Abstract
Purpose:
Ipilimumab induces durable remissions in about 15–20% of patients with metastatic melanoma. However, reliable predictors for response to ipilimumab are currently lacking. Whole-body metabolic tumor volume (wMTV) has been shown to be a strong prognostic factor in a variety of malignancies treated by chemotherapy, but little data has been reported for patients treated by immunotherapy. The purpose of this study was to investigate the prognostic value of wMTV and other metabolic parameters on baseline 18F-FDG PET/CT scans in patients with melanoma being treated with ipilimumab.
Methods:
The prognostic impact of wMTV, as well as mean standardized uptake values (SUVs) and total lesion glycolysis (TLG), was evaluated in 142 consecutive melanoma patients treated with single-agent ipilimumab therapy. Metabolic parameters were dichotomized by their respective medians and correlated with overall survival (OS). In addition, multivariate analyses of metabolic parameters with known clinical prognostic factors were performed.
Results:
Median OS for all patients was 14.7 months (95% CI, 10.45–18.93 months). wMTV was a strong independent prognostic factor for OS (p = 0.001). The median survival of patients with a metabolic volume above the median was 10.8 months (95% CI, 5.88–15.81 months) as compared to 26.0 months (95% CI, 3.02–49.15 months) for patients with an MTV below the median. A multivariate model including wMTV and known clinical prognostic factors, such as age and presence of brain metastases, further improved the identification of patient subgroups with different OS.
Conclusions:
wMTV appears to be a strong independent prognostic factor in melanoma patients treated with ipilimumab and can be determined semi-automatically from routine 18F-FDG PET/CT scans. wMTV, combined with clinical prognostic factors, could be used to personalize immunotherapy and future clinical studies.
Keywords: CTLA-4, metabolic tumor volume, ipilimumab, melanoma, PET
Introduction
Ipilimumab, an anti-cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) monoclonal antibody, significantly improves survival of patients with metastatic melanoma when compared to chemotherapy [1–3]. Importantly, ipilimumab not only improves the median survival of patients but can also induce long-term tumor remissions in about 15–20% of patients [4–6]. While this represents a major breakthrough for the treatment of metastatic melanoma, ipilimumab therapy also has known limitations. Ipilimumab is one of the so-called checkpoint inhibitors that block the action of negative regulators of the cellular immune response. Because of its mechanism of action, ipilimumab can also cause immune-related adverse events (irAEs). Severe irAEs—grade 3 or 4 as characterized by the Common Terminology Criteria for Adverse Events (CTCAE)—are observed in 20–30% of patients [1, 7]. Overall, treatment must be discontinued because of these side effects in approximately 20% of patients [7, 8].
Combining ipilimumab with the PD1 antibody nivolumab (an immune checkpoint inhibitor with a different mode of action than ipilimumab) significantly increases response rates and survival [5, 7]. In a randomized control trial of patients with metastatic or unresectable melanoma, the best overall response to ipilimumab and a combination of ipilimumab and nivolumab was 19% and 58%, respectively. Three-year survival rates were 58% and 34%, respectively. However, combination therapy also significantly increased the risk of irAEs. Grade 3 or 4 toxicity occurred in 59% of the patients treated with combination therapy, compared to 28% in patients receiving ipilimumab monotherapy [7]. Treatment-related adverse events led to discontinuation of combination therapy in 39% of patients. Interestingly, discontinuation of checkpoint inhibitor therapy due to irAEs is not necessarily associated with decreased efficacy. A pooled analysis of patients who discontinued ipilimumab and nivolumab combination therapy in phase II and III studies found that survival was not significantly different from patients who completed therapy. This raises the question of whether treatment may be stopped earlier without losing efficacy [9].
Thus, there is a clear clinical need for better selection of patients for single-agent versus combination immunotherapy, as well as for criteria for the optimal duration of immunotherapy. Despite significant efforts, no clinical, histological, or genetic parameters have been identified so far that reliably predict the success of ipilimumab therapy or other immunotherapies. The need for such parameters is further emphasized by the high costs of immunotherapy [10].
For several metastatic malignancies, metabolic tumor volume (MTV) derived from 18F-FDG PET/CT studies has been shown to be a strong prognostic factor for overall survival in patients treated with chemotherapy or radiotherapy [11–15]. However, only limited data exists on the prognostic relevance of MTV for immunotherapy. The purpose of this study was to investigate the prognostic value of metabolic parameters, including MTV on baseline18F-FDG PET/CT scans, in patients with metastatic melanoma undergoing ipilimumab immunotherapy. We also investigated whether metabolic parameters can be combined with known clinical prognostic parameters to more accurately predict patient outcome after ipilimumab therapy.
