Abstract
Sex differences in chronic pain and alcohol abuse are not well understood. The development of rodent models is imperative for investigating the underlying changes behind these pathological states. In the present study, we investigated whether hind paw treatment with the inflammatory agent Complete Freund’s Adjuvant (CFA) could generate hyperalgesia and alter alcohol consumption in male and female C57BL/6J mice. CFA treatment led to greater nociceptive sensitivity for both sexes in the Hargreaves test, and increased alcohol drinking for males in a continuous access two-bottle choice (CA2BC) paradigm. Regardless of treatment, female mice exhibited greater alcohol drinking than males. Following a 2-hour terminal drinking session, CFA treatment failed to produce changes in alcohol drinking, blood ethanol concentration (BEC), and plasma corticosterone (CORT) for both sexes. 2-hr alcohol consumption and CORT was higher in females than males, irrespective of CFA treatment. Taken together, these findings have established that male mice are more susceptible to escalations in alcohol drinking when undergoing pain, despite higher levels of total alcohol drinking and CORT in females. Furthermore, the exposure of CFA-treated C57BL/6J mice to the CA2BC drinking paradigm has proven to be a useful model for studying the relationship between chronic pain and alcohol abuse. Future applications of the CFA/CA2BC model should incorporate manipulations of stress signaling and other related biological systems to improve our mechanistic understanding of pain and alcohol interactions.
Introduction
Alcohol provides an accessible means of self-medication for pain suffering populations, with approximately 25% of chronic pain patients consuming alcohol for symptom relief (Riley and King, 2009). Although alcohol can act as a transient pharmacological intervention for pain, excessive drinking can lead to increased risk for addiction and related heath complications (Koob and Volkow, 2010). Chronic exposure to alcohol is especially detrimental for pain, with marked increases in sensitivity after drug cessation (Boissoneault et al., 2018; Dhir et al., 2005; Dina et al., 2000, 2007; Dodds et al., 1945; Edwards et al., 2012; Fu et al., 2015; Gatch and Lal, 1999; Jochum et al., 2010; Malec et al., 1987; Riley and King, 2009; Roltsch et al., 2017; Shumilla et al., 2005; Smith et al., 2015; Wolff et al., 1942). Clinical observations have suggested important differences in how men and women consume alcohol and experience the detrimental consequences of alcohol abuse when suffering from pathological pain. Among chronic pain patients, alcohol use is more prevalent in males (Brennan et al., 2011; Egli et al., 2012; Riley and King, 2009; Wilsnack et al., 2009). Females that habitually consume alcohol, however, are more susceptible to pathological pain (Boissoneault et al., 2018). To date, preclinical investigations have failed to model similar sex-specific patterns in drinking and pain, thus providing a major hurdle to studying the mechanisms of pain-related alcohol consumption (Smith et al., 2015; Yezierski and Hansson, 2018).
To address this deficit in preclinical models, the present study examines alcohol consumption in male and female C57BL/6J mice treated with Complete Freund’s Adjuvant (CFA), an antigen emulsion containing heat-dried Mycobacterium tuberculosis. CFA was selected to model chronic inflammatory pain, since it closely mimics the time course of persistent injury (Hargreaves et al., 1988; Larson et al., 1986; Ren and Dubner, 1999). Furthermore, postoperative pain, arthritis, and related inflammatory states have exhibited a close relationship with alcohol abuse (King et al., 2012). Therefore, we hypothesized that CFA-treated mice would show increased alcohol consumption compared to uninjured mice. To test this, we exposed saline- and CFA-treated mice of both sexes to a voluntary drinking paradigm (i.e. CA2BC with 20% ethanol [EtOH]) and measured alcohol consumption across a three-week timespan. Thermal nociceptive thresholds were assessed to ensure that CFA treatment altered pain sensitivity. Blood EtOH concentration (BEC) and plasma corticosterone (CORT) levels were analyzed to assess pharmacologically relevant drinking and alcohol-related stress signaling, two possible contributors to sex differences in alcohol and pain interactions (Edwards et al., 2012; Egli et al., 2012; Fu and Neugebauer, 2008; Heilig and Koob, 2007; Koob, 2013). Taken together, these experiments aim to successfully model sex differences in pain-related alcohol drinking for mice, with the hope that it can be applied towards mechanistic investigations of pain and alcohol interactions in the future.
Materials and Methods
Animals
A total of 32 male and female C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME) arrived at 6-weeks of age and were group-housed for one week to habituate to vivarium conditions. Following habituation, mice were individually housed in ventilated Plexiglas cages and given three days of acclimation in a 12-hr reverse light/dark cycle (12am-12pm) prior to the start of the experiment. Subjects were provided ad libitum access to water and Isopro RMH 3000 chow (Prolab, St. Louis, MO) for the duration of the experiment. All procedures were approved by the University of North Carolina at Chapel Hill Institutional Animal Care and Use Committee and were in accordance with the NIH Guide for Care and Use of Laboratory Animals.
Continuous access 2-bottle choice drinking
Male (N = 16) and female (N = 16) mice were given continuous access to 20% EtOH (w/v) and tap water for three weeks. EtOH solutions were prepared with tap water and 95% EtOH (Pharmaco-AAPER, Brookfield, CT), and delivered via sipper tubes made from 50 mL plastic tubes (Nalgene), rubber stoppers (Fisher Scientific, Agawam, MA), and sipper tubes (Ancare Corp., Bellmore, NY). Prior to EtOH exposure, subjects were given three days to adapt to drinking with sipper tubes. Individually housed mice were then given access to two sipper tubes per cage: one containing 20% EtOH and the other containing tap water. For the span of three weeks, EtOH and water bottles were weighed daily three hours into the dark cycle. Bottle placement (left vs. right) was alternated weekly to control for an inherent or developed side preference. To control for non-consumption-related fluid loss, a dummy cage containing identical sipper tubes was used to subtract weekly dripped fluid values from subject fluid consumption.
Chronic inflammatory pain
Prior to EtOH exposure, subjects were given a 50 μl subcutaneous injection of saline or Complete Freund’s Adjuvant (CFA; Sigma, St. Louis, MO) in the plantar surface of the right hind paw (n = 8). Drinking experiments started three days after paw injections, around the time that CFA exhibits maximum inflammatory hyperalgesia (Pitzer et al., 2016). To verify that changes in drinking behavior resulted from differences in pain sensitivity, thermal nociceptive thresholds of saline- and CFA-treated mice were assessed one day after the last 24-hr EtOH exposure using the Hargreaves test. Mice were placed in a plexiglas chamber elevated on a glass plate (IITC Life Science Inc., Woodland Hills, CA) and habituated to the behavioral apparatus for a minimum of 30 minutes. The mid-plantar surface of saline/CFA-treated and untreated hind paws was then exposed to three heat trials each and assessed for paw withdrawal latencies (PWL). The beam intensity was set to produce basal PWLs of approximately 4–6 seconds, with a maximal value of 20 seconds to prevent excessive tissue damage. All testing was conducted with investigators blinded to the experimental conditions.
Blood ethanol and corticosterone concentrations
Following pain sensitivity testing, mice were given four days to recover and re-establish CA2BC drinking behavior in preparation for blood EtOH and corticosterone measurements. On the final drinking day, mice were sacrificed 2 hours into the dark cycle, with trunk blood immediately collected in centrifuge tubes following decapitation. Blood samples were centrifuged at 4°C for 10 min at 3000 RPM, and the plasma was separated for storage at −80°C until analysis. To measure blood EtOH concentration (BEC; mg/dl), 5 μl plasma samples were administered through a Model AM1 Alcohol Analyser (Analox Instruments Ltd., Lunenburg, MA). To measure plasma corticosterone (CORT; ng/ml), 5 μl plasma samples were processed with a commercially available colorimetric ELISA kit (Arbor Assays; Ann Arbor, MI), according to the manufacturer’s instructions, with all samples run in duplicate.
Statistical analysis
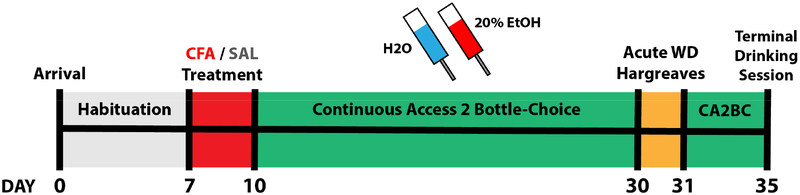
All statistical analyses were performed using Prism 6 (GraphPad Software Inc., La Jolla, CA). Analysis of drinking across sessions for saline- and CFA-treated mice (i.e. Treatment x Session for EtOH Intake, EtOH Preference Ratio, Water Intake, and Total Fluid Intake) was performed with a repeated measures analyses of variance (RM-ANOVA). Drinking values for each mouse were averaged across the three-week drinking period to compare Treatment x Sex with additional two-way ANOVAs. Differences in PWL were determined with a two-way ANOVA comparing Treatment x Paw. Post-hoc analyses with Sidak or Tukey adjustment were performed following significant main group effects. Data are reported as mean plus or minus the standard error of the mean (M ± SEM). Correlational analyses were conducted using linear regressions to assess the predictive relationship of EtOH intake and thermal nociceptive sensitivity, BEC, and CORT levels, with slopes and intercepts assessed for deviation from zero to determine statistical significance. See Fig. 1 for Experimental Timeline.
