Abstract
Supraphysiologic-dose anabolic-androgenic steroid (AAS) use is associated with physiologic, cognitive, and brain abnormalities similar to those found in people at risk for developing Alzheimer’s Disease and its related dementias (AD/ADRD), which are associated with high brain β-amyloid (Aβ) and hyperphosphorylated tau (tau-P) protein levels. Supraphysiologic-dose AAS induces androgen abnormalities and excess oxidative stress, which have been linked to increased and decreased expression or activity of proteins that synthesize and eliminate, respectively, Aβ and tau-P. Aβ and tau-P accumulation may begin soon after initiating supraphysiologic-dose AAS use, which typically occurs in the early 20s, and their accumulation may be accelerated by other psychoactive substance use, which is common among non-medical AAS users. Accordingly, the widespread use of supraphysiologic-dose AAS may increase the numbers of people who develop dementia. Early diagnosis and correction of sex-steroid level abnormalities and excess oxidative stress could attenuate risk for developing AD/ADRD in supraphysiologic-dose AAS users, in people with other substance use disorders, and in people with low sex-steroid levels or excess oxidative stress associated with aging.
Keywords: Aging, alcohol, Alzheimer’s disease, amyloid, anabolic-androgenic steroid, ApoE, Aquaporin 4, α-secretase, β-secretase, body-building, boldenone, cannabis, cocaine, dementia, estrogen, γ-secretase, GSK3β, heroin, homocysteine, hypogonadism, insomnia, insulin degrading enzyme, low-density lipoprotein receptor-related protein1, magnetic resonance imaging, magnetic resonance spectroscopy, menopause, methamphetamine, morphine, muscularity, N-acetylcysteine, nandrolone, neprilysin, neurodegeneration, Nrf2, opioid, oxidative stress, oxymetholone, performance-enhancing drugs, PET imaging, polydrug use, prealbumin, presenilin, protein phosphatase 2A, scyllo-inositol, stanozolol, tau, tobacco, sex-steroid, sleep disturbances, substance use disorder, testosterone, zinc
Graphical Abstract
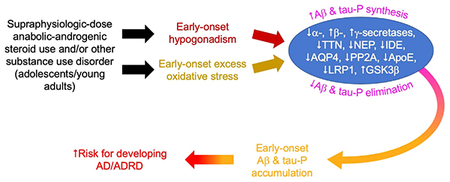
Supraphysiologic-dose anabolic-androgenic steroid use, which typically commences by the mid-20s, induces early-onset hypogonadism and excess oxidative stress. In turn, these abnormalities alter the function of many proteins involved in Aβ and tau-P synthesis and elimination. This could result in early-onset accumulation of these proteins in brain and increased risk for developing Alzheimer’s Disease and its related dementias.
1. Introduction
Anabolic-androgenic steroids (AAS) are a group of anabolic substances including the naturally occurring sex-steroid hormone testosterone (T) and synthetic T analogs designed for oral or injectable dosing. Synthetic AAS were developed in the 1930s (David et al., 1935; Wettstein, 1935) and by the 1950s, supraphysiologic-dose AAS were widely used by elite athletes (Kanayama et al., 2008, 2010; Pope et al., 2014a). By the 1980s, such use had spread to the general United States (US) population. Supraphysiologic-dose AAS are used almost entirely by men (98% of users) seeking to enhance muscularity. Few women use supraphysiologic-dose AAS because women rarely seek to become extremely muscular and because women are vulnerable to the masculinizing effects of AAS, including beard growth and virilization of secondary sexual characteristics (Kanayama et al. 2007, Kanayama and Pope, 2012a; Pope et al., 2014b). Accordingly, this review focuses primarily on the effects of supraphysiologic-dose (non-medical) AAS exposures in males, although some of the effects we discuss are directly relevant to AAS-nonusers and to women.
Currently, it is estimated that close to 4 million people in the US have used supraphysiologic-dose AAS and about one-third of users become AAS-dependent at some time (Brower, 2009; Kanayama et al., 2009a; Hildebrandt et al., 2011; Kanayama and Pope, 2012b; Pope et al., 2014a). There are nearly 100,000 new supraphysiologic-dose AAS users in the US each year (Kanayama and Pope, 2018) with a median age of initiation of 23 (Pope et al., 2014b; Sagoe et al., 2014). Surveys in other countries suggest that supraphysiologic-dose AAS use is a substantial global public health problem (Sagoe et al., 2014).
Long-term supraphysiologic-dose AAS exposures are associated with abnormalities in liver and kidney (Modlinski and Fields, 2006; Herlitz et al., 2010), endocrine (Tan and Scally, 2009; de Souza and Hallak, 2011; Coward et al., 2013; Kanayama et al., 2015; Rasmussen et al., 2016; Christou et al., 2017), and cardiovascular (Sullivan et al., 1998; Santora et al., 2006; Achar et al., 2010; Baggish et al., 2010, 2017; Thiblin et al,. 2015) systems. Additionally, recent studies from our laboratory and from other groups reporting on physiologic, cognitive, and brain abnormalities in long-term supraphysiologic-dose AAS users (Kanayama et al., 2013; Hildebrandt et al., 2014; Heffernan et al., 2015; Kaufman et al., 2015: Westlye et al., 2016; Bjørnebekk et al., 2017) detected abnormalities similar to those found in people diagnosed with or at risk for developing a family of dementias known as Alzheimer’s Disease and its related dementias (AD/ADRD). This suggests that such users may be at increased risk for developing dementia and, consequently, that as they reach the age of 75, the average age of diagnosis of late-onset AD (Barnes et al., 2015), they will experience a higher prevalence of AD/ADRD than the general population. We hypothesize that an increase in incidence of AD/ADRD diagnoses in supraphysiologic-dose AAS users has yet to be detected because the large majority remain under the age of 60 today (Kanayama et al., 2008, 2010) – too young to experience overt symptoms of late-onset AD/ADRD.
This review aims to synthesize published findings on the effects of supraphysiologic-dose AAS use and to highlight key deleterious effects induced by this type of AAS use, hypogonadism, and excess oxidative stress, which accelerate syntheses and brain accumulation of β-amyloid (Aβ, including Aβ40 and Aβ42 forms) and hyperphosphorylated tau (tau-P) proteins. These proteins likely have important causal roles in the pathophysiology of AD/ADRD (Götz and Ittner, 2008). Since supraphysiologic-dose AAS users also use other psychoactive substances, some of which have been independently associated with increased risk for AD/ADRD, we discuss how psychoactive substance use by AAS users and by people who do not take AAS could potentiate risk for developing AD/ADRD. Lastly, we discuss how existing and novel drug therapies, some of which already are being tested as treatments for substance use disorders, could reduce risks for developing AD/ADRD in supraphysiologic-dose AAS users and in other at-risk populations.
2. Supraphysiologic-dose AAS use patterns and effects on androgen levels
2.1. Human studies of AAS effects
Supraphysiologic doses of AAS are self-administered because they increase muscularity and strength. A study of the effects of T-enanthate (3mg/kg, once/week) given for 12 weeks to young/middle-aged men without prior illicit AAS use histories reported an increase in serum T levels to 1.7 μg/dL (normal physiologic range: approximately 0.3–0.9 μg/dL), together with increased muscle protein synthesis, but no increase in muscle volume (Griggs et al., 1989). Placebo-controlled laboratory studies in healthy men without supraphysiologic-dose AAS exposure histories documented that 10 weeks of supraphysiologic T (T-enanthate, 600 mg, intramuscular (IM) once/week, 7.5 mg/kg/week for an 80 kg subject), when combined with exercise, increased weightlifting capacities by more than 20% and increased triceps and quadriceps cross-sectional muscle areas by more than 13% (Bhasin et al., 1996). This AAS regimen increased serum total T concentrations to about 3 μg/dL, nearly 6 times higher than normal levels (Bhasin et al., 1996). Subsequent studies by this group reported that lower weekly T doses induced smaller strength and cross-sectional muscle area increases, and that under the conditions studied, supraphysiologic T promoted strength and muscle volume increases in a dose-related manner (Bhasin et al., 2001). In older men, T supplementation (T-enanthate, 25–600 mg IM, once/week) also increased muscularity in a dose-related manner (Bhasin et al., 2005).
2.2. Supraphysiologic AAS self-administration patterns
Supraphysiologic-dose AAS self-administration involves complex, customized patterns of AAS intake including concurrent use of supraphysiologic T plus other AAS to achieve even larger strength and muscle mass gains (e.g., Graham et al., 2008; Ip et al., 2011), a practice termed “stacking.” Illicit AAS users take substantially higher T doses than used clinically to treat male hypogonadism, which typically are ~100 mg/week (Surampudi et al., 2014), and many users take substantially higher AAS doses than those administered in the experimental studies described above. For example, self-reported weekly T or T-equivalent (via self-administration of multiple AAS) doses range from 250 to 5000 mg/week (Pope and Katz, 1994; Yu et al., 2014). Online surveys of large numbers of supraphysiologic dose AAS users have found that up to 60% of respondents reported taking more than 1000 mg/week of AAS (Parkinson and Evans, 2006; Ip et al., 2011; Westerman et al., 2016), or up to 10 mg/kg/week for a 100 kg subject. Thus, self-administration of extremely high AAS doses is prevalent. Systemic total T levels reported in such users range from concentrations of 2.8 μg/dL (Rasmussen et al., 2016), a level similar to those achieved in some laboratory studies, up to much higher levels exceeding 8 mg/dL (Hengevoss et al., 2015), ~16 times normal serum total T levels in men.
Users typically self-administer supraphysiologic-dose AAS in alternating cycles of AAS ingestion (“on-cycle”) and AAS abstinence (“off-cycle”) (Graham et al., 2008; Kanayama et al., 2009a). Users cycle off of AAS to reduce side effects and risks associated with prolonged supraphysiologic-dose AAS use, including infertility and gynecomastia (Pope and Katz, 1994; Christou et al., 2017). AAS on-cycle durations are typically on the order of several months but in long-term users, virtually continuous AAS use persisting for years has been reported (Brower, 2002; Kanayama et al., 2009a; Ip et al., 2011; Westerman et al., 2016). Those using supraphysiologic-dose AAS for years despite experiencing adverse medical or psychiatric consequences meet formal DSM criteria for AAS dependence (Kanayama et al., 2009b). Among supraphysiologic-dose AAS users as a whole, the prevalence of AAS dependence has been reported to range from 14% (Malone et al., 1995) to 57% (Brower et al., 1991), with a median prevalence across studies of 30% (Pope et al., 2014b).
2.3. Supraphysiologic AAS exposure-induced hypogonadism
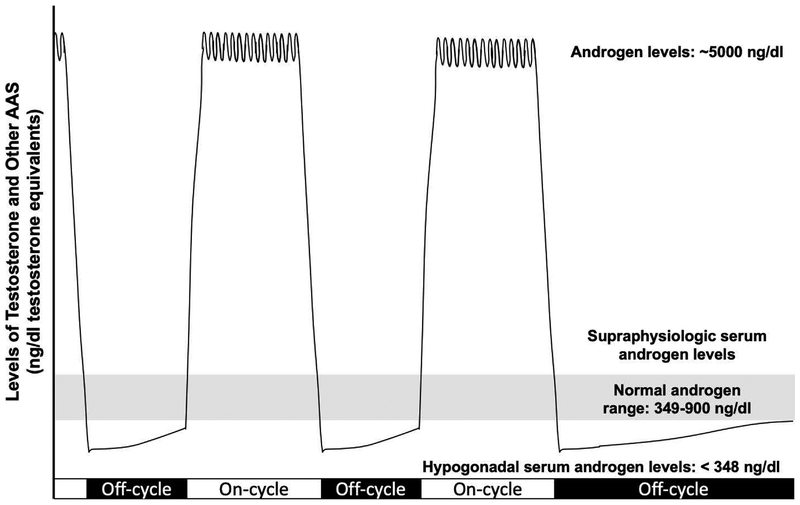
Although hypogonadism in men is rare under the age of 60 (Bhasin et al., 2011), supraphysiologic-dose androgen use results in feed-back suppression of the hypothalamic-pituitary-testicular (HPT) axis, which leads to decreased production of natural T and thereby induces hypogonadism. When male supraphysiologic-dose AAS users suspend AAS use, previously suppressed HPT axis function slowly returns in some users (Alèn et al., 1987). However, other users apparently do not spontaneously recover HPT axis function and their ability to produce T, resulting in a chronic hypogonadal state defined as a serum total T concentration of <348 ng/dL (Bhasin et al., 2011). AAS-induced hypogonadism has been described in case reports (reviewed by Kanayama et al., 2015; Rasmussen et al., 2016) and has been reported after as few as 12 weeks of supraphysiologic-dose AAS use (Martikainen et al., 1986). A study of all-cause male hypogonadism found that 20% of cases were associated with prior supraphysiologic-dose AAS use and that hypogonadism occurring before age 50 was strongly associated with prior supraphysiologic-dose AAS use (Coward et al., 2013). Among former users, hypogonadism is prevalent and persistent, ranging from 27% in subjects abstinent for 1.7 to 3.7 years (Rasmussen et al., 2016) to 53% in subjects abstinent for 3 months to over 10 years (Kanayama et al., 2015). Accordingly, a substantial proportion of supraphysiologic-dose AAS users experience very high systemic androgen levels (while on-cycle) and subphysiologic/hypogonadal systemic T levels for prolonged periods after suspending AAS use (Figure 1). Thus, most supraphysiologic-dose AAS users rarely experience physiologically normal (that is, eugonadal) androgen levels.
Figure 1:
Androgen Level Extremes During Supraphysiologic-dose AAS Cycling: Users Rarely Experience Physiologically-Normal (Eugonadal) Androgen Levels
T also is synthesized in brain. In male rat brain, T concentrations up 4 times higher than systemic T levels have been reported (Hojo et al., 2009; Caruso et al., 2010; Tobiansky et al., 2018). However, gonadectomy substantially depletes T levels in male rat whole brain cortex, medial frontal cortex, hippocampus, ventral tegmentum, nucleus accumbens, and cerebellum (Hojo et al., 2009; Caruso et al., 2010; Tobiansky et al., 2018). Brain T and DHT levels also decline with aging in men and in male mice (Rosario et al., 2009, 2011; Caruso et al., 2013). Thus, male systemic hypogonadism is accompanied by hypogonadal brain T levels. Importantly, as discussed below, significant departures from eugonadal T levels, systemically and in brain, can trigger deleterious effects, including increased Aβ and tau-P accumulations, which could increase risk for developing AD/ADRD.
3. Supraphysiologic-dose AAS exposures are associated with cognitive, cardiovascular, and sleep abnormalities similar to those found in people diagnosed with or at increased risk for developing AD/ADRD
3.1. Cognitive effects of supraphysiologic-dose AAS exposures
Chronic supraphysiologic-dose AAS use by men has been associated with the development of spatial memory impairments similar to those found in people with AD/ADRD. Using the Cambridge Neuropsychological Test Automated Battery (CANTAB) paired associates learning (PAL) and the pattern recognition memory (PRM) tasks, we detected visuospatial memory impairments in a British cohort of supraphysiologic-dose AAS users (Kanayama et al., 2013). Error numbers on these tasks were higher in men with higher self-reported lifetime cumulative AAS doses, suggesting a cumulative dose-effect relationship between supraphysiologic-dose AAS use and visuospatial memory impairment (Kanayama et al., 2013). The PAL and PRM tasks have been shown to differentiate individuals with mild dementia from healthy individuals and individuals with mild dementia those with AD, respectively (Swainson et al., 2001), suggesting that the cognitive abnormalities we found in supraphysiologic-dose AAS users could reflect increased risk for AD/ADRD. Another study found that on-cycle supraphysiologic-dose AAS users displayed a diminished ability to shift attention on a cognitive attention and set-shifting task as compared to off-cycle users, and also found that adolescent-onset supraphysiologic-dose AAS users displayed poorer spatial-planning efficiency on cognitive testing than individuals with adult onset use (Hildebrandt et al., 2014). A study of young-adult (18–30 years old) AAS-using respondents to an online questionnaire reported higher levels of retrospective and prospective memory and executive function deficits (Heffernan et al., 2015). Subsequently, we reported a possible impairment of CANTAB visuospatial memory performance on the PAL, albeit not reaching statistical significance (P = 0.052), in American middle-aged supraphysiologic-dose AAS users (Kaufman et al., 2015), consistent with our earlier finding in a British cohort (Kanayama et al., 2013).
Animal studies of the effects of supraphysiologic-dose AAS exposures in males report cognition abnormalities comparable to those found in humans. Nandrolone (15 mg/kg, subcutaneous: SC) given daily for 6 weeks impaired rat social memory (Kouvelas et al., 2008) and when given either daily for 2 weeks or every third day for 4 weeks reduced Morris Water Maze task spatial memory performance (Magnusson et al., 2009; Tanehkar et al., 2013). Morris Water Maze task spatial memory retention was impaired in rats given nandrolone or stanozolol (5 mg/kg, SC) for 4 weeks (Pieretti et al., 2013). Rats given chronic T (7.5 mg/kg/day, SC, 5 days/week) exhibited reversal learning and set shifting task deficits (Wallin and Wood, 2015).
3.2. Cognitive effects of low androgen levels
The cognitive effects of AAS-induced hypogonadism have not been assessed. However, low androgen levels accompanying other health conditions, including normal aging (van den Beld et al., 2000; Moffat et al., 2002; Muller et al., 2003; Bhasin et al., 2005, 2011; Rosario et a., 2011; Yeap et al., 2012; Caruso et al., 2013), have been associated with cognitive dysfunction. In otherwise healthy older men, nearly half of whom were hypogonadal, associations were found between serum T levels and working memory, although no associations were found between T levels and spatial cognition (Matousek and Sherwin, 2010). Men with low T levels due to idiopathic hypogonadotrophic hypogonadism were impaired on several tests of spatial cognition (Hier and Crowley, 1982). In individuals with prostate cancer, therapeutic androgen ablation was associated with impaired spatial cognition and working memory (Cherrier et al., 2003, 2009). Thus, a number of studies, but not all (e.g., Matousek and Sherman, 2010), report associations between low circulating T levels and impaired spatial cognition in men. Low serum T or bioavailable T levels in older men have been associated with increased risk for developing mild cognitive impairment or dementia (Chu et al., 2008, 2010; Carcaillon et al., 2014), and postmortem frontal cortex T levels are substantially lower in men diagnosed with mild cognitive impairment (MCI) or AD than T levels in men without these conditions (Rosario et al., 2004). Similarly, animal studies of castrated male rats have reported impaired spatial working memory (Gibbs and Johnson, 2008; McConnell et al., 2012; Locklear and Kritzer, 2014) and/or impaired memory retention (Sandstrom et al., 2006; Hawley et al., 2013; Locklear and Kritzer, 2014). Thus, hypogonadism, including that associated with supraphysiologic-dose AAS use, may increase risk for developing spatial memory and other cognitive impairments.
3.3. Cardiovascular abnormalities associated with supraphysiologic-dose AAS exposures
Supraphysiologic-dose AAS use doubles the risk for experiencing cardiovascular morbidity and mortality (Thiblin et al., 2015). Professional bodybuilders with supraphysiologic-dose AAS use histories averaging more than 12 years had increased coronary artery calcium levels (Santora et al., 2006). Subsequently, we reported reduced left ventricular ejection fractions and ventricular diastolic impairments in middle-aged long-term users of supraphysiologic-dose AAS (Baggish et al., 2010), effects that we and others confirmed in subsequent larger-scale studies (Angell et al., 2012; Luijkx et al., 2013; Baggish et al., 2017; Rasmussen et al., 2018a). Other groups also have reported finding right ventricular abnormalities (Angell et al., 2014; Alizade et al., 2016; Rasmussen et al., 2018a), apoptosis-related fibrotic changes in the heart (Cecchi et al., 2017), increased systolic blood pressure and aortic stiffness (Rasmussen et al., 2018b), and a pro-coagulant state (Chang et al., 2018) in long-term supraphysiologic-dose AAS users. In an echocardiography study in male rabbits, 6 months of supraphysiologic nandrolone administration (SC, 4 or 10 mg/kg, twice/week) was associated with impaired global myocardial performance in a dose-related manner (Vasilaki et al., 2016). These cardiovascular abnormalities have been independently linked to cognitive (including visuospatial memory) impairments, accelerated cognitive aging, dementia, and AD (Hoth et al., 2010; Jefferson et al., 2011; Quinn et al., 2011; Arangalage et al., 2015; Walker et al., 2017; Cui et al., 2018). Accordingly, supraphysiologic-dose AAS-induced cardiovascular abnormalities could amplify risk for developing AD/ADRD.
3.4. Sleep abnormalities associated with supraphysiologic-dose AAS exposures
Sleep disturbances, including insomnia, are among the most common symptoms reported by supraphysiologic-dose AAS users, occurring in 25–50% of cases (Korkia and Stimson, 1997; Bolding et al., 2002; Eklöf et al., 2003; Parkinson and Evans, 2006; Ip et al., 2011), and are more common among men who “stack” by using multiple AAS (Bolding et al., 2002). On-cycle AAS users exhibited lower sleep efficiency and increased wakefulness after sleep onset (Venâncio et al., 2008). In a laboratory study in healthy elderly men without prior AAS use histories, short-term high dose T (Sustanon, a mixture of T esters, 250–500 mg/week IM, for 2 weeks) reduced sleep time by an average of 1 hour (Liu et al., 2003a). Placebo-controlled studies in former supraphysiologic- dose AAS users or never-users found that 6 weeks of stepped T administration culminating in supraphysiologic T exposures (T-cypionate, 2 weeks at 150 mg IM once/week, 2 weeks at 300 mg once/week, 2 weeks at 600 mg once/week) induced manic or hypomanic symptoms in some subjects (Pope et al., 2000). Similarly, oral methyl-T (up to 240 mg/day) taken by healthy male AAS non-users for several days induced mania or hypomania in 2 (10%) of 20 subjects (Su et al., 1993). By reducing the need for sleep, mania/hypomania could contribute to sleep abnormalities in AAS users. Sleep disturbances have been linked to increased cortical and precuneus Aβ levels in healthy elderly adults (Spira et al., 2013) and sleep abnormalities worsen the pathophysiology underlying AD (Vanderheyden et al., 2018). Even one night of sleep deprivation of healthy young adults increased plasma and brain Aβ levels (Wei et al., 2017; Shokri-Kojori et al., 2018) and self-reported sleep duration in healthy subjects was associated with decreased precuneus Aβ levels (Shokri-Kojori et al., 2018). Accordingly, sleep disturbances in supraphysiologic-dose AAS users and in other groups may increase Aβ accumulation.
4. Brain structural, functional, and chemical effects of supraphysiologic-dose AAS exposures
4.1. Supraphysiologic-dose AAS exposures are associated with abnormal brain structure and functional connectivity
In a structural MRI study of long-term users of supraphysiologic-dose AAS (mean use duration = 9.3 years), we detected increased mean right amygdala volumes (Kaufman et al., 2015). The right lateralization of this structural abnormality prompted us to conduct a right amygdala seed-point analysis of resting state (task independent) functional MRI (fMRI) connectivity data collected from these same subjects. We found lower connectivity between the right amygdala and several cortical areas (dorsolateral prefrontal cortex, anterior cingulate cortex, superior frontal gyrus, precuneus, and cuneus), subcortical areas (caudate nucleus, putamen, nucleus accumbens, and hippocampus), and cerebellum. By contrast, few left amygdala connectivity differences were detected (Kaufman et al., 2015). Thus, both amygdala structural and functional connectivity abnormalities were right-lateralized. These findings are consistent with a human brain developmental study reporting that right amygdala volume in both sexes is sensitive to sex-steroid hormone levels and predicted by T levels (Herting et al., 2014), as well as with animal studies reporting that adult amygdala volume is sensitive to androgens (Cooke et al., 1999; Johnson et al., 2012), an effect limited to right amygdala in males (Johnson et al., 2012).