Materials and Methods
The institutional review board (IRB) approved this retrospective study and waived the informed consent requirement. The study was compliant with the Health Insurance Portability and Accountability Act. The hospital information system was searched for patients with metastatic melanoma who had been treated with ipilimumab and received a 18F-FDG PET/CT scan prior to therapy. The search window extended from 2010 to 2016 to ensure that the scans had been performed with similar PET/CT systems and that there was sufficient follow-up to assess overall survival. Exclusion criteria were as follows: (i) patients with only active brain metastasis; (ii) patients with no hypermetabolic lesion on the baseline 18F-FDG PET/CT; (iii) patients with advanced primary cancers other than melanoma; and (iv) patients with follow-up time of less than three months after starting ipilimumab therapy. For the included patients, the following parameters were recorded: age at treatment initiation, gender, serum lactate dehydrogenase (LDH) level, start date, type of other treatments (surgery, radiotherapy, and systemic chemotherapy), primary site of melanoma, status of BRAF mutation, reasons for discontinuation of ipilimumab therapy, and date and cause of death (whether disease-specific or not) or date of last documented visit.
18F-FDG PET/CT protocol
Before injection of 18F-FDG, all patients fasted for at least six hours. If plasma glucose levels were < 200 mg/dl, patients were injected with 444–555 MBq of IV radiotracer. After approximately 60–90 minutes uptake time, patients were scanned while in the supine position. In most cases, images were obtained from the skull vertex to the feet on PET/CT machines of the GE Discovery Series (VCT, ST, STE, 600, and 690). Cross-calibration between the dose calibrator and PET scanners were performed monthly. Low-dose CT images during PET/CT were used for attenuation correction of the PET emission scan and anatomical orientation. PET/CT images were reconstructed using an ordered-subset expectation maximization algorithm and a Gaussian filter using the standard manufacture-supplied reconstruction software. The acquisition and reconstruction parameters were harmonized to minimize the standardized uptake value (SUV) differences between scanners and keep them within 10% as tested using measurements of the IEC image quality phantom. Specifically, the scan duration per bed position for the first-generation bismuth germanate (BGO) scanners was set at 5 min, and at 3 min for the second-generation lutetium yttrium oxyorthosilicate (LYSO) detector scanners. Secondly, the number of iterations for image reconstruction was 4 (with 20 subsets and an 8 mm post-reconstruction transaxial Gaussian filter) for the BGO scanners compared to 2 iterations and 20 subsets for the LYSO scanners. This resulted in images of comparable smoothness and acceptable discrepancies (<10%) among SUV numbers in phantom studies.
Scans were generally acquired with an axial field of view from the vertex to the toes (n = 118). In 24 patients, only images from the base of the skull to the mid-thighs were obtained because no relevant lesions in the extremities were expected clinically.
Image analysis
One experienced physician, board-certified in radiology and nuclear medicine, reviewed all 18F-FDG PET/CT images on a GE Advantage Workstation using the PET VCAR software. The CT scan, along with clinical information from the patients’ files, was used to help differentiate between benign and treatment-related findings and metastatic disease. Image interpretations were confirmed by another board-certified nuclear medicine physician and any questionable findings were resolved by consensus among the two investigators.
18F-FDG PET/CT uptake was quantified by standardized uptake values normalized by lean body mass (SUL). As a measure of tumor metabolic activity, we determined the maximum SUL of all lesions in a patient (SULmax). To assess tumor burden, MTV was defined as the volume enclosed by a 42% isocontour around the maximum PET voxel of tumor lesions, as described previously [16]. Occasional manual adjustments were made if the volume defined by the 42% threshold extended beyond the lesion borders seen on CT. Whole-body MTV (wMTV) was defined as the sum of the individual MTVs of all lesions analyzed. Total lesion glycolysis (TLG) was obtained by multiplying the MTV of each focal lesion with the mean SUL in the MTV. Whole-body TLG (wTLG) was defined as the sum of TLGs of all lesions. In addition, we determined the sum of SULpeak for up to 5 target lesions (maximum of two per organ) as a simplified measure of tumor burden and metabolic activity. To measure SULpeak, a sphere or cube was drawn around the target lesions. Within this VOI, the AW software searches for the 1.0 cm3 sphere that encompasses the voxels with the highest average SUL [17].
Statistical analysis
Statistical analysis was performed by using SPSS (version 24) in all analyses. Continuous variables were summarized by median and interquartile range (IQR) or mean values and standard deviation (SD), and categorical variables were summarized by frequency and percentage. For continuous variables, statistical significance was determined using the student t-test. A P value of 0.05 or less was considered significant. The Kaplan-Meier method was used to determine whether there was an association between treatment response and OS. The log-rank test was used to evaluate differences between Kaplan-Meier curves. Bonferroni correction was used to adjust for multiple comparisons among risk groups. Univariate analysis was used to identify factors associated with OS. Factors that were identified as being significant by univariate analysis (p < 0.05) were then entered into a Cox multivariate regression analysis model. Then, forward stepwise multivariate regression analysis was carried out to identify factors that correlated with OS. In each step, the variables with p < 0.05 were entered and those with p > 0.10 were removed.
Results
Patient characteristics
Figure 1 shows the flow diagram of potentially eligible patients; overall, 142 patients were included (median age: 69 years, IQR: 60.25–77 years). In all patients, 3 mg/kg ipilimumab was administered as a one-hour infusion every 3 weeks for up to 4 cycles. Baseline 18F-FDG PET/CT scans were performed within 9 weeks before initiating ipilimumab (median: 3.4 weeks). Patient characteristics are shown in Table 1. Most patients were men with cutaneous melanoma who had undergone wide local excision and had wild-type BRAF tumors. Thirty-nine of 142 cases were treated with chemotherapy prior to ipilimumab therapy; the primary drugs were dacarbazine, temozolomide, or platinum-based chemotherapy. Twenty-seven of 134 tumors tested for BRAF V600 mutations had this mutation.