Figure 1.
Experimental timeline of CFA/CA2BC. Days are marked relative to arrival of subjects.
Results
CFA-treated mice exhibit greater alcohol drinking in a sex-dependent manner
EtOH Intake
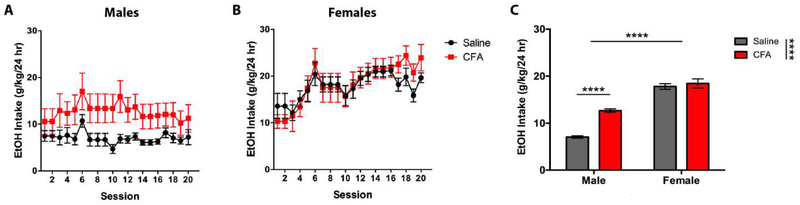
Male mice treated with CFA consumed a significantly greater amount of EtOH per day (g/kg/24hr) than saline-treated controls, while females did not exhibit any consumption differences across treatments (Fig. 2). A RM-ANOVA (Treatment x Session) revealed a main effect for Treatment [F(14, 266) = 4.768, p = 0.0465] and Session [F(19, 266) = 1.656, p = 0.0437] in males (Fig. 2A), and a main effect for Session [F(19, 266) = 9.470, p < 0.0001] in females (Fig. 2B), but no significant interaction for either sex. Sidak post-hoc analysis revealed no significant difference in EtOH intake between saline- and CFA-treated mice during individual drinking sessions of males or females. Mean drinking values for individual subjects were averaged across three weeks in their respective treatment and sex groupings. A two-way ANOVA (Treatment x Sex) revealed a main effect for Treatment [F(1, 76) = 26.04, p < 0.0001] and Sex [F(1, 76) = 179.6, p < 0.0001], and a significant interaction between Treatment and Sex [F(1, 76) = 16.45, p = 0.0001], where female mice exhibit greater EtOH intake than males, regardless of paw treatment (Fig. 2C). Tukey post-hoc analysis revealed a significant difference in EtOH intake between saline- and CFA-treated mice for males, but not females (Fig. 2C).
Figure 2.
Daily alcohol intake (g/kg/24 hr) of saline- or CFA-treated C57BL/6J mice over a 3-week continuous access regimen. Pain-related consumption of 20% EtOH (w/v) was assessed for (A) males (n = 8) and (B) females (n = 8) in 20 consecutive sessions. (C) Averages of daily intake were compared between saline- and CFA-treated males and females. Data are mean alcohol intake ± SEM. **** p < 0.0001 difference between groups.
EtOH Preference Ratio
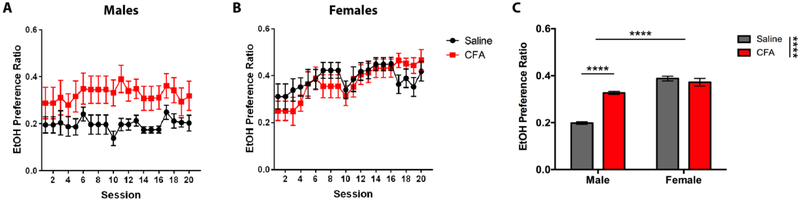
Preference for EtOH exhibited similar results, with CFA-treated male mice showing a significantly greater EtOH preference ratio than their saline-treated counterparts, while females did not exhibit a preference difference across treatments (Fig. 3). A RM-ANOVA (Treatment x Session) revealed a main effect for Treatment [F(1, 14) = 6.777, p = 0.0208] in males (Fig. 3A) and Session [F(19, 266) = 5.297, p < 0.0001] in females (Fig. 3B), but no significant interaction for either sex. Sidak post-hoc analysis showed no significant difference in EtOH preference ratio between saline- and CFA-treated mice during individual drinking sessions of males or females. A two-way ANOVA (Treatment x Sex) revealed a main effect for Treatment [F(1, 76) = 29.88, p < 0.0001] and Sex [F(1, 76) = 128.6, p < 0.0001], and a significant interaction between Treatment and Sex [F(1, 76) = 48.43, p < 0.0001], where EtOH preference ratio was greater in females than males for both treatments (Fig. 3C). Tukey post-hoc analysis showed a significant difference in EtOH preference ratio between saline- and CFA-treated mice for males, but not females (Fig. 3C).
Figure 3.
Daily alcohol preference ratio of saline- or CFA-treated C57BL/6J mice over a 3-week continuous access regimen. Pain-related alcohol preference was assessed for (A) males (n = 8) and (B) females (n = 8) in 20 consecutive sessions. (C) Averages of daily intake were compared between saline- and CFA-treated males and females. Data are mean alcohol preference ± SEM. **** p < 0.0001 difference between groups.
Water Intake
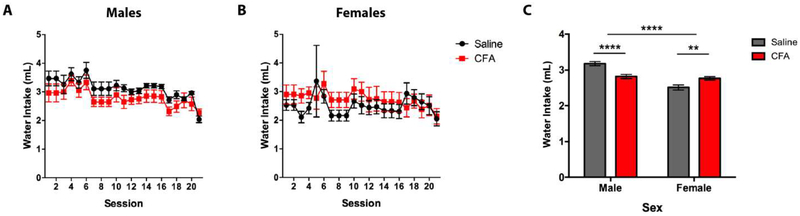
CFA-treated mice showed sex-specific effects for water intake, with CFA-treated males consuming less water than saline-treated controls, and CFA-treated females consuming more water than controls (Fig. 4). A RM-ANOVA (Treatment x Session) revealed a main effect for Session [F(19, 266) = 5.661, p = < 0.0001] in males (Fig. 4A), no main effect for Treatment or Session in females (Fig. 4B), and no significant interaction for either sex. Sidak post-hoc analysis showed no significant difference in water intake between saline- and CFA-treated mice during individual drinking sessions of males or females. A two-way ANOVA (Treatment x Sex) revealed a main effect for Sex [F(1, 76) = 37.04, p < 0.0001] but not Treatment [F(1, 76) = 0.8203, p = 0.3680], and a significant interaction between Treatment and Sex [F(1, 76) = 27.19, p < 0.0001] (Fig. 4C). Tukey post-hoc analysis showed a significant difference in water intake between saline- and CFA-treated mice for both males and females (Fig. 4C).
Figure 4.
Daily water intake (mL) of saline- or CFA-treated C57BL/6J mice over a 3-week continuous access regimen. Pain-related consumption of water was assessed for (A) males (n = 8) and (B) females (n = 8) in 20 consecutive sessions. (C) Averages of daily intake were compared between saline- and CFA-treated males and females. Data are mean alcohol preference ± SEM. ** and **** correspond to p < 0.005 and p < 0.0001 difference between groups.
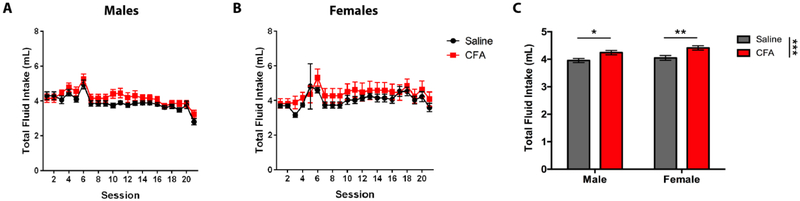
Total Fluid Intake
CFA-treated mice exhibit significantly greater total fluid intake than their saline-treated counterparts in both sexes (Fig. 5). A RM-ANOVA (Treatment x Session) revealed a main effect for Session in males [F(19, 266) = 13.35, p < 0.0001] (Fig. 5A) and females [F(19, 266) = 4.514, p < 0.0001] (Fig. 5B), but no significant interaction for either sex. Sidak post-hoc analysis demonstrated no significant difference in total fluid intake between saline- and CFA-treated mice during individual drinking sessions of males or females. A two-way ANOVA (Treatment x Sex) revealed a main effect for Treatment [F(1, 76) = 16.38, p = 0.0001], but no main effect for Sex [F(1, 76) = 2.742, p = 0.1018] or significant interaction between Treatment and Sex [F(1, 76) = 0.2358, p = 0.6287] (Fig. 5C). Tukey post-hoc analysis showed a significant difference in total fluid intake between saline- and CFA-treated mice for both males and females (Fig. 5C).
Figure 5.
Daily alcohol and water intake (mL) of saline- or CFA-treated C57BL/6J mice over a 3-week continuous access regimen. Pain-related consumption of fluids was assessed for (A) males (n = 8) and (B) females (n = 8) in 20 consecutive sessions. (C) Averages of daily intake were compared between saline- and CFA-treated males and females. Data are mean fluid intake ± SEM. *, **, and *** correspond to p < 0.05, p < 0.005, and p < 0.0005 difference between groups.
