anticoagulated, without a history of ulcer but who have some isolated risk factors.
‡Low risk: patients without risk factors.
ASA, acetylsalicylic acid.
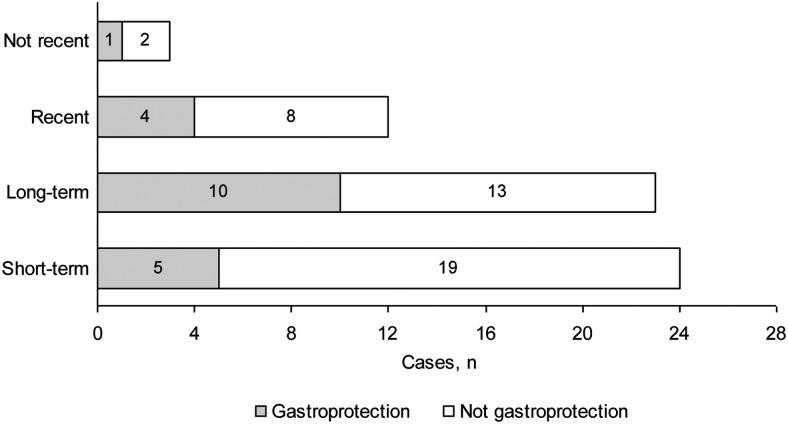
Figure 1 shows the distribution of detected cases in relation to the type of NSAID exposure and the use of gastroprotection. According to the emergency reports, in eight cases (12.9%) hospitalisation occurred after the ingestion of a single dose of the NSAID. In patients who did not use NSAIDs in the week before hospitalisation (‘recent’ and ‘not recent’ use), the average time from completion of NSAID treatment to hospitalisation for gastropathy was 19 days (range 7–53 days).
Figure 1.
Non-steroidal anti-inflammatory drug (NSAID) exposure and gastroprotection use. Short-term: patients who took NSAIDs in the week before hospitalisation but not in the 2–4 weeks before that; Long-term: patients who took NSAIDs at least 1–4 weeks before hospitalisation; Recent: patients who took NSAIDs in the 2–4 weeks before hospitalisation but not in the week before that; Not recent: patients who took NSAIDs but not in the 4 weeks before hospitalisation.
Patient-related risk factors and gastroprotection use
Forty-one patients (66.1%) had at least one of the relevant gastropathy risk factors for using a gastroprotective agent, 18 of whom (43.9%) had been prescribed gastroprotection during NSAID treatment. The electronic medication history showed that, in three of these 18 patients, some of the prescribed gastroprotective treatment containers were registered as ‘non-dispensed’. On the other hand, 21 patients (33.9%) had none of the relevant gastropathy risk factors, of whom two (9.5%) were using gastroprotection during NSAID treatment (table 3). In all cases the gastroprotective treatment was a PPI, with omeprazole most commonly used (55% of PPIs).
Table 3.
Indication and use of gastroprotective drugs in patients admitted for non-steroidal anti-inflammatory drug (NSAID)-induced gastropathy
| With gastroprotective criteria* |
No gastroprotective criteria |
||||||
|---|---|---|---|---|---|---|---|
| Mode of NSAID indication | Patients, n (%) | n (%) | With gastroprotection, n (%) | p Value | n (%) | With gastroprotection, n (%) | p Value |
| Medical prescription | 43 (69.4) | 34 (79.1) | 16† (47.1) | 0.345 | 9 (20.9) | 2 (22.2) | 0.086 |
| Self-medication | 19 (30.6) | 7 (36.8) | 2 (28.6) | 12 (63.2) | – | ||
*Patients with at least one of the following risk criteria (relevant risk factors): age >60 years (age risk factor); personal medical history of peptic ulcer with/without complication (history risk factor); and concomitant use of other NSAIDs, systemic corticosteroids, oral anticoagulants or platelet aggregation inhibitors (concomitant medication risk factor).
†In three of these patients some of the prescribed gastroprotective treatment containers were registered as ‘non-dispensed’ in the electronic medication history during NSAID intake.
Age
The mean±SD age of the patients was 62.8±22.1 years (median 70 years). Among the 37 patients (59.7%) aged >60 years, 18 (48.6%) were using a PPI. This was significantly higher than the percentage of patients aged ≤60 years who were using a PPI (table 4).
Table 4.
