Abstract
The western corn rootworm, Diabrotica virgifera virgifera LeConte (Coleoptera: Chrysomelidae), is an important economic pest of maize (Zea mays L.) in North America and Europe. Previous efforts to formulate an artificial diet for western corn rootworm larvae highlighted an important role of corn root powder, which had a significant positive impact on several larval developmental traits. Unfortunately, this ingredient is not available for purchase. Toward the goal of developing an artificial diet for western corn rootworm larvae with all ingredients readily accessible, we conducted research to isolate essential growth factors for larval development from corn root powder to improve the performance of diet without corn root powder. For all experiments, multiple life history parameters (survival, weight, and molting) were recorded from 15-d diet bioassays. Corn roots may contain factors that assist in larval growth, but some of these factors were not fully extracted by methanol and remained in the extracted root. Methanolic extracts significantly increased molting to second instar, but did not significantly increase survival, dry weight, or molting to third instar, suggesting the primary corn root substituents affecting these factors cannot be extracted or other extraction methods may be required to extract the essential factors from corn roots. We showed that whole corn root powder was best when used in combination with all the other nutrient sources in the published western corn rootworm formulation. Corn root powder made from proprietary seed and Viking seed has similar value.
Keywords: Diabrotica virgifera virgifera, artificial diet, corn root powder, corn root extraction
The western corn rootworm, Diabrotica virgifera virgifera LeConte, is an important insect pest of maize (Zea mays L.) in North America and Europe, causing more than $1 billion in crop losses and control costs annually (Metcalf 1986, Gray et al. 2009). Larvae feeding on roots of maize are responsible for most of the damage associated to this species (Branson and Krysan 1981, Moeser and Vidal 2005), whereas adult western corn rootworm feeding on silks, pollen, kernels, and foliage of maize plants may cause yield reduction if present at high densities before anthesis (Culy et al. 1992). Management of these pests has been a challenge to industry and corn growers because of the evolution of resistance to management tactics including chemical insecticides (Ball and Weekman 1963; Meinke et al. 1998; Pereira et al. 2015, 2017), crop rotation (Levine et al. 2002), and transgenic maize hybrids expressing insecticidal crystalline toxins from Bacillus thuringiensis (Bt) (Gassmann et al. 2011, Zukoff et al. 2016, Ludwick et al. 2017).
Monitoring programs to detect differences in susceptibility attributable to resistance development of western corn rootworm populations are mandated by the U.S. Environmental Protection Agency (EPA) (EPA 2016). Diet toxicity assays can be used as a tool for evaluating resistance. An artificial diet is a critical component of diet toxicity assays. An artificial diet capable of supporting larval growth and development that is similar to larvae reared on corn roots would be greatly beneficial for research programs and continuous rearing for western corn rootworm.
The first artificial diet for western corn rootworm larvae was developed by Pleau et al. (2002) which was a modification of a diet for southern corn rootworm, Diabrotica undecimpunctata howardi Barber (Coleoptera: Chrysomelidae). The southern corn rootworm formulation with wheat germ and casein as the major nutritional components was a modification of a diet developed for a lepidopteran species, pink bollworm, Pectinophora gossypiella (Saunders) (Lepidoptera: Gelechiidae) (Sutter et al. 1971, Rose and McCabe 1973, Marrone et al. 1985). The southern corn rootworm is a generalist feeding on over 100 different plants while western corn rootworm, from a practical standpoint, is nearly monophagous on corn roots, but can survive on a number of different grass species (Oyediran et al. 2004). Pleau et al. (2002) developed an initial formulation for western corn rootworm larvae by modifying the southern corn rootworm formulation to include corn root powder, removing formalin, and altering concentrations of several other ingredients including wheat germ, linseed oil, and potassium hydroxide. The initial western corn rootworm formulation doubled larval weight gain when compared to larvae reared on the southern corn rootworm formulation (Pleau et al. 2002).
We previously developed an improved artificial diet formulation for western corn rootworm larvae, hereafter referred to as ‘WCRMO-1’ which was an optimization of the composition of ingredients in the initial western corn rootworm formulation (Huynh et al. 2017). Our improved diet was created by optimizing the key ingredients (corn root powder, wheat germ, and casein) in the initial western corn rootworm formulation based on integrated evaluation of multiple larval developmental traits (weight, survival, and molting). The WCRMO-1 formulation supported approximately 99% of larvae for both molting and survival, and further doubled the weight of larvae compared to larvae reared on the initial western corn rootworm formulation after 11 d of feeding (Huynh et al. 2017).
Previous research in developing a diet for western corn rootworm larvae documented an important role of corn root powder as a key ingredient in the western corn rootworm diet. It had significant impact on overall larval performance (survival, weight, and molting) (Pleau et al. 2002, Huynh et al. 2017). Currently, corn root powder is the only ingredient that is not commercially available. Specialized equipment including a mill such as the one we use (ZM200, Retsch, Haan, Germany) and lyophilizer such as the benchtop pro (Omnitronics, SP Scientific, Stone Ridge, NY) is needed to make the powder. This equipment is expensive, limiting the use of corn root powder-based artificial diets for western corn rootworm larvae, and rendering it practically unavailable to smaller companies, academics, and government researchers. Rootworm research would be expedited if all of the ingredients in the rootworm diet could be purchased. The corn root powder used in our original diet (Huynh et al. 2017) was provided by Monsanto Company (now Bayer Crop Science, St. Louis, MO). In this study, we verified that the milling process developed to produce corn root powder at the Biological Control of Insects Research Laboratory (BCIRL) (USDA-ARS, Columbia, MO) could be used with proprietary seed from Monsanto. Further we verified that Viking seed can be substituted for Monsanto proprietary seed milled either by Monsanto or BCIRL.