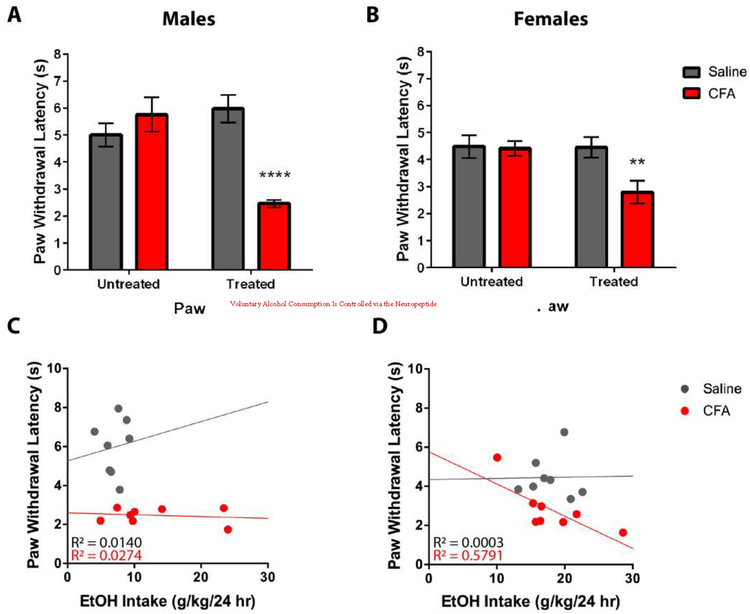
CFA-treated mice exhibit higher sensitivity to thermal nociception in both sexes
Male and female mice treated with CFA exhibit higher sensitivity to thermal nociception, as indicated by lower PWLs, when compared to saline-treated mice (Fig. 6). A RM-ANOVA (Treatment x Paw) exhibited a main effect for Treatment [F(1, 14) = 7.010, p = 0.0191] and Paw [F(1, 14) = 8.155, p = 0.0127], and a significant Treatment x Paw interaction [F(1, 14) = 27.46, p = 0.0001] in males (Fig. 6A), whereas a RM-ANOVA (Treatment x Paw) exhibited a main effect for Paw [F(1, 14) = 11.00, p = 0.0051], but not Treatment [F(1, 14) = 3.276, p = 0.0918], and a significant Treatment x Paw interaction [F(1, 14) = 10.15, p = 0.0066] in females (Fig. 6B). Sidak post-hoc analysis revealed a significant difference in PWLs between saline- and CFA-treated mice for treated paws, but not untreated paws, of both males (Fig. 6A) and females (Fig. 6B). To assess the relationship between pain and drinking, a linear regression was conducted for thermal nociceptive sensitivity of the treated paw as a predictor of EtOH intake. For males, saline-treated mice exhibited a non-significant regression equation of [F(1, 6) = 0.0855, p = 0.7798] with R2 of 0.0140 (Fig. 6C), whereas CFA-treated mice exhibited a non-significant regression equation of [F(1, 6) = 0.1691, p = 0.6952] with R2 of 0.0274 (Fig. 6C). For females, saline-treated mice exhibited a non-significant regression equation of [F(1, 6) = 0.0018, p = 0.9674] with R2 of 0.0003 (Fig. 6D), whereas CFA-treated mice exhibited a significant regression equation of [F(1, 6) = 8.254, p = 0.0283] with R2 of 0.5791 (Fig. 6D).
Figure 6.
Pain sensitivity of saline- and CFA-treated C57BL/6J mice was tested 24 hrs after last alcohol exposure. Using the Hargreaves test, paw withdrawal latency (s) was measured in response to a thermal nociceptive stimulus for substance-treated and untreated paws of (A) male and (B) female mice. Pain sensitivity was correlated with average daily alcohol intake in (C) males and (D) females. Data are mean paw withdrawal latency ± SEM. ** and **** correspond to p < 0.005 and p < 0.0001 difference between groups respectively.
CFA does not alter blood ethanol concentration and plasma corticosterone
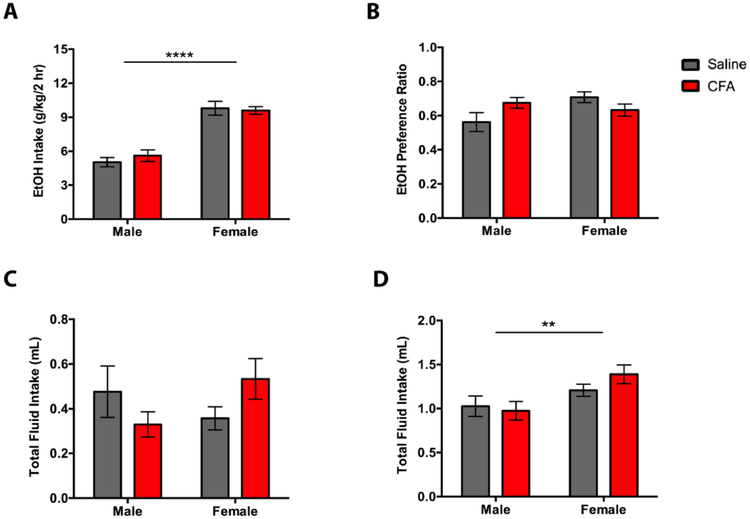
Female mice given 2-hr access to EtOH exhibit greater alcohol and total fluid consumption than males (Fig. 7). A two-way ANOVA (Treatment x Sex) for 2-hr EtOH intake revealed a main effect for Sex [F(1, 28) = 83.19, p < 0.0001], but not Treatment [F(1, 28) = 0.1574, p = 0.6945], and no significant Treatment x Sex interaction [F(1, 28) = 0.6611, p = 0.4230] (Fig. 7A). Tukey post-hoc analysis showed greater 2-hr EtOH intake in female mice, but no significant difference in drinking between saline- and CFA-treated mice (Fig. 7A). A two-way ANOVA (Treatment x Sex) for 2-hr EtOH preference ratio revealed no main effect for Treatment [F(1, 28) = 0.2239, p = 0.6397] or Sex [F(1, 28) = 1.673, p = 0.2064], and a significant Treatment x Sex interaction [F(1, 28) = 5.599, p = 0.0251] (Fig. 7B). Tukey post-hoc analysis showed no significant difference in EtOH preference ratio between treatments or sex (Fig. 7B). A two-way ANOVA (Treatment x Sex) for 2-hr water intake revealed no main effect for Treatment [F(1, 28) = 0.0328, p = 0.8574] or Sex [F(1, 28) = 0.2640, p = 0.6114], and no significant Treatment x Sex interaction [F(1, 28) = 3.800, p = 0.0613] (Fig. 7C). Tukey post-hoc analysis showed no significant difference in EtOH preference ratio between treatments or sex (Fig. 7C). A two-way ANOVA (Treatment x Sex) for 2-hr total fluid intake revealed a main effect for Sex [F(1, 28) = 8.726, p = 0.0063], but not Treatment [F(1, 28) = 0.4069, p = 0.5288], and no significant Treatment x Sex interaction [F(1, 28) = 1.341, p = 0.2566] (Fig. 7D). Tukey post-hoc analysis showed higher 2-hr total fluid intake in female mice, but no significant difference in drinking between saline- and CFA-treated mice (Fig. 7D).
Figure 7.
Averages of (A) alcohol intake (g/kg/2 hr), (B) preference ratio, (C) water intake (g/kg/2 hr), and (D) total fluid intake (mL) in saline- or CFA-treated C57BL/6J mice during a final 2-hr drinking session. Pain-related consumption of fluids was assessed for males (n = 8) and females (n = 8) on the 25th session of a continuous access regimen. Data are mean fluid intake/preference ratio ± SEM. Data are mean fluid intake/preference ratio ± SEM. ** and **** correspond to p < 0.005 and p < 0.0001 difference between groups respectively.
