Abstract
Objectives
Studies have shown that medication histories obtained by clinical pharmacists (CPs) are more complete, and that medication reviews by CPs reduce healthcare costs, drug-related readmissions and emergency readmissions. The aim of this study was to identify the consequences of delegating medication-related tasks from physicians to CPs.
Methods
An analytical study based on data from a prospective cluster randomised trial was performed. The intervention consisted of CPs obtaining medication history, performing medication reconciliation and medication review. The physician had to approve the prescriptions and assess changes proposed by the CP. The primary outcome measure was a comparison of changes in the Electronic Medication Module (EMM) and changes proposed by CPs.
Results
232 and 216 patients were included on control days (n=63) and intervention days (n=63). In total, 1018 changes were made in the control group (by physicians). In the intervention group 2123 changes were made, 1808 by CPs and 315 by physicians. In particular, the number of substitutions, registration of drugs and change of instructions for use (eg, administration times) differed between physicians and pharmacists. CPs made 341 written proposals in the intervention group and, of these, 22.9% (95% CI 18.7% to 27.8%) and 50.9% (95% CI 45.5% to 56.2%) were accepted by a physician at discharge from the acute admission unit (AAU) and hospital, respectively.
Conclusions
CPs updated the EMM more thoroughly than physicians, especially entering new prescriptions, substitutions and changing instructions for use. Half of the written proposals were accepted. The extent to which patients benefit from a CP intervention is unknown.
Keywords: Actue admission unit, medication review, Medication reconciliation, medication history, CLINICAL PHARMACY
EAHP Statement 4: Clinical Pharmacy Services
Introduction
Obtaining a comprehensive medication history is time consuming1–4 and requires use of more than one source.5 Physicians have the knowledge,6 but do not spend much time on collecting information about the patient's medication history.7 Moreover, several studies show that physician-obtained medication histories are often incomplete.1 2 6 In particular, omission of prescribed medicines is a common error.1–4 6 In comparison, studies have reported that clinical pharmacists (CPs) obtain more comprehensive medication histories than physicians.3 4 6 8 However, not all discrepancies are clinically significant and medication reconciliation at admission, by itself, may not improve patient outcomes without further intervention.9
Medication reviews by CPs have been shown to reduce healthcare costs and drug-related readmissions10 11 and emergency readmissions,12 which have led to an increase in CP-led medication reviews in acute admission units (AAU).11 13–15 In addition, it has been reported that 25% of all patients with a drug prescription could benefit from a CP-led medication review.15 Contemporary physicians have stated a need for external medication counselling.16
The acceptance rate of CP interventions varies considerably, ranging from 18% to 80%.11 13 17 18 One reason for this could be how interventions are communicated, which has been shown to influence the acceptance rate.11 13 19
An extended CP intervention consisting of medication history uptake, medication reconciliation and medication review has been found to be relevant, timely and useful to physicians.13 However, to the best of our knowledge, what distinguishes a CP intervention from physicians' usual routines when admitting patients has not been investigated. Therefore, the aim of this prospective and analytical study was to describe the consequences of delegating medication-related tasks from physicians to CPs in an AAU.
Methods
Study design
The study was designed as an analytical study based on a prospective cluster randomised trial where weekdays were randomised for either control or intervention using http://www.randomization.com.20
Participants
Patients were included on weekdays from 09.00 to 16.15 in the AAU at Randers Regional Hospital, Denmark from 22 October 2013 until 1 May 2014. Eligible for inclusion were medical or surgical patients aged ≥18 years, taking ≥4 drugs daily (including over-the-counter (OTC) drugs, herbals and supplements). Terminally ill or intoxicated patients and patients assigned to triage level 1—that is, immediate life-saving intervention21—were excluded.
Study setting
The emergency department at Randers Regional Hospital has an annual flow of approximately 32 000 patients of which around 9000 are observed in a nine-bed AAU. The unit receives both medical and surgical adult patients for short-term observation (4–12 hours).
