Abstract
Objective
To assess antibiotic usage in gastrointestinal disorders with respect to appropriateness, pattern of resistance, and incidence of adverse drug reactions (ADRs).
Methodology
Antibiotic prescribing in the gastroenterology department of a tertiary care hospital was evaluated using the Gyssens criteria and also by assessing drug related problems (DRPs) using the Pharmaceutical Care Network Europe V.6.2. A total of 173 patients were studied prospectively by a team of clinical pharmacists. Antibiotic susceptibility was prospectively studied; in addition, retrospective data on culture and sensitivity reports of commonly isolated organisms from 1 October 2012 to 30 September 2014 were collected to determine the resistance pattern in previous years. ADRs were evaluated using the Naranjo scale.
Results
Antibiotic therapy was appropriate in 60% of patients and inappropriate in the remaining patients due to incorrect decision, choice, and use. A total of 184 DRPs and 30 ADRs of antibiotics were identified. In the study patients, the most commonly isolated organism was Escherichia coli (27.3%) followed by Klebsiella pneumoniae (16.7%). Both E coli and K pneumoniae exhibited 100% resistance towards cefotaxime. There was an increase in the resistance of E coli and K pneumoniae against various antibiotics tested in 2013–2014 as compared to the previous year. An empirical antibiotic policy was developed which was endorsed by the gastroenterology department.
Conclusions
Although antibiotic therapy was appropriate in the majority of patients, irrational use occurred due to incorrect choice, improper dosage, and improper duration of therapy. E coli and K pneumoniae isolates showed an increase in resistance towards various antibiotics tested.
Keywords: antibiotic resistance, Adverse drug reactions, drug related problems, Gastrointestinal disorders, Gyssens criteria, PCNE classification
Introduction
Antibiotics play an important role in the management of various infections. However, over-utilisation of antibiotics is emerging as a major health problem.1 In fact, the 2014 WHO report on global surveillance of antimicrobial use revealed antibiotic resistance as a public health concern putting at risk the ability to treat common infections in the community and hospitals. Even though the development of antibiotic resistance is a natural phenomenon, certain physician practices accelerate its emergence and dissemination. Antibiotic misuse constitutes the primary cause of increased antibiotic resistance. Irrational use of antibiotics not only results in the steady increase in resistance but also increases the incidence of adverse drug reactions (ADRs), cost of therapy, duration of hospital stay as well as drug interactions, all of which ultimately lead to the failure of therapeutic regimens.2 Given the recent worldwide escalation in resistance and irrational use of antibiotics, the practical and essential approach is to control antibiotic use by developing and implementing antibiotic policies.
One of the areas where antibiotics are widely used as a prophylactic and treatment measure is in gastrointestinal (GI) disorders such as cholangitis, cholecystitis, gastroenteritis, pancreatitis, spontaneous bacterial peritonitis (SBP), and urinary tract infections (UTIs) associated with GI disorders. Hence, it would be worthwhile to assess antibiotic usage in these GI disorders with respect to appropriateness, pattern of resistance, and incidence of ADRs.
Methods
A prospective observational study was conducted on 173 patients who were admitted to the gastroenterology department of a tertiary care hospital in India with the diagnosis of cholangitis, cholecystitis, gastroenteritis, pancreatitis, spontaneous bacterial peritonitis or UTIs associated with GI disorders. The study was approved by the Institutional Review Board and informed signed consent was obtained from the study patients. Demographic details of the patients, pertinent laboratory data, and drug treatment details were collected from the hospital's digital information system and by direct review of the medical records of the admitted patients as well as by direct interview of the patients and caregivers using a pre-designed data collection form. Each case was meticulously examined and the patients were followed up on a daily basis from admission until discharge by a team of two dedicated clinical pharmacists. Assessments were made in terms of prescription pattern, appropriateness of antibiotic therapy, ADRs, and reasons for failure of initial therapy. The sensitivity pattern of organisms isolated was analysed. Systems involved in ADRs were classified according to the WHO System Organ Classification. Causality and severity assessment were performed by the Naranjo et al scale3 and Modified Hartwig and Siegel scale,4 respectively. For SBP, the initial antibiotic therapy was considered a failure when there was no improvement in clinical signs of infection and an inability to achieve at least a 25% decrease in ascitic fluid polymorphonuclear leucocytes after 48 h of antibiotic administration. For other indications, antibiotic therapy was considered a failure when there was no decrease in elevated inflammatory markers such as C-reactive protein (CRP) and no improvement in clinical signs of infection even after 48 h of antibiotic administration.