A subsequent large-scale structural MRI study of users of long-term supraphysiologic-dose AAS (mean use duration = 9.1 years) reported smaller cortical, gray matter, putamen, and corpus callosum volumes, but did not detect a total amygdala (right + left hemisphere) volume abnormality (Bjørnebekk et al., 2017). This study also reported widespread cortical thinning, including in the occipital pole (cuneus), precuneus, precentral gyrus, posterior cingulate gyrus, temporal lobe, and superior frontal cortex (Bjørnebekk et al., 2017). Greater cortical thinning was detected in long- versus short-term AAS users, suggesting a cumulative dose-effect relationship between AAS and cortical thickness (Bjørnebekk et al., 2017). This research group also analyzed resting state fMRI connectivity data from their subjects using a whole brain data-driven independent components analysis (ICA) approach (Westlye et al., 2016). Low amygdala connectivity with the default mode network (DMN) was found in current but not former AAS users, as was low connectivity between the dorsal attention network (DAN) and the superior frontal gyrus in former and current AAS users (Westlye et al., 2016). DMN-amygdala connectivities were reduced to a greater extent in AAS users taking higher versus lower AAS doses and in on- versus off-cycle AAS users, while DAN-superior frontal gyrus connectivities were reduced to a greater extent in dependent versus nondependent AAS users and in AAS users taking higher versus lower AAS doses (Westlye et al., 2016). Collectively, the structural and functional connectivity findings in supraphysiologic-dose AAS users implicate amygdala network disruption and widespread cortical thinning induced by long-term high-dose AAS use, possibly in a dose- or duration-dependent manner. Notably, the brain areas affected by AAS include regions in which the earliest increases in Aβ levels are reported, including amygdala (Sepulcre et al., 2013), precuneus (Gordon et al., 2018), and posterior cingulate cortex, which is an Aβ propagation hub (Sepulcre et al., 2018).
4.2. Supraphysiologic-dose AAS exposures are associated with neurochemical abnormalities
In our study of long-term AAS users cited above (Kaufman et al., 2015), we also acquired proton [1H] magnetic resonance spectroscopy (MRS) scans of dorsal anterior cingulate cortex. We detected an abnormally high glutamine/glutamate metabolite ratio (Kaufman et al., 2015). Because glutamine is a catabolite of glutamate, we interpreted the high glutamine/glutamate ratios in AAS users to reflect increased glutamate turnover (e.g., higher glutamate release and/or lower glutamate reuptake, leading to increased glutamate catabolism to glutamine). This interpretation is consistent with the finding that prolonged administration of a supraphysiologic dose of the AAS nandrolone (15 mg/kg, SC, 19 days) to male mice decreased expression of the astrocyte glutamate transporter (GLT-1), decreased hippocampal and cortical glutamate uptake, and increased hippocampal extracellular glutamate levels (Kalinine et al., 2014)—effects that likely increase glutamate availability for catabolism to glutamine. Nandrolone, like a number of other AAS, induces excess oxidative stress (see section 6.1 and Table 1), which reduces GLT-1 expression and function (Trotti et al., 1996, 1997; Blanc et al., 1998; Keller et al., 1997). High glutamate levels inhibit protein phosphatase 2A (Yi et al., 2005, 2008), an enzyme that reduces tau-P levels by dephosphorylation (see section 7.7), and thus high glutamate levels in AAS users could increase tau-P levels and risk for developing AD/ADRD.
Table 1.
Supraphysiologic doses of commonly-used AAS administered to animals induce widespread oxidative stress, as evidenced by oxidative small molecule, DNA, mRNA, protein, and lipid modifications and antioxidant enzyme expression changes
| Study/AAS/Dose | Model | Route/Duration | Tissue | Oxidative Stress Abnormalities |
|---|---|---|---|---|
| 1Nandrolone/20 mg kg−1 | Rats | SC, Single Depot/4 Weeks | Blood | ↑TBARS |
| 2Nandrolone/10 mg kg−1 | Rabbits | IM, Twice Weekly/6 Months | Blood | ↑TBARS |
| 3Stanozolol/2 or 5 mg kg−1 | Rats | IM, Weekly/8 Weeks | Erythrocyte | ↑GPx |
| 4Testosterone/≥500 nM | TM3 Leydig Cell culture | Culture Application, 1 Hour | Leydig Cell | ↑ROS ↑Lipid Peroxides |
| 5Testosterone/5 mg kg−1 | Rats | IM, Every 48 Hours/2 Weeks | Testicular | ↑TBARS, ↑AGEs, ↓TAC |
| 6Nandrolone/10 mg kg−1 | Rats | IM, Weekly/8 Weeks | Testicular | ↑MDA, ↑8-OHdG, ↓GSH, ↓iSOD |
| 7Nandrolone/5 mg kg−1 | Rats | IM, Twice Weekly/8 Weeks | Prostate | ↑NOX1, ↑Nrf2 |
| 8Boldenone or Stanozolol/1.25 or 5 mg kg−1 | Rats | IM, Weekly/12 Weeks for Low Dose or 4 Weeks for High Dose | Testicular | ↑ROS, ↑MDA |
| 9Oxymetholone or Methyltestosterone/100 uM | Rat 1° hepatocyte culture | Culture Application, 2–4 Hours | Hepatocyte | ↓GSH |
| 10Stanozolol/2 mg kg−1 | Rats | PO, 5 Days Each Week/8 Weeks | Liver | ↑TBARS, ↑GSH, ↑SOD, ↑CAT, ↑GPx |
| 11Nandrolone/10 mg kg−1 | Rats | IM, Weekly/8 Weeks | Liver; Kidney | ↑NOX1, ↓SOD, ↓CAT, ↓Thiols; ↑Carbonyls |
| 12Nandrolone/3.75 or 10 mg kg−1 | Mice | IM, Twice Weekly/7 Weeks | Kidney | ↑MDA, ↓GPx, ↓GR |
| 13Sustanon/10 mg kg−1 | Rats | IM, Weekly/8 Weeks | Liver | ↑SOD, ↑GPx, ↑GR |
| 14Boldenone or Stanozolol/1.25 or 5 mg kg−1 | Rats | IM, Weekly/12 Weeks for Low Dose or 4 Weeks for High Dose | Liver and Kidney | ↑ROS, ↑TBARS, ↓GSH |
| 15Testosterone/8 or 80 mg kg−1 | Rats | IM, Weekly/6 Weeks | Liver | ↑MDA, ↓SOD, ↓CAT, ↓GPx, ↓GR |
| 16Nandrolone/10 mg kg−1 | Rats | IM, Thrice Weekly/6 Weeks | Kidney | ↑8-OHdG |
| 16aStanozolol/20 mg kg−1 | LDLr−/− mice | SC, Weekly/8 Weeks | Liver | ↑TBARS, ↑AOPP |
| 17Stanozolol/2 mg kg−1 | Rats | PO, 5 Days Each Week/8 Weeks | Muscle | ↑SOD |
| 18Stanozolol/5 mg kg−1 | Rats | SC, 5 Days Each Week/6 Weeks | Heart | ↑SOD, ↑CAT |
| 11Nandrolone/10 mg kg−1 | Rats | IM, Weekly/8 Weeks | Heart | ↑NOX1 |
| 19Nandrolone/10 mg kg−1 | Rats | IM, Thrice Weekly/6 Weeks | Heart | ↑Oxidized LDL, ↑NOX1, ↑8-OHdG, ↑HCY |
| 20Stanozolol/5 mg kg−1 | Rats | SC, 5 Days Each Week/4 Weeks | Heart | ↑Carbonylation |
| 21Testosterone/100 nM | Neuronal N27 Cells* | Culture Application, 48 Hours | Neuronal | ↑Peroxides ↓Thiols |
| 22Nandrolone/10 mg kg−1 | Rats | IM, Weekly/8 Weeks | PF Cortex & Hippocampus | ↑MDA, ↓GPx |
| 23Nandrolone/15 mg kg−1 | Rats | SC, Every 3rd Day/30 Days | Brain | ↑MDA, ↓Nrf2, ↓CAT |
| 24Stanozolol/10 mg kg−1 | Rats | IM, Weekly/12 Weeks | Brain | ↑GPx |
| 25Nandrolone/1.875 mg kg−1 | Rats | IM, Twice Weekly/8 Weeks | PF Cortex, Striatal, Hipp., & Cb | ↑MDA |
| 26Boldenone or Stanozolol/1.25 or 5 mg kg−1 | Rats | IM, Weekly/12 Weeks for Low Dose or 4 Weeks for High Dose | Cortical & Hipp. | ↑ROS, ↑MDA, ↓GSH |
| 27Testosterone/20 mg kg−1 | Rats | SC, Weekly/6 Weeks | Hipp. | ↑TBARS, ↓SOD |
Sex of cells not specified.
AAS: anabolic-androgenic steroid; AGEs: advanced glycation endproducts; AOPP: Advanced oxidation protein products; CAT: catalase; Cb: cerebellum; GPx: glutathione peroxidase; GR: glutathione reductase; GSH: glutathione; HCY: homocysteine; Hipp.: hippocampus; IM: intramuscular; LDL: low density lipoprotein; LDLr−/−: Low density lipid receptor deficient; MDA: malondialdehyde; NOX1: NADPH Oxidase; Nrf2: Nuclear factor (erythroid-derived 2)-like 2; 8-OHdG: 8-hydroxy-2’-deoxyguanosine; PF: prefrontal; PO: oral; ROS: reactive oxygen species; SC: subcutaneous; SOD: superoxide dismutase; TAC: total antioxidant capacity; TBARS: thiobarbituric acid reacting substances.
Cited Studies:
We also found a low scyllo-inositol (sI) signal in AAS users (Kaufman et al., 2015). This finding was intriguing to us because sI complexes with and prevents clumping and accumulation of Aβ protein (McLaurin et al., 2000; Li et al., 2013a; Jin and Selkoe, 2015). Further, sI prevents tau hyperphosphorylation (Jin and Selkoe, 2015). These potentially beneficial effects have prompted development of sI and close sI analogs as potential treatments for AD/ADRD (Salloway et al., 2011; Morrone et al., 2016; Lee et al., 2017; Liu et al., 2018). A possible mechanism to explain the low sI signal in supraphysiologic-dose AAS users is that its synthetic enzyme, inositol epimerase, which converts myo-inositol to sI, may be inhibited by AAS. Inositol epimerase utilizes nicotinamide adenine dinucleotide phosphate (NADPH) as a cofactor for sI synthesis (Hipps et al., 1977) and NADPH thus could be depleted by AAS activation of NAPDH oxidase (NOX1) (Fu et al., 2013; Frankenfeld et al., 2014; Chignalia et al., 2015; Gomes et al., 2016; Tofighi et al., 2017; Table 1). Consistent with this possibility, AAS users in our MRS study exhibited a possible increase, albeit not statistically significant (P = 0.092), in anterior cingulate cortex myo-inositol levels (Kaufman et al., 2015), which could reflect a shift in the inositol epimerase equilibrium to favor myo-inositol over sI formation. Increased myo-inositol levels have been detected with MRS in association with increased Aβ plaque load (Voevodskaya et al., 2016). Thus, the low sI signal that we detected in supraphysiologic-dose AASusers may reflect sI depletion, which could accelerate Aβ and tau-P accumulation.
Another mechanism that could explain the finding of a low sI signal in AAS users is that they may have higher levels of Aβ protein. If so, a higher proportion of sI could be complexed with Aβ, which would result in an attenuation of the sI MRS signal by the so-called line-broadening effect (Fischer and Jardetzky, 1965). MRS line-broadening occurs when a relatively large molecule (e.g., Aβ monomer or oligomer, molecular weight ≅ 4,200 or multiples thereof) complexes with a relatively small one (e.g., sI, molecular weight = 180). During an MRS scan, this protein-protein interaction results in an acceleration of small molecule (e.g., sI) magnetic relaxation and an apparent reduction in its MRS signal. Molecular modeling studies suggest that sI complexes cooperatively with Aβ (Li and Pomès, 2013), meaning that several sI molecules may complex with each Aβ monomer or oligomer, which could substantially reduce the MRS signal intensity of sI. Importantly, the anterior cingulate cortex from which we obtained our sI measurement is an early region of Aβ accumulation in AD (Grothe et al., 2017; Gordon et al., 2018; Sepulcre et al., 2018), and so the low sI signal we found there in supraphysiologic-dose AAS users (Kaufman et al., 2015) could reflect a line-broadening effect resulting from higher Aβ levels. This possibility is supported by preclinical studies reporting that acute or short-term administration of supraphysiologic-dose AAS increase brain Aβ levels (Wahjoepramono et al., 2008; Ma and Liu, 2015; and see section 5 below). Thus, MRS measurement of sI could be very sensitive as a screen for detecting early increases in Aβ levels without the use of ionizing radiation.
By contrast, an MRS study reported higher brain sI levels in people with more advanced dementia (e.g., mild AD versus mild cognitive impairment) (Griffith et al., 2007). Although this finding would appear to be at odds with the line-broadening effect noted above, myo-inositol levels and sI levels tend to increase with dementia severity (Griffith et al., 2007), whereas brain Aβ levels tend to stabilize or even decline with dementia severity (Villemagne et al., 2013; Mishra et al., 2018). The net result of these effects could be higher sI/Aβ ratios in people with greater dementia severities, which would be accompanied by less sI line-broadening and a greater sI MRS signal. Although further studies are necessary to clarify whether the low sI signal we found in AAS users reflects low sI concentration, a low sI/Aβ ratio, or a combination of these effects, any of these scenarios – via reduction of sI availability to complex with and help eliminate Aβ ‒ could lead to or reflect increased Aβ burdens and risk for developing AD/ADRD. Because sI also prevents clumping of other neurotoxic proteins, including α-synuclein and Huntingtin proteins associated with the development of Parkinson’s and Huntington’s diseases, respectively (Vekrellis et al., 2009; Lai et al., 2014), the low sI signal we found in long-term supraphysiologic-dose AAS users could indicate that they are at increased risk for developing other neurodegenerative disorders.
Collectively, the cognitive, physiological, cardiovascular, sleep and neural disturbances induced by supraphysiologic-dose AAS use, which also have been reported in people diagnosed with or at increased risk for developing AD/ADRD, suggest that supraphysiologic-dose AAS users may be at increased risk for developing dementias. Below, we describe additional molecular effects of AAS that could increase risk for developing AD/ADRD.
5. Abnormal androgen levels increase Aβ levels and Aβ toxicity
To our knowledge, no human studies have been published that describe effects of supraphysiologic-dose androgen exposures on Aβ or tau-P levels. In animals, a single IM dose of the commonly used AAS 17β-trenbolone (up to 5 mg/kg) given to male rats induces rapid, dose-related plasma, whole brain, and hippocampal Aβ42 increases (Ma and Liu, 2015). Similarly, supraphysiologic T (20 mg/kg/day, 4–6 weeks) given to castrated male guinea pigs increases CSF and plasma Aβ40 levels (Wahjoepramono et al., 2008). Thus, the available evidence suggests that supraphysiologic-dose AAS administration rapidly increases brain, CSF, and plasma Aβ levels.
Similarly, low T levels, which are prevalent in older men (van den Beld et al., 2000; Moffat et al., 2002; Muller et al., 2003; Bhasin et al., 2005; Rosario et al., 2011; Yeap et al., 2012) and in hypogonadal former AAS users (Kanayama et al., 2015; Rasmussen et al., 2016), are associated with elevated Aβ levels. Decreased serum T levels are associated with increased plasma Aβ40 levels in older men with memory loss or dementia (Gillett et al., 2003), and decreased plasma free T levels are associated with increased plasma Aβ42 in older men with memory problems (Verdile et al., 2014). In postmortem frontal cortex samples from men with AD-related neuropathological changes, decreased T levels were associated with increased Aβ42 levels (Rosario et al., 2011). In aged male rats, decreased levels of the potent androgen 5α-dihydro-T (DHT) were associated with increased whole brain Aβ40 levels (Rosario et al., 2009).
More extreme androgen reductions, induced by therapeutic castration of men, substantially increased plasma Aβ40 levels (Gandy et al., 2001; Almeida et al., 2004) and nearly doubled the risk for developing AD within four years (Nead et al., 2016; Jhan et al., 2017). Similarly, brain, CSF, and plasma Aβ levels were elevated in castrated male rats, guinea pigs, and Alzheimer’s model 3xTg mice, and androgen normalization with T or DHT attenuated Aβ increases in these models (Ramsden et al., 2003; Rosario et al., 2006, 2010, 2012; Wahjoepramono et al., 2008). T and DHT treatments also substantially reduced tau-P deposits in castrated mice (Rosario et al., 2010), possibly by inhibiting glycogen synthase kinase 3β, which phosphorylates tau protein (see section 6.3).
These effects also occur in neuronal culture preparations, in which androgens reduce Aβ levels and its toxicity. T supplementation (0.2–1.0 μM) of murine N2a neuroblastoma cells or primary cortical neuronal cultures increases production of the soluble amyloid precursor protein-α (sAPPα), a nonamyloidogenic (α-secretase) enzyme product, and decreases Aβ release (Gouras et al., 2000). Low-dose T (10 nM) also increases sAPPα production in hypothalamic GT1–7 cells (Goodenough et al., 2000) and lowers Aβ42 levels in hippocampal neurons (Ma and Liu, 2015). Addition of up to 100 nM T or DHT to hippocampal neuronal cultures attenuates toxicity of a shortened Aβ fragment (Aβ25–35) (Pike, 2001). Similarly, in cortical neuronal cultures, 10 nM T protects against Aβ25–35 toxicity in a flutamide- (androgen receptor antagonist) and formestane- (aromatase inhibitor) sensitive manner (Caraci et al., 2011). By contrast, the potent AAS methandrostenolone supplemented at higher concentration (100 nM) exacerbates Aβ25–35 toxicity (Caraci et al., 2011). Collectively, these studies illustrate that Aβ and tau-P levels and Aβ toxicity are moderated by androgen levels and are increased in the presence both of abnormally high and low androgen concentrations.
6.0. Abnormal androgen levels and sleep disturbances increase oxidative stress
6.1. Supraphysiologic-dose AAS exposures increase oxidative stress
Generation of oxidative stress is a normal cellular signaling mechanism linked to a number of key biological processes including synaptic plasticity, learning, and memory (Kishida and Klann, 2007; Hidalgo et al., 2016; Kumar et al., 2018a). However, in healthy subjects, oxidative stress generally is maintained within a narrow range to minimize its deleterious effects (Yan, 2014), including mitochondrial impairments associated with increased Aβ and tau-P levels and with the development of AD/ADRD (Zhu et al., 2005; Grimm et al., 2016). Several of the most commonly used AAS induce excess oxidative stress in men and/or in male animals, as well as in cell culture models. In healthy men without AAS use histories, a single supraphysiologic dose of T (T-enanthate, 500 mg, IM) decreases urine total antioxidant capacity (TAC), reflecting increased systemic oxidative stress (Skogastierna et al., 2014). Among strength-trained men with histories of supraphysiologic-dose AAS use for at least 1 year, blood biomarkers indicative of elevated oxidative stress are found (Arazi et al., 2017a), including 8-hydroxy-2’-deoxyguanosine: (8-OHdG), a DNA oxidation product, malondialdehyde (MDA), a lipid peroxidation product, catalase (CAT), which neutralizes hydrogen peroxide (H2O2), and glutathione peroxidase (GPx), which utilizes glutathione (GSH), the most abundant endogenous small molecule antioxidant (Wu et al., 2004), to neutralize oxidative species. Homocysteine (HCY), levels of which are elevated in active long-term supraphysiologic-dose AAS users (Graham et al., 2006), is rapidly oxidized (Starkebaum and Harlan, 1986; Blom, 2000) and in brain, HCY increases levels of reactive oxygen species (ROS, which mediate oxidative stress), MDA, and protein carbonyls (oxidized proteins), while decreasing GSH levels (Kumar et al., 2018b). HCY levels also are elevated in female to male transsexuals therapeutically administered high-dose androgens (250 mg of Sustanon administered biweekly for 4 months) (Giltay et al., 1998) and in men with Klinefelter’s syndrome administered high-dose androgens (250 mg of Sustanon biweekly for 6 months) (Yesilova et al., 2004), suggesting that supraphysiologic androgen levels increase HCY levels. High HCY levels also are associated with AD/ADRD (Clarke et al., 1998; McCaddon et al., 1998; Seshadri et al., 2002; Genedani et al., 2004; Agnati et al., 2005; Guidi et al., 2006; Cascalheira et al., 2009).
The pro-oxidative effects of supraphysiologic-dose AAS reported in humans are consistent with findings in numerous animal studies of the effects of supraphysiologic-dose AAS given over a wide range of exposure conditions (except for years-long durations). Animal studies report abnormal levels of many different biomarkers of oxidative stress including thiobarbituric acid reacting substances (TBARS, lipid peroxidation byproducts), lipid peroxides, advanced glycation endproducts (AGEs), advanced oxidation protein products (AOPP), superoxide dismutase (SOD, which neutralizes superoxide anion), nicotinamide adenine dinucleotide phosphate (NADPH) Oxidase 1 (NOX1, the primary producer of ROS in some tissues and a depletor of inositol epimerase cofactor (Hipps et al., 1977)), Nuclear factor (erythroid-derived 2)-like 2 (Nrf2, the master antioxidant response transcription factor), and glutathione reductase (GR, which regenerates GSH from its oxidized form, glutathione disulfide, GSSG). Results from animal studies are summarized below in brief and additional study details are provided in Table 1.
Supraphysiologic doses of T in the forms of T, methyl-T, or Sustanon increase oxidative stress biomarkers in male reproductive tissues or cells (Hwang et al., 2011; Tóthová et al., 2013), in liver or hepatocytes (Welder et al., 1995; Arazi et al., 2017b; Sadowska-Krępa et al., 2017), in cultured dopaminergic cells (Cunningham et al., 2009) and in hippocampus (Joksimović et al., 2017). Supraphysiologic-dose boldenone increases oxidative stress in male reproductive tissues (Bueno et al., 2017b), in liver (Dornelles et al., 2017), and in brain cortex and hippocampus (Bueno et al., 2017a). Supraphysiologic-dose nandrolone increases oxidative stress in blood (Nikolic et al., 2016), in male reproductive tissues (Ahmed, 2015; Gomes et al., 2016) in liver (Frankenfeld et al., 2014), kidney (Frankenfeld et al., 2014; Riezzo et al., 2014; Tofighi et al., 2018), and cardiac tissues (Frankenfeld et al., 2014; Tofighi et al., 2017), as well as in prefrontal cortex and hippocampus (Tugyan et al., 2013; Turillazzi et al., 2016), striatum and cerebellum (Turillazzi et al., 2016), and in whole brain (Ahmed and El-Awdan, 2015). Supraphysiologic-dose oxymetholone increases oxidative stress in liver cells (Welder et al., 1995). Supraphysiologic-dose stanozolol increases oxidative stress in blood (Tahernejad et al., 2017), liver (Pey et al., 2003; Dornelles et al., 2017), muscle (Delgado et al., 2010) and cardiac (Barbosa Dos Santos et al., 2013; Kara et al., 2018) tissues, and in brain cortex, hippocampus, and whole brain (Camiletti-Moirón et al., 2015; Bueno et al., 2017a). Collectively, these findings indicate that supraphysiologic-dose AAS increase oxidative stress in blood, brain, and throughout the body. Comparable increases in oxidative stress biomarkers have been reported in association with low T levels, as described below.
6.2. Low T levels are associated with elevated oxidative stress
Low systemic and brain T levels are common in older men (van den Beld et al., 2000; Moffat et al., 2002; Muller et al., 2003; Bhasin et al., 2005; Rosario et al., 2011; Yeap et al., 2012) and are associated with excess oxidative protein modifications in cultured human fibroblasts and in postmortem human brain (Oliver et al., 1987, Smith et al., 1991). Low systemic T levels also are associated with excess oxidative stress in middle-aged men with erectile dysfunction and in young men with congenital hypogonadotrophic hypogonadism (Yasuda et al., 2008; Haymana et al., 2017).
