Abstract
Until the last decade, studies of the timing of early symptom emergence in autism spectrum disorder (ASD) relied upon retrospective methods. Recent investigations, however, are raising significant questions about the accuracy and validity of such data. Questions about when and how behavioral signs of autism emerge may be better answered through prospective studies, in which infants are enrolled near birth and followed longitudinally until the age at which ASD can be confidently diagnosed or ruled out. This review summarizes the results of recent studies that utilized prospective methods to study infants at high risk of developing ASD due to family history. Collectively, prospective studies demonstrate that the onset of ASD involves declines in the rates of key social and communication behaviors during the first years of life for most children. This corpus of literature suggests that regressive onset patterns occur much more frequently than previously recognized and may be the rule rather than the exception.
1. Introduction
The onset of behavioral signs of autism spectrum disorder (ASD) is usually conceptualized as occurring in one of two ways: an early onset pattern, in which children demonstrate delays and deviances in social and communication development early in life, and a regressive pattern, in which children develop largely as expected for some period and then experience a substantial decline in or loss of previously developed skills. While it was long believed that the majority of children with ASD demonstrated an early onset pattern, more recent studies suggest that regressive onset occurs more frequently than previously recognized (Brignell et al., 2017; Hansen et al., 2008; Kern, Geier & Geier, 2015; Pickles et al., 2009; Shumway et al., 2011; Thurm et al., 2014; for a review, see meta-analysis by Barger, Campbell & McDonough, 2013). Studies occasionally also identify a third onset pattern, that of developmental stagnation or plateau (Shumway et al., 2011), that is characterized by intact early skills that fail to progress or transform into more advanced developmental achievements. This onset pattern is distinct from regression, in that the child does not lose acquired skills, but instead fails to make expected gains.
1.1. Methods for Measuring Onset Patterns
The most common procedure for collecting information about the timing of early symptoms is retrospective parent report. A number of factors can influence report validity, including awareness of the child’s eventual diagnosis and knowledge of developmental milestones. It has long been understood that retrospective reports are subject to problems of memory and interpretation (Finney, 1981; Henry et al., 1994; Pickles et al., 1996), including in studies of ASD (Andrews et al., 2002). Multiple studies have documented the ways in which recall problems and other biases can influence parent report. Changes in recall occur over time, with past events often reported to occur more recently, closer to the time of recollection, than they actually took place, an error called forward telescoping (Loftus & Marburger, 1983). Studies of children with ASD have demonstrated significant forward telescoping in parent report of milestones, resulting in parents being less likely to report regression and more likely to report early delays as their children grow older (Hus et al., 2011; Lord et al., 2004). A recent study from our research team (Ozonoff et al., 2018a) conducted longitudinal interviews with parents about onset of ASD symptoms when their child was 2-3 years old (Time 1) and approximately 6 years old (Time 2). Significant forward telescoping was found in both age of regression and age when milestones were achieved. The correspondence between Time 1 and Time 2 parent report of onset was low (kappa=.38). One-quarter of the sample changed onset categories, most often due to parents not recalling a regression at Time 2 that they had reported at Time 1.
Analysis of home movies of children later diagnosed with ASD is another retrospective method used in research studies to study symptom emergence (Goldberg et al., 2008; Palomo et al., 2006). Video analysis may be a more objective procedure for documenting early symptoms than parent recall (Werner & Dawson, 2005) but it is labor-intensive and subject to other limitations, such as selective recording (e.g., tendency of parents to film positive behaviors). In a study from our team that compared classification of onset based on coding of family movies to onset type as recalled by parents (Ozonoff et al., 2011a), less than half of children whose home video displayed clear evidence of a major decline in social and communication behavior were reported to have had a regression by parents. Similarly, only 40% of participants with clear evidence of early delays and little evidence of skill decline on video were reported by parents to show an early onset pattern.
1.2. Prospective Studies of Onset
Questions about when and how behavioral signs of autism emerge may be better answered through prospective investigations, in which infants are recruited and enrolled near birth, prior to the advent of parent concerns, and then followed longitudinally through the window of developmental risk, until the age at which ASD can be confidently diagnosed or ruled out, usually 36 months. A few large general population cohorts have been studied prospectively to examine onset patterns (Brignell et al., 2017; Havdahl et al., 2018) but this study design is inefficient, since fewer than 2 in 100 participants will develop ASD (Centers for Disease Control and Prevention, 2018), making it difficult to achieve an appropriate sample size. Additionally, large prospective cohort studies must, of necessity, rely upon parent questionnaires and rarely provide the opportunity for in-person clinical assessments to verify diagnosis or onset pattern.
For this reason, most prospective investigations utilize high-risk samples in order to increase the number of ASD outcomes that are informative for study. The most widely used high-risk group has been later-born siblings of children with ASD, who are known to be at higher ASD risk than the general population (Constantino et al., 2010). Most investigations compare high-risk infants to lower-risk participants with no known family history of ASD in first-, second-, and sometimes third-degree relatives. This study design improves on retrospective methods in a number of important ways. Serial comprehensive assessments, in standardized testing contexts, are used to document the timing of symptom emergence, thus avoiding reliance on potentially fallible parent recall or non-representative home video. Assessments can utilize a wide range of tools, including eye tracking, EEG, and imaging, allowing broader investigations of symptom onset and testing of specific hypotheses. And while most retrospective studies recruit samples through clinics, which may influence the results by including more severely affected children, infant sibling studies avoid such potential biases by ascertaining participants via family history alone.
Several recent papers provide comprehensive reviews of the infant sibling literature (Bolte et al., 2013; Jones et al., 2014; Pearson et al., 2018; Szatmari et al., 2016). Here we focus on research reports of greatest relevance to symptom emergence, specifically those that study infants beginning in the first year of life on measures that are appropriate for examining potential skill decline over time. Using a variety of different prospective methods, these studies have reported largely intact early development, followed by developmental declines and onset of symptoms around the first birthday and in the second year of life. For example, Zwaigenbaum et al. (2005), using the Autism Observation Scale for Infants (AOSI), reported no differences at 6 months between infants subsequently diagnosed with ASD and both high- and low-risk infants without ASD outcomes; significant group differences emerged at 12 months and increased over time. This pattern on the AOSI was later replicated by an independent research team (Gammer et al., 2015). Wan et al. (2013) found that infant-parent interaction quality at 6-10 months did not predict which children would be diagnosed with ASD at age 3, but by 12-15 months, such variables were significantly associated with diagnostic outcome. Similar findings of lack of early group differences (or lack of early predictive ability), followed by later divergence from typically developing infants, have been reported by Landa and Garrett-Mayer (2006) using the Mullen Scales of Early Learning, Rozga et al. (2011) in joint attention, Bedford et al. (2012) on a gaze-following eye-tracking task, Elsabbagh et al. (2013) on a gap-overlap attention task, and Wolff et al. (2014) studying repetitive behavior. In an incisive recent review that attempts to reconcile retrospective and prospective studies of regression and explore how study design affects the likelihood of capturing regression, Pearson et al. (2018) conclude that, among infants who later develop ASD, “the majority show declining fixation of eyes, gaze to faces, and social engagement, from typical levels in early infancy (2-6 months) to significantly reduced levels by 24-36 months (p. 14).”