Fig. 1.
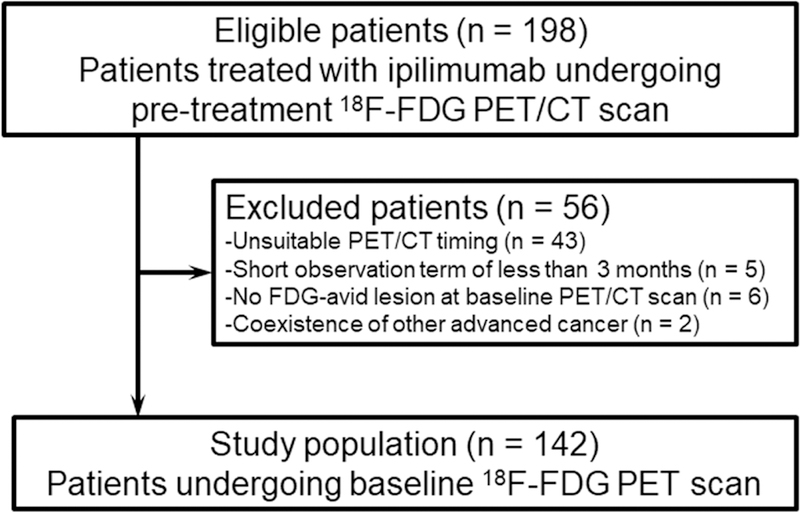
Flow diagram of study patients.
Table 1.
Demographic and disease characteristics of the patients at ipilimumab therapy
| Characteristic | Ipilimumab (n = 142) |
|---|---|
| Median age (range) | 69 (28–90) |
| Age ≥ 75 years (%) | 41 (28.9) |
| Male sex (%) | 83 (58.5) |
| Primary site (%) | |
| Cutaneous | 100 (70.4) |
| Mucosal* | 20 (14.1) |
| Uveal** | 9 (6.3) |
| Unknown | 13 (9.2) |
| Elevated LDH level over ULN (%) | 38 (26.8) |
| Prior surgery (%)† | 125 (88.0) |
| Prior radiotherapy (%) | 35 (24.6) |
| BRAF V600 mutations (%) | |
| Positive | 27 (19.0) |
| Negative | 107 (75.4) |
| Unknown** | 8 (5.6) |
| Active brain metastases (%) | 22 (15.5) |
| Status of ipilimumab therapy (%) | |
| Discontinuation (<4 cycles) | 35 (24.6) |
| Completion (4 cycles) | 107 (75.4) |
| Interval from PET to treatment (%) | |
| > 4 weeks | 43 (30.3) |
| ≤ 4 weeks | 99 (69.7) |
| Line of previous systemic therapy (%) | |
| 0 | 103 (72.5) |
| 1 | 35 (24.6) |
| 2 | 4 (2.8) |
| Type of previous systemic chemotherapy | |
| Alkylating agents or platinum-based chemotherapy | 25 |
| BRAF or MEK inhibitors or both | 7 |
| Others | 7 |
LDH: lactate dehydrogenase, ULN: upper limit of the normal range
Vagina 10, Maxillary sinus 5, GI tract 2, Oral mucosa 1, Esophagus 1, Anus 1
Seven cases with uveal melanoma harboring GNAQ mutate positive.
Most of these operations were wide local excision to cutaneous primary lesions
Thirty-five patients received fewer than 4 cycles of ipilimumab; these cases were defined as discontinuation. The reasons for discontinuation of ipilimumab were as follows: 20 rapid progression of disease, 13 severe immune-related adverse events (irAE) (8 colitis, 2 hypophysitis, 2 aseptic meningitis, and 1 hepatitis), 1 ileus requiring surgery, and 1 cardiovascular event. Patient survival was similar for those with and without severe irAE (p=0.655).
Association of 18F-FDG PET/CT parameters and clinical factors with OS
All patients were observed for at least 3 months from the start date of ipilimumab. At the time of data cutoff for the analysis, 95 deaths had occurred. The median OS for all 142 patients was 14.7 months (95% CI, 10.4–18.9 months).
