Abstract
Background
Directional, deep-brain stimulation may prove beneficial for targets (1) thinner along the lead trajectory, and (2) whose borders are not easily visible by neuroimaging. When targeting the ventral intermediate (VIM) nucleus of the thalamus for essential tremor, even baseline ataxia may be exacerbated by medial spread of current and antidromic stimulation of vestibular-cerebellar-thalamic afferents.
Case Report
The present patient with essential tremor developed refractory head tremor leading to implantation of bilateral St. Jude/Abbott segmented leads into the VIM to afford additional programming options.
Discussion
Video evidence showed that directional stimulation did not exacerbate ataxia beyond baseline, whereas nondirectional stimulation exacerbated ataxia consistently. We discuss how this programming advantage may help address a common complication from DBS implantation for essential tremor patients.
Keywords: segment, DBS, essential tremor, ataxia, head tremor
Introduction
Segmented deep-brain stimulation (DBS) leads offer a volume of tissue activation (VTA) that is constrained compared with that offered by nonsegmented leads.1 The application of directional stimulation may prove beneficial for DBS targets that are (1) thinner on a parallel plane to the lead trajectory, and (2) whose borders are not easily visible by standard neuroimaging modalities. One such target is the ventral intermediate (VIM) nucleus of the thalamus for essential tremor. Unintended spread of current to pathways adjacent to the VIM may lead to involuntary limb and/or facial muscle contraction from lateral spread (corticospinal tracts), uncomfortable paraesthesias from posterior spread (ventral caudal, Vc thalamic nucleus), and ataxia from medial and posterior spread (possibly from climbing cerebello-thalamic fibers). Although ataxia is a common clinical feature in patients with essential tremor plus,2 as suggested above, it may be exacerbated by spread of electrical current away from the VIM, especially with bilateral DBS implantation.3–5
Here, we present a case of a patient who underwent bilateral VIM DBS implantation with segmented electrodes for severe, medically refractory head tremor, with improvement of head tremor, while segmented stimulation dramatically reduced gait ataxia.
Initial presentation
NB is a 72-year-old right-handed female with multiple vascular risk factors, chronic obstructive pulmonary disease (COPD), anxiety, depression, and a family history of action tremor. Tremor began in her 30s in the right arm before later spreading to the left, exacerbated by reaching for distant objects. Impairment due to tremor began at the age of 54 when she was first diagnosed with essential tremor. Primidone and topiramate helped attenuate limb tremor; however, at the age of 60, a “no-no” head tremor developed that was medically refractory.
This significant, medically refractory head tremor led to the decision to undergo bilateral VIM DBS implantation. Per report, she had pre-procedural falls blamed on her suboptimal balance, one of which led to significant impairment of function due to a left wrist fracture requiring reconstructive surgery.
Movement disorders examination (Video 1)
She had normal facies and normal speech volume. Handwriting was illegible due to tremor without micrographia. Her tone was normal, without hypertrophy of limbs and cervical muscles. Froment’s sign was absent. There was no rest tremor, but postural and intention tremors of the arms were evident: 3–10 cm amplitude and 5–8 Hz on the right, with over 10 cm amplitude and 5–8 Hz on the left. A “no-no” head tremor without clear null position was evident at 3–10cm in amplitude and 3–5 Hz frequency for over 80% of the visit. Mild voice tremor was evident. She had normal rapid alternating movements throughout without breakdown. Foot tap was slow. To stand, she pushed off of the armrests of a low chair. Gait was unsteady with good stride and arm swing. She was unable to tandem-walk due to moderate-to-severe gait ataxia with severely widened base, developing titubation and worsened head tremor. Minimal limb ataxia was appreciated in all extremities.
Pre-operative testing
Pre-operative neuropsychological testing showed severely impaired processing speed, attention, and cognitive flexibility, while all other abilities were intact and within expectations for her. Although the patient had baseline history of anxiety and panic attacks, these were felt to be under good control prior to surgery, which was corroborated during neuropsychological assessment. Preoperative brain MRI demonstrated a minimal degree of diffuse cortical atrophy and cerebellar volume, which all appeared to be within expectations for the patient’s age. There was also evidence of moderate nonspecific patchy cerebral white matter T2 FLAIR hyperintensities considered to represent sequelae of microvascular ischemia without abnormal enhancement or abnormal signal from other cortical/subcortical structures.