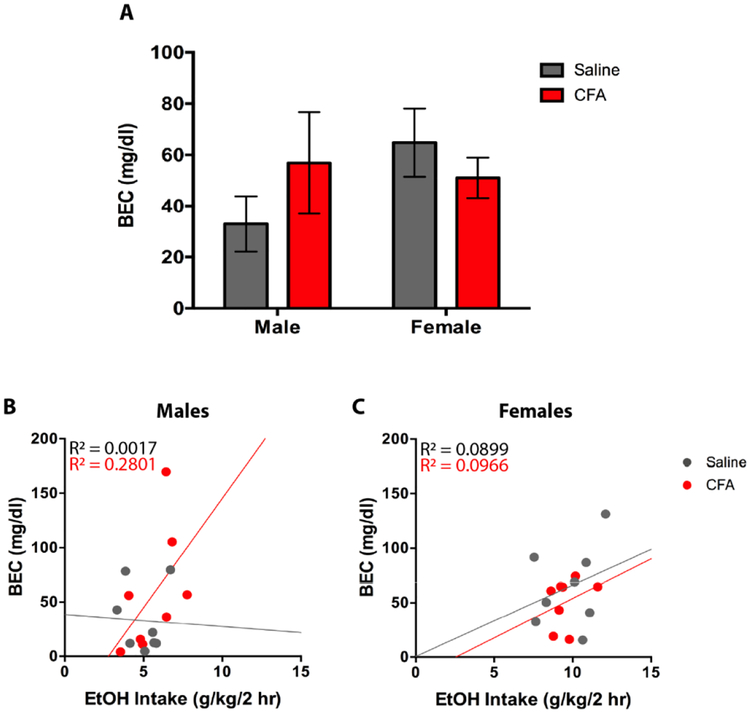
Following 2-hr access to EtOH, plasma samples were collected to measure BEC and CORT levels. For BEC, a two-way ANOVA (Treatment x Sex) revealed no main effect for Treatment [F(1, 28) = 0.1365, p = 0.7146] and Sex [F(1, 28) = 0.8967, p = 0.3518], and no Treatment x Sex interaction [F(1, 28) = 1.890, p = 0.1801] (Fig. 8A). Tukey post-hoc analysis revealed no significant difference in BEC between treatments or sex (Fig. 8A). To assess the relationship between drinking and BEC amongst paw treatment groups, a linear regression was conducted for 2-hr EtOH intake as a predictor of BEC. For males, saline-treated mice exhibited a non-significant regression equation of [F(1, 6) = 0.0104, p = 0.9220] with R2 of 0.0017 (Fig. 8B), whereas CFA-treated mice exhibited a non-significant regression equation of [F(1, 6) = 2.334, p = 0.1774] with R2 of 0.2801 (Fig. 8B). For females, saline-treated mice exhibited a non-significant regression equation of [F(1, 6) = 0.5927, p = 0.4706] with R2 of 0.0899 (Fig. 8C), whereas CFA-treated mice exhibited a non-significant regression equation of [F(1, 6) = 0.6417, p = 0.4536] with R2 of 0.0966 (Fig. 8C).
Figure 8.
(A) Blood ethanol content (mg/dl) of saline- and CFA-treated C57BL/6J mice was measured 2-hrs into the final alcohol exposure day in male and female mice. BEC was correlated with average daily alcohol intake in (B) males and (C) females. Data are mean BEC ± SEM.
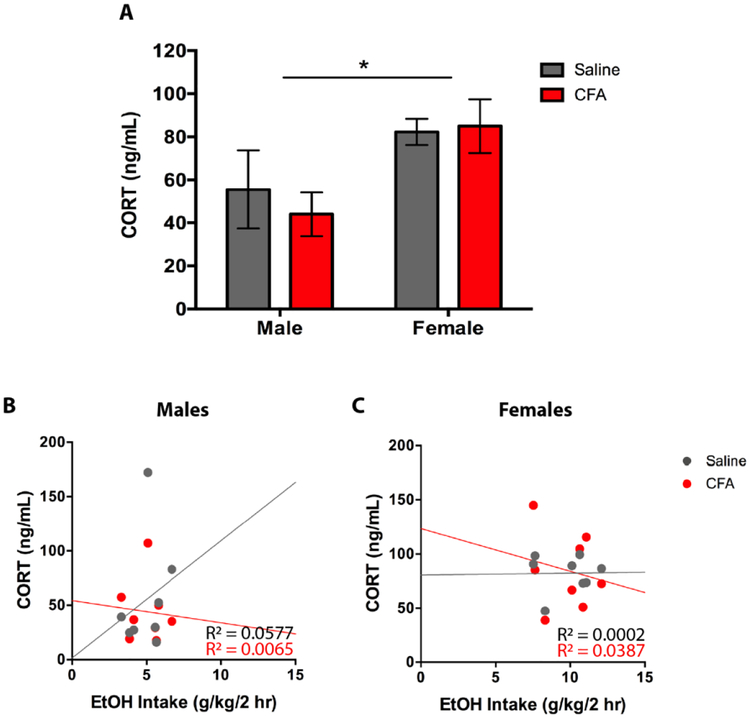
For CORT, a two-way ANOVA (Treatment x Sex) revealed a main effect for Sex [F(1, 28) = 7.298, p = 0.0116], but not Treatment [F(1, 28) = 0.1243, p = 0.7270], and no Treatment x Sex interaction [F(1, 28) = 0.3202, p = 0.5760] (Fig. 9A). Tukey post-hoc analysis revealed greater CORT levels in female mice, but no significant difference between saline- and CFA-treated mice (Fig. 9A). To assess the relationship between drinking and CORT amongst paw treatment groups, a linear regression was conducted for 2-hr EtOH intake as a predictor of CORT. For males, saline-treated mice exhibited a non-significant regression equation of [F(1, 6) = 0.3674, p = 0.5666] with R2 of 0.0577 (Fig. 9B), whereas CFA-treated mice exhibited a non-significant regression equation of [F(1, 6) = 0.0396, p = 0.8487] with R2 of 0.0065 (Fig. 9B). For females, saline-treated mice exhibited a non-significant regression equation of [F(1, 6) = 0.0016, p = 0.9691] with R2 of 0.0002 (Fig. 9C), whereas CFA-treated mice exhibited a non-significant regression equation of [F(1, 6) = 0.2297, p = 0.6487] with R2 of 0.0387 (Fig. 9C). Taken together, these findings suggest a weak relationship between 2-hr EtOH intake and BEC/CORT.
Figure 9.
(A) Plasma corticosterone (CORT [ng/mL]) of saline- and CFA-treated C57BL/6J mice was measured 2-hrs into the final alcohol exposure day in male and female mice. CORT levels were correlated with average daily alcohol intake in (B) males and (C) females. Data are mean CORT ± SEM. * p < 0.05 difference between groups.
Discussion
In this study, we describe sex differences in alcohol drinking following the induction of inflammatory pain. Male CFA-treated mice consumed more alcohol and exhibited higher preference for the drug than saline-treated controls, while female CFA-treated mice only showed greater water intake than controls. Characteristic of C57BL/6J mice with continuous access to high concentrations of alcohol (Jury et al., 2017; Middaugh et al., 1999; Smith et al., 2015), drinking levels differed between sexes, with female mice exhibiting greater EtOH consumption and preference than male mice, regardless of saline or CFA treatment. These findings suggest that chronic inflammatory pain increases alcohol drinking in males, despite higher overall drinking in females. Similar clinical observations have been made in chronic pain patients, with men more commonly self-medicating with alcohol than women (Riley and King, 2009). This pain-related alcohol consumption is greater in males throughout the lifetime, with its peak at early adulthood (Brennan et al., 2011; Riley and King, 2009; Wilsnack et al., 2009). By pairing CFA treatment and CA2BC drinking, our model was able to replicate these human patterns of voluntary drinking in injured young adult male mice, providing evidence that chronic inflammatory pain can potentiate alcohol drinking in a sex-specific manner for rodents.
Although this finding is reflective of clinical reports, it is notably inconsistent with another study on CFA-treated mice that found no effect on pain-related alcohol consumption (Smith et al., 2015). The discrepancy between findings may be due to differences in experimental design. For example, escalating concentrations of alcohol were utilized in Smith et al. (2015), while fixed concentrations were used in the present study. Mice given escalating concentrations had CFA administered while being exposed to a lower percentage of alcohol (10%), so consumption may not have produced comparable analgesia to that of a fixed high concentration of alcohol (20%). Theoretically, this would result in a higher dynamic range of alcohol consumption for the fixed concentration paradigm, which could explain why there are more pronounced effects of pain-induced drinking in the present study. Alternatively, the use of differently aged mice (i.e. 30–33 weeks old in Smith et al. [2015] versus 7–10 weeks old in the present study) could have altered alcohol consumption as well, since younger rodents drink more than fully developed adults (Holstein et al., 2011; Schramm-Sapyta et al., 2014; Vetter-O’Hagen et al., 2009). Divergences in pain severity following CFA treatment could also explain the inconsistency in findings. Male mice treated with a high volume of CFA (i.e. 50 μl in the present study) exhibited increased alcohol consumption relative to saline-treated controls, while low volume (i.e. 10 μl in Smith et al. [2015]) exposure did not alter consumption relative to pre-CFA levels. This suggests that high and low volumes of CFA treatment result in different severities of pain, which can then determine the extent of changes observed with alcohol drinking. If pain severity were to contribute to these consumption differences, a comparison of nociceptive sensitivity should reveal higher pain sensitivity in CFA-treated mice from the present study compared to those in Smith et al. (2015). The use of different behavioral assays at non-parallel time points and a lack of parallel control groups (i.e. Pre-CFA mice in Smith et al. [2015] versus saline-treated mice in the present study), however, makes it difficult to make a direct comparison. Future studies should examine how the severity of chronic inflammatory pain contributes to sex-specific patterns of drinking with more uniform measures of thermal and mechanical nociception and control groups. Determining the extent that alcohol concentration and age can alter pain-related drinking may further clarify any remaining drinking differences between the two studies.
