Abstract
Objectives
Patients can benefit from the coadministration of several medications because of the shorter infusion time and more rapid administration. The use of extemporaneously prepared admixtures of dexamethasone sodium phosphate (DSP) and 5-HT3 receptor antagonists (5-HT3RAs) must be supported by sufficient documentation of their compatibility. The objective of this study was to comprehensively investigate the compatibility of DSP with 5-HT3RAs in infusion solutions.
Methods
Admixtures of DSP with six different 5-HT3RAs (ondansetron hydrochloride, tropisetron hydrochloride, dolasetron mesylate, azasetron hydrochloride, palonosetron hydrochloride and ramosetron hydrochloride) were prepared in non-polyvinyl chloride (non-PVC) infusion bags filled with 5% glucose or 0.9% NaCl. Bags were stored at ambient temperature (25±2°C) without protection from light. Samples were taken immediately after preparation (0 hour) and at predetermined intervals (12, 24 and 48 hours after preparation). Particulate matter of admixtures was inspected visually and particles were counted with a particle counter. The pH of each sample was also determined. Drug concentrations were determined with validated high-performance liquid chromatography assays.
Results
No visible haze or particulate formation, colour change or gas evolution and no notable changes in pH were observed, and particulate matter was acceptable up to 48 hours. All preparations maintained more than 90.0% of the initial concentration over the study period.
Conclusions
All the admixtures of DSP and the 5-HT3RAs studied were compatible and stable for at least 48 hours in a 5% glucose injection or a 0.9% NaCl injection stored in non-PVC infusion bags under ambient conditions.
Keywords: compatibility, dexamethasone sodium phosphate, 5-HT3 receptor antagonists, admixture, chemotherapy-induced nausea and vomiting
EAHP Statement 4: Clinical Pharmacy Services.
Introduction
Chemotherapy-induced nausea and vomiting (CINV) is one of the greatest problems for patients with cancer receiving treatment.1 Adequately controlled CINV can improve their functional activity and quality of life, decrease their use of healthcare resources, and enhance their adherence to treatment.1 5-HT3 receptor antagonists (5-HT3RAs) offer potent antiemetic effects by binding and inhibiting the 5-HT3 receptor.1–3 5-HT3RAs approved in America, Europe, Japan and China among other countries include granisetron hydrochloride, ondansetron hydrochloride, tropisetron hydrochloride, dolasetron mesylate, azasetron hydrochloride, palonosetron hydrochloride and ramosetron hydrochloride.1–4 Corticosteroids, another kind of effective antiemetic agent, are always used in combination with 5-HT3RAs.1–3
A number of large clinical trials have established that coadministration of dexamethasone sodium phosphate (DSP) and 5-HT3RAs improves the management of CINV.1–5 For instance, DSP with ondansetron hydrochloride is more efficacious than ondansetron hydrochloride monotherapy in protecting patients from cisplatin-induced nausea and vomiting.5 DSP combined with granisetron hydrochloride has been found to be effective for the prevention of vomiting induced by moderately emetogenic chemotherapy.6 Furthermore, the regimen of 5-HT3RAs plus DSP is recommended for CINV by the European Society of Medical Oncology (ESMO), the Multinational Association of Supportive Care in Cancer (MASCC) and the National Comprehensive Cancer Network (NCCN).2 7
Patients can benefit from the coadministration of several medications because of the shorter infusion time and more rapid administration. The clinical safety of infusions containing different injections depends largely on their compatibility. Although there have been several reports on the compatibility of DSP with 5-HT3RAs in infusion solutions,8 the available evidence is not yet conclusive. Up to now, the compatibility of DSP with tropisetron hydrochloride, dolasetron mesylate, azasetron hydrochloride and ramosetron hydrochloride has not been reported. The purpose of this study was to assess the compatibility of DSP with common 5-HT3RAs in infusion bags under ambient conditions.
Methods
Materials
All formulations were obtained commercially in China (table 1). The infusion bags filled with 5% glucose or 0.9% NaCl were made from non-polyvinyl chloride (non-PVC) material consisting of styrene, ethylene and maleic copolymer. Reference standards (chemical purity >99.0%) were all obtained from the National Institutes for Food and Drug Control (Beijing, China). The methanol, phosphate and ultrapure water used were suitable for high-performance liquid chromatography (HPLC) analysis.
