Abstract
Objective
Incorrect storage and handling of refrigerated medicines may result in destruction of medicines and financial loss for hospitals. At the Medicine Information Centre we receive and answer queries on drug-related issues. In this study we aimed to investigate and quantify savings made following advice supplied by the Medicine Information Centre in reply to queries regarding the incorrect storage of refrigerated medicines.
Methods
A retrospective study was conducted by systematically reviewing each drug when the cold chain had been impaired, in order to determine whether the drug could continue to be used, possibly with a shortened expiry date. Thus, by examining all cases of incorrect storage, the value of drugs that pharmacists advised could be used despite a broken cold chain, could be estimated.
Results
The Medicine Information Centre dealt with 171 cases concerning incorrect storage in 2013. Data show that advice from Medicine Information Centre pharmacists resulted medicine cost savings of DKK 13 million (approx. €1.7 million) in hospitals in the Capital Region for that year.
Conclusions
Substantial savings can be made by seeking the advice of a team of information pharmacists regarding the incorrect storage of medicines.
Keywords: DRUG STORAGE AND DISTRIBUTION, CLINICAL PHARMACY
Introduction
Refrigerated medicines are frequently disposed of in hospitals because of incorrect storage despite Good Distribution Practice guidelines.1 However, the incorrect storage and handling of refrigerated medicinal products may result in their unnecessary destruction and consequent financial loss for the hospital. It is often difficult to access information about whether such drugs can still be used, and so such medicines are often unnecessarily discarded.
In the Capital Region of Copenhagen a significant number of refrigerated medicines are sold each year, with turnover reaching DKK 1.419 million (€ 189 million) in 2013.
The 11 pharmacists working at the Medicine Information Centre receive and answer queries regarding medicine-related issues from healthcare professionals employed in the Capital Region. Our main objective is to help ensure rational drug use and patient safety while avoiding unnecessary costs associated with patient treatment. All queries are documented in a national question and answer database called SAID. Approximately 25% of all inquiries in SAID involve questions about the storage and stability of medicines. The vast majority of these inquiries concern the incorrect storage of refrigerated medicines.
A guide has been developed to ensure high quality, consistent and systematic assessment of issues regarding incorrect storage of medicinal products. It includes a list of questions concerning all relevant information about the incident. The guide also indicates which information sources must be consulted, for example, the internal list (including statements from the manufacturers), the summary of product characteristics and the SAID database. The manufacturer is contacted when other sources are insufficient. The guide also contains general points about the future storage and handling of affected medicines.2 This procedure ensures that a medicine that has been degraded or otherwise affected by incorrect storage is not used for patient treatment, as well as preventing unnecessary disposal of often expensive medicines.
Inexpensive refrigerated medicines costing less than DKK 300 (€40) are discarded without further assessment as the cost of case handling would exceed the actual cost of the medicine.
To our knowledge, there have been no similar studies previously, and the published literature is sparse.3 However, we are aware that similar issues are handled by hospital pharmacies in other countries (Study visit to Northwick Park Hospital, London Medicines Information Service, Pharmacy, September 2012).
The Medicine Information Centre conducts an annual audit of queries on refrigerated medicines discarded due to incorrect storage. In a systematic review, each drug where the cold chain was broken was assessed in order to determine whether the medicine could have continued to be used, possibly with a reduced shelf life. The aim of this study was to investigate and quantify cost savings following advice from the Medicine Information Centre regarding the incorrect storage of refrigerated medicines.
Methods
A retrospective study using the Danish database ‘The Hospital Pharmacies Information Databases’ (SAID) was conducted in order to estimate the amount of money saved in 2013. SAID contains all documented inquiries about medicine information, including incorrect storage of medicine since the database was set up. Thus, examination of all incorrect storage cases allowed estimation of the value of medicine that, following pharmacist advice, could continue to be used despite a broken/impaired cold chain.
Search criteria
SAID is used by all hospital pharmacies in Denmark for documenting answers to queries. An inquiry entered in SAID is categorized under predetermined headings such as ‘Adverse Effects’ and ‘Incorrect Storage’. Data categorized as ‘Incorrect Storage’ were collected from 1 January 2013 to 31 December 2013.