DBS surgical details
St. Jude/Abbott segmented DBS leads (Model #6173) were chosen to provide additional postoperative programming options. An initial target was planned by splitting the AC/PC line into 12 equal segments, then measuring 3.5 segments posterior to the mid-commissural point. Lateral targeting was determined by taking half the width of the 3rd ventricle at the AC/PC plane and adding 11 mm. This pointed to a position just anterior to the Vc nuclei at the inferior-most point of the VIM nuclei bilaterally using the Shaltenbrand & Wahren atlas. The atlas was previously modified to conform to the patient’s visible anatomy. A slight medial trajectory was planned in order to target the head within its somatotopic representation within the VIM, with final coordinates on the left brain 13.27 mm lateral to the AC/PC line, 7.22 mm posterior to the mid commissural point, and 0.48 mm superior to the AC/PC plane. On the right brain, final coordinates were 13.69 mm lateral to the AC/PC line, 7.73 mm posterior to the mid commissural point, and 0.34 mm superior to the AC/PC plane.
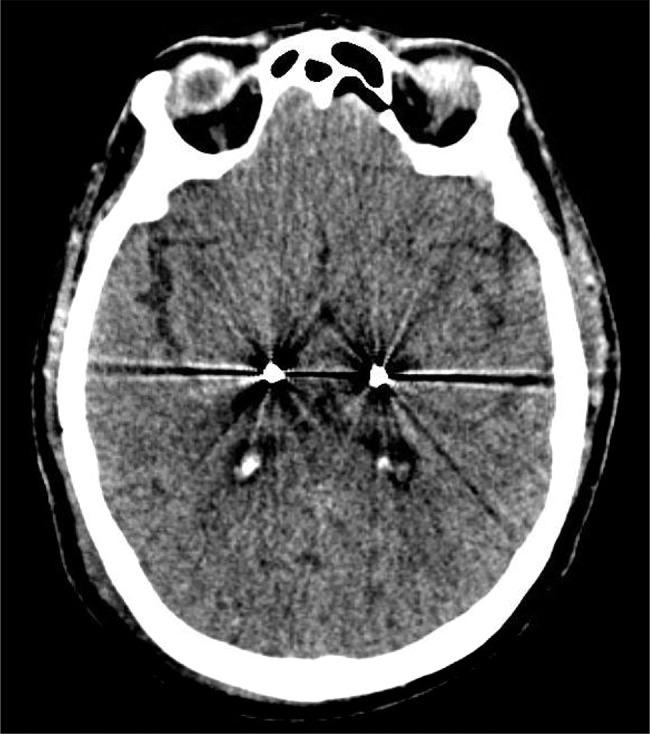
Single tract microelectrode recording (MER) and intraoperative stimulation were performed, aiding the decision for orientation of the segmented contacts. MER faithfully identified tremor cells and kinesthetic cells of the jaw bilaterally. Given that MER confirmed proper medial position, segmented contacts were oriented to project a single segment (“A”) laterally on each side, perpendicular to the mid-sagittal plane, under the hypothesis that lateral spread of current was prudent for ataxia reduction compared to partial anterior or posterior spread. Lateral orientation of segment “A” was confirmed via intraoperative fluoroscopy. With electrode insertion, tremor suppression was achieved at low settings bilaterally (1 mA on the left; 1.5 mA on the right), with supratherapeutic side effects bilaterally of slurred speech and tingling. A postoperative CT scan was performed and Stealth software used to compare with pre-operative images, demonstrating that lead placement after fixation remained excellent (Figure 1).
Figure 1. Confirming Final Electrode Placement. Post-operative CT scan showing position of contacts later chosen as active cathodes for stimulation.