Table 1.
Drugs studied for compatibility of dexamethasone sodium phosphate with 5-HT3 receptor antagonists
| Drug | Formulation | Specification | Excipient | Manufacturer | Lot number |
|---|---|---|---|---|---|
| Dexamethasone sodium phosphate | Powder for injection | 5 mg | Mannitol, water for injection | BBCA Pharmaceutical Co (Anhui, China) | 150130-1 |
| Ondansetron hydrochloride | Injection | 8 mg/4 mL | Citric acid, sodium citrate, NaCl | Ningbo Team Pharmaceutical Co (Zhejiang, China) | 140294A02 |
| Tropisetron hydrochloride | Infusion | 5 mg/100 mL | NaCl, HCl | Qilu Pharmaceutical Co (Shangdong, China) | 3C14111803 |
| Dolasetron mesylate | Injection | 12.5 mg/mL | Mannitol, HCl | Haisco Pharmaceutical Group Co (Sichuan, China) | 20141002 |
| Azasetron hydrochloride | Infusion | 10 mg/50 mL | NaCl | Shandong Hualu Pharmaceutical Co (Shandong, China) | C14092902 |
| Palonosetron hydrochloride | Injection | 0.25 mg/5 mL | NaCl, citric acid, sodium citrate, sodium calcium edetate | Hangzhou Jiuyuan Gene Engineering Co (Zhejiang, China) | 20150102 |
| Ramosetron hydrochloride | Powder for injection | 0.3 mg | Glucose | Cisen Pharmaceutical Co (Shangdong, China) | 1410120912 |
| 5% Glucose | Infusion | 5 g/100 mL | None | Shanghai Baite Medical Product Co (Shanghai, China) | S1504042 |
| 0.9% NaCl | Infusion | 0.9 g/100 mL | None | Shanghai Baite Medical Product Co (Shanghai, China) | S1504103 |
| Water for injection | Injection | 5 mL | None | Hubei Kelun Pharmaceutical Co (Hubei, China) | C140812E |
The 5-HT3RAs infusions, ondansetron hydrochloride, dolasetron mesylate, palonosetron hydrochloride, ramosetron hydrochloride, were prepared by transferring the injections to 100 mL 5% glucose or 0.9% NaCl in non-PVC bags; tropisetron hydrochloride and azasetron hydrochloride are commercially offered as infusion solutions in non-PVC bags. The appropriate amount of DSP, prepared for injection by dissolving powder in water, was then added to each 5-HT3RA infusion. Finally, admixtures of DSP and each 5-HT3RA were prepared. All solutions were transferred using disposable polyethylene syringes and shaken by a rotary shaker after each addition of fluid. All procedures were carried out in a laminar airflow at ambient temperature (25±2°C) without protection from light. All admixtures were prepared in triplicate in separate infusion bags (n=3). In each case, the final nominal concentrations of DSP and 5-HT3RAs corresponded to those used in daily medical practice (table 2). Admixtures were stored at ambient temperature (25±2°C) without protection from light. Samples were collected immediately after preparation (0 hour) and at predetermined intervals (12, 24 and 48 hours after preparation).
Table 2.
