Abstract
Whether sunscreen use affects melanoma risk has been widely studied with contradictory results. To answer this question we performed a systematic review of all published studies, accounting for sources of heterogeneity and bias. We searched for original articles investigating the sunscreen‐melanoma association in humans to February 28, 2018. We then used random‐effects meta‐analysis to combine estimates of the association, stratified by study design. Stratified meta‐analysis and meta‐regression were used to identify sources of heterogeneity. We included 21,069 melanoma cases from 28 studies published 1979–2018: 23 case–control (11 hospital‐based, 12 population‐based), 1 ecological, 3 cohort and 1 randomised controlled trial (RCT). There was marked heterogeneity across study designs and among case–control studies but adjustment for confounding by sun exposure, sunburns and phenotype systematically moved estimates toward decreased melanoma risk among sunscreen users. Ever‐ vs. never‐use of sunscreen was inversely associated with melanoma in hospital‐based case–control studies (adjusted odds ratio (OR) = 0.57, 95%confidence interval (CI) 0.37–0.87, p heterogeneity < 0.001), the ecological study (rate ratio = 0.48, 95%CI 0.35–0.66), and the RCT (hazard ratio (HR) = 0.49, 95%CI 0.24–1.01). It was not associated in population‐based case–control studies (OR = 1.17, 95%CI 0.90–1.51, p heterogeneity < 0.001) and was positively associated in the cohort studies (HR = 1.27, 95%CI 1.07–1.51, p heterogeneity = 0.236). The association differed by latitude (p interaction = 0.042), region (p interaction = 0.008), adjustment for naevi/freckling (p interaction = 0.035), and proportion of never‐sunscreen‐users (p interaction = 0·012). Evidence from observational studies on sunscreen use and melanoma risk was weak and heterogeneous, consistent with the challenges of controlling for innate confounding by indication. The only RCT showed a protective effect of sunscreen.
Keywords: sunscreen, melanoma, skin cancer, meta‐analysis, sun protection
Short abstract
What's new?
Effectiveness of sunscreen in reducing UV‐induced skin damage has been proven in experimental studies, but effectiveness in reducing melanoma in humans remains inconclusive. This is the first meta‐analysis to analyze data from four study designs, stratify hospital‐ and population‐based case–control studies, and include as many as five prospective studies. Evidence from observational studies on the sunscreen‐melanoma association was heterogeneous, consistent with the challenges of controlling for innate confounding by indication. The only randomized controlled trial showed a protective effect. Public health recommendations should place greater emphasis on the proper use of sunscreen in conjunction with other means of sun protection.
Abbreviations
- CI
confidence interval
- GRADE
grading of recommendations assessment, development and evaluation
- HR
hazard ratio
- N
Nord
- NOS
Newcastle‐Ottawa scale
- OR
odds ratio
- p
p Value
- RCT
randomised controlled trial
- RR
rate ratio
- SE
summary estimate
- SPF
sun protection factor
- USA
United States of America
- UV
ultraviolet
Introduction
Cutaneous melanoma is the leading cause of skin cancer death,1 accounting for 1–2% of all cancer deaths.2, 3 In 2015, melanoma occurred in 351,880 people and resulted in 59,782 deaths worldwide.4
The aetiology of cutaneous melanoma (hereafter termed melanoma) is a complex interaction of genetic, epigenetic and environmental risk factors.5, 6 Melanoma is mainly caused by ultraviolet (UV) radiation exposure in sun‐sensitive subjects and it is estimated that more than 85% of melanoma cases in Europe are attributed to sun exposure.7, 8, 9, 10 Genomic sequencing confirms that the majority of the mutations in melanomas are caused by UV radiation.11, 12 It follows that melanoma is preventable through reduction of UV exposure, making primary prevention highly cost‐effective.10, 13 Use of sunscreen is generally regarded as a major primary prevention measure alongside seeking shade, wearing protective clothes, and avoiding sunbeds,14, 15, 16, 17 and is a popular method of sun protection.18 However effectiveness of sunscreen to reduce UV‐induced damage to the skin has been proven only in experimental studies,19 and evidence of its effectiveness in preventing melanoma in humans is inconclusive. Only one randomised controlled trial (RCT) of daily sunscreen application to prevent skin cancer has been performed, showing a reduced risk of melanoma (hazard ratio = 0.50, p value = 0.051) in those randomly assigned to daily compared to discretionary sunscreen use.20, 21 The compliance to daily sunscreen application was approximately 75%; the majority of participants in the discretionary sunscreen group either did not apply sunscreen (38%) or applied at most once or twice a week (35%).21 All other studies of sunscreen and melanoma risk have been observational, mainly case–control, yielding contradictory results.22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40
The main problem with investigating this question with observational studies is confounding by indication, i.e. sunscreen users tend to be more susceptible to melanoma and more exposed to the sun than non‐users a priori.41 The contradictory and heterogeneous results of previous systematic reviews reflect this problem.42, 43, 44, 45, 46, 47, 48 In the current study we aimed to overcome these known limitations by performing in‐depth statistical analyses, comparing different patterns of sunscreen use and identifying the major sources of heterogeneity. Furthermore we wanted to update the field with new evidence.
Specifically, we aimed to 1) systematically summarise the existing literature on sunscreen use and melanoma in humans; 2) investigate the effect of ever‐ vs. never‐use on melanoma risk; 3) assess the effect of different levels and patterns of sunscreen use; 4) identify sources of bias and between‐study heterogeneity; and 5) describe the relationship between site of sunscreen application and site of melanoma.
Methods
The study protocol of this systematic review (PROSPERO ID: CRD4201706398049) was written according to PRISMA‐P50, 51 and the reporting in this article follows the PRISMA recommendations.52
Data sources and searches
We searched the electronic databases PubMed (including Medline), Embase and Cochrane Database of Systematic Reviews with search terms adapted for each of them (Supporting Information Appendix I ). In addition, we searched the protocol database PROSPERO to identify relevant ongoing reviews and screen their reference lists. To ensure literature saturation we also screened the reference lists of relevant published reviews.
Study selection
We included all original articles published by 28.02.2018 in peer‐reviewed journals arising from case–control studies, ecological studies (population‐level rather than individual‐level observational studies), cohort studies, intervention studies and clinical trials performed in humans with melanoma as endpoint and sunscreen use as exposure. We only included studies where the exposure clearly preceded the outcome. We had no restrictions regarding length of follow‐up or language.
Studies on childhood melanoma were included in the qualitative synthesis but excluded from the meta‐analyses because UV exposure does not seem to be a risk factor in the aetiology of melanoma occurring before 15 years of age.53
All records from the literature research were imported into EndNote (Thomson Reuters, version X8), de‐duplicated and then imported to Microsoft Excel (version 2010) to perform the selection process. Study selection was performed by two independent reviewers (CSR and JSS) by first screening titles and abstracts, then screening full texts. We calculated the proportion of agreement between the two reviewers for each of the two selection steps. Discrepancies were solved by discussion between the two reviewers. References were excluded based on the hierarchical exclusion criteria displayed in Figure 1 .
Figure 1.
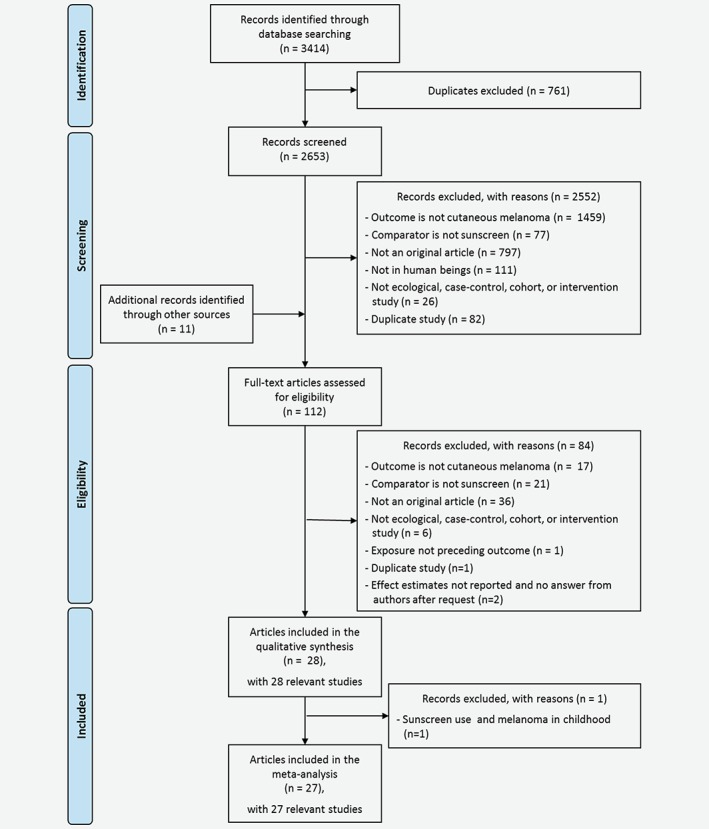
Flow diagram on inclusion of studies. The figure shows the process of selecting eligible studies for the current review and meta‐analysis. [Color figure can be viewed at wileyonlinelibrary.com]
Data extraction and quality assessment
Data were extracted using a data extraction form54 (Supporting Information Appendix II ) after piloting the process with three studies of different design and publication year. Data extraction was performed by CSR and the estimates of interest were double‐checked by MBV. Discrepancies were discussed among a subgroup of the authors until consensus was reached. We contacted study authors and requested the estimate of interest if it was not reported but the respective analysis was described. If necessary, additional articles from the same study were used to complete data extraction.