2. Findings from the University of California Davis Infant Sibling Study
In our laboratory, we have taken the analytic approach of growth curve modeling to examine directly the evidence of longitudinal developmental change in the first three years of life. Between 2003 and 2015, the UC Davis Infant Sibling Study recruited three cohorts of later-born siblings, each composed of 50 low-risk and 100 high-risk infants. Participants were tested as early as 6 months of age and then seen every 3 to 6 months until their 3rd birthday (up to 7 in-person evaluations). They have since been followed into school age and tested at approximately three-year intervals. The oldest children from Cohort 1 are now 16 years of age and the retention rate is over 80%. At each infant and preschool visit, a battery of age-appropriate standardized tests and experimental tasks was administered that measured language, cognition, social, communication, motor, and many other domains. Approximately 20% of the high-risk infants were later diagnosed with ASD (Ozonoff et al., 2011b). Diagnoses of ASD were made at any point that a child met criteria (mean age 24.2 months) but a full diagnostic assessment was completed on all children, regardless of previous findings, at 36 months by examiners unaware of family risk or prior assessment results. In the following sections, we summarize several studies from these cohorts that consistently demonstrate declining trajectories across a variety of different measures and developmental domains.
The phenomenon of regression is defined by loss or significant decrease in already-acquired skills. Thus, a critical methodological issue in prospective studies that wish to examine onset patterns is selection of which behaviors to measure. They must be 1) developmentally appropriate across the full age window of risk and 2) robustly present, at high frequency, in the first year of life. Such behaviors have the capacity to decrease and are therefore of highest relevance to the study of onset patterns. Measures that focus on socio-communicative behaviors that have not yet emerged in the first year of life, such as joint attention, imitation, and verbal communication, will be less useful for testing hypotheses about declining capacities. The behaviors our laboratory has focused on, including gaze to faces and eyes of others, shared affect, and social interest/engagement, are well developed in the first year of life (Inada et al., 2010) and therefore of highest relevance in the prospective measurement of regression.
2.1. Behavioral Coding of Social-Communication Rates
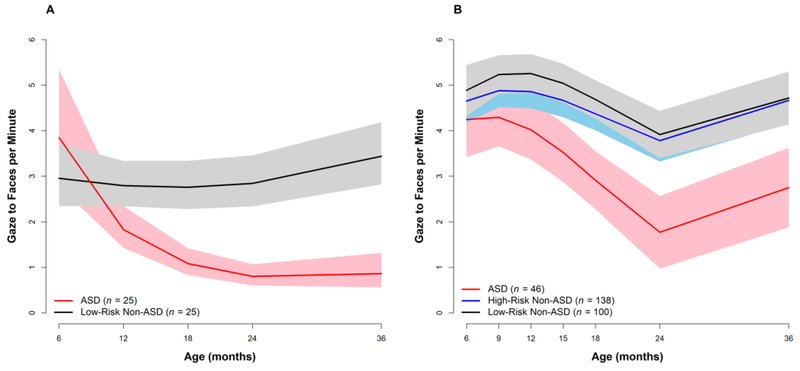
Our first exploration of longitudinal change in early social and communicative behaviors used video recordings of participants interacting with examiners during structured developmental testing (Ozonoff et al., 2010). Research assistants, unaware of family risk group or diagnostic outcome, were trained to 90% reliability to detect three behaviors: gaze to an adult’s face, smiles at an adult that were paired with eye contact, and vocalizations directed at an adult that were accompanied by eye contact. Rate per minute of eye gaze, shared affect, and directed vocalizations of the 25 children in Cohort 1 with outcomes of ASD were compared to those of 25 children who did not have ASD outcomes randomly selected from the low-risk group. The two groups behaved similarly at 6 months: frequencies of none of the three behaviors differed between the groups and effect sizes were in the small range. Over time, the Low-Risk (LR) Non-ASD group had a significant increase in social smiles and directed vocalizations, while maintaining the same consistently high level of gaze to faces. In the ASD group, in contrast, the rates of all three behaviors dramatically decreased overtime. Figure 1A displays longitudinal trajectories of eye contact rate per minute, showing comparable values between groups at 6 months, followed by group differences that became statistically significant by 12 months and persisted and widened over time. These longitudinal decreases in the rates of key social and communicative behaviors provided the first prospectively measured evidence consistent with a regressive onset pattern.
Figure 1:
Declining trajectories of gaze to eyes in children developing ASD, coded from a videotaped interaction with an examiner. Panel A: Cohort 1, Panel B: Cohorts 2 and 3.
We have since replicated these findings (see Figure 1B) using the same methods in an independent sample of 46 infants later diagnosed with ASD from Cohorts 2 and 3 of our longitudinal project. In this analysis (Gangi et al., in preparation), a third group, composed of high-risk (HR) infants who did not have ASD outcomes, was also included. This group was not different from the LR Non-ASD group in the frequency of gaze to adult faces at any age and did not show any evidence of decline in development, which was evident only in the participants developing ASD, replicating our 2010 study.
2.2. Observer Ratings of Social Engagement
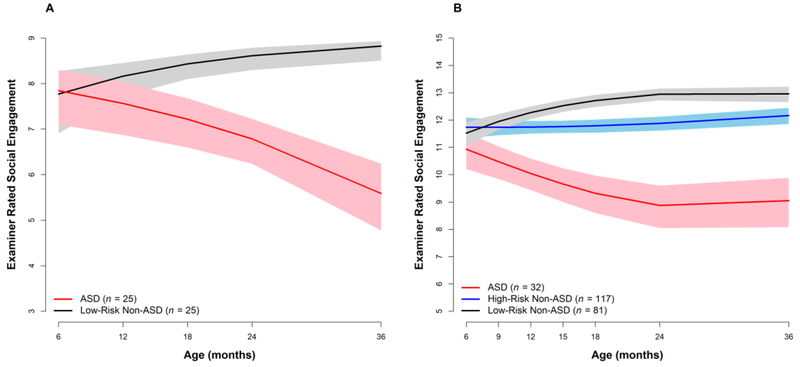
Coding behavior frequencies from video is time-consuming, labor-intensive, expensive, and not transferable to clinical contexts, so our research program has also sought to establish whether declining trajectories are evident using other methodological approaches. At the end of each visit, examiners rate the frequency of eye contact, shared affect, and overall social engagement (number of social initiations and social responses) made by the infant throughout the session, across all tasks, using a 3-point scale (1 = rare, 2 = occasional, 3 = frequent). These scores are summed to create a composite that ranges from 3 to 9. As reported in Ozonoff et al. (2010) and depicted in Figure 2A, these examiner ratings of social engagement showed similar longitudinal patterns to the social behaviors coded from video. There were no group differences in the 6-month examiner ratings; however, while the LR Non-ASD group showed a significant increase in social engagement ratings over time, reaching close to the maximum score by 36 months, the children in the ASD outcome group had a strong decline in social engagement ratings over the same time period.