In animals, older male rhesus macaques with low T levels have elevated blood oxidative stress biomarkers and older females with low estradiol (E2) levels exhibit similar effects (Ibáñez-Contreras et al., 2018). Orchidectomy lowers and elevates serum and testicular antioxidant and oxidative stress levels in rat, respectively (Deyhim et al., 2006, 2007; Bae et al., 2017); it increases brain and serum oxidative stress in rats (Pintana et al., 2015; Meydan et al., 2010); and it can be attenuated by daily low dose (0.5 mg/kg, SC) T administration (Meydan et al., 2010). Collectively, the literature indicates that both high and low departures from the eugonadal state induce excess oxidative stress.
6.3. Low sex-steroid hormone levels increase activity of glycogen synthase kinase 3β (GSK3β), an enzyme at the nexus of oxidative stress, Aβ, and tau-P regulation
Glycogen synthase kinase 3β (GSK3β) is a key enzyme involved in cell death and survival pathways (Maurer et al., 2014) and in the formation of amyloid plaques and tau tangles (Llorens-Martín et al., 2014) Brain GSK3β levels are increased in people with AD (Leroy et al., 2007). GSK3β activity is suppressed by normal androgen levels, while abnormally low T levels increase GSK3β activity (Papasozomenos, 1997; Papasozomenos and Shanavas, 2002; Gu et al., 2014; Maurer et al., 2014; Duran et al., 2016). A GSK3β target relevant to the regulation of oxidative stress is the master antioxidant transcription factor Nrf2, which is inhibited by GSK3β (Salazar et al., 2006; Rojo et al., 2008; Correa et al., 2011; Rada et al., 2012; Zou et al., 2013; Shang et al., 2015; Chen et al., 2016; Pang et al., 2016; Ebrahimi et al., 2018). Thus, in the presence of low T (e.g., in aging men (van den Beld et al., 2000; Moffat et al., 2002; Muller et al., 2003; Bhasin et al., 2005; Rosario et al., 2011; Yeap et al., 2012) and perhaps in hypogonadal off-cycle AAS users (Kanayama et al., 2015; Rasmussen et al., 2016)), high GSK3β activity inhibits Nrf2, leading to increased oxidative stress. Low Nrf2 protein and mRNA levels have been reported in rodent brain and spinal cord with aging (Suh et al., 2004; Duan et al., 2009; Zhang et al., 2013, 2016; Cui et al., 2017), which could underlie the age-associated reduction in Nrf2 antioxidant response efficiency (Zhang et al., 2015). Similarly, low brain Nrf2 expression occurs in hypogonadal 21-month-old male rats, an effect countered by low dose T administration (Zhang et al., 2016; Cui et al., 2017), while chemical castration of rats lowers testicular Nrf2 protein expression (Bae et al., 2017). Because excess oxidative stress itself increases GSK3β protein expression (Shin et al., 2006; Barbisan et al., 2018), excess oxidative stress may act in a feed-forward manner to increase GSK3β activity, inhibit Nrf2 activity, and further increase oxidative stress. GSK3β also upregulates Aβ production (Ryder et al., 2003) and it increases tau hyperphosphorylation (Papasozomenos, 1997; Papasozomenos and Shanavas, 2002). Accordingly, higher GSK3β activity accompanying low T levels in aging or other conditions associated with hypogonadism could increase oxidative stress and Aβ and tau-P levels, which could increase risk for developing AD/ADRD. Because Nrf2 not only attenuates oxidative stress but also reduces tau-P levels (Jo et al., 2014), GSK3β-induced Nrf2 downregulation in the presence of low T could increase tau-P levels.
Substantially less is known about how supraphysiologic-dose androgens affect GSK3β or Nrf2 activity, although supraphysiologic-dose nandrolone administration downregulates brain Nrf2 protein expression (Ahmed and El-Awdan, 2015). It remains to be determined whether nandrolone’s effect generalizes to other AAS.
E2 also inactivates GSK3β (Cardona-Gomez et al., 2004; Goodenough et al., 2005; Chen et al., 2013) and thus may enhance Nrf2 activity and inhibit Aβ and tau-P formation. Thus, the E2 decline in postmenopausal women, which occurs much more rapidly than the gradual T decline in otherwise healthy older men (Grimm et al., 2016), could more strongly disinhibit GSK3β activity in women than men, leading to more rapid Aβ increases and the higher AD/ADRD prevalence observed in women (Grimm et al., 2016). Abnormally low E2 levels also are found males after surgical or chemical castration (Resko et al., 2000; Almeida et al., 2004; Salminen et al., 2005; Hojo et al., 2009; Guerrieri et al., 16), in older men or males with low T levels (van den Beld et al., 2000; Rosario et al., 2009; Yeap et al., 2012), and in supraphysiologic-dose AAS users soon after suspending AAS use (Alèn et al., 1987). Accordingly, GSK3β activity and its deleterious effects may be increased in hypogonadal men due to depletion of T and E2. Collectively, the effects described above illustrate important interrelationships among T, E2, oxidative stress, Aβ, and tau-P levels, which are regulated by GSK3β activity, and indicate that physiological levels of T or E2 may help limit oxidative stress, Aβ, and tau-P formation.
6.4. Sleep disturbances increase oxidative stress
Sleep disturbances, independent of AAS exposures, increase oxidative stress. Individuals with insomnia exhibit abnormally elevated oxidative stress (Gulec et al., 2012; Liang et al., 2013a; Zhao et al., 2017) and even brief (overnight) sleep deprivation in healthy young adults is sufficient to increase systemic oxidative stress levels (Trivedi et al., 2017; Wei et al., 2017). Sleep studies in rodents also report increases in brain or liver oxidative stress after short- (3–4 day) (D’Almeida et al., 1998; Silva et al., 2004; Singh et al., 2008) and longer-duration (5–21 days) deprivation (Ramanathan et al., 2002; Chang et al., 2008; Süer et al., 2011; Feng et al., 2016), while eye movement (REM) sleep deprivation in rats for 6 weeks increases hippocampal oxidative stress (Alzoubi et al., 2012). Sleep deprivation upregulates GSK3β activity (Xia et al., 2018), which could be a basis for sleep deprivation-induced oxidative stress, Aβ, and tau-P increases. Together, these human and animal findings support the possibility that sleep disturbances, which are common in supraphysiolgic-dose AAS users (see section 3.4), contribute to AAS-induced oxidative stress, Aβ, and tau-P increases.
7. Androgen abnormalities and excess oxidative stress alter activities of a number of proteins involved in Aβ synthesis and tau-P turnover, and may increase Aβ and tau-P accumulation
Many proteins are involved in the regulation of Aβ and tau-P syntheses and elimination. Androgens and oxidative stress alter the expression and/or function of a number of these proteins including the amyloidogenic β- and γ-secretases (including Presenilins 1 and 2: PS1 and PS2). Androgens and oxidative stress also alter the expression and/or function of proteins involved in directing amyloid precursor protein (APP) toward the nonamyloidogenic processing pathway (α-secretase) and of proteins mediating turnover or elimination of Aβ or tau-P including transthyretin, insulin-degrading enzyme, neprilysin, the aquaporin 4 water channel, protein phosphatase 2A, apolipoprotein E, and the low-density lipoprotein receptor-related protein-1. The effects of androgens and excess oxidative stress on these proteins are outlined below.
7.1. α- and β-secretases
Under normal conditions, amyloid precursor protein (APP) is catabolized primarily by the α-secretase enzyme (Bouillot et al., 1996) to form soluble amyloid precursor protein-α (sAPPα), which is nonamyloidogenic and neuroprotective. Physiologic-dose T administered to hypogonadal male guinea pigs increases α-secretase activity and sAPPα synthesis and decreases Aβ synthesis and release (Gouras et al., 2000; Goodenough et al., 2000; Wahjoepramono et al., 2008; Ma and Liu, 2015). The apparent inverse relationship between sAPPα and Aβ production may result from sAPPα inhibition of amyloidogenic β-secretase (Obregon et al., 2012; Deng et al., 2015) and from β-secretase product Aβ42 inhibition of the ADAM10 α-secretase (Chinchalongporn et al., 2018). Excess oxidative stress also reduces α-secretase expression, increases β-secretase activity, and leads to higher bsecretase mRNA levels and higher Aβ40 and Aβ42 levels (Tamagno et al., 2008; Oda et al., 2010; Arimon et al., 2015; Hernández-Zimbrón and Rivas-Arancibia, 2015). In postmortem AD brain, the higher oxidative stress associated higher β-secretase activity (Tayler et al., 2010). Aβ42 itself increases oxidative stress (Huang et al., 1999; Yatin et al., 1999; Kim et al., 2003; Boyd-Kimball et al., 2005) and may thus act in a feed-forward manner to increase oxidative stress and Aβ levels. Aβ also upregulates GSK-3β activity resulting in greater amyloid plaque formation, tau hyperphosphorylation (Terwel et al., 2008; Hu et al., 2009; DaRocha-Souto et al., 2012; Deng et al., 2015; Chinchalongporn et al., 2018), and Nrf2 inhibition (see section 6.3), with the latter effect potentially amplifying oxidative stress and Aβ and tau-P formation.
7.2. γ-secretase
The γ-secretase enzyme is a complex of proteins involved in Aβ processing including presenilins 1 and 2 (PS-1, PS-2). In young gonadectomized male mice, T replacement suppresses PS-1 mRNA and protein levels (Ghosh and Thakur, 2008) and in aged male hpg (lifelong hypogonadal) mice, hippocampal PS-1 and Aβ42 levels are elevated (Drummond et al., 2012). These findings suggest that in males, subphysiologic T levels may increase PS-1 expression or activity and promote Aβ accumulation. GSK3β also increases PS-1 activity leading to higher Aβ42 levels (Ryder et al., 2003), and thus hypogonadism, by upregulating GSK3β, may increase PS-1 activity. Excess oxidative stress increases activities and alters efficiencies of the γ-secretase and its components, leading to higher Aβ42 levels (Tamagno et al., 2008; Arimon et al., 2015; Hernández-Zimbrón and Rivas-Arancibia, 2015).
7.3. Transthyretin
Transthyretin (TTR), also known as prealbumin, is a serum and CSF transport protein that complexes with, catabolizes, and helps eliminate Aβ (Schwarzman et al., 1994; Alshehri et al., 2015; Silva et al., 2017). TTR levels are reduced by 40% after androgen ablation therapy in individuals with prostate cancer (Wang et al., 2012) and are substantially reduced in gonadectomized male mice (Reuter and Kennes, 1966). By contrast, in individuals with spinal cord injury, therapeutic oral administration of the AAS oxandrolone (~1.75 mg/kg/week) increases serum TTR levels (Bauman et al., 2013) as does low-dose T administration to gonadectomized male mice (Reuter and Kennes, 1966). TTR most effectively complexes with and eliminates Aβ monomers and oligomers by assembling into tetramers (Li et al., 2013a), a conformation that is inhibited by excess oxidative stress (Zhao et al., 2013). Notably, the proportion of oxidized CSF TTR in individuals with AD and MCI is nearly twice that found in healthy individuals (Poulsen et al., 2014). Further, serum TTR levels are 16% lower in individuals with AD (Elovaara et al., 1986) and low CSF TTR levels are associated with increased dementia severity in individuals with AD (Riisøen, 1988). Thus, low androgen levels and excess oxidative stress may impair TTN expression and its ability to complex with and catabolize Aβ.
7.4. Neprilysin
Neprilysin (NEP, also known as neutral endopeptidase) is a metallo-endopeptidase that inactivates several peptide hormones and that catabolizes soluble Aβ40 and soluble and insoluble Aβ42 (Iwata et al., 2000, 2001; Eckman et al., 2006). Low-dose DHT (10 nM) rapidly increases NEP expression in hippocampal neuronal culture in an androgen receptor-dependent manner (Yao et al., 2008) and in transgenic AD mice, aromatase knockout (effectively doubling endogenous T levels) increases NEP expression and decreases Aβ pathology and cognitive dysfunction (McAllister et al., 2010). By contrast, gonadectomy substantially reduces brain NEP expression in male rats, an effect that is normalized by DHT treatment or by treatment with NEP28, a selective androgen receptor modulator (SARM) that also reduces brain Aβ40 levels (Yao et al., 2008; Akita et al., 2013). Co-treatment of male gonadectomized 3xTg AD mice with the SARM ACP-105 and with AC-186, a selective estrogen receptor β modulator (SERM), increases NEP, decreases brain Aβ levels, and improves cognition (George et al., 2013). Oxidative stress also inhibits NEP activity (Shinall et al., 2005; Wang et al., 2010), an effect that is attenuated by the antioxidant N-acetylcysteine (Wang et al., 2010). Thus, physiologic androgen levels upregulate, and oxidative stress inhibits NEP activity.
7.5. Insulin degrading enzyme
Insulin degrading enzyme (IDE) is a peptidase that complexes with and catabolizes Aβ (Kurochkin and Goto, 1994). In prostate cells, nuclear IDE expression and function are lowered by castration, an effect counteracted by T treatment (Udrisar et al., 2005). In aromatase knockout AD mice with elevated endogenous T levels, IDE is upregulated and Aβ pathology and cognitive dysfunction are decreased (McAllister et al., 2010). In gonadectomized male 3xTg AD mice, the SARM ACP-105 and the SERM AC-186 increase IDE, decrease brain Aβ levels, and improve cognition (George et al., 2013). Further, oxidative stress inhibits IDE activity (Shinall et al., 2005; Yui et al., 2015). Thus, IDE activity is upregulated by T and downregulated by hypogonadism and oxidative stress.
7.6. Aquaporin 4
Aquaporin-4 (AQP4) is an astrocyte water channel protein involved in glymphatic system fluid movements that participate in Aβ clearance (Iliff et al., 2012; Yang et al., 2012). In cultured astrocytes, physiologic T (100 nM) doubles AQP4 protein expression while E2 is without effect, indicating that AQP4 is upregulated by physiologic levels of androgens (Gu et al., 2003). By contrast, supraphysiologic T (500 nM) reduces astrocyte AQP protein expression (Gu et al., 2003). Although the effects of orchidectomy on astrocyte AQP4 expression have yet to be described, orchidectomy decreases kidney AQP4 channel expression in male rats (Loh et al., 2017). Thus, it appears that eugonadal androgen levels are needed for normal AQP4 channel expression and function. While oxidative stress reportedly upregulates AQP4 channel expression (Bi et al., 2017), OS also liberates zinc ion (Zn2+), an inhibitor of AQP4 channel function (Yukutake et al., 2009), from redox-sensitive intracellular Zn2+ sequestration sites (Kröncke and Klotz, 2009; Furuta et al., 2017). Amyloid plaques contain high Zn2+ concentrations and Zn2+ levels are high postmortem in hippocampal neurons from individuals with AD (Lovell et al., 1998; Suh et al., 2000). Accordingly, abnormal androgen levels and excess oxidative stress directly, and indirectly via Zn2+ mobilization, limit Aβ elimination by AQP4 channels.
7.7. Protein phosphatase 2A
Protein phosphatase 2A (PP2A) dephosphorylates and regulates activities of a number of proteins including tau-P (Goedert et al., 1992; Gong et al., 2000) and GSK3β (Qian et al., 2010). GSK-3β also phosphorylates and downregulates PP2A (Qian et al., 2010; Yao et al., 2012). Excess oxidative stress inhibits PP2A (Foley et al., 2007, 2011; Raghuraman et al., 2009) as does Zn2+ (Sun et al., 2012; Xiong et al., 2013), perhaps by upregulating GSK3β activity (Kwon et al., 2015). PP2A expression also is inhibited by excess glutamate (Yi et al., 2005, 2008). PP2A is inhibited by the pro-oxidant molecule HCY (Vafai and Stock, 2002; Sontag et al., 2007; Zhang et al., 2008; Shirafuji et al., 2018), the levels of which are elevated in active long-term supraphysiologic-dose AAS users (Graham et al., 2006), and HCY rapidly decreases and increases rat brain PP2A and tau-P levels, respectively (Luo et al., 2007). High systemic HCY levels are associated with AD/ADRD (Clarke et al., 1998; McCaddon et al., 1998; Seshadri et al., 2002; Genedani et al., 2004; Agnati et al., 2005; Guidi et al., 2006; Cascalheira et al., 2009) and PP2A activity and hippocampal mRNA levels are low in AD (Gong et al., 1995; Vogelsberg-Ragaglia et al., 2001; Sontag et al., 2004). Although it has yet to be determined whether physiologic concentrations of androgens alter PP2A expression or function, this effect is hypothesized since androgens inhibit GSK3β activity (see section 6.3); and E2, via ERα receptors, increases brain PP2A activity (Ueda et al., 2018). E2 and E2 analogs that do not bind to estrogen receptors also preserve PP2A levels under conditions of excess glutamate (Yi et al., 2005, 2008). Therefore, the rapid E2 declines that occur in postmenopausal women (Grimm et al., 2016) could facilitate tau-P accumulation. Thus, AAS-induced oxidative stress and hypogonadism (through GSK-3β upregulation), as well as hypogonadism in women, likely inhibit PP2A activity and increase tau-P levels.
7.8. Apolipoprotein E
Apolipoprotein E (ApoE) complexes with Aβ and plays a role in its elimination (Strittmatter et al., 1993; Verghese et al., 2013). The ApoE ε4 gene polymorphism is associated with increased risk for developing AD/ADRD (Corder et al., 1993). ApoE genotype determines Aβ accumulation in humans and in mice, with highest Aβ levels occurring in people possessing an ε4 allele (Castellano et al., 2011). In human ApoE ε4 carriers, T levels are associated with hippocampal volume and verbal episodic memory recall, suggesting that androgens modify risk for AD/ADRD in ApoE ε4 carriers (Panizzon et al., 2010, 2014). In otherwise healthy elderly male ApoE ε4 carriers, T levels are lower than in noncarriers and the combination of low T and ApoE ε4 increases risk for AD (Hogervorst et al., 2002). In cultured microglial cells, oxidative stress activates and reduces microglial ApoE levels and Aβ uptake, respectively (Feng et al., 2017), which could reduce microglial clearance of Aβ. Lipid peroxidation products of oxidative stress crosslink ε3 and ε4 proteins in culture, which reduces their ability to catalyze Aβ turnover (Montine et al. 1996). Thus, ApoE interactions with Aβ may be impaired by low T levels and/or high oxidative stress, resulting in increased Aβ accumulation.
7.9. Low-Density Lipoprotein Receptor-related protein-1
The low-density lipoprotein receptor-related protein-1 (LRP1) complexes with and helps clear Aβ both by transporting it out of the brain and into the blood and by transporting Aβ out of the blood and into the liver (Kang et al., 2000; Shibata et al., 2000; Liu et al., 2017b). A soluble fragment of LRP1 (sLRP1), which is excreted into the blood, complexes with and delivers Aβ to the liver for elimination, thereby preventing circulating Aβ from entering or re-entering the brain (Sagare et al., 2007). LRP1 also alters neuronal localization of β-secretase and enhances its degradation, which reduces neuronal Aβ synthesis capacity (Tanokashira et al., 2015). Acute induction of oxidative stress in mice decreases LRP1-dependent Aβ42 transport out of the brain (Erickson et al., 2012). One night of total sleep deprivation in humans, which increases oxidative stress (see section 3.4), decreases sLRP levels and increases plasma Aβ40 levels (Wei et al., 2017). Although the effects of androgen abnormalities on LRP1 expression and function have not been reported, ovariectomy lowers liver LRP1 mRNA levels and E2 supplementation attenuates this effect (Ngo Sock et al., 2014), perhaps by activating ERα receptors (Hwang et al., 2015). In people with AD, sLRP1 binding is low and sLRP1 is substantially oxidized (Sagare et al., 2007) as is hippocampal LRP1 (Owen et al., 2010). Individuals with mild cognitive impairment also exhibit low hippocampal LRP1 density (Sultana et al., 2010). Thus, excess oxidative stress and hypogonadal E2 levels impair the Aβ-clearing effects of LRP1/sLRP1 and may increase risk for developing AD/ADRD.
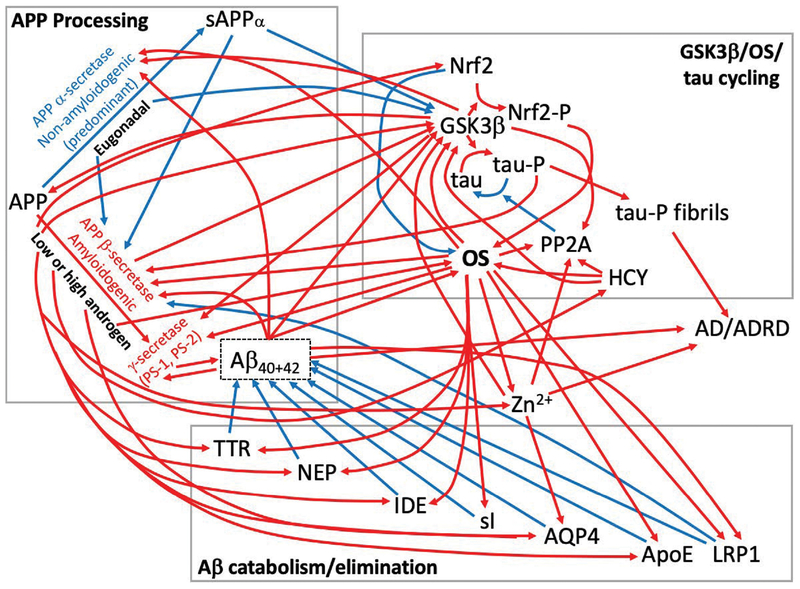
Collectively, these subsections demonstrate that many proteins are involved in a complex orchestration of Aβ and tau-P syntheses and elimination, which when out of balance as a consequence of abnormal sex-steroid levels and/or excess oxidative stress (Figure 2, red pathways), favors Aβ and tau-P accumulation and increases risk for developing AD/ADRD.
Figure 2:
Effects of abnormal androgen levels and excess oxidative stress (OS) on beta amyloid (Aβ) and hyperphosphorylated tau (tau-P) protein syntheses & elimination. In the figure, blue paths represent non-amyloidogenic and tau-phosphorylating pathways, whereas red paths represent amyloidogenic and tau-phosphorylating pathways. Three key systems that are involved in the control of Aβ and tau-P levels are affected by abnormal androgen levels (comprising both supraphysiologic and hypogonadal levels) and/or by excess OS. The upper left box illustrates the fate of amyloid precursor protein (APP), the precursor for Aβ. Under eugonadal conditions, APP is predominantly processed by the nonamyloidogenic α-secretase enzyme to form soluble APPα, which inhibits Aβ synthesis. Under supraphysiologic or subphysiologic androgen conditions, APP is processed to a greater extent by the amyloidogenic β-secretase enzyme to form Aβ. Excess OS tends to inhibit and activate the nonamyloidogenic and the amyloidogenic APP processing pathways, respectively. The upper right box illustrates GSK3β/Nrf2/tau protein cycling. GSK3β is inhibited by sAPPα and upregulated by Aβ, and so departures from eugonadism increase GSK3β activity. This leads to increased phosphorylation of tau protein and accumulation of tau-P, together with increased phosphorylation and downregulation of Nrf2, the master antioxidant transcription factor, which reduces antioxidant defenses. As noted above, an oxidizing environment increases amyloidogenic processing. The bottom box shows a number of proteins and a small molecule (scyllo-inositol, sI) that normally catabolize Aβ and/or facilitate its elimination. However, under supraphysiologic or subphysiologic androgen conditions, the Aβ-neutralizing effects of these proteins are attenuated. Excess OS also attenuates the function of many of these proteins and of sI. The proteins and small molecules within each of these 3 systems interact such that when concurrently present, abnormal androgen levels and excess OS likely potentiate Aβ and tau-P production and accumulation – as displayed in the red paths in the figure. These effects, if induced at an early age as a consequence of nonmedical AAS (or other drug) use, could lead to premature accumulation of Aβ and tau-P and increased risk for developing AD/ADRD. Legend: Aβ: beta-amyloid protein; AD/ADRD: Alzheimer’s Disease and its Related Dementias; APP: amyloid precursor protein; AQP4: Aquaporin 4 protein; GSK3β: Glycogen synthase kinase 3β; HCY: Homocysteine; IDE: Insulin degrading enzyme; LRP1: Low-density lipoprotein receptor-related protein1 and its soluble form (sLRP1); NEP: Neprilysin; Nrf2: Nuclear factor (erythroid-derived 2)-like 2; OS: Oxidative stress; -P: Phosphorylated protein; PP2A: Protein Phosphatase 2A; PS-1/2: Presenilins 1,2; sAPPα: Soluble APPα; sI: scyllo-inositol; tau: Tau protein; TTR: Transthyretin (prealbumin); Zn2+: Zinc ion.