Control/standard care
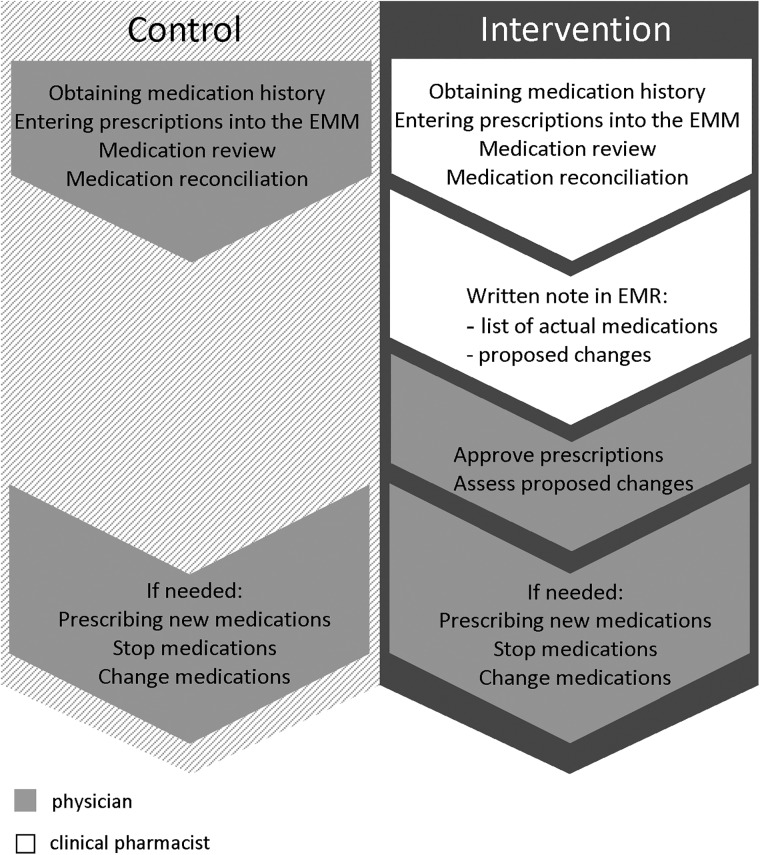
After triage performed by a nurse, the patient was examined by a physician (see figure 1 for usual routines regarding medication-related tasks).
Figure 1.
Allocation of tasks in the control and intervention groups. In the control group all medication-related tasks are performed by the physician. In the intervention group the tasks are divided between clinical pharmacist and physician. EMM, Electronic Medication Module; EMR, Electronic Medication Record.
Interventions
Two CPs working at the hospital pharmacy carried out the interventions. Physicians' usual routines for medication-related tasks are depicted in figure 1.
The CP medication history was based on a minimum of two sources of information—for example, shared medication record (electronic), medication list or telephone calls to the patient's general practitioner (GP). Whenever possible, one of the sources was an interview with the patient, relatives or both. Medication reconciliation was done by compiling all available information in the medication history and comparing this with the prescriptions in the Electronic Medication Module (EMM). The EMM is a module in the Electronic Medication Record (EMR) containing a list of all actual medications and information on doses, administration routes and exact time of prescription, administration and probable discontinuation.
The medication review followed the five steps described by Graabæk et al:14 (1) collection of clinical patient data; (2) collection of information on the patient's medical treatment; (3) patient interview; (4) critical examination of the patient's medication; and (5) recommendations for the hospital physician. A precondition for this approach was access to a clinical treatment plan (made by a physician) for the patients' present hospitalisation. Due to the study design, this was not an option in our study (see figure 1). Another exception was that the CPs did not obtain information about previous allergies.
The CPs communicated proposed changes in drug therapy to the physician as a written note in the EMR. Subsequently, the physicians had to assess and approve or decline the presented proposals (see figure 1). The CPs were allowed to perform the following changes without writing a proposal: (1) time of administration due to pharmacological issues (eg, some interactions, side effects, absorption); and (2) generic substitution to cheaper drugs in the EMM as required by Danish law.
Physicians could prescribe, stop or change medication as usual.
Data collection
All changes in the EMM made by physicians and CPs as well as the proposed changes were collected from the EMR and EMM by the first author (KBL) a few days after the intervention.
All changes were labelled according to the Anatomical Therapeutic Chemical Classification System (ATC) with a level 2 code corresponding to the anatomical and therapeutic main groups of the drug.22 In addition, it was noted whether the change involved a drug available OTC.