Use of gastroprotection, transfusion requirements and length of hospitalisation with regard to gastropathy risk factors in patients admitted for non-steroidal anti-inflammatory drug (NSAID)-induced gastropathy
| Risk factor | Patients, n (%) | Gastroprotection use, n (%) | p Value | Blood transfusion, n (%) | p Value | Mean (SD) hospital stay, days | p Value |
|---|---|---|---|---|---|---|---|
| Age | |||||||
| >60 years | 37 (59.7) | 18 (48.6) | 0.001 | 21 (56.8) | 0.026 | 6.1 (4.5) | 0.022 |
| ≤60 years | 25 (40.3) | 2 (8.0) | 7 (28.0) | 4.1 (1.9) | |||
| Sex | |||||||
| Men | 32 (51.6) | 8 (25.0) | 0.207 | 16 (50.0) | 0.429 | 5.9 (4.6) | 0.145 |
| Women | 30 (48.4) | 12 (40.0) | 12 (40.0) | 4.5 (2.5) | |||
| Concomitant medication* | |||||||
| Yes | 24 (38.7) | 13 (54.2) | 0.003 | 14 (58.3) | 0.098 | 5.6 (3.0) | 0.548 |
| No | 38 (61.3) | 7 (18.4) | 14 (36.8) | 5.0 (4.2) | |||
| History† | |||||||
| Yes | 9 (14.5) | 6 (66.7) | 0.017 | 5 (55.5) | 0.498 | 5.8 (2.9) | 0.659 |
| No | 53 (85.5) | 14 (26.4) | 23 (43.4) | 5.2 (3.9) | |||
| Comorbidities‡ | |||||||
| Yes | 15 (24.2) | 9 (60.0) | 0.008 | 12 (80.0) | 0.002 | 7.2 (2.9) | 0.021 |
| No | 47 (75.8) | 11 (23.4) | 16 (34.0) | 4.5 (3.8) | |||
*Concomitant use of systemic corticosteroids, oral anticoagulants, clopidogrel or other NSAIDs (including ASA as antiplatelet).
†Personal medical history of peptic ulcer with/without complication (gastrointestinal bleeding, pyloric stenosis and perforation).
‡Cerebrovascular disease, chronic obstructive lung disease, cirrhosis, coronary disease, heart failure or renal failure.
ASA, acetylsalicylic acid.
Concomitant medication
Twenty-four patients (38.7%) were taking concomitant risk medications: 12 (19.4%) low-dose ASA (100–300 mg/day), 8 (12.9%) other NSAIDs, 6 (9.7%) systemic corticosteroids, 5 (8.1%) clopidogrel and 2 (3.2%) acenocoumarol. Of these 24 patients, 13 (54.2%) were using a PPI. This percentage was significantly higher than in patients not taking concomitant risk medication (table 4).
History
Among the nine patients (14.5%) with a history of peptic ulcer with or without complications, six (66.7%) were using a PPI. This percentage was significantly higher than in patients without a history of peptic ulcer (table 4).
Associated comorbidities
Fifteen patients (24.2%) had severe comorbidities, nine of whom (60.0%) were using a PPI. This percentage was significantly higher than in patients without a severe comorbidity (table 4).
There were no significant differences in the use of gastroprotection between previously considered risk factors.
Outcome
All cases were recovered and discharged for medical follow-up to primary healthcare services. The length of hospital stay was 5.3±3.8 days. Age >60 years and the presence of severe comorbidities were risk factors for a longer hospital stay and for blood transfusion (table 4). Twenty-eight patients (45.2%) required a blood transfusion (21 (33.9%) patients, 2 units; 5 (8.1%) patients, 4 units; and 2 (3.2%) patients, 10 units).
Information about H. pylori status during hospitalisation was available in 31 patients, 10 of which (32.3%) were positive. All of them were treated with omeprazole, clarithromycin and amoxicillin (or metronidazole).
