Abstract
Background:
Many different oligosynaptic reflexes are known to originate in the lower brainstem which share phenomenological and neurophysiological similarities.
Objective:
To evaluate and discuss the differences and aberrancies among these reflexes, which are hard to discern clinically using neurophysiological investigations with the help of a case report.
Methods:
We describe the clinical and neurophysiological assessment of a young man who had a childhood history of opsoclonus-myoclonus syndrome with residual mild ataxia and myoclonic jerks in the distal extremities presenting with subacute onset total body jerks sensitive to sound and touch (in a limited dermatomal distribution), refractory to medications.
Results:
Based on clinical characteristics and insights gained from neurophysiological testing we could identify a novel reflex of caudal brainstem origin.
Conclusions:
The reflex described is likely an exaggerated normal reflex, likely triggered by a dolichoectatic vertebral arterial compression and shares characteristics of different reflexes known to originate in caudal brainstem, which subserve distinctive roles in human postural control.
Many different oligo-synaptic reflexes are known to originate from lower brainstem, such as the spino-bulbo-spinal reflex (SBS reflex), trigeminocervical reflex/head retraction reflex, and the startle reflex. They share many phenomenological similarities as they are clinically characterized by fairly symmetrical activation of the different muscles, originating most consistently in the muscles innervated by lower brainstem with a rostro-caudal propagation, making them clinically similar[1–5]. These reflexes also share many physiological similarities as they are all proposed to originate in the caudal brainstem reticular formation and are propagated up the brainstem and down the spinal cord by relatively slowly conducting efferent pathways; approximately 30 m/sec[1, 4, 6]. However, these reflexes are also different in terms of the afferent stimuli which induce them and their associations with certain neurological disorders. Differences are also noted in terms of the pattern of activation of different muscle groups (e.g. flexors vs extensors) and aberrancies in these patterns when they are abnormally exaggerated[4, 7–10]. The differences and aberrancies among these reflexes, though hard to discern clinically, can be identified using neurophysiological investigations which can provide useful pathophysiologic insights to guide treatment.
The best described of these reflexes in humans is the startle reflex, which is most extensively studied with auditory stimuli, though it can be elicited by visual, somatic or vestibular stimulation[1, 7, 11–13]. Exaggerated startle reflexes can be characterized by features such as excessive and more widespread muscle activation, excessive EMG bursts and amplitudes, lower thresholds for response and impaired habituation [1, 7, 14]. Both normal and exaggerated startle responses originate in the caudal pontine reticular nucleus and transmitted via efferent pathways which are similar to the other reflexes noted above[1, 3, 4]. SBS reflex is physiologically characterized by an early component that involves the local mono-synaptic reflex arc. The reflex further propagates along the spinal cord via two distinct pathways that have different conduction velocities. A slower conducting propriospinal pathway limited to and having reciprocal connections within the spinal cord and another faster conducting afferent pathway having a relay center in cadual brainstem reticular formation that is the site of origin of efferent pathways giving rise to the late component of the SBS reflex, similar to startle reflex. The late reflex discharges of SBS reflex are more consistently noted with stimulation of purely cutaneous nerves compared to motor/mixed nerves, suggestive of a highly specialized function of this reflex pathway involved in postural reflexes. Most of our understanding about SBS reflex comes from animal studies and very little is known about the normal and exaggerated physiology of this distinctive reflex in humans [3, 15]. Though startle and SBS reflex share certain clinical and physiological characteristics, they have distinguishing features which suggest they mediate different aspects of motor control. They are also distinct compared to those involved in the transmission of reticular and cortical myoclonus; which are common conditions in the clinical differential diagnosis[12, 16, 17]. A summary of the comparative clinical and physiological characterisitics of some of the reflexes of caudal brainstem origin and myoclonic disorders is presented in Table 1[3, 12, 17–20].