The appropriateness of antibiotic therapy was evaluated using the method developed by Gyssens et al5 and also by assessing drug related problems (DRPs) of antibiotics as per the Pharmaceutical Care Network Europe (PCNE)6 V.6.2. DRPs due to drug interactions were analysed using the Lexicomp drug interaction checker (UpToDate (http://www.uptodate.com), and category C (monitor the therapy), D (consider therapy modification) and category X (avoid combinations) interactions were considered.
The antibiotic consumption was calculated to defined daily dose (DDD)/100 bed days according to the anatomic therapeutic chemical/DDD index from the WHO collaborating centre for drug statistics methodology.7 Antibiotic susceptibility was prospectively studied during the study period from 1 October 2014 to 31 May 2015. In addition, in order to study any change in the resistance pattern of most commonly isolated organisms in the previous 2 years, retrospective data of culture and sensitivity reports of commonly isolated organisms during 1 October 2012 to 30 September 2014 were collected. All the collected data were compiled using Microsoft Excel and analysed using the Statistical Package for the Social Sciences (SPSS) software V.20. Descriptive statistics such as frequencies, mean±SD and median with range were calculated for relevant parameters. Pearson's χ2 test was used to calculate the p values and a value of p<0.05 was taken as significant.
Results
A total of 173 patients received antibiotic therapy for 183 indications. Antibiotics were administered to the inpatients for the treatment of cholangitis and cholecystitis (9, 4.9% each), gastroenteritis (3, 1.6%), pancreatitis (10, 5.5%), spontaneous bacterial peritonitis (51, 27.9%) and UTIs associated with GI disorders (101, 55.2%). Eighty-eight (48%) of total indications were treated with 2–3 antibiotics and 111 (60.6%) indications were treated with parenteral therapy. The duration of hospital stay (16.6±8.1 days) and duration of administration of antibiotics (15±7.9 days) were higher in cases of cholangitis. For the various indications treated, antibiotic use was appropriate in 55.5% cases of cholangitis, 44.5% cases of cholecystitis, 33.3% cases of gastroenteritis, 50% cases of pancreatitis, 80.4% cases of SBP, and 64.4% of UTI cases. The most commonly prescribed empirical antibiotic class was the cephalosporins for all the indications, but in the case of gastroenteritis combinations of fluoroquinolones and nitroimidazoles were most commonly used.
The mean age of the patients was 54.8±13.2 years (median age 57 years, range 17–85 years) and 74% of the total study patients were males. One hundred and forty-three (82.7%) patients were discharged with oral antibiotics. The initial antibiotic therapy was a failure in 106 (61.3%) patients. Antibiotic therapy was appropriate only in 104 (60%) patients as judged by the Gyssens criteria5 (table 1). Among the factors contributed to inappropriate antibiotic use, incorrect use was the major factor.
Table 1.
Appropriateness of antimicrobial therapy (AMT) based on the Gyssens criteria5
| Criteria | No. (%) of patients (n=173) |
|---|---|
| Appropriate AMT | 104 (60.1) |
| Inappropriate AMT | 69 (39.9) |
| Incorrect decision | |
| No infection and no AMT needed but AMT given | 10 |
| No AMT given for prophylaxis but AMT needed | 3 |
| Incorrect choice | 21 |
| Incorrect use | |
| Improper dosage | 20 |
| Improper timing | 2 |
| Improper administration | 3 |
One hundred and eighty-four DRPs of antibiotics were identified during the study period. The major cause of DRP was inappropriate drug selection followed by inappropriate dose selection (table 2). More than half (117, 59.2%) of the DRPs occurred in patients with UTIs.