Corn roots not only contain feeding stimulants for western corn rootworm larvae, which are a combination of three sugars (glucose, fructose, and sucrose in specific ratios) plus one free fatty acid (oleic or linoleic acid) (Bernklau and Bjostad 2008), but also contain host recognition cues (Bernklau et al. 2015) and repellents (Bernklau et al. 2016). In an artificial diet context, corn roots likely contain ingredients that serve as needed micronutrient sources, token stimuli, or cryptic nutrients making natural host plant-derived materials desirable in a diet (Cohen 2015). Toward the goal of developing an accessible diet for western corn rootworm larvae, we tested the use of corn root powder in diet formulations for western corn rootworm larvae and attempted extracting bioactive factors from corn roots based on an integrated evaluation of three life history parameters (survival, weight, and molting).
Materials and Methods
Insects
Western corn rootworm eggs (a nondiapausing strain) were obtained from the USDA-ARS laboratories in Columbia, MO and Brooking, SD. Egg plates (western corn rootworm eggs in Petri dishes with 70 mesh sieved soil) were incubated in an incubator at 25°C in complete darkness. Once the first hatch of eggs was initiated, the soil was removed by rinsing with water in a 60-mesh sieve (Hogentogler & Co. Inc., Columbia, MD). The eggs free of soil were poured into a beaker and surface-treated using a procedure described by Pleau et al. (2002). Briefly, the eggs were exposed to undiluted Lysol (Reckitt Benckiser, LLC, Parsippany, NJ) for 3 min, and then triple rinsed with distilled water after removing undiluted Lysol. Next, the eggs were treated with 10% formalin (HT501128, Sigma-Aldrich, St. Louis, MO) for 3 min, and then triple rinsed with distilled water after removing formalin. Finally, the eggs were distributed to a coffee filter paper (Pure Brew, Rockline Industries, Sheboygan, WI) using a 1-ml disposable pipette (13-711-9a, Fisher Scientific, Pittsburg, PA). The coffee paper was dried for 2 min and then placed inside a 16-oz cup with a lid (LG8RB-0090 & DM16R-0090, Solo Cup Company, Lake Forest, IL). For ventilation, several holes were made in the lid using a number zero insect pin. The egg container was kept at 25°C in darkness. Neonates (within 24 h and hatching within 2 d) were used for insect bioassays.
Corn Root Extraction
Untreated, dry Viking seeds (Viking 60-01N, Albert Lea Seed, Albert Lea, MN) were soaked for 24 h in tap water, rinsed, and germinated on moist blotter paper (Steel Blue, Anchor Paper Company, St. Paul, MN). Germinating seeds were kept at 25°C in closed polyethylene containers (20 × 40 × 5 cm). Roots (6 g fresh weight) were harvested from 5-d-old germinating seeds, placed in a 150-ml Erlenmeyer flask, and 100 ml of methanol (HPLC grade, A452-1, Fisher Scientific) was added. After 1 h, the liquid was filtered (Whatman no. 1, 1004-090, Springfield Mill, Maidstone, Kent, United Kingdom) into a 500-ml round bottom flask and the extract was concentrated to approximately 1 ml using a rotary evaporator. The concentrated liquid was transferred to a clean glass vial (4 ml, 66011-041, VWR International, West Chester, PA), the flask was rinsed twice with 1.5 ml methanol to collect remaining residue from the walls of the flask and the rinses were added to the concentrated solution. The extract was evaporated under a stream of nitrogen to remove the methanol (approximately 0.5 ml final volume). The extracted roots were air-dried overnight, weighed, and the extract concentration was adjusted (with methanol) to 0.1 g equivalents dry root per ml. Agar (5 g, A7002, Sigma-Aldrich) was placed in a glass container and the extract (2.5 ml total) was added 0.5 ml at a time, shaking well after each addition. The agar treated with extracted corn roots was spread out on a Teflon sheet and allowed to dry 48 h before being stored in an air-tight container.
Corn Root Powder
In order to evaluate both the milling process and the maize lines, corn root powder was produced by Monsanto and was also produced by the BCIRL (USDA-ARS). Each milling operation used both Monsanto proprietary seed and Viking seed to do so. Corn root powder provided by Monsanto from Viking seed and Monsanto seed was used 7 mo and 12 mo after receipt, respectively, whereas corn root powder made by BCIRL from Viking seed and Monsanto seed was used 3 mo and 1 mo, respectively, after being produced. All were stored at −20°C until use. Briefly, at BCIRL, corn seeds (500 g) were washed in 1,000 ml beaker via exposure to 6% NaClO (10% Clorox Regular Bleach, Clorox, Oakland, CA) solution plus 0.01% Tween 20 (BP337, Fisher Scientific) for 15 min, triple rinsed with distilled water, and then incubated at 24°C for 24 h. The seeds were placed in a straight line on top of germination paper (SD3815L, Anchor Paper Company) with 50 seeds per paper and the paper was folded over the seeds, rolled tightly, and secured with a rubber band below the seed line to make a ‘bundle’. The bundles were placed with the seed at the top in a 5-gallon bucket (40 bundles per bucket) containing distilled water to encourage downward growth of root tissue. The bucket was kept in an incubator at 27°C with RH of 70% and watered every 2 d. After 7 d, corn roots were collected by cutting the bundles below the rubber band with a guillotine-type paper cutter. The corn roots were then cut into pieces less than 2 cm using a cutter (27K0020, American Metalcraft, Inc., Franklin Park, IL), placed in a tray, immediately frozen at −80°C, and then dried in a lyophilizer (benchtop pro with omnitronics, SP Scientific, Stone Ridge, NY) at −70°C for 7 d. After 7 d of lyophilizing, the dry roots were milled into powder using an ultra-centrifugal mill (ZM200, Retsch, Haan, Germany) at 14,500 rpm.