Discussion
Since the advent of lesion-based therapy for movement disorders, successful reduction of motor symptomatology has highly correlated with both accurate target localization as well as accurate lesion creation.6 Deep-brain stimulation surgery is no exception, but at least has offered the ability to modify the lesion effect as an advantage over traditional lesion creation.7 Despite controlling for a number of variables through improved technologies such as enhanced neuroimaging, advances in planning software, and advents in microelectrode recording, stimulating electrodes may ultimately be implanted away from the intended target by varying distances unintended at the time of planning.
Recent publications are starting to shed light on the potential pathophysiologic mechanisms underlying the development of essential tremor. Within this research is an understanding that degeneration and abnormalities of cerebellar fibers seem to stand out as early and continuous aspects of disease development.8 Perhaps, for this reason, it is not surprising that essential tremor patients also are often known to have concomitant evidence of cerebellar ataxia, a phenomenon now classified among the ET plus syndromes.9 However, patients are not always aware of this problem given that tremor may be much more severe and impairing compared with discoordination of movement and gait. Ataxia as a symptom becomes even more relevant in the event that we successfully reduce the patient’s tremor amplitude via lesion-based therapy: invariably, we will not be able to reduce baseline ataxia, but instead with DBS could spread current away from the VIM nucleus and thus worsen ataxia with subsequent increased morbidity from falls and discoordination of movement of the limbs. This ultimately leads to inadvertent replacement of one disability for another, from tremor to ataxia, after having undergone surgery.
Conventionally, after implantation of DBS electrodes into the VIM, one could map out the contacts on the DBS electrode and identify not only which contact might best reduce the amplitude of tremor, but concomitantly which contacts cause unintended and irreversible side effects. Given the somewhat columnar shape of the VIM and its proximity to a number of tracts and nuclei of the thalamus, there are a number of potential adverse effects that can develop from stimulation away from intended targets. Spread of current laterally can lead to corticospinal tract activation and subsequent involuntary muscle contraction of the face, arm, and/or leg, possibly also inducing forms of dysarthria. More posterior stimulation could activate the ventral caudal nucleus of the thalamus and lead to significant paresthesias. Similarly, posterior and medial stimulation could activate cerebello-thalamic tracts, and, as previously suggested, lead to limb and/or gait ataxia. Such phenomena have been described more frequently in bilateral DBS implantation.10
The advent of segmented DBS electrodes introduces the potential principle of directional lead stimulation. When considering the volume of tissue activation (VTA) created by traditional nonsegmented electrodes, computer models predict that segmented electrodes can not only significantly reduce the size of the VTA, but can also direct current away from unintended targets adjacent to the electrode, especially at lower current.11 Keeping these principles in mind, theoretically, this technology offers an advantage over traditional stimulation methods especially for targets that are not easily visualized such as the VIM, despite technological advances in planning software and the use of microelectrode recording.
To the author’s knowledge, the case presented is the first published report attempting to test the theory that the advantages from directional stimulation may be used to specifically reduce the risk of worsening baseline ataxia after otherwise successful implantation of DBS electrodes into the VIM for essential tremor. Where possible, efforts were made to account for variables such as identifying the post-operative position of the electrodes with neuroimaging, identifying the orientation of the segmented contacts, and utilizing microelectrode recording data to help decide how to orient the contacts. Through monopolar survey, it was identified that gait and limb ataxia significantly worsened from baseline by 1.5 mA (if not lower) on most contacts despite head tremor and limb tremor improvement (Table 1). As such, for purposes of this testing session all amplitudes were maximized at the same current in an effort to control for all electrical properties except the VTA (which is dependent in large part on the chosen cathodes and anodes). Methodical testing of all 16 available contacts (eight per side) ultimately led to successful reduction of limb and head tremor while minimizing the development of ataxia after directing current away from medial structures (Videos 2–5). It should be noted that limb tremor was not optimally controlled in Video 5 while offering best ataxia reduction. However, later programming revealed best balance between limb tremor and ataxia reductions at 2.0 mA on each side, without otherwise altering the chosen cathodes and anodes for stimulation from the same video.