Discussion
Continuous assessment of the quality of provided healthcare includes risk management plans and detection of improvement points. The integral risk management plan of drugs of Francesc de Borja Hospital (Gandia, Valencia, Spain) includes the systematic detection of those events that cause hospitalisations, of special interest being those considered preventable and which therefore could have been avoided. In this regard, NSAIDs are associated with a broad spectrum of GI complications, and UGIB is especially important because of its severity.5–7 9 19 20
In assessing the risk of gastropathy, factors to be considered include older age, a history of peptic ulcer and concomitant use with other NSAIDs, oral anticoagulants, antiplatelet drugs or systemic corticosteroids.1 8–13 18 21 With regard to older age, there are different recommendations. Some authors have established age ≥65 years as a risk factor for the onset of GI complications in patients who take NSAIDs,1 7 13 21 while other authors consider an age >60 years to be a risk factor.12 18 Age >60 years was the most prevalent risk factor in our study (37 cases, 59.7%), followed by the use of concomitant medication (24 cases, 38.7%), severe comorbidity (15 cases, 24.2%) and a history of peptic ulcer (9 cases, 14.5%). All of these were associated with the use of PPIs.
Concerning the patient’s sex, 32 cases (51.6%) were men and 30 (48.4%) were women. There were no differences in the use of gastroprotection between the two sexes. These findings are consistent with previous studies in which sex was not a determining risk factor for NSAID-induced gastropathy or for indications for gastroprotection.7 12
Furthermore, H. pylori infection is an independent risk factor for these complications.12 13 22 Its determination is not routinely performed in clinical practice in all patients with GI risk. In fact, information about H. pylori status during hospitalisation was available only in 31 patients. Moreover, there is controversy about the need for its eradication before starting treatment with NSAIDs.11
Only 43.9% of patients who met the relevant criteria (at least a grade B recommendation according to the categories of recommendation grades proposed by the US Agency for Health Care Policy and Research) for gastroprotection as recommended by the AEG and SER12 and in the therapeutic decision algorithm of the Valencian Health System18 were using PPIs. Although this is an improvement in the use of gastroprotection compared with previous studies carried out in the same context,6 7 there was not a significant suitability for using anti-ulcer drugs by medical prescription in patients with gastroprotective criteria compared with other self-medication cases. Likewise, it is not known whether patients who met the relevant criteria for gastroprotection and did not use it (56.1% of patients) were a rare exception or whether they reflect a general misuse of gastroprotective treatment, as indicated in some previous studies.14 15
Other authors suggest different recommendations based on the GI risk profile (established according to the presence of the above factors): avoid treatment with NSAIDs in high-risk patients and, if necessary, use a coxib associated with a PPI; in medium-risk patients coxibs or classical NSAIDs with a PPI may be used; and in low-risk patients any NSAID is acceptable.1 21 These recommendations are based on studies in which a lower risk of UGIB has been established for coxibs than for classical NSAIDs.23 Conversely, other authors have not confirmed that greater selectivity for coxibs confers a lower risk of UGIB.24 In our study seven hospital admissions (11.3%) occurred after taking celecoxib, three of them in patients with medium or low GI risk, even with gastroprotection. Because of the lack of a control group, we cannot set a higher or lower risk of gastropathy for coxibs than for classical NSAIDs. However, these results suggest that the risk of these drugs should not be underestimated.
In addition, the risk depends on the NSAID used, the dosage and duration of use.19 20 Hospital admissions for ibuprofen were the most prevalent in our study. Sixteen of the 28 cases had a low GI risk profile (one with gastroprotection). The dose used in most cases was 600 mg/8 hours. Various studies indicate that ibuprofen is an NSAID associated with a low risk of serious upper GI tract complications, but the relative risk is four times greater for doses ≥1800 mg/day than for doses <1200 mg/day.19 20 For this reason, in most European countries, contrary to what happens in Spain, the usual marketed dose is 400 mg. This dose achieves the maximum peak analgesic effect, but higher doses only slightly enhance its duration of action and can negatively affect its risk-benefit ratio.25 26 This may require the need to adjust to lower doses of ibuprofen or to use gastroprotection regardless of the GI risk profile when high doses (≥1800 mg/day) are needed.
