Abstract
Heart failure (HF) is a clinical syndrome characterized by impaired ability of the heart to fill or eject blood. HF is rather prevalent and it represents the foremost reason of hospitalization in the United States. The costs linked to HF overrun those of all other causes of disabilities, and death in the United States and all over the developed as well as the developing countries which amplify the supreme significance of its prevention. Protein kinase (PK) A plays multiple roles in heart functions including, contraction, metabolism, ion fluxes, and gene transcription. Altered PKA activity is likely to cause the progression to cardiomyopathy and HF. Thus, this review is intended to focus on the roles of PKA and PKA-mediated signal transduction in the healthy heart as well as during the development of HF. Furthermore, the impact of cardiac PKA inhibition/activation will be highlighted to identify PKA as a potential target for the HF drug development.
Keywords: Heart failure, Cardiac hypertrophy, Cardiac muscle, PKA, PKA inhibitors
Introduction
The heart is no bigger than the size of a human adult fist, yet, this small organ beats more than two and a half billion times in an average lifetime without ever pausing to rest, in order to provide the contractile power needed for life. This remarkable muscle can remodel histologically, and cope functionally through various regulatory mechanisms. Often only when these mechanisms are no longer sufficiently adapting, the cardiac output that is sufficient to fulfill the body’s need for oxygen, the disease manifests and the patient is diagnosed with HF. Many different underlying pathological conditions can result in HF including hypertension, ischemia, diabetes, idiopathic cardiomyopathy, congenital cardiovascular defects, and valvular diseases. However, the most common etiologies of HF are the coronary artery diseases and myocardial infarction (MI) (1).
HF affects more than 5 million adult Americans and this number is expected to increase to be > 8 million in 2030 (2,3). Fifty percent of patients with HF are readmitted to the hospital within 6 months of discharge (4) and half of them die within 5 years of diagnosis (2). In 2011, 1 in 9 death certificates (284.388 deaths) in the United States mentioned HF. HF was the underlying cause in 58.309 of those deaths. The total estimated cost for HF in 2012 was $30.7 billion, and of this total 68% was attributable to direct medical costs (5). Estimates show that by 2030 the total cost of HF will increase almost 127% from 2012 to roughly be $69.7 billion, which means approximately $244 for every American adult (5). In fact, the costs linked to HF overrun those of all other causes of disabilities and death in the United States and all over the developed as well as the developing countries which amplify the supreme significance of its prevention (6).
Among the molecular alterations occurring during HF development that result in aggravation of the disease are the changes in protein kinase (PK) activities. Autophosphorylation, oxidative modifications, and interaction with non-enzymatic proteins are examples of these changes that can occur specifically during HF, as reviewed by Lorenz K, et al. (7). This makes the PKs an attractive target for therapeutic intervention.
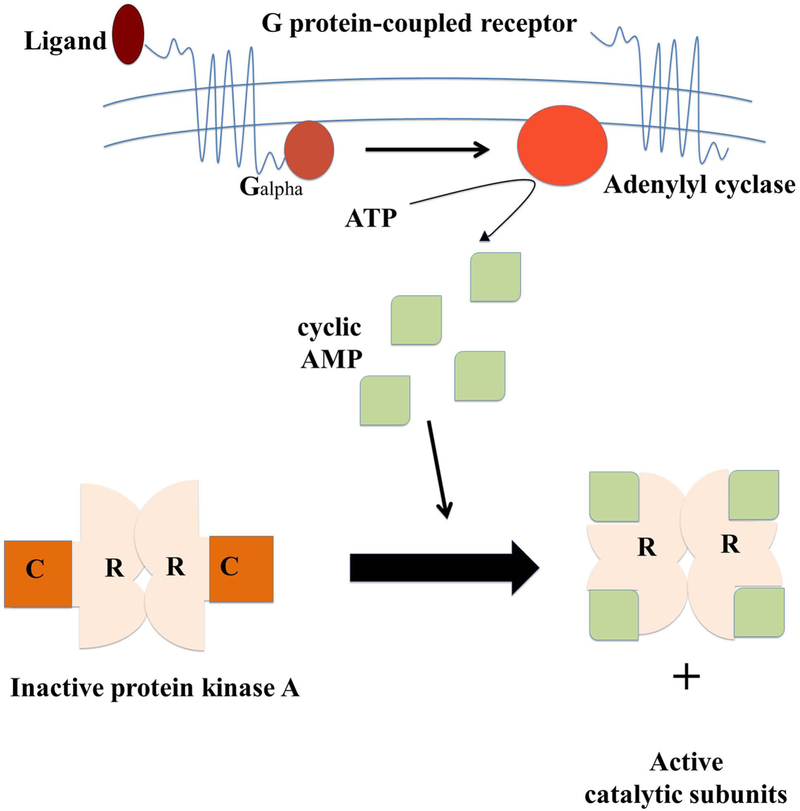
One of these kinases is the PKA (cAMP-dependent protein kinase), a kinase that is strongly implicated in the progression of HF and other cardiac diseases. PKA is a serine/threonine kinase that is activated by cyclic adenosine monophosphate (cAMP). It is composed of two regulatory subunits and two catalytic subunits. There are 4 isoforms of the regulatory subunits (RIα, RIβ, RIIα, RIIβ) and 3 isoforms of the catalytic subunits (Cα, Cβ, Cγ), each of which has different patterns of tissue expression and subcellular localization (8,9). PKA is considered to be the most common downstream effector system for cAMP. In the absence of cAMP PKA is a heterotetramer of two identical catalytic subunits (PKA-C) and two identical regulatory subunits (PKA-R). However, in the presence of cAMP the regulatory subunits bind to cAMP and the catalytic subunits are released from the holoenzyme, allowing phosphorylation of target substrates. This process involves binding of an extracellular ligand to a G protein-coupled receptor, which through G proteins regulates one of several isoforms of the adenylyl cyclase leading to the generation of cAMP (Figure 1).
Figure 1.