Diet Preparation
The diet was poured as described by Huynh et al. (2017). All dry ingredients, i.e., casein (1100, Bio-Serv, Flemington, NJ), cholesterol (C8503, Sigma-Aldrich), cellulose (3425, Bio-Serv), corn root powder (BCIRL and Monsanto), methyl paraben (H5501, Sigma-Aldrich), salt mix (F8680, Bio-Serv), sorbic acid (S1626, Sigma-Aldrich), sucrose (04821721, MP Biomedicals, Santa Ana, CA), vitamin mix (V1007, Sigma-Aldrich), and wheat germ (1661, Bio-Serv), were weighed and put in a plastic bag, except for agar (A7002, Sigma-Aldrich). Agar solution (agar plus distilled water) in a 600-ml beaker was boiled in a microwave for 2 min, and then poured into a blender (Hamilton Beach, Inc., Model 51101BZ) in a biosafety cabinet (Nuaire, Plymouth, MN). The dry ingredients were added into the blender and mixed thoroughly for 30 s. Next, liquid components, i.e., linseed oil (430021, Sigma-Aldrich), wheat germ oil (W1000, Sigma-Aldrich), streptomycin (612240500, Across, Morris Plains, NJ), chlortetracycline (C4881, Sigma-Aldrich), and green food coloring (Bulter, Lancaster, PA), were added into the blender and mixed thoroughly for 30 s. The pH of the diet was titrated to pH of 9 by an addition of KOH 10% (w/v) (P250, Fisher Scientific) and monitored using indicator strips (Whatman). The diet mixture was then poured into a low-sided 700-ml beaker placed on a hot plate (Cimarec, Thermo Scientific) at 65°C and mixed using a stir bar. The mixture was dispensed into a 96-well plate (3370, Corning Inc., Corning, NY) using a repeater pipette (200 µl per well) (Eppendorf repeater plus). The diet plate was kept in a biological cabinet for 10 min to allow excess moisture to evaporate, then stored in a refrigerator at 4°C and used for assays within a week. Bio-Serv has sold their insect diets to Frontier Agricultural Sciences and all Bio-Serv ingredients can be found at http://www.insectrearing.com/products/indiets.html.
Larval Artificial Diet Bioassays
Bioassays were conducted as described in Huynh et al. (2017). All materials used in larval handling were sterilized via exposure to UV light for 10 min in a biological cabinet. One neonate larva (<24 h after hatching) was infested to each well using a fine paintbrush (size #00). A sealing film (TSS-RTQ-100, Excel Scientific, Inc., Victorville, CA) was used to cover the plate and one hole per well for exchange air ventilation was made using an insect pin (size #0). The plates were kept at 25°C in darkness for 15 d. Larval weight, survival, and evidence of diet contamination were recorded at 15 d. For dry weight, surviving larvae were collected and pooled per replicate into 95% ethanol, and then dried at 50°C for 2 d in an oven (602752, Blue M Therm Dry Bacteriological Incubator). Dry larvae for each replicate were weighed using a micro balance (MSU6.6S-000-DM, Sartorius Lab Instruments GmbH & Co. KG, Goettingen, Germany). To determine differences in larval molting to second instar and third instar, larval molting to second instar was recorded at 10 d and larval molting to third instar, and larval incomplete molting to second instar (defined as an inability to complete removal of larval skin) were recorded at 15 d.
Diet Bioassays With Corn Root Extracts
To determine if essential nutrients in corn roots could be extracted, the WCRMO-1 diet (Huynh et al. 2017) was modified. The WCRMO-1 diet includes 17 diet ingredients (e.g., casein, cellulose, wheat germ, vitamin mix, salt mix, oil components, and preservatives) plus corn root powder. In formulations with dry corn root, extracted corn roots, and corn root powder, these ingredients were added to WCRMO-1 diet without corn root powder at the level of 1.5% (w/w), the same proportion of corn root powder in the WCRMO-1 diet. Other ingredients were the same as in the WCRMO-1 formulation (Huynh et al. 2017). In a formulation with agar treated with extracts from corn roots, the treated agar containing methanolic root extract was added at the level of 1.5% (w/w) to the WCRMO-1 diet without corn root powder and agar. Other ingredients of this formulation were the same as in the WCRMO-1 formulation, except for an increase in proportion of water by 1.5% (w/w). The original diet containing corn root powder was used as a control and all treatments were tested for their effect on larval performance (survival, weight, and molting).
Diet Bioassays With Corn Root Powder as a Major Nutritional Source
Corn root powder was previously demonstrated as the key ingredient that was used in combination with major nutritional components (e.g., wheat germ, casein, sucrose, linseed oils) (Pleau et al. 2002, Huynh et al. 2017). To determine if changing corn root powder alone while eliminating other macronutrient sources could be used for western corn rootworm larval growth, we varied corn root powder at varying concentrations namely, 0, 1, 1.5, 3, 6, and 10% w/w and added vitamin mix, diet preservatives, food coloring, agar, and water. Other nutritional components (wheat germ, casein, sucrose, and lipid components) were excluded. The maximum concentration of corn root powder in these agar-based diets was 10% w/w. The amount of other ingredients were the same in the WCRMO-1 diet (Huynh et al. 2017), except for the proportions of water that were changed to accommodate changes in the proportions of corn root powder (Table 1).
Table 1.