Table 1. Monopolar Symptom Threshold Assessment. Through this Monopolar Survey, Thresholds for Tremor Amplitude Improvement and the Development of Irreversible, Stimulation-induced Side Effects were Identified per Traditional Contact Assessment. Segmented Monopolar Survey was not Conducted.
| Improvement Threshold* | Irreversible SE Threshold+ | SE Description+ | |
|---|---|---|---|
| Left VIM | |||
| 4 | 1.0 mA | 1.2 mA | Limb ataxia |
| 3 | 0.6 mA | 1.2 mA | Limb ataxia |
| 2 | 1.2 mA | 1.0 mA | Limb ataxia and tingling |
| 1 | >2.0 mA ˆ | 1.0 mA | Tingling |
| Right VIM | |||
| 12 | 0.8 mA | 1.2 mA | Limb ataxia |
| 11 | 0.6 mA | 1.2 mA | Limb ataxia |
| 10 | 1.4 mA | 1.6 mA | Limb ataxia and tingling |
| 9 | >2.0 mA ˆ | 1.4 mA | Tingling |
Improvement defined as any clinically evident limb tremor amplitude reduction.
SE: side effect.
^Tremor reduction not achieved prior to intolerable SE.
Video 1. Presurgical Assessment of Tremor. Off primidone >48 hours. Stimulation Setting − Left VIM: N/A; Right VIM: N/A. Observations − Head tremor nearly 100% of time, 3−5 cm amplitude. +Jaw tremor. Bilateral hand tremor 3−10 cm postural, 1−3 cm intention. Moderately wide base with constant mild gait ataxia. Tandem documented as constant moderate deviation but not filmed. Video 2. Omnidirectional Bipolar Volume of Tissue Activation (VTA). Off primidone >7 days. Typical bipolar configuration, nonsegmented. Stimulation Setting − Left VIM: Contacts: 3(−) 1(+), Current: 1.5 mA, Pulse Width: 90 μs, Freq: 170 Hz; Right VIM: Contacts: 11(−) 9(+), Current: 1.5 mA, Pulse Width: 90 μs, Freq: 170 Hz. Observations − Head tremor 25−50% of time, 1−3 cm amplitude. No jaw tremor. Bilateral hand tremor 1−3 cm postural and intention. Mildly widened base with frequent gait ataxia, with frequent moderate tandem deviation. Video 3. Directional Monopolar VTA. Off primidone >7 days. Typical monopolar configuration, segmented, left VIM contact A points laterally. Stimulation Setting − Left VIM: Contacts: 3A(−) Case(+), Current: 1.2 mA, Pulse Width: 90 μs, Freq: 170 Hz; Right VIM: Contacts: 11A(−) Case (+), Current: 1.2 mA, Pulse Width: 90 μs, Freq: 170 Hz. Observations − Head tremor >75% of time, 3−5 cm amplitude. +Jaw tremor. Bilateral hand tremor 3−10 cm posture and intention. Moderately wide base, constant mild tandem deviation. Video 4. Directional, Medial Bipolar VTA. Off primidone >7 days. Segmented bipolar configuration, left VIM contact B points medially/posteriorly. Stimulation Setting − Left VIM: Contacts: 3B(−) 1(+), Current: 1.5 mA, Pulse Width: 90 μs, Freq: 170 Hz; Right VIM: Contacts: 11A(−) 9(+), Current: 1.5 mA, Pulse Width: 90 μs, Freq: 170 Hz. Observations − Head tremor 25−50% of time, 1−3 cm amplitude. No jaw tremor. Bilateral hand tremor 1−3 cm postural, 3−10 cm intention. Narrowed base but constant gait/lower limb ataxia, with constant moderate tandem deviation. Video 5. Directional, Lateral Bipolar VTA. Off primidone >7 days. Segmented bipolar configuration, left VIM contact A points laterally. Stimulation Setting − Left VIM: Contacts: 3A(−) 1(+), Current: 1.5 mA, Pulse Width: 90 μs, Freq: 170 Hz; Right VIM: Contacts: 11A(−) 9(+), Current: 1.5 mA, Pulse Width: 90 μs, Freq: 170 Hz. Observations − Head tremor <25% of time, <1 cm amplitude. No jaw tremor. Bilateral hand tremor 1−3 cm postural and intention. Mildly widened base with rare gait ataxia, with occasional mild tandem deviation.