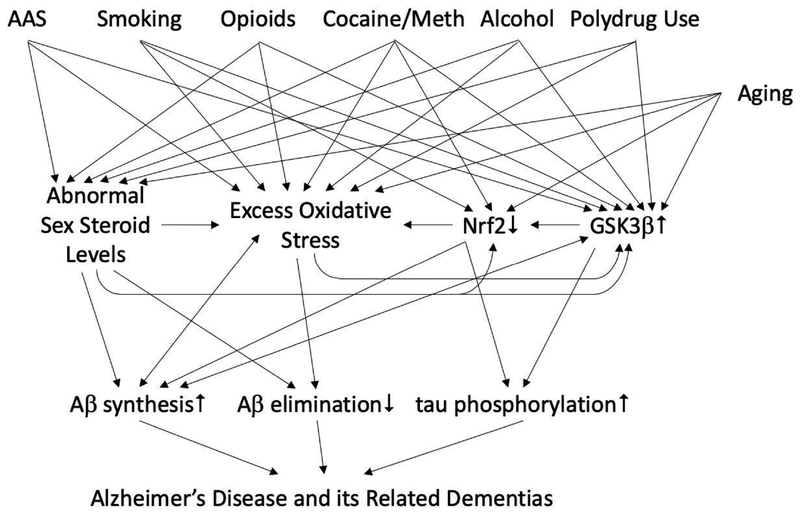
8. Other abused substances induce androgen abnormalities, excess oxidative stress, increased GSK3β activity, and may increase risk for developing AD/ADRD
Supraphysiologic-dose AAS users typically use other psychoactive substances including alcohol, tobacco, methamphetamine, cocaine, opioids, and cannabis (Skarberg et al., 2009; Ip et al., 2011; Lindqvist et al., 2013; Sagoe et al., 2015; Kanayama et al., 2018). Several of these substances have been independently linked to increased risk for developing AD/ADRD and several induce excess oxidative stress, and/or hypogonadism, and/or activate GSK3β. Known effects of several of these substances that could modify risk for developing AD/ADRD in supraphysiologic-dose AAS users and non-users alike are outlined below.
8.1. Alcohol Use Disorder
Alcohol use disorder has been established as a risk factor for AD/ADRD (Schwarzinger et al., 2018). Alcohol drinking typically commences by age 18 and the likelihood of developing alcohol use disorder substantially increases in people who consumed their first drink by 16 years of age (Hingson et al., 2006). In rats, chronic alcohol drinking increases brain expression of APP, β-secretase, and nicastrin (a component of the γ-secretase complex) (Kim et al., 2011) and alcohol withdrawal increases PS-1 expression and Aβ40 and Aβ42 levels (Ryou et al., 2017). Chronic alcohol drinking in mice increases brain levels of Aβ40 and Aβ42 (Gong et al., 2017) and in a mouse AD model, alcohol increases oxidative stress of APP, β-secretase, Aβ40 and Aβ42, and amyloid plaque (Huang et al., 2018a). Several blood oxidative stress biomarkers are abnormal in individuals with alcohol use disorder (Lecomte et al., 1994; Kalousová et al., 2004; Chen et al., 2011; Sandhya et al., 2016) and rats or mice chronically exposed to alcohol or its withdrawal exhibit brain, liver, and systemic biomarkers indicative of excess oxidative stress (Montoliu et al., 1994; Sun et al., 2001; Aydin et al., 2002; Das et al., 2007; Waly et al., 2011; Zeng et al., 2014; Gong et al., 2017; Ryou et al., 2017). Inflammatory processes, some of which are mediated by excess oxidative stress, also have been implicated as linking alcohol use to AD (Venkataraman et al., 2017). Acute and chronic heavy alcohol exposures decrease plasma T levels (Mendelson and Mello, 1974, Mendelson et al., 1978; Gordon et al., 1976; Persky et al., 1977) while chronic heavy alcohol use has been associated with hypogonadism (Castilla-García et al., 1987) and menstrual cycle dysfunction (Mello et al., 1989). Prolonged heavy alcohol exposure increases liver and kidney GSK3β expression and/or activity (Li et al., 2013b, Zeng et al., 2014; Wang et al., 2017). While light to moderate alcohol drinking by humans may be protective by increasing TTR expression (Jono et al., 2016), heavy alcohol use decreases TTR levels (Kalousová et al., 2004).
8.2. Tobacco Use Disorder
The association between tobacco smoking and dementia has been confirmed by a recent meta-analysis reporting a 50% increase in risk for developing AD/ADRD among tobacco smokers (Niu et al., 2018). Regular tobacco smoking typically is established before the age of 20 and in some smokers before they reach 15 years of age (Kendler et al., 2013). Tobacco use disorder has been associated with cortical thinning in brain areas that exhibit early atrophy in AD (Durazzo et al., 2018) and with higher brain levels of Aβ and higher risk for developing AD/ADRD (Durazzo et al., 2014a; Zhong et al., 2015). Smoking and having an ApoE ε4 genotype appear to exert additive effects on brain Aβ levels (Durazzo et al., 2016b). Conversely, tobacco smoking cessation reduces risk for developing AD/ADRD (Choi et al., 2018). Tobacco smoke is a potent oxidative stressor (Pryor and Stone, 1993) that induces systemic oxidative stress (Sandhya et al., 2016) and depletes Nrf2 expression (Naha et al., 2018). Smoking-induced oxidative stress has been demonstrated in humans by quantifying a CSF biomarker, F2-isoprostane (iPF2α-III), levels of which are elevated in both cognitively normal elderly smokers and in probable AD smokers (Durazzo et al., 2014b, 2016a). Cigarette smoke extracts increase GSK3β activity and reduce Nrf2 expression and function (Borgas et al., 2016; Ebrahimi et al., 2018). Tobacco smoke contains high concentrations of cadmium and smoking substantially increases systemic cadmium levels (McKelvey et al., 2007). Cadmium in turn induces oxidative stress and activates GSK3β activity (Wang et al., 2009) and inhibits PP2A activity (Chen et al., 2008). Other tobacco smoke components, especially metal ions and polycyclic aromatic hydrocarbons, stimulate Aβ aggregation (Wallin et al., 2017). Tobacco smoke also triggers NOX activity (Kim et al., 2014), which as noted above lowers NADPH levels and could lower inositol epimerase activity and sI synthesis (Hipps et al., 1977). In this regard, chronic smokers have low blood sI levels (Gu et al., 2016). Since systemic sI concentration predicts brain sI concentration (Liang et al., 2013b), it is likely that smokers also have low brain sI concentrations, which could lead to the higher levels of Aβ reported in smokers (Durazzo et al., 2014a).
Nicotine itself seems unlikely to mediate the harmful effects of tobacco smoking on AD/ADRD risk because it does not enhance Aβ accumulation (Wallin et al., 2017). In fact, nicotine may help eliminate Aβ by increasing choroid plexus TTR secretion (Li et al., 2000) and the nicotine metabolite cotinine is both neuroprotective against Aβ and inhibits GSK3β activity (Echeverria et al., 2011, Echeverria and Zeitlin, 2012). Yet, AD mice exposed chronically to cigarette smoke exhibit higher Aβ and tau-P burdens despite having high serum cotinine levels (Moreno-Gonzalez et al., 2013). Smoking also moderately elevates blood T levels in men (Muller et al., 2003; Svartberg and Jorde, 2007), which as noted above could reduce risk for developing AD/ADRD. These data suggest that any beneficial effects of tobacco smoking on AD/ADRD risk are outweighed by smoking’s deleterious effects. Moreover, concurrent use of alcohol and nicotine exacerbates oxidative stress (Sandhya et al., 2016) and thus could amplify Aβ and tau-P increases.
8.3. Opioid Use Disorder
Because late-onset AD/ADRD diagnoses on average occur in the 8th decade of life (Villemagne et al., 2013; Barnes et al., 2015), the early mortality associated with opioid use disorder (Smyth et al., 2006; Degenhardt et al., 2011; Veldhuizen and Callaghan 2014; Gomes et al., 2018; Ma et al., in press) may explain why to date, a link between opioid use disorder and AD/ADRD has not been established (Veldhuizen and Callaghan 2014). Yet, as medication-assisted treatment for opioid use disorder becomes more widely available (Alderks17), early mortality rates in individuals with opioid use disorder are likely to decline (Ma et al., in press) and numbers of individuals with opioid use disorder living into their 8th decade likely will increase. Nonmedical opioid use, like supraphysiologic-dose AAS use, is initiated on average by the age of 23 and can rapidly progress to opioid use disorder (OUD) (Wu et al., 2011; Woodcock et al., 2015). Accordingly, reports from several groups that levels of AT8 antibody, a biomarker of tau-P, are elevated in postmortem brain in illicit opioid users, even in users under age 30 (Ramage et al., 2005; Anthony et al., 2010; Kovacs et al., 2015; Flanagan et al., 2018), as are levels of APP (Ramage et al., 2005), suggest that individuals with opioid use disorder are at increased risk for developing AD/ADRD.
Opioids increase oxidative stress in brain, blood, and body (Zhou et al., 2000; Qiusheng et al., 2005; Arana et al., 2006; Xu et al., 2006; Pereska et al., 2007; Pérez-Casanova et al., 2008; Doyle et al., 2009; Ndengele et al., 2009; Abdel-Zahar et al., 2010; Kovatsi et al., 2010a, 2010b; Cemek et al., 2011; Gutowicz et al., 2011; Sumathi et al., 2011; Deng et al., 2012; Ghazavi et al., 2013; Soykut et al., 2013; Fan et al., 2015; Motaghinejad et al., 2015; Samarghandian et al., 2015; Yun et al., 2015, 2017; Lauro et al., 2016; Najafi et al., 2017; Mansouri et al., 2018) and thus could increase Aβ and tau-P syntheses and accumulations via multiple mechanisms described above. In neuronal culture, morphine upregulates GSK3β activity (Masvekar et al., 2015), which could contribute to increased tau-P levels in opioid users. Opioids also suppress T levels in men (Mendelson et al., 1975; Bliesener et al., 2005; Hallinan et al., 2009; Duarte et al., 2013; Cepeda et al., 2015; Huang et al., 2016a; Raheem et al., 2017; Rubinstein and Carpenter, 2017; Yee et al., 2018) and E2 levels in women (Daniell, 2008). The prevalence of opioid-induced hypogonadism has been estimated at greater than 50%, prompting the recommendation that opioid users be screened for hypogonadism (Hsieh et al., 2018). In men, the medication-assisted treatment pharmacotherapy methadone is more likely than buprenorphine to blunt T levels (Bliesener et al., 2005; Hallinan et al., 2009; Yee et al., 2018), meaning that the type of medication-assisted treatment administered could affect risk for developing AD/ADRD. Hypogonadism, by increasing GSK3β activity (Papasozomenos, 1997; Papasozomenos and Shanavas, 2002; Cardona-Gomez et al., 2004; Goodenough et al., 2005; Chen et al., 2013; Gu et al., 2014; Maurer et al., 2014; Duran et al., 2016), could contribute to the high brain GSK3β and AT-8 levels found postmortem in opioid users (Ramage et al., 2005; Anthony et al., 2010; Kovacs et al., 2015; Flanagan et al., 2018). A potential analgesic benefit of correcting opioid-induced hypogonadism is illustrated in studies reporting that androgen supplementation given to males with pain reduces both hyperalgesia (Basaria et al., 2015) and opioid analgesic requirements (Raheem et al., 2017), suggesting that androgen normalization could reduce opioid analgesic requirements, risk for developing opioid use disorder, and perhaps risk for developing AD/ADRD.
Consistent with the possibility that heavy use of opioids influences risk for AD/ADRD is a report that dementia prevalence is elevated in prescription opioid users taking the highest cumulative opioid doses (Dublin et al., 2015). By contrast, a population-based study in Finland did not detect an association between past prescription opioid use and dementia (Taipale et al., 2017). However, since no studies to date have been designed to determine whether individuals with opiod use disorder are at increased risk for developing AD/ADRD, studies assessing a potential association are needed. Because supraphysiologic-dose AAS use may represent a gateway to opioid use in some individuals (Kanayama et al., 2018), it also will be important to determine whether polydrug use involving both of these substances increases risk for developing AD/ADRD.
8.4. Methamphetamine and Cocaine Use Disorders
Methamphetamine and cocaine use disorders also may modify risk for developing AD/ADRD by lowering sex-steroid hormone levels or by increasing oxidative stress levels or GSK3β activity. Age of first regular use of methamphetamine and cocaine have been estimated to average 21 and 26 years old, respectively (Hser et al., 2008). Methamphetamine lowers plasma T levels in rats (Lin et al., 2014) and its use is associated with abnormal menstrual cycles in women (Shen et al., 2014). Methamphetamine increases systemic oxidative stress (Melo et al., 2010; Huang et al., 2013, 2018b; Walker et al., 2014; Najafi et al., 2017; Zhang et al., 2018) cardiac, retinal, and testicular oxidative stress (Lord et al., 2010; Melo et al., 2010; Lin et al., 2014), and brain oxidative stress (Mirecki et al., 2004; Banerjee et al., 2010; Jang et al., 2017). Chronic methamphetamine reduces Nrf2 activity in lung (Bai et al., 2017). Methamphetamine upregulates GSK3β expression in nucleus accumbens core, an effect linked to drug-induced sensitization (Xu et al., 2011). Methamphetamine also increases Aβ and tau levels in cultured vascular endothelial cells and in brain (Liu et al., 2017a), and in cultured neurons it upregulates GSK3β and enhances tau hyperphosphorylation (Xu et al., 2018).
Chronic cocaine administration substantially reduces plasma T levels in young adult male rats (Berul and Harclerode, 1989; Budziszewska et al., 1996; Sarnyai et al., 1998; Chin et al., 2002; Alves et al., 2014) and in men (Wisniewski et al., 2007), although normal T levels were reported in cocaine-dependent subjects during inpatient detoxification (Mendelson et al., 1988). Cocaine increases oxidative stress in blood (Winhusen et al., 2013; Walker et al., 2014; Zaparte et al., 2015), heart (Devi and Chan, 1999; Isabelle et al., 2007; Fan et al., 2009; Pomierny-Chamioło et al., 2013; Frustaci et al., 2015), kidney (Pomierny-Chamioło et al., 2013; Kowalczyk-Pachel et al., 2016), liver (Pomierny-Chamioło et al., 2013), and in a number of brain areas (Dietrich et al., 2005; Muriach et al., 2010; Pomierny-Chamioło et al., 2013; Jang et al., 2015; López-Pedrajas et al., 2015; Vitcheva et al., 2015; Beiser et al., 2017), which could increase Aβ and tau-P levels by multiple mechanisms noted above. Chronic cocaine exposures upregulate total GSK (GSK3α and β) activity in amygdala (Perrine et al., 2008) and nucleus accumbens core GSK3β activity, an effect related to cocaine-induced locomotor sensitization (Xu et al., 2009). Higher tau-P levels are found in cortex, hippocampus, and striatum in rats administered cocaine for several days (Liu et al., 2003b).
8.5. Cannabis Use Disorder
The main psychoactive component of cannabis, Δ9-tetrahydrocannabinol (THC), increases brain oxidative stress in a dose-related manner in rats (Wolff et al., 2015) and cannabis use disorder in humans is associated with excess systemic oxidative stress (Bayazit et al., 2017). However, the available evidence suggests that cannabinoids actually may reduce Aβ and tau-P accumulation. THC inactivates GSK3β in several brain regions including hippocampus (Ozaita et al., 2007), which could increase Nrf2 activity and oxidative stress-buffering capacity while also attenuating tau hyperphosphorylation. Cannabinoid activation of CB2 receptors enhances Aβ turnover (Aso et al., 2016). Further, a cannabis constituent without psychoactive effects, cannabidiol (CBD), attenuates Aβ-induced GSK3β activation and tau hyperphosphorylation in cultured PC12 cells (Esposito et al., 2006). Co-administration of THC and CBD more effectively attenuates AD-like phenotypes in a mouse AD model than administration of either compound alone (Aso et al., 2015). Accordingly, cannabinoids have been proposed as potential treatments for AD (Watt and Karl, 2017).
Collectively, the studies cited above demonstrate that the most common substance use disorders, with the apparent exception of cannabis, either have been linked to increased risk for developing AD/ADRD or, by triggering hypogonadism, excess oxidative stress, and/or GSK3β activation, have the potential to increase risk for developing AD/ADRD. Use of these substances, which begins typically during adolescence or early adulthood, may constitute an early risk factor for AD/ADHD. Concurrent use of these substances by supraphysiologic-dose AAS users may potentiate Aβ and tau-P accumulations and exacerbate risk for developing AD/ADRD. Figure 3 is a diagram representing the proposed role of supraphysiologic-dose AAS use in relation to other substance use disorders and biological mediators in the development of AD/ADRD.
Figure 3:
Model for supraphysiologic AAS use, other substance use disorders, and biological mediators in the causation of Alzheimer’s Disease and its Related Dementias. AAS use and other substance use disorders, which develop by young adulthood, induce premature hypogonadism and/or excess oxidative stress, effects that are associated with increased Aβ and tau-P burdens in later life. Accordingly, substance use disorders may constitute early, modifiable causal factors for the development of AD/ADRD. Treatments that optimize sex steroid hormone levels, that reduce excess oxidative stress, or that inhibit GSK3β activity may exert powerful multi-targeting benefits on key mediators in the causal pathway to AD/ADRD. Legend: AAS: supraphysiologic-dose anabolicandrogenic steroid abuse; Aβ: beta-amyloid protein; AD/ADRD: Alzheimer’s Disease and its Related Dementias; GSK3β: Glycogen synthase kinase 3β; Meth: methamphetamine; Nrf2: Nuclear factor (erythroid-derived 2)-like 2.
9. Summary and Future Directions
The literature included in this review demonstrates that use of supraphysiologic doses of AAS to enhance muscularity and strength increases risk for developing a number of serious health consequences, including possibly AD/ADRD. By inducing androgen abnormalities and excess oxidative stress, supraphysiologic-dose AAS exposures may modify expression and/or function of many proteins involved in Aβ and tau-P syntheses and elimination in ways that likely accelerate Aβ and tau-P accumulation. Animal studies reporting that supraphysiologic doses of AAS given acutely (Ma and Liu, 2015) or over the short term (Wahjoepramono et al., 2008) rapidly increase Aβ levels suggest that the complex systems that regulate Aβ and tau-P levels may be impaired soon after the initiation of supraphysiologic-dose AAS use. Since human supraphysiologic-dose AAS use commences at a median age of about 23 (Pope et al., 2014b; Sagoe et al., 2014), Aβ burdens could begin to increase in such users by their third decade of life, two or more decades earlier than in the general population, in whom Aβ levels typically begin to increase in the 5th decade of life (Villemagne et al., 2013). Given that the balance between Aβ synthesis and elimination slightly favors elimination in middle age but transitions to accumulation with aging (Bateman et al., 2006; Patterson et al., 2015), lifestyle factors that enable premature Aβ accumulation, including several substance use disorders and possibly supraphysiologic-dose AAS use, have the potential to increase risk for developing AD/ADRD. To date, it remains to be determined whether supraphysiologic-dose AAS use increases Aβ and tau-P levels. Accordingly, we plan to conduct imaging studies to determine whether supraphysiologic-dose AAS users exhibit higher Aβ and tau-P burdens than age-matched individuals who do not use AAS.
The literature included in this review also spotlights how two relatively common physiologic abnormalities that occur as a consequence of normal aging in men and in women, namely, sex-steroid level declines and oxidative stress elevations, can increase Aβ and tau-P accumulations. Accordingly, early diagnosis and correction of sex-steroid and oxidative stress abnormalities, which may be important common biological sources of risk similar to genetic factors related to lipid metabolism (Sepulcre et al., 2018), could delay or prevent the development of AD/ADRD, especially in people with substance use disorders. Brain biomarkers such as scyllo-inositol, which complexes with Aβ (McLaurin et al., 2000; Li and Pomès 2013; Jin and Selkoe, 2015) and which can be measured noninvasively without using ionizing radiation (Griffith et al., 2007; Liang et al., 2013b; Sarma et al., 2014; Kaufman et al., 2015), could be assessed early on in posterior cingulate cortex, an Aβ propagation hub (Sepulcre et al., 2018), to detect early increases in Aβ levels and predict who might benefit most from early interventions. In this regard, posterior cingulate cortex sI MRS signal is low in middle-aged people with obstructive sleep apnea (Sarma et al., 2014), a condition that has been associated with increased brain Aβ burdens and that is suspected to enhance risk for developing AD/ADRD (Elias et al., 2018; Sharma et al., 2018).
The potential benefits of therapeutic approaches that normalize androgen levels in men are manifold. Normalizing androgen levels attenuates CSF and plasma Aβ elevations (Ramsden et al., 2003; Rosario et al., 2006, 2010, 2012; Wahjoepramono et al., 2008; McAllister et al., 2010) and lowers tau-P levels (Papasozomenos, 1997; Papasozomenos and Shanavas, 2002; Rosario et al., 2010), likely by altering the activities of a number of regulatory proteins (Figure 2) including NEP (Yao et al., 2008; McAllister et al., 2010), AQP4 (Gu et al., 2003), PS-1 (Ghosh and Thakur, 2008), TTR (Reuter and Kennes, 1966; Gonçalves et al., 2008), IDE (Udrisar et al., 2005; McAllister et al., 2010), GSK3β (Papasozomenos, 1997; Papasozomenos and Shanavas, 2002; Gu et al., 2014; Maurer et al., 2014; Duran et al., 2016), and PP2A (Yi et al., 2005, 2008; Ueda et al., 2018). Recent studies indicate that physiological concentrations of androgens upregulate microglial uptake and elimination of Aβ and reduce Aβ-induced inflammatory responses (Yao et al., 2017). Some of these molecular effects of androgens could underlie the improved spatial memory and cognition reported in hypogonadal men or male rodents administered supplemental androgens (Janowsky et al., 1994; Sandstrom et al., 2006; McAllister et al., 2010; Spritzer et al., 2011; McConnell et al., 2012; Hawley et al., 2013; Locklear and Krtizer, 2014). E2 normalization may exert comparable effects in hypogonadal women (Perianes-Cachero et al., 2015), perhaps in part by attenuating an age-related increase in GSK3β activity reported in female mice (Krishnankutty et al., 2017) and by stimulating or preserving expression of PP2A (Yi et al., 2005, 2008; Ueda et al., 2018). T supplementation also increases Nrf2 protein expression and/or function after castration or with aging (Zhang et al., 2016; Bae et al., 2017; Cui et al., 2017) as does E2 supplementation, in part by inhibiting GSK3β activity (Wu et al., 2014; Li et al., 2017). Yet, to date, pharmacotherapy with T or E2 as a cognitive preservation/enhancement strategy has been associated with marginal benefits, except in women taking E2 for greater than 10 years or in women who started taking E2 near the onset of menopause (Shao et al., 2012; Huang et al., 2016b; Imtiaz et al., 2017). However, these studies have involved subjects aged 55 or older, an age by when Aβ and tau-P proteins likely have been accumulating for a decade or more (Villemagne et al., 2013). Since buildup of Aβ or tau-P could limit cognitive benefits of T or E2 supplementation, these studies may not have detected the full impact of sex-steroid supplementation. Accordingly, it seems that future studies are warranted to assess whether earlier initiation of T or E2 supplementation improves or preserves cognition.