For each study we extracted the following estimates on the association of sunscreen use and melanoma, if reported: a) ever‐ vs. never‐use of sunscreen from minimally adjusted model; b) ever‐ vs. never‐use of sunscreen from maximally adjusted model; c) three‐level estimate of sunscreen use from maximally adjusted models for frequency of use, sun protection factor (SPF) used and duration of use (Supporting Information Table 1). The minimally and maximally adjusted model was the model with no or only basic adjustment and the model with most variables included, respectively, in the original study. We chose the ever‐ vs. never‐use label because most underlying studies analysed ever‐ vs. never‐use or use vs. no use of sunscreen based on their questionnaires. In addition, we extracted bibliographic and demographic information of the studies, assessment of sunscreen use, and study quality to identify sources of heterogeneity. Study quality was assessed based on the Cochrane Handbook‘s tool for assessing risk of bias54 and the Newcastle‐Ottawa Scale (NOS).55 Level of bias (high, medium, low) was rated by the data extractor (CSR) after reading the methods part of the study and blinded toward the study results.
Data synthesis and analysis
All analyses were performed in STATA (StataCorp LP, Release 14.1). In the analysis of ever‐ vs. never‐use of sunscreen we used the method of Hamling and colleagues to aggregate estimates if more than two categories of sunscreen use were reported.56 For example, if a study reported an estimate with three categories of sunscreen use: never, sometimes, and often, we aggregated ‘sometimes’ and ‘often’ into ever‐use. This was done to make the estimates across studies more comparable. Without this aggregation we would end up pooling estimates across studies where some estimates reflected the effect of the highest sunscreen category vs. no sunscreen use, while others reflected ever‐ vs. never use. The same method was used to change the reference category, if necessary. To investigate three‐level, different patterns and high sunscreen use, we extracted all estimates with at least three categories on frequency of sunscreen use, SPF used, and duration of use. For each study, the lowest and highest categories were categorised as lowest and highest groups, respectively and all intermediate categories were aggregated.57
We performed random‐effects meta‐analysis58 stratified by study design for the minimally and maximally adjusted estimates of ever‐ vs. never‐use of sunscreen, and for each three‐level variable on sunscreen use, comparing the intermediate to the lowest level and the highest to the lowest level. Heterogeneity between studies was tested with the Q‐test.59 The I2‐index was used to quantify the extent of heterogeneity, with I2‐values >50%, and > 75% being indicative of moderate and high heterogeneity, respectively.54 We included one case‐cohort study that was analysed together with the cohort studies because it was conducted prospectively.
To explore sources of heterogeneity we performed random‐effects meta‐analyses stratified by important variables predefined in the protocol, and univariable random‐effects meta‐regression analyses, on the maximally adjusted ever‐ vs. never‐use estimate. We considered the following variables: study design; year of the end of the data collection (1975–1984, 1985–1999, 2000–2012); mean latitude (>42°N, ≤42°N); region; most frequent melanoma site in the study population (trunk, head/neck, lower limbs); duration of sunscreen use (not specified, specified period, lifetime); whether sunscreen use was assessed in detail or not; level of bias (high, medium, low); whether or not the estimate of interest was adjusted for nevi and/or freckles, history of sunburn, or sun exposure; and, the proportion of participants with blond/red hair (<30%, ≥30%), blue/green eyes (<50%, ≥50%), history of sunburn (<75%, ≥75%), and who never used sunscreen (<55%, ≥55%). The cut‐offs in the proportions were chosen based on the distribution of the respective characteristic across the studies. We used tau‐squared to estimate the remaining between‐study variance in the meta‐regression model by residual maximum likelihood.58
Publication bias was investigated by the funnel plot and Egger's regression test for the maximally‐adjusted ever‐never estimates.60 We used contour‐enhanced funnel plots to define regions of the plot in which a new study would have to be located to change the statistical significance of the meta‐analysis and thereby assess the robustness of the current meta‐analysis.61
Grading of the evidence
The confidence in the cumulative evidence was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system.62 GRADE rates the quality of evidence across the domains risk of bias, consistency, directness, precision, and publication bias and rates it into one of the four categories high (further research is very unlikely to change our confidence in our effect estimate), moderate (further research is likely to change our confidence in our effect estimate), low (further research is very likely to change our effect estimate), or very low (our effect estimate is very uncertain).
Results
Study selection
We identified 3,414 records in the three databases Pubmed (n = 1,054), Embase (n = 2,132), and Cochrane (n = 228), of which 761 were duplicates and 2,552 were rated as ineligible on first screening by two reviewers (agreement = 95%; Fig. 1). Eleven studies were identified through other sources resulting in the assessment of 112 full‐texts, of which 84 (agreement = 89%) were excluded, leaving 28 studies included in the qualitative synthesis and 27 studies in the meta‐analysis after exclusion of the childhood melanoma study.32
Characteristics of included studies
The 28 articles (11 hospital‐based case–control studies,22, 23, 31, 33, 34, 35, 37, 39, 63, 64, 65 12 population‐based case–control studies,24, 25, 26, 27, 28, 29, 30, 32, 36, 38, 40, 66 one ecological study,67 three cohort studies (one of them a case‐cohort study),68, 69, 70 and one RCT21) were published between 1979 and 2018, included 208 to 178,155 participants and 33 to 11,535 melanoma cases: in total, 21,069 melanoma cases, who originated from Australia (n = 4), Europe (n = 16), Brazil (n = 2) and the USA (n = 6; Table 1). The median latitude of the study locations was 43°N (range − 30°S‐65°N). On average, 21% of participants (range 9–61%) were blond or red‐haired, 47% (range 19–86%) blue or green eyed, 48% (range 28–70%) had freckles, and 55% (range 24–85%) were fair‐skinned (Supporting Information Table 2). Most studies only assessed sunscreen use or sunscreen use frequency (Table 1). Fourteen studies defined a timeframe for the sunscreen use,21, 24, 25, 29, 32, 35, 36, 38, 40, 63, 65, 66, 67, 68 eight studies assessed the SPF used,21, 35, 36, 37, 39, 40, 66, 69 three the reapplication,40, 65, 66 three the body sites or body coverage,21, 36, 40 two the product used,35, 69 two the thickness,21, 40 and one study the reasons for sunscreen use.36
Table 1.
Overview of the studies included (n = 28)
| First author (year) | Data collection | Country | Matching1 | Total no. of participants | No. of cases | Proportion of males (%) | Age range at dx (mean) | Sunscreen information assessed2 |
|---|---|---|---|---|---|---|---|---|
| Hospital‐based case–control studies | ||||||||
| Klepp (1979)22 | 1974–1975 | Norway | Unmatched | 209 | 78 | 61 | >20 (nr) | Questionnaire: sunscreen use frequency during solar irradiation |
| Graham (1985)23 | 1974–1980 | USA | Unmatched | 420 | 218 | 100 | nr (nr) | Interview: sunscreen use |
| Ródenas (1996)31 | 1989–1993 | Spain | Unmatched | 243 | 105 | 35 | 20–79 (52) | Interview: sunscreen use frequency |
| Wolf (1998)33 | 1993–1994 | Austria | Unmatched | 512 | 193 | 42 | 18–89 (54) | Questionnaire: sunscreen use frequency before formation of melanoma |
| Espinosa A. (1999)34 | 1990–1994 | Spain | Individual (age, sex) | 351 | 116 | 47 | 21–87 (56) | Questionnaire: sunscreen use |
| Naldi (2000)35 | 1992–1995 | Italy | Unmatched | 1,080 | 542 | 42 | nr (nr) | Interview: sunscreen use frequency and duration, product type used, SPF used |