Figure 2:
Declining trajectories of social engagement in children developing ASD, as rated by examiners unaware of risk group or outcome. Panel A: Cohort 1, Panel B: Cohorts 2 and 3.
This finding was recently replicated in an independent group of 32 infants with ASD outcomes from Cohorts 2 and 3 of our sample (Ozonoff et al., 2018b). We used the same examiner rating variable (this time with an expanded 5-point scale) and compared the ASD group to both a low-risk and a high-risk group without ASD. Again, all three groups had comparable levels of social engagement based on examiner scores at 6 months of age. The ASD group then demonstrated a decrease in scores with age, while the HR Non-ASD group showed stable high scores over time and the LR Non-ASD group demonstrated increasing scores longitudinally. By 12 months, the two Non-ASD groups demonstrated significantly higher rates of social engagement, as judged by examiners, than the ASD group and these differences widened over time, as can be seen in Figure 2B. Along with our 2010 paper, these findings demonstrate that the declines in the frequency of social and communication behaviors detected through more labor-intensive video coding methods are also detectable through much simpler methods that would be feasible for broader use, such as brief observational ratings of social engagement by clinical professionals.
2.3. Longitudinal Parent Ratings of Social Behavior
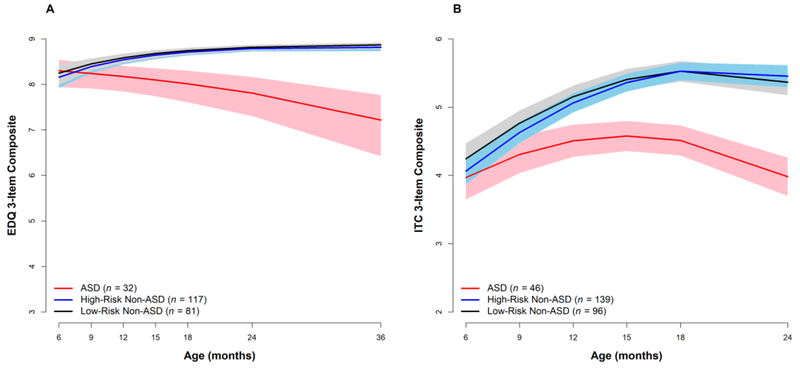
The question remained, however, whether such findings could be an artifact or byproduct of the assessment context with an unfamiliar examiner. For both clinical use and future development of screening methods, it is critical to also establish whether parent ratings are sensitive to the developmental decline phenomenon we have reported. In a recent study (Ozonoff et al., 2018b), we examined parent prospective ratings of the same early-appearing socio-communicative behaviors measured in the video and examiner ratings. Parents in our study completed the Early Development Questionnaire (EDQ; Ozonoff, Williams & Landa, 2005) prior to each visit. The EDQ consists of 45 questions about the child’s current functioning in social, communication, and repetitive behavior domains. Each item is rated on a 4-point frequency scale (0=behavior never occurs, 3=behavior often occurs). Three items, comparable in content to the video codes and examiner ratings, were summed: item 1 (“my child looks at me during social interactions”), item 4 (“my child smiles back at me when I smile at him/her”), and item 13 (“when I call my child’s name, he/she looks at me right away”). In addition to being parallel to the behaviors rated by examiners, these items were selected because they represent early-appearing behaviors that are relevant and developmentally appropriate across all ages of the study (6-36 months). In contrast to other EDQ items that measure later-developing skills (e.g., joint attention, language), the items selected for the composite measure behaviors present in the first year of life (Inada et al., 2010). As with the behaviors we selected for coding and examiner observational ratings, it was critical that the behaviors rated by parents have the potential to demonstrate decreases over time as ASD signs emerge. The composite variable, quantifying parent report of the frequency of key early social behaviors, had a potential range of 0 – 9. On the parent-rated EDQ, there were again no group differences at 6 months. As with the other measures, the ASD group showed a decline in levels of social engagement with age, while both the high-risk and low-risk Non-ASD groups demonstrated gains in social engagement over time. The ASD group’s scores were significantly lower than both Non-ASD groups by 12 months and the differences increased with age, demonstrating the same declining trajectory as evident in the coded behavior and examiner ratings (see Figure 3A).
Figure 3:
Declining trajectories of social engagement in children developing ASD, as rated by parents, Cohorts 2 and 3. Panel A: Early Development Questionnaire, Panel B: Infant Toddler Checklist.
Employing a similar approach, we replicated the ability of parent report to capture the decline in social and communication development using a standardized, normed measure (Parikh et al., 2018), the Infant-Toddler Checklist (ITC), a 24-item parent questionnaire from the Communication and Social Behavior Scales (CSBS; Wetherby & Prizant, 2002). This instrument is normed from 6 to 24 months and includes questions that span this developmental range, from early-appearing behaviors like social smiling to those that emerge at older ages, such as spoken language and pretend play. We created a composite of three items that represent behaviors typically present in the first year of life (item 2: “when your child plays with toys, does he/she look at you to see if you are watching?”; item 3: “does your child smile or laugh while looking at you?”; item 19: “when you call your child’s name, does he/she respond by looking or turning toward you?”). We then compared growth trajectories in infants subsequently diagnosed with ASD (n=46) to the HR Non-ASD (n=139) and LR Non-ASD groups (n=96). There were no group differences on the 3-item ITC composite at 6 months of age; however, over time, the ASD group showed a decline in scores, while the two Non-ASD groups demonstrated gains (see Figure 3B). This resulted in the ASD group having significantly lower scores at 24 months than both comparison groups.
These studies from our lab show that children with ASD, as a group, evidence declines in development from 6 to 36 months. Such declines are seen only in the ASD group and not in comparison samples, even those with elevated genetic risk or other developmental concerns. Findings of declining trajectories have since been replicated by other independent research teams. Landa et al. (2013) examined growth trajectories in infants later diagnosed with ASD and Non-ASD participants. Approximately half of the children with ASD, labeled the Early-ASD group, demonstrated differences from the Non-ASD cases at 14 months but the other half (Later-ASD group) did not diverge from typical infants until 24 months. The Later-ASD group demonstrated a steep decline in shared positive affect, as measured by the CSBS (Wetherby & Prizant, 2002), between 14 and 24 months. Jones and Klin (2013) conducted a prospective eye-tracking study with high- and low-risk infants to assess attention to eyes. The authors reported that very early in development (i.e., first two months of life), both low-risk and high-risk infants displayed high levels of attention to eyes, with no group differences. However, high-risk infants who were later diagnosed with ASD began to demonstrate a steady decline in looking at eyes at four months, reaching a level that was approximately half that of low-risk infants by 24 months. What was most predictive of a later ASD outcome was not the amount of visual fixation on eyes displayed at any particular age, but the overall declining trajectory over time. This study found that the majority of infants developing ASD demonstrated this declining pattern.
