Abstract
Purpose:
To evaluate venous thromboembolism (VTE) rates and risk factors following inpatient pediatric surgery.
Methods:
153,220 inpatient pediatric surgical patients were selected from the 2012–2015 NSQIP-P database. Demographic and perioperative variables were documented. Primary outcome was VTE requiring treatment within 30 postoperative days. Secondary outcomes included length of stay (LOS) and 30-day mortality. Prediction models were generated using logistic regression. Mortality and time to VTE were assessed using Kaplan-Meier survival analysis.
Results:
305 patients (0.20%) developed 296 venous thromboses and 12 pulmonary emboli (3 cooccurrences). Median time to VTE was 9 days. Most VTEs (81%) occurred pre-discharge. Subspecialties with highest VTE rates were cardiothoracic (0.72%) and general surgery (0.28%). No differences were seen for elective vs. urgent/emergent procedures (p=0.106). All-cause mortality VTE patients was 1.2% vs. 0.2% in patients without VTE (p<0.001). After stratifying by American Society of Anesthesiologists (ASA) class, no mortality differences remained when ASA < 3. Preoperative, postoperative, and total LOS were longer for patients with VTE (p<0.001 for each). ASA ≥ 3, preoperative sepsis, ventilator dependence, enteral/parenteral feeding, steroid use, preoperative blood transfusion, gastrointestinal disease, hematologic disorders, operative time, and age were independent predictors (C-statistic=0.83).
Conclusions:
Pediatric post-surgical patients have unique risk factors for developing VTE.
Keywords: NSQIP, VTE, postoperative, risk factors, mortality, thrombosis Level of Evidence: Level II
1.1. INTRODUCTION
Venous thromboembolism (VTE) is a rare complication of pediatric surgery with significant associated morbidity and mortality.[1] VTE occurs in approximately 40 per 10,000 pediatric admissions.[2, 3] The risk of in-hospital death is significantly increased in pediatric patients with VTE, with an adjusted relative risk greater than 6 compared to children without VTE.[3] Furthermore, cost attributable to pediatric VTEs in the U.S. has been estimated at $20,000 per event, or over $90 million annually.[1]
Pediatric VTE has predominately been studied in the context of chronic illnesses (e.g., cancer) and known inciting events (e.g., central venous catheter [CVC] use, trauma). Studies investigating VTE following pediatric surgery have focused on the trauma surgery population and have identified older age and injury severity as risk factors for postoperative VTE development.[4–7] However, data on VTE following non-trauma inpatient surgery are lacking. Developing a better understanding of surgery-related VTE could identify patients at highest risk in order to improve guidelines on VTE prophylaxis, diagnosis, and treatment in children.
1.2. MATERIAL AND METHODS
1.2.1. Study Design and Data Source
This is a cohort study of data prospectively collected in The American College of Surgeons (ACS) National Surgical Quality Improvement Program-Pediatric (NSQIP-P) dataset. The Institutional Review Board at the authors’ institution exempts NSQIP from IRB approval.[8]
Following alpha and beta phase testing,[9–11] NSQIP-P was officially expanded in 2012. Previous studies have demonstrated consistency between variables collected in NSQIP and NSQIP-P relative to medical record review.[12–14] The ACS trains Surgical Clinical Reviewers (SCRs) at each institution and completes inter-rater reliability (IRR) audits on collected data. Overall IRR disagreement rates are approximately 2% (confirmed in alpha phase testing).[9]
SCRs use an 8-day cycle to sample surgical cases from the hospital’s operative log.[15–18] All cases under the NSQIP-P procedure list are included if they fall in the 8-day cycle, do not exceed the 35 consecutive case limit within an 8-day cycle, and do not violate the following exclusion criteria: patient age ≥18 years, trauma surgery (except isolated long bone fractures), return to the operating room for complications related to a prior procedure, organ transplantation, and additional procedure performed by a different surgical team under the same anesthetic.[15–18]
1.2.2. Cohort Selection
NSQIP-P datasets from years 2012–2015 were combined yielding an initial cohort of 267,289 cases. Outpatient procedures were excluded, yielding 153,220 remaining inpatient surgeries. The following specialties/subspecialties are included in NSQIP-P: pediatric surgery/general surgery, orthopedics, cardiovascular/thoracic, neurosurgery, otolaryngology, urology, plastic surgery, and gynecology.
1.2.3. Primary and Secondary Outcomes
The primary outcome of interest was VTE requiring treatment (anticoagulation, placement of vena cava filter, or clipping of the vena cava) within 30 postoperative days. VTE includes venous thrombosis (VT) of the deep or superficial venous systems and pulmonary embolism (PE). The VT definition requires imaging confirmation by duplex ultrasound, venogram, or computed tomography (CT) scan. Internal jugular catheter clots, peripherally inserted central catheter (PICC) clots, and portal vein clots are all examples of VTE events that would be included in the NSQIP-P VT definition. The PE definition requires radiological confirmation of PE via a ventilation-perfusion scan interpreted as high probability of pulmonary embolism, a positive chest CT, trans-esophageal echocardiogram, pulmonary arteriogram, or CT angiogram. Timing to VTE was defined as the number of days from operation to diagnosis with zero days being a VTE that occurred the day of operation. Secondary outcomes included postoperative length of stay (LOS) and any-cause 30-day postoperative mortality.
1.2.4. Variables of Interest
Demographic, comorbidity, operative, and postoperative variables of interest were selected for analysis. For more specific variable details including definitions for each NQSIP-P variable referenced herein, please reference the NSQIP-P user guides. [15–18]
1.2.5. Statistical Analysis
Descriptive statistics of overall VTE frequency and frequency by variable of interest were generated. Contingency tables were generated for categorical variables with corresponding unadjusted odds ratios (OR), 95% confidence intervals (CI), and p values using univariate logistic regression. Univariate binary logistic regression was used to analyze VTE risk for continuous and LOS variables with calculation of corresponding unadjusted ORs, 95% CIs, and p values. Median (with interquartile range [IQR]) time from surgery to VTE event was calculated. Survival analyses were performed for VTE and mortality using Kaplan Meier estimates and log-rank tests. Statistical significance within univariate tests was set at p<0.05. All tests for statistical significance were 2-tailed.
Multivariate binary logistic regression models were fitted using variables deemed significant on univariate testing (p<0.05) with calculation of corresponding adjusted ORs, 95% CIs, and p values. Adjusted models were generated for the following sub-stratified analyses: overall cohort, patient age <3 years, age > 15 years, pre-discharge VTE, and post-discharge VTE. Patients were only included in the models if all independent variables in the model existed for that patient. A receiver operating characteristic (ROC) curve was generated for each adjusted model with subsequent calculation of the area under the curve (AUC) or C-statistic, 95% CI for the AUC, and p values. Statistical significance for the adjusted models was set at p<0.05. All statistical analyses were performed using IBM SPSS v. 23.
1.3. RESULTS
1.3.1. Overall VTE Rates
Of 153,220 unique inpatient pediatric patients, 305 (0.20%) had a VTE requiring treatment within 30 postoperative days (Table 1). VT occurred after 296 cases, and PE occurred after 12 cases. Three patients had VT with subsequent PE. No patient had multiple VTs or PEs. There was no significant difference in VTE rate between years of operation.