Table 2.
Drug related problems (DRPs) based on the Pharmaceutical Care Network Europe (PCNE) V.6.2 classification scheme
| Classification of DRPs | DRP code V 6.2 |
Cause | No. (%) of DRPs (n=184) |
|---|---|---|---|
| Drug selection | C1.1 | Inappropriate drug | 39 (21.1) |
| C1.2 | No indication for drug | 19 (9.6) | |
| C1.3 | Inappropriate combination of drug, drugs and food | 30 (15.2) | |
| C1.4 | Inappropriate duplication of therapeutic group or active ingredient | 3 (1.6) | |
| C1.8 | Synergistic/preventive drug required and not given | 5 (2.6) | |
| Drug form | C2.1 | Inappropriate drug form | 30 (15.2) |
| Dose selection | C3.1 | Drug dose too low | 5 (2.5) |
| C3.4 | Dosage regimen too frequent | 4 (2) | |
| C3.7 | Deterioration/improvement of disease state requiring dosage adjustment | 34 (17.1) | |
| Treatment duration | C4.1 | Duration of treatment too short | 4 (2) |
| C4.2 | Duration of treatment too long | 11 (5.9) |
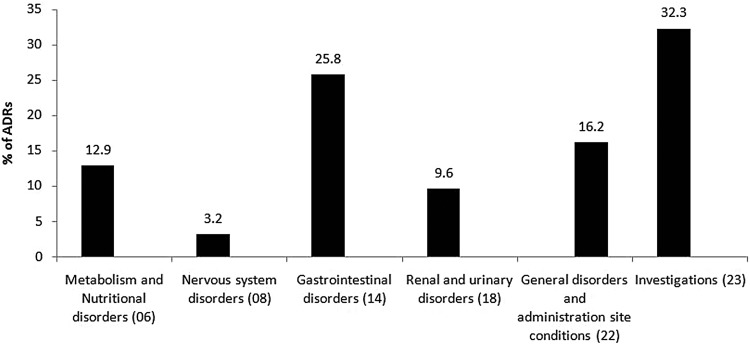
A total of 31 ADRs caused by antibiotics were identified in 30 patients. Ten (33.3%) of the ADRs were classified under the WHO SOC criteria ‘investigations’ (figure 1). Most of the ADRs (24, 80%) were ‘moderate’ in severity. Seventeen (56.7%) ADRs were ‘probable’, 11 (36.6%) ‘possible’, and 2 (6.7%) ‘definite’. Eighteen (60%) ADRs were caused by cephalosporins followed by penicillins (4, 13.3%). In 20 (66.6%) cases of ADRs, treatment with the antibiotic causing the ADR was continued with conservative management.
Figure 1.
Adverse drug reactions (ADRs) of antibiotics in the study patients based on WHO System Organ Classification (31 ADRs in 30 patients).
The most commonly isolated organism was Escherichia coli (41, 27.3%) followed by Klebsiella pneumoniae (25, 16.7%). E coli showed 100% sensitivity towards nitrofurantoin and colistin during the prospective study period. Sensitivity of K pneumoniae isolates ranged from 4–62% among the antibiotics commonly administered. Both E coli and K pneumoniae exhibited 100% resistance towards cefotaxime. Even though there was a general increase in the resistance of E coli and K pneumoniae against various antibiotics tested, a significant increase was seen in the case of E coli towards amikacin, cotrimoxazole, levofloxacin, piperacillin–tazobactam, cefoperazone–sulbactam, and nitrofurantoin (see online supplementary table S1), and in the case of K pneumoniae towards piperacillin–tazobactam, cefoperazone–sulbactam, cotrimoxazole, and ciprofloxacin (see online supplementary table S2) in 2013–2014 as compared to the previous year.