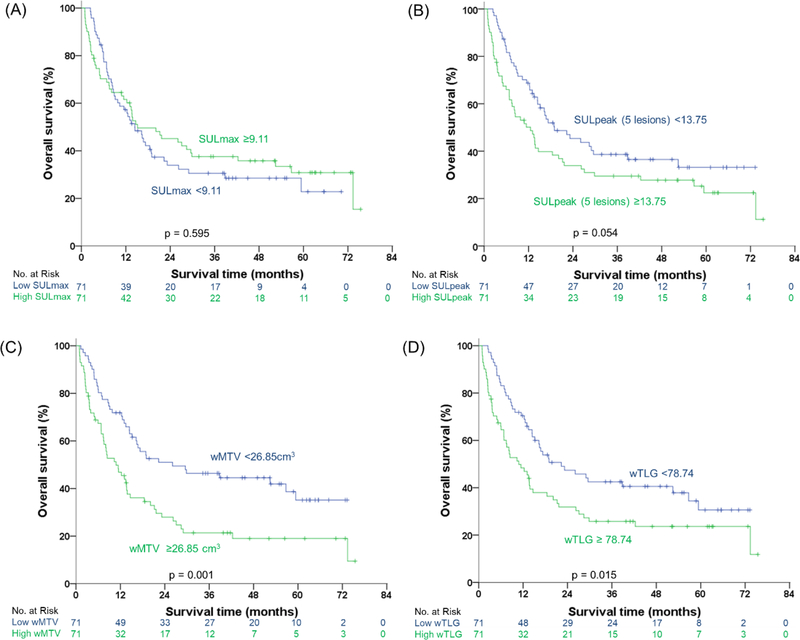
The median values for SULmax, sum of SULpeak, wTMV, and wTLG were 9.11 (IQR: 6.14–13.67), 13.75 (IQR: 6.42–24.13), 26.85 cm3 (IQR: 8.21–54.85 cm3), and 78.74 (IQR: 25.60–365.60). wMTV and wTLG were significantly associated with OS (Fig. 2). The median OS of patients with wMTV above the median was 10.84 months (95% CI, 5.88–5.81 months) as compared to 26.09 months (95% CI, 3.02–49.15 months) for patients with a wMTV below the median (p = 0.002, hazard ratio 1.9). The corresponding one-, two-, and three-year survival rates were 47% vs. 72%, 28% vs. 51%, and 21% vs. 46%, respectively.
Fig. 2.
Kaplan-Meier estimations of overall survival in different groups. Curves of overall survival in (A) SULmax, (B) sum of five highest SULpeak lesions, (C) wMTV, and (D) wTLG.
The prognostic value of wTLG was similar to that of wMTV. The median OS for patients with wTLG above the median was 10.84 months (95% CI, 5.50–16.19 months) as compared to 22.34 months (95% CI, 9.36–35.32 months) for patients with wTLG below the median (Fig. 2). SULmax was not a prognostic parameter (p = 0.60, HR = 0.90, Fig. 2). Differences in survival between patients with a sum of SULpeak above and below the median were not significant, but a trend was noted (p = 0.056, HR = 1.48, Fig. 2).
Combining PET parameters and clinical factors
In a univariate Cox proportional hazards model, age, lines of previous chemotherapy, primary site of melanoma, elevated LDH level, and active brain metastases were also significantly associated with OS (Table 2). In a multivariate analysis including the significant clinical and PET parameters, wMTV (HR, 1.845 95% CI, 1.180–2.883; p = 0.007) remained a significant independent factor associated with OS (Table 2). While the wMTV was larger in patients undergoing PET ≤4 weeks prior to starting therapy (mean ± SD, 91.81 ± 153.07 cm3 vs. 43.42 ± 88.42 cm3; p = 0.019), this time interval was not a prognostic factor (p = 0.096) in the univariate analysis.
Table 2.
Factors associated with overall survival in patients with advanced melanoma
| Hazard ratio | 95% CI | P value | |
|---|---|---|---|
| Univariate analysis | |||
| Age | |||
| ≥ 75 years | 2.018 | 1.324 – 3.077 | 0.001 |
| < 75 years | 1.000 (ref) | ||
| Sex | |||
| Women | 0.793 | 0.526 – 1.194 | 0.267 |
| Men | 1.000 (ref) | ||
| Lines of previous chemotherapy | |||
| ≥ 1 | 1.727 | 1.130 – 2.640 | 0.012 |
| 0 | 1.000 (ref) | ||
| Primary site of melanoma | |||
| Others and unknown | 1.645 | 1.068 – 2.531 | 0.024 |
| Cutaneous | 1.000 (ref) | ||
| BRAF V600 mutation | |||
| Present | 0.954 | 0.565 –1.611 | 0.861 |
| Absent | 1.000 (ref) | ||
| Prior surgery | |||
| Yes | 0.592 | 0.335 – 1.046 | 0.071 |
| No | 1.000 (ref) | ||
| Elevated LDH level over ULN | |||
| Yes | 2.671 | 1.724 – 4.140 | <0.001 |
| No | 1.000 (ref) | ||
| Active brain metastases | |||
| Present | 2.800 | 1.684 – 4.657 | < 0.001 |