We used the Pharmaceutical Care Network Europe (PCNE) classification system V6.223 designed for classification of drug-related problems to categorise the changes proposed by CPs. The PCNE defines a drug-related problem as an event or circumstance involving drug therapy that actually or potentially interferes with desired health outcomes. We used the PCNE classification translated into Danish by Nielsen et al.13 The original P codes P2.1, P2.2 and P2.3 dealt with ‘adverse drug events’. However, we used these codes for ‘potential adverse drug events’ (pADEs), defined as drug-related incidents that could potentially result in an injury.
The classification of PCNE codes was performed by the first author (KBL) and a trained CP (CAS).
We determined the physicians' acceptance of the proposed changes as the proportion of ‘totally solved’ outcomes (O codes) classified according to the PCNE classification. The outcomes of proposed changes were assessed shortly after discharge from the hospital by review of the EMM and EMR.
Outcomes
Outcome measures in the present analytical study are listed in table 1. Note that some outcomes are only relevant for the intervention group.
Table 1.
Outcomes
| Outcome | Group |
|---|---|
| Overall number of changes | I, C |
| Distribution of all changes | I, C |
| Number of patients with no changes by physicians | I, C |
| Acceptance rate: performed and proposed changes | I |
| Distribution of P codes of proposed changes | I |
| Distribution of ATC codes of proposed changes | I |
ATC, Anatomical Therapeutic Chemical Classification System; C, control group; I, intervention group; P, Pharmaceutical Care Network Europe (PCNE) P category.
Data analysis
All data were analysed using STATA V.13 (Stata Corp, College Station, Texas, USA). Descriptive statistics were calculated for variables of interest using counts and percentages unless otherwise specified and were presented with 95% CIs.
For dichotomous outcomes, differences between study groups were tested with χ2 tests.
Results
Baseline data
Baseline characteristics were equally distributed among patients in the study groups (table 2).
Table 2.
Baseline characteristics and results from original trial
| Control group | Intervention group | p Value | |
|---|---|---|---|
| Patient level | |||
| Patients, n (%) | 232 (51.8) | 216 (48.2) | – |
| Age, mean (SD) | 69.8 (12.7) | 70.9 (13.8) | 0.36 |
| Gender, men (%) | 107 (46.1) | 109 (50.5) | 0.36 |
| Patient category, medical (%) | 161 (69.4) | 161 (74.5) | 0.23 |
| Admitted to hospital ward (%) | 188 (81.0) | 183 (84.7) | 0.10 |
| Discharged to GP from AAU (%) | 44 (19.0) | 33 (15.3) | 0.10 |
| Cluster level | |||
| Number of days, n (%) | 63 (50.0) | 63 (50.0) | – |
| Results from original trial 20 | |||
| Length of stay, min (95% CI) | 339 (322 to 357) | 342 (323 to 362) | 0.83 |
| No of drugs/patient (SD) | 8.8 (4.24) | 10.0 (4.03) | 0.002 |
| No of information sources* (95% CI) | – | 3.0 (2.9 to 3.1) | – |
*Number of information sources for medication history uptake; only compiled in intervention group (among clinical pharmacists).
AAU, acute admission unit.
Changes in EMM
Overall, physicians made 1018 changes in the control group and 2123 changes in the intervention group (1808 by CPs and 315 by physicians), as shown in table 3. Of 315 changes made by physicians in the intervention group, 198 were new prescriptions—a task restricted to physicians only. Physicians entered fewer drugs in the EMR, made fewer substitutions and removed fewer inappropriate or duplicated drugs from the EMR in the intervention group than in the control group.
Table 3.
Changes in Electronic Medication Module (EMM)
| Control group | Intervention group |
||
|---|---|---|---|
| Physician | Clinical pharmacist | Physician | |
| Total | |||
| All changes | 1018 | 1808 | 315 |
| Changes/patient | 4.4 | 8.4 | 1.5 |
| Changes/drug | 0.5 | 0.83 | 0.15 |
| Number of patients with no change in EMM | 21 | 0 | 75 |
| Type of changes | |||
| New prescription* | 256 | 198 | |
| Registration of drug† | 240 | 646 | 3 |
| Substitution (synonym or analogue) | 134 | 546 | 26 |
| Dosage changed | 84 | 172 | 20 |
| Formulation changed | 6 | 18 | 8 |
| Instructions for use changed‡ | 9 | 105 | 0 |
| Pausing of dose | 50 | 52 | 43 |
| Drug stopped§ | 236 | 307 | 16 |
| Other intervention | 3 | 4 | 1 |
*Only physicians were allowed to prescribe new drugs.