Table 1:
Summary of comparative clinical and physiologic characteristics of jerk-like movements
| Reflex Characteristics | Startle Reflex | Spino-Bulbo-Spinal | Vestibulospinal Reflex | Audiospinal Reflex | Electrical Blink Reflex | Trigeminocervical Reflex | Propriospinal Myoclonus | Reticular Myoclonus | Cortical Myoclonus |
|---|---|---|---|---|---|---|---|---|---|
| Phenomenology | Bilaterally | Total body jerk- | Postural | Facilitation of | Eye blink in | Neck withdrawal | Arrhythmic, | Spontaneous | Spontaneous |
| synchronous flexor | like movements | adjustments | ongoing | response to | and adoption of a | usually | jerks involving | jerks more | |
| response to a | beginning at the | and sway to | motor activity | electrical | defensive posture | flexor, brief | the entire body | commonly | |
| startling stimulus | level of | counteract | and | stimulation of | upon noxious | jerks | which can also | involving the | |
| cutaneous | changes in | monosynaptic | supra-orbital | stimulation in the | involving the | be evoked by | face and distal | ||
| stimulation | head and neck | spinal | branch of V1 | supra/infra | trunk, hips, | somatosensory | upper | ||
| position/ | reflexes in | division of | orbital trigeminal | knees in a | stimuli to distal | extremities | |||
| galvanic | response to | trigeminal | nerve distribution | fairly uniform | extremities | ||||
| vestibular | an auditory | nerve | pattern | ||||||
| stimulation | stimulus | ||||||||
| Reflex Physiology | Early eyelid blink | Early component | Short latency | A | Early | Early | Craniocaudal | Craniocaudal | Cortical onset |
| followed most | involving | reflex | conditioning | oligosynaptic | oligosynaptic | EMG | EMG discharge | short lasting | |
| consistently by | segmental reflex | response, | auditory | short latency | component | discharge | beginning at the | EMG bursts | |
| SCM with | followed by late | followed by a | stimulus | R1 component | mainly | usually | lower medulla | (simultaneous | |
| subsequent cranio- | component | medium | causes most | ipsilaterally | characterized by | beginning at | agonist- | ||
| caudal | mediated via | latency sway | consistently | followed by | drop in EMG | the level of | antagonist | ||
| propagation | cranio-caudal | response; | an initial | polysynaptic R2 | activity in | the | discharge) with | ||
| propagation | medium | facilitation | component | tonically active | abdominal | a cortical EEG | |||
| originating in | latency/ sway | followed by a | causing | neck muscles | muscles; | correlate on | |||
| caudal | responses can | period of | bilateral blink | (most | restricted to | back averaging. | |||
| brainstem | be influenced | inhibition of | consistently | spinal cord | |||||
| by other | the spinal | SCM), followed | propriospinal | ||||||
| sensory inputs | mono- | by late Splenius | pathways | ||||||
| synaptic | muscle activation | ||||||||
| reflex arc | mediated via polysynaptic pathways | ||||||||
| Afferent Stimulus | Auditory; can also | Cutaneous nerve | Postural | Auditory | Electrical | Noxious | Spontaneous; | Spontaneous; | Spontaneous; |
| be elicited by | stimulation | changes; | stimulation of | stimulation in | but can be | can be induced | but can be | ||
| visual, somatic and | Vestibular | supra-orbital | trigeminal nerve | induced by | by | triggered by | |||
| vestibular | stimulation | nerve | distribution in the | tactile or | somatosensory | tactile or other | |||
| stimulation | face | auditory | stimuli, touch or | somatosensory | |||||
| stimuli | muscle stretch | stimuli more | |||||||
| of distal | commonly to | ||||||||
| extremities | distal upper extremities. | ||||||||
| Pattern of Muscle activation; Flexors vs Extensors | Mainly flexors (but extensors also noted to be activated) | Mainly flexors (but extensor activation noted; mainly based on animal studies) | Adaptive response to counteract the head and neck postural changes | Both flexors and extensors | Bilateral blink | Mainly neck extensors | Mainly flexors | Both flexors and extensors | Mainly involving distal upper extremities and face (which have a large cortical representation) |
| Site of origin | Caudal pontine reticular formation (nucleus reticularlis pontis caudalis) | Medullary reticular formation | Lateral Vestibular Nucleus (medullary reticular formation) | Brainstem reticular formation | Trigeminal and facial nuclei in pons and descending tract of spinal trigeminal nucleus in medulla for R2 component | Descending tract of spinal trigeminal nucleus and spinal nucleus of accessory nerve | Limited to spinal cord (note that most cases are functional) | Medullary reticular formation (likely nucleus reticularis gigantocellularis) | Cerebral cortex |
| Velocity of conduction of bulbospinal efferent volley | ~30 m/sec | ~30 m/sec | ~30 m/sec | ~100 m/sec | NA | NA | 5–15 m/sec | > 50 m/sec | ~100 m/sec |
| EMG burst duration | >70 msec | > 70 msec | >70 msec | >70 msec | >70 msec | >70 msec | ~150–450 msec (can be longer) | <50 msec | < 50 msec |
Here we present a case of a young man who had a childhood history of opsoclonus-myoclonus syndrome with residual mild ataxia and myoclonic jerks in the extremities. He presented to us with symptoms characterized by total body jerks sensitive to sound and touch, refractory to medications.