| Absent | 1.000 (ref) | ||
| Interval from PET to treatment | |||
| > 4 weeks | 0.670 | 0.419 – 1.073 | 0.096 |
| ≤ 4 weeks | 1.000 (ref) | ||
| SULmax | |||
| Greater than median | 0.896 | 0.598 – 1.344 | 0.596 |
| Less than median | 1.000 (ref) | ||
| Sum of SULpeak 5 lesions | |||
| Greater than median | 1.484 | 0.990 – 2.225 | 0.056 |
| Less than median) | 1.000 (ref) | ||
| wMTV | |||
| Greater than median | 1.929 | 1.282 – 2.904 | 0.002 |
| Less than median | 1.000 (ref) | ||
| wTLG | |||
| Greater than median | 1.644 | 1.097 – 2.465 | 0.016 |
| Less than median | 1.000 (ref) | ||
| Multivariate analysis | |||
| Age | |||
| ≥ 75 years | 2.147 | 1.361 – 3.385 | 0.001 |
| < 75 years | 1.000 (ref) | ||
| Lines of previous chemotherapy | |||
| ≥ 1 | 1.810 | 1.160 – 2.823 | 0.009 |
| 0 | 1.000 (ref) | ||
| Elevated LDH level over ULN | |||
| Yes | 2.039 | 1.269 – 3.277 | 0.003 |
| No | 1.000 (ref) | ||
| Active brain metastases | |||
| Present | 3.421 | 1.967 – 5.949 | <0.001 |
| Absent | 1.000 (ref) | ||
| Wmtv | |||
| Greater than median | 2.015 | 1.287 – 3.154 | 0.002 |
| Less than median | 1.000 (ref) | ||
LDH: lactate dehydrogenase, ULN: upper limit of the normal range, wMTV: whole-body metabolic tumor volume, wTLG: whole-body total lesion glycolysis, CI: confidence interval
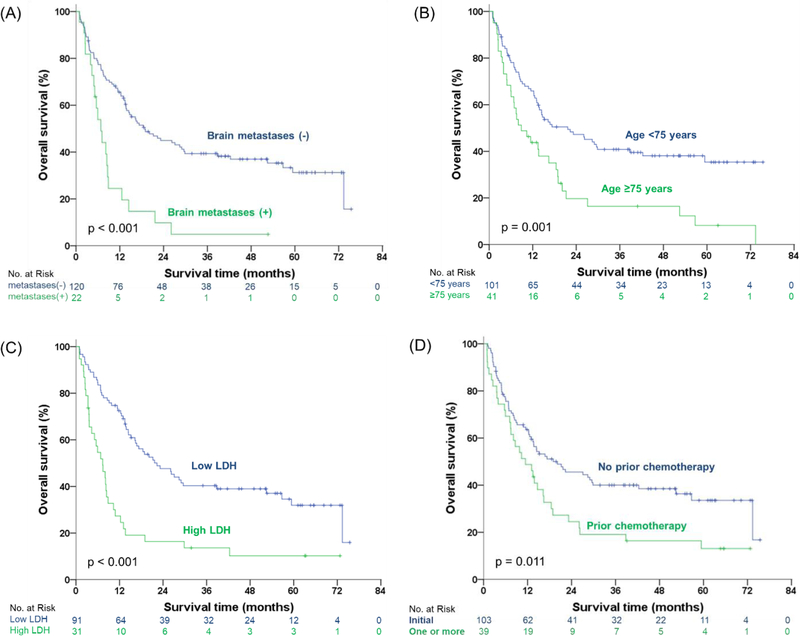
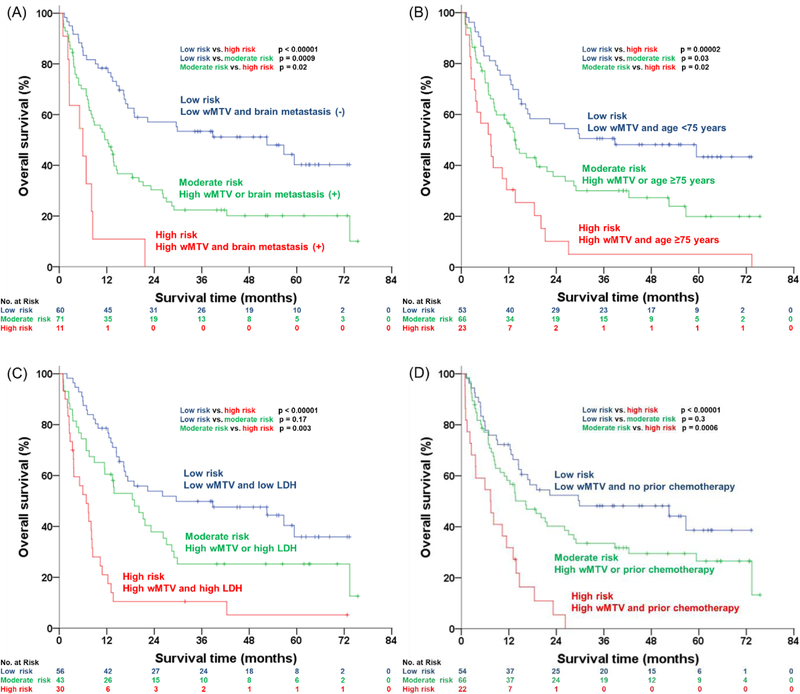
Figure 3 shows the survival curves of four other baseline clinical factors, except wMTV, which remained significant after multivariate analysis. Median OS of good vs. poor prognostic groups dichotomized for brain metastases, age, LDH, and lines of prior chemotherapy were 18.89 vs. 6.80 months, 21.65 vs. 8.87 months, 22.34 vs. 7.36 months, and 20.14 vs. 11.43 months, respectively. Combining these parameters with wMTV further improved patient stratification. Specifically, we identified the following risk groups: (i) low risk: patients with low wMTV and favorable clinical risk factors; (ii) moderate risk: low wMTV and unfavorable clinical risk factors or high wMTV and favorable clinical risk factors; and (iii) high risk: high wMTV and unfavorable clinical risk factors. The survival curves for these three risk groups are shown in Figure 4. The high-risk groups showed statistically significantly different OS for all clinical factors studied and stratified patient survival better than wMTV or the clinical parameters alone. Median OS for all patients with high wMTV was 10.84 months, but if wMTV was associated with brain metastases, age greater than 75 years, high LDH, or two or more lines of chemotherapy, OS decreased to 5.95, 7.46, 6.80, and 7.36 months, respectively. Conversely, median OS for all patients with low wMTV was 20.09 months, and if it was associated with no brain metastases, age of less than 75 years, low LDH, and no prior chemotherapy, it increased to 52.45, 38.80, 52.47, and 29.83 months, respectively.