In conclusion, this video case report aims to illustrate an example of how segmented DBS programming can offer tremor control whilst minimizing ataxia after bilateral VIM implantation. We suggest that careful surgical planning and methodical implementation of a clinical and surgical workflow to ensure high degrees of targeting accuracy as well as MER information can be useful to ultimately tailor segmented DBS electrode placement, orientation, and ultimate stimulation for essential tremor patients who might otherwise experience ataxic side effects from stimulation due to the narrow shape of the VIM.
Footnotes
Funding: No specific funding was received for this work.
Financial Disclosures: The author declares he has no commercial associations at the time of this submission.
Conflicts of Interest: The author reports no conflict of interest.
Ethics Statement: This study was reviewed by the authors' institutional ethics committee and was considered exempted from further review.
References
- 1.Pollo C, Kaelin-Lang A, Oertel MF, Stieglitz L, Taub E, Fuhr P, et al. Directional deep brain stimulation: an intraoperative double-blind pilot study. Brain. 2014;137:2015–2026. doi: 10.1093/brain/awu102. [DOI] [PubMed] [Google Scholar]
- 2.Bhatia KP, Bain P, Bajaj N, Elble RJ, Hallett M, Louis ED, et al. Consensus statement on the classification of tremors. From the Task Force on Tremor of the International Parkinson and Movement Disorder Society. Mov Disord. 2018;33:75–87. doi: 10.1002/mds.27121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fasano A, Herzog J, Raethjen J, Raethjen J, Rose FEM, Muthuraman M, et al. Gait ataxia in essential tremor is differentially modulated by thalamic stimulation. Brain. 2010;133:3635–3648. doi: 10.1093/brain/awq267. [DOI] [PubMed] [Google Scholar]
- 4.Groppa S, Herzog J, Falk D, Riedel C, Deuschl G, Volkmann J. Physiologic and anatomical decomposition of subthalamic neurostimulation effects in essential tremor. Brain. 2014;137:109–121. doi: 10.1093/brain/awt304. [DOI] [PubMed] [Google Scholar]
- 5.Reich MM, Brumberg J, Pozzi NG, Marotta G, Roothans J, Åström M, et al. Progressive gait ataxia following deep brain stimulation for essential tremor: adverse effect or lack of efficacy? Brain. 2016;139:2948–2956. doi: 10.1093/brain/aww223. [DOI] [PubMed] [Google Scholar]
- 6.Peng S, Levin D, Ramirez-Zamora A, Chockalingam A, Feustel PJ, Durphy J, et al. A comparison of unilateral deep brain stimulations (DBS), simultaneous bilateral DBS, and staged bilateral DBS lead accuracies. Neuromodulation. 2017;20:478–483. doi: 10.1111/ner.12588. [DOI] [PubMed] [Google Scholar]
- 7.Dwarakanath S, Zafar A, Yadav R, et al. Does lesioning surgery have a role in the management of multietiological tremor in the era of deep brain stimulation? Clin Neurol Neurosurg. 2014;125:131–136. doi: 10.1016/j.clineuro.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 8.Lin C, Louis ED, Faust PL, Koeppen AH, Vonsattel JG, Kuo S. Abnormal climbing fibre-Purkinje cell synaptic connections in the essential tremor cerebellum. Brain. 2014;137:3149–3159. doi: 10.1093/brain/awu281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Louis ED. The evolving definition of essential tremor: What are we dealing with? Parkinsonism Relat D. 2018;46:S87–S91. doi: 10.1016/j.parkreldis.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitchell KT, Larson P, Starr PA, et al. Parkinsonism Relat D. 2018. Benefits and risks of unilateral and bilateral ventral intermediate nucleus deep brain stimulation for axial essential tremor symptoms. [DOI] [PubMed] [Google Scholar]
- 11.Rebelo P, Green AL, Aziz TZ, et al. Thalamic Directional Deep Brain Stimulation for tremor: Spend less, get more. Brain Stim. 2018;11:600–606. doi: 10.1016/j.brs.2017.12.015. [DOI] [PubMed] [Google Scholar]