ejhpharm-2015-000818supp001.pdf (158.8KB, pdf)
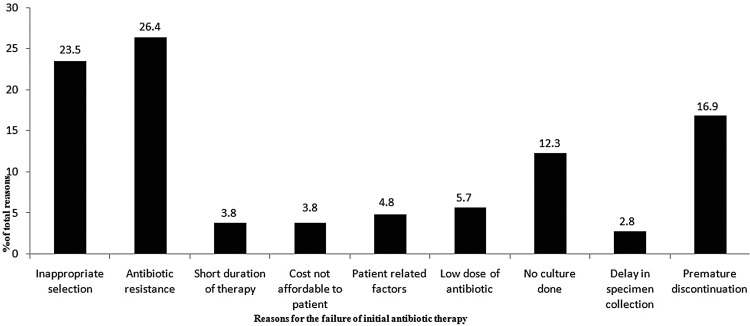
In our study the departmental antibiotic consumption was estimated to be 117.04 DDDs/100 bed days and the total antibiotic consumption was 2208.6 DDDs. In 26.4% of cases, reason for the failure of initial antibiotic therapy was resistance towards these agents (figure 2). Based on the outcome of evaluation and the local sensitivity pattern, an empirical antibiotic policy was developed by the clinical pharmacists in consultation with the physicians of the department.
Figure 2.
Reasons for failure of initial antibiotic therapy in the study patients (106 failures occurred in 106 patients).
Discussion
The major consideration for the proper use of antibiotics is to select an agent with optimal activity at the proper dose and dose interval for the appropriate duration of time. In this study, however, 69 (40%) patients received inappropriate antibiotic therapy as per the criteria developed by Gyssens et al.5 SBP, cholangitis, and UTIs associated with GI disorders were the clinical conditions most linked with inappropriate antibiotic use. During this study, a considerable number of DRPs and ADRs were identified.
Another important problem in antibiotic use was resistance of the organisms towards various antibiotics. E coli and K pneumoniae accounted for the majority of the Gram-negative organisms isolated during the study period. Analysis of the sensitivity and resistance pattern showed E coli was fully susceptible to colistin and nitrofurantoin. On the other hand, susceptibility of K pneumoniae isolates ranged from 4–62% among the commonly administered antibiotics. Both the bacteria exhibited 100% resistance towards cefotaxime. Data collected retrospectively showed that there was an increase in the resistance pattern of E coli and K pneumoniae towards most of the antibiotics tested.
Though UTI is not a GI disorder, approximately 50% of the patients had UTI in conjunction with other GI conditions such as SBP (11.8%), cholangitis (2.9%), cholecystitis (2.9%), pancreatitis (0.9%), and cirrhosis (81.4%). Hence it was decided to evaluate antibiotic usage in these UTI patients.
Usually females are more prone to UTI and cholecystitis,8 9 but in our study there was a male predominance for all the indications of antibiotic therapy, which may be due to there being more male patients in our study. Parenteral antibiotic therapy was prescribed for the majority of infections; however, when the patient's condition improves and he or she is able to tolerate oral medications it is possible to convert parenteral medications into oral form, which can reduce the cost of treatment and the complications resulting from parenteral therapy.10 11 This was reflected in our study in which the majority of our patients were switched from parenteral to oral therapy.
A study by Berrington12 in the UK showed that antibiotic consumption in the gastroenterology department of a 950-bed secondary care hospital was 438.5 DDDs/1000 bed days; however, in our study the antibiotic consumption was 2.6 times more than this study. ‘Inappropriate antibiotic selection’ was the major DRP according to PCNE classification. One of ‘the inappropriate selections’ observed in the majority of the patients was the prescribing of linezolid after the culture and sensitivity reports, in spite of the availability of other antibiotics to which the isolated organism was sensitive. Being a reserved antibiotic, linezolid is recommend13 14 only in cases of vancomycin–ampicillin resistance. Linezolid is now restricted to consultants in our institution after the feedback of this study.