| Bakos (2002)37 | 1995–1998 | Brazil | Individual (age, sex, Ethnic group, region) | 309 | 103 | nr | 20–84 (53) | Questionnaire: sunscreen use, SPF used |
| Nikolaou (2008)64 | 2000–2004 | Greece | Individual (age, sex) | 400 | 200 | 49 | 19–84 (53) | Interview: sunscreen use |
| Klug (2010)39 | 1991–1992 | USA | Frequency (age, sex, Ethnic group, study site) | 1,662 | 717 | 55 | 20–79 (nr) | Interview: sunscreen use, sunscreen use ≥8 SPF, regular use ≥8 SPF |
| Luiz (2012)63 | 2004–2008 | Brazil | Frequency (age, sex) | 424 | 202 | 50 | 15–79 (48) | Interview: sunscreen use frequency in childhood, lifetime sunscreen use frequency |
| Vranova (2012)65 | 2010–2011 | Czech Republic | Frequency (age) | 518 | 216 | 46 | nr (54) | Questionnaire: sunscreen use frequency in childhood, sunscreen use frequency in adulthood, number of sunscreen applications when sunbathing |
| Population‐based case–control studies | ||||||||
| Holman (1986)24 | 1980–1981 | Australia | Individual (age, sex, electoral subdivision) | 1,014 | 507 | 46 | 10–79 (nr) | Interview: sunscreen use frequency and duration |
| Østerlind (1988)25 | 1982–1985 | Denmark | Frequency (age, sex) | 1,400 | 474 | 41 | 20–79 (52) | Interview: sunscreen use frequency and duration |
| Beitner (1990)26 | 1978–1983 | Sweden | Individual (age, sex) | 1,028 | 523 | 45 | nr (nr) | Questionnaire: sunscreen use frequency |
| Herzfeld (1993)27 | 1982–1983 | USA | Unmatched | 739 | 324 | 100 | >18 (nr) | Interview: sunscreen use frequency |
| Autier (1995)28 | <1990 | France, Germany, Belgium | Individual (municipality) | 856 | 418 | nr | nr (nr) | Questionnaire: sunscreen use |
| Holly (1955)29 | nr | USA | Frequency (age) | 1,382 | 452 | 0 | 25–59 (42) | Questionnaire: sunscreen use frequency in 5 years previously |
| Westerdahl (1995)30 | 1988–1990 | Sweden | Individual (age, sex, parish) | 1,040 | 400 | 49 | 15–75 (nr) | Questionnaire: sunscreen use frequency when spending time in the sun |
| Whiteman3 (1997)32 | 1994 | Australia | Individual (sex, school, grade) | 208 | 52 | nr | 3–14 (nr) | Questionnaire: sunscreen use frequency at school and on holidays in childhood |
| Westerdahl (2000)36 | 1995–1997 | Sweden | Individual (age, sex, parish) | 1,449 | 558 | 50 | 16–80 (nr) | Questionnaire: sunscreen use frequency, regular use, age at first use, SPF used, body parts applied, reasons for sunscreen use |
| Youl4 (2002)38 | 1987–1994 | Australia | Individual (age, sex, region) | 406 | 201 | 50 | 15–19 (17) | Interview: sunscreen use frequency at school, at home, on holidays for ages 5–10, 10–15, ≥15 years |
| Lazovich (2011)40 | 2004–2009 | USA | Frequency (age, sex) | 2,268 | 1,167 | 40 | 25–59 (nr) | Interview: lifetime sunscreen use frequency during outdoor activities, SPF used, thickness applied, amount of skin covered, reapplication, routine use |
| Savoye (2018)66 | 1989–2008 | France | Individual (age, birth county, education) | 1,219 | 366 | 0 | nr (57) | Questionnaire: sunscreen use at ages <15, 15–25, >25 years, SPF used, re‐application |
| Prospective ecological study | ||||||||
| Kojo (2006)67 | 1920–1985 | Finland | na | 11,535 | 11,535 | 47 | nr (nr) | Sales of sunscreen preparations 5 and 10 years before diagnosis |
| Prospective cohort studies | ||||||||
| Cho (2005)68 | 1976–2000 | USA | na | 178,1555 | 5355 | 325 | nr (53) | Questionnaire: sunscreen use frequency at the pool or beach as a teenager and in the past summer |
| Ghiasvand (2016)69 | 1991–2012 | Norway | na | 143,844 | 722 | 0 | 42–83 (60) | Questionnaire: sunscreen use in low and high latitudes, SPF used, brands of sunscreen used |
| Stenehjem6 (2017)70 | 1999–2012 | Norway | na | 1,755 | 112 | 100 | 33–84 (58) | Questionnaire: present sunscreen use frequency |
| Randomised controlled trial | ||||||||
| Green (2011)21 | 1992–2006 | Australia | na | 1,621 | 33 | 44 | nr (nr) | Intervention to daily apply sunscreen on head, neck, arms and hands, weight of returned sunscreen bottles, questionnaire on weekly sunscreen use frequency |
Abbreviations: dx, diagnosis; na, not applicable; nr, not reported; no., number; SPF, sun protection factor.
Only relevant for case–control studies; variables given as reported in the underlying article.
This column gives an overview of the sunscreen information assessed in the study. The detailed descriptions of the sunscreen estimates used in the meta‐analyses are given in Table 2 and Supporting Information Table 4.
Sunscreen and melanoma in childhood.
Sunscreen and melanoma in adolescence.
Data received upon author request with some differences to the article cited.
Case‐cohort study design.
Methodological quality of included studies
The methodological quality of the case–control studies was very heterogeneous with 11 hospital‐based case–control studies based on non‐representative cases and controls (Supporting Information Table 3). The ecological study, cohort studies and RCT fulfilled almost all of the methodological requirements.54, 55
The method and detail of assessment of sunscreen use also varied greatly between the studies (Table 2); the same was true for the level of adjustment of the “maximally‐adjusted” estimate, though most studies adjusted in some way for UV exposure and some host factors of participants.
Table 2.
Description of the two‐level estimates extracted for each study (described exactly as reported in the articles)
| First author (Publ. year) | Estimate reported in the publication | Aggregated1 two‐level estimate | Effect measure | Minimally adjusted estimate (95% CI) | Adjustment of minimally adjusted estimate2 | Maximally adjusted estimate (95% CI) | Adjustment of maximally adjusted estimate2 |
|---|---|---|---|---|---|---|---|
| Hospital‐based case–control studies | |||||||
| Klepp (1979)22 | Use of any kind of sun lotion/oil during solar irradiation: almost never ‐ very rarely ‐ sometimes ‐ quite often ‐ always | Use of any kind of sun lotion/oil during solar irradiation: almost never ‐ ever | OR | 2.05 (1.06–4.03) | None | nr | |
| Graham (1985)23 | Use of sun screening lotion: no ‐ yes | Use of sun screening lotion: no ‐ yes | OR | 2.20 (1.20–4.10) | Age | nr | |
| Ródenas (1996)31 | Sunscreen use: never ‐ sometimes ‐ always | Sunscreen use: never ‐ ever | OR | 0.38 (0.20–0.70) | None | 0.43 (0.21–0.90) | Age, skin colour, skin type, recreational sun exposure, occupational sun exposure, nevi |
| Wolf (1998)33 | Use of sunscreens: never ‐ rarely ‐ often | Use of sunscreens: never ‐ ever | OR | 1.74 (1.18–2.57) | Age, sex | 2.15 (1.37–3.37) | Age, sex, skin colour, sunbaths, sunburns |
| Espinosa A. (1999)34 | Use of sunscreens: no ‐ yes | Use of sunscreens: no ‐ yes | OR | 0.38 (0.28–0.63)3 | None | 0.45 (0.33–0.67)3 | Skin type, freckles, age |
| Naldi (2000)35 | Sunscreen use: never ‐ sometimes ‐ often | Sunscreen use: never ‐ ever | OR | 1.14 (0.89–1.45) | None | 0.90 (0.68–1.18) | Age, sex, demographic area, education, skin colour, eye colour, hair colour, freckles, nevi, sunburns, tanning pattern, sunny holiday weeks per year |
| Bakos (2002)37 | Sunscreen use habit: never ‐ SPF <8, SPF 8–15, SPF 15+ | Sunscreen use habit: never ‐ ever (all SPF) | OR | 0.46 (0.29–0.74)3 | None | 0.34 (0.18–0.63)3 | Eye colour, hair colour, photo‐ type, freckles, nevi, dysplastic nevi, physical protection, sunburn |
| Nikolaou (2008)64 | Sunscreen use: never/rarely ‐ during summer/sunny months | Sunscreen use: never/rarely ‐ during summer/sunny months | OR | 0.56 (0.34–0.90) | Conditional regression | 0.37 (0.14–0.98) | Age, gender, phototype, skin colour, outdoor leisure activities, weeks/year of sun exposure, sunburns <20 years of age, common nevi, atypical nevi, lentigenes |
| Klug (2010)39 | Sunscreen use: no use ‐ ever use | Sunscreen use: no use ‐ ever use | OR | 1.05 (0.82–1.35) | Matched logistic regression analysis | 0.90 (0.70–1.19) | Gender, age, study site, Ethnic group, ambient resident UV, hours outdoors, tan type, sunburns, gender, age group, study site |
| Luiz (2012)63 | Lifetime sunscreen use: never/almost never ‐ occasionally ‐ modified ‐ often | Lifetime sunscreen use: never/almost never ‐ ever | OR | 0.53 (0.22–1.24) | Age, sex, education | 0.34 (0.11–1.01) | Age, sex, education, ethnicity, eye colour, history of pigmented lesion removal, sunburns age 5–19, severe lifetime sunburns |