We additionally showed that female C57BL/6J mice exhibit greater alcohol consumption than males, an observation that is common among female rodents exposed to voluntary drinking paradigms (Blanchard and Glick, 2002; Cailhol and Mormède, 2001; Chester et al., 2006; Doremus et al., 2005; Jury et al., 2017; Lancaster et al., 1996; Lê et al., 2001; Middaugh et al., 1999; Smith et al., 2015; Truxell et al., 2007). Moreover, our female drinking data matches the maximal average consumption values previously reported in C57BL/6J mice undergoing continuous access to ≥ 10% alcohol (Jury et al., 2017; Middaugh et al., 1999; Smith et al., 2015). With such high baseline alcohol consumption, it is possible that saline-treated females have already reached maximal drinking in our study, so the addition of pain would not increase drinking in females. Although this ceiling effect is possible, it is unlikely, considering that daily EtOH intake in females was around 10–12 g/kg during the first four sessions and escalated to 20–25 g/kg by the last session. If pain were able to drive alcohol drinking in females, early session EtOH intake should have increased up to two times in CFA-treated mice, but that is not the case. Therefore, sex differences in basal alcohol consumption were not likely to prevent pain-related drinking increases for females.
Differences in inflammatory response and alcohol analgesia may provide a better explanation for sex-specific patterns in drinking. CFA and related inflammatory agents cause female rodents to develop hyperalgesia more rapidly than males, with females being less prone to attenuation of inflammation by alcohol and other analgesic drugs (Alfonso-Loeches et al., 2013; Coleman and Crews, 2018; Cook and Nickerson, 2005; Pascual et al., 2017). Thus, it is possible that CFA-treated females are less susceptible to escalated alcohol consumption relative to saline-treated controls because the drug provides inadequate analgesia to promote further drinking. Although not measured in the present study, ongoing investigations with this model should assess the potential impact of sex differences in alcohol analgesia and its effects on pain-related drinking.
Sex-specific patterns in pain-driven consumption were observed for water as well. CFA-treated males and females exhibited divergent water consumption patterns, with CFA-treated males reducing intake and CFA-treated females increasing intake relative to their saline-treated counterparts. The consumption of more alcohol and less water by CFA-treated males likely reflects increases in alcohol preference following repeated drug exposure. Although reductions in water intake is not characteristic for escalations in voluntary alcohol consumption (Griffin, 2014; Hwa et al., 2011; Tordoff and Bachmanov, 2008), it has been reported that mice treated with pro-inflammatory agents exhibit increased alcohol intake and reduced water intake when given access to CA2BC (Biesmans et al., 2013; Blednov et al., 2011; Stein et al., 1988). This is consistent with parallel studies showing that the impairment of pro-inflammatory cytokines reduces alcohol preference (Blednov et al., 2005). In agreement with the literature, our findings show that increases in alcohol intake and decreases in water intake are expected outcomes of heightened inflammatory states.
Inflammation-driven changes in alcohol and water consumption were previously reported for both male and female mice (Blednov et al., 2011), in contrast to the CFA-treated females in the present study that consumed comparable amounts of alcohol and more water than saline-treated controls. This increase in water intake following CFA treatment may be due to alterations in body water composition, a major contributor to sex differences in alcohol consumption (Ely, 1999). Considering the high level of alcohol consumption in females, it is possible that inflammation enhances the diuretic properties of alcohol and the higher rate of fluid loss is compensated for by consuming greater quantities of water (Crow, 1968; Strauss et al., 1950). This effect may be restricted to CFA and other long-lasting inflammatory agents, since acute inflammation reduces water intake, but attenuates over time in female mice (Melgar et al., 2007). The time course of water intake from acute to chronic inflammation has yet to be investigated for both sexes, but this knowledge will likely be important for understanding how relative fluid intake determines alcohol drinking behavior.
Sex differences in gustation may also explain the observed drinking patterns, since intake profiles differ by fluid type. It has been theorized that C57BL/6J mice are more motivated to consume alcohol for its sweet tasting components (Alexander A. Bachmanov et al., 1996; Blednov et al., 2007), but sex does not appear to influence preference for saccharin or alcohol (A. A. Bachmanov et al., 1996). However, it is still possible that inflammation alters the gustatory components of alcohol relative to water in a sex-specific manner (Kumarhia et al., 2016; Steen et al., 2010). More research assessing the relationship of sex, inflammation, gustation, and fluid intake is required to understand CFA-mediated effects on alcohol and water intake in CA2BC.
Three weeks after paw treatment, pain testing revealed that CFA-treated mice had higher thermal nociceptive sensitivity than saline-treated controls for both males and females. Considering the timing of the test, it is unclear whether this difference in sensitivity reflects the contributions of pain to drinking or drinking to pain. This is an important distinction, as cessation of alcohol intake following repeated drug exposure can exacerbate pain sensitivity. This phenomenon, commonly referred to as EtOH withdrawal-induced hyperalgesia (EIH), has been reported in mice (Dhir et al., 2005; Smith et al., 2016), as well as rats (Dina et al., 2000, 2007; Edwards et al., 2012; Fu et al., 2015; Gatch and Lal, 1999; Malec et al., 1987; Roltsch et al., 2017; Shumilla et al., 2005) and humans (Boissoneault et al., 2018; Dodds et al., 1945; Jochum et al., 2010; Riley and King, 2009; Wolff et al., 1942), using a variety of alcohol exposure models. In our CA2BC paradigm, it is unclear whether there were pathological shifts in sensitivity following three weeks of alcohol exposure. The lack of sensitivity differences following increased drinking in CFA-treated males, along with the non-significant regression of Drinking x Pain suggests that the extent of alcohol consumption had little effect on predicting pain sensitivity (and vice versa) in all groups. This result is similar to that of other continuous access experiments in mice, which also failed to produce EIH (Smith et al., 2015). The exception to this finding is CFA-treated females: despite comparable drinking patterns to saline-treated controls, CFA-treated females exhibited a significant negative correlation for Drinking x Pain, where injured females that drink more alcohol experience higher pain sensitivity. This result is reminiscent of clinical data showing that there is higher EIH severity and prevalence in females, with women being more likely to report significant recurrent pain and concurrent chronic pain conditions (Boissoneault et al., 2018). Despite our findings closely following predicted results from the literature, not having nociceptive sensitivity measures prior to EtOH exposure makes it impossible to conclude whether or not these data indicate a causal null effect of alcohol consumption on pain. Although a limitation in the study, it does not change our interpretation that male-specific increases in alcohol consumption follow the induction of chronic inflammatory pain. However, future experiments should be mindful of establishing baseline nociceptive sensitivity prior to the start of alcohol exposure when studying pain-related alcohol use.
Following 2 hours of CA2BC, females consumed more alcohol than males. Contrary to the increased drinking phenotype seen in CFA-treated males during 24-hr alcohol access, no treatment effects were observed. These results suggest that the terminal drinking session did not last long enough to capture peak alcohol consumption (i.e. 4+ hours into the dark cycle) (Smith et al., 2015). Although increases for alcohol drinking in the 2-hr CA2BC were specific to females, no sex effects were found for BEC. By contrast, a previous evaluation of BEC showed no sex differences for alcohol consumption after 30-minute access to 12% EtOH, but higher BECs in females (Middaugh et al., 1999). Although a common result for humans when both sexes are allowed an equal dosing of orally administered EtOH (Ammon et al., 1996; Sutker et al., 1983), such results are less common in rodents (Ho et al., 1989). This difference may be due to feeding manipulations coinciding with BEC measurements after drinking, as suggested in Middaugh et al. (1999). Although caloric mediation of alcohol consumption is well established in the literature (Rodgers et al., 1963; Rodgers, 1966; Thiele et al., 2012), sex differences in prandial metabolism and alcohol consumption are thought to be negligible, as thirst is more strongly motivating for alcohol drinking than caloric need in both sexes (Middaugh et al., 1999). Paradoxically, higher BECs in females are accompanied by enhancements in alcohol metabolism (Baraona et al., 2001; Collins et al., 1975; Mumenthaler et al., 1999), and may explain why female mice that consume a greater amount of alcohol do not produce higher BECs than males.
Similar to alcohol consumption patterns in the 2-hr CA2BC paradigm, CORT was not affected by CFA, but differed by sex. Specifically, female CORT levels were found to be higher than that of males. Higher CORT release following alcohol exposure has previously been observed in female rodents, with deletion of circulating sex steroids attenuating the difference (Rivier, 1993). However, increased levels of CORT in female mice may not be the result of the drug, as alcohol drinking does not produce differences in CORT relative to consumption of water in males or females (Finn et al., 2004). Although CFA-treated males did not drink more in the 2-hr terminal drinking session, the literature would predict that an increase in drinking after 24-hr access would still not potentiate CORT release in the males, despite showing pain-related increases in alcohol consumption. This suggests that CORT is basally higher in females, and not susceptible to changes following CFA treatment in both sexes. Previous work has found that inflammatory pain increases CORT, but this phenotype has only been observed in male rats and has yet to be investigated in combination with drinking (Butkevich et al., 2013; Harper et al., 2001; Pitcher et al., 2018), suggesting that our findings for CORT are specific to CFA/CA2BC-exposed C57BL/6J mice.