Regarding ASA-associated gastropathy, an increased risk of UGIB with low doses (75–325 mg/day) used in the prevention of cardiovascular events has been described.27 28 Age ≥69 years has been proposed as a sufficient risk factor to associate gastroprotection with ASA at these doses.6 Four cases (6.4%) used ASA 100 mg/day without combination with other NSAIDs. The mean age of these patients was 79.0±7.7 years and only two used gastroprotection. This risk is increased further in patients taking low-dose ASA along with other NSAIDs,22 27 and gastroprotective treatment is equally recommended in the case of classic NSAIDs or coxibs.12 In 12 cases (19.3%) ASA (100–300 mg/day) was used with other NSAIDs, and only five were using gastroprotection. These results suggest the lack of gastroprotection previously described in patients with GI risk who take ASA at an antiaggregant dosage.6 29
On the other hand, the GI risk seems to be more strongly related to the dose than to the duration of ASA use.28 This contrasts with the availability of OTC medicines that contain doses of ASA higher than doses used in cardiovascular prevention. In previous studies it was found that 22.1% of detected hospitalisations for NSAID-induced UGIB were by OTC medicines that contained doses of ASA above 325 mg in patients not using any gastroprotection.5 In our study four cases (6.4%) were related to OTC medicines that contained 500 mg ASA. Two of them had a medium/high GI risk profile (both were using a PPI) and the other two had a low GI risk profile (neither was using gastroprotection). However, there remains a contradistinction between the capacity of pharmacists to dispense OTC medicines that contain ASA (or other NSAIDs) and the legal capacity of the same professional to dispense the necessary gastroprotective treatment to prevent the serious side effects that can be produced by these drugs.
Mention should also be made of hospital admissions for metamizole-induced gastropathy, classified by some authors as an agent belonging to the group of NSAIDs.30 Leaving aside discussions on its classification as a NSAID, gastroprotection is recommended in patients at risk.12 Of the three detected cases, all were patients with a high GI risk profile and only one was using gastroprotection. This reinforces the advisability of taking preventive measures in this patient profile.
Concerning the duration of treatment with NSAIDs, we have defined four exposure types following the model used by Lewis et al.19 Twenty-four patients (38.7%) took NSAIDs in the week before hospitalisation (but not in the 2–4 weeks before that) and eight (12.9%) were admitted after the ingestion of a single dose. Of these 24 patients, only five (20.8%) were using gastroprotection. These findings reinforce the need to assess the use of gastroprotection regardless of the time for which NSAIDs are used because the risk of gastropathy starts from the first day of administration, it is maintained throughout the time it is consumed and there is a maximum risk during the first week of treatment.12 19 Moreover, the risk remains until 2 months after the end of NSAID treatment.12 20 In our study the average time from the completion of NSAID treatment to hospital admission for gastropathy in patients not receiving NSAIDs at admission was 19 days (range 7–53 days).
Another important aspect to emphasise, aside from the correct prescription of NSAIDs and gastroprotective treatment, is patient adherence to treatment. In the electronic medication history of three of the 20 patients (15%) prescribed gastroprotective treatment, some of the prescribed containers were described as ‘non-dispensed’ during NSAID treatment. All three patients had gastroprotective criteria. Although the presence of dispensing records does not ensure adherence to treatment, the absence of these records may be indicative that it can be compromised. We must therefore keep working to improve adherence to treatment, especially in patients with a medium/high GI risk profile.
Finally, among other limitations it should be noted that this is a descriptive retrospective observational study in which the included cases have been identified on the basis of encoded data in healthcare reports, which may have been prone to omission errors in coding. Moreover, precise information was not available for one or more clinical parameters in some cases, such as NSAID schedules, real adherence to treatment, H. pylori assessment and smoking and alcohol consumption. This might have resulted in an underestimation of the gastropathy risk.
Conclusion
Serious events of NSAID-induced gastropathy require admission to hospital. Gastroprotection is the most rational preventive measure in patients with GI risk factors. In detected hospital admissions, all the relevant criteria for gastroprotection were associated with PPI use. However, the use of PPIs did not depend on the mode by which NSAIDs were indicated (medical prescription or self-medication) and the percentage without gastroprotection was not negligible in detected cases with gastroprotective criteria. Feedback of our results to health area agents and the implementation of corrective measures can serve as a basis on which to reinforce the safe use of NSAIDs.
What this paper adds.
What is already known on this subject
Non-steroidal anti-inflammatory drugs (NSAIDs) are one of the most consumed drugs and their therapeutic effects have mainly been associated with the risk of developing gastrointestinal adverse events such as gastropathy.
NSAIDs are components of many over-the-counter medicines.
A gradual increase in anti-ulcer drug consumption has been found, which may be related to their use for prophylaxis of NSAID-induced gastropathy.
What this study adds
All relevant criteria for gastroprotection were associated with the use of gastroprotective treatment in detected hospital admissions for NSAID-induced gastropathy.