Fig. 3.
Kaplan-Meier estimations of overall survival in different groups. Curves of overall survival in (A) brain metastases, (B) age, (C) LDH, and (D) prior chemotherapy.
Fig. 4.
Kaplan-Meier estimates of overall survival according to wMTV combined with the four independent prognostic factors categorized into three groups: (A) brain metastases, (B) age, (C) LDH, and (D) prior chemotherapy.
Discussion
To our knowledge, this is the first study to evaluate the prognostic value of wMTV in patients with melanoma undergoing immunotherapy. Our findings indicate that wMTV prior to ipilimumab therapy is an independent prognostic factor in patients with advanced melanoma that significantly adds to known clinical prognostic factors such as LDH and the presence of brain metastases.
The prognostic value of wMTVs on baseline PET/CT in patients treated with chemotherapy or radiotherapy has been extensively studied for various malignancies, including lymphoma [18], breast cancer [19], non-small cell lung cancer [20], and head and neck cancer [21]. Most studies have indicated that high wMTV or wTLG are strongly correlated with poor outcome. In contrast, data on the prognostic value of wMTV or wTLG in malignant melanoma or in patients treated with immunotherapy is limited. Son et al. have published on the relationship between wMTV on pre-treatment FDG PET/CT in a heterogenous group of 41 patients with cutaneous melanoma and tumor stages [11]. wMTV was found to be a significant prognostic factor in a multivariate analysis adjusting for clinical factors such as tumor stage. Here we confirm the prognostic value of FDG PET/CT in an almost three-fold larger patient population of patients with advanced melanoma treated with ipilimumab and demonstrate that a group with high wMTV above the cutoff of 27 cm3 had a significantly shorter median OS of 10.84 months. Differences in the results are probably related to different baseline patient characteristics and methodological differences.
The strong prognostic value of wMTV in patients treated with ipilimumab suggests several potential future clinical applications in patients with melanoma undergoing immunotherapy. As shown in Figure 2, the difference in median OS between the higher and lower wMTV groups was approximately 15 months. The difference in median OS between these two groups is larger than the difference in median OS in a study comparing ipilimumab plus dacarbazine with single agent dacarbazine [2]. The three-year OS rates of patients with low and high wMTV were 46% and 21%, respectively. The difference between those two survival rates is similar to the difference of three-year survival rates of the combination therapy of ipilimumab/nivolumab and ipilimumab monotherapy (53% vs. 32%) [7]. This indicates that wMTV should be considered for patient stratification in randomized clinical trials because imbalances in wMTV could clearly confound comparisons of treatment groups. Currently, clinical factors such as LDH levels and history of brain metastases are used to balance the treatment arms in randomized clinical trials [5]. However, our data show that wMTV is an independent prognostic factor that can improve patient stratification.
Clinical factors combined with wMTV could also be used to select specific patient populations for experimental therapies. Patients with poor clinical prognostic factors and high wMTV were characterized by very low OS rates. For example, median OS of patients with brain metastases and high wMTV was 5.9 months, and all patients died within two years after starting ipilimumab therapy. Thus, these patients may be candidates for combination immunotherapy or experimental therapies [22]. Conversely, patients with low wMTV and favorable clinical prognostic factors showed an excellent prognosis after ipilimumab therapy and thus may be potential candidates for less intensive therapies with a lower risk for irAEs. Of course, these hypotheses for future applications of wMTV in clinical practice and research must be properly tested in prospective clinical trials.