Activation and inactivation mechanism of cAMP-dependent protein kinase A (PKA). When a G protein-coupled receptor is activated by its extracellular ligand, a conformational change is induced in the receptor, catalyzes the conversion of ATP into cyclic adenosine monophosphate (cAMP) increasing cAMP levels. Four cAMP molecules are required to activate a single PKA enzyme. This is done by two cAMP molecules binding to each of the two regulatory subunits (R) on a PKA enzyme causing the subunits to detach exposing the two (now activated) catalytic subunits (C). Next the catalytic subunits can go on to phosphorylate other proteins.
PKA plays multiple roles in heart function regulation including, contraction, metabolism, ion fluxes, and gene transcription. Altered PKA activity is likely to cause the progression to cardiomyopathy and HF. Thus, this review is intended to focus on the roles of PKA, and PKA-mediated signal transduction in the healthy heart as well as during the development of HF. Furthermore, the impact of cardiac PKA inhibition/activation will be highlighted to identify PKA as a potential target for the HF drug development.
Protein Kinase A (PKA) in Healthy and Failing Heart
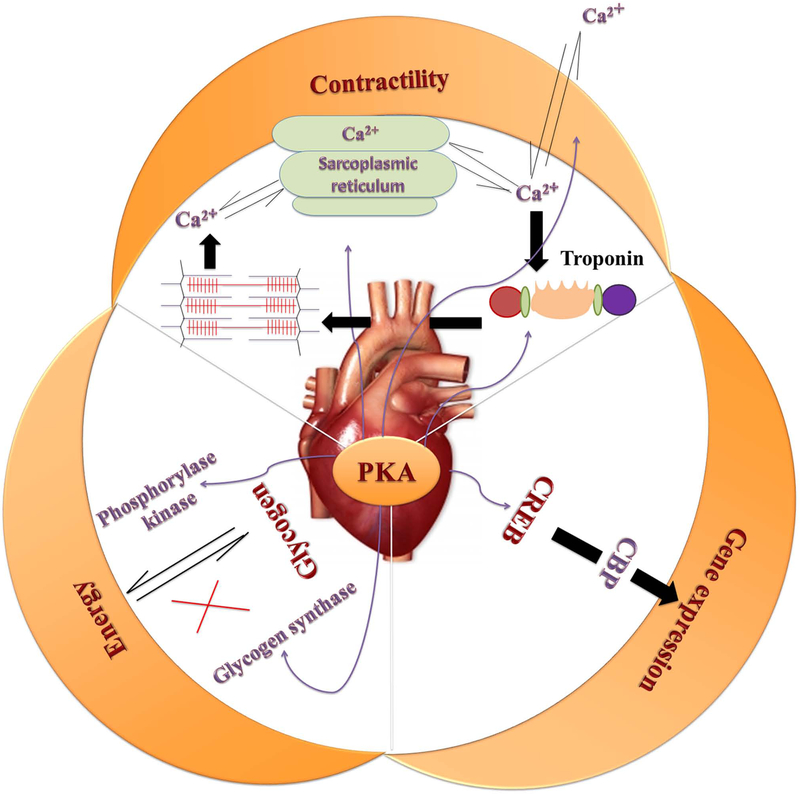
Normally, PKA plays multiple roles in the heart function. Phosphorylation of PKA in cardiomyocytes regulates many processes including metabolism, transcription of numerous genes, ion fluxes, and contraction (Figure 2) (10). Regarding metabolism and energy production in muscle cells, both phosphorylation of phosphorylase kinase by PKA as well as calcium ions released from the sarcoplasmic reticulum during muscle contraction (11–13) play an important role in stimulating glycogen breakdown into free glucose. Muscle cells generally breakdown glycogen to provide energy during bursts of activity (14). In the meanwhile, phosphorylation of glycogen synthase, a key enzyme in glycogenesis, by PKA (15,16) leads to its deactivation and inhibits conversion of glucose into glycogen. Also, binding of norepinephrine to the β-adrenergic receptors (βAR) in the heart activates the cAMP-PKA pathway, leading to the phosphorylation of multiple target proteins (17,18). PKA phosphorylates and activates the transcription factor cAMP regulatory element binding proteins (CREB), which subsequently stimulates gene transcription (19,20). This allows interaction with the co-activator CREB binding protein, which in turn binds to components of the basal transcriptional machinery and affects the transcriptional function (21–23). Additionally, PKA has several substrates in the cardiomyocytes that influence contractility in response to activated βAR signaling. Activation of PKA-RIIα by βAR signaling phosphorylates many of the target proteins involved in the excitation-contraction (E–C) coupling mechanism such as, cardiac troponin I (cTnI), cardiac myosin binding protein C (cMyBPC), phospholamban (PLB), L-type calcium channel, phosphodiesterase (PDE)4D3, CREB and the ryanodine receptor (RyR2) to modify their function and/or activity (24–26). PKA increases the Ca2+current, SR Ca2+uptake and release, SR Ca2+content, and the dissociation of Ca2+ from the myofilaments which facilitate the contraction and relaxation of the heart through phosphorylation of these and other related substrates (Figure 3) (27,28). In this context, It has been documented that abnormal handling of Ca2+ at any of these steps could lead to cardiac dysfunction and HF (29).
Figure 2.
Role of protein kinase A (PKA) in normal heart function. Phosphorylation of PKA in cardiomyocytes regulates many processes including metabolism, transcription of numerous genes such as transcription factor cAMP regulatory element binding proteins (CREB), ion fluxes and contraction.
Figure 3.
Role of activated Protein kinase A (PKA) in the excitation-coupling mechanism and cardiac contraction and relaxation. When PKA is activated by β-adrenergic receptors (βAR) signaling it phosphorylates many components increasing intracellular Ca2+ such as L-type calcium channel, ryanodine receptor (RyR2), and cardiac myosin binding protein (cMyBP). These phosphorylated components in turn accelerate the kinetics of cross-bridge cycling and the heart contracts faster and stronger. Whereas, phosphorylation of phospholamban (PLB) by PKA increases sarcoplasmic reticulum (SR) Ca2+uptake and typically results in enhanced cardiac relaxation. Similarly, PKA phosphorylates troponin I (cTnI), which decreases myofilaments Ca2+ sensitivity and accelerates relaxation as well.