Admixture of dexamethasone sodium phosphate (DSP) with selected 5-HT3 receptor antagonists (5-HT3RAs)
| 5-HT3RA | Solvent | Nominal concentration (μg/mL) |
||
|---|---|---|---|---|
| Code of admixtures | DSP | 5-HT3RAs | ||
| A | Ondansetron hydrochloride | 5% glucose | 100.0 | 80.0 |
| B | Ondansetron hydrochloride | 5% glucose | 200.0 | 80.0 |
| C | Ondansetron hydrochloride | 0.9% NaCl | 100.0 | 80.0 |
| D | Ondansetron hydrochloride | 0.9% NaCl | 200.0 | 80.0 |
| E | Tropisetron hydrochloride and 0.9% NaCl | None | 100.0 | 50.0 |
| F | Tropisetron hydrochloride and 0.9% NaCl | None | 200.0 | 50.0 |
| G | Dolasetron mesylate | 5% glucose | 100.0 | 1000.0 |
| H | Dolasetron mesylate | 5% glucose | 200.0 | 1000.0 |
| I | Dolasetron mesylate | 0.9% NaCl | 100.0 | 1000.0 |
| J | Dolasetron mesylate | 0.9% NaCl | 200.0 | 1000.0 |
| K | Azasetron hydrochloride and 0.9% NaCl | None | 100.0 | 200.0 |
| L | Azasetron hydrochloride and 0.9% NaCl | None | 200.0 | 200.0 |
| M | Palonosetron hydrochloride | 5% glucose | 100.0 | 2.5 |
| N | Palonosetron hydrochloride | 5% glucose | 200.0 | 2.5 |
| O | Palonosetron hydrochloride | 0.9% NaCl | 100.0 | 2.5 |
| P | Palonosetron hydrochloride | 0.9% NaCl | 200.0 | 2.5 |
| Q | Ramosetron hydrochloride | 5% glucose | 100.0 | 3.0 |
| R | Ramosetron hydrochloride | 5% glucose | 200.0 | 3.0 |
| S | Ramosetron hydrochloride | 0.9% NaCl | 100.0 | 3.0 |
| T | Ramosetron hydrochloride | 0.9% NaCl | 200.0 | 3.0 |
Physical and chemical analysis
The physical properties of the above samples were visually inspected for particles, discoloration and bubbles against ambient light and dark backgrounds at each test time point. Particulate matter was counted with a particle counter (GWF-8JA particle detector; Tianjin Tianhe Analytical Instrument Co, Tianjin, China). In addition, the pH of each sample was determined with a pH meter (Seveneasy S20 pH Meter; Mettler Toledo (Shanghai) Co, Shanghai, China). The concentrations of DSP and 5-HT3RAs were determined using seven validated HPLC assays described below, which were based on previous studies with slight modification.9 10 The initial concentration of DSP and 5-HT3RAs was defined as 100%, and subsequent sample concentrations were expressed as a percentage of the initial concentration. Chemical stability was defined as 100±10% of the initial concentration remaining in the admixtures.
HPLC analysis was carried out using a Shimadzu HPLC system (Kyoto, Japan) composed of a DGU-20A5R degasser, an LC-16 quaternary gradient pump, an SIL-16 autosampler, a CTO-10A column temperature oven, and an SPD-16UV detector. The column was an Intersil ODS-3 C18 column (4.6 mm×150 mm, 5 μm) (Shimadzu, Japan) fitted with a guard column. Samples were injected into an autosampler and isocratically eluted with a solvent system containing solvent A (10 mM phosphate aqueous solution, pH 7.4) and solvent B (methanol) at a ratio of 40/60 (v/v) at a flow rate of 1.0 mL/min. The detection wavelength was 240 nm. The data were processed using LC solution V.1.21 software from Shimadzu.