†Entering of drugs appearing in the medication history but not in the EMM.
‡Primarily changes in timing of administration and removal of self-administration.
§Primarily removal of inappropriate or duplicated drugs and drugs registered in EMM but not in medication history.
In some cases physicians did not make any changes in patient's medication at all (besides approving CP changes and proposals). The percentage of patients in whom the physician made no changes in the EMM differed significantly (p<0.01) between the study groups. In the control group this comprised 21 of 232 patients (9.1% ( 95% CI 5.7% to 13.5%)) compared with 75 of 216 patients (34.7% ( 95% CI 28.4% to 41.5%)) in the intervention group.
The CPs registered significantly more new drugs than physicians (p<0.01). Drugs available OTC accounted for 28.4% (95% CI 22.8% to 34.5%) of all the drugs registered by physicians compared with 39.6% (95% CI 35.7% to 43.7%) among the CPs.
Almost all changes in the EMM performed by CPs (98.2% (95% CI 97.3% to 98.6%)) were accepted by physicians.
Proposed changes
For the 216 patients in the intervention group, the CPs communicated a total of 341 drug changes to the physician as a written note in the EMR, corresponding to an average of 1.58 proposals/patient. Table 4 shows the distribution of the proposed changes on the PCNE P categories.
Table 4.
Number of proposed changes
| P category | Total |
|---|---|
| No/not optimal/wrong effect of drug treatment (P1.1–1.3) | 72 |
| Untreated indication (P1.4) | 39 |
| Potential adverse drug event (P2.1–2.3) | 149 |
| Drug treatment more costly than necessary (P3.1) | 11 |
| Unnecessary drug treatment (P3.2) | 36 |
| Patient dissatisfied with therapy (P4.1) | 21 |
| Unclear problem/complaint (P4.2) | 13 |
| All | 341 |
P, Pharmaceutical Care Network Europe (PCNE) P category.
Of the proposed changes, 22.9% ( 95% CI 18.7% to 27.8%) were accepted by the physicians in the AAU. However, for hospitalised patients, some of the proposed changes were acknowledged by physicians in other departments before discharge resulting in an acceptance rate of 50.9% ( 95% CI 45.5% to 56.2%). There was no statistically difference in acceptance rate between medical and surgical physicians (53.5% vs 41.3%, p=0.62).
The non-accepted proposals covered a wide range of different pharmacological issues—for example, changing a pain treatment from a non-steroidal anti-inflammatory to paracetamol; lowering drug doses in case of impaired kidney function; highlighting interactions between different drugs and warfarin that could result in an increased risk of bleeding.
The distribution of causes (C category of the PCNE classification) for the proposed changes of ‘potential adverse drug event’ (P2.1–P2.3) showed that more than 80% consisted of the categories ‘inappropriate drug’ (C1.1), ‘inappropriate combination of drugs, or drugs and food’ (C1.3) and ‘drug dose too high’ (C3.2).
Table 5 shows the distribution of ATC codes for proposed changes in the categories ‘potential adverse drug events’ and ‘no/not optimal/wrong effect of drug treatment’. In addition, the table provides examples of proposed changes written by the CPs.
Table 5.