A recent publication investigated the prognostic value of tumor burden as assessed by CT scans in melanoma patients treated with the PD1 antibody pembrolizumab [23]. The baseline CT scans of patients treated with pembrolizumab in the KEYNOTE-1 study were retrospectively analyzed. The sum of the maximum diameters of target lesions on the baseline scan (baseline tumor size, BTS) was used as an index of tumor burden. A BTS above the median was associated with significantly worse OS. Thus, tumor burden appears to not only be a prognostic factor for patients treated with ipilimumab, but also for other immunotherapies. Conceptually, wMTV is a better marker for tumor burden than BTS because it includes all metastases in a patient, whereas BTS is based on up to five index lesions. Furthermore, many metastases, such as bone metastases, are not measurable on CT. Selection of target lesions is also to a significant extent subjective and not only based on lesion size, but also on how well the lesions are delineated on CT. In addition, a one-dimensional measurement of lesions on CT does not capture the actual tumor volume; irregularly shaped lesions with the same maximum diameter may have very different volumes. In contrast, wMTV from FDG PET/CT is a true measure of tumor volume. Because of the high metabolic activity of malignant melanoma and the resulting high image contrast, wMTV was determined semi-automatically within 15 minutes per patient using commercially available software packages in the present study. Semi-automatic delineation of lesions on whole-body CT is technically more challenging due to the much lower contrast between tumor and normal tissues on CT images. Future comparative studies are required to determine if these principal advantages of wMTV as compared to BTS translate into clinically relevant differences in the prognostic value of these two parameters.
We recognize several limitations of this study. FDG PET/CT scans were performed at the discretion of the referring physician, and the patient population may not be representative of the overall patient population eligible for immunotherapy. The methodology for tumor volume measurements (including all voxels that show at least 42% of the maximum FDG uptake in the tumor) has been used in several previous studies, but more sophisticated approaches may provide more accurate volume measurements, especially for tumors with relatively low FDG uptake. Nevertheless, we believe that the wMTV determined with our straightforward approach provides a good estimate for whole-body tumor burden and allows investigators to quantitatively compare tumor burden between individual patients. Finally, further studies are needed to evaluate the prognostic value of wMTV in patients receiving PD1-directed immunotherapy, which has emerged as the most common treatment for metastatic melanoma in recent years.
In conclusion, our retrospective analysis indicates that tumor burden of patients with advanced melanoma as quantified by 18F-FDG PET/CT is a strong independent prognostic factor for OS after immunotherapy with ipilimumab. Combined with clinical prognostic factors, 18F-FDG PET/CT can potentially identify patient groups with markedly different prognosis. Thus, future prospective validation of 18F-FDG PET/CT for this application is warranted. Ideally, this evaluation should be performed as part of prospective randomized controlled trials to determine if tumor burden is not only a prognostic factor, but whether it can also predict the success or failure of specific therapies.
Acknowledgments
Funding: This work was funded in part by the NIH/NCI Cancer Center Support Grant (P30 CA008748).
Footnotes
Conflict of Interest: Dr. Jedd Wolchok is a consultant for: Adaptive Biotech; Advaxis; Amgen; Apricity; Array BioPharma; Ascentage Pharma;Astellas; Beigene; Bristol Myers Squibb; Celgene; Chugai; Elucida; Eli Lilly; F Star; Genentech; Imvaq; Kleo Pharma; MedImmune; Merck; Neon Therapeutics; Ono; Polaris Pharma; Polynoma; Psioxus; Puretech; Recepta; Trienza; Sellas Life Sciences; Serametrix; Surface Oncology; Syndax. Research support: Bristol Myers Squibb; Medimmune; Merck Pharmaceuticals; Genentech. Equity in: Potenza Therapeutics; Tizona Pharmaceuticals; Adaptive Biotechnologies; Elucida; Imvaq; Beigene; and Trieza. Dr. Wolfgang Weber received research support from Ipsen, Piramal, Blue Earth Diagnostics, and Bristol-Myers Squibb. He has served as a consultant for Progenics Pharmaceuticals Inc., Endocyte, Merck, Bayer, and Blue Earth Diagnostics. All other authors declare that they have no conflict of interest.
References
- 1.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. The New England journal of medicine 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robert C, Thomas L, Bondarenko I, O’Day S, Weber J, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. The New England journal of medicine 2011;364:2517–26. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 3.McDermott D, Haanen J, Chen TT, Lorigan P, O’Day S. Efficacy and safety of ipilimumab in metastatic melanoma patients surviving more than 2 years following treatment in a phase III trial (MDX010–20). Annals of oncology : official journal of the European Society for Medical Oncology 2013;24:2694–8. doi: 10.1093/annonc/mdt291. [DOI] [PubMed] [Google Scholar]
- 4.Kohn CG, Zeichner SB, Chen Q, Montero AJ, Goldstein DA, Flowers CR. Cost-Effectiveness of Immune Checkpoint Inhibition in BRAF Wild-Type Advanced Melanoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2017;35:1194–202. doi: 10.1200/jco.2016.69.6336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. The New England journal of medicine 2015;372:2006–17. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schadendorf D, Hodi FS, Robert C, Weber JS, Margolin K, Hamid O, et al. Pooled Analysis of Long-Term Survival Data From Phase II and Phase III Trials of Ipilimumab in Unresectable or Metastatic Melanoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2015;33:1889–94. doi: 10.1200/jco.2014.56.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. The New England journal of medicine 2017;377:1345–56. doi: 10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horvat TZ, Adel NG, Dang TO, Momtaz P, Postow MA, Callahan MK, et al. Immune-Related Adverse Events, Need for Systemic Immunosuppression, and Effects on Survival and Time to Treatment Failure in Patients With Melanoma Treated With Ipilimumab at Memorial Sloan Kettering Cancer Center. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2015;33:3193–8. doi: 10.1200/jco.2015.60.8448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schadendorf D, Wolchok JD, Hodi FS, Chiarion-Sileni V, Gonzalez R, Rutkowski P, et al. Efficacy and Safety Outcomes in Patients With Advanced Melanoma Who Discontinued Treatment With Nivolumab and Ipilimumab Because of Adverse Events: A Pooled Analysis of Randomized Phase II and III Trials. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2017;35:3807–14. doi: 10.1200/jco.2017.73.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beasley D The cost of cancer: new drugs show success at a steep price. Reuters; 2017.