Arribas et al15 conducted a study to measure appropriateness of antibiotic therapy in patients with UTI in an emergency ward of a tertiary care hospital. Approximately 20.5% of patients had inappropriate empirical antibiotic therapy as judged by the culture and sensitivity tests; however, inappropriate antibiotic use was higher in our patients, which may be due to changes in the methodology. Arribas et al15 measured appropriateness with only one criterion which specifies that the empiric antibiotic therapy was appropriate if the isolated organism was susceptible to the antibiotic administered. In our study appropriateness was judged using the Gyssen et al criteria.5 The major factors influencing inappropriate antibiotic therapy were improper dosage, timing, administration, and duration of therapy. About 57% of the cases of incorrect use were due to incorrect dosage, mainly attributed to failure of dosage adjustment in renal failure patients. Hence, physician education is extremely important to reduce irrational use of antibiotics.
The documented adverse effects of antibiotics mainly affect the GI system and skin,16 17 but in our case the majority of antibiotics caused ‘investigation-related’ adverse effects. For example, cefoperazone–sulbactam caused elevation of prothrombin time/international normalised ratio (PT/INR) in the majority of patients (figure 1).
The antimicrobial resistance pattern might increase or decrease with time. In our study, E coli and K pneumonia had developed 100% resistance towards cefotaxime since 2012 when the GI disorders of the gastroenterology department were taken separately. But our hospital-wide antibiotic susceptibility pattern showed 27% and 24% sensitivity, respectively, for 3714 isolates of E coli and 3757 K pneumoniae isolates towards cefotaxime. Sheikhbahaei et al18 conducted a study on SBP patients in 2014 and noticed a significant increase in the resistance of organisms towards cefotaxime, from 62.5% to 85.7%. In our study, E coli and K pneumoniae isolates showed increased resistance to meropenem (see online supplementary tables S1 and S2). A study19 conducted in India showed a significant increase in meropenem resistance towards K pneumoniae isolates. Another study20 also showed 22.1% of total isolates as being resistant to meropenem.
The current American21 and European guidelines22 recommend third generation cephalosporins, especially cefotaxime, as the first-line treatment for SBP. Recent studies23 24 have reported the emergence of resistance to third generation cephalosporins at rates of 21–41%. In contrast, a study25 conducted in India suggested that cefotaxime could still be the choice of primary empiric antibiotic therapy for SBP. Due to high resistance, ascitic fluid isolates are not commonly tested for cefotaxime sensitivity currently in our institution, and those isolates which are tested occasionally have shown complete resistance to cefotaxime. Other cephalosporins (ceftriaxone, cefoperazone, and cefixime) show susceptibility of <6% towards most of the E coli isolates. So we recommend cefoperazone–sulbactam as first line and piperacillin–tazobactam as second line for the treatment of SBP in our clinical setting; this is because 65% of E coli isolates were found to be susceptible to cefoperazone–sulbactam and 53% of isolates were susceptible to piperacillin–tazobactam. Even though colistin is 100% susceptible, it was not recommended as it is a reserved antibiotic.
Our study led to the development of an empirical antibiotic policy (see online supplementary table S3) which was developed by the clinical pharmacists in consultation with the physicians based on sensitivity patterns of bacterial isolates and was endorsed by the gastroenterology department. It is recommended to conduct an antibiotic stewardship programme to evaluate the effectiveness of the policy and an interventional study using a locally established guideline for increasing appropriateness of antibiotic therapy. Addition of a dedicated ‘antibiotic pharmacist’ to the healthcare team has shown to be of benefit to patients by reducing medication errors, reducing length of hospital stay, increasing savings on antibiotic costs, encouraging the use of oral medications, and ensuring the appropriate choice of drugs.26 Our study also suggests that clinical pharmacists can play an active role in monitoring the appropriateness of antibiotic use and help to develop guidelines for antibiotic use.