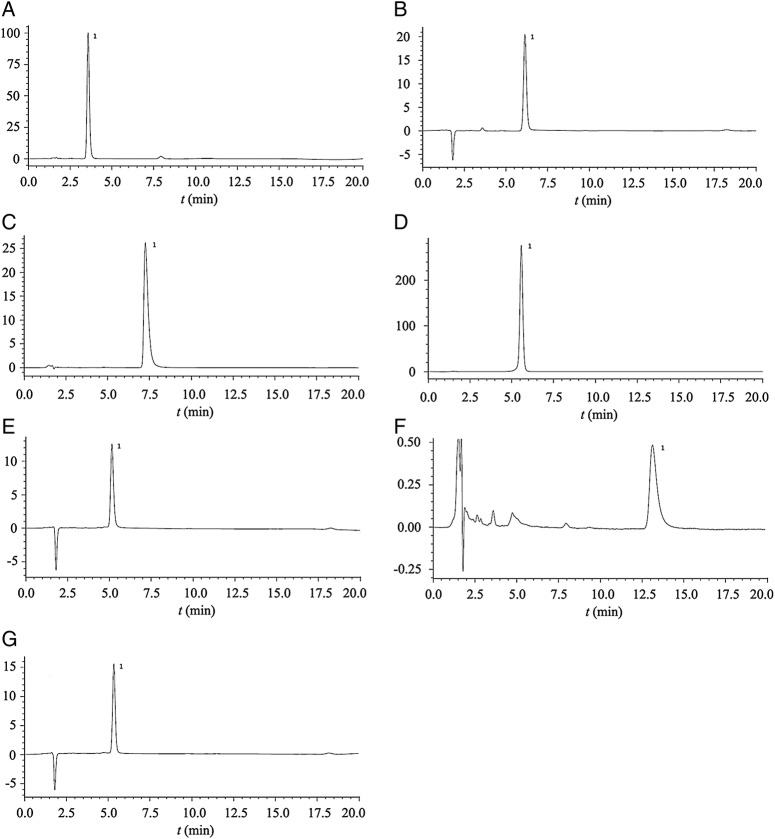
The HPLC methods all provided good baseline separation of DSP and each 5-HT3RA (figure 1), revealing them to be highly specific for the drugs studied. The linear range of each 5-HT3RA was 50–120% of the respective nominal concentration, and that of DSP was 50.0–250.0 ng/mL. The methods were highly linear, with the correlation coefficient (R2) always exceeding 0.999 over the linear range. Quality control (QC) samples were set at low QC (150% lower limit of linear ranges), median QC (nominal concentration of each drug in admixtures) and high QC (80% higher limit of linear ranges) (n=6). The accuracy was expressed as QC recovery. The accuracies of DSP and 5-HT3RA determinations were all within 100.0±3.0% of the nominal concentration. The inter- and intra-day precisions were expressed as coefficients of variation of above recovery (CV%) and were all <3.0%. The QC samples were stored at ambient temperature for 48 hours, and the final recoveries were all within 100.0±3.0%. The validation studies confirmed the accuracy, precision and stability of the methods to be high, making them suitable for study of the chemical stability of admixtures of DSP with 5-HT3RAs.
Figure 1.
Chromatograms. (A) Dexamethasone sodium phosphate; (B) ondansetron hydrochloride; (C) tropisetron hydrochloride; (D) dolasetron mesylate; (E) azasetron hydrochloride; (F) palonosetron hydrochloride; (G) ramosetron hydrochloride. Peak 1 represents the correspondent component. The retention time was 3.6, 6.1, 7.3, 5.6, 5.2, 13.1 and 5.3 min, respectively.
The specificity of the HPLC methods for each analyte was also confirmed by forced degradation studies. QC samples of each analyte were degraded at 60°C for 5 hours with 0.1 M HCl, 0.1 M NaOH and 3% H2O2, respectively. Degradation samples were analysed using the above HPLC methods. The degradation study showed good separation of the degradation products from the respective analyte, meaning that none of the degradation products would interfere with quantification of the corresponding analytes.
Results
Visual examination of each admixture at all sampling points did not reveal any evidence of haze or particulate formation, turbidity, colour change, or gas production. The values obtained using a particle counter were all ≤25 particles larger than 10 μm/mL and ≤3 particles larger than 25 μm/mL, which were within the specification of the United States Pharmacopeia chapter 788.11 The pH values of admixtures prepared with 0.9% NaCl were 0–0.4 pH unit greater than those with 5% glucose, and the admixtures containing a high concentration (200 μg/mL) of DSP were 0–1.2 pH unit greater than those containing a low concentration (100.0 μg/mL) of DSP. pH changes in all admixtures were <0.2 pH unit (table 3).