Table 1.
Overall 30-day VTE frequencies within NSQIP-P by year of operation
| Year | N Hospitals | N Inpatient Cases | N VT | N PE | N VTE* | % VTE per procedure | VTE Rate 95% CI (lower-upper) |
|---|---|---|---|---|---|---|---|
| 2012 | 50 | 29,620 | 55 | 3 | 57 | 0.19% | 0.14–0.24% |
| 2013 | 56 | 36,539 | 63 | 5 | 68 | 0.19% | 0.14–0.23% |
| 2014 | 64 | 37,718 | 68 | 2 | 69 | 0.18% | 0.14–0.23% |
| 2015 | 80 | 49,343 | 110 | 2 | 111 | 0.22% | 0.18–0.27% |
| TOTAL | 153,220 | 296 | 12 | 305 | 0.20% | 0.18–0.22% |
3 patients had co-occurrences of VT with subsequent PE. There was no significant difference between years (p=0.3).
VTE = venous thromboembolism; NSQIP-P = National Surgical Quality Improvement Program-Pediatric; VT = venous thrombosis; PE = pulmonary embolism; CI = confidence interval
1.3.2. Surgical Subspecialty
The four specialties with the highest VTE rates were cardiothoracic, general/pediatric surgery, otolaryngology, and neurosurgery (Table 2). The specialties with the lowest VTE rates were plastic surgery, urology, and orthopedics. Gynecology had no VTEs. General/pediatric surgery had both the greatest number of surgical cases (74,446) and the greatest number of VTEs (211) of any specialty.
Table 2.
VTE rate sorted by surgical subspecialty.
| Subspecialty | N Procedures | N VTE | % VTE | VTE Rate 95% CI (lower-upper) |
|---|---|---|---|---|
| Cardiothoracic surgery | 277 | 2 | 0.72% | 0.00–1.72% |
| General/pediatric surgery | 74446 | 211 | 0.28% | 0.25–0.32% |
| Otolaryngology | 8297 | 15 | 0.18% | 0.09–0.27% |
| Neurosurgery | 22385 | 38 | 0.17% | 0.12–0.22% |
| Orthopedics | 29285 | 30 | 0.10% | 0.07–0.14% |
| Urology | 9711 | 7 | 0.07% | 0.02–0.13% |
| Plastic surgery | 8514 | 2 | 0.02% | 0.00–0.06% |
| Gynecology | 305 | 0 | 0.00% | NA |
| TOTAL | 153,220 | 305 | 0.20% | 0.18–0.22% |
VTE = venous thromboembolism; CI = confidence interval; NA = not applicable
1.3.3. Time to VTE
Median time from surgery to VTE was 9 (IQR: 4–16) days. Median time to VT was 9 (IQR: 4–16) days, whereas median time to PE was 5 (IQR: 2.25–9) days. Seventy percent of VTEs occurred within the first two weeks postoperatively. Fifty (16.4%) VTE events occurred post-discharge, and post-discharge VTE events differed significantly from pre-discharge VTE in timing to event (p<0.001).
1.3.4. Individual Procedures
Of 640 CPT codes in the NSQIP-P dataset, the top procedures by VTE rate are predominately general/pediatric surgery or cardiovascular-thoracic procedures (Table 3). Approximately 70% of the procedures in Table 3 are gastrointestinal or thoracic procedures. The procedures listed in Table 3 account for 128 VTEs (42% of total).
Table 3.
Top individual procedures sorted by VTE rate.
| CPT Code | Description | N Cases | N (%) VTE* |
|---|---|---|---|
| 49610 | First stage repair of omphalocele | 30 | 2 (6.7) |
| 62220 | Creation of CSF shunt; ventriculo-atrial, -jugular, or -auricular | 48 | 2 (4.2) |
| 44150 | Complete colectomy with ileostomy by abdominal approach | 97 | 4 (4.1) |
| 32607 | Unilateral thoracoscopy with biopsy of lung infiltrate | 122 | 4 (3.3) |
| 44143 | Partial colectomy with end colostomy and closure of distal segment | 167 | 5 (3.0) |
| 47600 | Cholecystectomy | 105 | 3 (2.9) |
| 32601 | Diagnostic thoracoscopy of mediastinal space | 106 | 3 (2.8) |
| 44187 | Surgical laparoscopy with jejunostomy or ileostomy | 106 | 3 (2.8) |
| 44020 | Enterotomy of small intestine for biopsy or foreign body removal | 75 | 2 (2.7) |
| 31600 | Tracheostomy | 180 | 4 (2.2) |
| 32652 | Surgical thoracoscopy with complete pulmonary decortication | 251 | 5 (2.0) |
| 44211 | Surgical laparoscopy with complete colectomy, proctectomy, ileoanal anastomosis, creation of ileal reservoir, and loop ileostomy | 151 | 3 (2.0) |
| 44210 | Surgical laparoscopy with complete colectomy and ileostomy | 209 | 4 (1.9) |
| 49421 | Open insertion of tunneled intraperitoneal catheter for dialysis | 259 | 4 (1.5) |
| 27030 | Arthrotomy of hip with drainage for infection | 340 | 5 (1.5) |
| 38510 | Open biopsy or excision of cervical lymph nodes | 210 | 3 (1.4) |
| 44120 | Resection and anastomosis of small intestine | 1283 | 18 (1.4) |
| 47100 | Wedge biopsy of liver | 240 | 3 (1.3) |
| 39503 | Repair of hernia of diaphragm with creation of ventral hernia | 729 | 9 (1.2) |
| 44160 | Partial colectomy with removal of terminal ileum and ileocolostomy | 459 | 5 (1.1) |
| 44125 | Resection of small intestine with enterostomy | 603 | 5 (0.8) |
| 32480 | Single lobe lobectomy | 390 | 3 (0.8) |
| 49000 | Exploratory celiotomy | 925 | 7 (0.8) |
| 44126 | Enterectomy, resection of small intestine for congenital atresia | 310 | 2 (0.6) |
| 31601 | Tracheostomy, under 2 years of age | 787 | 5 (0.6) |
| 38120 | Laparoscopy with splenectomy | 488 | 3 (0.6) |
| 44055 | Correction of malrotation by lysis of duodenal band | 1262 | 7 (0.6) |
| 44050 | Laparotomy and reduction of intussusception or volvulus | 543 | 3 (0.6) |
| 44130 | Anastomosis of intestine with cutaneous enterostomy | 374 | 2 (0.5) |
Minimum 2 VTEs per individual procedure were required to be included in this table. Procedures were included if the VTE rate was greater than or equal to 0.5%.
