Abstract
Objectives
Sotalol hydrochloride (SOT) is an antiarrhythmic β-blocker which is highly effective for the treatment of supraventricular tachycardia in children. However, a licensed paediatric dosage form with sotalol is not currently available in Europe. The aim of this work was to formulate paediatric oral solutions with SOT 5 mg/mL for extemporaneous preparation in a hospital pharmacy with the lowest possible amount of excipients and to determine their stability.
Methods
Three aqueous solutions were formulated. One preparation without any additives for neonates and two preparations for children from 1 month of age were compounded using citric acid to stabilise the pH value, potassium sorbate 0.1% w/v as a preservative, and simple syrup or sodium saccharin as a sweetener. The samples were stored at room temperature and in a refrigerator, respectively, and the content of SOT and potassium sorbate was determined simultaneously using a validated high performance liquid chromatography method at different time points over 180 days.
Results
At least 95% of the initial sotalol concentration remained throughout the 180-day study period in all three preparations at both temperatures. The content of potassium sorbate decreased by 17% with sodium saccharin stored at room temperature.
Conclusions
The three proposed oral aqueous solutions of SOT for neonates and infants were stable for 180 days. Storage in a refrigerator is preferred, particularly with sodium saccharin. The additive-free solution of SOT can be autoclaved to ensure microbiological stability and used particularly for neonates and in emergency situations.
Keywords: PAEDIATRICS, CARDIOLOGY, sotalol hydrochloride, oral liquid
Introduction
Sotalol hydrochloride (SOT) is an anti-arrhythmic β-blocker which is well tolerated and highly effective for the treatment of ventricular and supraventricular tachycardia in children.1 The British National Formulary recommends sotalol should be administered to children in an initial oral dose of 1 mg/kg twice daily, increased as necessary every 3–4 days to a maximum of 4 mg/kg twice daily.2 Recently, age-specific dosage guidelines for sotalol were developed by Läer et al3 to ensure safe and effective anti-arrhythmic therapy in children, especially neonates and infants.
Sotalol is commercially available in tablet dosage forms for adults in four strengths: 80, 120, 160 and 240 mg.1 However, the lack of marketed low-dose paediatric products means extemporaneous preparation is often necessary. Extemporaneous preparations for paediatric use must be formulated in accordance with the guidelines of the European Medicines Agency.4 5 Compounding should be restricted to an approved institution, for example, a hospital pharmacy.
In general, there are three basic approaches to the pharmacy preparation of paediatric dosage forms.
The preparation of capsules from licensed tablets or from the active substance is time-consuming for pharmacists and inconvenient for caregivers. As a small child is unable to swallow capsules, they should be opened and mixed with baby food or a beverage before administration. The advantage of this method is relatively good chemical and microbiological stability without the need to add preservatives.
The preparation of a suspension from licensed tablets or a solution from licensed injection is a simple way to prepare an oral liquid preparation. Commercial tablets should be crushed to a fine powder and mixed with a suitable vehicle; commercial injections could be diluted with water. Excipients improving stability and palatability should be added. However, the stability of the final product is not ensured due to the presence of other excipients in licensed medicines and their potential interactions with vehicles. Above all, there is a high risk of an inaccurate dose in the case of suspensions and drugs with a narrow therapeutic range, particularly in children.6
The preparation of an aqueous oral solution from the active substance is the best method if the active ingredient is of the required pharmacopoeial quality and soluble in water.
In all these circumstances, the pharmacist should pay attention to the stability of the active pharmaceutical substance for the labelled time period, excipient safety and tolerability, particularly for very young children, and expected duration of treatment.7 Special attention must be given to formulations for neonates to whom no preservatives, antioxidants or hyperosmotic solutions should be administered.5
Regarding the paediatric use of SOT, some suspensions prepared from commercial tablets are referred to in the literature as being stable for a maximum of 90 days.8–11 The presence of many different additives in tablets as well as in commercial vehicles (ORA-Sweet, ORA-Plus), sedimentation and possible dose inaccuracy make suspensions a less suitable dosage form for infants.