| Vranova (2012)65 | Use of the sunscreen in the adulthood: never ‐ occasionally ‐ regularly | Use of the sunscreen in the adulthood: never ‐ ever | OR | 0.63 (0.36–1.12)4 | None | 0.19 (0.09–0.43)4 | Freckles/nevi, sunburns in childhood, sunscreen in childhood, sunbathing in adulthood, sun exposure, time of day of sun exposure, holidays at seaside, holidays in mountains, solarium use |
| Population‐based case–control studies | |||||||
| Holman (1986)24 | Use of sunscreens: never ‐ <10 years ‐ ≥10 years | Use of sunscreens: never ‐ ever | OR | nr | 1.11 (0.82–1.49) | Age, sex, electoral subdivision, chronic and acute skin reaction to sunlight, hair colour, ethnic origin, age at arrival in Australia | |
| Østerlind (1988)25 | Use of sunscreens: never ‐ <10 years ‐ ≥10 years | Use of sunscreens: never ‐ ever | OR | 1.23 (0.98–1.55)4 | None | nr | |
| Beitner (1990)26 | Employment of sun protection agents: never ‐ rarely ‐ often/very often | Employment of sun protection agents: never ‐ ever | OR | nr | 1.59 (1.17–2.15)3 | Age, sex, hair colour | |
| Herzfeld (1993)27 | Using sunscreens: no ‐ yes | Using sunscreens: no ‐ yes | OR | 0.81 (0.58–1.12) | None | nr | |
| Autier (1995)28 | Regular sunscreen use: never ‐ ever | Regular sunscreen use: never ‐ ever | OR | 1.59 (1.18–2.14) | Conditional regression | 1.50 (1.09–2.06) | Age, sex, hair colour, holiday weeks in sunny resorts, municipality |
| Holly (1995)29 | Use of sunscreen 5 years before diagnosis: never ‐ sometimes ‐ almost always | Use of sunscreen 5 years before diagnosis: never ‐ ever | OR | 0.67 (0.51–0.87)4 | None | 0.52 (0.37–0.73) | Sunburns ≤12 years, skin reaction to sun, hair colour, nevi, complexion, maternal ethnicity, history of skin cancer, age |
| Westerdahl (1995)30 | Use of sunscreens: never ‐ sometimes ‐ almost always | Use of sunscreens: never ‐ ever | OR | 1.65 (1.24–2.20) | Matched analysis | 1.47 (1.08–2.01) | Sunburns, sunbathing in summer, outdoor employment in summer, nevi, hair colour, eye colour, freckling, age, gender, parish |
| Whiteman5 (1997)32 | Sunscreen use at school: never/rarely ‐ sometimes ‐ often ‐ always | Sunscreen use at school: never/rarely ‐ ever | OR | 1.73 (0.97–3.08) | Matched analysis | 1.01 (0.50–2.05) | Tanning ability, freckling, nevi, sex, school, grade |
| Westerdahl (2000)36 | Use of sunscreens: never ‐ sometimes ‐ always initially of the year then sometimes ‐ always | Use of sunscreens: never ‐ ever | OR | 1.35 (1.08–1.69) | Conditional regression | 1.30 (0.90–1.90) | Hair colour, sunburns, sunbathing in summer, duration of sunbathing, age, sex, parish |
| Youl6 (2002)38 | Average lifetime index of sunscreen use at home: never/rarely ‐ sometimes ‐ often/always | Average lifetime index of sunscreen use at home: never/rarely – ever | OR | 1.05 (0.63–1.74) | Conditional regression | nr | |
| Lazovich (2011)40 | Routine sunscreen use: nonusers in both decades ‐ middle ‐ high in both decades | Routine sunscreen use: nonusers in both decades ‐ users in both decades | OR | 1.33 (0.91–1.95) | Age, gender | 1.12 (0.78–1.62) | Age, gender, phenotype risk score, moles, income, education, family history, sunburns, sun exposure, solarium use |
| Savoye (2018)66 | Sunscreen use since age 25: no protection ‐ SPF <8 ‐ SPF 8–15 ‐ SPF >15 | Sunscreen use since age 25: no protection ‐ SPF <8/SPF 8‐15/SPF >15 | OR | 1.71 (1.29–2.27) | Conditional regression | 1.50 (1.10–2.06) | Skin sensitivity, nevi, freckling, eye colour, skin colour, hair colour, hours of recreational sun exposure, recreational UV score, sunburns >25 years, age, birth county, education |
| Prospective ecological study | |||||||
| Kojo (2006)67 | Rate ratio for CM per 1 euro increase per capita in sunscreen sales | Rate ratio per 1 euro increase per capita in sunscreen sales | RR | nr | 0.48 (0.35–0.66) | Age, gender, 10 year lag time, sunny resort holidays, holiday duration | |
| Prospective cohort studies | |||||||
| Cho7 (2005)68 | Percent of time of sunscreen use when outside at the pool or beach in the past summer: 0–25 ‐ 50 ‐ 75 ‐ 100 | Percent of time sunscreen used outside at the pool or beach in past summer: 0 ‐ ≥25 | HR | 1.66 (1.41–1.96) | Age | 1.42 (1.21–1.68) | Age, alcohol consumption, sunburns, childhood reaction to sun, hair colour, smoking, BMI, exercise, UV flux, moles, caffeine, family history of CM |
| Ghiasvand (2016)69 | Sunscreen use from time‐dependent analysis: never ‐ ever | Sunscreen use from time‐dependent analysis: never ‐ ever | HR | 1.45 (1.11–1.90) | Age, calendar year | 1.13 (0.85–1.50) | Age, calendar year, hair colour, freckles, ambient UV, weeks sunbathing, sunburns, solarium use |
| Stenehjem8 (2017)70 | Present sunscreen use: never/rarely ‐ often ‐ almost always | Present sunscreen use: never/rarely ‐ often/almost always | HR | 1.11 (0.69–1.76) | Age | 1.10 (0.77–1.57) | Age, benzene exposure, education |
| Randomised controlled trial | |||||||
| Green (2011)21 | Random assignment to daily or discretionary sunscreen application to head and arms | Sunscreen application to head and arms: daily ‐ discretionary | HR | 0.50 (0.24–1.02) | 0.49 (0.24–1.02) | Sex, skin type, nevi, history of cancer, sun exposure | |
Abbreviations: BMI, body mass index; CI, confidence interval; CM, cutaneous melanoma; HR, hazard ratio; nr, not reported; OR, odds ratio; Publ., publication; SPF, sun protection factor; RR, rate ratio; RCT, randomised controlled trial; UV, ultraviolet.
If sunscreen exposure was reported in more than two categories they were aggregated into two categories (ever‐ vs. never‐use).
As reported by the authors.
Estimate from individual‐matched case–control study that did not take the matching into account in the statistical analysis, or did not report it.
Estimate from frequency‐matched case–control study that did not adjust for the matching variables in the statistical analysis, or did not report it.
Sunscreen and melanoma in childhood.
Sunscreen and melanoma in adolescence.
Estimates received upon author request because they were not reported in the cited article.
Case‐cohort study design.
Ever sunscreen use and melanoma risk
The forest plot of minimally‐adjusted estimates showed substantial heterogeneity both within hospital‐based (I 2 = 86%, p < 0.001) and population‐based case–control studies (I 2 = 80%, p < 0.001), and between the different study designs (Fig. 2).
Figure 2.
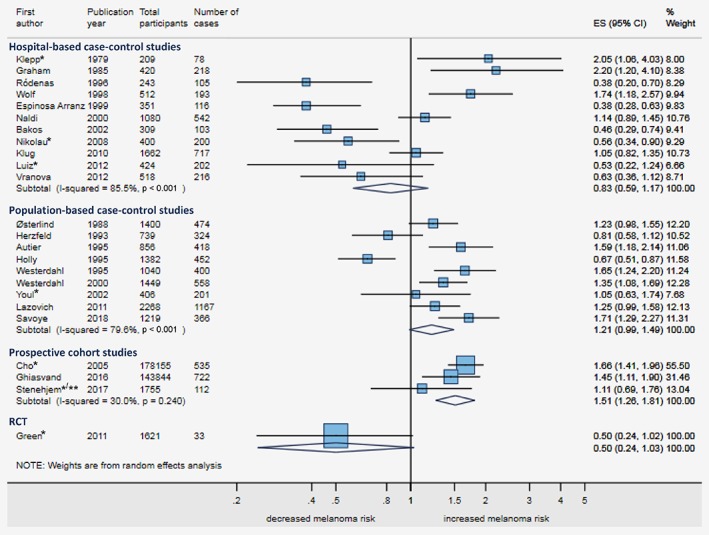
Forest plot for ever‐ vs. never‐use of sunscreen and melanoma risk, minimally adjusted estimates stratified by study design. The figure shows the forest plot for melanoma risk comparing ever‐ vs. never‐use of sunscreen for all studies that reported a minimally adjusted estimate, stratified by study design. The estimates of the case–control studies are reported in odds ratios with 95% confidence intervals (CIs); and, the estimates of the cohort studies and the RCT as hazard ratios with 95% CIs. Minimal adjustment of some estimates (e.g. age and sex) and exact definition of the estimates is described in Table 2. Abbreviations: CI, confidence interval; ES, effect size; RCT, randomised controlled trial. * Not ever‐ vs. never‐use of sunscreen; see Table 2 for the exact definition of the estimate. **Case‐cohort study. [Color figure can be viewed at wileyonlinelibrary.com]
The forest plot of maximally‐adjusted estimates showed that adjustment moved most estimates toward a more reduced risk of melanoma among sunscreen users (Figs. 2 and 3) though substantial heterogeneity remained (Fig. 3), especially within case–control studies (I 2 = 86%, p < 0.001 for hospital‐based; 81%, p < 0.001 for population‐based) but also between study designs. We found an inverse sunscreen‐melanoma association in hospital‐based case–control studies (summary odds ratio (OR) = 0.57, 95%CI 0.37–0.87), the ecological study (rate ratio (RR) = 0.48, 95%CI 0.35–0.66), and the RCT (hazard ratio (HR) = 0.49, 95%CI 0.24–1.01). No association was found on summarising results from population‐based case–control studies (OR = 1.17, 95%CI 0.90–1.51) and a positive sunscreen‐melanoma association was seen on summarising the three cohort studies (HR = 1.27, 95%CI 1.07–1.51).
Figure 3.