The high percentage without gastroprotection in detected cases, especially when there are criteria to use it, can serve as a basis on which to evaluate the need to implement corrective measures to reinforce the safe use of NSAIDs.
Footnotes
Competing interests: None declared.
Ethics approval: Ethics approval was obtained from the Teaching, Investigation and Ethics Commission of Gandia Health Department.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Loza E. AINes en la práctica clínica: lo que hay que saber. Inf Ter Sist Nac Salud 2011;35:88–95 [Google Scholar]
- 2. De Abajo FJ, del Pozo JG, del Pino A [Trends of non-steroidal anti-inflammatory drugs use in Spain, 1990 through 2003]. (In Spanish). Aten Primaria 2005;36:424–33. 10.1157/13081056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Montaño Alonso A, Torelló Iserte J, Castillo Ferrando JR, et al. [The knowledge and attitude of consumers in relation to the use of NSAIDs. An intervention study]. (In Spanish) Aten Primaria 1997;20:114, 116–20. [PubMed] [Google Scholar]
- 4. Blower AL, Brooks A, Fenn GC, et al. Emergency admissions for upper gastrointestinal disease and their relation to NSAID use. Aliment Pharmacol Ther 1997;11:283–91. 10.1046/j.1365-2036.1997.d01-604.x [DOI] [PubMed] [Google Scholar]
- 5. Marco JL, Boscá B. Ingresos hospitalarios por hemorragia digestiva alta asociada a especialidades farmacéuticas publicitarias. Pharm Care Esp 2003;5:112–13. [Google Scholar]
- 6. Marco JL, Boscá B, Real M, et al. [Non-steroidal anti-inflammatory high digestive bleeding hospital income]. (In Spanish) Seguim Farmacoter 2004;2:217–27. [Google Scholar]
- 7. Marco JL, Amariles P, Boscá B, et al. Risk factors associated with NSAID-induced upper gastrointestinal bleeding resulting in hospital admissions: a cross-sectional, retrospective, case series analysis in Valencia, Spain. Curr Ther Res Clin Exp 2007;68:107–19. 10.1016/j.curtheres.2007.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. García Rodríguez LA, Jick H. Risk of upper gastrointestinal bleeding and perforation associated with individual non-steroidal anti-inflammatory drugs. Lancet 1994;343:769–72. 10.1016/S0140-6736(94)91843-0 [DOI] [PubMed] [Google Scholar]
- 9. Gutthann SP, García Rodríguez LA, Raiford DS. Individual nonsteroidal antiinflammatory drugs and other risk factors for upper gastrointestinal bleeding and perforation. Epidemiology 1997;8:18–24. 10.1097/00001648-199701000-00003 [DOI] [PubMed] [Google Scholar]
- 10. Agrawal N. Risk factors for gastrointestinal ulcers caused by nonsteroidal anti-inflammatory drugs (NSAIDs). J Fam Pract 1991;32:619–24. [PubMed] [Google Scholar]
- 11. Rodríguez C. [Risk factors of gastric disease by NSAIDS]. (In Spanish). Rev Esp Reumatol 2000;27:9–14 [Google Scholar]
- 12. Lanas A, Martín-Mola E, Ponce J, et al. [Clinical strategy to prevent the gastrointestinal adverse effects of nonsteroidal anti-inflammatory agents]. (In Spanish). Gastroenterol Hepatol 2003;26:485–502. 10.1016/S0210-5705(03)70400-2 [DOI] [PubMed] [Google Scholar]
- 13. Vallès R, Franzi A, Ferro JJ. Condiciones clínicas y terapéuticas que requieren gastroprotección. FMC 2014;21:528–33 [Google Scholar]
- 14. Sánchez JI, Larrabe J, Óscar J, et al. [Prescription of non-steroidal anti-inflammatory and stomach protector drugs. How they fit primary care quality criteria]. (In Spanish). Aten Primaria 1997;20:127–32. [PubMed] [Google Scholar]
- 15. Carrillo P, Amado E, de la Fuente JA, et al. [Prescribing non-steroidal anti-inflammatory drugs and gastrointestinal protection in primary care]. (In Spanish). Aten Primaria 2008;40:559–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Agencia Española de Medicamentos y Productos Sanitarios. Utilización de medicamentos antiulcerosos en España durante el período 2000-2012. 2014. http://www.aemps.gob.es/medicamentosUsoHumano/observatorio/docs/antiulcerosos.pdf (accessed 12 Aug 2016).