The aim of our research was to formulate extemporaneous paediatric solutions of SOT 5 mg/mL for two different paediatric groups: neonates to 1 month of age (without any additives) and infants (with the lowest possible amount of excipients) and to evaluate their stability under two different conditions of storage (refrigerated and room temperature) throughout the 180-day study period. In the unpreserved solution, the influence of autoclaving on the stability of SOT was also investigated. High performance liquid chromatography (HPLC) was used to simultaneously estimate the concentrations of SOT and potassium sorbate (PS) in the preserved preparations.
Materials and methods
Materials
SOT, PS, citric acid monohydrate, sodium saccharin and simple syrup (64% w/w, preservative-free) of pharmaceutical quality were used. Water for injection (WFI) was used throughout the study as a solvent.
Methods
Sample preparation
Sotalol samples were carefully prepared in University Hospital Motol in Prague.
Solution S1 5 mg/mL was prepared by dissolving 0.50 g of SOT in WFI and made up to 100 mL under aseptic conditions. One sample (S1aut) was filled into a infusion glass bottle, stoppered and crimped, and sterilised in a laboratory autoclave at 121°C for 20 min.
Solution S2 5 mg/mL was made by dissolving 0.50 g of SOT, 0.10 g of PS and 0.08 g of citric acid in an appropriate amount of WFI. Then, 20 g of simple sucrose syrup (64% w/w) was added and the solution was made up to 100 mL (ie, 105 g) with WFI.
Solution S3 5 mg/mL was prepared by dissolving 0.50 g of SOT, 0.10 g of PS, 0.08 g of citric acid and 0.10 g of sodium saccharin in WFI and made up to 100 mL.
The composition of solutions S1, S2 and S3 is shown in table 1.
Table 1.
Composition and properties of sotalol hydrochloride solutions
| S1 (g) | S2 (g) | S3 (g) | |
|---|---|---|---|
| Sotalol hydrochloride | 0.50 | 0.50 | 0.50 |
| Citric acid | – | 0.08 | 0.08 |
| Potassium sorbate | – | 0.10 | 0.10 |
| Simple syrup | – | 20.0 | – |
| Sodium saccharin | – | – | 0.10 |
| Water for injection to | 100.0 mL | 100.0 mL (=105.0 g) | 100.0 mL |
| Density* (g/mL) | 0.9997 | 1.0500 | 1.0008 |
| Osmolality (mOsmol/kg) | 49 | 497 | 60 |
| pH | 5.43–5.87 | 4.16–4.19 | 4.14–4.19 |
| Taste | Slightly bitter | Sweet | Sweet, slightly bitter |
*At 20±0.1°C.
Measurement of density, osmolality and pH value
The density of the preparations was measured at 20±0.1°C using a DMA 4100M density meter (Anton Paar, Austria). The osmolality of the solutions was measured using an automatic semi-micro osmometer (Knauer, Germany) calibrated in accordance with Ph. Eur. 8.0 (2.2.35. Osmolality). Density and osmolality were measured five times in each formulation.
pH was measured under stabilised conditions using a pH metre (pH 212 meter, Hanna instruments, Germany) with a combined pH electrode. Samples were measured at 7, 14, 30, 60, 90, 120, 150 and 180 days.
Instrumentation and analytical conditions
A stability-indicating HPLC assay was developed for simultaneous determination of SOT and PS by Matysova et al.12 Briefly, determination of SOT and PS was performed on an HPLC system with an absorbance UV detector. Separation was achieved using an Ascentis Express C18 (100×4.6 mm, particles 2.7 μm; Supelco, USA) column. Linear gradient elution was used.
Stability method and sample analysis
All preparations (S1, S2 and S3) were prepared in duplicate with the same composition. Each solution was divided into four amber glass bottles (50 mL). Samples were stored at room temperature (25±2°C) or in a refrigerator (5±3°C) and protected from light; that is, two samples from each batch were stored at each of the experimental conditions (n=4).
The concentration of SOT in all preparations and of the preservative, PS, in preparations S2 and S3 was evaluated at the beginning of the stability assay (t0, an initial content of 100%) and at the time points mentioned above. Each sample was measured in triplicate.