Without pre-treatment CORT levels, it is difficult to determine whether these female-specific elevations in stress are entirely due to sex differences in basal CORT. It is important to consider the alternate possibility that these changes are driven by differences in stress reactivity. It is well-established that maladaptive responses to stress promote alcohol drinking and relapse behavior (Becker et al., 2011; Keyes et al., 2012; Koob, 2001; Koob and Kreek, 2007). Previous studies have linked these behaviors to the sex-dependent expression of stress markers, with females exhibiting higher glucocorticoid levels and alcohol consumption following exposure to various stressors (Cozzoli et al., 2014; Haleem et al., 1988; Heinsbroek et al., 1991; Kudielka and Kirschbaum, 2005; Yoshimura et al., 2003). Considering that female mice exhibit a more sensitive and sustained stress response compared to males (Blanchard and Glick, 2002; Brown and Grunberg, 1995; Hermes et al., 2006; Palanza et al., 2001), it is possible that early experimental stressors such as isolated housing and paw injections increased CORT levels and alcohol consumption for female mice in the present study (Hermes et al., 2006; Lopez et al., 2011; Moriya et al., 2015). This would be contingent on early experimental stressors upregulating CORT responses for over three weeks in female mice, an improbable but untested concept. Future studies using CFA/CA2BC should more closely investigate this relationship between sex differences in stress reactivity and alcohol drinking.
In conclusion, male and female mice exhibit distinct alcohol drinking behavior when undergoing pain. Compared to saline-treated controls, CFA-treated males show greater alcohol consumption, while CFA-treated females show a closer relationship between alcohol consumption and pain sensitivity. Regardless of pain state, females exhibited higher levels of total alcohol drinking and corticosterone compared to males. These findings reflect sex-specific trends in alcohol use reported by chronic pain populations and may speak to the validity of our CFA/CA2BC model in mice. Mechanistic contributions to sex differences in pain-related alcohol drinking will be required in future applications of the model, with special attention reserved for the role of stress signaling in pain and alcohol interactions.
Highlights.
CFA treatment increases alcohol drinking for male, but not female mice in a continuous access two-bottle choice (CA2BC) paradigm.
Female mice exhibit higher levels of alcohol drinking and CORT than males, regardless of CFA treatment.
The combination of CFA treatment and the CA2BC drinking paradigm in C57BL/6J mice proves to be a useful model for studying the relationship between chronic inflammatory pain and alcohol use.
Acknowledgments
Funding Sources
Research reported in this publication was supported by the following grants from the National Institutes of Health: P60 AA011605 (TLK), R01 AA019454 (TLK), U01 AA020911 (TLK), T32 NS007431 (VHM), F31 AA027436 (VHM), T32 AA007573 (LSH), T32 GM007040 (WY), and F31 AA027129 (WY). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Cited
- Alfonso-Loeches S, Pascual M, Guerri C, 2013. Gender differences in alcohol-induced neurotoxicity and brain damage. Toxicology 311, 27–34. 10.1016/j.tox.2013.03.001 [DOI] [PubMed] [Google Scholar]
- Ammon E, Schäfer C, Hofmann U, Klotz U, 1996. Disposition and first-pass metabolism of ethanol in humans: Is it gastric or hepatic and does it depend on gender? Clin. Pharmacol. Ther 59, 503–513. 10.1016/S0009-9236(96)90178-2 [DOI] [PubMed] [Google Scholar]
- Bachmanov AA, Reed DR, Tordoff MG, Price RA, Beauchamp GK, 1996. Intake of Ethanol, Sodium Chloride, Sucrose, Citric Acid, and Quinine Hydrochloride Solutions by Mice: A Genetic Analysis. Behav. Genet 26, 563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmanov Alexander A., Tordoff MG, Beauchamp GK, 1996. Ethanol Consumption and Taste Preferences in C57BL/6ByJ and 129/J Mice. Alcohol. Clin. Exp. Res 20, 201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraona E, Abittan CS, Dohmen K, Moretti M, Pozzato G, Chayes ZW, Schaefer C, Lieber CS, 2001. Gender Differences in Pharmacokinetics of Alcohol. Alcohol. Clin. Exp. Res 25, 502–507. 10.1111/j.1530-0277.2001.tb02242.x [DOI] [PubMed] [Google Scholar]
- Becker HC, Lopez MF, Doremus-Fitzwater TL, 2011. Effects of stress on alcohol drinking: a review of animal studies. Psychopharmacology (Berl.) 218, 131–156. 10.1007/s00213-011-2443-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biesmans S, Meert TF, Bouwknecht JA, Acton PD, Davoodi N, De Haes P, Kuijlaars J, Langlois X, Matthews LJR, Ver Donck L, Hellings N, Nuydens R, 2013. Systemic Immune Activation Leads to Neuroinflammation and Sickness Behavior in Mice [WWW Document]. Mediators Inflamm 10.1155/2013/271359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard BA, Glick SD, 2002. Sex Differences in Mesolimbic Dopamine Responses to Ethanol and Relationship to Ethanol Intake in Rats, in: Recent Developments in Alcoholism, Recent Developments in Alcoholism Springer, Boston, MA, pp. 231–241. 10.1007/0-306-47138-8_15 [DOI] [PubMed] [Google Scholar]
- Blednov Y, Bergeson S, Walker D, Ferreira V, Kuziel W, Harris R, 2005. Perturbation of chemokine networks by gene deletion alters the reinforcing actions of ethanol. Behav. Brain Res 165, 110–125. 10.1016/j.bbr.2005.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Benavidez JM, Geil C, Perra S, Morikawa H, Harris RA, 2011. Activation of inflammatory signaling by lipopolysaccharide produces a prolonged increase of voluntary alcohol intake in mice. Brain. Behav. Immun 25, S92–S105. 10.1016/j.bbi.2011.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Walker D, Martinez M, Levine M, Damak S, Margolskee RF, 2007. Perception of sweet taste is important for voluntary alcohol consumption in mice. Genes Brain Behav 0, 070321054409001-??? 10.1111/j.1601-183X.2007.00309.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boissoneault J, Lewis B, Nixon SJ, 2018. Characterizing Chronic Pain and Alcohol Use Trajectory Among Treatment Seeking Alcoholics. Alcohol 10.1016/j.alcohol.2018.05.009 [DOI] [PubMed] [Google Scholar]
- Brennan PL, Schutte KK, Moos BS, Moos RH, 2011. Twenty-Year Alcohol-Consumption and Drinking-Problem Trajectories of Older Men and Women*. J. Stud. Alcohol Drugs 72, 308–321. 10.15288/jsad.2011.72.308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KJ, Grunberg NE, 1995. Effects of housing on male and female rats: Crowding stresses males but calms females. Physiol. Behav 58, 1085–1089. 10.1016/0031-9384(95)02043-8 [DOI] [PubMed] [Google Scholar]
- Butkevich IP, Mikhailenko VA, Bagaeva TR, Vershinina EA, Aloisi AM, Otellin VA, 2013. Inflammatory Pain and Corticosterone Response in Infant Rats: Effect of 5-HT1A Agonist Buspirone Prior to Gestational Stress [WWW Document]. Mediators Inflamm 10.1155/2013/915189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cailhol S, Mormède P, 2001. Sex and Strain Differences in Ethanol Drinking: Effects of Gonadectomy. Alcohol. Clin. Exp. Res 25, 594–599. 10.1111/j.1530-0277.2001.tb02255.x [DOI] [PubMed] [Google Scholar]
- Chester JA, Rausch EJ, June HL, Froehlich JC, 2006. Decreased reward during acute alcohol withdrawal in rats selectively bred for low alcohol drinking. Alcohol 38, 165–172. 10.1016/j.alcohol.2006.06.009 [DOI] [PubMed] [Google Scholar]
- Coleman LG, Crews FT, 2018. Innate Immune Signaling and Alcohol Use Disorders, in: SpringerLink, Handbook of Experimental Pharmacology Springer, Berlin, Heidelberg, pp. 1–28. 10.1007/164_2018_92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins AC, Yeager TN, Lebsack ME, Panter SS, 1975. Variations in alcohol metabolism: Influence of sex and age. Pharmacol. Biochem. Behav 3, 973–978. 10.1016/0091-3057(75)90004-0 [DOI] [PubMed] [Google Scholar]
- Cook CD, Nickerson MD, 2005. Nociceptive Sensitivity and Opioid Antinociception and Antihyperalgesia in Freund’s Adjuvant-Induced Arthritic Male and Female Rats. J. Pharmacol. Exp. Ther 313, 449–459. 10.1124/jpet.104.077792 [DOI] [PubMed] [Google Scholar]