Components in diets used to rear western corn rootworm larvae in corn root powder experiment
| Components | Amount |
|---|---|
| Variable components | |
| 1. Corn root powder | 0–9 g |
| 2. Distilled water | 75–84 ml |
| Constant components | |
| 3. Agar | 1.5 g |
| 4. Chlortetracycline (10 mg/ml) | 6.4 mg |
| 5. Food coloring | 6.4 mg |
| 6. Methyl paraben | 0.1 g |
| 7. Potassium hydroxide (10%) | 3.5 ml |
| 8. Sorbic acid | 6.4 mg |
| 9. Streptomycin (12.8 mg/ml) | 6.4 mg |
| 10. Vanderzant vitamin mix | 0.90 g |
Diet Bioassays With Corn Root Powder From Different Seeds and Suppliers
To verify the milling process producing corn root powder between Monsanto and BCIRL and the substitution of Viking seed for Monsanto proprietary seed, four diets were made incorporating different seed sources and seed varieties. Both Monsanto and BCIRL made root powder from both Monsanto proprietary seed and Viking seed. The four sources of root powder were compared for their support of larval performance (survival, weight, and molting). The proportions of corn root powder and remaining ingredients were the same as in WCRMO-1 diet (Huynh et al. 2017).
Statistical Analysis
The experiments were designed as a randomized complete block design. Each formulation tested (treatment) was randomly assigned in a 12-well row of the 96-well plate and replicated at least four times in different diet plates. Each group of 12 larvae (replication) was randomly assigned a different row within each 96-well plate (1 larva per well). The plates were randomly assigned a different portion of a growth chamber. The block (the plate) was considered as a specific portion of the growth chamber. Each group of 12 larvae per plate was used to calculate percentages of survival and molting for that block or replication. Live larvae per each block were pooled and were averaged to calculate larval weight for that block. Larval performance (larval survival, weight, molt to second instar, incomplete molt to second instar, and molt to third instar) was analyzed as a randomized complete block design using PROC MIXED in SAS.
Results
Diet Bioassays With Corn Root Extracts
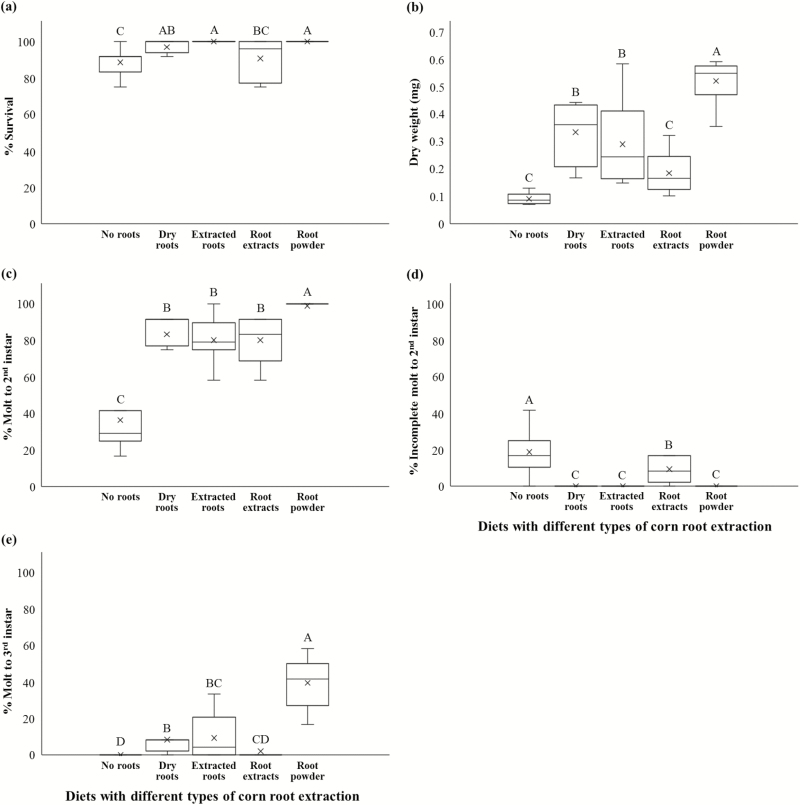
Adding extracted roots, dry roots, or corn root powder to the base diet for western corn rootworm (Huynh et al. 2017) significantly improved all measured developmental traits of larval performance compared to larvae reared on the diet with 0% root powder (Fig. 1). This included survivorship (P = 0.0022, F4, 28 = 5.46, Fig. 1a), dry weight (P < 0.0001, F4, 28 = 21.76, Fig. 1b), percent molting to second instar (P < 0.0001, F4, 28 = 22.31, Fig. 1c), reduced incomplete molting (P < 0.0001, F4, 28 = 13.91, Fig. 1d), and molting to the third instar (P < 0.0001, F4, 28 = 22.49, Fig. 1e). Methanolic root extracts did not have the same level of improvement as dry roots, root powder, or roots that had previously been extracted. Only for molting to the second instar and reduced incomplete molting were corn root extracts significantly better than diet alone (Figs. 1c and d). Root powder was significantly better than dry roots for dry weight (Fig. 1b), molting to second instar (Fig 1c), and molting to the third instar (Fig. 1e). Root extracts were significantly worse than dry roots and extracted roots for dry weight (Fig. 1b), incomplete molting (Fig. 1d), and molting to the third instar (Fig. 1e).
Fig. 1.
Survival (a), average dry weight (b), molt to second instar (c), incomplete molt to second instar (d), molt to third instar (e) of western corn rootworm larvae reared on WCRMO-1 diet (Huynh et al. 2017) containing different types of corn root preparations for 15 d. Tukey’s box plots with median (black line), the 25th and 75th percentiles (bottom and top of box, respectively), and the 5th and 95th percentiles (whiskers) are shown. Boxes with different letters are significantly different (P < 0.05).