In HF, deregulated Ca2+ fluxes as well as Ca2+ utilization due to altered PKA have been reported (30–32). For example, several reports have shown that in failing human hearts PLB phosphorylation as well as the SR Ca2+ were reduced resulting in decreased Ca2+ affinity of the SR Ca2+ pump and calcium current ICa-triggered Ca2+-induced Ca2+-release from the SR Ca2+stores (33–38). Since PLB is phosphorylated by PKA (39,40), PKA-PLB interaction is proposed to be a potential target for drug development in the treatment of HF (41). In addition, phosphorylation of cMyBP, a downstream target of PKA, has been shown to be decreased in patients with atrial fibrillation, hypertrophic cardiomyopathy, and HF (42–44). Likewise, decreased expression of PKA regulatory subunits RI and RII in HF decreases the affinity of the troponin complex for Ca2+, increases baseline myofibrillar Ca2+ sensitivity resulting in cardiomyopathy in humans, due to reduced phosphorylation of cTnI and other targets (31).
On the other hand, activation of PKA has been shown to have a synergistic effect on PKC-induced stimulation of Raf-1 and mitogen-activated protein kinases in rat cardiomyocytes (45,46), which are involved in the development of cardiac hypertrophy (47). Moreover, alterations in muscle-specific A-kinase anchoring protein (AKAP) was found to affect the onset of cardiac hypertrophy (48). In view of the fact that AKAP binds to PKA regulatory subunits such as RIIβ in order to regulate its interactions and its downstream targets, it is more likely support the involvement of PKA in the development of cardiac hypertrophy. Consistent with that finding, PKA under the influence of AKAP-Lbc, an AKAP, phosphorylates the protein tyrosine phosphatase Shp2 (PTPN11) at Thr73 and Ser189, which was found to be contributing to β-AR-induced cardiomyocyte hypertrophy (49). Also, Wang J, et al. (30) have shown that the activity and protein level of PKA in HF is significantly increased compared to non-failing hearts. Increased PKA activity could lead to hyperphosphorylation of downstream targets such as RyRs resulting in loss of E–C coupling gain, both in failing human hearts (50) and in transgenic mice with dilated cardiomyopathy, arrhythmia, and sudden death (32). In this latter study, prolonged activation of PKA results also in hyperphosphorylation of PLB (32), yet, there are contradictory reports concerning hyperphosphorylation of PLB as we mentioned above (33–38).
Impact of Activating and Deactivating Protein Kinas A (PKA) Signaling Pathway on the Development of Cardiomyopathy
Table 1 summarizes past studies on the effect of different PKA inhibitors on the heart. Altered PKA signaling has been found to be involved in a number of physiological problems leading to hypertrophy (48). Since hypertrophy increases the risk of HF, prevention or reversal of this maladaptive phenotype has thus been proposed to treat HF (51). In this regard, Enns LC, et al. (52) have found that deletion of RIIβ regulatory subunit of PKA resulted in a cardio-protective effect against age-related pathologies, including cardiac hypertrophy and cardiac dysfunction. Similarly, C57/BL6J male mice lacking the PKA catalytic Cβ subunits have been found to resist cardiac hypertrophy induced by angiotensin II (53). In another study, using a PDE4 novel activator, UCR1C, was found to inhibit nuclear PKA activity and attenuate cardiomyocyte hypertrophy (54). Beside, Huang T-S, et al. (55) found that inhibition of PKA activity using H89 significantly inhibited thyrotropin-induced expression of HMG-CoA reductase, a known regulator of the expression of some HF marker proteins, such as β-myosin heavy chain and brain natriuretic peptide (56). Astoundingly, this latter study indicated for the first time the involvement of PKA signaling pathway in the development of HF associated with hypothyroidism (55). Likewise, increased Adrenomedullin levels in patients with HF, hypertension, and MI (57–60) have been linked to increased PKA activation (61). Inhibiting the PKA by KT5720 effectively attenuated the negative ionotropic and lusitropic effects of adrenomedullin and improved cardiac function (62). Moreover, HF-induced myocardial depression was suggested to be mediated, at least in part, by inducible nitric oxide (NO) synthase (63–69). KT5720, a PKA inhibitor, has been reported to block the interleukin-1β-induced NO formation, potentially reducing the effect of damage caused by NO synthase formation and preventing HF (70). Furthermore, both PKA inhibitors, H89 and KT5720, exhibited a protective effect against NO-induced cytotoxicity in H9c2 cardiac cells (71). It has also been found that H89 partially inhibited the proliferation of T cells when used before stimulation with β1-Adrenoreceptor autoimmune antibodies (72). Given that the activation of T lymphocytes as well as increased inflammatory cytokines have been reported to be involved in the development of dilated cardiomyopathy and chronic HF (73,74) it may be concluded that inhibition of PKA could play an important role in attenuation of dilated cardiomyopathy and prevention of HF. Notably, prolonged exposure to PKA activators was found to stimulate the production of intracellular reactive oxygen species, which in turn significantly impaired the HERG K+ channel function, a critical regulator of cardiac action potential repolarization (75–77), possibly leading to electrical disturbance in failing hearts (78,79). These effects were efficiently prevented by PKA inhibitor, H89 (79). Likewise, cAMP/PKA pathway through stimulation of β2-AR was demonstrated to inhibit the rectifier potassium current (Ikr) and resulted in action potential prolongation in ventricular myocytes of guinea pigs with HF. These inhibitory effects were fully prevented by intracellular application of Rp-cAMPS, competitive inhibitor of cAMP-dependent protein kinases, and partly attenuated by PKA inhibitor (80). Furthermore, PKA inhibition has been revealed to decrease cell death occurring in I/R and HF (81–86), and hence exerts protective effect against I/R injury (87–90). In an experimentally-induced ischemia model in rabbits, PKA inhibitor, H89, reversed the phosphorylation and kinetic properties of cytochrome C oxidase and thus protected the heart against ischemic injury (91). Inhibition of PKA has also been shown to have a great impact on improving cardiac function after MI. A PKA specific inhibition gene named PKI-GFP was reported to block the β-adrenergic agonist–induced myocyte death and improve cardiac function after MI (92). Interestingly, inhibition of PKA in this latter study was superior to a β1-blocker, metoprolol, in improving cardiac function, which suggests that selective inhibition of PKA could be an effective therapy in HF.