VTE = venous thromboembolism; CPT = current procedural terminology; CSF = cerebrospinal fluid
1.3.5. Demographics
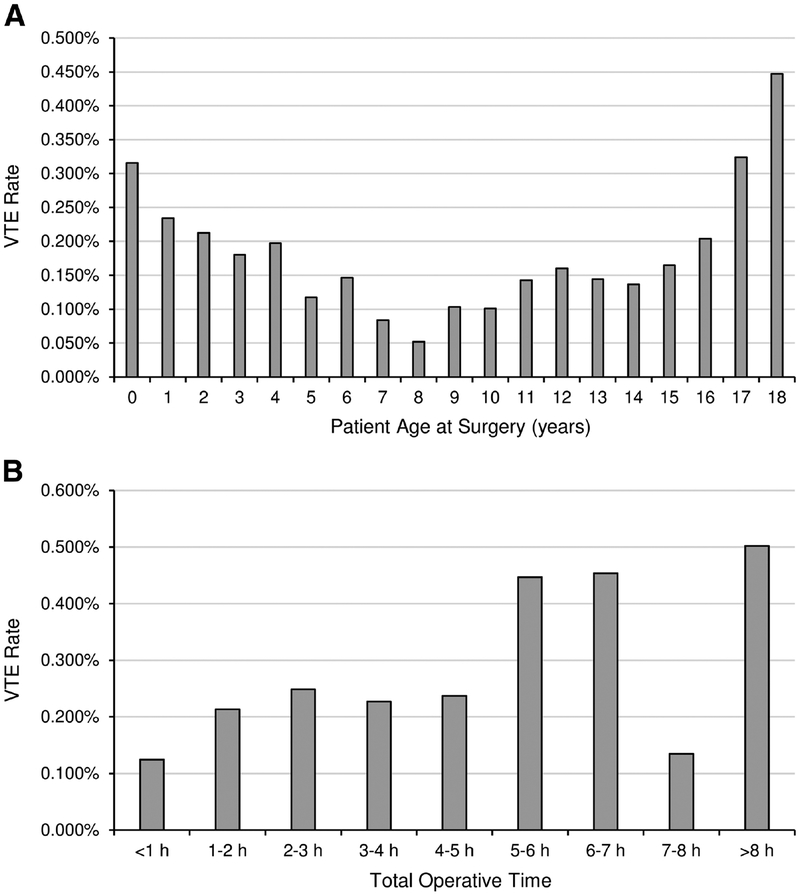
Patient age was significantly associated with VTE on univariate analysis (Table 4). VTE risk was greatest in patients < 3 years or > 15 years of age at surgery (Fig. 1A). No differences were seen between sexes. Black race was the only race significantly associated with VTE on univariate analysis.
Table 4.
Patient demographic and operative variables corresponding univariate analyses via binary logistic regression.
| Demographic Variable | VTE Group (n=305) | No VTE Group (n=152,915) | p value | Unadjusted OR | 95% CI (Lower-Upper) |
|---|---|---|---|---|---|
| Age at surgery, n (%) | |||||
| <1 year | 115 (0.28) | 40463 (99.72) | <0.001 | 2.59 | 1.83–3.65 |
| 1–3 years | 39 (0.25) | 15681 (99.75) | <0.001 | 2.26 | 1.47–3.48 |
| 3–10 years | 45 (0.11) | 40925 (99.89) | Reference cate; | gory | |
| 10–15 years | 53 (0.15) | 35816(99.85) | 0.14 | 1.35 | 0.91–2.00 |
| 15–18 years | 53 (0.26) | 20030 (99.74) | <0.001 | 2.40 | 1.62–3.58 |
| Gender, n (%) | |||||
| Male | 155 (0.19) | 83370(99.81) | 0.21 | 0.86 | 0.69–1.08 |
| Female | 150 (0.22) | 69545 (99.78) | Reference category | ||
| Race, n (%) | |||||
| White | 208 (0.19) | 107168(99.81) | Reference category | ||
| Black | 54 (0.26) | 20586 (99.74) | 0.05 | 1.35 | 1.00–1.82 |
| Other* | 6 (0.11) | 5670 (99.89) | 0.14 | 0.55 | 0.24–1.23 |
| Unknown or not reported | 40 (0.20) | 19510(99.80) | 0.75 | 1.06 | 0.75–1.48 |
| Operative time in hours**, n (%) | |||||
| Less than 1 hour | 70 (0.12) | 58543 (99.88) | Reference | ||
| 1 hour – 5 hours | 191 (0.23) | 83683 (99.77) | <0.001 | 1.91 | 1.45–2.51 |
| Greater than 5 hours | 44 (0.41) | 10689 (99.59) | <0.001 | 3.44 | 2.36–5.02 |
| Emergent or urgent operation§, n (%) | 122 (0.23) | 54078 (99.77) | 0.09 | 1.22 | 0.97–1.53 |
| Prior operation in 30 days prior to index surgery†, n (%) | 17 (1.02) | 1652 (98.98) | <0.001 | 6.14 | 3.67–10.25 |
| ASA Class, n (%) | |||||
| ASA 1 (Normal healthy patient) | 7 (0.02) | 31854(99.98) | Reference | ||
| ASA 2 (Mild systemic disease) | 44 (0.07) | 65757 (99.93) | 0.01 | 3.04 | 1.37–6.76 |
| ASA 3 (Severe systemic disease) | 153 (0.32) | 47364 (99.68) | <0.001 | 14.7 | 6.89–31.4 |
| ASA 4 (Severe, life-threatening systemic disease) | 89 (1.21) | 7259 (98.79) | <0.001 | 55.8 | 25.8–120.5 |
| ASA 5 (Moribund) | 10 (2.66) | 366 (97.34) | <0.001 | 124.3 | 47.1–328.4 |
Includes Native American, Native Hawaiian/Pacific Islander, and Asian
Unadjusted odds ratio is with respect to per hour increase in operative time.
vs. elective operations
Data on prior surgery unavailable for 87,061 cases (only available for 66,159 cases). This variable was not included in the multivariate model.
VTE = venous thromboembolism; ASA = American Society of Anesthesiologists; SD = standard deviation; OR = odds ratio; CI = confidence interval
Figure 1.
VTE rate by (A) patient age at surgery and (B) total operative time.
1.3.6. Operative Variables
Longer operative time, ASA classification, and prior operation in 30 days were all significantly associated with higher VTE rate (Table 4, Fig. 1B). Procedures lasting > 5 hours had over three times the VTE rate vs. procedures < 1 hour (0.41% vs. 0.12%). Greater ASA classification corresponded to higher VTE risk, with ASA 3 being the first classification to surpass the general study population VTE rate. Prior operation within 30 days was significantly associated with VTE; however, 87,061 cases (57%) had no data on prior operations. Therefore, this variable was excluded from the multivariate models. Case status (elective vs. urgent or emergent procedure) VTE rate was no different between groups.
1.3.7. Comorbidities
The strongest effect sizes (unadjusted OR>5) among comorbidities were observed for preoperative blood transfusion, ventilator dependence, steroid use, nutritional support, and hematologic comorbidities (Table 5). Comorbidities that were not significantly associated with VTE were neuromuscular disorder, cerebral palsy, and weight for age z-score.
Table 5.