A limitation of our study is that the outcome of antibiotic therapy was not assessed.
Conclusion
Though antibiotic therapy was appropriate in the majority of patients in our study, irrational use occurred due to incorrect decision, choice, and use. There was an increase in the resistance of E coli and K pneumoniae in the year 2013–2014 as compared to the previous year. Although cefotaxime is recommended as the drug of choice for SBP by the American Association for the Study of Liver Diseases, in our setting cefoperazone–sulbactam is a better choice because of the development of 100% resistance towards cefotaxime by the causative bacteria. Rational use of antibiotics is needed to prevent further development of antibiotic resistance.
Key messages.
What is already known on this subject
Misuse of antibiotics can increase adverse drug reactions, duration of hospital stay, and cost of therapy.
Irrational use of antibiotics results in a steady increase in antibiotic resistance.
What this study adds
Inappropriate use of antibiotics continues to occur and monitoring of antibiotic usage by clinical pharmacists can help identify irrational use.
Incorrect use of antibiotics such as improper dosage, timing, administration, and duration of treatment are factors contributing to inappropriate use of antibiotics.
Even those antibiotics recommended as drugs of choice in certain infections can become ineffective due to development of resistance by the causative bacteria.
Our study led to the development of an empirical antibiotic policy which was endorsed by the gastroenterology department.
Footnotes
Contributors: GM collected the data. EJ, RPV and GM were involved in analysis and interpretation of results and preparation of the manuscript.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: Institutional Review Board of AIMS.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Davies J, Davies D. Origin and evolution of antibiotic resistance. Microbiol Mol Biol Rev 2010;74:417–33. 10.1128/MMBR.00016-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sutradhar KB, Saha A, Nazz HH, et al. Irrational use of antibiotics and antibiotic resistance in southern rural Bangladesh: perspectives from both the physicians and patients. Anns Res Rev Biol 2014;4:1421–30. 10.9734/ARRB/2014/8184 [DOI] [Google Scholar]
- 3.Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981;30:239–45. 10.1038/clpt.1981.154 [DOI] [PubMed] [Google Scholar]
- 4.Hartwig SC, Siegel J, Schneider PJ. Preventability and severity assessment in reporting adverse drug reactions. Am J Hosp Pharm 1992;49:2229–32. [PubMed] [Google Scholar]
- 5.Gyssens IC, van den Broek PJ, Kullberg BJ, et al. Optimizing antimicrobial therapy. A method for antimicrobial drug use evaluation. J Antimicrob Chemother 1992;30:724–7. 10.1093/jac/30.5.724 [DOI] [PubMed] [Google Scholar]
- 6. Pharmaceutical Care Network Europe [homepage on the Internet] The PCNE Classification for drug-related problems V 6.2. (accessed 8 Aug 2014). (Updated 14 January 2010). http://www.pcne.org/sig/drp/documents/PCNE%20classification%20V6-2.pdf.