Table 1.
Different protein kinase A (PKA) inhibitors and their effects on heart function
| Inhibitor | Model | Pathway/Target | Outcomes | Reference |
|---|---|---|---|---|
| H89 | Humans (The IgG fractions from β1-AA -positive DCM patients and corresponding receptor agonists were added to rats myocytes) | Inhibit proliferation of T cells induced by β1-AA | May attenuates development of HF due to dilated DCM | (72) |
| (RV trabeculae muscles from non-failing and failing hearts | Determine the ex-vivo effect of PKA inhibitor on ktr | No significant effect | (97) | |
| (LV trabeculae muscles from non-failing and failing hearts) | Investigate the ex-vivo effect of PKA inhibitor on developed force and kinetics | No significant effect | (98) | |
| H89 | Isolated Rabbit hearts (Langendorff preparation) | Reverse the phosphorylation and kinetic properties of cytochrome C oxidase | Protects heart against ischemic heart injury | (91) |
| H89 | Rats (In vivo treatment then ventricle was isolated) Mice (In vivo treatment then ventricle was isolated) H9c2 cardiac cells | Inhibit TSH-induced expression of HMGCR and BNP | May attenuate TSH-induced HF | (55) |
| H89 | HEK293 cells | Inhibited ROS production by PKA | Reserve the HERG K+ channel function which plays a critical role in cardiac AP repolarization | (79) |
| H89 | Isolated rat hearts Rats myocytes H9c2 cardiac cells | Blunt the phosphorylation and assembly of the proteasome | Impairment of proteasome activity may contribute to the progression of cardiac dysfunction and I/R | (94) |
| H89 and KT5720 | H9c2 cardiac cells | Block the IL-1 induced NO-formation | Protective effect against NO-induced cytotoxicity | (71) |
| KT5720 | Rabbit papillary muscles | Inhibit the negative ionotropic and lusitropic effects of ADM | Protect the heart against the deleterious effects of highly activated PKA by ADM in case of HF, hypertension, and MI | (62) |
| KT5720 and Rp-cAMPs | Guinea pig ventricular myocytes | Prevent the inhibitory effect of β2-AR stimulation on Ikr | Prevent delay in cardiac repolarization | (80) |
| KT5720 | Isolated rat hearts Rat myocytes H9c2 cardiac cells | Inhibit GHRH | Prevent GHRH induced protective benefits on cardiac performance in I/R injury | (87) |
| PKI-GFP | Mice (In vivo treatment) AFVMs | Inhibit β-A agonists-induced myocyte death | Improve cardiac function after MI | (92) |
| UCR1C | Neonatal rat ventricular myocytes | Inhibit nuclear PKA activity | Attenuate cardiomyocyte hypertrophy | (54) |
β1- ADM, adrenomedullin; ADM, Adrenomedullin; AFVMs, Adult feline ventricular myocytes; AP, Action potential; β1-AA, β1- Adrenoreceptor autoimmune antibodies; β2-AR, β2 adrenergic receptor; BNP, Brain natriuretic peptide; DCM, Diastolic cardiomyopathy; GHRH, Growth hormone releasing hormone; HERG K+, Human ether-a-go-go-related gene potassium; HF, heart failure; HMGCR, 3-hydroxy-3-methyl-glutaryl-CoA reductase; Ikr, Rectifier K+ channel; IL-1, interleukin-1; I/R, Ischemic/Reperfusion; ktr, rate of tension redevelopment MI, myocardial infarction; NO, nitric oxide; PKA, protein kinase A; ROS, Reactive oxygen species; TSH, Thyrotropin.
At variance with the above mentioned studies, Ha CH, et al. (93) reported that PKA attenuates angiotensin II-induced rat cardiomyocyte hypertrophy through inhibiting histone deacetylase 5 pathway. In addition, NO-induced cardiomyocytes apoptosis was suggested to be protected by PDE4 inhibitor, roflumilast, via dual activation of the PKA and Epac pathways (88). Finally, PKA inhibitors were shown to prevent the growth hormone releasing hormone-induced protective benefits on cardiac performance in I/R injury (87). Similarly, PKA has also been shown to enhance the phosphorylation and assembly of the proteasome in-vivo, and this effect was blunted by administration of PKA inhibitor, H89 (94). Of note, it has been reported that impairment of proteasome activity may contribute to the progression of cardiac dysfunction and I/R (95,96). Our own recent data on ex vivo human cardiac trabeculae showed that PKA inhibition by the H-89 did not significantly affect the rate of tension redevelopment (ktr) at either L90 (90% of optimal length) or at Lopt (optimal length) (97). Not only that, inhibition of PKA also could not significantly affect either contractile force or kinetics parameters of isolated muscles, despite the fact that this inhibitor was used at a concentration higher than the reported IC50s and Kis. However, several factors such as selectivity, concentration, and treatment time, which are related to this PKA inhibitor that is used in our experiments according to previous studies still require further exploration (98).
Conclusion and Future Directions
In conclusion, PKA is a key regulator of heart function in health and disease. Although inconsistency exists in literature about the exact role of PKA in the development of cardiomyopathy most of these reports show PKA inhibition as a potential target in the treatment of cardiac hypertrophy, cardiac dilation, I/R, MI and HF. Yet, the vast majority of studies have been done in isolated cardiomyocytes or under other in-vitro settings (Table 1). Therefore, it is required to validate the results of these studies in-vivo in both small and large animal models. Further validation of the effect of PKA inhibitors in human heart diseases through clinical trials is a prerequisite before final approval of these drugs as therapies in HF. Now, this can be feasibly accomplished through taking advantage of new development in drug delivery system with the practicability of selective and direct drug targeting to the heart (99).