Like sex-steroid hormones, antioxidants exert pleiotropic beneficial effects on AD-related proteins and the potential benefits of attenuating oxidative stress are manifold. For example, the antioxidant N-acetylcysteine (NAC), which has been evaluated as a potential treatment for AD, substance use disorders, and other brain disorders (Uys et al., 2011; Deepmala et al., 2015; Hara et al., 2017; Womersley et al., in press), inhibits oxidative stress-induced PS-1 upregulation (Oda et al., 2010) and oxidative stress- and Aβ-induced NEP downregulation (Wang et al., 2010). An analog of NAC with greater brain bioavailability, N-acetylcysteine amide, normalizes oxidative stress-induced γ-secretase dysfunction and attenuates oxidative stress-induced Aβ42/Aβ40 ratio elevations (Arimon et al., 2015). Long-term dietary supplementation with an antioxidant mixture including NAC normalizes aging-induced oxidative stress, cortical β-secretase and NEP activity, and Aβ42 levels, and also has been shown to improve spatial memory (Sinha et al., 2010, 2016). NAC attenuates excess oxidative stress-induced PP2A inhibition (Chen et al., 2008; Raghuraman et al., 2009) and inhibits Zn2+ activation of GSK3β and tau hyperphosphorylation (Kwon et al., 2015). NAC also converts TTR into its native state (Henze et al., 2013), which could increase its Aβ-complexing/elimination capacity. Yet, NAC is relatively ineffective at neutralizing key oxidative species including superoxide anion and H2O2 (Aruoma et al., 1989; Winterbourn and Metodiewa, 1999; Schneider et al., 2005), brain uptake of NAC is low because it is lipophilic, and NAC is actively extruded from brain (Clark et al., 2017). Thus, other antioxidants may be even more effective than NAC, such as activators of the master antioxidant transcription factor Nrf2, which as noted above is downregulated by abnormal sex-steroid levels, excess oxidative stress, high GSK3β activity, and during aging (see section 6.3). Nrf2 activators broadly attenuate many different sources of oxidative stress including superoxide anion and H2O2 by increasing activities of hundreds of antioxidant and detoxifying proteins (Hybertson et al., 2011; Gorrini et al., 2013; Ma, 2013; Cuadrado et al., 2018). Given the wide range of oxidative stress biomarkers altered by AAS (Table 1), broad spectrum antioxidants such as Nrf2 activators may be especially beneficial in supraphysiologic-dose AAS users and in other groups at risk for developing AD/ADRD. This hypothesis is supported by the observations that genetic knockout of Nrf2 exacerbates Aβ and tau-P burdens and spatial cognition impairments in AD mice (Branca et al., 2017; Rojo et al., 2017) and by the finding that the nutriceutical Nrf2 activator sulforaphane decreases β-secretase and PS-1 expression, Aβ and tau-P burdens, and improves cognition in AD mice (Hou et al., 2018; Lee et al., 2018). In non-AD models, sulforaphane inhibits GSK3β activity (Wang et al., 2016) and increases AQP4 expression (Zhao et al., 2005). In neuroblastoma cells, the Nrf2 activator dimethylfumarate reduces Aβ42 levels and tau hyperphosphorylation (Campolo et al., 2018) and the Nrf2 activator carnosic acid increases ADAM10 (nonamyloidogenic APP processing) and IDE mRNA expression and reduces Aβ42 protein levels (Meng et al., 2013). Thus, Nrf2 activators, through multiple mechanisms, could slow or prevent the development of AD/ADRD. By attenuating excess oxidative stress, antioxidants also may be able to enhance neural plasticity, which is impaired both in dementias and in addiction disorders (Kishida and Klann, 2007; Massaad and Klann, 2011; Uys et al., 2011, 2014; Hidalgo and Arias-Cavieres, 2016; Kumar et al., 2018a; Womersley et al., in press), and may reduce stimulant- or alcohol-seeking (Jang et al., 2015, 2017; Beiser et al., 2017; Quintanilla et al., 2018) or opioid tolerance or withdrawal symptoms (Abdel-Zaher et al., 2010).
GSK3β inhibitors also have been evaluated in cell culture, animal, and human AD/ADRD models and several inhibitors exert promising effects. For example, in cultured cells or in transgenic mice, several different types of GSK3β inhibitors including lithium (Li), inhibited Aβ42 or tau-P accumulation, accelerated Aβ42 clearance, upregulated LRP1 expression, and/or reduced spatial memory deficits (Ryder et al., 2003; Hu et al., 2009; Lin et al., 2016; Habib et al., 2017; Pan et al., 2018). In humans, low dose Li administered daily for 1 year significantly lowered CSF concentrations of tau-P, induced a trend level decline in CSF Aβ42 concentration, and improved cognitive function in individuals with mild cognitive impairment (Forlenza et al., 2011). A subsequent 15-month Li microdosing study reported stabilization of cognitive function in individuals with AD (Nunes et al., 2013). Like antioxidants, GSK3 inhibitors also exert beneficial effects on substance use-related behaviors including cocaine or methamphetamine hyperactivity or locomotor sensitization (Miller et al., 2009; Xu et al., 2009, 2011), cocaine cue memory reconsolidation (Shi et al., 2014), cannabis withdrawal signs (Rahimi et al., 2014), opioid-induced tolerance (Parkitna et al., 2006) or hyperalgesia (Yuan et al., 2013; Li et al., 2014), ketamine self-administration (Huang et al., 2015), and alcohol preference and withdrawal symptoms (van der Vaart et al., 2018). Accordingly, GSK3 inhibitors under development for substance use disorders also may have potential for reducing risk for developing AD/ADRD.
By reducing the presence or severity of lifestyle and health conditions (including supraphysiologic-dose AAS use and other substance use disorders) and/or by initiating treatment that slows accumulation of Aβ and tau-P starting in middle age, when Aβ and tau-P protein burdens begin to build (or earlier in substance users), it may be possible to slow or prevent the development of AD/ADRD. Because so many proteins involved in regulating Aβ and tau-P accumulation are responsive to sex-steroid or oxidative stress levels or GSK3β activity, the latter of which is modulated by sexsteroids and oxidative stress, the most effective interventions likely will include multi-targeting strategies that modulate these and downstream regulatory (e.g., Nrf2) pathways. Multi-targeting therapeutic strategies are increasingly being pursued because at least theoretically, they are likely to be comparably if not more effective than agents that selectively target any one protein involved in a pathophysiologic cascade (Cavalli et al., 2008; Agatonovic-Kustrin et al., 2018; Gameiro et al., 2017; Gong et al., 2018; Knez et al., 2018; Oset-Gasque et al., 2018; Reis et al., 2018). Multi-targeting strategies, by enhancing neural plasticity and learning and/or by reducing drug-seeking, drug-tolerance, or drug-withdrawal symptoms, also may improve treatment outcomes in people suffering from substance use disorders.
10. Conclusions
A number of lifestyle and health conditions – including smoking, excessive alcohol intake, hypertension, obesity, diabetes, depression, physical inactivity, and social isolation – have been identified as presumed causal factors for the development of AD/ADRD, and the co-occurrence of these and other causal factors may amplify risk for developing dementia (Song et al., 2014; Livingston et al., 2017). We propose that supraphysiologic-dose AAS use, which may induce hypogonadism and excess oxidative stress, also is a causal factor for AD/ADRD, particularly when occurring in adolescents and young adults, perhaps along with other substance use disorders. If this hypothesis proves correct, it follows that the public health burden of supraphysiologic-dose AAS use and other substance use disorders may be greater than previously recognized. It also follows that persistent AAS users may benefit from multi-targeting treatment strategies, as outlined above, that may slow accumulation of Aβ and tau-P and delay or prevent development of AD/ADRD.
Highlights.
Supraphysiologic-dose anabolic-androgenic steroid (sAAS) use starts by the mid-20s
sAAS use occurs primarily in men aiming to increase muscularity
sAAS use causes health problems including hypogonadism & excess oxidative stress
These effects may increase synthesis & decrease elimination of Aβ & tau-P proteins
sAAS use may cause early Aβ & tau-P increases and may enhance risk for dementia
Acknowledgment:
The authors dedicate this work to the memory of J. Eric N. Jensen, Ph.D.
Funding: This work was supported in part by a National Institutes of Health grant [DA041866].
Role of the Funding Source: The sponsors did not have any role in manuscript design, or interpretation of research reports cited by this review, or manuscript writing, or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests: Drs. Kaufman and Kanayama report no conflicts of interest. Dr. Hudson has received grant support and consulting fees from Shire and Sunovion. During the last five years, Dr. Pope has received research grant support from Shire and Sunovion, and has testified as an expert witness in two cases involving illicit androgen use.
References
- Abdel-Zaher AO, Abdel-Rahman MS, ELwasei FM. Blockade of nitric oxide overproduction and oxidative stress by Nigella sativa oil attenuates morphine-induced tolerance and dependence in mice. Neurochem Res. 2010. October;35(10):1557–65. doi: 10.1007/s11064-010-0215-2. [DOI] [PubMed] [Google Scholar]
- Achar S, Rostamian A, Narayan SM. Cardiac and metabolic effects of anabolic-androgenic steroid abuse on lipids, blood pressure, left ventricular dimensions, and rhythm. Am J Cardiol. 2010. September 15;106(6):893–901. doi: 10.1016/j.amjcard.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agatonovic-Kustrin S, Kettle C, Morton DW. A molecular approach in drug development for Alzheimer’s disease. Biomed Pharmacother. 2018. October;106:553–565. doi: 10.1016/j.biopha.2018.06.147. [DOI] [PubMed] [Google Scholar]
- Agnati LF, Genedani S, Rasio G, Galantucci M, Saltini S, Filaferro M, Franco R, Mora F, Ferré S, Fuxe K. Studies on homocysteine plasma levels in Alzheimer’s patients. Relevance for neurodegeneration. J Neural Transm (Vienna). 2005. January;112(1):163–9. [DOI] [PubMed] [Google Scholar]
- Ahmed MA, El-Awdan SA. Lipoic acid and pentoxifylline mitigate nandrolone decanoate-induced neurobehavioral perturbations in rats via re-balance of brain neurotransmitters, up-regulation of Nrf2/HO-1 pathway, and down-regulation of TNFR1 expression. Horm Behav. 2015. July;73:186–99. doi: 10.1016/j.yhbeh.2015.07.007. [DOI] [PubMed] [Google Scholar]
- Ahmed MA. Amelioration of nandrolone decanoate-induced testicular and sperm toxicity in rats by taurine: effects on steroidogenesis, redox and inflammatory cascades, and intrinsic apoptotic pathway. Toxicol Appl Pharmacol. 2015. February 1;282(3):285–96. doi: 10.1016/j.taap.2014.12.007. [DOI] [PubMed] [Google Scholar]
- Akita K, Harada K, Ichihara J, Takata N, Takahashi Y, Saito K. A novel selective androgen receptor modulator, NEP28, is efficacious in muscle and brain without serious side effects on prostate. Eur J Pharmacol. 2013. November 15;720(1–3):107–14. doi: 10.1016/j.ejphar.2013.10.042. [DOI] [PubMed] [Google Scholar]
- Alderks CE. Trends in the Use of Methadone, Buprenorphine, and Extended-Release Naltrexone at Substance Abuse Treatment Facilities: 2003–2015 (Update) The CBHSQ Report. Rockville (MD): Substance Abuse and Mental Health Services Administration (US); 2013-. [PubMed] [Google Scholar]
- Alèn M, Rahkila P, Reinilä M, Vihko R. Androgenic-anabolic steroid effects on serum thyroid, pituitary and steroid hormones in athletes. Am J Sports Med. 1987. Jul-Aug;15(4):357–61. [DOI] [PubMed] [Google Scholar]
- Alizade E, Avci A, Tabakcı MM, Toprak C, Zehir R, Acar G, Kargin R, Emiroğlu MY, Akçakoyun M, Pala S. Comparison of Right Ventricle Systolic Function between Long-Term Anabolic-Androgenic Steroid User and Nonuser Bodybuilder Athletes: A Study of Two-Dimensional Speckle Tracking Echocardiography. Echocardiography 2016. August;33(8):1178–85. doi: 10.1111/echo.13243. [DOI] [PubMed] [Google Scholar]
- Almeida OP, Waterreus A, Spry N, Flicker L, Martins RN. One year follow-up study of the association between chemical castration, sex hormones, beta-amyloid, memory and depression in men. Psychoneuroendocrinology 2004. September;29(8):1071–81. [DOI] [PubMed] [Google Scholar]
- Alshehri B, D’Souza DG, Lee JY, Petratos S, Richardson SJ. The diversity of mechanisms influenced by transthyretin in neurobiology: development, disease and endocrine disruption. J Neuroendocrinol. 2015. May;27(5):303–23. doi: 10.1111/jne.12271. [DOI] [PubMed] [Google Scholar]
- Alves CJ, Magalhães A, Melo P, de Sousa L, Tavares MA, Monteiro PR, Summavielle T. Long-term effects of chronic cocaine exposure throughout adolescence on anxiety and stress responsivity in a Wistar rat model. Neuroscience 2014. September 26;277:343–55. doi: 10.1016/j.neuroscience.2014.07.008. [DOI] [PubMed] [Google Scholar]
- Alzoubi KH, Khabour OF, Rashid BA, Damaj IM, Salah HA. The neuroprotective effect of vitamin E on chronic sleep deprivation-induced memory impairment: the role of oxidative stress. Behav Brain Res. 2012. January 1;226(1):205–10. doi: 10.1016/j.bbr.2011.09.017. [DOI] [PubMed] [Google Scholar]
- Angell PJ, Chester N, Green DJ, Shah R, Somauroo J, Whyte G, George K. Anabolic steroid use and longitudinal, radial, and circumferential cardiac motion. Med Sci Sports Exerc. 2012. April;44(4):583–90. doi: 10.1249/MSS.0b013e3182358cb0. [DOI] [PubMed] [Google Scholar]
- Angell PJ, Ismail TF, Jabbour A, Smith G, Dahl A, Wage R, Whyte G, Green DJ, Prasad S, George K. Ventricular structure, function, and focal fibrosis in anabolic steroid users: a CMR study. Eur J Appl Physiol. 2014. May;114(5):921–8. doi: 10.1007/s00421-014-2820-2. [DOI] [PubMed] [Google Scholar]
- Anthony IC, Norrby KE, Dingwall T, Carnie FW, Millar T, Arango JC, Robertson R, Bell JE. Predisposition to accelerated Alzheimer-related changes in the brains of human immunodeficiency virus negative opiate abusers. Brain. 2010. December;133(Pt 12):3685–98. doi: 10.1093/brain/awq263. [DOI] [PubMed] [Google Scholar]
- Arana C, Cutando A, Ferrera MJ, Gómez-Moreno G, Worf CV, Bolaños MJ, Escames G, Acuña-Castroviejo D. Parameters of oxidative stress in saliva from diabetic and parenteral drug addict patients. J Oral Pathol Med. 2006. October;35(9):554–9. [DOI] [PubMed] [Google Scholar]
- Arangalage D, Ederhy S, Dufour L, Joffre J, Van der Vynckt C, Lang S, Tzourio C, Cohen A. Relationship between cognitive impairment and echocardiographic parameters: a review. J Am Soc Echocardiogr. 2015. March;28(3):264–74. doi: 10.1016/j.echo.2014.11.009. [DOI] [PubMed] [Google Scholar]
- Arazi H, Mohammadjafari H, Asadi A. Use of anabolic androgenic steroids produces greater oxidative stress responses to resistance exercise in strength-trained men. Toxicol Rep. 2017a. June 8;4:282–286. doi: 10.1016/j.toxrep.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arazi H, Rahmati S, Ghafoori H. The interaction effects of resistance training and sustanon abuse on liver antioxidant activities and serum enzymes in male rats. Interv Med Appl Sci. 2017b. September;9(3):178–183. doi: 10.1556/1646.9.2017.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimon M, Takeda S, Post KL, Svirsky S, Hyman BT, Berezovska O. Oxidative stress and lipid peroxidation are upstream of amyloid pathology. Neurobiol Dis. 2015. December;84:109–19. doi: 10.1016/j.nbd.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aruoma OI, Halliwell B, Hoey BM, Butler J. The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic Biol Med. 1989;6(6):593–7. [DOI] [PubMed] [Google Scholar]
- Aso E, Sánchez-Pla A, Vegas-Lozano E, Maldonado R, Ferrer I. Cannabis-based medicine reduces multiple pathological processes in AβPP/PS1 mice. J Alzheimers Dis. 2015;43(3):977–91. doi: 10.3233/JAD-141014. [DOI] [PubMed] [Google Scholar]
- Aso E, Andrés-Benito P, Carmona M, Maldonado R, Ferrer I. Cannabinoid Receptor 2 Participates in Amyloid-β Processing in a Mouse Model of Alzheimer’s Disease but Plays a Minor Role in the Therapeutic Properties of a Cannabis-Based Medicine. J Alzheimers Dis. 2016;51(2):489–500. doi: 10.3233/JAD-150913. [DOI] [PubMed] [Google Scholar]
- Aydin S, Ozaras R, Uzun H, Belce A, Uslu E, Tahan V, Altug T, Dumen E, Senturk H. N-acetylcysteine reduced the effect of ethanol on antioxidant system in rat plasma and brain tissue. Tohoku J Exp Med. 2002. October;198(2):71–7. [DOI] [PubMed] [Google Scholar]
- Bae WJ, Zhu GQ, Choi SW, Jeong HC, Bashraheel F, Kim KS, Kim SJ, Cho HJ, Ha US, Hong SH, Lee JY, Oh HA, Koo HC, Kim DR, Hwang SY, Kim SW. Antioxidant and Antifibrotic Effect of a Herbal Formulation In Vitro and in the Experimental Andropause via Nrf2/HO-1 Signaling Pathway. Oxid Med Cell Longev. 2017;2017:6024839. doi: 10.1155/2017/6024839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggish AL, Weiner RB, Kanayama G, Hudson JI, Picard MH, Hutter AM Jr, Pope HG Jr. Long-term anabolic-androgenic steroid use is associated with left ventricular dysfunction. Circ Heart Fail. 2010. July;3(4):472–6. doi: 10.1161/CIRCHEARTFAILURE.109.931063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggish AL, Weiner RB, Kanayama G, Hudson JI, Lu MT, Hoffmann U, Pope HG Jr. Cardiovascular Toxicity of Illicit Anabolic-Androgenic Steroid Use. Circulation 2017. May 23;135(21):1991–2002. doi: 10.1161/CIRCULATIONAHA.116.026945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y, Wang Y, Liu M, Gu YH, Jiang B, Wu X, Wang HL. Suppression of nuclear factor erythroid-2-related factor 2-mediated antioxidative defense in the lung injury induced by chronic exposure to methamphetamine in rats. Mol Med Rep. 2017. May;15(5):3135–3142. doi: 10.3892/mmr.2017.6356. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Zhang X, Manda KR, Banks WA, Ercal N. HIV proteins (gp120 and Tat) and methamphetamine in oxidative stress-induced damage in the brain: potential role of the thiol antioxidant N-acetylcysteine amide. Free Radic Biol Med. 2010. May 15;48(10):1388–98. doi: 10.1016/j.freeradbiomed.2010.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbisan F, Azzolin VF, Monteiro GC, Teixeira CF, Mastella MH, Buefno V, Duarte MMMF, Wagner G, do Prado-Lima PAS, Ribeiro EE, da Cruz IBM. Genetic or pharmacological superoxide-hydrogen peroxide imbalances modulate the in vitro effects of lithium on glycogen synthase kinase-3β. Gene 2018. May 20;655:48–55. doi: 10.1016/j.gene.2018.02.046. [DOI] [PubMed] [Google Scholar]
- Barbosa Dos Santos G, Machado Rodrigues MJ, Gonçalves EM, Cintra Gomes Marcondes MC, Areas MA. Melatonin reduces oxidative stress and cardiovascular changes induced by stanozolol in rats exposed to swimming exercise. Eurasian J Med. 2013. October;45(3):155–62. doi: 10.5152/eajm.2013.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes J, Dickerson BC, Frost C, Jiskoot LC, Wolk D, van der Flier WM. Alzheimer’s disease first symptoms are age dependent: Evidence from the NACC dataset. Alzheimers Dement. 2015. November;11(11):1349–57. doi: 10.1016/j.jalz.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basaria S, Travison TG, Alford D, Knapp PE, Teeter K, Cahalan C, Eder R, Lakshman K, Bachman E, Mensing G, Martel MO, Le D, Stroh H, Bhasin S, Wasan AD, Edwards RR. Effects of testosterone replacement in men with opioid-induced androgen deficiency: a randomized controlled trial. Pain 2015. February;156(2):280–8. doi: 10.1097/01.j.pain.0000460308.86819.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman RJ, Munsell LY, Morris JC, Swarm R, Yarasheski KE, Holtzman DM. Human amyloid-beta synthesis and clearance rates as measured in cerebrospinal fluid in vivo. Nat Med. 2006. July;12(7):856–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman WA, Spungen AM, Collins JF, Raisch DW, Ho C, Deitrick GA, Nemchausky BA, Goetz LL, Park JS, Schwartz M, Merritt JL, Jayawardena V, Sandford P, Sabharwal S, Holmes SA, Nasar F, Sasaki R, Punj V, Zachow KF, Chua WC, Thomas MD, Trincher RC. The effect of oxandrolone on the healing of chronic pressure ulcers in persons with spinal cord injury: a randomized trial. Ann Intern Med. 2013. May 21;158(10):718–26. doi: 10.7326/0003-4819-158-10-201305210-00006. [DOI] [PubMed] [Google Scholar]
- Bayazit H, Selek S, Karababa IF, Cicek E, Aksoy N. Evaluation of Oxidant/Antioxidant Status and Cytokine Levels in Patients with Cannabis Use Disorder. Clin Psychopharmacol Neurosci. 2017. August 31;15(3):237–242. doi: 10.9758/cpn.2017.15.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beiser T, Numa R, Kohen R, Yaka R. Chronic treatment with Tempol during acquisition or withdrawal from CPP abolishes the expression of cocaine reward and diminishes oxidative damage. Sci Rep. 2017. September 11;7(1):11162. doi: 10.1038/s41598-017-11511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berul CI, Harclerode JE. Effects of cocaine hydrochloride on the male reproductive system. Life Sci. 1989;45(1):91–5. [DOI] [PubMed] [Google Scholar]