Table 3.
pH values of admixtures
| Admixture code* | Storage time (hours) |
|
|---|---|---|
| 0 | 48 | |
| A | 4.7±0.0 | 4.6±0.0 |
| B | 5.8±0.0 | 5.7±0.0 |
| C | 5.3±0.0 | 5.2±0.0 |
| D | 5.9±0.1 | 5.8±0.0 |
| E | 6.8±0.1 | 6.6±0.1 |
| F | 6.9±0.0 | 6.8±0.0 |
| G | 4.8±0.0 | 4.7±0.0 |
| H | 5.1±0.0 | 5.1±0.0 |
| I | 5.0±0.0 | 5.0±0.0 |
| J | 5.4±0.0 | 5.4±0.0 |
| K | 3.9±0.0 | 3.7±0.0 |
| L | 5.0±0.0 | 4.6±0.0 |
| M | 5.5±0.1 | 5.5±0.0 |
| N | 6.3±0.0 | 6.3±0.0 |
| O | 6.1±0.0 | 6.1±0.0 |
| P | 6.4±0.0 | 6.4±0.0 |
| Q | 6.2±0.0 | 6.1±0.0 |
| R | 6.7±0.0 | 6.6±0.0 |
| S | 6.9±0.0 | 6.8±0.0 |
| T | 7.1±0.0 | 7.0±0.0 |
Results are expressed as mean±SD (n=3).
*Codes of admixtures are noted in table 2.
The chemical stability of DSP with 5-HT3RAs in infusion solutions is shown in table 4 and online supplementary material after storage at ambient temperature. Throughout the 48 hour storage period, the initial concentrations of the analytes all remained at 100.0%±10.0%. No abnormal peaks were observed on chromatography of each admixture during the study period.
Table 4.
Chemical stability of dexamethasone sodium phosphate (DSP) with different 5-HT3 receptor antagonists (5-HT3RAs) in infusion solutions
| Admixture code* | Initial concentration (μg/mL) |
% of initial concentration remaining after 48 hours |
||
|---|---|---|---|---|
| DSP | 5-HT3RA | DSP | 5-HT3RA | |
| A | 91.8±1.0 | 74.5±0.9 | 101.2±0.1 | 101.5±0.2 |
| B | 181.3±2.1 | 72.2±0.8 | 101.2±0.1 | 101.4±0.2 |
| C | 92.7±1.1 | 74.5±0.9 | 101.6±0.2 | 99.9±0.1 |
| D | 179.6±1.8 | 72.4±0.5 | 99.1±0.1 | 99.9±0.1 |
| E | 99.1±0.4 | 50.5±0.5 | 99.2±0.1 | 99.3±0.2 |
| F | 198.3±2.2 | 50.1±0.4 | 100.5±0 | 99.0±0.4 |
| G | 91.4±1.2 | 924.6±8.5 | 99.6±0.8 | 99.7±1.0 |
| H | 185.1±1.1 | 918.1±7.9 | 100.0±0.6 | 98.8±0.3 |
| I | 91.2±0.9 | 916.6±10.1 | 99.1±0.7 | 99.0±0.5 |
| J | 188.2±1.5 | 934.6±10.6 | 98.8±0.5 | 99.1±0.7 |
| K | 98.9±0.5 | 198.7±2.6 | 97.5±0.5 | 99.3±0.4 |
| L | 197.7±2.4 | 197.9±2.3 | 97.4±0.4 | 99.7±0.4 |
| M | 90.2±2.1 | 2.3±0.1 | 98.9±0.9 | 100.1±0.3 |
| N | 181.4±2.9 | 2.2±0.1 | 99.5±1.2 | 99.8±0.3 |
| O | 90.8±1.3 | 2.3±0.0 | 98.7±1.2 | 99.2±1.0 |
| P | 182.1±2.6 | 2.3±0.1 | 99.1±1.6 | 99.4±1.1 |
| Q | 88.5±0.9 | 2.7±0.1 | 99.3±1.0 | 100.2±0 |
| R | 183.1±1.5 | 2.7±0.1 | 98.3±0.9 | 99.5±0 |
| S | 91.3±0.8 | 2.8±0.0 | 98.6±0.3 | 100.2±0.5 |
| T | 180.2±2.4 | 2.7±0.1 | 101.6±0.3 | 100.7±0 |
Results are expressed as mean±SD (n=3).