Samples of solution S1aut were stored in an autoclave bottle under the same storage conditions as above. The concentration of SOT was evaluated before sterilisation in an autoclave, after sterilisation (t0) and then at 7, 14 and 30 days.
Data analysis
At each time point, the percentage of the actual initial concentration remaining was calculated for sotalol and PS (n=4). Stability was defined as the retention of at least 95% of the initial concentration of sotalol and 90% of PS.
Results
Table 1 shows the composition and the properties: the average of five measurements of density and osmolality, the relative SD of which was less than 1%, and the taste of the prepared solutions. In our opinion, both solutions formulated with a sweetener tasted sweet, while solution S3 containing sodium saccharin had a slightly bitter aftertaste. Table 1 also gives the pH values measured at the stability study time points. The pH of the aqueous solution of sotalol S1 without additives varied between 5.43 and 5.87; the average pH value of 4.15 in the buffered solutions with preservative (S2, S3) remained practically unchanged throughout the stability study.
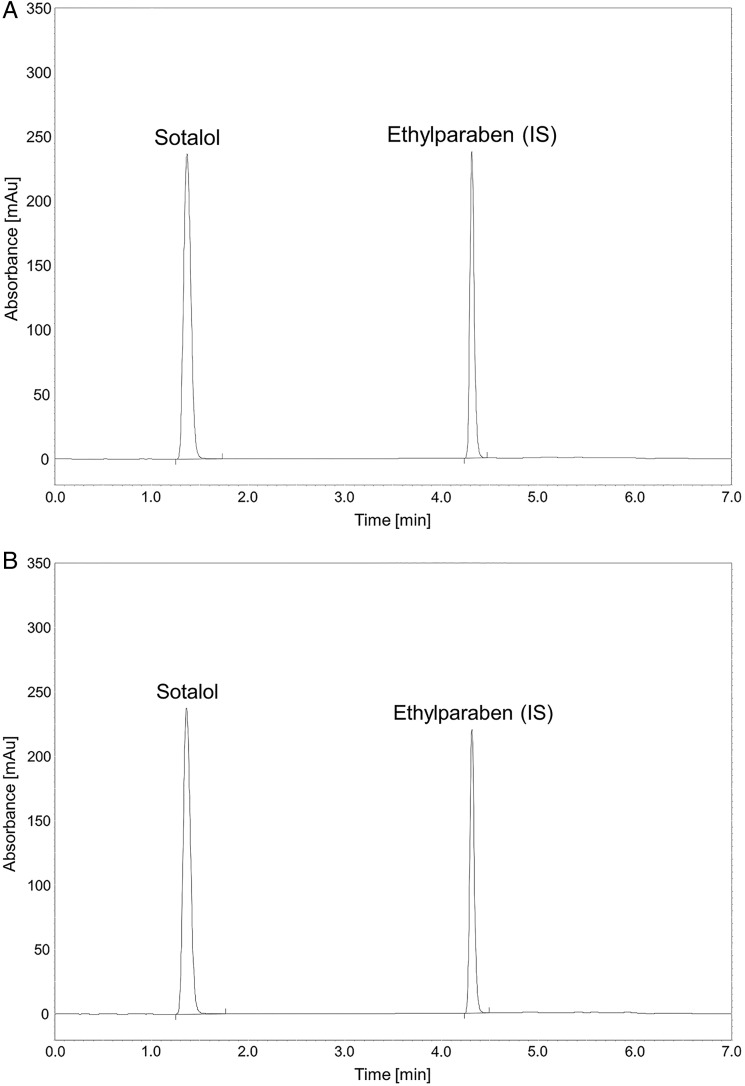
In figure 1, the HPLC chromatograms of sample S1aut before (A) and after (B) autoclaving are compared. The lack of change in the retention time of sotalol demonstrated that autoclaving did not influence SOT stability. The concentration of sotalol before and after autoclaving was unchanged at 5.17±0.11 mg/mL and therefore taken to be equal to the initial value (t0).
Figure 1.