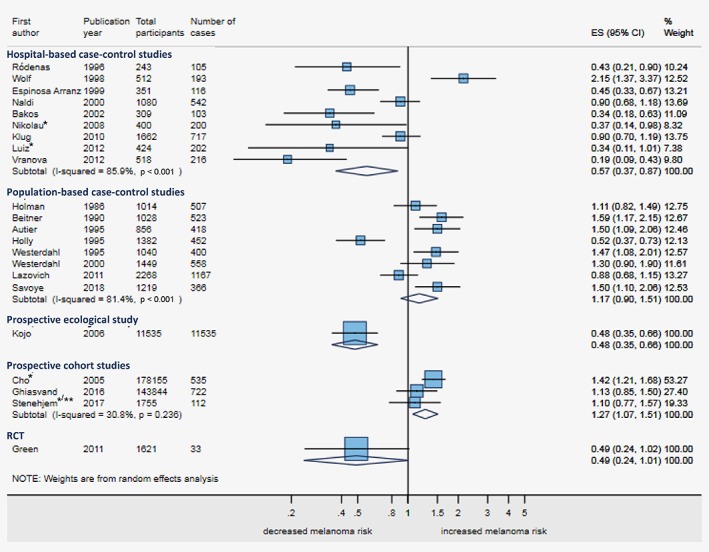
Forest plot for ever‐ vs. never‐use of sunscreen and melanoma risk, maximally adjusted estimates stratified by study design. The figure shows the forest plot for melanoma risk comparing ever‐ vs. never‐use of sunscreen for all studies that reported a maximally adjusted estimate, stratified by study design. The estimates of the case–control studies are reported as odds ratios with 95% confidence intervals (CIs); the estimates of the cohort studies and the RCT as hazard ratios with 95% CIs; and, the estimate of the ecological study as rate ratio with 95% CI. Adjustment and exact definition of the estimates is described in Table 2. Abbreviations: CI, confidence interval; ES, effect size; RCT, randomised controlled trial. *Not ever‐ vs. never‐use of sunscreen; see Table 2 for the exact definition of the estimate. **Case‐cohort study. [Color figure can be viewed at wileyonlinelibrary.com]
Three‐level estimates of sunscreen use and melanoma risk
Sixteen studies reported at least a three‐level estimate on the frequency of sunscreen use (never, sometimes, often/always),22, 24, 25, 26, 29, 30, 31, 33, 35, 36, 38, 40, 63, 68, 69, 70 six studies distinguished low from high SPF sunscreen use (compared to no use),35, 36, 37, 40, 66, 69 and four studies distinguished short‐ from long‐term use of sunscreen (compared to no use)24, 25, 35, 36 (Supporting Information Table 4). We did not observe a trend or U‐shaped association comparing the intermediate‐ and high‐users of sunscreen to the non‐users for each of the three‐level estimates (Supporting Information Fig. 1). The summary estimates comparing sometimes‐ to never‐use were 1.07 (95%CI 0.80–1.42) in the hospital‐based case–control studies, 1.13 (95%CI 0.98–1.30) in the population‐based case–control studies, and 1.38 (95%CI 1.17–1·62) in the cohort studies. The summary estimates comparing often/always‐ to never‐use were 1.01 (95%CI 0.38–2.67) in the hospital‐based case–control studies, 1.01 (95%CI 0.67–1.52) in the population‐based case–control studies, and 1.32 (95%CI 1.10–1.59) in the cohort studies (Supporting Information Fig. 1A).
Sources of heterogeneity
The association between sunscreen use and melanoma from stratified analyses is presented in Table 3 and Supporting Information Figure 2. Studies conducted in lower latitudes showed an inverse association between sunscreen use and melanoma (summary estimate = 0.64, 95%CI 0.47–0.89 for studies ≤42°N) but there was no association in studies from higher latitudes (summary estimate = 1.09, 95%CI 0.83–1.44, p interaction = 0·042). Further statistically significant interactions were observed between the association of sunscreen use and 1) the region of the study (p interaction = 0.008); 2) adjustment for nevi and/or freckles (with an inverse association only in studies adjusting; p interaction = 0.035); and, 3) the proportion of sunscreen users in the study (with an inverse association of sunscreen use and melanoma only in studies where ≥55% of participants never used sunscreen; p interaction = 0.012). Remaining between‐study variance was generally high after all stratifications (0.131 ≤ tau‐squared ≤ 0.492).
Table 3.
Association between sunscreen use and melanoma from stratified analyses
| No1 | Estimate | 95% CI | p 2 | Tau2 3 | |
|---|---|---|---|---|---|
| Study design | 0.069 | 0.221 | |||
| Hospital‐based case–control studies | 9 | 0.57 | 0.37–0.87 | ||
| Population‐based case–control studies | 8 | 1.17 | 0.91–1.51 | ||
| Ecological study | 1 | 0.48 | 0.35–0.66 | ||
| Cohort studies | 3 | 1.27 | 1.07–1.51 | ||
| Randomised controlled trial | 1 | 0.49 | 0.24–1.01 | ||
| Year of the end of data collection | 0.3194 | 0.320 | |||
| 1975–1984 | 2 | 1.33 | 0.93–1.89 | ||
| 1985–1999 | 10 | 0.86 | 0.61–1.21 | ||
| 2000–2012 | 9 | 0.82 | 0.60–1.13 | ||
| Mean latitude of the study | 0.042 | 0.248 | |||
| > 42° N | 11 | 1.09 | 0.83–1.44 | ||
| ≤ 42° N | 11 | 0.64 | 0.47–0.89 | ||
| Region of the study | 0.008 | 0.131 | |||
| Northern Europe | 6 | 1.10 | 0.78–1.57 | ||
| Northern America | 4 | 0.89 | 0.59–1.34 | ||
| Eastern Europe | 1 | 0.19 | 0.09–0.42 | ||
| Western Europe | 3 | 1.61 | 1.32–1.97 | ||
| Southern Europe | 4 | 0.55 | 0.33–0.89 | ||
| Southern America | 2 | 0.34 | 0.20–0.59 | ||
| Australia | 2 | 0.79 | 0.36–1.74 | ||
| Most frequent melanoma site | 0.825 | 0.256 | |||
| Trunk | 8 | 0.72 | 0.49–1.05 | ||
| Head/neck | 3 | 0.93 | 0.57–1.54 | ||
| Lower limbs | 2 | 0.74 | 0.29–1.90 | ||
| Duration of sunscreen use | 0.482 | 0.313 | |||
| Not specified (general habit) | 11 | 0.94 | 0.69–1.28 | ||
| Specified period | 10 | 0.81 | 0.60–1.10 | ||
| Lifetime | 1 | 0.34 | 0.11–1.03 | ||
| More detailed assessment than “sunscreen yes‐no” | 0.493 | 0.319 | |||
| No (only sunscreen yes‐no) | 10 | 0.93 | 0.66–1.32 | ||
| Yes (more than sunscreen yes‐no) | 12 | 0.80 | 0.60–1.05 | ||
| Level of bias | 0.884 | 0.345 | |||
| High | 6 | 0.76 | 0.42–1.40 | ||
| Medium | 12 | 0.84 | 0.64–1.12 | ||
| Low | 4 | 1.02 | 0.73–1.41 | ||
| Adjusted for nevi/freckling | 0.035 | 0.238 | |||
| No | 8 | 1.25 | 0.99–1.56 | ||
| Yes | 14 | 0.69 | 0.51–0.92 | ||
| Adjusted for history of sunburn | 0.587 | 0.323 | |||
| No | 6 | 0.95 | 0.63–1.44 | ||
| Yes | 16 | 0.82 | 0.64–1.05 | ||
| Adjusted for sun exposure | 0.253 | 0.295 | |||
| No | 6 | 0.64 | 0.38–1.09 | ||
| Yes | 16 | 0.95 | 0.77–1.18 | ||
| Proportion with blond/red hair | 0.150 | 0.411 | |||
| < 30% | 10 | 0.65 | 0.44–0.97 | ||
| ≥ 30% | 3 | 1.24 | 0.80–1.93 | ||
| Proportion with blue/green eyes | 0.326 | 0.492 | |||
| < 50% | 7 | 0.57 | 0.35–0.93 | ||
| ≥ 50% | 4 | 0.93 | 0.48–1.79 | ||
| Proportion with history of sunburn | 0.406 | 0.429 | |||
| < 75% | 6 | 0.62 | 0.33–1.15 | ||
| ≥ 75% | 7 | 0.98 | 0.72–1.31 | ||
| Proportion of never5 sunscreen user | 0.012 | 0.164 | |||
| < 55% | 13 | 1.03 | 0.83–1.28 | ||
| ≥ 55% | 4 | 0.42 | 0.32–0.55 | ||
Abbreviations: CI, confidence interval; No, number; p, p value.
Number of studies in each group.
p Value for interaction from univariable meta‐regression model.
Remaining between‐study variance estimated by residual maximum likelihood.
p Value for trend.
A few studies included rare sunscreen users in the “never user” category. See Table 2 for the exact definition of the sunscreen variable.
Site of sunscreen application and site of melanoma
Two studies21, 36 assessed the body site of sunscreen application but neither related this to the site of melanoma.
Meta bias and quality of the cumulative evidence
The funnel plot (Supporting Information Fig. 3) shows the effect estimates from the individual studies against the precision of the studies (standard error in reversed scale), placing the largest studies toward the top. In the absence of bias and between‐study heterogeneity, the plot would have resembled a symmetric inverted funnel, while our plot showed evidence of asymmetry confirmed by an Egger's test for small‐study effects (p = 0.010). The funnel plot with contours of statistical significance (Supporting Information Fig. 4) shows which combinations of effect size and standard error would be required in an additional study, to change or maintain the statistical significance of the current summary estimate. In our meta‐analysis, the plot showed that all of the current studies were lying in the area where future studies (if lying in the same area) would change the current effect estimate toward a significantly positive association between sunscreen use and melanoma risk (significant effect estimate >1).
The GRADE assessment resulted in an overall very low quality of evidence from the case–control studies, ecological study and cohort studies, and in a moderate quality of evidence from the RCT (Supporting Information Table 5).
Discussion
We assessed the sunscreen‐melanoma association in 21,068 melanoma patients based on 28 studies in this comprehensive systematic review. The main body of evidence came from observational studies with high between‐study heterogeneity. We found an inverse association between sunscreen use and melanoma in hospital‐based case–control studies, the ecological study and the RCT. There was no association in the population‐based case–control studies and positive association between sunscreen use and melanoma in the cohort studies. No clear pattern resulted when comparing the few studies that reported three‐level estimates of sunscreen use regarding frequency of use, SPF of sunscreen used or duration of use. The association between sunscreen use and melanoma differed by latitude, region, adjustment for nevi/freckling, and proportion of never sunscreen users.