Diet Bioassays With Corn Root Powder as the Major Nutritional Source
Survival
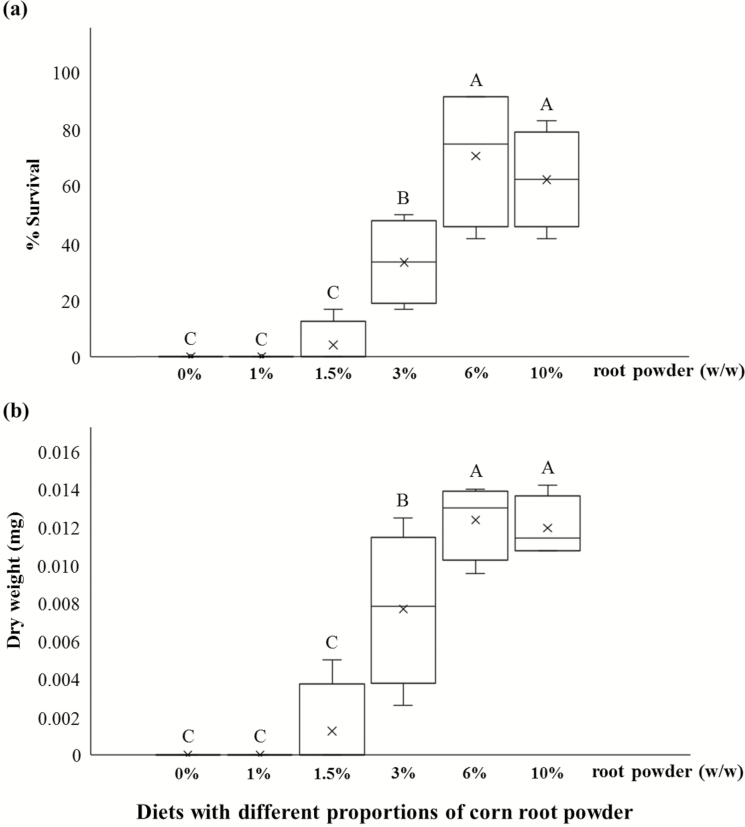
Diets containing 1.5% corn root powder in combination with other macronutrients (Fig. 1a) had close to 100% survivorship, which was higher than the larval survivorship on the diets containing only corn root powder as the major nutritional source (Fig. 2a). When corn root powder was utilized as the main nutrient source of diets, an increase in proportions of the powder resulted in a significant improvement in survival (P < 0.0001, F5, 15 = 33.41, Fig. 2a). No larvae survived when reared on diets with corn root powder at 0 and 1% while only 4.1% larvae survived on a diet with 1.5% corn root powder to 15 d. Diets with corn root powder at 6 and 10% had greater larval survival compared to the other diets tested (Fig. 2a).
Fig. 2.
Survival (a), and average dry weight (b) of western corn rootworm larvae reared on diet formulations containing corn root powder as the major macronutrient component for 15 d. Diets included corn root powder plus additions of other ingredients including vitamin mix, cholesterol, and diet preservatives (Table 1). Boxes with different letters are significantly different (P < 0.05).
Dry weight
Larval dry weight at 15 d exhibited a sigmoid response, which is typical of dose response, ranging from 0.001 mg per larva for the diet with 1.5% corn root powder up to 0.012 mg per larva for the diet with 6% corn root powder (Fig. 2b). This was a 12-fold increase, but still at least 43-fold lower when compared to larvae feeding on a diet with corn root powder combined with other macronutrient ingredients (e.g., wheat germ, casein, sucrose) (Fig. 1b). Diets with corn root powder at 6 and 10% had significantly higher larval weight than lower percent corn root powder (P < 0.0001, F5, 15 = 38.48). Larval weight for the diet with corn root powder at 3% was significantly higher than that for diets with corn root powder at 1.5%. No larvae molted to the second instar within 15 d.