(A) High performance liquid chromatography chromatogram of sotalol hydrochloride in sample S1aut before sterilisation. (B) HPLC chromatogram of sotalol hydrochloride in sample S1aut after sterilisation.
Table 2 shows the percentage±SD of the initial concentration of SOT in solutions S1, S2 and S3 (n=4) stored under various conditions as mentioned above. The first row gives the amount of SOT in milligrams per millilitre at the beginning of the study (t0=100%). SOT demonstrated good stability in the preparations, with final content being within ±5% of the initial concentration after 180 days of storage at cold or room temperature. Chromatograms showed no evidence of degradation products throughout 6-month stability study.
Table 2.
The percentage content of sotalol hydrochloride during the stability study at cold and room temperature*
| Time point (day) | Cold (5±3°C) | Room (25±2°C) | ||||
|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S1 | S2 | S3 | |
| 0 (100%) | 5.17±0.11 mg/mL | 5.19±0.03 mg/mL | 5.19±0.05 mg/mL | 5.17±0.11 mg/mL | 5.19±0.03 mg/mL | 5.19±0.05 mg/mL |
| 7 | 101.10±1.37 | 99.03±0.93 | 100.89±0.88 | 100.37±0.89 | 99.52±0.74 | 100.19±0.93 |
| 14 | 96.72±0.48 | 99.58±1.29 | 98.48±0.23 | 98.32±0.73 | 100.22±0.69 | 98.12±0.34 |
| 30 | 100.65±0.66 | 100.55±1.16 | 101.20±0.33 | 98.91±1.01 | 99.69±1.18 | 99.0±0.39 |
| 60 | 98.41±0.32 | 98.75±1.12 | 99.36±0.80 | 98.78±0.59 | 99.29±0.69 | 98.79±0.84 |
| 90 | 98.75±0.28 | 99.02±0.89 | 98.84±0.63 | 99.04±0.30 | 99.54±0.29 | 99.27±0.40 |
| 120 | 98.58±0.97 | 98.99±0.66 | 99.22±0.56 | 98.39±0.60 | 98.89±0.39 | 98.27±0.92 |
| 150 | 97.33±0.67 | 99.17±0.83 | 98.62±0.74 | 97.84±0.34 | 99.23±0.70 | 98.27±0.25 |
| 180 | 99.29±0.83 | 98.85±0.91 | 101.14±0.91 | 100.07±0.52 | 99.27±0.81 | 98.97±1.06 |
*Mean±SD of determinations for four samples (n=4).
The results for PS are presented in table 3. The remaining percentage content of PS was within ±5% of the initial PS concentration for solutions S2 and S3 stored in a refrigerator for 180 days. At room temperature, the percentage of PS declined slowly, remaining within ±5% for 60 days, within ±10% for 90 days, and then decreasing further, particularly for S3. Nevertheless, no detectable changes in colour, odour or taste were observed in any formulation.
Table 3.
The percentage content of potassium sorbate during the stability study at cold and room temperature*
| Time point (day) | Cold (5±3°C) | Room (25±2°C) | ||
|---|---|---|---|---|
| S2 | S3 | S2 | S3 | |
| 0 (100%) | 1.03±0.02 mg/mL | 1.04±0.01 mg/mL | 1.03±0.02 mg/mL | 1.04±0.01 mg/mL |
| 7 | 98.65±1.99 | 102.55±1.90 | 99.34±1.82 | 101.42±1.92 |
| 14 | 99.04±2.02 | 99.49±1.46 | 99.39±0.81 | 98.24±1.92 |
| 30 | 99.17±2.25 | 99.94±1.64 | 99.56±0.84 | 97.69±1.38 |
| 60 | 98.20±1.31 | 98.54±2.45 | 97.45±0.99 | 95.34±0.89 |
| 90 | 97.46±1.65 | 98.83±0.89 | 97.99±0.51 | 94.28±0.67 |
| 120 | 98.53±1.42 | 98.09±1.58 | 96.13±0.68 | 89.94±1.34 |
| 150 | 97.95±0.58 | 99.34±1.73 | 94.34±0.79 | 86.76±1.51 |
| 180 | 98.37±0.32 | 98.78±1.19 | 92.60±0.75 | 83.42±0.75 |
*Mean±SD of determinations for four samples (n=4).