- Bhasin S, Storer TW, Berman N, Callegari C, Clevenger B, Phillips J, Bunnell TJ, Tricker R, Shirazi A, Casaburi R. The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N Engl J Med. 1996. July 4;335(1):1–7. [DOI] [PubMed] [Google Scholar]
- Bhasin S, Woodhouse L, Casaburi R, Singh AB, Bhasin D, Berman N, Chen X, Yarasheski KE, Magliano L, Dzekov C, Dzekov J, Bross R, Phillips J, Sinha-Hikim I, Shen R, Storer TW. Testosterone dose-response relationships in healthy young men. Am J Physiol Endocrinol Metab. 2001. December;281(6):E1172–81. [DOI] [PubMed] [Google Scholar]
- Bhasin S, Woodhouse L, Casaburi R, Singh AB, Mac RP, Lee M, Yarasheski KE, Sinha-Hikim I, Dzekov C, Dzekov J, Magliano L, Storer TW. Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J Clin Endocrinol Metab. 2005. February;90(2):678–88. [DOI] [PubMed] [Google Scholar]
- Bhasin S, Pencina M, Jasuja GK, Travison TG, Coviello A, Orwoll E, Wang PY, Nielson C, Wu F, Tajar A, Labrie F, Vesper H, Zhang A, Ulloor J, Singh R, D’Agostino R, Vasan RS. Reference ranges for testosterone in men generated using liquid chromatography tandem mass spectrometry in a community-based sample of healthy nonobese young men in the Framingham Heart Study and applied to three geographically distinct cohorts. J Clin Endocrinol Metab. 2011. August;96(8):2430–9. doi: 10.1210/jc.2010-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi C, Tham DKL, Perronnet C, Joshi B, Nabi IR, Moukhles H. The Oxidative Stress-Induced Increase in the Membrane Expression of the Water-Permeable Channel Aquaporin-4 in Astrocytes Is Regulated by Caveolin-1 Phosphorylation. Front Cell Neurosci. 2017. December 20;11:412. doi: 10.3389/fncel.2017.00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjørnebekk A, Walhovd KB, Jørstad ML, Due-Tønnessen P, Hullstein IR, Fjell AM. Structural Brain Imaging of Long-Term Anabolic-Androgenic Steroid Users and Nonusing Weightlifters. Biol Psychiatry 2017. August 15;82(4):294–302. doi: 10.1016/j.biopsych.2016.06.017. [DOI] [PubMed] [Google Scholar]
- Blanc EM, Keller JN, Fernandez S, Mattson MP. 4-hydroxynonenal, a lipid peroxidation product, impairs glutamate transport in cortical astrocytes. Glia 1998. February;22(2):149–60. [DOI] [PubMed] [Google Scholar]
- Bliesener N, Albrecht S, Schwager A, Weckbecker K, Lichtermann D, Klingmüller D. Plasma testosterone and sexual function in men receiving buprenorphine maintenance for opioid dependence. J Clin Endocrinol Metab. 2005. January;90(1):203–6. [DOI] [PubMed] [Google Scholar]
- Blom HJ. Consequences of homocysteine export and oxidation in the vascular system. Semin Thromb Hemost. 2000;26(3):227–32. [DOI] [PubMed] [Google Scholar]
- Bolding G, Sherr L, Elford J. Use of anabolic steroids and associated health risks among gay men attending London gyms. Addiction 2002. February;97(2):195–203. [DOI] [PubMed] [Google Scholar]
- Borgas D, Chambers E, Newton J, Ko J, Rivera S, Rounds S, Lu Q. Cigarette Smoke Disrupted Lung Endothelial Barrier Integrity and Increased Susceptibility to Acute Lung Injury via Histone Deacetylase 6. Am J Respir Cell Mol Biol. 2016. May;54(5):683–96. doi: 10.1165/rcmb.2015-0149OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouillot C, Prochiantz A, Rougon G, Allinquant B. Axonal amyloid precursor protein expressed by neurons in vitro is present in a membrane fraction with caveolae-like properties. J Biol Chem. 1996. March 29;271(13):7640–4. [DOI] [PubMed] [Google Scholar]
- Boyd-Kimball D, Castegna A, Sultana R, Poon HF, Petroze R, Lynn BC, Klein JB, Butterfield DA. Proteomic identification of proteins oxidized by Abeta(1–42) in synaptosomes: implications for Alzheimer’s disease. Brain Res. 2005. May 24;1044(2):206–15. [DOI] [PubMed] [Google Scholar]
- Branca C, Ferreira E, Nguyen TV, Doyle K, Caccamo A, Oddo S. Genetic reduction of Nrf2 exacerbates cognitive deficits in a mouse model of Alzheimer’s disease. Hum Mol Genet. 2017. December 15;26(24):4823–4835. doi: 10.1093/hmg/ddx361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brower KJ, Blow FC, Young JP, Hill EM. Symptoms and correlates of anabolic-androgenic steroid dependence. Br J Addict. 1991. June;86(6):759–68. [DOI] [PubMed] [Google Scholar]
- Brower KJ. Anabolic steroid abuse and dependence. Curr Psychiatry Rep. 2002. October;4(5):377–87. [DOI] [PubMed] [Google Scholar]
- Brower KJ. Anabolic steroid abuse and dependence in clinical practice. Phys Sportsmed. 2009. December;37(4):131–40. doi: 10.3810/psm.2009.12.1751. [DOI] [PubMed] [Google Scholar]
- Budziszewska B, Jaworska-Feil L, Lasoń W. The effect of repeated amphetamine and cocaine administration on adrenal, gonadal and thyroid hormone levels in the rat plasma. Exp Clin Endocrinol Diabetes 1996;104(4):334–8. [DOI] [PubMed] [Google Scholar]
- Bueno A, Carvalho FB, Gutierres JM, Lhamas C, Andrade CM. A comparative study of the effect of the dose and exposure duration of anabolic androgenic steroids on behavior, cholinergic regulation, and oxidative stress in rats. PLoS One 2017a. June 8;12(6):e0177623. doi: 10.1371/journal.pone.0177623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueno A, Carvalho FB, Gutierres JM, Lhamas CL, Brusco I, Oliveira SM, Amaral MG, Dorneles G, Sorraila J, Duarte MM, de Andrade CM. Impacts of dose and time of boldenone and stanazolol exposure in inflammatory markers, oxidative and nitrosative stress and histopathological changes in the rat testes. Theriogenology 2017b. March 1;90:101–108. doi: 10.1016/j.theriogenology.2016.11.024. [DOI] [PubMed] [Google Scholar]
- Camiletti-Moirón D, Aparicio VA, Nebot E, Medina G, Martínez R, Kapravelou G, Andrade A, Porres JM, López-Jurado M, Aranda P. High-intensity Exercise Modifies the Effects of Stanozolol on Brain Oxidative Stress in Rats. Int J Sports Med. 2015. November;36(12):984–91. doi: 10.1055/s-0035-1548941. [DOI] [PubMed] [Google Scholar]
- Campolo M, Casili G, Lanza M, Filippone A, Paterniti I, Cuzzocrea S, Esposito E. Multiple mechanisms of dimethyl fumarate in amyloid β-induced neurotoxicity in human neuronal cells. J Cell Mol Med. 2018. February;22(2):1081–1094. doi: 10.1111/jcmm.13358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraci F, Pistarà V, Corsaro A, Tomasello F, Giuffrida ML, Sortino MA, Nicoletti F, Copani A. Neurotoxic properties of the anabolic androgenic steroids nandrolone and methandrostenolone in primary neuronal cultures. J Neurosci Res. 2011. April;89(4):592–600. doi: 10.1002/jnr.22578. [DOI] [PubMed] [Google Scholar]
- Carcaillon L, Brailly-Tabard S, Ancelin ML, Tzourio C, Foubert-Samier A, Dartigues JF, Guiochon-Mantel A, Scarabin PY. Low testosterone and the risk of dementia in elderly men: Impact of age and education. Alzheimers Dement. 2014. October;10(5 Suppl):S306–14. doi: 10.1016/j.jalz.2013.06.006. [DOI] [PubMed] [Google Scholar]
- Cardona-Gomez P, Perez M, Avila J, Garcia-Segura LM, Wandosell F. Estradiol inhibits GSK3 and regulates interaction of estrogen receptors, GSK3, and beta-catenin in the hippocampus. Mol Cell Neurosci. 2004. March;25(3):363–73. [DOI] [PubMed] [Google Scholar]
- Caruso D, Pesaresi M, Maschi O, Giatti S, Garcia-Segura LM, Melcangi RC. Effect of short-and long-term gonadectomy on neuroactive steroid levels in the central and peripheral nervous system of male and female rats. J Neuroendocrinol. 2010. November;22(11):1137–47. doi: 10.1111/j.1365-2826.2010.02064.x. [DOI] [PubMed] [Google Scholar]
- Caruso D, Barron AM, Brown MA, Abbiati F, Carrero P, Pike CJ, Garcia-Segura LM, Melcangi RC. Age-related changes in neuroactive steroid levels in 3xTg-AD mice. Neurobiol Aging 2013. April;34(4):1080–9. doi: 10.1016/j.neurobiolaging.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascalheira JF, João SS, Pinhanços SS, Castro R, Palmeira M, Almeida S, Faria MC, Domingues FC. Serum homocysteine: interplay with other circulating and genetic factors in association to Alzheimer’s type dementia. Clin Biochem. 2009. June;42(9):783–90. doi: 10.1016/j.clinbiochem.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Castellano JM, Kim J, Stewart FR, Jiang H, DeMattos RB, Patterson BW, Fagan AM, Morris JC, Mawuenyega KG, Cruchaga C, Goate AM, Bales KR, Paul SM, Bateman RJ, Holtzman DM. Human apoE isoforms differentially regulate brain amyloid-β peptide clearance. Sci Transl Med. 2011. June 29;3(89):89ra57. doi: 10.1126/scitranslmed.3002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilla-García A, Santolaria-Fernández FJ, González-Reimers CE, Batista-López N, González-García C, Jorge-Hernández JA, Hernández-Nieto L. Alcohol-induced hypogonadism: reversal after ethanol withdrawal. Drug Alcohol Depend. 1987. November 30;20(3):255–60. [DOI] [PubMed] [Google Scholar]
- Cavalli A, Bolognesi ML, Minarini A, Rosini M, Tumiatti V, Recanatini M, Melchiorre C. Multi-target-directed ligands to combat neurodegenerative diseases. J Med Chem. 2008. February 14;51(3):347–72. doi: 10.1021/jm7009364. Erratum in: J Med Chem. 2008 Apr 10;51(7):2326. [DOI] [PubMed] [Google Scholar]
- Cecchi R, Muciaccia B, Ciallella C, Di Luca NM, Kimura A, Sestili C, Nosaka M, Kondo T. Ventricular androgenic-anabolic steroid-related remodeling: an immunohistochemical study. Int J Legal Med. 2017. November;131(6):1589–1595. doi: 10.1007/s00414-017-1589-3. [DOI] [PubMed] [Google Scholar]
- Cepeda MS, Zhu V, Vorsanger G, Eichenbaum G. Effect of Opioids on Testosterone Levels: Cross-Sectional Study using NHANES. Pain Med. 2015. December;16(12):2235–42. doi: 10.1111/pme.12843. [DOI] [PubMed] [Google Scholar]
- Cemek M, Büyükokuroğlu ME, Hazman O, Konuk M, Bulut S, Birdane YO. The roles of melatonin and vitamin E plus selenium in prevention of oxidative stress induced by naloxone-precipitated withdrawal in heroin-addicted rats. Biol Trace Elem Res. 2011. July;142(1):55–66. doi: 10.1007/s12011-010-8744-8. [DOI] [PubMed] [Google Scholar]
- Chang HM, Mai FD, Chen BJ, Wu UI, Huang YL, Lan CT, Ling YC. Sleep deprivation predisposes liver to oxidative stress and phospholipid damage: a quantitative molecular imaging study. J Anat. 2008. March;212(3):295–305. doi: 10.1111/j.1469-7580.2008.00860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S, Rasmussen JJ, Frandsen MN, Schou M, Johansen ML, Faber J, Münster AB, Sidelmann JJ, Kistorp C. Procoagulant State in Current and Former Anabolic Androgenic Steroid Abusers. Thromb Haemost. 2018. April;118(4):647–653. doi: 10.1055/s-0038-1636540. [DOI] [PubMed] [Google Scholar]
- Chen L, Liu L, Huang S. Cadmium activates the mitogen-activated protein kinase (MAPK) pathway via induction of reactive oxygen species and inhibition of protein phosphatases 2A and 5. Free Radic Biol Med. 2008. October 1;45(7):1035–44. doi: 10.1016/j.freeradbiomed.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Chen CH, Pan CH, Chen CC, Huang MC. Increased oxidative DNA damage in patients with alcohol dependence and its correlation with alcohol withdrawal severity. Alcohol Clin Exp Res. 2011. February;35(2):338–44. doi: 10.1111/j.1530-0277.2010.01349.x. [DOI] [PubMed] [Google Scholar]
- Chen CS, Tseng YT, Hsu YY, Lo YC. Nrf2-Keap1 antioxidant defense and cell survival signaling are upregulated by 17β-estradiol in homocysteine-treated dopaminergic SH-SY5Y cells. Neuroendocrinology 2013;97(3):232–41. doi: 10.1159/000342692. [DOI] [PubMed] [Google Scholar]
- Chen X, Liu Y, Zhu J, Lei S, Dong Y, Li L, Jiang B, Tan L, Wu J, Yu S, Zhao Y. GSK-3β downregulates Nrf2 in cultured cortical neurons and in a rat model of cerebral ischemia-reperfusion. Sci Rep. 2016. February 3;6:20196. doi: 10.1038/srep20196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherrier MM, Rose AL, Higano C. The effects of combined androgen blockade on cognitive function during the first cycle of intermittent androgen suppression in patients with prostate cancer. J Urol. 2003. November;170(5):1808–11. [DOI] [PubMed] [Google Scholar]
- Cherrier MM, Aubin S, Higano CS. Cognitive and mood changes in men undergoing intermittent combined androgen blockade for non-metastatic prostate cancer. Psychooncology 2009. March;18(3):237–47. doi: 10.1002/pon.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chignalia AZ, Oliveira MA, Debbas V, Dull RO, Laurindo FR, Touyz RM, Carvalho MH, Fortes ZB, Tostes RC. Testosterone induces leucocyte migration by NADPH oxidase-driven ROS- and COX2-dependent mechanisms. Clin Sci (Lond). 2015. July;129(1):39–48. doi: 10.1042/CS20140548. [DOI] [PubMed] [Google Scholar]
- Chin J, Sternin O, Wu HB, Burrell S, Lu D, Jenab S, Perrotti LI, Quiñones-Jenab V. Endogenous gonadal hormones modulate behavioral and neurochemical responses to acute and chronic cocaine administration. Brain Res. 2002. July 26;945(1):123–30. [DOI] [PubMed] [Google Scholar]
- Chinchalongporn V, Shukla M, Govitrapong P. Melatonin ameliorates Aβ(42)-induced alteration of βAPP-processing secretases via the melatonin receptor through the Pin1/GSK3β/NF-κB pathway in SH-SY5Y cells. J Pineal Res. 2018. May;64(4):e12470. doi: 10.1111/jpi.12470. [DOI] [PubMed] [Google Scholar]
- Choi D, Choi S, Park SM. Effect of smoking cessation on the risk of dementia: a longitudinal study. Ann Clin Transl Neurol. 2018. September 5;5(10):1192–1199. doi: 10.1002/acn3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christou MA, Christou PA, Markozannes G, Tsatsoulis A, Mastorakos G, Tigas S. Effects of Anabolic Androgenic Steroids on the Reproductive System of Athletes and Recreational Users: A Systematic Review and Meta-Analysis. Sports Med. 2017. September;47(9):1869–1883. doi: 10.1007/s40279-017-0709-z. [DOI] [PubMed] [Google Scholar]
- Chu LW, Tam S, Lee PW, Wong RL, Yik PY, Tsui W, Song Y, Cheung BM, Morley JE, Lam KS. Bioavailable testosterone is associated with a reduced risk of amnestic mild cognitive impairment in older men. Clin Endocrinol (Oxf). 2008. April;68(4):589–98. [DOI] [PubMed] [Google Scholar]
- Chu LW, Tam S, Wong RL, Yik PY, Song Y, Cheung BM, Morley JE, Lam KS. Bioavailable testosterone predicts a lower risk of Alzheimer’s disease in older men. J Alzheimers Dis. 2010;21(4):1335–45. [DOI] [PubMed] [Google Scholar]
- Clark RSB, Empey PE, Bayır H, Rosario BL, Poloyac SM, Kochanek PM, Nolin TD, Au AK, Horvat CM, Wisniewski SR, Bell MJ. Phase I randomized clinical trial of N-acetylcysteine in combination with an adjuvant probenecid for treatment of severe traumatic brain injury in children. PLoS One 2017. July 7;12(7):e0180280. doi: 10.1371/journal.pone.0180280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke R, Smith AD, Jobst KA, Refsum H, Sutton L, Ueland PM. Folate, vitamin B12, and serum total homocysteine levels in confirmed Alzheimer disease. Arch Neurol. 1998. November;55(11):1449–55. [DOI] [PubMed] [Google Scholar]
- Cooke BM, Tabibnia G, Breedlove SM. A brain sexual dimorphism controlled by adult circulating androgens. Proc Natl Acad Sci U S A. 1999. June 22;96(13):7538–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 1993. August 13;261(5123):921–3. [DOI] [PubMed] [Google Scholar]
- Correa F, Mallard C, Nilsson M, Sandberg M. Activated microglia decrease histone acetylation and Nrf2-inducible anti-oxidant defence in astrocytes: restoring effects of inhibitors of HDACs, p38 MAPK and GSK3β. Neurobiol Dis. 2011. October;44(1):142–51. doi: 10.1016/j.nbd.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coward RM, Rajanahally S, Kovac JR, Smith RP, Pastuszak AW, Lipshultz LI. Anabolic steroid induced hypogonadism in young men. J Urol. 2013. December;190(6):2200–5. doi: 10.1016/j.juro.2013.06.010. [DOI] [PubMed] [Google Scholar]
- Christou MA, Christou PA, Markozannes G, Tsatsoulis A, Mastorakos G, Tigas S. Effects of Anabolic Androgenic Steroids on the Reproductive System of Athletes and Recreational Users: A Systematic Review and Meta-Analysis. Sports Med. 2017. September;47(9):1869–1883. doi: 10.1007/s40279-017-0709-z. [DOI] [PubMed] [Google Scholar]
- Cuadrado A, Manda G, Hassan A, Alcaraz MJ, Barbas C, Daiber A, Ghezzi P, León R, López MG, Oliva B, Pajares M, Rojo AI, Robledinos-Antón N, Valverde AM, Guney E, Schmidt HHHW. Transcription Factor NRF2 as a Therapeutic Target for Chronic Diseases: A Systems Medicine Approach. Pharmacol Rev. 2018. April;70(2):348–383. doi: 10.1124/pr.117.014753. [DOI] [PubMed] [Google Scholar]
- Cui R, Kang Y, Wang L, Li S, Ji X, Yan W, Zhang G, Cui H, Shi G. Testosterone Propionate Exacerbates the Deficits of Nigrostriatal Dopaminergic System and Downregulates Nrf2 Expression in Reserpine-Treated Aged Male Rats. Front Aging Neurosci. 2017. May 31;9:172. doi: 10.3389/fnagi.2017.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui C, Sekikawa A, Kuller LH, Lopez OL, Newman AB, Kuipers AL, Mackey RH. Aortic Stiffness is Associated with Increased Risk of Incident Dementia in Older Adults. J Alzheimers Dis. 2018;66(1):297–306. doi: 10.3233/JAD-180449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham RL, Giuffrida A, Roberts JL. Androgens induce dopaminergic neurotoxicity via caspase-3-dependent activation of protein kinase Cdelta. Endocrinology 2009. December;150(12):5539–48. doi: 10.1210/en.2009-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Almeida V, Lobo LL, Hipólide DC, de Oliveira AC, Nobrega JN, Tufik S. Sleep deprivation induces brain region-specific decreases in glutathione levels. Neuroreport 1998. August 24;9(12):2853–6. [DOI] [PubMed] [Google Scholar]
- Daniell HW. Opioid endocrinopathy in women consuming prescribed sustained-action opioids for control of nonmalignant pain. J Pain 2008. January;9(1):28–36. [DOI] [PubMed] [Google Scholar]
- DaRocha-Souto B, Coma M, Pérez-Nievas BG, Scotton TC, Siao M, Sánchez-Ferrer P, Hashimoto T, Fan Z, Hudry E, Barroeta I, Serenó L, Rodríguez M, Sánchez MB, Hyman BT, Gómez-Isla T. Activation of glycogen synthase kinase-3 beta mediates β-amyloid induced neuritic damage in Alzheimer’s disease. Neurobiol Dis. 2012. January;45(1):425–37. doi: 10.1016/j.nbd.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das SK, Hiran KR, Mukherjee S, Vasudevan DM. Oxidative stress is the primary event: Effects of ethanol consumption in brain. Indian J Clin Biochem. 2007. March;22(1):99–104. doi: 10.1007/BF02912890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza GL, Hallak J. Anabolic steroids and male infertility: a comprehensive review. BJU Int. 2011. December;108(11):1860–5. doi: 10.1111/j.1464-410X.2011.10131.x. [DOI] [PubMed] [Google Scholar]
- David K, Dingemanse E, Freud J, Laquer E. Uber Krystallinisches mannliches Hormon Hoden (Testosteron), wirksamer als aus Harn oder aus Cholesterin Bereitetes Androsteron. Zeit Physiol Chem 1935;233:281–282. 10.1515/bchm2.1935.233.5-6.281. [DOI] [Google Scholar]
- Deepmala, Slattery J, Kumar N, Delhey L, Berk M, Dean O, Spielholz C, Frye R. Clinical trials of N-acetylcysteine in psychiatry and neurology: A systematic review. Neurosci Biobehav Rev. 2015. August;55:294–321. doi: 10.1016/j.neubiorev.2015.04.015. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Bucello C, Mathers B, Briegleb C, Ali H, Hickman M, McLaren J. Mortality among regular or dependent users of heroin and other opioids: a systematic review and meta-analysis of cohort studies. Addiction 2011. January;106(1):32–51. doi: 10.1111/j.1360-0443.2010.03140.x [DOI] [PubMed] [Google Scholar]
- Delgado J, Saborido A, Megías A. Prolonged treatment with the anabolic-androgenic steroid stanozolol increases antioxidant defences in rat skeletal muscle. J Physiol Biochem. 2010. March;66(1):63–71. doi: 10.1007/s13105-010-0010-1. [DOI] [PubMed] [Google Scholar]
- Deng Y, Bu Q, Hu Z, Deng P, Yan G, Duan J, Hu C, Zhou J, Shao X, Zhao J, Li Y, Zhu R, Zhao Y, Cen X. (1) H-nuclear magnetic resonance-based metabonomic analysis of brain in rhesus monkeys with morphine treatment and withdrawal intervention. J Neurosci Res. 2012. November;90(11):2154–62. doi: 10.1002/jnr.23109. [DOI] [PubMed] [Google Scholar]
- Deng J, Habib A, Obregon DF, Barger SW, Giunta B, Wang YJ, Hou H, Sawmiller D, Tan J. Soluble amyloid precursor protein alpha inhibits tau phosphorylation through modulation of GSK3β signaling pathway. J Neurochem. 2015. November;135(3):630–7. doi: 10.1111/jnc.13351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deyhim F, Lopez E, Gonzalez J, Garcia M, Patil BS. Citrus juice modulates antioxidant enzymes and lipid profiles in orchidectomized rats. J Med Food 2006. Fall;9(3):422–6. [DOI] [PubMed] [Google Scholar]
- Deyhim F, Patil BS, Villarreal A, Lopez E, Garcia K, Rios R, Garcia C, Gonzales C, Mandadi K. Cranberry juice increases antioxidant status without affecting cholesterol homeostasis in orchidectomized rats. J Med Food 2007. March;10(1):49–53. [DOI] [PubMed] [Google Scholar]
- Devi BG, Chan AW. Effect of cocaine on cardiac biochemical functions. J Cardiovasc Pharmacol. 1999. January;33(1):1–6. [DOI] [PubMed] [Google Scholar]