Distribution of ATC codes for proposed changes labelled ‘potential adverse drug events’ (P2.1–2.3) and ‘no/not optimal/wrong effect of drug treatment’ (P1.1–P1.3)
| ATC code | No/not optimal/wrong effect of drug treatment (P1.1–1.3) | Potential adverse drug event (P2.1–2.3) | Example of proposed change in medication |
|---|---|---|---|
| A02 | 5 | 7 | Esomeprazole is recommended for patient with feeding tube instead of pantoprazole (P1.1) |
| A06 | 7 | 2 | Consider discontinuation of laxative in patient with diarrhoea (P1.3) |
| A12 | 5 | 6 | Patient treated with alendronate should also be treated with calcium and vitamin D (P1.2) |
| B01 | 2 | 22 | Interaction: citalopram and warfarin. Increased risk of bleeding (P2.1) |
| C01 | 5 | 6 | Recommend change in timing of administration of isosorbid dinitrate to reduce risk for tolerance (reduced therapeutic effect) (P1.2) |
| C03 | 7 | 10 | Spironolactone reduces clearance of digoxin (around 25%) (P2.1) |
| C07 | 1 | 7 | Consider dose too high of metoprolol due to interaction with sertraline (P2.1) |
| C09 | 1 | 10 | Patient with hyperkalaemia. This is a common side effect of losartan (P2.1) |
| C10 | 1 | 8 | Rosuvastatin is contraindicated due to severe renal impairment (P2.3) |
| J01 | 8 | 6 | Prophylactic nitrofurantoin is recommended to be administered at night (P1.2) |
| M01 | 3 | 13 | For patient treated with NSAID, prophylactic proton pump inhibitor is recommended (P2.1) |
| N02 | 13 | 30 | Co-codamol is prescribed for patient with allergy to paracetamol (P2.2) |
| N03 | 1 | 8 | Dose of pregabalin too high in patient with renal impairment (P2.1) |
| N05 | 1 | 15 | Dose of zopiclone too high in elderly patient (P2.1) |
| N06 | 2 | 23 | Concomitant use of venlafaxine and methadone increases risk of QT-interval prolongation (P2.1) |
| R03 | 9 | 1 | Dose of tiotropium (Respimat) too low (5 μm daily is recommended) (P1.2) |
| Other | 13 | 26 | Pramipexole is recommended instead of quinine for restless legs syndrome (P1.2) |
| Total* | 84 | 200 |
*One written clinical pharmacist (CP) proposal can deal with more than one Anatomical Therapeutic Chemical Classification System (ATC) code due to drug–drug interactions resulting in a larger number of total ATC codes than written CP proposals.
NSAID, non-steroidal anti-inflammatory drug.
Discussion
Overall, CPs made more changes than physicians (1808 vs 1018). In particular, CPs made more substitutions, registered more drugs in the EMM and changed instructions for use more frequently. Moreover, the number of patients in which the physician made no changes in the EMM differed significantly (21 patients (control) vs 75 patients (intervention)).
In line with several other study findings suggesting that CPs conduct more comprehensive medication histories than physicians,1–4 6 24 we found in the original trial20 that physicians identified fewer drugs per patient than CPs (8.8 vs 10.0 drugs). As a consequence, CPs registered more drugs in the EMM, as we found in the present study. However, not all the difference in the number of drugs registered could be explained by this. One additional explanation could be that CPs separated the medication review process into more steps than physicians in order to register every prescription correctly—for example, CPs registered the correct brand name of a drug and substituted to the available brand whereas physicians would register the available brand first. Whether these extra steps have any impact for the patient is difficult to determine since errors can occur in both working procedures. Another cause could be physicians giving lower priority to drugs available OTC than CPs, as we found (28% vs 40% of drugs entered in the EMM). Other studies have shown that OTC drugs are often omitted in medication histories.25 Despite the fact that OTC drugs seldom cause serious adverse drug events, clinically significant interactions between OTC drugs and prescribed drugs do occur,26 suggesting that these drugs should be addressed upon admission.
In general, CPs updated the EMM more thoroughly according to administrative standards. The extent to which this has an impact on quality of patient care cannot be deduced from our findings. However, a more complete EMM will presumably result in easier working procedures for nurses when administering medications, since nurses in the AAU are not allowed, for example, to substitute generic medication.
Interestingly, physicians only changed instructions for use of a drug nine times compared with 105 times by CPs. Examples of important instructions for use could be equal distribution of some antibiotic doses over the day or administration of alendronic acid a minimum of 30 min before other drugs, beverages or food to optimise the treatment effect.
In the intervention group there were significantly more patients for whom the physician made no changes in the EMM. Furthermore, physicians made far fewer changes in the EMM in the intervention group than in the control group as a result of CP intervention. This indicates that physicians were relieved from some tasks and, thus, could concentrate on tasks related to the patient's actual illness. However, the downside could be that physicians pay less attention to medication, leading to unawareness of their responsibility for the patient's overall medication treatment. This could explain why we found fewer new prescriptions in the intervention group than in the control group. On the other hand, this difference could also be due to CPs updating the EMM in the intervention group to a greater extent and registering more drugs than physicians in the control group, as discussed above.