Diet bioassays with corn root powder from different seeds and suppliers
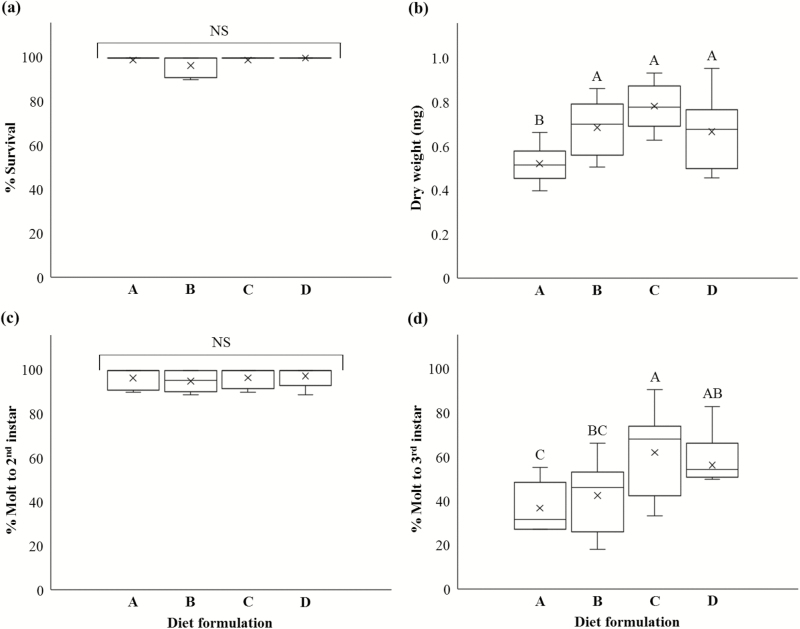
Larval survivorship to 15 d was high on all diets with corn root powder produced from different seeds or millers (Fig. 3a) and there were no significant differences in survivorship among all diets (P = 0.1884, F3, 21 = 1.75). For larval dry weight at 15 d, there were no significant differences among the diets with Viking seed milled by BCIRL or Monsanto and the diet with Monsanto seed milled by BCIRL. The diet with Monsanto seed milled by Monsanto had significantly lower larval weight (0.52 mg per larva) than other diets (P = 0.0028, F3, 21 = 6.49), which ranged from 0.67 mg per larva to 0.78 mg per larva (Fig. 3b). Percentages of larvae molted to the second instar within 10 d on all diets were high, ranging from 95.1 to 97.5%. There was no significant difference between diets (P = 0.7994, F3, 21 = 0.34) (Fig. 3c). The percentages of larvae molted to the third instar within 15 d were significantly different among diets (P = 0.0191, F3, 21 = 4.12). The diet with Monsanto seed milled by BCIRL produced significantly more third instar larvae compared to the diets with Monsanto and Viking seeds milled by Monsanto, whereas the percentage of the third instar larvae reared on the diet with Viking seed milled by BCIRL was significantly higher than that for the diet with Monsanto seed milled by Monsanto (Fig. 3d). Overall, the diets differing in corn root powder sources exhibited no significant difference in larval survivorship to 15 d and molting to the second instar. The differences were seen in larval weight and molting to the third instar after 15 d of feeding. Corn root powder made by BCIRL from Monsanto seed and Viking seed was used 1 mo and 3 mo after being produced, respectively, while corn root powder provided by Monsanto from Monsanto seed and Viking seed was used at 12 mo and 7 mo after received, respectively (stored at −20°C).
Fig. 3.
Survival (a), average dry weight (b), molt to second instar (c), molt to third instar (d) of western corn rootworm larvae reared on diet formulations differing in corn root powder sources for 15 d. Diet A and diet B contained corn root powder provided by Monsanto from Monsanto seed and Viking seed, respectively, while corn root powder made by BCIRL from Monsanto seed and Viking seed was included in diet C and diet D, respectively. Other ingredients in all diets were same as in WCRMO-1 formulation (Huynh et al. 2017). Boxes with different letters are significantly different (P < 0.05).
Discussion
We demonstrated that factors from corn roots significantly increase survivorship, larval weight, and molting (Fig. 1). Adding corn root powder, dry corn roots, or even remnant roots after extraction to a base diet for western corn rootworm significantly improved all measured developmental traits of larval performance compared to larvae reared on the diet without corn roots (Fig. 1). Overall, dry roots and extracted roots were better than the methanolic extracts of these roots, although there was no significant difference between the extract and the same mass of the corn root tissue from which they were extracted when evaluating molting to the second instar (Fig. 1c). Larval survivorship, weight, and molting to the third instar were better with extracted corn root remnants than with the methanolic extracts, indicating that the solvent did not extract all relevant nutrients (Figs. 1a, b, and e). Corn root powder was better than the dried corn roots for most factors evaluated, but may or may not have been directly comparable because these samples were made from different drying methods.
Our results suggest it is not possible to quickly isolate and identify essential factors in corn roots that assist in growth and development. Several insect species have been reared successfully on artificial diets by adding natural host material into a base diet consisting of major nutritional and textural features (e.g., soy flour, wheat germ, or casein) (Lindig and Malone 1973, Ito et al. 1975, Brun et al. 1993, Blossey et al. 2000). Corn roots contain host recognition cues and feeding stimulants, but also have a repellent compound for western corn rootworm larvae (Bernklau and Bjostad 2008; Bernklau et al. 2015, 2016). Since corn root powder is the only ingredient in the western corn rootworm formulation that is not commercially available, the identification of the essential corn root factors could assist in formulation of an open access diet for which all of ingredients could be purchased.
Corn root powder was previously optimized in combination with key diet components (wheat germ and casein) in the western corn rootworm formulation (Huynh et al. 2017). Our current results revealed that corn root powder alone without the addition of other macronutrient sources (e.g., wheat germ, casein, sucrose, linseed oil, wheat germ oil) failed to develop a diet that supports larval growth successfully compared to a diet with these factors. The maximum amount of corn root powder that was able to add to agar-based corn root powder diets after eliminating several nutritional components (i.e., wheat germ, casein, sucrose, linseed oil, wheat germ oil, cellulose, and salt mix) from the WCRMO-1 (Huynh et al. 2017) was 10% w/w. Larvae survived without molting to 15 d when reared on the agar-based corn root powder diets containing 1.5–10% corn root powder, but performed best at 6% (Fig. 2). The poor performance of larvae fed the agar-based corn root powder diets is not currently understood. The drying process may affect nutritional factors such as proteins. Inadequate dietary protein can cause inhibition of larval molting due to insufficient production of ecdysteroid in the hemolymph of larvae in other species (Hamano et al. 1994).
All diets with differing corn root powder sources supported over 95% of larvae for survival and molting to the second instar to 10 d of feeding (Fig. 3). Data for larval weight at 10 d were not available because weight data were only recorded at the end of experiments (15 d). These results suggest the milling process to making corn root powder was not significantly different between Monsanto and BCIRL, and that Viking seed can be substituted for the industry proprietary seed. Interestingly, the differences in the third instar for weight and molting were seen from differing corn root powder. There was an inverse correlation between time of storage and larval performance. Corn root powder from Monsanto seed and Viking seed provided by Monsanto was used at 12 mo and 7 mo of receipt, respectively. The diets with these corn root powders exhibited lower support of larval performance compared to the diets with corn root powder used within 3 mo of storage (Fig. 3).