*Codes of admixtures are noted in table 2.
ejhpharm-2016-000980supp001.pdf (232.4KB, pdf)
Discussion
In this comprehensive study on the compatibility of DSP with six different 5-HT3RAs in infusion solutions, no evidence of incompatibility was observed. Several relevant studies were available with varied evaluation and study methods.8–10 12–18 As the compatibility of DSP with granisetron hydrochloride has already been reported and verified by several studies,9 12 13 we did not include it in our study. PVC or unknown bags were used in studies of compatibility of DSP with other 5-HT3RAs in infusion solutions.10 12–18 Our study demonstrated the compatibility of DSP with palonosetron hydrochloride, which is consistent with the report of Trissel and Zhang.14 The compatibility of DSP with ondansetron hydrochloride was also investigated by Evrard et al,10 15 16 but pH values and drug concentrations were not determined in some of these studies.15 16 The compatibility of fosaprepitant with intravenous 5-HT3RAs (ondansetron, granisetron, palonosetron and tropisetron) and corticosteroids (DSP or methylprednisolone sodium succinate) was studied by Sun et al,17 but the chemical stability of 5-HT3RAs and corticosteroids was not investigated except for fosaprepitant. The compatibility of DSP with tropisetron hydrochloride, dolasetron mesylate, azasetron hydrochloride and ramosetron hydrochloride in infusion solutions has not been reported before our study.
Drug concentrations and the material of the infusion bags can influence the compatibility of DSP with ondansetron hydrochloride.10 18 Admixture of DSP (67 μg/mL) with ondansetron hydrochloride (1.07 mg/mL) was incompatible after 3 days in a syringe.10 In our study, the concentrations of admixtures (ondansetron hydrochloride 80.0 μg/mL, DSP 100.0 μg/mL or 200.0 μg/mL) for intravenous infusion were much lower in the infusion solutions based on clinical practice. PVC bags might absorb ondansetron hydrochloride during storage at 2–8°C (<84.7% remaining) or 15–25°C (<88.2% remaining) filled with 5% glucose.18 Our results show that non-PVC bags do not absorb ondansetron hydrochloride (98.6–103.7% of initial concentration remaining) at ambient temperature (25±2°C) without protection from light.
Although our study supports the compatibility and stability of DSP with 5-HT3RAs in infusion solutions, there are several limitations to the study. First, the sterility of these admixtures was not tested. According to the United States Pharmacopeia chapter 797 guidelines, low-risk compounded sterile products are permissible within a maximum-use period of 48 hours for non-refrigerated samples.19 Second, the investigated duration in other reports was much longer than the 48 hours used in our study.12 17 However, infusions are always used immediately after preparation, so our research may provide valuable evidence on compatibility of DSP with 5-HT3RAs in infusion solutions.
Conclusion
All the admixtures of DSP with 5-HT3RAs were compatible and stable for at least 48 hours in non-PVC infusion bags using 5% glucose or 0.9% NaCl as diluent under ambient conditions.
Key messages.
What is already known on this subject?
There have been several reports on the compatibility of dexamethasone sodium phosphate (DSP) with 5-HT3 receptor antagonists (5-HT3RAs) in infusion solutions, but the available evidence is not yet conclusive.
The use of extemporaneously prepared admixtures of DSP with 5-HT3RAs must be supported by sufficient documentation of their compatibility.
What this study adds?
This study provides valuable evidence on the compatibility of dexamethasone sodium phosphate with 5-HT3 receptor antagonists in infusion solutions.
Footnotes
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: All data from our study are included.