Case Report
29-year-old, right-handed man with a childhood history of opsoclonus-myoclonus syndrome(OMS), attributed to viral encephalitis. He underwent treatment with immunosuppressive therapy with significant improvements. He was left with deficits in fine motor control, ataxia, tremors and myoclonic jerks (mainly involving his extremities), cognitive impairment and behavioral problems. However, over the last 2 years he started to develop total body jerks mainly triggered by loud noises, but also caused by touch in the back of his neck or a pat on his shoulders. The jerks were greatly disabling resulting in falls and requiring him to be accompanied by someone always for safety. He had mild spasticity, distal weakness with associated fine motor difficulties in the left upper extremity. He had left more than right dysmetria, dysdiadochokinesia, wide based gait with associated difficulties with tandem gait, and a hypoactive gag reflex. At rest his head was held tilted to the right side, slightly flexed and any sudden sound or touch in the restricted region of the back of his head/neck and between shoulder blades triggered total body jerks characterized by head retraction, backwards jerking of the right shoulder, followed by extensor posturing of the trunk which did not habituate even after multiple trials (Video 1). These jerky movements could also be elicited by passive head extension. Head retraction reflex with tapping in the mid-face was negative [5, 21]. Additionally, he had mild postural tremors with associated myoclonic intrusions which were residual and stable since his childhood OMS. He was being treated with a combination of Levetiracetam, Valproic acid and Clonazepam with suboptimal benefit.
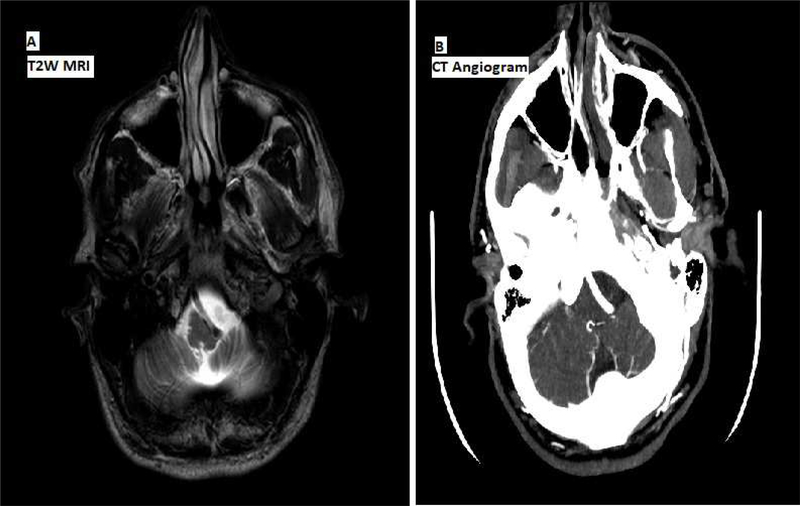
Diagnostic workup included normal routine EEG, somatosensory evoked potentials without any giant waves for the early components and normal clinical blink reflex study. Brain MRI showed dolichoectatic vertebral arterial compression at cervical-medullary junction without any evidence of myelomalacia or aneurysmal dilatation per CT angiogram (Figure 1).