As previously mentioned, CPs were allowed to perform a number of changes in the EMM. Physicians rarely opposed these changes. However, for written proposals the acceptance rate in our study was 22.9% in the AAU and 50.9% at discharge from other hospital departments. There is no consensus in the literature on how to measure physicians' acceptance rate, making the credibility of comparison low. A crude comparison of the acceptance rate in our study with findings in the literature placed it in the middle of the range of rates from comparable studies.11 13 17–19
In general, the acceptance rate varies with the route by which the proposed CP interventions are communicated11 13 19 and the category of the proposal.18 A large-scale questionnaire among physicians demonstrated that physicians preferred to receive proposals as notes in the EMR,16 so we chose this route in our study.
Since many of the proposals were accepted after the patients were transferred to another hospital department, it questions whether patients being discharged directly from the AAU have unsolved potential drug-related problems. Unfortunately, GPs did not have access to the written CP proposals and therefore were not informed about these potential drug-related problems. Further investigations are needed in order to illustrate the extent of this problem.
More conditions may have contributed to our low acceptance rate. First, the length of stay in the AAU is short (average 340 min), therefore many of the proposals might not have been read or dealt with in the AAU. Second, physicians admitting patients in the AAU are often junior physicians with short clinical experience who possibly do not feel confident to consider some of the CP's proposed changes. On the other hand, junior physicians have been shown to be confident in their prescribing skills.27 Third, due to overload of notes in the EMR, the physicians may unknowingly have disregarded some of the written proposals. Fourth, there might be some hesitation to change the patient's regular drug prescriptions.16 A reason could be that this demands knowledge of how to identify and correct non-optimal prescriptions, especially in drug prescriptions related to other medical specialities.16 Finally, psychological reactance might play a role. This is a well-established theory explaining that we often choose to do the opposite of what is recommended. This is also present for physicians' acceptance of CP recommendations28 and could play a role here.
Finally, the ideal time and place for completing medication review should be discussed. The low acceptance of CP proposals in the AAU suggests that medication review would be more efficiently performed on other hospital wards. On the other hand, this would lead to duplication of work—that is, two interviews with the same focus (one in the AAU and one in the hospital ward)—since a gold standard medication review compromises an interview with the patient.14 In addition, it will preclude patients who are discharged directly home from the AAU from having a thorough medication review.
In order to prevent potential drug-related problems it has been suggested that medication reviews should be supplemented with additional approaches.29 A large-scale American study found that consultative activities—for example, CPs taking part in ward rounds—resulted in the interception of more medication errors than medication reviews.29 A combination of CP medication reviews and consultations may therefore be preferred.
Additionally, implementation of a CP intervention in the AAU demands evidence on which patients can focus.
Our study indicated that the big difference between the two professions can be viewed as follows: physicians have their main focus on the patient and look at the medication through the patient while CPs have their main focus on the medication and look at the patient through the medication. This suggests that academic professions can complement each other in a fruitful and productive way.
Study limitations
The generalisability of the study is somewhat limited due to the single-centre focus, the recruitment of patients during office hours only and the alternative interpretation of the PCNE classification. Moreover, the classification of potential drug-related problems was primarily carried out in consensus by two persons only, which increases the uniformity of the classification but introduces a possible source of error.
The number of published studies using the PCNE classification system is limited, particularly in acute care settings. However, studies have found the system to be useful and a practical tool in hospital settings.13 30 Additionally, one Danish study13 performed an inter-rater reliability study of the PCNE classification as a tool for CP interventions in acute wards and found substantial agreement for P, C and I codes (they did not test O codes). This indicates that the PCNE classification system is applicable in AAUs.
Unfortunately, the system is not entirely clear. In the classification scheme a headline is ‘Adverse drug reaction (ADR)’ (P2) and the elaboration is ‘patient suffers, or will possibly suffer, from an adverse drug event (ADE)’. We used the codes for potential ADEs and not only in cases of actual ADEs. This interpretation resulted in a higher number of proposals classified with P2 codes in our study compared with other studies.
Conclusions
CPs updated the EMM more thoroughly than physicians, especially entering new prescriptions, substitutions and changing instructions for use. Half of the written proposals were accepted.
A CP intervention can thus reduce administrative tasks for physicians, ensure a more updated EMM and increase the focus on potential drug-related problems. The extent to which patients benefit from a CP intervention is unknown.
Key messages.
What is already known on this subject?
Several studies have shown that clinical pharmacists (CPs) obtain more comprehensive medication histories than physicians.
CP interventions have positive effects on medication use, health service use and costs.