Heart failure represents the foremost reason of hospitalization in the United States.
PKA is a key regulator of heart function in health and disease.
Most of the studies show PKA as a promising target for heart failure drug development.
It is required to validate the results of all studies in-vivo in both animal and human.
Selectivity, concentration, and treatment time of PKA inhibitors should be considered.
Acknowledgments
Funding was supported by NIH RC1HL099538 and NIH R01HL113084 (to PMLJ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors have no conflicts to disclose
References
- 1.Gheorghiade M, Bonow RO. Chronic heart failure in the United States a manifestation of coronary artery disease. Circulation 1998;97:282–289. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics−−2014 update: a report from the American Heart Association. Circulation 2014;129:e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heidenreich PA. Placing a Value on New Technologies. Circulation 2013;127:2375–2377. [DOI] [PubMed] [Google Scholar]
- 4.Krumholz HM, Chen Y-T, Wang Y, et al. Predictors of readmission among elderly survivors of admission with heart failure. Am Heart J 2000;139:72–77. [DOI] [PubMed] [Google Scholar]
- 5.Heidenreich PA, Albert NM, Allen LA, et al. Forecasting the Impact of Heart Failure in the United States A Policy Statement From the American Heart Association. Circulation: Heart Failure 2013;6:606–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosamond W, Flegal K, Friday G, et al. Heart Disease and Stroke Statistics-2007 Update. Circulation 2006. [DOI] [PubMed] [Google Scholar]
- 7.Lorenz K, Stathopoulou K, Schmid E, et al. Heart failure-specific changes in protein kinase signalling. Pflügers Archiv-European Journal of Physiology 2014;466:1151–1162. [DOI] [PubMed] [Google Scholar]
- 8.Seasholtz AF, Gamm DM, Ballestero RP, et al. Differential expression of mRNAs for protein kinase inhibitor isoforms in mouse brain. Proc Natl Acad Sci USA 1995;92:1734–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brandon EP, Idzerda RL, McKnight GS. PKA isoforms, neural pathways, and behaviour: making the connection. Curr Opin Neurobiol 1997;7:397–403. [DOI] [PubMed] [Google Scholar]
- 10.Walsh DA, Van Patten SM. Multiple pathway signal transduction by the cAMP-dependent protein kinase. The FASEB Journal 1994;8:1227–1236. [DOI] [PubMed] [Google Scholar]
- 11.Brushia RJ, Walsh DA. Phosphorylase kinase: the complexity of its regulation is reflected in the complexity of its structure. Front Biosci 1999;4:D618–D41. [DOI] [PubMed] [Google Scholar]
- 12.Cohen P The role of protein phosphorylation in neural and hormonal control of cellular activity. 1982. [DOI] [PubMed]
- 13.Heilmeyer LM. Molecular basis of signal integration in phosphorylase kinase. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research 1991;1094:168–174. [DOI] [PubMed] [Google Scholar]
- 14.Kollberg G, Tulinius M, Gilljam T, et al. Cardiomyopathy and exercise intolerance in muscle glycogen storage disease 0. N Engl J Med 2007;357:1507–1514. [DOI] [PubMed] [Google Scholar]
- 15.Huang T-S, Krebs EG. Amino acid sequence of a phosphorylation site in skeletal muscle glycogen synthetase. Biochem Biophys Res Commun 1977;75:643–650. [DOI] [PubMed] [Google Scholar]
- 16.Proud CG, Rylatt DB, Yeaman SJ, et al. Amino acid sequences at the two sites on glycogen synthetase phosphorylated by cyclic AMP-dependent protein kinase and their dephosphorylation by protein phosphatase-III. FEBS letters 1977;80:435–442. [DOI] [PubMed] [Google Scholar]
- 17.Molinoff PB. α-and β-Adrenergic Receptor Subtypes. Drugs 1984;28:1–15. [DOI] [PubMed] [Google Scholar]
- 18.Buxton I, Brunton LL. Beta-adrenergic receptor subtypes and subcellular compartmentation of cyclic AMP and cyclic AMP-dependent protein kinase in rabbit cardiomyocytes. Biochem Int 1985;11:137–144. [PubMed] [Google Scholar]
- 19.Boutillier A, Barthel F, Roberts J, et al. Beta-adrenergic stimulation of cFOS via protein kinase A is mediated by cAMP regulatory element binding protein (CREB)-dependent and tissue-specific CREB-independent mechanisms in corticotrope cells. J Biol Chem 1992;267:23520–23526. [PubMed] [Google Scholar]
- 20.Roseboom PH, Klein DC. Norepinephrine stimulation of pineal cyclic AMP response element-binding protein phosphorylation: primary role of a beta-adrenergic receptor/cyclic AMP mechanism. Mol Pharmacol 1995;47:439–449. [PubMed] [Google Scholar]
- 21.Meinkoth JL, Alberts AS, Went W, et al. Signal transduction through the cAMP-dependent protein kinase. Reversible Protein Phosphorylation in Cell Regulation: Springer 1993. pp. 179–86. [DOI] [PubMed] [Google Scholar]
- 22.Goldman PS, Tran VK, Goodman RH. The multifunctional role of the co-activator CBP in transcriptional regulation. Recent Prog Horm Res 1996;52:103–119;discussion 19–20. [PubMed] [Google Scholar]
- 23.Sassone-Corsi P Coupling gene expression to cAMP signalling: role of CREB and CREM. Int J Biochem Cell Biol 1998;30:27–38. [DOI] [PubMed] [Google Scholar]
- 24.Keely S Activation of cAMP-dependent protein kinase without a corresponding increase in phosphorylase activity. Res Commun Chem Pathol Pharmacol 1977;18:283–290. [PubMed] [Google Scholar]