Patient pre-existing comorbidities with corresponding univariate analyses via Chi-square tests.
| Comorbidity*, n (%) | VTE group (n=305) | Non VTE Group (n=152,915) | P value | Unadjusted OR | 95% CI (Lower-Upper) |
|---|---|---|---|---|---|
| Ventilator dependent in 48 hours before surgery | 88(1.17) | 7421 (98.83) | <0.001 | 7.95 | 6.20–10.20 |
| Hematologic disorder | 63 (0.84) | 7481 (99.16) | <0.001 | 5.06 | 3.83–6.68 |
| Neuromuscular disorder | 27 (0.27) | 9994 (99.73) | 0.10 | 1.39 | 0.94–2.06 |
| Cerebral palsy | 21 (0.28) | 7591 (99.72) | 0.14 | 1.42 | 0.91–2.21 |
| Parenteral or enteral nutrition required at surgery | 140 (0.70) | 19848 (99.30) | <0.001 | 5.69 | 4.54–7.13 |
| Prematurity history (gestational age at birth <37 weeks gestation) | 77 (0.32) | 23988 (99.68) | <0.001 | 1.83 | 1.41–2.38 |
| Current malignancy or current treatment for malignancy | 33 (0.57) | 5779 (99.43) | <0.001 | 3.09 | 2.15–4.44 |
| Weight-for-age z-score > 2 (categorical) | 18(0.17) | 10847 (99.83) | 0.50 | 0.82 | 0.51–1.33 |
| Gastrointestinal disease | 159 (0.41) | 39018(99.59) | <0.001 | 3.18 | 2.54–3.98 |
| Tracheostomy present at time of surgery | 13 (0.54) | 2403 (99.46) | 0.001 | 2.79 | 1.60–4.87 |
| Sepsis, SIRS, or septic shock in 48 h preoperatively | 64 (0.40) | 16094 (99.60) | <0.001 | 2.26 | 1.71–2.98 |
| Blood transfusion in 48 h preoperatively | 38(1.41) | 2664 (98.59) | <0.001 | 8.03 | 5.70–11.30 |
| Chronic seizure disorder | 52 (0.46) | 11234(99.54) | <0.001 | 2.59 | 1.92–3.50 |
| Oral or parenteral steroid course in 30 d preoperatively | 61 (1.04) | 5795 (98.96) | <0.001 | 6.35 | 4.79–8.41 |
For all binary predictor variables, the “no” or null state is the reference category for the given unadjusted odds ratios. Please refer to Supplement S1 and the NSQIP-P user guides for specifics of comorbidity variable descriptions.
VTE = venous thromboembolism; OR = odds ratio; CI = confidence interval; SIRS=systemic inflammatory response syndrome
1.3.8. All-Cause Mortality and Length of Stay
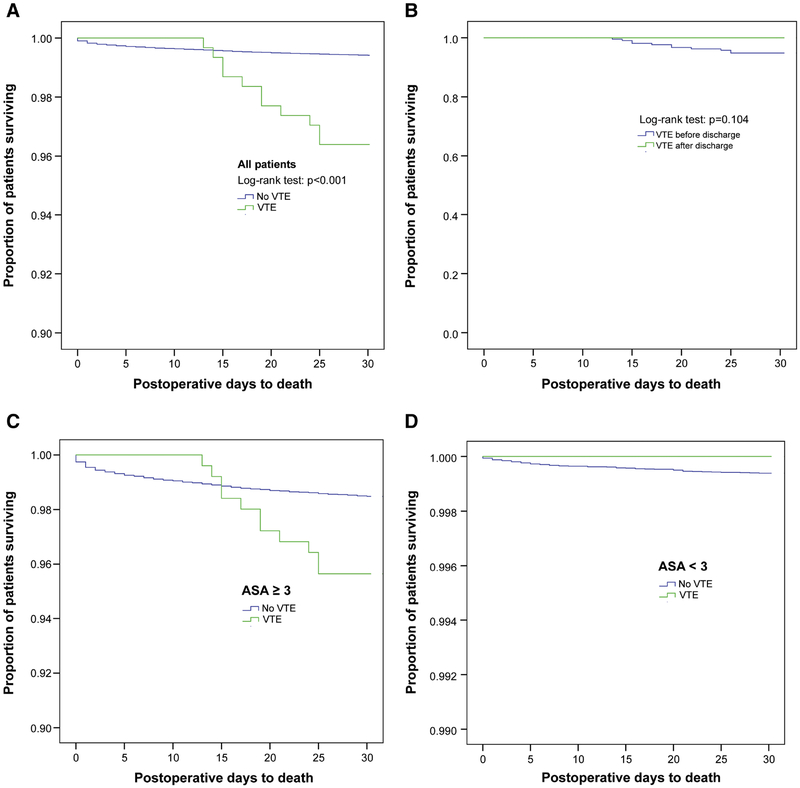
Of the 305 children with VTE, eleven (1.2%) died of any cause within 30 postoperative days. Median time from VTE to death was 6 (IQR: 3–11) days. All 11 children with VTE who died had a VT, not PE. Children with VTE had a significant increase in mortality vs. children without VTE (1.2% vs. 0.2%, p<0.001, OR 6.3, 95% CI 3.5–11.6) (Fig. 2A). No mortality differences were observed for pre- vs. post-discharge VTE (Fig. 2B). For children with ASA class <3, no mortality differences were seen when comparing VTE status (Fig. 2C and 2D).
Figure 2.
Mortality-free survival curves for (A) all patients, (B) pre- vs. post- discharge VTE, (C) patients with ASA class ≥ 3, and (D) patients with ASA class <3. Survival analyses were performed using Kaplan-Meier estimates and log-rank tests.
Preoperative LOS (12.5 ± 29.2 days for VTE vs. 4.2 ± 19.6 days for no VTE; OR 1.008, 95% CI 1.005–1.010, p<0.001), postoperative LOS (26.8 ± 24.1 days vs. 5.6 ± 11.1 days; OR 1.039, 95% CI 1.036–1.043, p<0.001), and total LOS (33.9 ± 30.2 days vs. 7.8 ± 17.3 days; OR 1.016, 95% CI 1.015–1.018, p<0.001) were all significantly longer in children with VTE.
1.3.9. Multivariate Logistic Regression
In the overall cohort model, the predictors with greatest effect size (adjusted OR>2) included ASA classification ≥ 3, preoperative sepsis/SIRS/septic shock, ventilator dependence, and parenteral/enteral nutrition (Table 6). Other significant VTE risk factors in the overall cohort model included preoperative blood transfusion, patient age, and operative time. Prematurity history, although associated with VTE on univariate analysis, was associated with lower VTE rate multivariate analysis. No differences were observed for current malignancy, chronic seizure disorder, preoperative length of stay, or tracheostomy.
Table 6.