- Dietrich JB, Mangeol A, Revel MO, Burgun C, Aunis D, Zwiller J. Acute or repeated cocaine administration generates reactive oxygen species and induces antioxidant enzyme activity in dopaminergic rat brain structures. Neuropharmacology 2005. June;48(7):965–74. [DOI] [PubMed] [Google Scholar]
- Dixit S, Hecht S, Concoff A. Cardiovascular risk factors in football players. Curr Sports Med Rep. 2011. Nov-Dec;10(6):378–82. doi: 10.1249/JSR.0b013e31823a362e. [DOI] [PubMed] [Google Scholar]
- Dornelles GL, Bueno A, de Oliveira JS, da Silva AS, França RT, da Silva CB, Machado MS, Petry LD, Abdalla FH, Lhamas CL, de Andrade CM. Biochemical and oxidative stress markers in the liver and kidneys of rats submitted to different protocols of anabolic steroids. Mol Cell Biochem. 2017. January;425(1–2):181–189. doi: 10.1007/s11010-016-2872-1. [DOI] [PubMed] [Google Scholar]
- Doyle T, Bryant L, Batinic-Haberle I, Little J, Cuzzocrea S, Masini E, Spasojevic I, Salvemini D. Supraspinal inactivation of mitochondrial superoxide dismutase is a source of peroxynitrite in the development of morphine antinociceptive tolerance. Neuroscience 2009. December 1;164(2):702–10. doi: 10.1016/j.neuroscience.2009.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond ES, Martins RN, Handelsman DJ, Harvey AR. Altered expression of Alzheimer’s disease-related proteins in male hypogonadal mice. Endocrinology 2012. June;153(6):2789–99. doi: 10.1210/en.2011-2003. [DOI] [PubMed] [Google Scholar]
- Duan W, Zhang R, Guo Y, Jiang Y, Huang Y, Jiang H, Li C. Nrf2 activity is lost in the spinal cord and its astrocytes of aged mice. In Vitro Cell Dev Biol Anim. 2009. Jul-Aug;45(7):388–97. doi: 10.1007/s11626-009-9194-5. Epub 2009 May 19. [DOI] [PubMed] [Google Scholar]
- Duarte RV, Raphael JH, Labib M, Southall JL, Ashford RL. Prevalence and influence of diagnostic criteria in the assessment of hypogonadism in intrathecal opioid therapy patients. Pain Physician 2013. January;16(1):9–14. [PubMed] [Google Scholar]
- Dublin S, Walker RL, Gray SL, Hubbard RA, Anderson ML, Yu O, Crane PK, Larson EB. Prescription Opioids and Risk of Dementia or Cognitive Decline: A Prospective Cohort Study. J Am Geriatr Soc. 2015. August;63(8):1519–26. doi: 10.1111/jgs.13562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran J, Oyarce C, Pavez M, Valladares D, Basualto-Alarcon C, Lagos D, Barrientos G, Troncoso MF, Ibarra C, Estrada M. GSK-3β/NFAT Signaling Is Involved in Testosterone-Induced Cardiac Myocyte Hypertrophy. PLoS One 2016. December 15;11(12):e0168255. doi: 10.1371/journal.pone.0168255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Mattsson N, Weiner MW; Alzheimer’s Disease Neuroimaging Initiative. Smoking and increased Alzheimer’s disease risk: a review of potential mechanisms. Alzheimers Dement. 2014a. June;10(3 Suppl):S122–45. doi: 10.1016/j.jalz.2014.04.009. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Mattsson N, Weiner MW, Korecka M, Trojanowski JQ, Shaw LM; Alzheimer’s Disease Neuroimaging Initiative. History of cigarette smoking in cognitively-normal elders is associated with elevated cerebrospinal fluid biomarkers of oxidative stress. Drug Alcohol Depend. 2014b. September 1;142:262–8. doi: 10.1016/j.drugalcdep.2014.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Korecka M, Trojanowski JQ, Weiner MW, O’ Hara R, Ashford JW, Shaw LM; Alzheimer’s Disease Neuroimaging Initiative. Active Cigarette Smoking in Cognitively-Normal Elders and Probable Alzheimer’s Disease is Associated with Elevated Cerebrospinal Fluid Oxidative Stress Biomarkers. J Alzheimers Dis. 2016a. July 25;54(1):99–107. doi: 10.3233/JAD-160413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Mattsson N, Weiner MW; Alzheimer’s Disease Neuroimaging Initiative. Interaction of Cigarette Smoking History With APOE Genotype and Age on Amyloid Level, Glucose Metabolism, and Neurocognition in Cognitively Normal Elders. Nicotine Tob Res. 2016b. February;18(2):204–11. doi: 10.1093/ntr/ntv075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Meyerhoff DJ, Yoder KK. Cigarette smoking is associated with cortical thinning in anterior frontal regions, insula and regions showing atrophy in early Alzheimer’s Disease. Drug Alcohol Depend. 2018. November 1;192:277–284. doi: 10.1016/j.drugalcdep.2018.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Mattsson N, Weiner MW; Alzheimer’s Disease Neuroimaging Initiative. Interaction of Cigarette Smoking History With APOE Genotype and Age on Amyloid Level, Glucose Metabolism, and Neurocognition in Cognitively Normal Elders. Nicotine Tob Res. 2016b. February;18(2):204–11. doi: 10.1093/ntr/ntv075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Meyerhoff DJ, Yoder KK. Cigarette smoking is associated with cortical thinning in anterior frontal regions, insula and regions showing atrophy in early Alzheimer’s Disease. Drug Alcohol Depend. 2018. November 1;192:277–284. doi: 10.1016/j.drugalcdep.2018.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahimi KB, Cano M, Rhee J, Datta S, Wang L, Handa JT. Oxidative Stress Induces an Interactive Decline in Wnt and Nrf2 Signaling in Degenerating Retinal Pigment Epithelium. Antioxid Redox Signal. 2018. August 1;29(4):389–407. doi: 10.1089/ars.2017.7084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverria V, Zeitlin R, Burgess S, Patel S, Barman A, Thakur G, Mamcarz M, Wang L, Sattelle DB, Kirschner DA, Mori T, Leblanc RM, Prabhakar R, Arendash GW. Cotinine reduces amyloid-β aggregation and improves memory in Alzheimer’s disease mice. J Alzheimers Dis. 2011;24(4):817–35. doi: 10.3233/JAD-2011-102136. [DOI] [PubMed] [Google Scholar]
- Echeverria V, Zeitlin R. Cotinine: a potential new therapeutic agent against Alzheimer’s disease. CNS Neurosci Ther. 2012. July;18(7):517–23. doi: 10.1111/j.1755-5949.2012.00317.x. Epub 2012 Apr 25. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckman EA, Adams SK, Troendle FJ, Stodola BA, Kahn MA, Fauq AH, Xiao HD, Bernstein KE, Eckman CB. Regulation of steady-state beta-amyloid levels in the brain by neprilysin and endothelin-converting enzyme but not angiotensin-converting enzyme. J Biol Chem. 2006. October 13;281(41):30471–8. [DOI] [PubMed] [Google Scholar]
- Eklöf AC, Thurelius AM, Garle M, Rane A, Sjöqvist F. The anti-doping hot-line, a means to capture the abuse of doping agents in the Swedish society and a new service function in clinical pharmacology. Eur J Clin Pharmacol. 2003. November;59(8–9):571–7. [DOI] [PubMed] [Google Scholar]
- Elias A, Cummins T, Tyrrell R, Lamb F, Dore V, Williams R, Rosenfeld JV, Hopwood M, Villemagne VL, Rowe CC. Risk of Alzheimer’s Disease in Obstructive Sleep Apnea Syndrome: Amyloid-β and Tau Imaging. J Alzheimers Dis. 2018;66(2):733–741. doi: 10.3233/JAD-180640. [DOI] [PubMed] [Google Scholar]
- Elovaara I, Maury CP, Palo J. Serum amyloid A protein, albumin and prealbumin in Alzheimer’s disease and in demented patients with Down’s syndrome. Acta Neurol Scand. 1986. September;74(3):245–50. [DOI] [PubMed] [Google Scholar]
- Erickson MA, Hansen K, Banks WA. Inflammation-induced dysfunction of the low-density lipoprotein receptor-related protein-1 at the blood-brain barrier: protection by the antioxidant N-acetylcysteine. Brain Behav Immun. 2012. October;26(7):1085–94. doi: 10.1016/j.bbi.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito G, De Filippis D, Carnuccio R, Izzo AA, Iuvone T. The marijuana component cannabidiol inhibits beta-amyloid-induced tau protein hyperphosphorylation through Wnt/beta-catenin pathway rescue in PC12 cells. J Mol Med (Berl). 2006. March;84(3):253–8. [DOI] [PubMed] [Google Scholar]
- Fan L, Sawbridge D, George V, Teng L, Bailey A, Kitchen I, Li JM. Chronic cocaine-induced cardiac oxidative stress and mitogen-activated protein kinase activation: the role of Nox2 oxidase. J Pharmacol Exp Ther. 2009. January;328(1):99–106. doi: 10.1124/jpet.108.145201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan R, Schrott LM, Snelling S, Ndi J, Arnold T, Korneeva NL. Chronic oxycodone induces integrated stress response in rat brain. BMC Neurosci. 2015. September 16;16:58. doi: 10.1186/s12868-015-0197-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L, Wu HW, Song GQ, Lu C, Li YH, Qu LN, Chen SG, Liu XM, Chang Q. Chronical sleep interruption-induced cognitive decline assessed by a metabolomics method. Behav Brain Res. 2016. April 1;302:60–8. doi: 10.1016/j.bbr.2015.12.039. [DOI] [PubMed] [Google Scholar]
- Feng CZ, Yin JB, Yang JJ, Cao L. Regulatory factor X1 depresses ApoE-dependent Aβ uptake by miRNA-124 in microglial response to oxidative stress. Neuroscience 2017. March 6;344:217–228. doi: 10.1016/j.neuroscience.2016.12.017. [DOI] [PubMed] [Google Scholar]
- Fischer JJ, Jardetzky O. Nuclear Magnetic Relaxation study of intermolecular complexes. The mechanism of penicillin binding to serum albumin. J Am Chem Soc. 1965. July 20;87:3237–44. [DOI] [PubMed] [Google Scholar]
- Flanagan ME, Larson EB, Walker RL, Keene CD, Postupna N, Cholerton B, Sonnen JA, Dublin S, Crane PK, Montine TJ. Associations between Use of Specific Analgesics and Concentrations of Amyloid-β 42 or Phospho-Tau in Regions of Human Cerebral Cortex. J Alzheimers Dis. 2018;61(2):653–662. doi: 10.3233/JAD-170414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley TD, Petro LA, Stredny CM, Coppa TM. Oxidative inhibition of protein phosphatase 2A activity: role of catalytic subunit disulfides. Neurochem Res. 2007. November;32(11):1957–64. [DOI] [PubMed] [Google Scholar]
- Foley TD, Melideo SL, Healey AE, Lucas EJ, Koval JA. Phenylarsine oxide binding reveals redox-active and potential regulatory vicinal thiols on the catalytic subunit of protein phosphatase 2A. Neurochem Res. 2011. February;36(2):232–40. doi: 10.1007/s11064-010-0310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forlenza OV, Diniz BS, Radanovic M, Santos FS, Talib LL, Gattaz WF. Disease-modifying properties of long-term lithium treatment for amnestic mild cognitive impairment: randomised controlled trial. Br J Psychiatry 2011. May;198(5):351–6. doi: 10.1192/bjp.bp.110.080044. [DOI] [PubMed] [Google Scholar]
- Frankenfeld SP, Oliveira LP, Ortenzi VH, Rego-Monteiro IC, Chaves EA, Ferreira AC, Leitão AC, Carvalho DP, Fortunato RS. The anabolic androgenic steroid nandrolone decanoate disrupts redox homeostasis in liver, heart and kidney of male Wistar rats. PLoS One 2014. September 16;9(9):e102699. doi: 10.1371/journal.pone.0102699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frustaci A, Russo MA, Morgante E, Scopelliti F, Aquilano K, Ciriolo MR, Grande C, Verardo R, Chimenti C. Oxidative myocardial damage in human cocaine-related cardiomyopathy. Eur J Heart Fail. 2015. March;17(3):283–90. doi: 10.1002/ejhf.231. [DOI] [PubMed] [Google Scholar]
- Fu Y, Lu Y, Liu EY, Zhu X, Mahajan GJ, Lu D, Roman RJ, Liu R. Testosterone enhances tubuloglomerular feedback by increasing superoxide production in the macula densa. Am J Physiol Regul Integr Comp Physiol. 2013. May 1;304(9):R726–33. doi: 10.1152/ajpregu.00341.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta T, Mukai A, Ohishi A, Nishida K, Nagasawa K. Oxidative stress-induced increase of intracellular zinc in astrocytes decreases their functional expression of P2X7 receptors and engulfing activity. Metallomics 2017. December 1;9(12):1839–1851. doi: 10.1039/c7mt00257b. [DOI] [PubMed] [Google Scholar]
- Gameiro I, Michalska P, Tenti G, Cores Á, Buendia I, Rojo AI, Georgakopoulos ND, Hernández-Guijo JM, Teresa Ramos M, Wells G, López MG, Cuadrado A, Menéndez JC, León R. Discovery of the first dual GSK3β inhibitor/Nrf2 inducer. A new multitarget therapeutic strategy for Alzheimer’s disease. Sci Rep. 2017. March 31;7:45701. doi: 10.1038/srep45701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandy S, Almeida OP, Fonte J, Lim D, Waterrus A, Spry N, Flicker L, Martins RN. Chemical andropause and amyloid-beta peptide. JAMA 2001. May 2;285(17):2195–6. [DOI] [PubMed] [Google Scholar]
- Genedani S, Rasio G, Cortelli P, Antonelli F, Guidolin D, Galantucci M, Fuxe K, Agnati LF. Studies on homocysteine and dehydroepiandrosterone sulphate plasma levels in Alzheimer’s disease patients and in Parkinson’s disease patients. Neurotox Res. 2004;6(4):327–32. [DOI] [PubMed] [Google Scholar]
- George S, Petit GH, Gouras GK, Brundin P, Olsson R. Nonsteroidal selective androgen receptor modulators and selective estrogen receptor β agonists moderate cognitive deficits and amyloid-β levels in a mouse model of Alzheimer’s disease. ACS Chem Neurosci. 2013. December 18;4(12):1537–48. doi: 10.1021/cn400133s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazavi A, Mosayebi G, Solhi H, Rafiei M, Moazzeni SM. Serum markers of inflammation and oxidative stress in chronic opium (Taryak) smokers. Immunol Lett. 2013. June;153(1–2):22–6. doi: 10.1016/j.imlet.2013.07.001. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Thakur MK. PS1 expression is downregulated by gonadal steroids in adult mouse brain. Neurochem Res. 2008. March;33(3):365–9. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Johnson DA. Sex-specific effects of gonadectomy and hormone treatment on acquisition of a 12-arm radial maze task by Sprague Dawley rats. Endocrinology. 2008. June;149(6):3176–83. doi: 10.1210/en.2007-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillett MJ, Martins RN, Clarnette RM, Chubb SA, Bruce DG, Yeap BB. Relationship between testosterone, sex hormone binding globulin and plasma amyloid beta peptide 40 in older men with subjective memory loss or dementia. J Alzheimers Dis. 2003. August;5(4):267–9. [DOI] [PubMed] [Google Scholar]
- Giltay EJ, Hoogeveen EK, Elbers JM, Gooren LJ, Asscheman H, Stehouwer CD. Effects of sex steroids on plasma total homocysteine levels: a study in transsexual males and females. J Clin Endocrinol Metab. 1998. February;83(2):550–3. [DOI] [PubMed] [Google Scholar]
- Goedert M, Cohen ES, Jakes R, Cohen P. p42 MAP kinase phosphorylation sites in microtubule-associated protein tau are dephosphorylated by protein phosphatase 2A1. Implications for Alzheimer’s disease [corrected]. FEBS Lett. 1992. November 2;312(1):95–9. Erratum in: FEBS Lett 1992 Nov 23;313(2):203. [DOI] [PubMed] [Google Scholar]
- Gomes FC, Chuffa LG, Scarano WR, Pinheiro PF, Fávaro WJ, Domeniconi RF. Nandrolone decanoate and resistance exercise training favor the occurrence of lesions and activate the inflammatory response in the ventral prostate. Andrology 2016. May;4(3):473–80. doi: 10.1111/andr.12162. [DOI] [PubMed] [Google Scholar]
- Gomes T, Tadrous M, Mamdani MM, Paterson JM; Juurlink DN. The Burden of Opioid-Related Mortality in the United States. JAMA Netw Open 2018. June 1; 1(2):e180217 Accessed Aug. 27, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves I, Alves CH, Quintela T, Baltazar G, Socorro S, Saraiva MJ, Abreu R, Santos CR. Transthyretin is up-regulated by sex hormones in mice liver. Mol Cell Biochem. 2008. October;317(1–2):137–42. doi: 10.1007/s11010-008-9841-2. [DOI] [PubMed] [Google Scholar]
- Gong CX, Shaikh S, Wang JZ, Zaidi T, Grundke-Iqbal I, Iqbal K. Phosphatase activity toward abnormally phosphorylated tau: decrease in Alzheimer disease brain. J Neurochem. 1995. August;65(2):732–8. [DOI] [PubMed] [Google Scholar]
- Gong CX, Lidsky T, Wegiel J, Zuck L, Grundke-Iqbal I, Iqbal K. Phosphorylation of microtubule-associated protein tau is regulated by protein phosphatase 2A in mammalian brain. Implications for neurofibrillary degeneration in Alzheimer’s disease. J Biol Chem. 2000. February 25;275(8):5535–44. [DOI] [PubMed] [Google Scholar]
- Gong YS, Hu K, Yang LQ, Guo J, Gao YQ, Song FL, Hou FL, Liang CY. Comparative effects of EtOH consumption and thiamine deficiency on cognitive impairment, oxidative damage, and β-amyloid peptide overproduction in the brain. Free Radic Biol Med. 2017. July;108:163–173. doi: 10.1016/j.freeradbiomed.2017.03.019. [DOI] [PubMed] [Google Scholar]
- Gong CX, Liu F, Iqbal K. Multifactorial Hypothesis and Multi-Targets for Alzheimer’s Disease. J Alzheimers Dis. 2018;64(s1):S107–S117. doi: 10.3233/JAD-179921. [DOI] [PubMed] [Google Scholar]
- Goodenough S, Engert S, Behl C. Testosterone stimulates rapid secretory amyloid precursor protein release from rat hypothalamic cells via the activation of the mitogen-activated protein kinase pathway. Neurosci Lett. 2000. December 15;296(1):49–52. [DOI] [PubMed] [Google Scholar]
- Goodenough S, Schleusner D, Pietrzik C, Skutella T, Behl C. Glycogen synthase kinase 3beta links neuroprotection by 17beta-estradiol to key Alzheimer processes. Neuroscience 2005;132(3):581–9. [DOI] [PubMed] [Google Scholar]
- Gordon GG, Altman K, Southren AL, Rubin E, Lieber CS. Effect of alcohol (ethanol) administration on sex-hormone metabolism in normal men. N Engl J Med. 1976. October 7;295(15):793–7. [DOI] [PubMed] [Google Scholar]
- Gordon BA, Blazey TM, Su Y, Hari-Raj A, Dincer A, Flores S, Christensen J, McDade E, Wang G, Xiong C, Cairns NJ, Hassenstab J, Marcus DS, Fagan AM, Jack CR Jr, Hornbeck RC, Paumier KL, Ances BM, Berman SB, Brickman AM, Cash DM, Chhatwal JP, Correia S, Förster S, Fox NC, Graff-Radford NR, la Fougère C, Levin J, Masters CL, Rossor MN, Salloway S, Saykin AJ, Schofield PR, Thompson PM, Weiner MM, Holtzman DM, Raichle ME, Morris JC, Bateman RJ, Benzinger TLS. Spatial patterns of neuroimaging biomarker change in individuals from families with autosomal dominant Alzheimer’s disease: a longitudinal study. Lancet Neurol. 2018. March;17(3):241–250. doi: 10.1016/S1474-4422(18)30028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorrini C, Harris IS, Mak TW. Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov. 2013. December;12(12):931–47. doi: 10.1038/nrd4002. [DOI] [PubMed] [Google Scholar]
- Götz J, Ittner LM. Animal models of Alzheimer’s disease and frontotemporal dementia. Nat Rev Neurosci. 2008. July;9(7):532–44. doi: 10.1038/nrn2420. [DOI] [PubMed] [Google Scholar]
- Gouras GK, Xu H, Gross RS, Greenfield JP, Hai B, Wang R, Greengard P. Testosterone reduces neuronal secretion of Alzheimer’s beta-amyloid peptides. Proc Natl Acad Sci U S A 2000. February 1;97(3):1202–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham MR, Grace FM, Boobier W, Hullin D, Kicman A, Cowan D, Davies B, Baker JS. Homocysteine induced cardiovascular events: a consequence of long term anabolic-androgenic steroid (AAS) abuse. Br J Sports Med. 2006. July;40(7):644–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham MR, Davies B, Grace FM, Kicman A, Baker JS. Anabolic steroid use: patterns of use and detection of doping. Sports Med. 2008;38(6):505–25. [DOI] [PubMed] [Google Scholar]
- Griffith HR, den Hollander JA, Stewart CC, Evanochko WT, Buchthal SD, Harrell LE, Zamrini EY, Brockington JC, Marson DC. Elevated brain scyllo-inositol concentrations in patients with Alzheimer’s disease. NMR Biomed. 2007. December;20(8):709–16. [DOI] [PubMed] [Google Scholar]
- Griggs RC, Kingston W, Jozefowicz RF, Herr BE, Forbes G, Halliday D. Effect of testosterone on muscle mass and muscle protein synthesis. J Appl Physiol (1985). 1989. January;66(1):498–503. [DOI] [PubMed] [Google Scholar]
- Grimm A, Mensah-Nyagan AG, Eckert A. Alzheimer, mitochondria and gender. Neurosci Biobehav Rev. 2016. August;67:89–101. doi: 10.1016/j.neubiorev.2016.04.012. Review. [DOI] [PubMed] [Google Scholar]
- Grothe MJ, Barthel H, Sepulcre J, Dyrba M, Sabri O, Teipel SJ; Alzheimer’s Disease Neuroimaging Initiative. In vivo staging of regional amyloid deposition. Neurology 2017. November 14;89(20):2031–2038. doi: 10.1212/WNL.0000000000004643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu F, Hata R, Toku K, Yang L, Ma YJ, Maeda N, Sakanaka M, Tanaka J. Testosterone up-regulates aquaporin-4 expression in cultured astrocytes. J Neurosci Res. 2003. June 15;72(6):709–15. [DOI] [PubMed] [Google Scholar]
- Gu S, Honisch S, Kounenidakis M, Alkahtani S, Alarifi S, Alevizopoulos K, Stournaras C, Lang F. Membrane androgen receptor down-regulates c-src-activity and beta-catenin transcription and triggers GSK-3beta-phosphorylation in colon tumor cells. Cell Physiol Biochem. 2014;34(4):1402–12. doi: 10.1159/000366346. [DOI] [PubMed] [Google Scholar]
- Gu F, Derkach A, Freedman ND, Landi MT, Albanes D, Weinstein SJ, Mondul AM, Matthews CE, Guertin KA, Xiao Q, Zheng W, Shu XO, Sampson JN, Moore SC, Caporaso NE. Cigarette smoking behaviour and blood metabolomics. Int J Epidemiol. 2016. October;45(5):1421–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrieri GM, Wakim PG, Keenan PA, Schenkel LA, Berlin K, Gibson CJ, Rubinow DR, Schmidt PJ. Sex differences in visuospatial abilities persist during induced hypogonadism. Neuropsychologia 2016. January 29;81:219–29. doi: 10.1016/j.neuropsychologia 2015.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidi I, Galimberti D, Lonati S, Novembrino C, Bamonti F, Tiriticco M, Fenoglio C, Venturelli E, Baron P, Bresolin N, Scarpini E. Oxidative imbalance in patients with mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging 2006. February;27(2):262–9. [DOI] [PubMed] [Google Scholar]