Comparison with previous meta‐analyses
Our study is the first systematic review and meta‐analysis to present results from four different study designs, the first to include five prospective studies, and the first to stratify the case–control studies into hospital‐based and population‐based studies. Five meta‐analyses of the association of sunscreen use and melanoma have been published (in 200243, 200344, 200745, 201546, and 201848). Only Dennis and colleagues (2003)44 aggregated three‐level estimates of sunscreen use into ever‐ vs. never‐use, as we did, but the final estimate (pooled OR = 1.0, 95%CI 0.8–1.2, from 18 case–control studies) was unadjusted for confounding factors. Consistent with our findings, they showed that adjustment moved estimates toward a reduced risk of melanoma in sunscreen users, by pooling only the nine studies that adjusted for sun sensitivity (OR = 0.8, 95%CI 0.6–1.0).44 Similar to our approach, Dennis and colleagues tried to go beyond “ever‐use” of sunscreen and pooled 12 case–control studies that reported at least a three‐level estimate on the frequency of sunscreen use (aggregated by ordered regression models) but found no association.44
Despite high heterogeneity, the other four meta‐analyses pooled results using quite different definitions of sunscreen use into one estimate (for example always‐ vs. never‐use and ever‐ vs. never‐use), across very different study designs or different types of skin cancer, and across estimates from adjusted and unadjusted models. The earliest meta‐analysis (2002)43 included 11 case–control studies but pooled only the four registry‐based, resulting in no association (OR = 1.01). Gorham and colleagues (2007)45 included 17 case–control studies with a pooled OR = 1.2 (95%CI 0.9–1.6). Similar to our review, they found statistically significant interaction with study latitude. Xie and colleagues (2015)46 included 21 studies and calculated a summary estimate of 1.15 (95%CI 0.91–1.44; I2 = 84%, p heterogeneity < 0.001). This review46 also tried to identify sources of heterogeneity by meta‐regression but found no significant interactions. The most recent meta‐analysis (2018)48 included 30 studies but only 25 were related to melanoma. They included only two prospective studies compared to five in our review, included cross‐sectional study designs and calculated a summary estimate despite high heterogeneity (summary estimate = 1.08, 95%CI 0.91–1.29, including melanoma and other skin cancers). It is not possible to directly compare the aggregated estimates of association from these previous meta‐analyses with our sorted and stratified estimates.
Interpretation of results
When interpreting our results, we needed to account for the different levels of evidence of the study designs included in our meta‐analyses. In the hierarchy of strength of evidence, ecological studies are the weakest, and cohort studies and RCTs are the strongest.71 Our funnel plot showed small‐study effects, meaning that the results in small studies differed from the results in large studies. We suspect that this funnel plot asymmetry is due to poor methodological quality in small studies rather than publication bias.60 This supports the fact that our results need to be interpreted taking the methodological quality and level of evidence into account as was done in the GRADE assessment.
Careful interpretation of the results of the observational studies is essential because of their multiple methodological limitations when assessing the sunscreen‐melanoma association: recall bias (in the case–control studies); ecological fallacy (in the ecological study, where we do not know whether the specific individuals who used sunscreen were those with lower incidence of melanoma because the association was measured at the population level); difficulty in meaningfully assessing sunscreen use by ad hoc questionnaires; and, by far the most concerning, residual confounding since the determinants of sunscreen use and melanoma (susceptibility to sunburn and high sun exposure) are almost inseparable in observational studies.41 Furthermore, in their large population‐based cohort study,69 Ghiasvand and colleagues found significant differences between sunscreen users and non‐users in regard to phenotype and sun exposure. Our review highlights the profound influence of residual confounding by showing that increasing adjustment systematically moved effect estimates toward a more reduced risk of melanoma among sunscreen users. The problems incorporated in observational studies have also led to an overall very low quality of evidence in the GRADE rating.72 To overcome this problem we suggest performing cohort studies that also explore reasons for sunscreen use and non‐use, and how sunscreen users’ behaviour differs from that of non‐users,73 or analysing cohort studies using newer statistical methods (for example inverse probability weighting of using sunscreen) that can adjust for confounding by indication and mimic an RCT design.74 In observational studies, “treatment selection” (sunscreen use in our case) is often influenced by subject characteristics. As a result, baseline characteristics of subjects using sunscreen differ systematically from those not using sunscreen. A propensity score such as inverse probability weights is the probability of using sunscreen conditional on observed baseline characteristics. Applying such weights allows one to analyse an observational (nonrandomized) study so that it mimics an RCT by balancing the distribution of observed baseline covariates between sunscreen users and non‐users.75
The strongest existing evidence comes from the one RCT, as suggested by the pyramid of evidence.76 The RCT was performed in an Australian population with high year‐round sun exposure and skin cancer awareness.21, 77 There is therefore a need for additional high‐quality, large RCTs in countries of higher latitude, but these are highly unlikely to be conducted because of ethical constraints (vulnerable study participants cannot be denied regular use of sunscreen) and the need to enrol extremely large numbers of participants in order to prospectively assess the rare outcome of melanoma.19 However, future RCTs could examine intermediate endpoints (biomarkers, genetic mutations) to improve the evidence‐base for sunscreen use.19
Because of the imprecise definition of ever‐ vs. never‐use of sunscreen and highly variable assessment of sunscreen use across studies, we compared the studies reporting at least three‐levels and different patterns of sunscreen use. Unfortunately very few studies reported such estimates, and therefore we could not provide evidence about what pattern of use would be most effective and whether there is a discernible trend with increasing frequency of sunscreen use. We generally observed that very few studies assessed sunscreen use behaviour in depth such as exploring thickness of sunscreen applied, re‐application or proportion of body covered with sunscreen. Such information would be crucial to assess in future research in relation to melanoma risk since we know that most people do not apply sunscreen properly.78, 79
Of further concern is the high heterogeneity between studies that could not be fully explained by the variables we investigated in the meta‐regression analysis (see also heterogeneity between study participants in Supporting Information Table 2). We found a more protective effect of sunscreens in lower latitudes and Southern countries. This might be due to sun exposure being more homogeneous in these studies (everybody is exposed to some degree) and to sunscreen use being regarded as a routine preventive measure rather than being regarded as a means to prolong sun exposure by some at higher latitudes.80, 81 It would therefore be important to distinguish between studies where sunscreen was used for intentional sun exposure and tan acquisition versus for protection from sun damage. This was not possible with currently available evidence. Also, people from lower and higher latitude might differ in their interpretation of frequencies of sunscreen use. For example higher latitude participants might consider “often” using sunscreen means applying on sunny days, whereas lower latitude participants may think of “often” using sunscreen as daily application.
We further found an inverse association between sunscreen use and melanoma in studies where the estimate was adjusted for number of naevi and/or freckling, while no association was found in studies without such adjustment. This might be due to the fact that number of naevi/freckling are especially important predictors of melanoma,82 and self‐reported assessment of number of naevi/freckling as confounding factor might be more valid than other factors (e.g. sun exposure or sunburns long time ago).83, 84 We found an inverse association of sunscreen use and melanoma in studies with a high proportion of never sunscreen users. This makes sense because of a better contrast between sunscreen users and non‐users, revealing the effect of sunscreen in populations where the majority is not using it.
Strengths and limitations
This systematic review has several strengths. Compared to previous reviews, it adds several new studies and study designs, including three large cohort studies, and performs in‐depth statistical analyses. We have extracted a variety of descriptive variables to identify sources of heterogeneity. To make the sunscreen variable as comparable as possible between studies, we attempted to aggregate or transform the estimates into ever‐ vs. never‐use of sunscreen in order to combine the studies, but this inherited the weakness that the sunscreen measure was very broad, further obscuring any true effect of sunscreen.
Other limitations include the relatively low number of eligible studies, especially intervention studies and studies reporting three‐level estimates on sunscreen use, the difference in study designs, and the between‐study heterogeneity. Because of the high heterogeneity we could not calculate an overall summary estimate. Due to the limited number of studies we could not perform multivariable meta‐regression analysis, and were forced to collapse the meta‐regression and stratified meta‐analysis over the different study designs. Also, we could not identify enough studies to answer our last research question on a possible relationship between body sites of sunscreen application and of melanoma. Furthermore, we used the label ever‐ vs. never‐use because never or no use were the terms mostly used in the original studies included in the meta‐analysis. This might be somewhat misleading as the never‐users probably include some who used sunscreen rarely.
Conclusion
We found overall weak and heterogeneous published evidence for an association between sunscreen use and melanoma. Observational studies showed an inverse association in hospital‐based case–control studies and the ecological study, no association in population‐based case–control studies and a positive association in the three cohort studies. A protective effect of sunscreen was found in the only RCT performed. We therefore advocate for studies examining intermediate (biological) endpoints to be used in high‐quality RCTs. The effectiveness of sunscreen to reduce UV radiation to the skin has been proven after acute exposure in human studies and in experimental studies.19 In our review, this translated into a reduced melanoma risk in the long‐term for only some studies and we attribute this to residual confounding of observational studies and the misuse of sunscreen to increase rather than decrease sun exposure in some high latitude populations. Public health recommendations should place greater emphasis on the proper use of sunscreen (for sun protection vs. to prolong time in the sun) in conjunction with other means of sun protection.