- Cozzoli DK, Tanchuck-Nipper MA, Kaufman MN, Horowitz CB, Finn DA, 2014. Environmental stressors influence limited-access ethanol consumption by C57BL/6J mice in a sex-dependent manner. Alcohol Fayettev. N 48, 741–754. 10.1016/j.alcohol.2014.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow LT, 1968. Diencephalic influence in alcohol diuresis. Physiol. Behav 3, 319–322. 10.1016/0031-9384(68)90107-8 [DOI] [Google Scholar]
- Dhir A, Naidu PS, Kulkarni SK, 2005. Protective effect of cyclooxygenase-2 (COX-2) inhibitors but not non-selective cyclooxygenase (COX)-inhibitors on ethanol withdrawal-induced behavioural changes. Addict. Biol 10, 329–335. 10.1080/13556210500352964 [DOI] [PubMed] [Google Scholar]
- Dina OA, Barletta J, Chen X, Mutero A, Martin A, Messing RO, Levine JD, 2000. Key Role for the Epsilon Isoform of Protein Kinase C in Painful Alcoholic Neuropathy in the Rat [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dina OA, Gear RW, Messing RO, Levine JD, 2007. Severity of alcohol-induced painful peripheral neuropathy in female rats: Role of estrogen and protein kinase (A and Cε). Neuroscience 145, 350–356. 10.1016/j.neuroscience.2006.11.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds EC, Lawson W, Simpson SA, Williams PC, 1945. Testing diphenylethylamine compounds for analgesic action. J. Physiol 104, 47–51. 10.1113/jphysiol.1945.sp004105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Rajendran P, Spear LP, 2005. Factors Influencing Elevated Ethanol Consumption in Adolescent Relative to Adult Rats. Alcohol. Clin. Exp. Res 29, 1796–1808. 10.1097/01.alc.0000183007.65998.aa [DOI] [PubMed] [Google Scholar]
- Edwards S, Vendruscolo LF, Schlosburg JE, Misra KK, Wee S, Park PE, Schulteis G, Koob GF, 2012. Development of mechanical hypersensitivity in rats during heroin and ethanol dependence: Alleviation by CRF1 receptor antagonism. Neuropharmacology 62, 1142–1151. 10.1016/j.neuropharm.2011.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egli M, Koob GF, Edwards S, 2012. Alcohol dependence as a chronic pain disorder. Neurosci. Biobehav. Rev 36, 2179–2192. 10.1016/j.neubiorev.2012.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ely M, 1999. GENDER DIFFERENCES IN THE RELATIONSHIP BETWEEN ALCOHOL CONSUMPTION AND DRINK PROBLEMS ARE LARGELY ACCOUNTED FOR BY BODY WATER. Alcohol Alcohol 34, 894–902. 10.1093/alcalc/34.6.894 [DOI] [PubMed] [Google Scholar]
- Finn DA, Sinnott RS, Ford MM, Long SL, Tanchuck MA, Phillips TJ, 2004. Sex differences in the effect of ethanol injection and consumption on brain allopregnanolone levels in C57BL/6 mice. Neuroscience 123, 813–819. 10.1016/j.neuroscience.2003.11.017 [DOI] [PubMed] [Google Scholar]
- Fu R, Gregor D, Peng Z, Li J, Bekker A, Ye J, 2015. Chronic intermittent voluntary alcohol drinking induces hyperalgesia in Sprague-Dawley rats 9. [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Neugebauer V, 2008. Differential mechanisms of CRF1 and CRF2 receptor functions in the amygdala in pain-related synaptic facilitation and behavior. J. Neurosci. Off. J. Soc. Neurosci 28, 3861–3876. 10.1523/JNEUROSCI.0227-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Lal H, 1999. Effects of Ethanol and Ethanol Withdrawal on Nociception in Rats Gatch - 1999 - Alcoholism: Clinical and Experimental Research - Wiley Online Library [WWW Document] URL https://onlinelibrary.wiley.com/doi/epdf/10.1111/j.1530-0277.1999.tb04118.x (accessed 7.22.18). [PubMed]
- Griffin WC, 2014. Alcohol dependence and free-choice drinking in mice. Alcohol 48, 287–293. 10.1016/j.alcohol.2013.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haleem DJ, Kennett G, Curzon G, 1988. Adaptation of female rats to stress: shift to male pattern by inhibition of corticosterone synthesis. Brain Res 458, 339–347. 10.1016/0006-8993(88)90476-3 [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J, 1988. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 32, 77–88. 10.1016/0304-3959(88)90026-7 [DOI] [PubMed] [Google Scholar]
- Harper RP, Kerins CA, McIntosh JE, Spears R, Bellinger LL, 2001. Modulation of the inflammatory response in the rat TMJ with increasing doses of complete Freund’s adjuvant. Osteoarthritis Cartilage 9, 619–624. 10.1053/joca.2001.0461 [DOI] [PubMed] [Google Scholar]
- Heilig M, Koob GF, 2007. A key role for corticotropin-releasing factor in alcohol dependence. Trends Neurosci 30, 399–406. 10.1016/j.tins.2007.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinsbroek RPW, Van Haaren F, Feenstra MGP, Endert E, Van de Poll NE, 1991. Sexand time-dependent changes in neurochemical and hormonal variables induced by predictable and unpredictable footshock. Physiol. Behav 49, 1251–1256. 10.1016/0031-9384(91)90359-V [DOI] [PubMed] [Google Scholar]
- Hermes GL, Rosenthal L, Montag A, McClintock MK, 2006. Social isolation and the inflammatory response: sex differences in the enduring effects of a prior stressor. Am. J. Physiol.-Regul. Integr. Comp. Physiol 290, R273–R282. 10.1152/ajpregu.00368.2005 [DOI] [PubMed] [Google Scholar]
- Ho A, Chin AJ, Dole VP, 1989. Early Experience and the Consumption of Alcohol by Adult C57BL/6J Mice [DOI] [PubMed] [Google Scholar]
- Holstein SE, Spanos M, Hodge CW, 2011. Adolescent C57BL/6J Mice Show Elevated Alcohol Intake, but Reduced Taste Aversion, as Compared to Adult Mice: A Potential Behavioral Mechanism for Binge Drinking: ELEVATED ALCOHOL INTAKE IN ADOLESCENCE. Alcohol. Clin. Exp. Res 35, 1842–1851. 10.1111/j.1530-0277.2011.01528.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa LS, Chu A, Levinson SA, Kayyali TM, DeBold JF, Miczek KA, 2011. Persistent Escalation of Alcohol Drinking in C57BL/6J Mice With Intermittent Access to 20% Ethanol. Alcohol. Clin. Exp. Res 35, 1938–1947. 10.1111/j.1530-0277.2011.01545.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jochum T, Boettger MK, Burkhardt C, Juckel G, Bär K-J, 2010. Increased pain sensitivity in alcohol withdrawal syndrome. Eur. J. Pain 14, 713–718. 10.1016/j.ejpain.2009.11.008 [DOI] [PubMed] [Google Scholar]
- Jury NJ, DiBerto JF, Kash TL, Holmes A, 2017. Sex differences in the behavioral sequelae of chronic ethanol exposure. Alcohol 58, 53–60. 10.1016/j.alcohol.2016.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes K, Hatzenbuehler M, Grant BF, Hasin DS, 2012. Stress and Alcohol. Alcohol Res. Curr. Rev 34, 391–400. [PMC free article] [PubMed] [Google Scholar]
- King WC, Chen J-Y, Mitchell JE, Kalarchian MA, Steffen KJ, Engel SG, Courcoulas AP, Pories WJ, Yanovski SZ, 2012. Prevalence of Alcohol Use Disorders Before and After Bariatric Surgery. JAMA 307 10.1001/jama.2012.6147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G, 2001. Drug Addiction, Dysregulation of Reward, and Allostasis. Neuropsychopharmacology 24, 97–129. 10.1016/S0893-133X(00)00195-0 [DOI] [PubMed] [Google Scholar]
- Koob G, Kreek MJ, 2007. Stress, Dysregulation of Drug Reward Pathways, and the Transition to Drug Dependence. Am. J. Psychiatry 164, 1149–1159. 10.1176/appi.ajp.2007.05030503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, 2013. Addiction is a Reward Deficit and Stress Surfeit Disorder. Front. Psychiatry 4 10.3389/fpsyt.2013.00072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND, 2010. Neurocircuitry of Addiction. Neuropsychopharmacology 35, 217–238. 10.1038/npp.2009.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C, 2005. Sex differences in HPA axis responses to stress: a review. Biol. Psychol 69, 113–132. 10.1016/j.biopsycho.2004.11.009 [DOI] [PubMed] [Google Scholar]
- Kumarhia D, He L, McCluskey LP, 2016. Inflammatory stimuli acutely modulate peripheral taste function. J. Neurophysiol 115, 2964–2975. 10.1152/jn.01104.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster FE, Brown TD, Coker KL, Elliott JA, Wren SB, 1996. Sex Differences in Alcohol Preference and Drinking Patterns Emerge during the Early Postpubertal Period in Sprague-Dawley Rats. Alcohol. Clin. Exp. Res 20, 1043–1049. 10.1111/j.1530-0277.1996.tb01945.x [DOI] [PubMed] [Google Scholar]