Figure 1.
A) T2 weighted MRI axial view showing compression of the medulla (compressing the medullary pyramids and olives predominantly on the left side) by a dolichoectatic vertebral artery without any evidence of myelomalacia. B) CT angiogram showing brainstem vascular compression without any evidence of aneurysmal dilatation.
Poly EMG recordings were performed to assess the pattern and latency of propagation of the discharge with surface EMG recordings. The signal was amplified using Nihon Kohden amplifier, bandpass filter was set at 10 to 1000 Hz. Responses were studied to acoustic stimuli of 50 ms duration, 120 dB intensity given through headphones and to tactile stimuli to the back of the head/neck region by tapping over a surface electrode to mark the stimulus for assessment of onset latency. EMG data were reviewed, traces with artifacts rejected, and the signal was rectified and averaged from the acoustic/tactile stimuli. Data were analyzed offline using Spike software (Cambridge Electronic Designs). For the blink reflex recovery cycle, surface EMG was recorded over both orbicularis oculi muscles with electrical stimulation applied to the supraorbital nerve in the supraorbital notch with a bipolar stimulating electrode. Single and double electrical pulses were given with the following interstimulus intervals (ISI): 200ms, 300ms, 500ms, 1000ms, 3000ms. Data were rectified and area under the curve was obtained from the R2 responses. The recovery cycle was calculated as the ratio between conditioned and unconditioned R2 for each ISI.
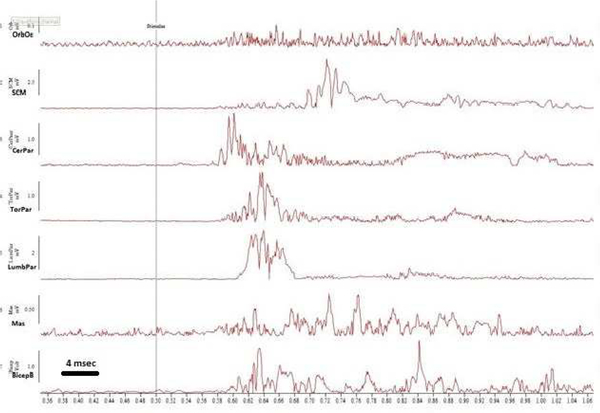
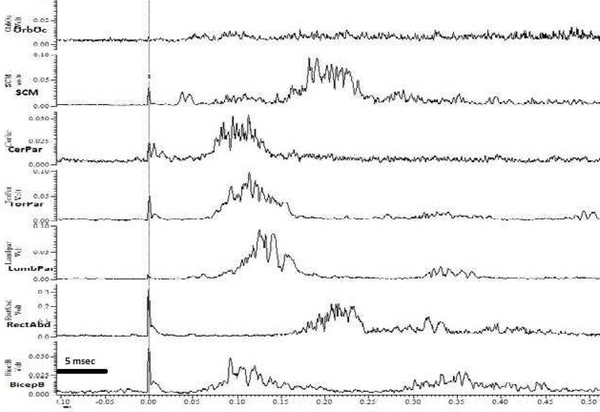
With the auditory stimulus, there was a consistent and reproducible pattern of activation beginning with the SCM, closely followed by the cervical paraspinals and the masseter with an average latency of 78 ms that propagated rostrally to the orbicularis oculi and caudally to the thoracic paraspinals and limb muscles (Figure 2a). Onset latencies and velocity of propagation were consistent with that of a startle reflex[11, 12]with no habituation, suggestive of an exaggerated startle reflex[7, 13]. However, the early EMG component in orbicularis oculi, sometimes referred to as the auditory blink was not seen, and there was a predominant extensor muscle group activation(Figure 2a)[12]. The body region for tactile induced jerks was limited to a restricted distribution involving the back of the neck and nape region. Using tactile stimuli, a similar pattern of EMG activation was noted with the most consistent onset noted in cervical paraspinal muscles with an average onset latency of 64 ms with subsequent rostro-caudal propagation similar to the auditory stimulus induced reflex. However, with tactile induced jerks there was an early small EMG component noted in SCM with an average latency around 30 ms followed by another small component with an average latency of 73 ms. Additionally, there was another major late EMG component noted in head and trunk flexors that followed the paraspinal muscles (head and trunk extensors); this SCM EMG component was noted to have an average latency of 150 ms that was followed by rectus abdominus EMG component with an average latency of 170 ms (Figure 2b). The blink reflex recovery cycle showed about 50% suppression of R2 at 200ms suggestive of some brainstem hyper-excitability.