Diet assays for western corn rootworm larvae are typically limited to 10 d (Pleau et al. 2002, Siegfried et al. 2005, Ludwick et al. 2018). Here we extended the bioassay period to 15 d, which allowed the detection of differences in multiple larval development stages. Data collection from the 5-d and 6-d diet bioassays for western corn rootworm initially included larval weight and survival while ignoring the information about molting (Pleau et al. 2002, Siegfried et al. 2005). The limited bioassay duration was possibly due to contamination, which was reported as a major issue of diet bioassays (Pereira et al. 2016). By minimizing contamination through clean laboratory practices as described by Huynh et al. (2017) and Ludwick et al. (2018), the length of diet bioassays for western corn rootworm testing was extended to 10 d (Ludwick et al. 2018) or 11 d (Huynh et al. 2017), which allowed the determination of differences in larval performance (weight, molt, and survival) to the second instar. In this study, we observed <1% contamination during all experiments. Similarly, low contamination rates were reported in Huynh et al. (2017), Ludwick et al. (2018), and Meihls et al. (2018). The ability to conduct an extended bioassay would facilitate experiments that require longer periods (e.g., dsRNA) and would also allow for continuous rearing of western corn rootworm which optimizes labor and other costs for rearing while avoiding possible physical damage and contamination due to larval manual transfers.
Acknowledgments
We thank Julie Barry (USDA-ARS Plant Genetics Research Unit, Columbia, MO) and James Smith (USDA-ARS BCIRL, Columbia, MO) for technical assistance. The authors would like to thank Dr. Deborah Finke (University of Missouri, Columbia, MO) for comments that improved the text. Research for this study, in part, was funded by Monsanto Corn Rootworm Knowledge Grants Program. Mention of trade names or commercial products is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the USDA. USDA is an equal opportunity provider and employer. This article reports the results of research only. Mention of a proprietary product does not constitute an endorsement or recommendation for its use by the USDA or the University of Missouri.
References Cited
- Ball H. J., and Weekman G. T.. . 1963. Differential resistance of corn rootworms to insecticides in Nehraska and adjoining States. J. Econ. Entomol. 56: 553–555. [Google Scholar]
- Bernklau E. J., and Bjostad L. B.. . 2008. Identification of feeding stimulants in corn roots for western corn rootworm (Coleoptera: Chrysomelidae) larvae. J. Econ. Entomol. 101: 341–351. [DOI] [PubMed] [Google Scholar]
- Bernklau E. J., Hibbard B. E., Dick D. L., Rithner C. D., and Bjostad L. B.. . 2015. Monogalactosyldiacylglycerols as host recognition cues for Western corn rootworm larvae (Coleoptera: Chrysomelidae). J. Econ. Entomol. 108: 539–548. [DOI] [PubMed] [Google Scholar]
- Bernklau E. J., Hibbard B. E., Norton A. P., and Bjostad L. B.. . 2016. Methyl anthranilate as a repellent for Western corn rootworm larvae (Coleoptera: Chrysomelidae). J. Econ. Entomol. 109: 1683–1690. [DOI] [PubMed] [Google Scholar]
- Blossey B., Eberts D., Morrison E., and Hunt T. R.. . 2000. Mass rearing the weevil Hylobius transversovittatus (Coleoptera: Curculionidae), biological control agent of Lythrum salicaria, on semiartificial diet. J. Econ. Entomol. 93: 1644–1656. [DOI] [PubMed] [Google Scholar]
- Branson T. F., and Krysan J. L.. . 1981. Feeding and oviposition behavior and life cycle strategies of Diabrotica: an evolutionary view with implications for pest management. Environ. Entomol. 10: 826–831. [Google Scholar]
- Brun L. O., Gaudichon V., and Wigley P.. . 1993. An artificial diet for continuous rearing of the coffee berry borer, Hypothenemus hampei (Ferrari) (Coleoptera: Scolytidae). Int. J. Trop. Insect Sci. 14: 585–587. [Google Scholar]
- Cohen A. C. 2015. Insect diets: science and technology, 2nd ed. Taylor & Francis Group, Abingdon, Oxfordshire, United Kingdom. [Google Scholar]
- Culy M. D., Edwards C. R., and Cornelius J. R.. . 1992. Effect of silk feeding by western corn rootworm (Coleoptera: Chrysomelidae) on yield and quality of inbred corn in seed corn production fields. J. Econ. Entomol. 85: 2440–2446. [Google Scholar]
- (EPA) U.S. Environmental Protection Agency. 2016. EPA docket for corn rootworm resistance management and framework for Bt corn. InUSEP Agency (ed.). United States Environmental Protection Agency, Washington, DC. [Google Scholar]
- Gassmann A. J., Petzold-Maxwell J. L., Keweshan R. S., and Dunbar M. W.. . 2011. Field-evolved resistance to Bt maize by western corn rootworm. PLoS One. 6: e22629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray M. E., Sappington T. W., Miller N. J., Moeser J., and Bohn M. O.. . 2009. Adaptation and invasiveness of Western corn rootworm: intensifying research on a worsening pest. Annu. Rev. Entomol. 54: 303–321. [DOI] [PubMed] [Google Scholar]