2.4. Growth Curve Modeling Approaches to Determining Onset Classifications
In aggregate, the studies reviewed up to this point converge on the conclusion that longitudinal decreases in key social behaviors are a signature of the early emergence of ASD. But these data do not clarify how widespread such phenomena are within ASD and whether the group-level findings are driven by extreme outliers or characterize a majority of young children developing ASD. In our lab, we have approached this issue analytically using multivariate Latent Class Analysis (LCA; Muthén 2004), permitting us to identify distinct subgroups of children based on their longitudinal patterns on multiple measures of social communication. This technique does not rely on preconceived notions or poorly defined definitions of onset phenomena, but instead uses statistical modeling to empirically derive the optimum number of classes described by the patterns of performance demonstrated in the measures.
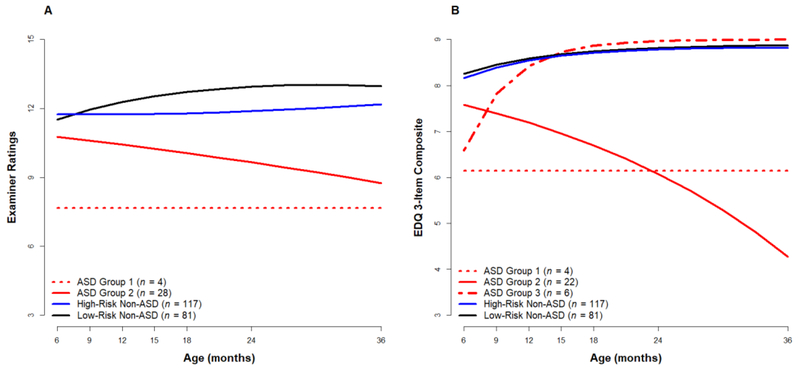
Data from a recent paper (Ozonoff et al., 2018b) address the question of how widespread the declining trajectories pattern is within a group of 32 infants subsequently diagnosed with ASD. We employed latent class growth models to examine potential within-group variation in onset patterns, using both examiner ratings of social engagement and parent ratings from the EDQ (see sections 2.2 and 2.3 for instrument descriptions). Best model fit for examiner ratings was a two-group solution. Using their highest posterior group probability, the 32 participants were classified into two trajectories (see Figure 4A, which also presents the Low- and High-Risk Non-ASD groups as contrasts). Only a small proportion of the ASD cases (n = 4; 13%) were assigned to an Early Onset/No Regression group by the latent class analyses, based on examiners prospectively reporting low levels of social behavior at all ages. The vast majority (n = 28; 88%) were classified by these analyses into a Regression group, in which examiners prospectively rated initially high levels of social engagement that dropped significantly over time.
Figure 4:
Latent classes of social engagement, demonstrating declining trajectories in the majority of children developing ASD, Cohorts 2 and 3. Panel A: Examiner ratings, Panel B: Parent ratings.
The best fit for the parent EDQ 3-item composite in the latent class models was a three-group solution: Group 1, an Early Onset trajectory, Group 2, a Declining trajectory, and Group 3, an Improving trajectory (see Figure 4B). Parents prospectively reported low levels of social engagement at all ages for Group 1, which again made up a small minority of the sample (n = 4; 13% of the sample). The majority of the sample (n = 22; 69%) was classified in Group 2; these children were prospectively reported by parents to show high rates of social engagement early in life, which significantly declined over time. Parents of children in Group 3 (n = 6; 19%) prospectively reported low levels of skills at early ages that then significantly increased over time.
2.5. Concordance Between Retrospective and Prospective Onset Classifications
In several of our studies, we have examined the correspondence between prospectively- and retrospectively-defined onset patterns and in each case have found them to be quite poor. For example, in our initial paper (Ozonoff et al., 2010), we compared onset classifications based upon coded frequencies of social and communicative behaviors to onset classifications employing retrospective parent report on the Autism Diagnostic Interview-Revised (ADI-R; Le Couteur et al., 2003). Using prospective observational data, 86% of the ASD sample showed decreasing rates of eye contact, social smiles, and vocalizations over time, but by parental recall using the ADI-R, only 17% of the children were classified as having regressive onset. In a more recent study (Ozonoff et al., 2018b), 69% of parents rated their child in a manner consistent with regression on a prospective questionnaire (the EDQ, described in section 2.3), but only 29% rated that same child as losing skills using a retrospective measure (the ADI-R). Parents were able to implicitly identify the changes in their child’s development over time when making ratings of the frequencies of current behaviors, but often did not explicitly label these changes as skill loss or regression when asked in a more categorical way. These results were particularly striking since both relied upon parental observations of the child. Similar findings of low concordance between retrospective and prospective methods of defining onset were reported by Landa and colleagues (2013). And a recent large general population study (Havdahl et al., 2018) found similar under-reporting of losses based on a retrospective parent interview. Of parents who prospectively reported a loss, defined as rating certain social behaviors as present at 18 months but absent at 36 months, only a striking 2% of them recalled such a decline, or labeled it as a loss, when asked at age 3.
3. Conclusions and Theoretical Implications
A number of conclusions can be drawn from the collective body of work reviewed in this paper.
3.1. Onset Involves Declining Social Development
ASD emerges over the first two years of life and is not present “from the beginning of life” as stated by Kanner (1943, p. 242) in his seminal paper. For many years, it was presumed that ASD signs were present, but were just challenging to measure, from birth. Diagnostic criteria for ASD were developed at a time when children with autism were rarely, if ever, identified in infancy and thus many symptoms in the DSM and ICD criteria, such as delays or deficits in gestures, language, imitation, and pretense, are less relevant to the first year of life. As we have emphasized in this review, one key to understanding early symptom emergence is to focus on very early-appearing social behaviors, those that are robustly present in early infancy, such as social interest, shared affect, gaze to faces and eyes, and response to name. When such a methodological approach is taken, there is clear evidence, across multiple methods, replicated by independent research teams, of declining social behavior over time, after a period of relatively typical development. There is convergence across studies of a lack of group differences from comparison samples without ASD before 9 months of age, followed by statistically significant differences starting at 12 months that widen over time. Logically, if certain skills are evident at typical rates at an early point in development and then those same skills, defined and measured the same way later in development, have substantially diminished, resulting in statistically significant differences from typical infants, a loss or regression of some magnitude must have occurred.
This paper advocates for taking a dimensional approach and using trajectories to identify patterns of onset. We are not arguing, however, for a fundamental reconsideration of the use of the word “regression.” The Merriam-Webster definition of the word regression is “a trend or shift toward a lower or less perfect state, such as (a) progressive decline of a manifestation of disease or (b) gradual loss of acquired skills.” This aptly describes the loss of established skills (e.g., eye contact, response to name, social interest) that occurs during the declines in social development described in this paper.