- Larson AA, Brown DR, El-Atrash S, Walser MM, 1986. Pain threshold changes in adjuvant-induced inflammation: A possible model of chronic pain in the mouse. Pharmacol. Biochem. Behav 24, 49–53. 10.1016/0091-3057(86)90043-2 [DOI] [PubMed] [Google Scholar]
- Lê AD, Israel Y, Juzytsch W, Quan B, Harding S, 2001. Genetic Selection for High and Low Alcohol Consumption in a Limited-Access Paradigm. Alcohol. Clin. Exp. Res 25, 1613–1620. 10.1111/j.1530-0277.2001.tb02168.x [DOI] [PubMed] [Google Scholar]
- Lopez MF, Doremus-Fitzwater TL, Becker HC, 2011. Chronic social isolation and chronic variable stress during early development induce later elevated ethanol intake in adult C57BL/6J mice. Alcohol 45, 355–364. 10.1016/j.alcohol.2010.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malec D, Kotlińska J, Langwiński R, 1987. Cross-tolerance between morphine and ethanol and their antinociceptive effects. J. Stud. Alcohol 48, 507–510. 10.15288/jsa.1987.48.507 [DOI] [PubMed] [Google Scholar]
- Melgar S, Bjursell M, Gerdin A-K, Svensson L, Michaëlsson E, Bohlooly-Y M, 2007. Mice with experimental colitis show an altered metabolism with decreased metabolic rate. Am. J. Physiol.-Gastrointest. Liver Physiol 292, G165–G172. 10.1152/ajpgi.00152.2006 [DOI] [PubMed] [Google Scholar]
- Middaugh LD, Kelley BM, Bandy A-LE, McGroarty KK, 1999. Ethanol Consumption by C57BL/6 Mice: Influence of Gender and Procedural Variables 9. [DOI] [PubMed] [Google Scholar]
- Moriya Y, Kasahara Y, Hall FS, Sakakibara Y, Uhl GR, Tomita H, Sora I, 2015. Sex differences in the effects of adolescent social deprivation on alcohol consumption in -opioid receptor knockout mice. Psychopharmacology (Berl.) 232, 1471–1482. 10.1007/s00213-014-3784-y [DOI] [PubMed] [Google Scholar]
- Mumenthaler MS, Taylor JL, O’Hara R, Yesavage JA, 1999. Gender Differences in Moderate Drinking Effects 23, 10. [PMC free article] [PubMed] [Google Scholar]
- Palanza P, Gioiosa L, Parmigiani S, 2001. Social stress in mice: Gender differences and effects of estrous cycle and social dominance. Physiol. Behav., Social Stress: Acute and Long-term Effects on Physiology & Behavior 73, 411–420. 10.1016/S0031-9384(01)00494-2 [DOI] [PubMed] [Google Scholar]
- Pascual M, Montesinos J, Marcos M, Torres J-L, Costa-Alba P, García‐García F, Laso F-J, Guerri C, 2017. Gender differences in the inflammatory cytokine and chemokine profiles induced by binge ethanol drinking in adolescence. Addict. Biol 22, 1829–1841. 10.1111/adb.12461 [DOI] [PubMed] [Google Scholar]
- Pitcher MH, Tarum F, Lehmann M, Bushnell MC, 2019. Persistent inflammatory pain alters sexually-motivated behavior in male rats. Behav. Brain Res 356, 380–389. 10.1016/j.bbr.2018.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitzer C, Kuner R, Tappe-Theodor A, 2016. Voluntary and evoked behavioral correlates in neuropathic pain states under different social housing conditions. Mol. Pain 12, 174480691665663 10.1177/1744806916656635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren K, Dubner R, 1999. Inflammatory Models of Pain and Hyperalgesia. ILAR J 40, 111–118. 10.1093/ilar.40.3.111 [DOI] [PubMed] [Google Scholar]
- Riley JL, King C, 2009. Self-report of alcohol use for pain in a multi-ethnic community sample. J. Pain Off. J. Am. Pain Soc 10, 944–952. 10.1016/j.jpain.2009.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier C, 1993. Female Rats Release More Corticosterone Than Males in Response to Alcohol: Influence of Circulating Sex Steroids and Possible Consequences for Blood Alcohol Levels. Alcohol. Clin. Exp. Res 17, 854–859. 10.1111/j.1530-0277.1993.tb00853.x [DOI] [PubMed] [Google Scholar]
- Rodgers DA, 1966. Factors Underlying Differences in Alcohol Preference Among Inbred Strains of Mice: Psychosom. Med 28, 498–513. 10.1097/00006842-196607000-00028 [DOI] [Google Scholar]
- Rodgers DA, McClearn GE, Bennett EL, Hebert M, 1963. Alcohol preference as a function of its caloric utility in mice. J. Comp. Physiol. Psychol 56, 666–672. 10.1037/h0040350 [DOI] [PubMed] [Google Scholar]
- Roltsch EA, Impastato RA, Gilpin NW, 2017. Intra‐cerebral and intra-nasal melanocortin-4 receptor antagonist blocks withdrawal hyperalgesia in alcohol-dependent rats - Roltsch Hellard - 2017 - Addiction Biology - Wiley Online Library [WWW Document] URL https://onlinelibrary-wiley-com.libproxy.lib.unc.edu/doi/epdf/10.1111/adb.12360 (accessed 7.22.18). [DOI] [PubMed]
- Schramm-Sapyta NL, Francis R, MacDonald A, Keistler C, O’Neill L, Kuhn CM, 2014. Effect of sex on ethanol consumption and conditioned taste aversion in adolescent and adult rats. Psychopharmacology (Berl.) 231, 1831–1839. 10.1007/s00213-013-3319-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumilla JA, Liron T, Mochly-Rosen D, Kendig JJ, Sweitzer SM, 2005. Ethanol Withdrawal–Associated Allodynia and Hyperalgesia: Age-Dependent Regulation by Protein Kinase Cϵ and γ Isozymes. Pain Forum 6, 535–549. 10.1016/j.jpain.2005.03.005 [DOI] [PubMed] [Google Scholar]
- Smith ML, Hostetler CM, Heinricher MM, Ryabinin AE, 2016. Social transfer of pain in mice | Science Advances [WWW Document] URL http://advances.sciencemag.org/content/2/10/e1600855 (accessed 7.22.18). [DOI] [PMC free article] [PubMed]
- Smith ML, Li J, Ryabinin AE, 2015. Increased Alcohol Consumption in Urocortin 3 Knockout Mice Is Unaffected by Chronic Inflammatory Pain. Alcohol Alcohol. Oxf. Oxfs 50, 132–139. 10.1093/alcalc/agu084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen PW, Shi L, He L, McCluskey LP, 2010. Neutrophil responses to injury or inflammation impair peripheral gustatory function. Neuroscience 167, 894–908. 10.1016/j.neuroscience.2010.02.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein C, Millan MJ, Herz A, 1988. Unilateral inflammation of the hindpaw in rats as a model of prolonged noxious stimulation: Alterations in behavior and nociceptive thresholds. Pharmacol. Biochem. Behav 31, 445–451. 10.1016/0091-3057(88)90372-3 [DOI] [PubMed] [Google Scholar]
- Strauss MB, Rosenbaum JD, Nelson WP, 1950. THE EFFECT OF ALCOHOL ON THE RENAL EXCRETION OF WATER AND ELECTROLYTE 1. J. Clin. Invest 29, 1053–1058. 10.1172/JCI102336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutker PB, Tabakoff B, Goist KC, Randall CL, 1983. Acute alcohol intoxication, mood states and alcohol metabolism in women and men. Pharmacol. Biochem. Behav 18, 349–354. 10.1016/0091-3057(83)90198-3 [DOI] [PubMed] [Google Scholar]
- Thiele TE, Koh MT, Pedrazzini T, 2012. Voluntary Alcohol Consumption Is Controlled via the Neuropeptide Y Y1 Receptor 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordoff MG, Bachmanov AA, 2008. Influence of the Number of Alcohol and Water Bottles on Murine Alcohol Intake 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truxell EM, Molina JC, Spear NE, 2007. Ethanol Intake in the Juvenile, Adolescent, and Adult Rat: Effects of Age and Prior Exposure to Ethanol. Alcohol. Clin. Exp. Res 31, 755–765. 10.1111/j.1530-0277.2007.00358.x [DOI] [PubMed] [Google Scholar]
- Vetter-O’Hagen C, Varlinskaya E, Spear L, 2009. Sex Differences in Ethanol Intake and Sensitivity to Aversive Effects during Adolescence and Adulthood. Alcohol Alcohol 44, 547–554. 10.1093/alcalc/agp048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilsnack RW, Wilsnack SC, Kristjanson AF, Vogeltanz-Holm ND, Gmel G, 2009. Gender and alcohol consumption: patterns from the multinational GENACIS project. Addiction 104, 1487–1500. 10.1111/j.1360-0443.2009.02696.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff HG, Hardy JD, Goodell H, 1942. Studies on Pain: Measurement of the Effect of Ethyl Alcohol on the Pain Threshold and on the “Alarm” Reaction. J. Pharmacol. Exp. Ther 75, 38–49. [Google Scholar]
- Yezierski RP, Hansson P, 2018. Inflammatory and Neuropathic Pain From Bench to Bedside: What Went Wrong? J. Pain 19, 571–588. 10.1016/j.jpain.2017.12.261 [DOI] [PubMed] [Google Scholar]
- Yoshimura S, Sakamoto S, Kudo H, Sassa S, Kumai A, Okamoto R, 2003. Sex-differences in adrenocortical responsiveness during development in rats. Steroids 68, 439–445. 10.1016/S0039-128X(03)00045-X [DOI] [PubMed] [Google Scholar]