Multivariate binary logistic regression analysis for the overall cohort.†
| Variable | Model C-Statistic (95% CI) | p value | Adjusted OR | 95% CI lower bound | 95% CI upper bound |
|---|---|---|---|---|---|
| Entire Cohort Analysis (N=135,964; 273 VTEs) | 0.83 (0.81–0.86) | ||||
| ASA Class ≥ 3 (vs. ASA <3) | 0.001 | 4.09 | 2.83 | 5.91 | |
| Sepsis, SIRS, or septic shock in 48 h preoperatively | 0.001 | 2.72 | 1.97 | 3.76 | |
| Ventilator dependent in 48 h preoperatively | 0.001 | 2.33 | 1.68 | 3.24 | |
| Parenteral or enteral nutrition required at surgery | 0.001 | 2.01 | 1.48 | 2.74 | |
| Oral or parenteral steroid course in 30 d preoperatively | 0.001 | 1.92 | 1.39 | 2.67 | |
| Blood transfusion in 48 h preoperatively | 0.02 | 1.64 | 1.09 | 2.47 | |
| Gastrointestinal disease | 0.003 | 1.51 | 1.15 | 1.98 | |
| Hematologic disorder | 0.02 | 1.50 | 1.08 | 2.09 | |
| Operative time (h) | 0.001 | 1.14 | 1.08 | 1.20 | |
| Patient age squared (y) | 0.03 | 1.002 | 1.000 | 1.003 | |
| Prematurity history (gestational age <37 wks at birth) | 0.03 | 0.72 | 0.54 | 0.97 |
Only independently significant (p<0.05 in multivariate logistic regression) variables are shown. Only variables significant on univariate analyses (p<0.05) were included in the multivariate models. Patients were only included for analysis if all variables in the model were present for that patient. No model had more than 20 total predictor variables analyzed.
Adjusted odds ratio with respect to per hour increase in operative time.
VTE = venous thromboembolism; OR = odds ratio; CI = confidence interval; ASA = American Society of Anesthesiologists; SIRS = systemic inflammatory response syndrome
In the subgroup analyses, several differences were observed between age groups (age ≤ 3 years vs. age ≥ 15 years) and between pre- vs. post-discharge VTE (Supplement S1). Generally, the statistical models were not as accurate at predicting VTEs in the older patient group and in the post-discharge VTE group, nor were there as many independently significant variables identified in these groups. All subgroup analyses had C-statistics > 0.75.
1.4. DISCUSSION
We report VTE rates following inpatient pediatric surgery using a validated national database. Procedures and subspecialties with the highest VTE rates were identified. We have also identified risk factors that are associated with increased VTE risk. We report that VTE-associated mortality, although small, is significantly greater than patients without VTE.
1.4.1. Overall VTE Rates and Timing to VTE
The overall VTE rate is low and consistent with prior literature on pediatric VTE. [2, 3, 19, 20] Several publications have reported VTE rates in the hospitalized pediatric population [2, 3, 21–23] and in the intensive care population.[20, 24, 25] To date, no study has investigated the pediatric inpatient surgical population as a whole while comparing rates between procedures and subspecialties.
Several studies have reported increasing incidence of pediatric VTE over time, likely owing to the increased burden of intervention and higher screening rates. [1, 6, 26] We observed a slight increase over the years evaluated (2012–2015), however this difference was insignificant. We speculate that inpatient pediatric VTE is a multi-hit problem: patients who are already critically ill (e.g., septic, intubated, with central lines placed, etc.) who also have a surgical procedure are at increased risk for VTE vs. those who are only critically ill or are only having a procedure without underlying critical illness. Although NSQIP itself cannot be used to examine this hypothesis since inpatients not undergoing surgery are excluded, this idea is supported by the literature showing that inpatient pediatric inpatients who are critically ill are at higher risk for VTE vs. the general inpatient population and by studies showing that postoperative pediatric patients are at increased risk for VTE vs. patients who did not undergo a procedure. [1, 3, 23, 26, 28, 31]
Median time to VTE was nine days postoperatively, seventy percent of VTE events occurred within two weeks, PE typically occurred before VT, and nearly one in five VTEs occurred post-discharge. Collectively, these data are unsurprising and consistent with prior literature discussing timing to pediatric VTE.
1.4.2. VTE Rates by Surgical Subspecialty and Procedure
We report that the VTE rate is highest in cardiothoracic procedures followed by general surgery, otolaryngology, and neurosurgery. We suspect our findings partially result from the acuity of the patient population since sicker patients require longer LOS, often require long-term CVCs, and have reduced mobility, all of which have been implicated as VTE risk factors.[2, 20, 21, 27–29]
Patients with congenital heart disease have been reported as having high VTE rates in the literature, owing to the need for surgical intervention, alterations in blood flow, and need for CVCs.[30, 31] VTE rates after congenital cardiac surgery have been reported from 3.9% to 31%, much higher than most other surgical subspecialties in our database and in the literature.[32, 33] Four of the top procedures in the present analysis were colectomies, although the gastrointestinal procedure with the highest VTE rate was first stage omphalocele repair. Gonzalez-Hernandez et al. discuss the relationship between gastrointestinal disease, CVC usage, and VTE.[34] Necrotizing entercolitis and gastroschisis were the most common diagnoses associated with CVC-related VTE in that analysis, and no patients diagnosed with omphalocele were included.[34] Antiel et al. noted 4% incidence of VTE in pediatric patients with ulcerative colitis undergoing colectomy, similar to our observed rate for complete colectomy (4.1%).[35]
Pediatric neurosurgical procedures in the present analysis had a VTE incidence of 0.17%. Sherrod et al. noted a slightly higher 0.4% incidence in 30-day readmission in a cohort of pediatric neurosurgical patients.[36] In one case series of 14 pediatric neurosurgical patients with VTE,[37] all had significant comorbidities, and over half had a history of trauma. Ventriculoatrial shunts had the second highest VTE rate in our dataset. This procedure involves placement of an intracardiac catheter, and many of the patients are the neonatal and premature population. This population, has a large comorbidity burden and have been noted to have a higher VTE rate than other age groups.[6, 23, 25, 29]
Pediatric orthopedic postoperative VTE rates range from 0.05–0.1%, similar to our findings of 0.1%.[38–40] Orthopedic procedures were not the procedures with the highest VTE rate, but they were among the procedures with the highest overall VTE number (CPT code 22804, posterior arthrodesis of 13 or more vertebral segments, had 10 reported VTE events). However, NSQIP excludes trauma with the exception of isolated long bone fractures. Therefore the database may be missing important information about VTE rates in children with polytrauma undergoing the orthopedic procedures. We were unable to find published VTE rates in pediatric otolaryngology, but our VTE incidence of 0.18% be explained by VTE rates after tracheostomies (2.2%), and prolonged mechanical ventilation is a known VTE risk factor.[6, 25, 41] No data has been published on VTE rates after pediatric plastic or urological surgical procedures, however VTE incidence in renal tumor patients is low (0.1%).[42]
1.4.3. VTE-associated Mortality
VTE was associated with increased mortality. Previous pediatric VTE studies have reported similar findings.[3, 25] However, the effect size of the mortality difference was small (1.2% vs. 0.2%), particularly when accounting for the denominator in the non-VTE group. Our data on survival by ASA classification support that patients with higher comorbidity burden are more likely to die postoperatively. Importantly, no patient with VTE and ASA < 3 died in 30 days postoperatively (see Figure 1D).
Surprisingly, all eleven patients with VTE who died had a VT. Prior literature suggests that PEs have greater mortality risk than VTs; [28, 43, 44] however, this was not observed here.