3.2. Regression in ASD is the Rule, not the Exception
The data summarized in this review suggest that a regressive pattern of onset is much more common than previously thought, the rule rather than the exception. While retrospective studies yielded regression estimates of 20-30%, prospective data put them much higher, in some studies well over 80%. We and others (Jones et al., 2014; Ozonoff et al., 2018b; Pearson et al., 2018) have suggested that the regressions reported by parents retrospectively on measures like the ADI-R represent just “the tip of the iceberg,” while prospective studies are able to capture earlier, more gradual, subtle changes that may be less noticeable in real-time observation. A hypothesis deriving from this supposition is that concordance between retrospective and prospective methods should be most frequent when the regression occurs later, is more drastic or severe, and involves loss of clearly defined skills like language. No published studies have yet examined this question and it would be a fruitful avenue for future investigation.
We propose that the way ASD starts, for all children, is through declines in early social and communication abilities. This presents a testable hypothesis: that all infants developing ASD lose some skills, but at different ages, some of which may be harder to detect with current measurement approaches than others. It may be difficult for parents to perceive and describe changing patterns of development that occur over many months during infancy, particularly when the period of normalcy is fairly brief. Our team (Ozonoff et al., 2010, 2011a) and others (Pearson et al., 2018; Rogers, 2009; Szatmari et al., 2016; Thurm et al., 2014) have suggested that onset is better thought of dimensionally, as a continuum of age when social and communication behaviors begin to diverge or decline, rather than a dichotomy (regression v. early onset). In a dimensional conceptualization of onset, at one end of the continuum lie children who display declines so early that they are difficult to measure and symptoms appear to have always been present. At the other end of the continuum are children who experience losses so late, when more skills have been acquired and thus there are more skills to lose, that the regression appears quite overt and dramatic. We propose that variable timing of these processes across children leads to symptoms exceeding the threshold for diagnosis at different points in the first 3 years of life, resulting in a distributed curve of onset timing.
3.3. Simplex v. Multiplex Samples
An important question to consider is whether regression in infant sibling samples is representative of regression in children with ASD who are the first in their families to be diagnosed with the condition. If symptoms emerge differently in multiplex and simplex families, then the insights about onset afforded by prospective research may not be applicable to the general population of children with ASD. For example, perhaps children in multiplex families are more likely to experience a regression than children from simplex families, accounting for the higher rates of decline apparent in prospective studies. We have no reason to believe this is the case. In fact, the rate of retrospectively-reported regression in multiplex families has been reported to be similar or lower than in simplex families (Boterberg et al., under review; Parr et al., 2011), failing to account for the high rates apparent in infant sibling studies, whose participants are, by definition, from multiplex families. A related issue is that parents participating in infant sibling investigations have an older child with ASD. It is possible that these parents may be different reporters than other parents, given their previous experience with ASD. This may make them more astute observers of development than parents in the general population and therefore more likely to recognize skill decline. This hypothesis, however, is not supported by the data presented earlier in this review in which parents in multiplex families also under-report skill loss (Landa et al., 2013; Ozonoff et al., 2010, 2018a, 2018b). Nevertheless, it is important to keep these cautions in mind in interpreting the extant data on onset patterns. Validating these results in different kinds of samples, such as community-based epidemiological cohorts or other high-risk groups like very preterm infants, will be critical.
3.4. Improving the Measurement of Onset
Collectively, the studies reviewed in this paper present significant concerns about the accuracy of the most widely used methods of measuring regression, that is, retrospective parent report, and argue against their widespread use. The challenge currently faced by the field is that there are no practical alternative strategies to parent report for characterizing onset status. The time-intensive process and cost of home videotape analysis is prohibitive for large samples. Future studies will continue to rely on retrospective data, of necessity, since inclusion criteria for most samples require a confirmed ASD diagnosis (i.e., not prospective data).
Several strategies have been proposed to improve reporting (Ayhan & Isiksal, 2005). To minimize comprehension or interpretation problems, it is recommended that further specific information about the behavior in question be provided. ASD screening instruments have begun to incorporate video to improve accuracy (Marrus et al., 2018; Smith et al., 2017) and this strategy could be adapted to improve reporting of onset patterns. For example, longitudinal video of a child experiencing skill loss could be shown to parents to illustrate the kinds of changes in behavior that define regression. To minimize recall problems, the simplest approach, and the one shown to have the best validity, is to ask respondents to consult relevant records prior to completing the interview (Ayhan & Isiksal, 2005). Parents could, for example, review entries in baby books or journals or watch home video of the child prior to the interview. Another approach is to link reporting to key events in the respondent’s life by creating a detailed timeline and context that assist recall of specific details (Loftus & Marberger, 1983). This method has already been used by Werner et al. (2005) to improve recall of early development in ASD and it could be further adapted for reporting about onset patterns. Whether these methods will enhance the validity of parent report of onset remains to be seen and would be a fruitful area of future study. For further discussion, see also Boterberg et al. (this issue).
3.5. Validity of Previous Studies of Regression
The studies reviewed in this paper call into serious question the validity of previous studies of regression, which have, of necessity and the lack of alternatives, relied upon retrospective measures. Refining methods of studying the onset of ASD has the potential to transform research programs on etiological factors that contribute to the development of ASD by providing more precise and accurate measurements of an important phenotype (Barbaresi, 2016; Thurm et al., 2018). A better understanding of the inflection points at which development diverges from a typical trajectory to an autism trajectory could be highly informative to the search for risk factors. Better measures of onset are urgently needed for etiologic studies, which have been hindered already by the tremendous heterogeneity of the autism phenotype (Constantino & Charman, 2016). Many recent studies have examined whether onset types are associated with potential etiologic factors and biological correlates, such as brain growth (Nordahl et al., 2011; Valvo et al., 2016), seizures (Barger et al., 2017), vaccinations (Goin-Kochel et al., 2016), gastrointestinal problems (Downs et al., 2014; Richler et al., 2006), immunological function (Scott et al., 2017; Wasilewska et al., 2012), and genetic and genomic variations (Goin-Kochel et al., 2017; Gupta et al., 2017; Parr et al., 2011), including mitochondrial and MeCP2 mutations (Shoffner et al., 2010; Veeraragavan et al., 2016; Xi et al., 2007). So far, none of these factors has been firmly associated with onset patterns. This may be due to the errors that are likely to have occurred in the classifications of onset done in these studies. Clearly, examining the biological underpinnings of an imprecise measure is problematic.
In a review of autism genetics, one of the major priorities identified for future research is the characterization of ASD subtypes to relate to genetic variations (Geschwind, 2011). As more and more risk genes for ASD are identified, the common molecular pathways that these genes share are becoming understood, with some expressed early in neurobiological development and others later (Konopka et al., 2012). A twin study (Hallmayer et al., 2011) suggested a greater role for environmental factors in ASD than previously appreciated. A more precise timing of first symptom emergence would enhance identification of etiological factors and when they might operate, with potential implications for intervention and prevention.