- 25.Solaro R, Moir A, Perry S. Phosphorylation of troponin I and the inotropic effect of adrenaline in the perfused rabbit heart 1976. [DOI] [PubMed]
- 26.Yoshida A, Takahashi M, Imagawa T, et al. Phosphorylation of ryanodine receptors in rat myocytes during β-adrenergic stimulation. Journal of biochemistry 1992;111:186–190. [DOI] [PubMed] [Google Scholar]
- 27.Dash R, Kadambi VJ, Schmidt AG, et al. Interactions between phospholamban and β-adrenergic drive may lead to cardiomyopathy and early mortality. Circulation 2001;103:889–896. [DOI] [PubMed] [Google Scholar]
- 28.Bers DM. Cardiac excitation–contraction coupling. Nature 2002;415:198–205. [DOI] [PubMed] [Google Scholar]
- 29.Epstein FH, Katz AM. Cardiomyopathy of overload: a major determinant of prognosis in congestive heart failure. N Engl J Med 1990;322:100–110. [DOI] [PubMed] [Google Scholar]
- 30.Wang J, Liu X, Arneja AS, et al. Alterations in protein kinase A and protein kinase C levels in heart failure due to genetic cardiomyopathy. Can J Cardiol 1999;15:683–690. [PubMed] [Google Scholar]
- 31.Zakhary DR, Moravec CS, Stewart RW, et al. Protein kinase A (PKA)-dependent troponin-I phosphorylation and PKA regulatory subunits are decreased in human dilated cardiomyopathy. Circulation 1999;99:505–510. [DOI] [PubMed] [Google Scholar]
- 32.Antos CL, Frey N, Marx SO, et al. Dilated cardiomyopathy and sudden death resulting from constitutive activation of protein kinase A Circ Res 2001;89:997–1004. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt U, Hajjar RJ, Kim CS, et al. Human heart failure: cAMP stimulation of SR Ca2+-ATPase activity and phosphorylation level of phospholamban. Am J Physiol 1999;277:H474–H480. [DOI] [PubMed] [Google Scholar]
- 34.Schwinger RH, Münch G, Bölck B, et al. Reduced Ca 2+-sensitivity of SERCA 2a in failing human myocardium due to reduced serin-16 phospholamban phoshorylation. J Mol Cell Cardiol 1999;31:479–491. [DOI] [PubMed] [Google Scholar]
- 35.Dash R, Frank KF, Carr AN, et al. Gender influences on sarcoplasmic reticulum Ca 2+-handling in failing human myocardium. J Mol Cell Cardiol 2001;33:1345–1353. [DOI] [PubMed] [Google Scholar]
- 36.Piacentino V, Weber CR, Chen X, et al. Cellular basis of abnormal calcium transients of failing human ventricular myocytes. Circ Res 2003;92:651–658. [DOI] [PubMed] [Google Scholar]
- 37.Hasenfuss G, Reinecke H, Studer R, et al. Relation between myocardial function and expression of sarcoplasmic reticulum Ca (2+)-ATPase in failing and nonfailing human myocardium. Circ Res 1994;75:434–442. [DOI] [PubMed] [Google Scholar]
- 38.Schwinger RH, Böhm M, Schmidt U, et al. Unchanged protein levels of SERCA II and phospholamban but reduced Ca2+ uptake and Ca2+-ATPase activity of cardiac sarcoplasmic reticulum from dilated cardiomyopathy patients compared with patients with nonfailing hearts. Circulation 1995;92:3220–3228. [DOI] [PubMed] [Google Scholar]
- 39.Phrommintikul A, Chattipakorn N. Roles of cardiac ryanodine receptor in heart failure and sudden cardiac death. Int J Cardiol 2006;112:142–152. [DOI] [PubMed] [Google Scholar]
- 40.Mattiazzi A, Hove-Madsen L, Bers DM. Protein kinase inhibitors reduce SR Ca transport in permeabilized cardiac myocytes. Am J Physiol 1994;267:H812–H20. [DOI] [PubMed] [Google Scholar]
- 41.Schmitt JP, Ahmad F, Lorenz K, et al. Alterations of phospholamban function can exhibit cardiotoxic effects independent of excessive sarcoplasmic reticulum Ca2+-ATPase inhibition. Circulation 2009;119:436–444. [DOI] [PubMed] [Google Scholar]
- 42.El-Armouche A, Boknik P, Eschenhagen T, et al. Molecular determinants of altered Ca2+ handling in human chronic atrial fibrillation. Circulation 2006;114:670–680. [DOI] [PubMed] [Google Scholar]
- 43.Jacques AM, Messer AE, Gallon CE, et al. Myosin binding protein C phosphorylation in normal, hypertrophic and failing human heart muscle. J Mol Cell Cardiol 2008;45:209–216. [DOI] [PubMed] [Google Scholar]
- 44.Van Dijk SJ, Dooijes D, dos Remedios C, et al. Cardiac myosin-binding protein C mutations and hypertrophic cardiomyopathy haploinsufficiency, deranged phosphorylation, and cardiomyocyte dysfunction. Circulation 2009;119:1473–1483. [DOI] [PubMed] [Google Scholar]
- 45.Yamazaki T, Komuro I, Zou Y, et al. Protein Kinase A and Protein Kinase C Synergistically Activate theRaf-1 Kinase/Mitogen-activated Protein Kinase Cascade in Neonatal Rat Cardiomyocytes. J Mol Cell Cardiol 1997;29:2491–2501. [DOI] [PubMed] [Google Scholar]
- 46.Yamazaki T, Komuro I, Zou Y, et al. Norepinephrine induces the raf-1 kinase/mitogen-activated protein kinase cascade through both α1-and β-adrenoceptors. Circulation 1997;95:1260–1268. [DOI] [PubMed] [Google Scholar]
- 47.Chang S, McKinsey TA, Zhang CL, et al. Histone deacetylases 5 and 9 govern responsiveness of the heart to a subset of stress signals and play redundant roles in heart development. Molecular and cellular biology 2004;24:8467–8476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McConnachie G, Langeberg LK, Scott JD. AKAP signaling complexes: getting to the heart of the matter. Trends in molecular medicine 2006;12:317–323. [DOI] [PubMed] [Google Scholar]