Figure 2a.
Rectified surface EMG traces of 7 muscles to the acoustic stimulus. 1.OrbOc: Orbicularis Oculi; 2.SCM: Sternocleidomastoid;3.CerPar: Cervical Paraspinal; 4.TorPar:Thoracic paraspinal; 5.LumbPar: Lumbar Paraspinal; 6.Mas: Masseter; 7.BicepB: Biceps brachi.
Figure 2b.
Average rectified surface EMG traces of 7 muscles with tactile stimulus. 1.OrbOc: Orbicularis Oculi; 2.SCM: Sternocleidomastoid; 3.CerPar: Cervical Paraspinal; 4.TorPar:Thoracic paraspinal; 5.LumbPar: Lumbar Paraspinal; 6.RectAbd: Rectus abdominus; 7.BicepB: Biceps brachi.
Discussion
The case highlights the importance and utility of objective neurophysiological techniques in identification, localization of the site of origin and providing insights into the potential pathophysiologic processes implicated in movement disorders. Considering the childhood history of OMS, the likely etiology for the total body jerks was initially thought to be reticular myoclonus, based on similar site of pathology implicated for both these disorders, namely the caudal pontine nucleus[16, 22, 23]. On clinical examination, there were findings noted to be localizing to the medullary region (hypoactive gag reflex), which were new. Additionally, the phenomenology of the new movement disorder which was characterized by non-habituating sensitivity to sound and touch mainly in the back of the neck and nape region along with the absence of head retraction reflex was suggestive of an exaggerated startle reflex[5, 7, 9, 24, 25]. A brainstem vascular compression from a dolichoectatic vertebral artery was noted at the level of the cervical medullary junction (Figure 1) which would explain the localizing signs; however, the site of compression was further distal to the caudal pontine region implicated in the pathogenesis of reticular myoclonus and startle reflex. Neurophysiological testing revealed non-habituating reflex similar to startle based on relatively low velocity of transmission and burst duration compared to reticular myoclonus, with the implicated site of origin being in the lower brainstem considering the first muscle being activated with subsequent cranio-caudal propagation. Another clinical finding noted in this case was the new head position which the patient adopted (holding his head titled to the right and slightly flexed) and triggering of the head jerks by passive head extension which suggested a potential dynamic vascular compression [26, 27]. We did not perform poly EMG recordings with passive head extension since the jerks noted clinically were limited to the neck paraspinal musculature and did not propagate, additionally artifacts were introduced to the recordings by passive head manipulation.
Neurophysiological and clinical characteristics of the reflex noted in the current patient shares features of some of the reflexes noted above. Clinically it is characterized by non-habituating jerks to auditory and tactile stimuli (in a limited dermatomal distribution) which are similar to exaggerated startle reflex; however, predominant extensor muscle activation and the absence of the early EMG component in orbicularis oculi associated with the auditory blink are notable differences[7, 12]. Additionally the dermatomal specificity of the tactile induced jerks suggest irritability in a local reflex arc leading to an exaggerated reflex upon stimulation only at that level, which also happens to be the site of dolichoectatic vascular compresson. The latencies and pattern of activation involving the early reflexive component at the same level of tactile stimulation followed by cranio-cadual propagation involving mainly the head and trunk extensors followed by late flexor EMG activation (likely stretch/propriospinal reflex mediated) is more compatible with a SBS reflex. Although the SBS reflex also involves predominant flexor activation in quadrupedal animals, it is poorly studied in humans, and extensor motor neuron activation has also been described in SBS reflex [3, 6, 28]. Considering the latencies of onset and activation pattern, the source of this aberrant reflex in our patient is in the lower medulla, likely triggered by dynamic vascular compression, distinguishing it from a startle reflex.