4. Clinical Implications
Finally, the studies reviewed here provide hope and promise for improvements in screening, early diagnosis, and treatment. Many prospective studies (e.g., Bosl et al., 2018; Jones & Klin, 2013; Ozonoff et al., 2010) used measures, such as behavioral coding, eye tracking, and EEG, that are expensive, labor intensive, and not practical for routine use. Studies reviewed in this paper, however, have demonstrated that prospective parent report can identify declining trajectories of development (Ozonoff et al., 2018b; Parikh et al., 2018), as long as the instruments focus on early-appearing social behaviors, present in the first year of life, that have the potential to demonstrate decreases over time as ASD signs emerge. Brief rating scales of this type, administered longitudinally at regular well-child health care visits, could provide a clinically feasible and cost-effective screening tool capable of detecting declines over time. We hypothesize that dynamic screening, which utilizes longitudinal screenings over time and comparison of scores across ages to identify declining trajectories, will improve identification over static, cross-sectional screenings examining whether a single score at a single age exceeds a cutoff. This approach has been successfully used in identifying Rett syndrome (RTT), where head circumference is normal at birth, followed by deceleration of head growth between 5 months and 4 years (Hagberg et al., 2001; Tarquinio et al., 2012). Through the development of RTT-specific growth references throughout early childhood, based on mapping head circumference trajectories, diagnosis of RTT has been possible at earlier ages (Schultz et al., 1993; Tarquinio et al., 2012). We (Ozonoff et al., 2010, 2018b) and others (Landa et al., 2013; Pearson et al., 2018; Thomas et al., 2009) have suggested that this kind of dimensional, trajectory-based methodological approach, percentiling social and communication milestones as we percentile other growth parameters, could be applied to detect ASD early.
Prospective studies have repeatedly demonstrated that developmental declines follow a period in the first year of life when socio-communicative skills are largely intact. Such early intact skills can be capitalized upon in treatment, presenting opportunities for preventive intervention when the brain is rapidly developing and maximally malleable. For many years, the holy grail has been finding a marker present prior to symptom emergence, thus affording the possibility of earlier, possibly preventative, treatment during the prodromal period. If the prospective methods described in this paper can be harnessed to identify infants at risk for ASD, during the decline of skills, rather than after the decline was over, it might be possible to disrupt these trajectories prior to the full onset of symptoms (Dawson, 2011). Children could be provided immediate access to infant interventions (Fein et al., 2016; Rogers et al., 2014), capitalizing on still-preserved skills and harnessing the brain plasticity of early infancy to improve outcomes, lessen disability, and perhaps, prevent the full disorder from developing.
Highlights.
Onset of ASD involves declines in the rates of key social and communication behaviors during the first and second years of life.
Onset patterns are difficult to measure using retrospective methods, such as parent recall and analysis of home movies. This calls into question the results of previous studies that relied upon retrospective measurement.
Prospective methods suggest that regressive onset patterns occur more frequently than previously realized. Regression in ASD is the rule, not the exception.
Acknowledgments
This work was supported by NIH grants R01 MH068398 (Ozonoff) and R01 MH099046 (Ozonoff). Thank you to Sofie Boterberg for her reading of an earlier version of this manuscript. We are deeply grateful to all the children and parents who participated in and showed sustained commitment to our longitudinal program of research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no competing interests to declare.
References
- Andrews N, Miller E, Taylor B, Lingam R, Simmons A, Stowe J, et al. (2002). Recall bias, MMR, and autism. Archives of Diseases of Childhood, 87, 493–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayhan HO, & Isiksal S (2005). Memory recall errors in retrospective surveys: A reverse record check study. Quality and Quantity, 38, 475–493. [Google Scholar]
- Barbaresi WJ (2016). Commentary: The meaning of “regression” in children with autism spectrum disorder: Why does it matter? Journal of Developmental and Behavioral Pediatrics, 37, 506–507. [DOI] [PubMed] [Google Scholar]
- Barger BD, Campbell JM, & McDonough JD (2013). Prevalence and onset of regression within autism spectrum disorders: A meta-analytic review. Journal of Autism and Developmental Disorders, 43, 817–828. [DOI] [PubMed] [Google Scholar]
- Barger BD, Campbell J, & Simmons C (2017). The relationship between regression in autism spectrum disorder, epilepsy, and atypical epileptiform EEGs: A meta-analytic review. Journal of Intellectual & Developmental Disability, 42, 45–60. [Google Scholar]
- Bedford R, Elsabbagh M, Gliga T, Pickles A, Senju A, et al. (2012). Precursors to social and communication difficulties in infants at-risk for autism: gaze following and attentional engagement. Journal of Autism and Developmental Disorders, 42, 2208–2218. [DOI] [PubMed] [Google Scholar]
- Bölte S, Marschik PB, Falck-Ytter T, Charman T, Roeyers H, et al. (2013) Infants at risk for autism: A European perspective on current status, challenges, and opportunities. European Child and Adolescent Psychiatry, 22, 341–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosl WJ, Tager-Flusberg H, & Nelson CA (2018). EEG analytics for early detection of autism spectrum disorder: A data-driven approach. Scientific Reports, 8, 6828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boterberg S, Chairman T, Marschik P, Bolte S, & Roeyers H (this issue). Regression in autism spectrum disorder: Characteristics, etiology, early development, and outcomes – A review of retrospective studies.
- Boterberg S, Van Coster R, & Roeyers H (under review). The clinical contribution of parent reported regression in autism spectrum disorder: Characteristics, early development, and later outcomes. [DOI] [PubMed]
- Brignell A, Williams K, Prior M, Donath S, Reilly S, et al. (2017). Parent-reported patterns of loss and gain in communication in 1- to 2-year-old children are not unique to autism spectrum disorder. Autism, 21, 344–356. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2018). Prevalence of autism spectrum disorder among children aged 8 years – Autism and developmental disabilities monitoring network, 11 sites, United States, 2014. MMWR Surveillance Summary, 67, 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, & Charman T (2016). Diagnosis of autism spectrum disorder: Reconciling the syndrome, its diverse origins, and variation in expression. Lancet Neurology, 15, 279–291. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Zhang Y, Frazier T, Abbacchi AM, & Law P (2010). Sibling recurrence and the genetic epidemiology of autism. American Journal of Psychiatry, 167, 1349–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G (2011). Editorial: Coming closer to describing the variable onset patterns in autism. Journal of the American Academy of Child and Adolescent Psychiatry, 50, 744–746. [DOI] [PubMed] [Google Scholar]
- Downs R, Perna J, Vitelli A, et al. (2014). Model-based hypothesis of gut microbe populations and gut-brain barrier permeability in the development of regressive autism. Medical Hypotheses, 83, 649–655. [DOI] [PubMed] [Google Scholar]