References
- 1.Hesketh PJ. Chemotherapy-induced nausea and vomiting. N Engl J Med 2008;358:2482–94. 10.1056/NEJMra0706547 [DOI] [PubMed] [Google Scholar]
- 2.Roila F, Herrstedt J, Aapro M, et al. . Guideline update for MASCC and ESMO in the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol 2010;21(Suppl 5):v232–43. 10.1093/annonc/mdq194 [DOI] [PubMed] [Google Scholar]
- 3.China Cancer Society Cancer Rehabilitation and Palliative Care Professional committee; China Clinical Oncology Institute of Antitumor Drug Safety Management Expert Committee. Guidelines for the prevention and treatment of vomiting associated with tumor therapy (2014 edition). J Clin Oncol (Chinese) 2014;19:263–73. [Google Scholar]
- 4.Hayakawa T, Sato M, Konaka M, et al. . Comparison of ramosetron and azasetron for prevention of acute and delayed cisplatin-induced emesis in lung cancer patients. Gan To Kagaku Ryoho 2006;33:633–8. [PubMed] [Google Scholar]
- 5.Roila F, Tonato M, Cognetti F, et al. . Prevention of cisplatin-induced emesis: a double-blind multicenter randomized crossover study comparing ondansetron and ondansetron plus dexamethasone. J Clin Oncol 1991;9:675–8. [DOI] [PubMed] [Google Scholar]
- 6.Italian Group for Antiemetic Research. Dexamethasone, granisetron, or both for the prevention of nausea and vomiting during chemotherapy for cancer. N Engl J Med 1995;332:1–5. 10.1056/NEJM199501053320101 [DOI] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network. NCCN Clinical Practice Guideline in Oncology: Antiemesis (version 1. 2015). http://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf (accessed 1 July 2015).
- 8.Longfield V. Compatibility and stability of 5-HT3 receptor antagonists: a pharmacology review. Oncol Nurs Forum 2002;29:1469–82. 10.1188/02.ONF.1469-1482 [DOI] [PubMed] [Google Scholar]
- 9.Pinguet F, Rouanet P, Martel P, et al. . Compatibility and stability of granisetron, dexamethasone, and methylprednisolone in injectable solutions. J Pharm Sci 1995;84:267–8. [DOI] [PubMed] [Google Scholar]
- 10.Evrard B, Ceccato A, Gaspard O, et al. . Stability of ondansetron hydrochloride and dexamethasone sodium phosphate in 0.9% sodium chloride injection and in 5% dextrose injection. Am J Health Syst Pharm 1997;54:1065–8. [DOI] [PubMed] [Google Scholar]
- 11.United States Pharmacopeial Convention. Particulate matter in injections (general information chapter 788). http://www.usp.org/sites/default/files/usp_pdf/EN/USPNF/788ParticulateMatter.pdf (accessed 3 May 2016).
- 12.Mayron D, Gennaro AR. Stability and compatibility of granisetron hydrochloride in i.v. solutions and oral liquids and during simulated Y-site injection with selected drugs. Am J Health Syst Pharm 1996;53:294–304. [DOI] [PubMed] [Google Scholar]
- 13.Chin A, Moon YS, Chung KC, et al. . Stability of granisetron hydrochloride with dexamethasone sodium phosphate for 14 days. Am J Health Syst Pharm 1996;53:1174–6. [DOI] [PubMed] [Google Scholar]
- 14.Trissel LA, Zhang Y. Compatibility and stability of Aloxi (palonosetron hydrochloride) admixed with dexamethasone sodium phosphate. Int J Pharmaceut Compound 2004;8:398–403. [PubMed] [Google Scholar]
- 15.Trissel LA, Tramonte SM, Grilley BJ. Visual compatibility of ondansetron hydrochloride with selected drugs during simulated Y-site injection. Am J Hosp Pharm 1991;48:988–92. [PubMed] [Google Scholar]
- 16.Beijnen JH, Koks CHW. Visual compatibility of ondansetron and dexamethasone. DICP 1991;25:869. [DOI] [PubMed] [Google Scholar]
- 17.Sun S, Schaller J, Placek J, et al. . Compatibility of intravenous fosaprepitant with intravenous 5-HT3 antagonists and corticosteroids. Cancer Chemother Pharmacol 2013;72:509–13. 10.1007/s00280-013-2201-2 [DOI] [PubMed] [Google Scholar]
- 18.Hagan RL, Mallett MS, Fox JL. Stability of ondansetron hydrochloride and dexamethasone sodium phosphate in infusion bags and syringes for 32 days. Am J Health Syst Pharm 1996;53:1431–5. [DOI] [PubMed] [Google Scholar]
- 19.Trissel LA. The new national standard for sterile preparation. Hospital Pharm 2004;39:900–4. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ejhpharm-2016-000980supp001.pdf (232.4KB, pdf)