1.4.4. VTE Risk Factors
Many of the risk factors reported here are well-known. Longer operative times have been associated with increased postoperative VTE risk.[45, 46] Younger and older pediatric patients in a bimodal distribution have the highest VTE risk in the literature relative to the early teen years similar to our findings.[28, 47] Ventilator dependence,[48] steroid use,[49] nutritional support,[34] seizure disorders,[50] and preoperative blood transfusions[5] have all been previously reported as VTE risk factors.
There were several pertinent negative results within the risk factor analysis. One surprising finding was a lack of difference observed for emergent/urgent vs. elective procedures since this has been reported as a VTE risk factor.[51] The following comorbidities were not independently associated with VTE despite previous studies implicating them as risk factors: neuromuscular disease,[52] cerebral palsy,[52] obesity,[53] and current malignancy/current treatment for malignancy.[54]
History of prematurity was independently associated with lower VTE rates despite being associated with higher VTE rates on univariate testing. One explanation for this finding is that accounting for patient age and comorbidities in the multivariable analysis attenuates the effect of prematurity on VTE risk.
1.4.5. Limitations
Limitations of the NSQIP-P dataset include limited follow up for capturing postoperative events including VTE and later VTE-associated complications. The NSQIP-P definition of VTE includes superficial and deep VTs, which may lead to an overestimation of clinically relevant VTEs in the current study due to inclusion of superficial VTs that may be insignificant but nevertheless present on imaging modalities that meet the NSQIP-P criteria for diagnosis. Only VTEs requiring treatment and VTEs meeting other previously mentioned guidelines are captured, potentially decreasing VTE capture sensitivity. Many VTEs are likely related to PICC lines and CVCs;[55] importantly, NSQIP-P does not capture whether these are used within a separate variable, limiting analysis of the contribution of PICC lines and CVCs to the VTEs observed in NSQIP. Binary categorical variables limit analysis of comorbidity severity (e.g., “hematologic disorder” variable), and certain conditions known to increase VTE risk are not included (e.g., pregnancy). Perioperative medication data is lacking, preventing analysis of anticoagulation used either prophylactically or therapeutically. VTE-related morbidity such as post-thrombotic syndrome is not captured. Individual hospital identifiers are removed, precluding analysis of hospital outliers or hospitals implementing routine VTE prophylaxis. The large number of patients lacking prior operation data (over 50%) precludes analysis of what may be a significant VTE risk factor. Traumatic cases (except long bone fractures) are excluded, which are known to have high VTE rates.
Limitations in the authors’ own analyses should be recognized. Our results may be too generalized to be applicable within subspecialties or within particular procedures given lack of stratified analyses by specialty/procedure. However, we attempted to overcome this to a degree by reporting VTE rates by subspecialty and procedure. Our statistical analysis risks type I error (observing a difference that doesn’t truly exist) due to multivariable analysis including a relatively high number of variables.
1.4.6. Future Directions
The most complete, evidenced-based clinical guideline on pediatric VTE treatment and prophylaxis to date comes from the American College of Chest Physicians (ACCP).[56] However this guideline is focused more on a general pediatric population and is lacking in recommendations for perioperative patients.
While no national guidelines exist currently for post-surgical patients, there are institutional guidelines and consensus statements published to aid decision-making. Jackson et al published an institutional guideline in 2008.[57] They considered orthopedic procedures to have the highest risk and used a cutoff of < or > 30 minutes for procedure length.[57] Our own analysis is inconsistent with orthopedic procedures being at greatest VTE risk and suggests operative times longer than 30 minutes are likely necessary to be concerning for VTE development.
Cincinnati Children’s Hospital published an institutional guideline for VTE prophylaxis in 2014.[58] A scoring system based on risk factors and immobility following surgery dictates prophylactic intervention. Similar to the Jackson et al. guideline, the only surgical procedures mentioned as risk factors are orthopedic.[58]
Clearly, future research should be directed at VTE risk associated with post-surgical pediatric patients. Our own analysis suggests that findings in the adult literature on postoperative VTE rates may not be applicable in the pediatric population and that the existing guidelines on pediatric VTE prophylaxis are limited in their utility.
1.5. CONCLUSION
This study successfully reports VTE risk in the pediatric inpatient surgical population with analyses of contributing comorbidities and VTE-associated mortality. We report VTE rates across surgical subspecialties. Individual surgical procedures with the highest VTE rates are reported here as well. These data may be used to identify pediatric patients at highest postoperative VTE risk and for developing guidelines on VTE prophylaxis and therapeutic intervention.
Supplementary Material
Acknowledgements:
We would like to thank the Children’s Hospital of Alabama NSQIP-P coordinators for their assistance with our many questions related to the use of the NSQIP-P dataset.
Dr. Rocque is supported by NIH Grant 1KL2TR001419.
The American College of Surgeons National Surgical Quality Improvement Program and the hospitals participating in the ACS NSQIP are the source of the data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.
Financial Disclosure: The authors have no financial relationships relevant to this article to disclose.
Abbreviations List:
- VTE
venous thromboembolism
- VT
venous thrombosis
- PE
pulmonary embolism
- CVC
central venous catheter
- ACS
American College of Surgeons
- NSQIP
National Surgical Quality Improvement Program
- NSQIP-P
National Surgical Quality Improvement Program-Pediatric
- SCR
surgical clinical reviewer
- CT
computed tomography
- PICC
peripherally inserted central catheter
- CDC
Centers for Disease Control and Prevention
- SIRS
systemic inflammatory response syndrome
- CPT
current procedural terminology
- ASA
American Society of Anesthesiologists
- CI
confidence interval
- ROC
receiver operating characteristic
- AUC
area under the curve
- OR
odds ratio
- DVT
deep venous thrombosis
- CSF
cerebrospinal fluid
- SD
standard deviation
- TPN
total parenteral nutrition
- NG
nasogastric
- PRBC
packed red blood cells
- LOS
length of stay
- ACCP
American College of Chest Physicians
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: The authors have no conflicts of interest relevant to this article to disclose.
REFERENCES
- [1].Kerlin BA. Current and future management of pediatric venous thromboembolism. Am J Hematol 2012;87 Suppl 1:S68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Vu LT, Nobuhara KK, Lee H, Farmer DL. Determination of risk factors for deep venous thrombosis in hospitalized children. J Pediatr Surg 2008;43(6):1095–9. [DOI] [PubMed] [Google Scholar]
- [3].Setty BA, O’Brien SH, Kerlin BA. Pediatric venous thromboembolism in the United States: a tertiary care complication of chronic diseases. Pediatr Blood Cancer 2012;59(2):258–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Allen CJ, Murray CR, Meizoso JP, Ray JJ, Neville HL, Schulman CI, et al. Risk factors for venous thromboembolism after pediatric trauma. J Pediatr Surg 2016;51(1):168–71. [DOI] [PubMed] [Google Scholar]
- [5].Connelly CR, Laird A, Barton JS, Fischer PE, Krishnaswami S, Schreiber MA, et al. A Clinical Tool for the Prediction of Venous Thromboembolism in Pediatric Trauma Patients. JAMA Surg 2016;151(1):50–7. [DOI] [PubMed] [Google Scholar]
- [6].Mahajerin A, Petty JK, Hanson SJ, Thompson AJ, O’Brien SH, Streck CJ, et al. Prophylaxis against venous thromboembolism in pediatric trauma: A practice management guideline from the Eastern Association for the Surgery of Trauma and the Pediatric Trauma Society. J Trauma Acute Care Surg 2017;82(3):627–36. [DOI] [PubMed] [Google Scholar]
- [7].Thompson AJ, McSwain SD, Webb SA, Stroud MA, Streck CJ. Venous thromboembolism prophylaxis in the pediatric trauma population. J Pediatr Surg 2013;48(6):1413–21. [DOI] [PubMed] [Google Scholar]
- [8].University of Alabama at Birmingham. Is IRB review required for use of public datasets?, http://www.uab.edu/research/administration/offices/IRB/FAQs/Pages/PublicDatasets.aspx.