A CP intervention consisting of medication history, medication reconciliation and medication review is relevant, timely and useful to physicians.
What this study adds?
CPs updated the Electronic Medication Module more thoroughly than physicians, especially entering of prescriptions, substitutions and changing instructions for use.
Physicians in the acute admission unit accepted only one in five proposals made by CPs. Half of the proposals were accepted before discharge from hospital.
Footnotes
Contributors: KBL, CAS, SAS and ML were responsible for the planning and design of the study. KBL and CAS were responsible for data collection and analysis. KBL and CAS wrote the first draft. SAS and ML provided critical revision. All authors read and approved the final manuscript.
Funding: This study was funded by the Research Center for Emergency Medicine at Aarhus University Hospital, Denmark, the Hospital Pharmacy of Aarhus and Randers Regional Hospital, Denmark.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Tam VC, Knowles SR, Cornish PL, et al. Frequency, type and clinical importance of medication history errors at admission to hospital: a systematic review. CMAJ 2005;173:510–15. 10.1503/cmaj.045311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. De Winter S, Spriet I, Indevuyst C, et al. Pharmacist- versus physician-acquired medication history: a prospective study at the emergency department. Qual Saf Health Care 2010;19:371–5. 10.1136/qshc.2009.035014 [DOI] [PubMed] [Google Scholar]
- 3. Gleason KM, McDaniel MR, Feinglass J, et al. Results of the Medications at Transitions and Clinical Handoffs (MATCH) study: an analysis of medication reconciliation errors and risk factors at hospital admission. J Gen Intern Med 2010;25:441–7. 10.1007/s11606-010-1256-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Reeder TA, Mutnick A. Pharmacist- versus physician-obtained medication histories. Am J Health Syst Pharm 2008;65:857–60. 10.2146/ajhp070292 [DOI] [PubMed] [Google Scholar]
- 5. World Health Organization. Assuring Medication Accuracy at Transitions in Care. Patient Safety Solution 2007. http://www.who.int/patientsafety/events/07/02_05_2007/en/ (accessed 15 Oct 2014).
- 6. Basey AJ, Krska J, Kennedy TD, et al. Prescribing errors on admission to hospital and their potential impact: a mixed-methods study. BMJ Qual Saf 2014;23:17–25. 10.1136/bmjqs-2013-001978 [DOI] [PubMed] [Google Scholar]
- 7. Ghazanfar MN, Honoré PH, Nielsen TR, et al. Hospital admission interviews are time-consuming with several interruptions. Dan Med J 2012;59:A4534. [PubMed] [Google Scholar]
- 8. Mergenhagen KA, Blum SS, Kugler A, et al. Pharmacist- versus physician-initiated admission medication reconciliation: impact on adverse drug events. Am J Geriatr Pharmacother 2012;10:242–50. 10.1016/j.amjopharm.2012.06.001 [DOI] [PubMed] [Google Scholar]
- 9. Kwan JL, Lo L, Sampson M, et al. Medication reconciliation during transitions of care as a patient safety strategy: a systematic review. Ann Intern Med 2013;158(5 Pt 2):397–403. 10.7326/0003-4819-158-5-201303051-00006 [DOI] [PubMed] [Google Scholar]
- 10. Graabaek T, Kjeldsen LJ. Medication reviews by clinical pharmacists at hospitals lead to improved patient outcomes: a systematic review. Basic Clin Pharmacol Toxicol 2013;112:359–73. 10.1111/bcpt.12062 [DOI] [PubMed] [Google Scholar]
- 11. Gillespie U, Alassaad A, Henrohn D, et al. A comprehensive pharmacist intervention to reduce morbidity in patients 80 years or older: a randomized controlled trial. Arch Intern Med 2009;169:894–900. 10.1001/archinternmed.2009.71 [DOI] [PubMed] [Google Scholar]
- 12. Christensen M, Lundh A. Medication review in hospitalised patients to reduce morbidity and mortality. Cochrane Database Syst Rev 2013;2:CD008986 10.1002/14651858.CD008986.pub2 [DOI] [PubMed] [Google Scholar]
- 13. Nielsen TR, Andersen SE, Rasmussen M, et al. Clinical pharmacist service in the acute ward. Int J Clin Pharm 2013;35:1137–51. [DOI] [PubMed] [Google Scholar]