The cases identified were separated into ‘Refrigerator Failure’ and ‘Incorrect Handling’ to distinguish between technical and human error, respectively. Examples of human error include refrigerated medicines not stored in a refrigerator but accidently left at room temperature, cool boxes not unpacked and refrigerator doors left open.
Collected information
Cases were separated into the two categories ‘Refrigerator Failure’ and ‘Incorrect Handling’, and information on the Anatomical Therapeutic Chemical (ATC) classification, the name of the medicinal product and if possible, the article number and number of packages stored incorrectly, was collected.
Estimating price
The item price of cases was determined if possible by reference to ApoVision, the storage logistics IT system. If the item price was unavailable, the price was estimated as follows:
If the number of packages involved was not mentioned, only one item was counted.
If the article number was not mentioned, the cheapest medicine was chosen from the list of possible preparations.
If the article number was given, but the price at the time of the event was missing, the price in April 2014 was used.
Consequently, the lowest possible price was estimated. (SAID, Sygehus Apotekernes Informations Database, Sygehusapotekerne I Danmark—national Q&A database. ApoVision, Region Hovedstadens varestyringssystem—Storage logistics system).
Results
The Medicine Information Centre dealt with 171 cases of incorrect storage in 2013. In 74 of these cases, all medicines concerned were discarded. In the remaining 97 cases, the Centre recommended that at least one or more of the medicines could be returned to stock as the shelf life was deemed by the pharmacist to be either shortened or unchanged. Information concerning packages with reduced shelf life, the number of packages, the article number and the prices of medicines returned to stock was collected. The results are shown in table 1.
Table 1.
Summary of results
| Refrigerator failure | Incorrect handling | |
|---|---|---|
| Number of cases | 34 | 63 |
| Number of units | 237 | 253 |
| Number of units with reduced shelf life (number of article, units and price known) | 58 (25%) | 17 (7%) |
| Number of units with unchanged shelf life (number of article, units and price known) | 22 (9%) | 61 (24%) |
| Article number unknown | 57 (24%) | 39 (15%) |
| Number of packages unknown | 77 (32%) | 123 (49%) |
| Price unknown | 23 (10%) | 13 (5%) |
Data were collected for 34 ‘Refrigerator Failure’ cases with 237 units, and 63 ‘Incorrect Handling’ cases with 253 units (a unit is one specific product (one or more packages) in one specific case). Five of 237 units among the ‘Refrigerator Failure’ cases and six of 253 units among the ‘Incorrect Handling’ cases were valued at between DKK 100 000 (approx. €13 000) and DKK 1 million (approx. €133 000) each.
One unit in an ‘Incorrect Handling’ case was valued at above DKK 8.5 million (approx. €1.13 million) and another case at above DKK 1 million (approx. €133 000).
DKK 13 million (approx. €1.7 million) was saved in 2013, where the values of medicines subject to ‘Refrigerator Failure’ and ‘Incorrect Handling’ were DKK 1.8 million (approx. €240 000) and DKK 11.2 million (approx. €1.5 million), respectively (see table 2).
Table 2.
Savings in DDK according to the Anatomical Therapeutic Chemical (ATC) classification system
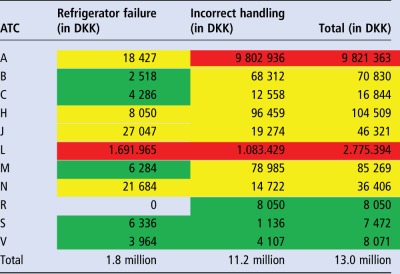 |
Groups D, G and P were under the minimum limit (less than DKK 1000) and were therefore excluded. Red indicates costs above DKK 100 001, yellow costs of DKK 10 001–100 000 and green costs of DKK 1000–10 000.
Refrigerator failure: in ATC group L, Avastin was common, with one case accounting for DKK 0.6 million; the remaining DKK 1.7 million was accounted for by many cases and different medicines.