- Elsabbagh M, Fernandes J, Webb SJ, Dawson G, Charman T, et al. (2013). Disengagement of visual attention in infancy is associated with emerging autism in toddlerhood. Biological Psychiatry, 74, 189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein D, Helt M, Brennan L, & Barton M (2016). The activity kit for babies and toddlers at risk: How to use everyday routines to build social and communication skills. New York, NY: Guilford Press. [Google Scholar]
- Finney HC (1981). Improving the reliability of retrospective survey measures: Results of a longitudinal field survey. Evaluation Review, 5, 207–229. [Google Scholar]
- Gammer I, Bedford R, Elsabbagh M, Garwood H, Pasco G, Tucker L, et al. (2015). Behavioral markers for autism in infancy: Scores on the Autism Observational Scale for Infants in a prospective study of at-risk siblings. Infant Behavior and Development, 38, 107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind DH (2011). Genetics of autism spectrum disorders. Trends in Cognitive Sciences, 15, 409–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goin-Kochel RP, Mire SS, Dempsey AG, et al. (2016). Parental report of vaccine receipt in children with autism spectrum disorder: Do rates differ by pattern of ASD onset? Vaccine, 34, 1335–1342. [DOI] [PubMed] [Google Scholar]
- Goin-Kochel RP, Trinh S, Barber S, & Bernier R (2017). Gene disrupting mutations associated with regression in autism spectrum disorder. Journal of Autism and Developmental Disorders, 47, 3600–3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg WA, Thorsen KL, Osann K, & Spence MA (2008). Use of home videotapes to confirm parental reports of regression in autism. Journal of Autism and Developmental Disorders, 38, 1136–1146. [DOI] [PubMed] [Google Scholar]
- Gupta AR, Westphal A, Yang DY, Sullivan CA, Eilbott J, et al. (2017). Neurogenetic analysis of childhood disintegrative disorder. Molecular Autism, 8, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg WA, Thorsen KL, Osann K, & Spence MA (2008). Use of home videotapes to confirm parental reports of regression in autism. Journal of Autism and Developmental Disorders, 38, 1136–1146. [DOI] [PubMed] [Google Scholar]
- Hagberg G, Stenbom Y, & Engerstrom IW (2001). Head grown in Rett syndrome. Brain Development, 23(Supplement), S227–S229. [DOI] [PubMed] [Google Scholar]
- Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, et al. (2011). Genetic heritability and shared environmental factors among twin pairs with autism. Archives of General Psychiatry, 68, 1095–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen RL, Ozonoff S, Krakowiak P, Angkustsiri K, Jones C, et al. (2008). Regression in autism: Prevalence and associated factors in the CHARGE study. Ambulatory Pediatrics, 8, 25–31. [DOI] [PubMed] [Google Scholar]
- Havdahl A, Bishop S, Farmer C, Schjolberg S, Bresnahan M, et al. (2018). Loss of social-communication skills and outcomes during childhood in a large general population cohort. Paper presented at the International Society for Autism Research meeting, Rotterdam. [Google Scholar]
- Henry B, Moffitt TE, Caspi A, Langley J, & Silva PA (1994). On the “remembrance of things past.” A longitudinal evaluation of the retrospective method. Psychological Assessment, 6, 92–101. [Google Scholar]
- Hus V, Taylor A, & Lord C (2011). Telescoping of caregiver report on the autism diagnostic interview-revised. Journal of Child Psychology and Psychiatry, 52, 753–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inada N, Kamio Y, & Koyama T (2010). Developmental chronology of preverbal social behaviors in infancy using the M-CHAT: Baseline for early detection of atypical social development. Research in Autism Spectrum Disorders, 4, 605–611. [Google Scholar]
- Jones Gliga, T., Bedford R, Charman T, & Johnson MH (2014). Developmental pathways to autism: A review of prospective studies of infants at risk. Neuroscience and Biobehavioral Reviews, 39, 1–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones W & Klin A (2013). Attention to eyes is present but in decline in 2 to 6 month old infants later diagnosed with autism. Nature, 504, 427–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner L (1943). Autistic disturbances of affective contact. Nervous Child, 2, 217–250. [PubMed] [Google Scholar]
- Kern JK, Geier DA, & Geier MR (2015). Evaluation of regression in autism spectrum disorder based on parental reports. North American Journal of Medical Sciences, 6, 41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka G, Wexler E, Rosen E, Mukamel Z, Osborn GE, et al. (2012). Modeling the functional genomics of autism using human neurons. Molecular Psychiatry, 17, 202–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landa R, & Garrett-Mayer E (2006). Development in infants with autism spectrum disorders: A prospective study. Journal of Child Psychology and Psychiatry, 47, 629–638. [DOI] [PubMed] [Google Scholar]
- Landa RJ, Stuart EA, Gross AL, & Faherty A (2013). Developmental trajectories in children with and without autism spectrum disorders: The first 3 years. Child Development, 84, 429–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeCouteur A, Lord C, & Rutter M (2003). Autism Diagnostic Interview-Revised (ADI-R). Los Angeles: Western Psychological Services. [Google Scholar]
- Loftus EF, & Marburger W (1983). Since the eruption of Mt. St. Helens, has anyone beaten you up? Improving the accuracy of retrospective reports with landmark events. Memory & Cognition, 11, 114–120. [DOI] [PubMed] [Google Scholar]
- Lord C, Shulman C, & DiLavore P (2004). Regression and word loss in autistic spectrum disorders. Journal of Child Psychology and Psychiatry, 45, 936–955. [DOI] [PubMed] [Google Scholar]
- Marrus N, Kennon-McGill S, Harris B, Zhang Y, Glowinski AL, et al. (2018). Use of a video scoring anchor for rapid serial assessment of social communication in toddlers. Journal of Visualized Experiments, 133, 57041. doi: 10.3791/57041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthen B (2004). Latent variable analysis: growth mixture modeling and related techniques for longitudinal data In Kaplan D (Ed.), Handbook of Quantitative Methodology for the Social Sciences (pp. 345–368). Thousand Oaks, CA: Sage Publications. [Google Scholar]
- Nordahl CW, Lange N, Li DD, et al. (2011). Brain enlargement is associated with regression in preschool-age boys with autism spectrum disorders. Proceedings of the National Academy of Sciences, 108, 20195–20200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Williams BJ, & Landa R (2005). Parental report of the early development of children with regressive autism: The “delays-plus-regression” phenotype. Autism, 9, 495–520. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Iosif A, Baguio F, Cook IC, Hill MM, et al. (2010). A prospective study of the emergence of early behavioral signs of autism. Journal of the American Academy of Child and Adolescent Psychiatry, 49, 258–268. [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Iosif A, Young GS, Hepburn S, Thompson M, et al. (2011a). Onset patterns in autism: Correspondence between home video and parent report. Journal of the American Academy of Child and Adolescent Psychiatry, 50, 796–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Carter A, Messinger D, Yirmiya N, et al. (2011b). Recurrence risk for autism spectrum disorders: A Baby Siblings Research Consortium study. Pediatrics, 128, e488–e495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Li D, Deprey L, Hanzel EP, & Iosif A (2018a). Reliability of parent recall of ASD symptom onset and timing. Autism, 22, 891–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Gangi D, Hanzel EP, Hill A, Hill MM, et al. (2018b). Onset patterns in autism: Variation across informants, methods, and timing. Autism Research, 11, 788–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomo R, Belinchon M, & Ozonoff S (2006). Autism and family home movies: A comprehensive review. Journal of Developmental & Behavioral Pediatrics, 27(Supplement), S59–S68. [DOI] [PubMed] [Google Scholar]