- [9].Raval MV, Dillon PW, Bruny JL, Ko CY, Hall BL, Moss RL, et al. American College of Surgeons National Surgical Quality Improvement Program Pediatric: a phase 1 report. J Am Coll Surg 2011;212(1):1–11. [DOI] [PubMed] [Google Scholar]
- [10].Raval MV, Dillon PW, Bruny JL, Ko CY, Hall BL, Moss RL, et al. Pediatric American College of Surgeons National Surgical Quality Improvement Program: feasibility of a novel, prospective assessment of surgical outcomes. J Pediatr Surg 2011;46(1):115–21. [DOI] [PubMed] [Google Scholar]
- [11].Bruny JL, Hall BL, Barnhart DC, Billmire DF, Dias MS, Dillon PW, et al. American College of Surgeons National Surgical Quality Improvement Program Pediatric: a beta phase report. J Pediatr Surg 2013;48(1):74–80. [DOI] [PubMed] [Google Scholar]
- [12].Sellers MM, Merkow RP, Halverson A, Hinami K, Kelz RR, Bentrem DJ, et al. Validation of new readmission data in the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg 2013;216(3):420–7. [DOI] [PubMed] [Google Scholar]
- [13].Sharp NE, Knott EM, Iqbal CW, Thomas P, St Peter SD. Accuracy of American College of Surgeons National Surgical Quality Improvement Program Pediatric for laparoscopic appendectomy at a single institution. J Surg Res 2013;184(1):318–21. [DOI] [PubMed] [Google Scholar]
- [14].Sherrod BA, Arynchyna AA, Johnston JM, Rozzelle CJ, Blount JP, Oakes WJ, et al. Risk factors for surgical site infection following nonshunt pediatric neurosurgery: a review of 9296 procedures from a national database and comparison with a single-center experience. J Neurosurg Pediatr 2017;19(4):407–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].American College of Surgeons. User Guide for the 2012 ACS NSQIP Pediatric Participant Use Data File, https://www.facs.org/~/media/files/quality%20programs/nsqip/2012pedsuserguide.ashx; 2013.
- [16].American College of Surgeons. User Guide for the 2013 ACS NSQIP Pediatric Participant Use Data File (PUF), https://www.facs.org/~/media/files/quality%20programs/nsqip/acs_nsqip_puf_user_guide_2013.ashx; 2014.
- [17].American College of Surgeons. User Guide for the 2014 ACS NSQIP Pediatric Participant Use Data File (PUF), https://www.facs.org/~/media/files/quality%20programs/nsqip/peds_acs_nsqip_puf_userguide_2014.ashx; 2015.
- [18].American College of Surgeons. User Guide for the 2015 ACS NSQIP Pediatric Participant Use Data File (PUF) https://www.facs.org/~/media/files/quality%20programs/nsqip%20peds/peds_acs_nsqip_puf_userguide_2015.ashx; 2016.
- [19].Humes DJ, Nordenskjold A, Walker AJ, West J, Ludvigsson JF. Risk of venous thromboembolism in children after general surgery. J Pediatr Surg 2015;50(11):1870–3. [DOI] [PubMed] [Google Scholar]
- [20].Arlikar SJ, Atchison CM, Amankwah EK, Ayala IA, Barrett LA, Branchford BR, et al. Development of a new risk score for hospital-associated venous thromboembolism in critically-ill children not undergoing cardiothoracic surgery. Thromb Res 2015;136(4):717–22. [DOI] [PubMed] [Google Scholar]
- [21].Takemoto CM, Sohi S, Desai K, Bharaj R, Khanna A, McFarland S, et al. Hospital-associated venous thromboembolism in children: incidence and clinical characteristics. J Pediatr 2014;164(2):332–8. [DOI] [PubMed] [Google Scholar]
- [22].Rohrer MJ, Cutler BS, MacDougall E, Herrmann JB, Anderson FA Jr., Wheeler HB. A prospective study of the incidence of deep venous thrombosis in hospitalized children. J Vasc Surg 1996;24(1):46–9; discussion 50. [DOI] [PubMed] [Google Scholar]
- [23].Stein PD, Kayali F, Olson RE. Incidence of venous thromboembolism in infants and children: data from the National Hospital Discharge Survey. J Pediatr 2004;145(4):563–5. [DOI] [PubMed] [Google Scholar]
- [24].DeAngelis GA, McIlhenny J, Willson DF, Vittone S, Dwyer SJ 3rd, Gibson JC, et al. Prevalence of deep venous thrombosis in the lower extremities of children in the intensive care unit. Pediatr Radiol 1996;26(11):821–4. [DOI] [PubMed] [Google Scholar]
- [25].Higgerson RA, Lawson KA, Christie LM, Brown AM, McArthur JA, Totapally BR, et al. Incidence and risk factors associated with venous thrombotic events in pediatric intensive care unit patients. Pediatr Crit Care Med 2011;12(6):628–34. [DOI] [PubMed] [Google Scholar]
- [26].Raffini L, Huang YS, Witmer C, Feudtner C. Dramatic increase in venous thromboembolism in children’s hospitals in the United States from 2001 to 2007. Pediatrics 2009;124(4):1001–8. [DOI] [PubMed] [Google Scholar]
- [27].Radecki RT, Gaebler-Spira D. Deep vein thrombosis in the disabled pediatric population. Arch Phys Med Rehabil 1994;75(3):248–50. [DOI] [PubMed] [Google Scholar]
- [28].Faustino EV, Spinella PC, Li S, Pinto MG, Stoltz P, Tala J, et al. Incidence and acute complications of asymptomatic central venous catheter-related deep venous thrombosis in critically ill children. J Pediatr 2013;162(2):387–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Gray BW, Gonzalez R, Warrier KS, Stephens LA, Drongowski RA, Pipe SW, et al. Characterization of central venous catheter-associated deep venous thrombosis in infants. J Pediatr Surg 2012;47(6):1159–66. [DOI] [PubMed] [Google Scholar]
- [30].Silvey M, Brandao LR. Risk Factors, Prophylaxis, and Treatment of Venous Thromboembolism in Congenital Heart Disease Patients. Front Pediatr 2017;5:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hanson SJ, Punzalan RC, Christensen MA, Ghanayem NS, Kuhn EM, Havens PL. Incidence and risk factors for venous thromboembolism in critically ill children with cardiac disease. Pediatr Cardiol 2012;33(1):103–8. [DOI] [PubMed] [Google Scholar]
- [32].Faraoni D, Gardella KM, Odegard KC, Emani SM, DiNardo JA. Incidence and Predictors for Postoperative Thrombotic Complications in Children With Surgical and Nonsurgical Heart Disease. Ann Thorac Surg 2016;102(4):1360–7. [DOI] [PubMed] [Google Scholar]