Incorrect handling: in ATC group A, Fabrazyme and Replagal were common with a single random case accounting for DKK 8.5 million;
in ATC group L, MabThera and Herceptin were common, with many individual cases contributing to the total of DKK 1.1 million.
As mentioned above, one case alone was valued at DKK 8.5 million, which is not necessarily the case every year, but it shows the importance of the Medicine Information Centre because the potential for savings is significant.
For comparison, the total turnover of refrigerated medicines in 2013 in the Capital Region was DKK 1.419 million (approx. €189 million) corresponding to approximately 500 000 packages (Årsrapport 2013, Region Hovedstadens Lægemiddelkomité Drug and Therapeutic Committee). The amount saved was equal to 0.9% of annual turnover, keeping in mind that this is the lowest possible estimation.
In table 2 the costs of cases are further divided into ATC groups. The data show that ATC groups A and L were particularly involved in refrigerator storage cases in 2013.
Discussion
The results show that pharmacist advice given by the Medicine Information Centre allowed hospitals in the Capital Region to save medicines valued at DKK 13 million (approx. €1.7 million) in 2013.
The number of ‘Refrigerator Failure’ cases could be reduced with improved surveillance. Hospitals are aware of the extent of wastage of pharmaceuticals3 and so refrigerator alarms and loggers, as well as cool box trackers, are being increasingly used.
However, the main problem seems to be ‘Incorrect Handling’ of medicine. The costs of these cases at DKK 11.2 million are significantly higher than for ‘Refrigerator Failure’ cases, which were valued at DKK 1.8 million. This indicates that the focus should be on minimising human error.
This study uncovered a single random case which represented a very significant amount and highlights the potential for savings.
Possible risks associated with obtaining data from SAID were minimised by collecting data throughout an entire year (2013), so the results were not affected by seasonal changes.
Possible limitations of this study are:
‘Incorrect Storage’ cases miscategorised under other headings could have been identified by a complete search of the database.
Medicine prices may have changed during 2013 so the use of April 2014 prices could have resulted in incorrect estimates. However, none of the affected drugs came off patent in 2013, so the prices most probably varied little if at all.
In some cases, the medicine given a shortened shelf life may not have been used in time before it had to be discarded.
A huge quantity of refrigerated medicines is sold every year in the Capital Region of Copenhagen: turnover was DKK 1.419 million in 2013, with the top 20 most expensive refrigerated medicines accounting for almost DKK 800 million of that figure. This corresponds to 23% of all packages and 56% of refrigerated medicines delivered to hospitals.
Although refrigerated medicines constitute only a small proportion of all medicines sold, expensive medicines are often stored in cool boxes and refrigerators, and costs are incurred if they have to be discarded due to incorrect storage. The pharmacists in the Medicine Information Centre facilitated large cost savings in 2013.
Conclusion
In 2013, the Medicine Information Centre for the Capital Region facilitated a minimum saving of DKK 13 million (€1.7 million) on refrigerated medicines in hospitals. Savings can be made by consulting a pharmacist or a team of information pharmacists concerning the frequent incorrect storage of refrigerated medicine.
Key messages.
What is already known on this subject
Improper storage of medicines is frequent but the size of the problem has not previously been investigated or whether it is possible to save money by continuing to use improperly stored drugs.
The study helps to clarify that pharmaceutical assistance is important and results in cost savings.
What this study adds
Pharmacist advice in cases of incorrect storage can result in significant savings in medical costs for hospitals.
Improper storage of medicines is a problem, so it would be cost effective to develop systems to reduce human error associated with the improper storage of medicines.
Footnotes
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Official Journal of the European Union. European Commission guideline of 5 November 2013 on Good Distribution Practice of medicinal products for human use (2013/C 343/01).
- 2. Lone Schmidt-Petersen et al. Guide for handling queries about incorrect storage of medicine. Poster presented at the 2011 ASHP Midyear Clinical Meeting.
- 3.Hart RJ, Marshall FSV, Wastage of pharmaceuticals. Lancet, 1976;2:1239–40. [DOI] [PubMed] [Google Scholar]