- 49.Burmeister BT, Wang L, Gold MG, et al. Protein kinase A (PKA) phosphorylation of Shp2 inhibits its phosphatase activity and modulates ligand specificity. J Biol Chem 2015:jbc. M115. 642983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marx SO, Reiken S, Hisamatsu Y, et al. PKA phosphorylation dissociates FKBP12. 6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell 2000;101:365–376. [DOI] [PubMed] [Google Scholar]
- 51.Selvetella G, Hirsch E, Notte A, et al. Adaptive and maladaptive hypertrophic pathways: points of convergence and divergence. Cardiovasc Res 2004;63:373–380. [DOI] [PubMed] [Google Scholar]
- 52.Enns LC, Morton JF, Treuting PR, et al. Disruption of protein kinase A in mice enhances healthy aging. PLoS One 2009;4:e5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Enns LC, Bible KL, Emond MJ, et al. Mice lacking the Cβ subunit of PKA are resistant to angiotensin II-induced cardiac hypertrophy and dysfunction. BMC Res Notes 2010;3:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang L, Burmeister BT, Johnson KR, et al. UCR1C is a novel activator of phosphodiesterase 4 (PDE4) long isoforms and attenuates cardiomyocyte hypertrophy. Cellular Signalling 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang W, Xu J, Jing F, et al. Functional thyrotropin receptor expression in the ventricle and the effects on ventricular BNP secretion. Endocrine 2014;46:328–339. [DOI] [PubMed] [Google Scholar]
- 56.Mathiyalagan P, Chang L, Du X-J, et al. Cardiac ventricular chambers are epigenetically distinguishable. Cell Cycle 2010;9:612–617. [DOI] [PubMed] [Google Scholar]
- 57.Ishimitsu T, Nishikimi T, Saito Y, et al. Plasma levels of adrenomedullin, a newly identified hypotensive peptide, in patients with hypertension and renal failure. J Clin Invest 1994;94:2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nishikimi T, Saito Y, Kitamura K, et al. Increased plasma levels of adrenomedullin in patients with heart failure. J Am Coll Cardiol 1995;26:1424–1431. [DOI] [PubMed] [Google Scholar]
- 59.Jougasaki M, Wei C-M, McKinley LJ, et al. Elevation of circulating and ventricular adrenomedullin in human congestive heart failure. Circulation 1995;92:286–289. [DOI] [PubMed] [Google Scholar]
- 60.Yoshitomi Y, Nishikimi T, Kojima S, et al. Plasma levels of adrenomedullin in patients with acute myocardial infarction. Clin Sci (Lond) 1998;94:135–140. [DOI] [PubMed] [Google Scholar]
- 61.Nishida H, Sato T, Miyazaki M, et al. Infarct size limitation by adrenomedullin: protein kinase A but not PI3-kinase is linked to mitochondrial KCa channels. Cardiovasc Res 2008;77:398–405. [DOI] [PubMed] [Google Scholar]
- 62.Fontes-Sousa AP, Pires AL, Carneiro CS, et al. Effects of adrenomedullin on systolic and diastolic myocardial function. Peptides 2009;30:796–802. [DOI] [PubMed] [Google Scholar]
- 63.Mery P, Pavoine C, Belhassen L, et al. Nitric oxide regulates cardiac Ca2+ current. Involvement of cGMP-inhibited and cGMP-stimulated phosphodiesterases through guanylyl cyclase activation. J Biol Chem 1993;268:26286–26285. [PubMed] [Google Scholar]
- 64.Shah AM, Spurgeon HA, Sollott SJ, et al. 8-bromo-cGMP reduces the myofilament response to Ca2+ in intact cardiac myocytes. Circ Res 1994;74:970–978. [DOI] [PubMed] [Google Scholar]
- 65.Wahler GM, Dollinger SJ. Nitric oxide donor SIN-1 inhibits mammalian cardiac calcium current through cGMP-dependent protein kinase. Am J Physiol 1995;268:C45–C54. [DOI] [PubMed] [Google Scholar]
- 66.Olson RE. Myocardial metabolism in congestive heart failure. J Chronic Dis 1959;9:442–464. [DOI] [PubMed] [Google Scholar]
- 67.Weber K, Brilla CG. Pathological hypertrophy and cardiac interstitium. Fibrosis and reninangiotensin-aldosterone system. Circulation 1991;83:1849–1865. [DOI] [PubMed] [Google Scholar]
- 68.Saini HK, Xu Y-J, Zhang M, et al. Role of tumour necrosis factor-alpha and other cytokines in ischemia-reperfusion-induced injury in the heart. Exp Clin Cardiol 2005;10:213. [PMC free article] [PubMed] [Google Scholar]
- 69.Murray PA, Baig H, Fishbein MC, et al. Effects of exerimental right ventricular hypertrophy on myocardial blood flow in conscious dogs. J Clin Invest 1979;64:421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Oddis C, Simmons R, Hattler B, et al. Protein kinase A activation is required for IL-1-induced nitric oxide production by cardiac myocytes. Am J Physiol 1996;271:C429–C34. [DOI] [PubMed] [Google Scholar]
- 71.Chae H-J, Chae S-W, Kim H-R. Cyclic adenosine monophosphate inhibits nitric oxide-induced apoptosis of cardiac muscle cells in a c-Jun N-terminal kinase-dependent manner. Immunopharmacol Immunotoxicol 2004;26:249–263. [DOI] [PubMed] [Google Scholar]
- 72.Du Y, Yan L, Wang J, et al. β1-Adrenoceptor autoantibodies from DCM patients enhance the proliferation of T lymphocytes through the β1-AR/cAMP/PKA and p38 MAPK pathways. PloS one 2012;7:e52911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Noutsias M, Rohde M, Göldner K, et al. Expression of functional T‐cell markers and T‐cell receptor Vbeta repertoire in endomyocardial biopsies from patients presenting with acute myocarditis and dilated cardiomyopathy. Eur J Heart Fail 2011;13:611–618. [DOI] [PubMed] [Google Scholar]
- 74.Lindberg E, Andersson B, Hörnquist EH, et al. Impaired activation of IFN-γ+ CD4+ T cells in peripheral blood of patients with dilated cardiomyopathy. Cell Immunol 2010;263:224–229. [DOI] [PubMed] [Google Scholar]