- [33].Todd Tzanetos DR, Yu C, Hernanz-Schulman M, Barr FE, Brown NJ. Prospective study of the incidence and predictors of thrombus in children undergoing palliative surgery for single ventricle physiology. Intensive Care Med 2012;38(1):105–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gonzalez-Hernandez J, Daoud Y, Styers J, Journeycake JM, Channabasappa N, Piper HG. Central venous thrombosis in children with intestinal failure on long-term parenteral nutrition. J Pediatr Surg 2016;51(5):790–3. [DOI] [PubMed] [Google Scholar]
- [35].Antiel RM, Hashim Y, Moir CR, Rodriguez V, Elraiyah T, Zarroug AE. Intra-abdominal venous thrombosis after colectomy in pediatric patients with chronic ulcerative colitis: incidence, treatment, and outcomes. J Pediatr Surg 2014;49(4):614–7. [DOI] [PubMed] [Google Scholar]
- [36].Sherrod BA, Johnston JM, Rocque BG. Risk factors for unplanned readmission within 30 days after pediatric neurosurgery: a nationwide analysis of 9799 procedures from the American College of Surgeons National Surgical Quality Improvement Program. J Neurosurg Pediatr 2016;18(3):350–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Levy ML, Granville RC, Hart D, Meltzer H. Deep venous thrombosis in children and adolescents. J Neurosurg 2004;101(1 Suppl):32–7. [DOI] [PubMed] [Google Scholar]
- [38].Murphy RF, Naqvi M, Miller PE, Feldman L, Shore BJ. Pediatric orthopaedic lower extremity trauma and venous thromboembolism. J Child Orthop 2015;9(5):381–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Baker D, Sherrod B, McGwin G Jr., Ponce B, Gilbert S. Complications and 30-day Outcomes Associated With Venous Thromboembolism in the Pediatric Orthopaedic Surgical Population. J Am Acad Orthop Surg 2016;24(3):196–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Georgopoulos G, Hotchkiss MS, McNair B, Siparsky G, Carry PM, Miller NH. Incidence of Deep Vein Thrombosis and Pulmonary Embolism in the Elective Pediatric Orthopaedic Patient. J Pediatr Orthop 2016;36(1):101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Harris DA, Lam S. Venous thromboembolism in the setting of pediatric traumatic brain injury. J Neurosurg Pediatr 2014;13(4):448–55. [DOI] [PubMed] [Google Scholar]
- [42].Zamperlini-Netto G, Zanette A, Wehbi E, Williams S, Grant RM, Brandao LR. PO-60 – Renal tumors with extensive vascular disease: management challenges in a pediatric series from the Hospital for Sick Children. Thromb Res 2016;140 Suppl 1:S198–9. [DOI] [PubMed] [Google Scholar]
- [43].Biss TT, Brandao LR, Kahr WH, Chan AK, Williams S. Clinical features and outcome of pulmonary embolism in children. Br J Haematol 2008;142(5):808–18. [DOI] [PubMed] [Google Scholar]
- [44].Dijk FN, Curtin J, Lord D, Fitzgerald DA. Pulmonary embolism in children. Paediatr Respir Rev 2012;13(2):112–22. [DOI] [PubMed] [Google Scholar]
- [45].Bekelis K, Labropoulos N, Coy S. Risk of Venous Thromboembolism and Operative Duration in Patients Undergoing Neurosurgical Procedures. Neurosurgery 2017;80(5):787–92. [DOI] [PubMed] [Google Scholar]
- [46].Kim JY, Khavanin N, Rambachan A, McCarthy RJ, Mlodinow AS, De Oliveria GS Jr., et al. Surgical duration and risk of venous thromboembolism. JAMA Surg 2015;150(2):110–7. [DOI] [PubMed] [Google Scholar]
- [47].Van Arendonk KJ, Schneider EB, Haider AH, Colombani PM, Stewart FD, Haut ER. Venous thromboembolism after trauma: when do children become adults? JAMA Surg 2013;148(12):1123–30. [DOI] [PubMed] [Google Scholar]
- [48].Ibrahim EH, Iregui M, Prentice D, Sherman G, Kollef MH, Shannon W. Deep vein thrombosis during prolonged mechanical ventilation despite prophylaxis. Crit Care Med 2002;30(4):771–4. [DOI] [PubMed] [Google Scholar]
- [49].Waljee AK, Rogers MA, Lin P, Singal AG, Stein JD, Marks RM, et al. Short term use of oral corticosteroids and related harms among adults in the United States: population based cohort study. BMJ 2017;357:j1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Martz GU, Wilson DA, Malek AM, Selassie AW. Risk of venous thromboembolism in people with epilepsy. Epilepsia 2014;55(11):1800–7. [DOI] [PubMed] [Google Scholar]
- [51].Rogers SO Jr., Kilaru RK, Hosokawa P, Henderson WG, Zinner MJ, Khuri SF. Multivariable predictors of postoperative venous thromboembolic events after general and vascular surgery: results from the patient safety in surgery study. J Am Coll Surg 2007;204(6):1211–21. [DOI] [PubMed] [Google Scholar]
- [52].Heit JA. Epidemiology of venous thromboembolism. Nat Rev Cardiol 2015;12(8):464–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Stokes S, Breheny P, Radulescu A, Radulescu VC. Impact of obesity on the risk of venous thromboembolism in an inpatient pediatric population. Pediatr Hematol Oncol 2014;31(5):475–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Heit JA, Spencer FA, White RH. The epidemiology of venous thromboembolism. J Thromb Thrombolysis 2016;41(1):3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Schoot RA, Kremer LC, van de Wetering MD, van Ommen CH. Systemic treatments for the prevention of venous thrombo-embolic events in paediatric cancer patients with tunnelled central venous catheters. Cochrane Database Syst Rev 2013(9):CD009160. [DOI] [PubMed] [Google Scholar]
- [56].Monagle P, Chan AKC, Goldenberg NA, Ichord RN, Journeycake JM, Nowak-Gottl U, et al. Antithrombotic therapy in neonates and children: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141(2 Suppl):e737S–e801S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Jackson PC, Morgan JM. Perioperative thromboprophylaxis in children: development of a guideline for management. Paediatr Anaesth 2008;18(6):478–87. [DOI] [PubMed] [Google Scholar]
- [58].Cincinnati Children’s Hospital. Best Evidence Statement Venous Thromboembolism (VTE) Prophylaxis in Children and Adolescents, https://www.cincinnatichildrens.org/-/media/cincinnati%20childrens/home/service/j/anderson-center/evidence-basedcare/recommendations/type/venous%20thromboembolism%20best%20181.pdf?la=en; 2014. [accessed September 8, 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.