- 14. Graabæk T, Bonnerup DK, Kjeldsen LJ, et al. Pharmacist-led medication review in an acute admissions unit: a systematic procedure description. Eur J Hosp Pharm 2015;22:202–6. 10.1136/ejhpharm-2014-000507 [DOI] [Google Scholar]
- 15. Mogensen CB, Thisted AR, Olsen I. Medication problems are frequent and often serious in a Danish emergency department and may be discovered by clinical pharmacists. Dan Med J 2012;59:A4532. [PubMed] [Google Scholar]
- 16. Bonnerup DK, Lisby M, Eskildsen AG, et al. Medication counselling: physicians’ perspective. Basic Clin Pharmacol Toxicol 2013;113:425–30. 10.1111/bcpt.12111 [DOI] [PubMed] [Google Scholar]
- 17. Lisby MMHS, Bonnerup DKMP, Brock B, et al. Medication review and patient outcomes in an orthopedic department: a randomized controlled study. J Patient Saf. Published Online First: 4 Mar 2015 10.1097/PTS.0000000000000173 [DOI] [PubMed] [Google Scholar]
- 18. Gronkjaer LS, Jensen ML, Madsen H, et al. Successful implementation of pharmaceutical intervention at an acute medical admission unit. Ugeskr Laeg 2011;173:1353–5. [PubMed] [Google Scholar]
- 19. Taegtmeyer AB, Curkovic I, Rufibach K, et al. Electronic prescribing increases uptake of clinical pharmacologists’ recommendations in the hospital setting. Br J Clin Pharmacol 2011;72:958–64. 10.1111/j.1365-2125.2011.04032.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lind KB, Soerensen CA, Salamon SA, et al. Impact of clinical pharmacist intervention on length of stay in an acute admission unit: a cluster randomised study. Eur J Hosp Pharm 2016;23:171–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Agency for Healthcare Research and Quality. Emergency Severity Index (ESI). A triage tool for emergency department care. Version 4 2012. http://www.ahrq.gov/sites/default/files/wysiwyg/professionals/systems/hospital/esi/esihandbk.pdf [DOI] [PubMed] [Google Scholar]
- 22. WHO Collaborating Centre for Drug Statistics Methodology. Anatomical Therapeutic Chemical (ATC) classification system. Structure and principles. http://www.whocc.no/atc/structure_and_principles/ (accessed 3 Jun 2015).
- 23. Pharmaceutical Care Network Europe (PCNE). Classification system v6.2. http://www.pcne.org/upload/files/11_PCNE_classification_V6-2.pdf (accessed Apr 2015).
- 24. Vasileff HM, Whitten LE, Pink JA, et al. The effect on medication errors of pharmacists charting medication in an emergency department. Pharm World Sci 2009;31:373–9. 10.1007/s11096-008-9271-y [DOI] [PubMed] [Google Scholar]
- 25. Oborne CA, Luzac ML. Over-the-counter medicine use prior to and during hospitalization. Ann Pharmacother 2005;39:268–73. 10.1345/aph.1D160 [DOI] [PubMed] [Google Scholar]
- 26. Hersh EV, Pinto A, Moore PA. Adverse drug interactions involving common prescription and over-the-counter analgesic agents. Clin Ther 2007;29(Suppl):2477–97. 10.1016/j.clinthera.2007.12.003 [DOI] [PubMed] [Google Scholar]
- 27. Ryan C, Ross S, Davey P, et al. Junior doctors’ perceptions of their self-efficacy in prescribing, their prescribing errors and the possible causes of errors. Br J Clin Pharmacol 2013;76:980–7. 10.1111/bcp.12154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. de Almeida Neto AC, Chen TF. When pharmacotherapeutic recommendations may lead to the reverse effect on physician decision-making. Pharm World Sci 2008;30:3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Patanwala AE, Sanders AB, Thomas MC, et al. A prospective, multicenter study of pharmacist activities resulting in medication error interception in the emergency department. Ann Emerg Med 2012;59:369–73. 10.1016/j.annemergmed.2011.11.013 [DOI] [PubMed] [Google Scholar]
- 30. Lampert ML, Kraehenbuehl S, Hug BL. Drug-related problems: evaluation of a classification system in the daily practice of a Swiss university hospital. Pharm World Sci 2008;30:768–76. 10.1007/s11096-008-9213-8 [DOI] [PubMed] [Google Scholar]