Discussion
Approximately 14 000 capsules containing 5–30 mg of SOT were prepared in the hospital pharmacy of the University Hospital Motol in Prague in 2014 for paediatric patients. In cooperation with the children's heart centre at the same hospital, the aim of this work was to replace the preparation of SOT-containing capsules with extemporaneous 5 mg/mL oral solutions which would cover most paediatric needs in the hospital.
Oral paediatric solutions provide many benefits including easy and faster preparation in a hospital pharmacy and more flexible and accurate dosing. Unfortunately, aqueous solutions often have less stability and a short shelf-life, so preservatives must generally be added to multi-dose preparations. In addition, the pharmacist is responsible for the selection of suitable excipients safe for children in the targeted age groups. Adequate palatability also plays an important role in patient acceptability, with flavours or sweeteners often added to improve taste.7
SOT is a white powder, freely soluble in water and chemically stable at pH 4–5.9 13 PS is believed to be a safe antimicrobial preservative, is freely soluble in water and is generally used at 0.1–0.2% concentration in oral formulations.14 PS (in the form sorbic acid) displays highest antimicrobial efficacy at pH 4–5, the same pH as sotalol.15 Because SOT has a slightly bitter taste, sucrose syrup and/or sodium saccharin were used to improve the palatability of the S2 and S3 preparations, respectively.
A simple aqueous solution of SOT without any additives is proposed for neonates. Microbiological stability is ensured by the aseptic technique and final sterilisation of the product. Bacteria retention using a 0.22 μm membrane filter, sterilisation after compounding in an autoclave or a combination of both are the most common sterilisation methods employed in hospital pharmacies. The stability of solution S1 is documented in table 2. The effect of sterilisation in an autoclave at 121°C for 20 min on the concentration of SOT is shown in figure 1, where the HPLC sample chromatograms are compared before (A) and after (B) autoclaving. As can be seen, autoclaving did not influence the retention time of sotalol. The concentration of sotalol (5.17±0.11 mg/mL) before autoclaving was the same as that after autoclaving and therefore considered to be the initial value (t0). The percentage content of SOT remained within ±5% of the initial concentration during 30 days of storage at both cold and room temperature.
Solutions S2 and S3 were formulated with an antimicrobial agent and are proposed for children above 1 month of age. The results in table 2 document the good stability of SOT in all preparations tested. As can be seen in table 3, the concentration of PS remained within ±5% of its initial concentration for solutions S2 and S3 stored in a refrigerator for 6 months. However, the percentage content of PS decreased slowly at room temperature, declining finally below 90% of the original concentration after 90 days. This was noted particularly for solution S3.
Conclusions
Three aqueous oral solutions of SOT 5 mg/mL for antiarrhythmic therapy in children were formulated for extemporaneous preparation in a hospital pharmacy. Validated HPLC analysis demonstrated that the concentration of SOT in the formulations was in accordance with the criterion that at least 95% of the initial content should remain during storage at cold or room temperature throughout the 180-day study period.
The used excipients ensured stable pH and a more pleasant taste, while the preservative afforded sufficient antimicrobial stability in solutions S2 and S3 targeted at children aged 1 month and over. Storage in a refrigerator is preferred, and the solutions were stable for 180 days under this condition. Preparations should be stored in a brown glass container with a screw cap suitable for use with a graduated pipette for accurate oral dosing. The efficacy of PS 0.1% w/v in formulation S2, which is a better candidate for microbial contamination due to the content of sucrose syrup, was demonstrated by an accredited laboratory (Ph. Eur., 5.1.3 Efficacy of antimicrobial preservation).
In formulation S1aut, no effect of autoclaving on the stability of SOT was observed; the solution was stable for 30 days regardless of storage conditions. Although this preservative-free solution is particularly targeted at neonates, it could be prepared in advance in the pharmacy and stored until needed.
Key messages.