- Hamano K., Ikeda A., and Shen W.. . 1994. Relationship between food consumption and molting of the silkworm, Bombyx mori. Proc. Jpn. Acad., Series B. 70: 146–150. [Google Scholar]
- Huynh M. P., Meihls L. N., Hibbard B. E., Lapointe S. L., Niedz R. P., Ludwick D. C., and Coudron T. A.. . 2017. Diet improvement for western corn rootworm (Coleoptera: Chrysomelidae) larvae. PLoS One. 12: e0187997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T., Horie Y., and Nakasone S.. . 1975. Deterrent effect of soybean meal on feeding of the silkworm, Bombyx mori. J. Ins. Physiol. 21: 995–1006. [Google Scholar]
- Levine E., Spencer J. L., Isard S. A., Onstad D. W., and Gray M. E.. . 2002. Adaptation of the western corn rootworm to crop rotation: evolution of a new strain in response to a management practice. Am. Entomol. 48: 94–117. [Google Scholar]
- Lindig O., and Malone O.. . 1973. Oviposition of boll weevils fed diets containing germinated cottonseed puree or cottonseed meats puree. J. Econ. Entomol. 66: 566–567. [Google Scholar]
- Ludwick D. C., Meihls L. N., Ostlie K. R., Potter B. D., French L., and Hibbard B. E.. . 2017. Minnesota field population of western corn rootworm (Coleoptera: Chrysomelidae) shows incomplete resistance to Cry34Ab1/Cry35Ab1 and Cry3Bb1. J. Appl. Entomol. 141: 28–40. [Google Scholar]
- Ludwick D. C., Meihls L. N., Huynh M. P., Pereira A. E., French B. W., Coudron T. A., and Hibbard B. E.. . 2018. A new artificial diet for western corn rootworm larvae is compatible with and detects resistance to all current Bt toxins. Sci. Rep. 8: 5379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrone P. G., Ferri F. D., Mosley T. R., and Meinke L. J.. . 1985. Improvements in laboratory rearing of the southern corn rootworm, Diabrotica undecimpuncta howardi Barber (Coleoptera: Chrysomelidae), on an artificial diet and corn. J. Econ. Entomol. 78: 290–293. [Google Scholar]
- Meinke L. J., Siegfried B. D., Wright R. J., and Chandler L. D.. . 1998. Adult susceptibility of Nebraska western corn rootworm (Coleoptera: Chrysomelidae) populations to selected insecticides. J. Econ. Entomol. 91: 594–600. [Google Scholar]
- Meihls L. N., Huynh M. P., Ludwick D. C., Coudron T. A., French B. W., Shelby K., Hitchon A. J., Schaafsma A. W., Pereira A. E., and Hibbard B. E.. . 2018. Comparison of six artificial diets for support of western corn rootworm bioassays and rearing. J. Econ. Entomol. doi: 10.1093/jee/toy268 (online version without final page numbers). [DOI] [PubMed] [Google Scholar]
- Metcalf R. L. 1986. Preface, pp. vii–xv. InKrysan J. L. and Miller T. A. (eds.), Methods for the study of pest Diabrotica. Springer-Verlag, New York. [Google Scholar]
- Moeser J., and Vidal S.. . 2005. Nutritional resources used by the invasive maize pest Diabrotica virgifera virgifera in its new south-east-European distribution range. Ent. Exp. Appl. 114: 55–63. [Google Scholar]
- Oyediran I. O., Hibbard B. E., Clark T. L., and French B. W.. . 2004. Selected grassy weeds as alternate hosts of northern corn rootworm (Coleoptera: Chrysomelidae). Environ. Entomol. 33: 1497–1504. [DOI] [PubMed] [Google Scholar]
- Pereira A. E., Wang H., Zukoff S. N., Meinke L. J., French B. W., and Siegfried B. D.. . 2015. Evidence of field-evolved resistance to bifenthrin in Western corn rootworm (Diabrotica virgifera virgifera LeConte) populations in Western Nebraska and Kansas. PLoS One. 10: e0142299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira A. E., Carneiro N. P., and Siegfried B. D.. . 2016. Comparative susceptibility of southern and western corn rootworm adults and larvae to vATPase-A and Snf7 dsRNAs. J. RNAi Gene Silencing. 12: 528–535. [Google Scholar]
- Pereira A. E., Souza D., Zukoff S. N., Meinke L. J., and Siegfried B. D.. . 2017. Cross-resistance and synergism bioassays suggest multiple mechanisms of pyrethroid resistance in western corn rootworm populations. PLoS One. 12: e0179311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleau M. J., Huesing J. E., Head G. P., and Feir D. J.. . 2002. Development of an artificial diet for the western corn rootworm. Entomol. Exp. Appl. 105: 1–11. [Google Scholar]
- Rose R. I., and McCabe J. M.. . 1973. Laboratory rearing techniques for the southern corn rootworm. J. Econ. Entomol. 66: 398–400. [Google Scholar]
- Siegfried B. D., Vaughn T. T., and Spencer T.. . 2005. Baseline susceptibility of western corn rootworm (Coleoptera: Crysomelidae) to Cry3Bb1 Bacillus thuringiensis toxin. J. Econ. Entomol. 98: 1320–1324. [DOI] [PubMed] [Google Scholar]
- Sutter G. R., Krysan J. L., and Guss P. L.. . 1971. Rearing the southern corn rootworm on artificial diet. J. Econ. Entomol. 64: 65–67. [Google Scholar]
- Zukoff S. N., Zukoff A. L., Geisert R. W., and Hibbard B. E.. . 2016. Western corn rootworm (Coleoptera: Chrysomelidae) larval movement in eCry3.1Ab+mCry3A seed blend scenarios. J. Econ. Entomol. 109: 1834–1845. [DOI] [PubMed] [Google Scholar]