Exaggerated startle reflex has been previously reported as part of OMS syndrome[29]. Vascular compression has also been implicated to cause exaggerated startle reflex[26, 27]. To the best of our knowledge, this is the first report of a novel exaggerated ‘SBS-like’ reflex in humans, likely triggered by dynamic vascular compression, which also happens to be at the level in caudal brainstem where SBS reflex has been proposed to originate, based on animal studies. The current case highlights the importance of astute clinical examination combined with detailed neurophysiological testing in the identification of movement disorders and their pathophysiologic mechanisms, which have a bearing on treatment. Further exploration of the distinguishing characteristics, functions and aberrancies of the different postural reflexes originating in the cadual brainstem reticular formation is needed to gain more insights into human postural control. The vascular compression identified in this case may be further contributing towards the aberrant brainstem hyper excitability from baseline OMS, leading to poor response to pharmacological treatment. The vascular compression noted may be potentially amenable to decompression surgery, if symptoms become more disabling[26].
Supplementary Material
Different brainstem reflexes serve distinctive roles in postural and motor control
Phenomenological and physiological differences are hard to discern clinically
We describe a novel brainstem reflex in humans using neurophysiological techniques
Reflex shares characteristics with other normal reflexes of caudal brainstem origin
Acknowledgements:
This work was supported by the NINDS intramural program (including fellowship of SM).
Footnotes
Relevant Financial Disclosures and Conflict of Interest: None (for all co-authors)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brown P, Physiology of startle phenomena. Adv Neurol, 1995. 67: p. 273–87. [PubMed] [Google Scholar]
- 2.Shimamura M, (Studies on a Motor Reflex with Its Reflex Center in the Bulbar Reticular Formation (Spino-Bulbo-Spinal Reflex). No To Shinkei, 1963. 15: p. 1165–72. [PubMed] [Google Scholar]
- 3.Shimamura M, et al. , On the Spino-Bulbo-Spinal Reflex in Dogs, Monkeys and Man. Jpn J Physiol, 1964. 14: p. 411–21. [DOI] [PubMed] [Google Scholar]
- 4.Ertekin C, Celebisoy N, and Uludag B, Trigeminocervical reflexes elicited by stimulation of the infraorbital nerve: head retraction reflex. J Clin Neurophysiol, 2001. 18(4): p. 378–85. [DOI] [PubMed] [Google Scholar]
- 5.Wartenberg R, Head retraction reflex. Calif Med, 1949. 70(5): p. 382. [PMC free article] [PubMed] [Google Scholar]
- 6.Shimamura M, [The spino-bulbo-spinal reflex system]. Shinkei Kenkyu No Shimpo, 1967. 11(3): p. 452–7. [PubMed] [Google Scholar]
- 7.Dreissen YE, et al. , Exaggerated startle reactions. Clin Neurophysiol, 2012. 123(1): p. 34–44. [DOI] [PubMed] [Google Scholar]
- 8.Shimamura M, Aoki M, and Sato T, [Averaging analysis of the spino-bulbo-spinal (SBS) reflex in man]. Nihon Seirigaku Zasshi, 1967. 29(7): p. 324–5. [PubMed] [Google Scholar]
- 9.Berger C and Meinck HM, Head retraction reflex in stiff-man syndrome and related disorders. Mov Disord, 2003. 18(8): p. 906–11. [DOI] [PubMed] [Google Scholar]