- Parikh C, Iosif A, & Ozonoff S (2018). A longitudinal examination of onset patterns and developmental trajectories among infant siblings of children with autism spectrum disorder. Paper presented at the annual Gatlinburg Conference, San Diego CA. [Google Scholar]
- Parr JR, LeCouteur A, Baird G, Rutter M, Pickles A, et al. &the International Molecular Genetic Study of Autism Consortium (2011). Early developmental regression in ASD: Evidence from an international multiplex sample. Journal of Autism and Developmental Disorders, 41, 332–340. [DOI] [PubMed] [Google Scholar]
- Pearson N, Charman T, Happe F, Bolton PF, & McEwen FS (2018). Regression in autism spectrum disorder: Reconciling findings from retrospective and prospective research. Autism Research, 11, 1602–1620. [DOI] [PubMed] [Google Scholar]
- Pickles A, Pickering K, & Taylor C (1996). Reconciling recalled dates of developmental milestones, events and transitions: A mixed generalized linear model with random mean and variance functions. Journal of the Royal Statistical Society. Series A (Statistics in Society), 159(Part 2), 225–234. [Google Scholar]
- Pickles A, Simonoff E, Conti-Ramsden G, Falcaro M, Simkin Z, et al. (2009). Loss of language in early development of autism and specific language impairment. Journal of Child Psychology and Psychiatry, 50, 843–852. [DOI] [PubMed] [Google Scholar]
- Richler J, Luyster R, Risi S, Hsu W, Dawson G, et al. (2006). Is there a ‘regressive phenotype’ of autism spectrum disorder associated with the measles-mumps-rubella vaccine? A CPEA study. Journal of Autism and Developmental Disorders, 36, 299–316. [DOI] [PubMed] [Google Scholar]
- Rogers SJ (2009). What are infant siblings teaching us about autism in infancy? Autism Research, 2, 125–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SJ, Vismara L, Wagner AL, McCormick C, Young G, & Ozonoff S (2014). Autism treatment in the first year of life: A pilot study of Infant Start, a parent-implemented intervention for symptomatic infants. Journal of Autism and Developmental Disorders, 44, 2981–2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozga A, Hutman T, Young GS, Rogers SJ, Ozonoff S, Dapretto M, & Sigman M (2011). Behavioral profiles of affected and unaffected siblings of children with autism in the first year of life: Contributions of measures of mother-infant interaction and triadic communication. Journal of Autism and Developmental Disorders, 41, 287–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz RJ, Glaze DG, Motil KJ, Armstrong DD, del Junco DJ, et al. (1993). The pattern of growth failure in Rett syndrome. American Journal of Diseases of Children, 147, 633–637. [DOI] [PubMed] [Google Scholar]
- Scott O, Shi D, Andriashek D, Clark B, & Goez HR (2017). Clinical clues for autoimmunity and neuroinflammation in patients with autistic regression. Developmental Medicine & Child Neurology, 59, 947–951. [DOI] [PubMed] [Google Scholar]
- Shoffner J, Hyams L, Langley GN, Cossette S, Mylacraine L, et al. (2010). Fever plus mitochondrial disease could be risk factors for autistic regression. Journal of Child Neurology, 25, 429–434. [DOI] [PubMed] [Google Scholar]
- Shumway S, Thurm A, Swedo SE, Deprey L, Barnett LA, Amaral DG, Rogers SJ, & Ozonoff S (2011). Symptom onset patterns and functional outcomes in young children with autism spectrum disorders. Journal of Autism and Developmental Disorders, 41, 1727–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CJ, Rozga A, Matthews N, Oberleitner R, Nazneen N, et al. (2017). Investigating the accuracy of a novel telehealth diagnostic approach for autism spectrum disorder. Psychological Assessment, 29, 245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szatmari P, Chawarska K, Dawson G, Georgiades S, Landa R, et al. (2016). Prospective longitudinal studies of infant siblings of children with autism: Lessons learned and future directions. Journal of the American Academy of Child and Adolescent Psychiatry, 55, 179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarquinio DC, Motil KJ, Hou W, Lee HS, Glaze DG, et al. (2012). Growth failure and outcome in Rett syndrome: Specific growth references. Neurology, 79, 1653–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MSC, Annaz D, Ansari D, Scerif G, Jarrold C, Karmiloff-Smith A (2009). Using developmental trajectories to understand developmental disorders. Journal of Speech, Language, and Hearing Research, 52, 336–358. [DOI] [PubMed] [Google Scholar]
- Thurm A, Manwaring SS, Luckenbaugh DA, Lord C, & Swedo SE (2014). Patterns of skill attainment and loss in young children with autism. Development and Psychopathology, 26, 203–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurm A, Powell EM, Neul JL, Wagner A, & Zwaigenbaum L (2018). Loss of skills and onset patterns in neurodevelopmental disorders: Understanding the neurobiological mechanisms. Autism Research, 11, 212–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valvo G, Baldini S, Retico A, et al. (2016). Temporal lobe connects regression and macrocephaly to autism spectrum disorders. European Child and Adolescent Psychiatry, 25, 421–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeraragavan S, Wan YW, Connolly DR, Hamilton SM, Ward CS, et al. (2016). Loss of MeCP2 in the rat models regression, impaired sociability and transcriptional deficits of Rett syndrome. Human Molecular Genetics, 25, 3284–3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan MW, Green J, Elsabbagh M, Johnson M, Charman T, et al. (2013). Quality of interaction between at-risk infants and caregivers at 12-15 months is associated with 3-year autism outcome. Journal of Child Psychology and Psychiatry, 54, 763–771. [DOI] [PubMed] [Google Scholar]
- Wasilewska J, Kaczmarski M, Stasiak-Barmuta A, Tobolczyk J, & Kowalewska E (2012). Low serum IgA and increased expression of CD23 on B lymphocytes in peripheral blood in children with regressive autism aged 3-6 years old. Archives of Medical Science, 8, 324–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner E, & Dawson G (2005). Validation of the phenomenon of autistic regression using home videotapes. Archives of General Psychiatry, 62, 889–895. [DOI] [PubMed] [Google Scholar]
- Werner E, Dawson G, Munson J, & Osterling J (2005). Variation in early developmental course in autism and relation with behavioral outcome at 3-4 years of age. Journal of Autism & Developmental Disorders, 35, 337–350. [DOI] [PubMed] [Google Scholar]
- Wetherby A, & Prizant B (2002). Communication and symbolic behavior scales developmental profile—First normed edition. Baltimore: Paul H. Brookes. [Google Scholar]
- Wolff JJ, Botteron KN, Dager SR, Elison JT, Estes AM, et al. (2014). Longitudinal patterns of repetitive behavior in toddlers with autism. Journal of Child Psychology and Psychiatry, 55, 945–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi CY, Ma HW, Lu Y, Zhao YJ, Hua TY, Zhao Y, & Ji YH (2007). MeCP2 gene mutation analysis in autistic boys with developmental regression. Psychiatric Genetics, 17, 113–116. [DOI] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, & Szatmari P (2005). Behavioral manifestations of autism in the first year of life. International Journal of Developmental Neuroscience, 23, 143–152. [DOI] [PubMed] [Google Scholar]