- 75.Chiesa N, Rosati B, Arcangeli A, et al. A Novel Role for HERG K+ Channels: Spike‐Frequency Adaptation. J Physiol 1997;501:313–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang Z, Fermini B, Nattel S. Delayed rectifier outward current and repolarization in human atrial myocytes. Circ Res 1993;73:276–285. [DOI] [PubMed] [Google Scholar]
- 77.Wang Z, Fermini B, Nattel S. Rapid and slow components of delayed rectifier current in human atrial myocytes. Cardiovasc Res 1994;28:1540–1546. [DOI] [PubMed] [Google Scholar]
- 78.Yamagishi S-i, Edelstein D, Du X-l, et al. Leptin induces mitochondrial superoxide production and monocyte chemoattractant protein-1 expression in aortic endothelial cells by increasing fatty acid oxidation via protein kinase A. J Biol Chem 2001;276:25096–25100. [DOI] [PubMed] [Google Scholar]
- 79.Shu L, Zhang W, Su G, et al. Modulation of HERG K+ channels by chronic exposure to activators and inhibitors of PKA and PKC: actions independent of PKA and PKC phosphorylation. Cell Physiol Biochem 2012;32:1830–1844. [DOI] [PubMed] [Google Scholar]
- 80.Wang H, Chen Y, Zhu H, et al. Increased Response to β2-Adrenoreceptor Stimulation Augments Inhibition of IKr in Heart Failure Ventricular Myocytes. PloS one 2012;7:e46186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Freude B, Masters TN, Robicsek F, et al. Apoptosis is initiated by myocardial ischemia and executed during reperfusion. J Mol Cell Cardiol 2000;32:197–208. [DOI] [PubMed] [Google Scholar]
- 82.Scarabelli T, Stephanou A, Rayment N, et al. Apoptosis of endothelial cells precedes myocyte cell apoptosis in ischemia/reperfusion injury. Circulation 2001;104:253–256. [DOI] [PubMed] [Google Scholar]
- 83.Gottlieb RA, Burleson K, Kloner RA, et al. Reperfusion injury induces apoptosis in rabbit cardiomyocytes. J Clin Invest 1994;94:1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kajstura J, Cheng W, Reiss K, et al. Apoptotic and necrotic myocyte cell deaths are independent contributing variables of infarct size in rats. Lab Invest 1996;74:86–107. [PubMed] [Google Scholar]
- 85.Guerra S, Leri A, Wang X, et al. Myocyte death in the failing human heart is gender dependent. Circ Res 1999;85:856–866. [DOI] [PubMed] [Google Scholar]
- 86.Olivetti G, Abbi R, Quaini F, et al. Apoptosis in the failing human heart. N Engl J Med 1997;336:1131–1141. [DOI] [PubMed] [Google Scholar]
- 87.Granata R, Trovato L, Gallo MP, et al. Growth hormone-releasing hormone promotes survival of cardiac myocytes in vitro and protects against ischaemia–reperfusion injury in rat heart. Cardiovasc Res 2009;83:303–312. [DOI] [PubMed] [Google Scholar]
- 88.Kwak H-J, Park K-M, Choi H-E, et al. PDE4 inhibitor, roflumilast protects cardiomyocytes against NO-induced apoptosis via activation of PKA and Epac dual pathways. Cell Signal 2008;20:803–814. [DOI] [PubMed] [Google Scholar]
- 89.Robinet A, Hoizey G, Millart H. PI 3-kinase, protein kinase C, and protein kinase A are involved in the trigger phase of β1-adrenergic preconditioning. Cardiovasc Res 2005;66:530–542. [DOI] [PubMed] [Google Scholar]
- 90.Sichelschmidt OJ, Hahnefeld C, Hohlfeld T, et al. Trapidil protects ischemic hearts from reperfusion injury by stimulating PKAII activity. Cardiovasc Res 2003;58:602–610. [DOI] [PubMed] [Google Scholar]
- 91.Prabu SK, Anandatheerthavarada HK, Raza H, et al. Protein kinase A-mediated phosphorylation modulates cytochrome c oxidase function and augments hypoxia and myocardial ischemia-related injury. J Biol Chem 2006;281:2061–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang X, Szeto C, Gao E, et al. Cardiotoxic and cardioprotective features of chronic β-adrenergic signaling. Circ Res 2013;112:498–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ha CH, Kim JY, Zhao J, et al. PKA phosphorylates histone deacetylase 5 and prevents its nuclear export, leading to the inhibition of gene transcription and cardiomyocyte hypertrophy. Proc Natl Acad Sci USA 2010;107:15467–15472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Asai M, Tsukamoto O, Minamino T, et al. PKA rapidly enhances proteasome assembly and activity in in vivo canine hearts. J Mol Cell Cardiol 2009;46:452–462. [DOI] [PubMed] [Google Scholar]
- 95.Bulteau A-L, Lundberg KC, Humphries KM, et al. Oxidative modification and inactivation of the proteasome during coronary occlusion/reperfusion. J Biol Chem 2001;276:30057–30063. [DOI] [PubMed] [Google Scholar]
- 96.Tsukamoto O, Minamino T, Okada K-i, et al. Depression of proteasome activities during the progression of cardiac dysfunction in pressure-overloaded heart of mice. Biochem Biophys Res Commun 2006;340:1125–1133. [DOI] [PubMed] [Google Scholar]
- 97.Milani-Nejad N, Chung J-H, Canan BD, et al. Insights into length-dependent regulation of cardiac cross-bridge cycling kinetics in human myocardium. Arch Biochem Biophys 2016;601:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Saad NS, Elnakish MT, Brundage EA, et al. Assessment of PKA and PKC inhibitors on force and kinetics of non-failing and failing human myocardium. Life Sciences 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mayer CR, Bekeredjian R. Ultrasonic gene and drug delivery to the cardiovascular system. Adv Drug Deliv Rev 2008;60:1177–1192 [DOI] [PubMed] [Google Scholar]