- 17. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981;30:239–45. 10.1038/clpt.1981.154 [DOI] [PubMed] [Google Scholar]
- 18. Direcció General de Farmàcia i Productes Sanitaris, Generalitat Valenciana . Criterios de consenso por los que se establece el algoritmo de decisión terapéutica corporativo de la Agencia Valenciana de Salud, para el manejo y prescripción farmacológica en la prevención de la gastropatía por antiinflamatorios no esteroideos (AINE). 2013. http://www.san.gva.es/documents/152919/1092738/TRATAMIENTO+GASTROPATIA+POR+AINE.PDF (accessed 12 Aug 2016).
- 19. Lewis SC, Langman MJ, Laporte JR, et al. Dose-response relationships between individual nonaspirin nonsteroidal anti-inflammatory drugs (NANSAIDs) and serious upper gastrointestinal bleeding: a meta-analysis based on individual patient data. Br J Clin Pharmacol 2002;54:320–6. 10.1046/j.1365-2125.2002.01636.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hernández-Díaz S, Rodríguez LA. Association between nonsteroidal anti-inflammatory drugs and upper gastrointestinal tract bleeding/perforation: an overview of epidemiologic studies published in the 1990s. Arch Intern Med 2000;160:2093–9. 10.1001/archinte.160.14.2093 [DOI] [PubMed] [Google Scholar]
- 21. Marcén B, Sostres C, Lanas A [NSAID and gastrointestinal risk]. (In Spanish). Aten Primaria 2016;48:73–6 10.1016/j.aprim.2015.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lanas A. [Gastrointestinal bleeding associated with NSAIDs, antiplatelet therapy and anticoagulant agent]. (In Spanish). Gastroenterol Hepatol 2012;35(Suppl 1):35–42. [DOI] [PubMed] [Google Scholar]
- 23. Lanas A, García-Rodríguez LA, Arroyo MT, et al. Risk of upper gastrointestinal ulcer bleeding associated with selective cyclo-oxygenase-2 inhibitors, traditional non-aspirin non-steroidal anti-inflammatory drugs, aspirin and combinations. Gut 2006;55:1731–8. 10.1136/gut.2005.080754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Laporte JR, Ibáñez L, Vidal X, et al. Upper gastrointestinal bleeding associated with the use of NSAIDs: newer versus older agents. Drug Saf 2004;27:411–20. 10.2165/00002018-200427060-00005 [DOI] [PubMed] [Google Scholar]
- 25. Laska EM, Sunshine A, Marrero I, et al. The correlation between blood levels of ibuprofen and clinical analgesic response. Clin Pharmacol Ther 1986;40:1–7. 10.1038/clpt.1986.129 [DOI] [PubMed] [Google Scholar]
- 26. Seymour RA, Ward-Booth P, Kelly PJ. Evaluation of different doses of soluble ibuprofen and ibuprofen tablets in postoperative dental pain. Br J Oral Maxillofac Surg 1996;34:110–14. 10.1016/S0266-4356(96)90147-3 [DOI] [PubMed] [Google Scholar]
- 27. García Rodríguez LA, Lin KJ, Hernández-Díaz S, et al. Risk of upper gastrointestinal bleeding with low-dose acetylsalicylic acid alone and in combination with clopidogrel and other medications. Circulation 2011;123:1108–15. 10.1161/CIRCULATIONAHA.110.973008 [DOI] [PubMed] [Google Scholar]
- 28. Huang ES, Strate LL, Ho WW, et al. Long-term use of aspirin and the risk of gastrointestinal bleeding. Am J Med 2011;124:426–33. 10.1016/j.amjmed.2010.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Casado-Arroyo R, Scheiman JM, Polo-Tomas M, et al. Underutilization of gastroprotection for at-risk patients undergoing percutaneus coronary intervention: Spain compared with the United States. Aliment Pharmacol Ther 2010;32:689–95. 10.1111/j.1365-2036.2010.04393.x [DOI] [PubMed] [Google Scholar]
- 30. Arcila H, Barragán S, Borbolla JR, et al. Consenso de un grupo de expertos mexicanos. Eficacia y seguridad del Metamizol (Dipirona). Gac Med Mex 2004;140:99–101. [PubMed] [Google Scholar]