What is already known on this subject
Most sotalol hydrochloride preparations on the market are not suitable for small children.
Good stability of sotalol hydrochloride in an aqueous preparation has been shown.
Extemporaneous preparation of stable pharmaceutical products in pharmacies is essential if marketed paediatric products are lacking.
What this study adds
Three oral aqueous solutions of sotalol hydrochloride for neonates and infants were formulated and stability for 180 days was demonstrated in a validated high performance liquid chromatography assay.
Autoclaving had no effect on the stability of an additive-free aqueous solution of sotalol hydrochloride which can be used in particular for neonates and in emergency situations.
Footnotes
Funding: The authors gratefully acknowledge financial support by MH CZ–DRO, University Hospital Motol, Prague, Czech Republic 00064203, Charles University in Prague GAUK 1472213, and student grants SVV 260 183 and SVV 260 184. The study was co-financed by the European Social Fund and the state budget of the Czech Republic TEAB, project no. CZ.1.07/2.3.00/20.0235.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Sweetman SC, ed. Martindale: the complete drug reference. London: Pharmaceutical Press, 2011. [Google Scholar]
- 2.Paediatric Formulary Committee. British National Formulary for Children 2012–2013. London: Pharmaceutical Press, 2012. [Google Scholar]
- 3.Läer S, Elshoff JP, Meibohm B, et al. . Development of a safe and effective pediatric dosing regimen for sotalol based on population pharmacokinetics and pharmacodynamics in children with supraventricular tachycardia. J Am Coll Cardiol 2005;46:1322–30. 10.1016/j.jacc.2005.06.061 [DOI] [PubMed] [Google Scholar]
- 4.Draft guideline on pharmaceutical development of medicines for paediatric use. European Medicines Agency; http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2011/06/WC500107908.pdf (accessed 5 Apr 2015). [Google Scholar]
- 5.EMEA/536810/2008 Guideline on the investigation of medicinal products in the term and preterm neonate. 2010. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003750.pdf (accessed 5 Apr 2015).
- 6.Hurtado J, Moffett BS. Pediatric oral formulations: a continual challenge. Int J Pharm Compound 2007;11:17–19. [PubMed] [Google Scholar]
- 7.Tuleu C, Breitkreutz J. Educational paper: Formulation-related issues in pediatric clinical pharmacology. Eur J Pediatr 2013;172:717–20. 10.1007/s00431-012-1872-8 [DOI] [PubMed] [Google Scholar]
- 8.Nahata MC, Pai VB, eds. Pediatric drug formulations. 6th edn Cincinnati: Harvey Whitney Books, 2011. [Google Scholar]
- 9.Dupuis LL, James G, Bacola G. Stability of a sotalol hydrochloride oral liquid formulation. Can J Hosp Pharm 1988;41:121–3. [Google Scholar]
- 10.Nahata MC, Morosco RS. Stability of a sotalol in two liquid formulations at two temperatures. Ann Pharmacother 2003;37:506–9. 10.1345/aph.1C333 [DOI] [PubMed] [Google Scholar]
- 11.Sidhom MB, Rivera N, Almoazen N, et al. . Stability of sotalol hydrochloride in extemporaneously prepared oral suspension formulations. Int J Pharm Compound 2005;9:402–6. [PubMed] [Google Scholar]
- 12.Matysova L, Zahalkova O, Klovrzova S, et al. . Development of a gradient HPLC method for the simultaneous determination of sotalol and sorbate in oral liquid preparations using solid core stationary phase. J Anal Methods Chem 2015;2015:806736 10.1155/2015/806736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trissel LA, ed. Trissel's stability of compounded formulations. Washington: American Pharmacists Association, 2009. [Google Scholar]
- 14.Rowe RC, Sheskey PJ, Owen SC, eds. Handbook of pharmaceutical excipients. London: Pharmaceutical Press, 2006. [Google Scholar]
- 15.Denyer SP, Baird RM, eds. Guide to microbiological control in pharmaceuticals and medical devices. 2nd edn New York: CRC Press, Taylor & Francis, 2006. [Google Scholar]