- 10.Matsumoto J, et al. , Physiological abnormalities in hereditary hyperekplexia. Ann Neurol, 1992. 32(1): p. 41–50. [DOI] [PubMed] [Google Scholar]
- 11.Brown P, Neurophysiology of the startle syndrome and hyperekplexia. Adv Neurol, 2002. 89: p. 153–9. [PubMed] [Google Scholar]
- 12.Brown P, et al. , New observations on the normal auditory startle reflex in man. Brain, 1991. 114 ( Pt 4): p. 1891–902. [DOI] [PubMed] [Google Scholar]
- 13.Brown P, et al. , The hyperekplexias and their relationship to the normal startle reflex. Brain, 1991. 114 ( Pt 4): p. 1903–28. [DOI] [PubMed] [Google Scholar]
- 14.Dreissen YE and Tijssen MA, The startle syndromes: physiology and treatment. Epilepsia, 2012. 53 Suppl 7: p. 3–11. [DOI] [PubMed] [Google Scholar]
- 15.Shimamura M, [Longitudinal conduction systems serving spinal and brainstem coordination-spino-bulbo-spinal reflex]. Nihon Seirigaku Zasshi, 1971. 33(4): p. 225–33. [PubMed] [Google Scholar]
- 16.Hallett M, et al. , Reticular reflex myoclonus: a physiological type of human post-hypoxic myoclonus. J Neurol Neurosurg Psychiatry, 1977. 40(3): p. 253–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hallett M, Chadwick D, and Marsden CD, Cortical reflex myoclonus. Neurology, 1979. 29(8): p. 1107–25. [DOI] [PubMed] [Google Scholar]
- 18.Watson SR and Colebatch JG, EMG responses in the soleus muscles evoked by unipolar galvanic vestibular stimulation. Electroencephalogr Clin Neurophysiol, 1997. 105(6): p. 476–83. [DOI] [PubMed] [Google Scholar]
- 19.Wright CG and Barnes CD, Audio-spinal reflex responses in decerebrate and chloralose anesthetized cats. Brain Res, 1972. 36(2): p. 307–31. [DOI] [PubMed] [Google Scholar]
- 20.van der Salm SM, et al. , Propriospinal myoclonus: clinical reappraisal and review of literature. Neurology, 2014. 83(20): p. 1862–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ansink BJ, Head retraction reflex. Clin Neurol Neurosurg, 1983. 85(1): p. 71–2. [DOI] [PubMed] [Google Scholar]
- 22.Matsumoto H and Ugawa Y, [Paraneoplastic opsoclonus-myoclonus syndrome--a review]. Brain Nerve, 2010. 62(4): p. 365–9. [PubMed] [Google Scholar]
- 23.Leder RM, The opsoclonus-myoclonus syndrome. A review of the literature. Bull Los Angeles Neurol Soc, 1981. 46: p. 41–50. [PubMed] [Google Scholar]
- 24.Landis C and Hunt WA, The startle pattern. 1939, New York,: Farrar & Rinehart; x, 168 p. [Google Scholar]
- 25.Tijssen MA, Padberg GW, and van Dijk JG, The startle pattern in the minor form of hyperekplexia. Arch Neurol, 1996. 53(7): p. 608–13. [DOI] [PubMed] [Google Scholar]
- 26.Salvi F, et al. , Hypertension, hyperekplexia, and pyramidal paresis due to vascular compression of the medulla. Neurology, 2000. 55(9): p. 1381–4. [DOI] [PubMed] [Google Scholar]
- 27.Gambardella A, et al. , Hyperekplexia in a patient with a brainstem vascular anomaly. Acta Neurol Scand, 1999. 99(4): p. 255–9. [DOI] [PubMed] [Google Scholar]
- 28.Shimamura M, Mori S, and Yamauchi T, Effects of spino-bulbo-spinal reflex volleys on extensor motoneurons of hindlimb in cats. J Neurophysiol, 1967. 30(2): p. 319–32. [DOI] [PubMed] [Google Scholar]
- 29.Yonekawa T, et al. , Augmented startle responses in opsoclonus-myoclonus syndrome. Brain Dev, 2011. 33(4): p. 335–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.