- 11.Son SH, Kang SM, Jeong SY, Lee SW, Lee SJ, Lee J, et al. Prognostic Value of Volumetric Parameters Measured by Pretreatment 18F FDG PET/CT in Patients With Cutaneous Malignant Melanoma. Clinical nuclear medicine 2016;41:e266–73. doi: 10.1097/rlu.0000000000001205. [DOI] [PubMed] [Google Scholar]
- 12.Bastiaannet E, Hoekstra OS, de Jong JR, Brouwers AH, Suurmeijer AJ, Hoekstra HJ. Prognostic value of the standardized uptake value for (18)F-fluorodeoxyglucose in patients with stage IIIB melanoma. European journal of nuclear medicine and molecular imaging 2012;39:1592–8. doi: 10.1007/s00259-012-2182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma A, Mohan A, Bhalla AS, Sharma MC, Vishnubhatla S, Das CJ, et al. Role of Various Metabolic Parameters Derived From Baseline 18F-FDG PET/CT as Prognostic Markers in Non-Small Cell Lung Cancer Patients Undergoing Platinum-Based Chemotherapy. Clinical nuclear medicine 2018;43:e8–e17. doi: 10.1097/rlu.0000000000001886. [DOI] [PubMed] [Google Scholar]
- 14.Winther-Larsen A, Fledelius J, Sorensen BS, Meldgaard P. Metabolic tumor burden as marker of outcome in advanced EGFR wild-type NSCLC patients treated with erlotinib. Lung cancer (Amsterdam, Netherlands) 2016;94:81–7. doi: 10.1016/j.lungcan.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 15.Im HJ, Pak K, Cheon GJ, Kang KW, Kim SJ, Kim IJ, et al. Prognostic value of volumetric parameters of (18)F-FDG PET in non-small-cell lung cancer: a meta-analysis. European journal of nuclear medicine and molecular imaging 2015;42:241–51. doi: 10.1007/s00259-014-2903-7. [DOI] [PubMed] [Google Scholar]
- 16.Meignan M, Sasanelli M, Casasnovas RO, Luminari S, Fioroni F, Coriani C, et al. Metabolic tumour volumes measured at staging in lymphoma: methodological evaluation on phantom experiments and patients. European journal of nuclear medicine and molecular imaging 2014;41:1113–22. doi: 10.1007/s00259-014-2705-y. [DOI] [PubMed] [Google Scholar]
- 17.Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. Journal of nuclear medicine : official publication, Society of Nuclear Medicine 2009;50 Suppl 1:122s–50s. doi: 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schoder H, Moskowitz C. Metabolic Tumor Volume in Lymphoma: Hype or Hope? Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2016. doi: 10.1200/jco.2016.69.3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marinelli B, Espinet-Col C, Ulaner GA, McArthur HL, Gonen M, Jochelson M, et al. Prognostic value of FDG PET/CT-based metabolic tumor volumes in metastatic triple negative breast cancer patients. American journal of nuclear medicine and molecular imaging 2016;6:120–7. [PMC free article] [PubMed] [Google Scholar]
- 20.Salavati A, Duan F, Snyder BS, Wei B, Houshmand S, Khiewvan B, et al. Optimal FDG PET/CT volumetric parameters for risk stratification in patients with locally advanced non-small cell lung cancer: results from the ACRIN 6668/RTOG 0235 trial. European journal of nuclear medicine and molecular imaging 2017;44:1969–83. doi: 10.1007/s00259-017-3753-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pak K, Cheon GJ, Nam HY, Kim SJ, Kang KW, Chung JK, et al. Prognostic value of metabolic tumor volume and total lesion glycolysis in head and neck cancer: a systematic review and meta-analysis. Journal of nuclear medicine : official publication, Society of Nuclear Medicine 2014;55:884–90. doi: 10.2967/jnumed.113.133801. [DOI] [PubMed] [Google Scholar]
- 22.Warner AB, Postow MA. Combination Controversies: Checkpoint Inhibition Alone or in Combination for the Treatment of Melanoma? Oncology (Williston Park, NY) 2018;32:228–34. [PubMed] [Google Scholar]
- 23.Joseph RW, Elassaiss-Schaap J, Kefford RF, Hwu WJ, Wolchok JD, Joshua AM, et al. Baseline Tumor Size Is an Independent Prognostic Factor for Overall Survival in Patients With Melanoma Treated With Pembrolizumab. Clinical cancer research : an official journal of the American Association for Cancer Research 2018. doi: 10.1158/1078-0432.ccr-17-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]