Supporting information
Supplemental Figure 1 Forest plot comparing three levels of sunscreen use (no – medium – high) and melanoma risk, stratified by study design
Supplemental Figure 2: Sources of heterogeneity: forest plot of summary estimates from stratified meta‐analyses
Supplemental Figure 3: Funnel plot and Egger's regression to estimate publication bias
Supplemental Figure 4: Funnel plot with statistical significance contours
Supplemental Table 1 Rules applied to choose the minimally and maximally adjusted estimate to be included in the meta‐analysis
Supplemental Table 2: Skin cancer related characteristics of participants in the studies included (n = 28)
Supplemental Table 3: Description of the methodological quality of the studies included (n = 28)
Supplemental Table 4: Description of the three‐level estimates extracted for each study (described exactly as reported in the articles)*
Supplemental Table 5: GRADE evidence profile for sunscreen use and melanoma risk, stratified by study design
Appendix S1: Supporting Information
Appendix S2: Supporting Information
Conflict of interest: No conflict of interest for any of the authors.
CSR is the guarantor of the paper. CSR and MBV conceived and designed the study, CSR conducted the literature research, CSR and JSS conducted the study selection, CSR and MBV conducted the data extraction, CSR analysed the data, all authors interpreted the data, all authors wrote the paper, and all authors approved the final draft of the study. All authors had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis.
Transparency declaration: The study's guarantor (CSR) affirms that the study is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as registered have been explained in the PROSPERO registry.
The funding sources had no role in the study conception, literature research, data extraction, data analysis and interpretation, writing of the study or the decision to submit it for publication.
References
- 1. Ferlay J, Soerjomataram I, Ervik M, et al. Cancer incidence and mortality worldwide: IARC CancerBase no. 11 [internet]. Lyon, France: International Agency for Research on Cancer, 2012. [Google Scholar]
- 2. American Cancer Society . Cancer Facts & Figures 2012. Atlanta, GA: American Cancer Society, 2012. [Google Scholar]
- 3. SEER ‐ Surveillance . Epidemiology and end result program: turning cancer data into discovery: NCI ‐ National Cancer Institute, 2014; Available from https://seer.cancer.gov.
- 4. Karimkhani C, Green AC, Nijsten T, et al. The global burden of melanoma: results from global burden of disease study 2015. Br J Dermatol 2017;177:134–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ossio R, Roldan‐Marin R, Martinez‐Said H, et al. Melanoma: a global perspective. Nat Rev Cancer 2017;17:393–4. [DOI] [PubMed] [Google Scholar]
- 6. Berwick M, Buller DB, Cust A, et al. Melanoma Epidemiology and Prevention. Cancer Treat Res 2016;167:17–49. [DOI] [PubMed] [Google Scholar]
- 7. IARC , IARC Monographs on the Evaluation of Carcinogenic Risks to Humans ‐ Radiation. Lyon, France: International Agency for Cancer Research, 2012.
- 8. Lucas RM, McMichael AJ, Armstrong BK, et al. Estimating the global disease burden due to ultraviolet radiation exposure. Int J Epidemiol 2008;37:654–67. [DOI] [PubMed] [Google Scholar]
- 9. Parkin DM, Mesher D, Sasieni P. 13. Cancers attributable to solar (ultraviolet) radiation exposure in the UK in 2010. Br J Cancer 2011;105(Suppl 2):S66–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Whiteman DC, Wilson LF. The fractions of cancer attributable to modifiable factors: a global review. Cancer Epidemiol 2016;44:203–21. [DOI] [PubMed] [Google Scholar]
- 11. Sample A, He Y‐Y. Mechanisms and prevention of UV‐induced melanoma. Photodermatol Photoimmunol Photomed 2018;34:13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hodis E, Watson IR, Kryukov GV, et al. A landscape of driver mutations in melanoma. Cell 2012;150:251–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gordon LG, Scuffham PA, van der Pols JC, et al. Regular sunscreen use is a cost‐effective approach to skin cancer prevention in subtropical settings. J Invest Dermatol 2009;129:2766–71. [DOI] [PubMed] [Google Scholar]
- 14. Langer T, Follmann M. Das Leitlinienprogramm Onkologie (OL): Nukleus einer evidenzbasierten, patientenorientieren, interdisziplinären Onkologie? Zeitschrift fur Evidenz, Fortbildung und Qualitat im Gesundheitswesen 2015;109:437–44. [DOI] [PubMed] [Google Scholar]
- 15. Sun Exp Dermatol ‐ Recommendations , vol. 2016 USA: Centers for Disease Control and Prevention (CDC), Providing National and World Leadership to Prevent Workplace Illnesses and Injuries (NIOSH), 2016.
- 16.Sun Safety, vol. 2017 USA: Centres for Disease Control and Prevention (CDC), 2014.
- 17.Sun protection, vol. 2017: World Health Organization (WHO) INTERSUN programme, 2016.
- 18. Berwick M. The good, the bad, and the ugly of sunscreens. Clin Pharmacol Ther 2011;89:31–3. [DOI] [PubMed] [Google Scholar]
- 19. Olsen CM, Wilson LF, Green AC, et al. Prevention of DNA damage in human skin by topical sunscreens. Photodermatol Photoimmunol Photomed 2017;33:135–42. [DOI] [PubMed] [Google Scholar]
- 20. Iannacone MR, Hughes MC, Green AC. Effects of sunscreen on skin cancer and photoaging. Photodermatol Photoimmunol Photomed 2014;30:55–61. [DOI] [PubMed] [Google Scholar]
- 21. Green AC, Williams GM, Logan V, et al. Reduced melanoma after regular sunscreen use: randomized trial follow‐up. J Clin Oncol 2011;29:257–63. [DOI] [PubMed] [Google Scholar]
- 22. Klepp O, Magnus K. Some environmental and bodily characteristics of melanoma patients. A case‐control study. Int J Cancer 1979;23:482–6. [DOI] [PubMed] [Google Scholar]
- 23. Graham S, Marshall J, Haughey B, et al. An inquiry into the epidemiology of melanoma. Am J Epidemiol 1985;122:606–19. [DOI] [PubMed] [Google Scholar]
- 24. Holman CD, Armstrong BK, Heenan PJ. Relationship of cutaneous malignant melanoma to individual sunlight‐exposure habits. J Natl Cancer Inst 1986;76:403–14. [PubMed] [Google Scholar]
- 25. Osterlind A, Tucker MA, Stone BJ, et al. The Danish case‐control study of cutaneous malignant melanoma. II. Importance of UV‐light exposure. Int J Cancer 1988;42:319–24. [DOI] [PubMed] [Google Scholar]
- 26. Beitner H, Norell SE, Ringborg U, et al. Malignant melanoma: aetiological importance of individual pigmentation and sun exposure. Br J Dermatol 1990;122:43–51. [DOI] [PubMed] [Google Scholar]
- 27. Herzfeld PM, Fitzgerald EF, Hwang SA, et al. A case‐control study of malignant melanoma of the trunk among white males in upstate New York. Cancer Detect Prev 1993;17:601–8. [PubMed] [Google Scholar]
- 28. Autier P, Dore JF, Schifflers E, et al. Melanoma and use of sunscreens: an Eortc case‐control study in Germany, Belgium and France. The EORTC Melanoma Cooperative Group . Int J Cancer 1995;61:749–55. [DOI] [PubMed] [Google Scholar]
- 29. Holly EA, Aston DA, Cress RD, et al. Cutaneous melanoma in women. I. Exposure to sunlight, ability to tan, and other risk factors related to ultraviolet light. Am J Epidemiol 1995;141:923–33. [DOI] [PubMed] [Google Scholar]
- 30. Westerdahl J, Olsson H, Masback A, et al. Is the use of sunscreens a risk factor for malignant melanoma? Melanoma Res 1995;5:59–65. [DOI] [PubMed] [Google Scholar]
- 31. Rodenas JM, Delgado‐Rodriguez M, Herranz MT, et al. Sun exposure, pigmentary traits, and risk of cutaneous malignant melanoma: a case‐control study in a Mediterranean population. Cancer Causes Control 1996;7:275–83. [DOI] [PubMed] [Google Scholar]
- 32. Whiteman DC, Valery P, McWhirter W, et al. Risk factors for childhood melanoma in Queensland, Australia. Int J Cancer 1997;70:26–31. [DOI] [PubMed] [Google Scholar]
- 33. Wolf P, Quehenberger F, Mullegger R, et al. Phenotypic markers, sunlight‐related factors and sunscreen use in patients with cutaneous melanoma: an Austrian case‐control study. Melanoma Res 1998;8:370–8. [DOI] [PubMed] [Google Scholar]
- 34. Espinosa Arranz J, Sanchez Hernandez JJ, Bravo Fernandez P, et al. Cutaneous malignant melanoma and sun exposure in Spain. Melanoma Res 1999;9:199–205. [DOI] [PubMed] [Google Scholar]
- 35. Naldi L, Gallus S, Imberti GL, et al. Sunscreens and cutaneous malignant melanoma: an Italian case‐control study. Int J Cancer 2000;86:879–82. [DOI] [PubMed] [Google Scholar]
- 36. Westerdahl J, Ingvar C, Masback A, et al. Sunscreen use and malignant melanoma. Int J Cancer 2000;87:145–50. [DOI] [PubMed] [Google Scholar]
- 37. Bakos L, Wagner M, Bakos RM, et al. Sunburn, sunscreens, and phenotypes: some risk factors for cutaneous melanoma in southern Brazil. Int J Dermatol 2002;41:557–62. [DOI] [PubMed] [Google Scholar]
- 38. Youl P, Aitken J, Hayward N, et al. Melanoma in adolescents: a case‐control study of risk factors in Queensland, Australia. Int J Cancer 2002;98:92–8. [DOI] [PubMed] [Google Scholar]
- 39. Klug HL, Tooze JA, Graff‐Cherry C, et al. Sunscreen prevention of melanoma in man and mouse. Pigment Cell Melanoma Res 2010;23:835–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lazovich D, Vogel RI, Berwick M, et al. Melanoma risk in relation to use of sunscreen or other sun protection methods. Cancer Epidemiol Biomarkers Prev 2011;20:2583–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Green AC, Williams GM. Point: sunscreen use is a safe and effective approach to skin cancer prevention. Cancer Epidemiol Biomarkers Prev 2007;16:1921–2. [DOI] [PubMed] [Google Scholar]