- 7.WHO Collaborating Centre for Drug Statistics Methodology. Guidelines for ATC classification and DDD assignment 2013. Oslo: WHO; 2012. [Google Scholar]
- 8.Thamilselvi RT, Sinha P, Subrahmaniam PA, et al. A clinicopathological study of cholecystitis with special reference to analysis of cholelithiasis. IJBMS 2015;6:8–12. [Google Scholar]
- 9.Gupta K, Hootoon MT, Naber GK, et al. Guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 Update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious diseases. Clin Infect Dis 2011;52:e101–20. 10.1093/cid/cir102 [DOI] [PubMed] [Google Scholar]
- 10.Shrayteh ZM, Rahal MK, Malaeb DN. Practice of switch from intravenous to oral antibiotics. SpringerPlus 2014;3:717 10.1186/2193-1801-3-717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.James E, Cyria JM. Impact of educational interventions on the physicians for early switch over of parenteral drugs to oral therapy. Eur J Hosp Pharm 2015;22:176–8. 10.1136/ejhpharm-2014-000474 [DOI] [Google Scholar]
- 12.Berrington A. Antimicrobial prescribing in hospitals: be careful what you measure. J Antimicrob Chemother 2010;5:163–8. 10.1093/jac/dkp399 [DOI] [PubMed] [Google Scholar]
- 13.Ament PW, Jamshed N, Horne JP. Linezolid: its role in the treatment of gram positive, drug resistant bacterial infection. Am Fam Physician 2002;16:663–7. [PubMed] [Google Scholar]
- 14.Bain KT, Wittbrodt ET. Linezolid for the treatment of resistant gram positive organism. Ann Pharmacother 2001;35:566–75. 10.1345/aph.10276 [DOI] [PubMed] [Google Scholar]
- 15.Velasco Arribas M, Rubio Cirilo L, Casas Martín A. [Appropriateness of empirical antibiotic therapy in UTI in emergency room]. Rev Clin Esp 2010;210:11–16. 10.1016/j.rce.2009.06.009 [DOI] [PubMed] [Google Scholar]
- 16.Horen B, Montastruc JL, Lapeyre mestre M. Adverse drug reactions and off-label drug use in paediatric outpatients. Br J Clin Pharmacol 2002;54:665–70. 10.1046/j.1365-2125.2002.t01-3-01689.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shamna M, Dilip C, Ajmal M, et al. A prospective study on adverse drug reactions of antibiotics in a tertiary care hospital. Saudi Pharm J 2014;22:303–8. 10.1016/j.jsps.2013.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sheikhbahaei S, Abdollahi A, Hafezi-Nejad N, et al. Patterns of antimicrobial resistance in the causative organisms of spontaneous bacterial peritonitis: a single centre six years of experience of 1981 samples. Int J Hepatol 2014;2014:917856 10.1155/2014/917856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joseph Nm, Bhanupuriya B, Shewade DG, et al. Relationship between antimicrobial consumption and incidence of antimicrobial resistance in E. coli and K. pneumoniae isolates. J Clin Diagn Res 2015;9:6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta E, Mohanty S, Sood S, et al. Emerging resistance to carbapenems in a tertiary care hospital in north India. Indian J Med Res 2006;124:95–8. [PubMed] [Google Scholar]
- 21.Runyon BA. An introduction for the revised American Association for the Study of Liver Disease practice guidelines--Management of adult patients with ascites due to cirrhosis: update 2012. Hepatology 2013;57:1651–3. 10.1002/hep.26359 [DOI] [PubMed] [Google Scholar]
- 22.European Association of Study of Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis and hepato renal syndrome in cirrhosis. J Hepatol 2010;53:397–417. 10.1016/j.jhep.2010.05.004 [DOI] [PubMed] [Google Scholar]
- 23.Angeloni S, Leboffe C, Parente A, et al. Efficacy of current guidelines for the treatment of spontaneous bacterial peritonitis in clinical practice. World J Gastroenterol 2008;14:2757–62. 10.3748/wjg.14.2757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park MK, Lee JH, Byun YH, et al. Changes in the profile of causative agents and antibiotic resistance rate for spontaneous bacterial peritonitis: an analysis of cultured microorganisms in recent 12 years. Korean J Hepatol 2007; 13:370–7. 10.3350/kjhep.2007.13.3.370 [DOI] [PubMed] [Google Scholar]
- 25.Purohit HP, Malek SS, Desai KJ, et al. A study of bacteriological profile of ascitic fluid in suspected cases of spontaneous bacterial peritonitis at a tertiary care hospital in India. Int J Med Sci Public Health 2015;4:496–501. [Google Scholar]
- 26.Naomi F, Sue B, Diane AO. Pharmacists have critical role in conservation of effective antibiotics. Pharm J 2011;17:34. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ejhpharm-2015-000818supp001.pdf (158.8KB, pdf)