- 42. Bastuji‐Garin S, Diepgen TL. Cutaneous malignant melanoma, sun exposure, and sunscreen use: epidemiological evidence. Br J Dermatol 2002;146(Suppl 61):24–30. [DOI] [PubMed] [Google Scholar]
- 43. Huncharek M, Kupelnick B. Use of topical sunscreens and the risk of malignant melanoma: a meta‐analysis of 9067 patients from 11 case‐control studies. Am J Public Health 2002;92:1173–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dennis LK, Beane Freeman LE, Vanbeek MJ. Sunscreen use and the risk for melanoma: a quantitative review. Ann Intern Med 2003;139:966–78. [DOI] [PubMed] [Google Scholar]
- 45. Gorham ED, Mohr SB, Garland CF, et al. Do sunscreens increase risk of melanoma in populations residing at higher latitudes? Ann Epidemiol 2007;17:956–63. [DOI] [PubMed] [Google Scholar]
- 46. Xie F, Xie T, Song Q, et al. Analysis of association between sunscreens use and risk of malignant melanoma. Int J Clin Exp Med 2015;8:2378–84. [PMC free article] [PubMed] [Google Scholar]
- 47. IARC, IARC Handbook of Cancer Prevention. Sunscreens . International Agency for Research on Cancer World Health Organisation, Lyon, France: International Agency for Research on Cancer, 2001. [Google Scholar]
- 48. Silva ESD, Tavares R, Paulitsch FDS, et al. Use of sunscreen and risk of melanoma and non‐melanoma skin cancer: a systematic review and meta‐analysis. Eur J Dermatol 2018;28:186‐201. [DOI] [PubMed] [Google Scholar]
- 49. Rueegg C, Stenehjem J, Egger M, et al. Melanoma and sunscreen use ‐ systematic review and meta‐analysis. PROSPERO 2017:CRD42017063980. [Google Scholar]
- 50. Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015 statement. Syst Rev 2015;4:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015: elaboration and explanation. BMJ (Clinical research ed) 2015;349:g7647. [DOI] [PubMed] [Google Scholar]
- 52. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wallingford SC, Iannacone MR, Youlden DR, et al. Comparison of melanoma incidence and trends among youth under 25 years in Australia and England, 1990‐2010. Int J Cancer 2015;137:2227–33. [DOI] [PubMed] [Google Scholar]
- 54. Higgins J, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane collaboration, 2011; Available from http://handbook.cochrane.org. [Google Scholar]
- 55. Wells G, Shea B, O'Connell D, et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses, vol. 2017 Ottawa: Ottawa Hospital Research Institute, 2014. [Google Scholar]
- 56. Hamling J, Lee P, Weitkunat R, et al. Facilitating meta‐analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease category. Stat Med 2008;27:954–70. [DOI] [PubMed] [Google Scholar]
- 57. Pandey A, Garg S, Khunger M, et al. Dose response relationship between physical activity and risk of heart failure: a meta‐analysis. Circulation 2015;132:1786–94. [DOI] [PubMed] [Google Scholar]
- 58. Dersimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- 59. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- 60. Egger M, Davey Smith G, Schneider M, et al. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical research ed) 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Langan D, Higgins JPT, Gregory W, et al. Graphical augmentations to the funnel plot assess the impact of additional evidence on a meta‐analysis. J Clin Epidemiol 2012;65:511–9. [DOI] [PubMed] [Google Scholar]
- 62. Group GW . Grading quality of evidence and strength of recommendations. Br Med J 2004;328:1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Luiz OC, Gianini RJ, Goncalves FT, et al. Ethnicity and cutaneous melanoma in the city of Sao Paulo, Brazil: a case‐control study. PLoS One 2012;7:e36348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Nikolaou VA, Sypsa V, Stefanaki I, et al. Risk associations of melanoma in a southern European population: results of a case/control study. Cancer Causes Control 2008;19:671–9. [DOI] [PubMed] [Google Scholar]
- 65. Vranova J, Arenbergerova M, Arenberger P, et al. Incidence of cutaneous malignant melanoma in the Czech Republic: the risks of sun exposure for adolescents. Neoplasma 2012;59:316–25. [DOI] [PubMed] [Google Scholar]
- 66. Savoye I, Olsen CM, Whiteman DC, et al. Patterns of ultraviolet radiation exposure and skin cancer risk: the E3N‐SunExp study. J Epidemiol 2018;28:27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kojo K, Jansen CT, Nybom P, et al. Population exposure to ultraviolet radiation in Finland 1920‐1995: exposure trends and a time‐series analysis of exposure and cutaneous melanoma incidence. Environ Res 2006;101:123–31. [DOI] [PubMed] [Google Scholar]
- 68. Cho E, Rosner BA, Feskanich D, et al. Risk factors and individual probabilities of melanoma for whites. J Clin Oncol 2005;23:2669–75. [DOI] [PubMed] [Google Scholar]
- 69. Ghiasvand R, Weiderpass E, Green AC, et al. Sunscreen use and subsequent melanoma risk: a population‐based cohort study. J Clin Oncol 2017;185:147–56. [DOI] [PubMed] [Google Scholar]
- 70. Stenehjem JS, Robsahm TE, Bratveit M, et al. Ultraviolet radiation and skin cancer risk in offshore workers. Occup Med (Oxford, England) 2017;67:569–73. [DOI] [PubMed] [Google Scholar]
- 71. Ahrens W, Krickeberg K, Pigeot I. An Introduction to epidemiology In: Ahrens W, Pigeot I, eds Handbook of Epidemiologyed. Berlin: Springer, 2006:1–42. [Google Scholar]
- 72. Guyatt GH, Oxman AD, Schunemann HJ, et al. GRADE guidelines: a new series of articles in the journal of clinical epidemiology. J Clin Epidemiol 2011;64:380–2. [DOI] [PubMed] [Google Scholar]
- 73. Vainio H, Miller AB, Bianchini F. An international evaluation of the cancer‐preventive potential of sunscreens. Int J Cancer 2000;88:838–42. [DOI] [PubMed] [Google Scholar]
- 74. Hernan MA, Alonso A, Logan R, et al. Observational studies analyzed like randomized experiments: an application to postmenopausal hormone therapy and coronary heart disease. Epidimiology 2008;19:766–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Austin PC. An Introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar Behav Res 2011;46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Murad MH, Asi N, Alsawas M, et al. New evidence pyramid. Evidence Based Med 2016;21:125–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Green A, Battistutta D, Hart V, et al. The Nambour skin cancer and actinic eye disease prevention trial: design and baseline characteristics of participants. Control Clin Trials 1994;15:512–22. [DOI] [PubMed] [Google Scholar]
- 78. Vasicek BE, Szpunar SM, Manz‐Dulac LA. Patient knowledge of sunscreen guidelines and frequency of physician counseling: a cross‐sectional study. J Clin Aesthet Dermatol 2018;11:35–40. [PMC free article] [PubMed] [Google Scholar]
- 79. Olsen CM, Wilson LF, Green AC, et al. How many melanomas might be prevented if more people applied sunscreen regularly? Br J Dermatol 2018;178:140–7. [DOI] [PubMed] [Google Scholar]
- 80. Autier P, Boniol M, Dore JF. Sunscreen use and increased duration of intentional sun exposure: still a burning issue. Int J Cancer 2007;121:1–5. [DOI] [PubMed] [Google Scholar]
- 81. Autier P, Boniol M, Dore JF. Is sunscreen use for melanoma prevention valid for all sun exposure circumstances? J Clin Oncol 2011;29: e425‐6. [DOI] [PubMed] [Google Scholar]
- 82. Whiteman DC, Pavan WJ, Bastian BC. The melanomas: a synthesis of epidemiological, clinical, histopathological, genetic, and biological aspects, supporting distinct subtypes, causal pathways, and cells of origin. Pigment Cell Melanoma Res 2011;24:879–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Veierod MB, Parr CL, Lund E, et al. Reproducibility of self‐reported melanoma risk factors in a large cohort study of Norwegian women. Melanoma Res 2008;18:1–9. [DOI] [PubMed] [Google Scholar]
- 84. English DR, Armstrong BK, Kricker A. Reproducibility of reported measurements of sun exposure in a case‐control study. Cancer Epidemiol Biomarkers Prev 1998;7:857–63. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1 Forest plot comparing three levels of sunscreen use (no – medium – high) and melanoma risk, stratified by study design
Supplemental Figure 2: Sources of heterogeneity: forest plot of summary estimates from stratified meta‐analyses
Supplemental Figure 3: Funnel plot and Egger's regression to estimate publication bias
Supplemental Figure 4: Funnel plot with statistical significance contours
Supplemental Table 1 Rules applied to choose the minimally and maximally adjusted estimate to be included in the meta‐analysis
Supplemental Table 2: Skin cancer related characteristics of participants in the studies included (n = 28)
Supplemental Table 3: Description of the methodological quality of the studies included (n = 28)
Supplemental Table 4: Description of the three‐level estimates extracted for each study (described exactly as reported in the articles)*
Supplemental Table 5: GRADE evidence profile for sunscreen use and melanoma risk, stratified by study design
Appendix S1: Supporting Information
Appendix S2: Supporting Information