- Gulec M, Ozkol H, Selvi Y, Tuluce Y, Aydin A, Besiroglu L, Ozdemir PG. Oxidative stress in patients with primary insomnia. Prog Neuropsychopharmacol Biol Psychiatry 2012. June 1;37(2):247–51. doi: 10.1016/j.pnpbp.2012.02.011. [DOI] [PubMed] [Google Scholar]
- Gutowicz M, Kaźmierczak B, Barańczyk-Kuźma A. The influence of heroin abuse on glutathione-dependent enzymes in human brain. Drug Alcohol Depend. 2011. January 1;113(1):8–12. doi: 10.1016/j.drugalcdep.2010.06.020. [DOI] [PubMed] [Google Scholar]
- Habib A, Sawmiller D, Li S, Xiang Y, Rongo D, Tian J, Hou H, Zeng J, Smith A, Fan S, Giunta B, Mori T, Currier G, Shytle DR, Tan J. LISPRO mitigates β-amyloid and associated pathologies in Alzheimer’s mice. Cell Death Dis. 2017. June 15;8(6):e2880. doi: 10.1038/cddis.2017.279. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Hallinan R, Byrne A, Agho K, McMahon CG, Tynan P, Attia J. Hypogonadism in men receiving methadone and buprenorphine maintenance treatment. Int J Androl. 2009. April;32(2):131–9. [DOI] [PubMed] [Google Scholar]
- Hara Y, McKeehan N, Dacks PA, Fillit HM. Evaluation of the Neuroprotective Potential of NAcetylcysteine for Prevention and Treatment of Cognitive Aging and Dementia. J Prev Alzheimers Dis. 2017;4(3):201–206. doi: 10.14283/jpad.2017.22. [DOI] [PubMed] [Google Scholar]
- Hawley WR, Grissom EM, Martin RC, Halmos MB, Bart CL, Dohanich GP. Testosterone modulates spatial recognition memory in male rats. Horm Behav. 2013. April;63(4):559–65. doi: 10.1016/j.yhbeh.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Haymana C, Aydoğdu A, Soykut B, Erdem O, Ibrahimov T, Dinc M, Meric C, Basaran Y, Sonmez A, Azal O. Oxidative stress status in congenital hypogonadism: an appraisal. Toxicol Mech Methods. 2017. July;27(6):451–457. doi: 10.1080/15376516.2017.1320693. [DOI] [PubMed] [Google Scholar]
- Heffernan TM, Battersby L, Bishop P, O’Neill TS. Everyday memory deficits associated with anabolicandrogenic steroid use in regular gymnasium users. The Open Psychiatry J. 2015;9:1–16. doi: 10.2174/1874354401509010001. [DOI] [Google Scholar]
- Hengevoss J, Piechotta M, Müller D, Hanft F, Parr MK, Schänzer W, Diel P. Combined effects of androgen anabolic steroids and physical activity on the hypothalamic-pituitary-gonadal axis. J Steroid Biochem Mol Biol. 2015. June;150:86–96. doi: 10.1016/j.jsbmb.2015.03.003. [DOI] [PubMed] [Google Scholar]
- Henze A, Raila J, Scholze A, Zidek W, Tepel M, Schweigert FJ. Does N-acetylcysteine modulate post-translational modifications of transthyretin in hemodialysis patients? Antioxid Redox Signal. 2013. October 10;19(11):1166–72. doi: 10.1089/ars.2012.5125. [DOI] [PubMed] [Google Scholar]
- Herlitz LC, Markowitz GS, Farris AB, Schwimmer JA, Stokes MB, Kunis C, Colvin RB, D’Agati VD. Development of focal segmental glomerulosclerosis after anabolic steroid abuse. J Am Soc Nephrol. 2010. January;21(1):163–72. doi: 10.1681/ASN.2009040450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Zimbrón LF, Rivas-Arancibia S. Oxidative stress caused by ozone exposure induces β-amyloid 1–42 overproduction and mitochondrial accumulation by activating the amyloidogenic pathway. Neuroscience 2015. September 24;304:340–8. doi: 10.1016/j.neuroscience.2015.07.011. [DOI] [PubMed] [Google Scholar]
- Herting MM, Gautam P, Spielberg JM, Kan E, Dahl RE, Sowell ER. The role of testosterone and estradiol in brain volume changes across adolescence: a longitudinal structural MRI study. Hum Brain Mapp. 2014. November;35(11):5633–45. doi: 10.1002/hbm.22575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo C, Arias-Cavieres A. Calcium, Reactive Oxygen Species, and Synaptic Plasticity. Physiology (Bethesda) 2016. May;31(3):201–15. doi: 10.1152/physiol.00038.2015. [DOI] [PubMed] [Google Scholar]
- Hier DB, Crowley WF Jr. Spatial ability in androgen-deficient men. N Engl J Med. 1982. May 20;306(20):1202–5. [DOI] [PubMed] [Google Scholar]
- Hildebrandt T, Lai JK, Langenbucher JW, Schneider M, Yehuda R, Pfaff DW. The diagnostic dilemma of pathological appearance and performance enhancing drug use. Drug Alcohol Depend. 2011. March 1;114(1):1–11. doi: 10.1016/j.drugalcdep.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt T, Langenbucher JW, Flores A, Harty S, Berlin HA. The influence of age of onset and acute anabolic steroid exposure on cognitive performance, impulsivity, and aggression in men. Psychol Addict Behav. 2014. December;28(4):1096–104. doi: 10.1037/a0036482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingson RW, Heeren T, Winter MR. Age at drinking onset and alcohol dependence: age at onset, duration, and severity. Arch Pediatr Adolesc Med. 2006. July;160(7):739–46. [DOI] [PubMed] [Google Scholar]
- Hipps PP, Holland WH, Sherman WR. Interconversion of myo- and scyllo-inositol with simultaneous formation of neo-inositol by an NADP+ dependent epimerase from bovine brain. Biochem Biophys Res Commun. 1977. July 11;77(1):340–6. [DOI] [PubMed] [Google Scholar]
- Hogervorst E, Lehmann DJ, Warden DR, McBroom J, Smith AD. Apolipoprotein E epsilon4 and testosterone interact in the risk of Alzheimer’s disease in men. Int J Geriatr Psychiatry 2002. October;17(10):938–40. [DOI] [PubMed] [Google Scholar]
- Hojo Y, Higo S, Ishii H, Ooishi Y, Mukai H, Murakami G, Kominami T, Kimoto T, Honma S, Poirier D, Kawato S. Comparison between hippocampus-synthesized and circulation-derived sex steroids in the hippocampus. Endocrinology 2009. November;150(11):5106–12. doi: 10.1210/en.2009-0305. [DOI] [PubMed] [Google Scholar]
- Hoth KF, Poppas A, Ellison KE, Paul RH, Sokobin A, Cho Y, Cohen RA. Link between change in cognition and left ventricular function following cardiac resynchronization therapy. J Cardiopulm Rehabil Prev. 2010. Nov-Dec;30(6):401–8. doi: 10.1097/HCR.0b013e3181e1739a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou TT, Yang HY, Wang W, Wu QQ, Tian YR, Jia JP. Sulforaphane Inhibits the Generation of Amyloid-β Oligomer and Promotes Spatial Learning and Memory in Alzheimer’s Disease (PS1V97L) Transgenic Mice. J Alzheimers Dis. 2018;62(4):1803–1813. doi: 10.3233/JAD-171110. [DOI] [PubMed] [Google Scholar]
- Hser YI, Evans E, Huang D, Brecht ML, Li L. Comparing the dynamic course of heroin, cocaine, and methamphetamine use over 10 years. Addict Behav. 2008. December;33(12):1581–9. doi: 10.1016/j.addbeh.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh A, DiGiorgio L, Fakunle M, Sadeghi-Nejad H. Management Strategies in Opioid Abuse and Sexual Dysfunction: A Review of Opioid-Induced Androgen Deficiency. Sex Med Rev. 2018. October;6(4):618–623. doi: 10.1016/j.sxmr.2018.04.003. [DOI] [PubMed] [Google Scholar]
- Hu S, Begum AN, Jones MR, Oh MS, Beech WK, Beech BH, Yang F, Chen P, Ubeda OJ, Kim PC, Davies P, Ma Q, Cole GM, Frautschy SA. GSK3 inhibitors show benefits in an Alzheimer’s disease (AD) model of neurodegeneration but adverse effects in control animals. Neurobiol Dis. 2009. February;33(2):193–206. doi: 10.1016/j.nbd.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Atwood CS, Hartshorn MA, Multhaup G, Goldstein LE, Scarpa RC, Cuajungco MP, Gray DN, Lim J, Moir RD, Tanzi RE, Bush AI. The A beta peptide of Alzheimer’s disease directly produces hydrogen peroxide through metal ion reduction. Biochemistry 1999. June 15;38(24):7609–16. [DOI] [PubMed] [Google Scholar]
- Huang MC, Lin SK, Chen CH, Pan CH, Lee CH, Liu HC. Oxidative stress status in recently abstinent methamphetamine abusers. Psychiatry Clin Neurosci. 2013. February;67(2):92–100. doi: 10.1111/pcn.12025. [DOI] [PubMed] [Google Scholar]
- Huang X, Huang K, Zheng W, Beveridge TJ, Yang S, Li X, Li P, Zhou W, Liu Y. The effects of GSK-3β blockade on ketamine self-administration and relapse to drug-seeking behavior in rats. Drug Alcohol Depend. 2015. February 1;147:257–65. doi: 10.1016/j.drugalcdep.2014.10.028. [DOI] [PubMed] [Google Scholar]
- Huang G, Travison T, Maggio M, Edwards RR, Basaria S. Effects of testosterone replacement on metabolic and inflammatory markers in men with opioid-induced androgen deficiency. Clin Endocrinol (Oxf). 2016a. August;85(2):232–8. doi: 10.1111/cen.13049. [DOI] [PubMed] [Google Scholar]
- Huang G, Wharton W, Bhasin S, Harman SM, Pencina KM, Tsitouras P, Li Z, Hally KA, Asthana S, Storer TW, Basaria S. Effects of long-term testosterone administration on cognition in older men with low or low-to-normal testosterone concentrations: a prespecified secondary analysis of data from the randomised, double-blind, placebo-controlled TEAAM trial. Lancet Diabetes Endocrinol. 2016b. August;4(8):657–65. doi: 10.1016/S2213-8587(16)30102-4. [DOI] [PubMed] [Google Scholar]
- Huang D, Yu M, Yang S, Lou D, Zhou W, Zheng L, Wang Z, Cai F, Zhou W, Li T, Song W. Ethanol Alters APP Processing and Aggravates Alzheimer-Associated Phenotypes. Mol Neurobiol. 2018a. June;55(6):5006–5018. doi: 10.1007/s12035-017-0703-3. [DOI] [PubMed] [Google Scholar]
- Huang MC, Lai YC, Lin SK, Chen CH. Increased blood 8-hydroxy-2-deoxyguanosine levels in methamphetamine users during early abstinence. Am J Drug Alcohol Abuse 2018b;44(3):395–402. doi: 10.1080/00952990.2017.1344683. [DOI] [PubMed] [Google Scholar]
- Hwang TI, Liao TL, Lin JF, Lin YC, Lee SY, Lai YC, Kao SH. Low-dose testosterone treatment decreases oxidative damage in TM3 Leydig cells. Asian J Androl. 2011. May;13(3):432–7. doi: 10.1038/aja.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang CJ, Yun HM, Park KR, Song JK, Seo HO, Hyun BK, Choi DY, Yoo HS, Oh KW, Hwang DY, Han SB, Hong JT. Memory Impairment in Estrogen Receptor α Knockout Mice Through Accumulation of Amyloid-β Peptides. Mol Neurobiol. 2015. August;52(1):176–86. doi: 10.1007/s12035-014-8853-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hybertson BM, Gao B, Bose SK, McCord JM. Oxidative stress in health and disease: the therapeutic potential of Nrf2 activation. Mol Aspects Med. 2011. August;32(4–6):234–46. doi: 10.1016/j.mam.2011.10.006. [DOI] [PubMed] [Google Scholar]
- Ibáñez-Contreras A, Hernández-Arciga U, Poblano A, Arteaga-Silva M, Hernández-Godínez B, Mendoza-Cuevas GI, Toledo-Pérez R, Alarcón-Aguilar A, González-Puertos VY, Konigsberg M. Electrical activity of sensory pathways in female and male geriatric Rhesus monkeys (Macaca mulatta), and its relation to oxidative stress. Exp Gerontol. 2018. January;101:80–94. doi: 10.1016/j.exger.2017.11.003. [DOI] [PubMed] [Google Scholar]
- Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012. August 15;4(147):147ra111. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imtiaz B, Taipale H, Tanskanen A, Tiihonen M, Kivipelto M, Heikkinen AM, Tiihonen J, Soininen H, Hartikainen S, Tolppanen AM. Risk of Alzheimer’s disease among users of postmenopausal hormone therapy: A nationwide case-control study. Maturitas 2017. April;98:7–13. doi: 10.1016/j.maturitas.2017.01.002. [DOI] [PubMed] [Google Scholar]
- Ip EJ, Barnett MJ, Tenerowicz MJ, Perry PJ. The Anabolic 500 survey: characteristics of male users versus nonusers of anabolic-androgenic steroids for strength training. Pharmacotherapy 2011. August;31(8):757–66. doi: 10.1592/phco.31.8.757. [DOI] [PubMed] [Google Scholar]
- Isabelle M, Vergeade A, Moritz F, Dautréaux B, Henry JP, Lallemand F, Richard V, Mulder P, Thuillez C, Monteil C. NADPH oxidase inhibition prevents cocaine-induced up-regulation of xanthine oxidoreductase and cardiac dysfunction. J Mol Cell Cardiol. 2007. February;42(2):326–32. [DOI] [PubMed] [Google Scholar]
- Iwata N, Tsubuki S, Takaki Y, Watanabe K, Sekiguchi M, Hosoki E, Kawashima-Morishima M, Lee HJ, Hama E, Sekine-Aizawa Y, Saido TC. Identification of the major Abeta1–42-degrading catabolic pathway in brain parenchyma: suppression leads to biochemical and pathological deposition. Nat Med. 2000. February;6(2):143–50. [DOI] [PubMed] [Google Scholar]
- Iwata N, Tsubuki S, Takaki Y, Shirotani K, Lu B, Gerard NP, Gerard C, Hama E, Lee HJ, Saido TC. Metabolic regulation of brain Abeta by neprilysin. Science 2001. May 25;292(5521):1550–2. [DOI] [PubMed] [Google Scholar]
- Jang EY, Ryu YH, Lee BH, Chang SC, Yeo MJ, Kim SH, Folsom RJ, Schilaty ND, Kim KJ, Yang CH, Steffensen SC, Kim HY. Involvement of reactive oxygen species in cocaine-taking behaviors in rats. Addict Biol. 2015. July;20(4):663–75. doi: 10.1111/adb.12159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang EY, Yang CH, Hedges DM, Kim SP, Lee JY, Ekins TG, Garcia BT, Kim HY, Nelson AC, Kim NJ, Steffensen SC. The role of reactive oxygen species in methamphetamine self-administration and dopamine release in the nucleus accumbens. Addict Biol. 2017. September;22(5):1304–1315. doi: 10.1111/adb.12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowsky JS, Oviatt SK, Orwoll ES. Testosterone influences spatial cognition in older men. Behav Neurosci. 1994. April;108(2):325–32. [DOI] [PubMed] [Google Scholar]
- Jefferson AL, Himali JJ, Au R, Seshadri S, Decarli C, O’Donnell CJ, Wolf PA, Manning WJ, Beiser AS, Benjamin EJ. Relation of left ventricular ejection fraction to cognitive aging (from the Framingham Heart Study). Am J Cardiol. 2011. November 1;108(9):1346–51. doi: 10.1016/j.amjcard.2011.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhan JH, Yang YH, Chang YH, Guu SJ, Tsai CC. Hormone therapy for prostate cancer increases the risk of Alzheimer’s disease: a nationwide 4-year longitudinal cohort study. Aging Male 2017. March;20(1):33–38. doi: 10.1080/13685538.2016.1271782. [DOI] [PubMed] [Google Scholar]
- Jin M, Selkoe DJ. Systematic analysis of time-dependent neural effects of soluble amyloid β oligomers in culture and in vivo: Prevention by scyllo-inositol. Neurobiol Dis. 2015. October;82:152–163. doi: 10.1016/j.nbd.2015.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo C, Gundemir S, Pritchard S, Jin YN, Rahman I, Johnson GV. Nrf2 reduces levels of phosphorylated tau protein by inducing autophagy adaptor protein NDP52. Nat Commun. 2014. March 25;5:3496. doi: 10.1038/ncomms4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RT, Schneider A, DonCarlos LL, Breedlove SM, Jordan CL. Astrocytes in the rat medial amygdala are responsive to adult androgens. J Comp Neurol. 2012. August 1;520(11):2531–44. doi: 10.1002/cne.23061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joksimović J, Selaković D, Jakovljević V, Mihailović V, Katanić J, Boroja T, Rosić G. Alterations of the oxidative status in rat hippocampus and prodepressant effect of chronic testosterone enanthate administration. Mol Cell Biochem. 2017. September;433(1–2):41–50. doi: 10.1007/s11010-017-3014-0. [DOI] [PubMed] [Google Scholar]
- Jono H, Su Y, Obayashi K, Tanaka Y, Ishiguro A, Nishimura H, Shinriki S, Ueda M, Ikeda K, Yamagata K, Ichihara K, Ando Y; Scientific Committee for the Asia-Pacific Federation of Clinical Biochemistry. Sources of variation of transthyretin in healthy subjects in East and Southeast Asia: Clinical and experimental evidence for the effect of alcohol on transthyretin metabolism. Clin Chim Acta 2016. July 1;458:5–11. doi: 10.1016/j.cca.2016.04.011. [DOI] [PubMed] [Google Scholar]
- Kalinine E, Zimmer ER, Zenki KC, Kalinine I, Kazlauckas V, Haas CB, Hansel G, Zimmer AR, Souza DO, Müller AP, Portela LV. Nandrolone-induced aggressive behavior is associated with alterations in extracellular glutamate homeostasis in mice. Horm Behav. 2014. July;66(2):383–92. doi: 10.1016/j.yhbeh.2014.06.005. [DOI] [PubMed] [Google Scholar]
- Kalousová M, Zima T, Popov P, Spacek P, Braun M, Soukupová J, Pelinkova K, Kientsch-Engel R. Advanced glycation end-products in patients with chronic alcohol misuse. Alcohol Alcohol. 2004. Jul-Aug;39(4):316–20. [DOI] [PubMed] [Google Scholar]
- Kanayama G, Boynes M, Hudson JI, Field AE, Pope HG Jr. Anabolic steroid abuse among teenage girls: an illusory problem? Drug Alcohol Depend. 2007. May 11;88(2–3):156–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanayama G, Hudson JI, Pope HG Jr. Long-term psychiatric and medical consequences of anabolicandrogenic steroid abuse: a looming public health concern? Drug Alcohol Depend. 2008. November 1;98(1–2):1–12. doi: 10.1016/j.drugalcdep.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanayama G, Brower KJ, Wood RI, Hudson JI, Pope HG Jr. Anabolic-androgenic steroid dependence: an emerging disorder. Addiction 2009a. December;104(12):1966–78. doi: 10.1111/j.1360-0443.2009.02734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanayama G, Brower KJ, Wood RI, Hudson JI, Pope HG Jr. Issues for DSM-V: clarifying the diagnostic criteria for anabolic-androgenic steroid dependence. Am J Psychiatry 2009b. June;166(6):642–5. doi: 10.1176/appi.ajp.2009.08111699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanayama G, Hudson JI, Pope HG Jr. Illicit anabolic-androgenic steroid use. Horm Behav. 2010. June;58(1):111–21. doi: 10.1016/j.yhbeh.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanayama G, Pope HG Jr. Misconceptions about anabolic-androgenic steroid abuse. Psychiatric Annals 2012a. October 22;41(10):371–375. doi: 10.3928/00485713-20121003-05. [DOI] [Google Scholar]
- Kanayama G, Pope HG Jr. Illicit use of androgens and other hormones: recent advances. Curr Opin Endocrinol Diabetes Obes. 2012b. June;19(3):211–9. doi: 10.1097/MED.0b013e3283524008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanayama G, Kean J, Hudson JI, Pope HG Jr. Cognitive deficits in long-term anabolic-androgenic steroid users. Drug Alcohol Depend. 2013. June 1;130(1–3):208–14. doi: 10.1016/j.drugalcdep.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanayama G, Hudson JI, DeLuca J, Isaacs S, Baggish A, Weiner R, Bhasin S, Pope HG Jr. Prolonged hypogonadism in males following withdrawal from anabolic-androgenic steroids: an under-recognized problem. Addiction 2015. May;110(5):823–31. doi: 10.1111/add.12850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanayama G, Pope HG Jr. History and epidemiology of anabolic androgens in athletes and non-athletes. Mol Cell Endocrinol. 2018. March 15;464:4–13. doi: 10.1016/j.mce.2017.02.039. [DOI] [PubMed] [Google Scholar]
- Kanayama G, Pope HG, Hudson JI. Associations of anabolic-androgenic steroid use with other behavioral disorders: an analysis using directed acyclic graphs. Psychol Med. 2018. November;48(15):2601–2608. doi: 10.1017/S0033291718000508. [DOI] [PubMed] [Google Scholar]
- Kang DE, Pietrzik CU, Baum L, Chevallier N, Merriam DE, Kounnas MZ, Wagner SL, Troncoso JC, Kawas CH, Katzman R, Koo EH. Modulation of amyloid beta-protein clearance and Alzheimer’s disease susceptibility by the LDL receptor-related protein pathway. J Clin Invest. 2000. November;106(9):1159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kara M, Ozcagli E, Kotil T, Alpertunga B. Effects of stanozolol on apoptosis mechanisms and oxidative stress in rat cardiac tissue. Steroids 2018. June;134:96–100. doi: 10.1016/j.steroids.2018.02.004. [DOI] [PubMed] [Google Scholar]
- Kaufman MJ, Janes AC, Hudson JI, Brennan BP, Kanayama G, Kerrigan AR, Jensen JE, Pope HG Jr. Brain and cognition abnormalities in long-term anabolic-androgenic steroid users. Drug Alcohol Depend. 2015. July 1;152:47–56. doi: 10.1016/j.drugalcdep.2015.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller JN, Mark RJ, Bruce AJ, Blanc E, Rothstein JD, Uchida K, Waeg G, Mattson MP. 4-Hydroxynonenal, an aldehydic product of membrane lipid peroxidation, impairs glutamate transport and mitochondrial function in synaptosomes. Neuroscience 1997. October;80(3):685–96. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Myers J, Damaj MI, Chen X. Early smoking onset and risk for subsequent nicotine dependence: a monozygotic co-twin control study. Am J Psychiatry 2013. April;170(4):408–13. doi: 10.1176/appi.ajp.2012.12030321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HC, Yamada K, Nitta A, Olariu A, Tran MH, Mizuno M, Nakajima A, Nagai T, Kamei H, Jhoo WK, Im DH, Shin EJ, Hjelle OP, Ottersen OP, Park SC, Kato K, Mirault ME, Nabeshima T. Immunocytochemical evidence that amyloid beta (1–42) impairs endogenous antioxidant systems in vivo. Neuroscience 2003;119(2):399–419. [DOI] [PubMed] [Google Scholar]
- Kim SR, Jeong HY, Yang S, Choi SP, Seo MY, Yun YK, Choi Y, Baik SH, Park JS, Gwon AR, Yang DK, Lee CH, Lee SM, Park KW, Jo DG. Effects of chronic alcohol consumption on expression levels of APP and Aβ-producing enzymes. BMB Rep. 2011. February;44(2):135–9. doi: 10.5483/BMBRep.2011.44.2.135. [DOI] [PubMed] [Google Scholar]
- Kim M, Han CH, Lee MY. NADPH oxidase and the cardiovascular toxicity associated with smoking. Toxicol Res. 2014. September;30(3):149–57. doi: 10.5487/TR.2014.30.3.149. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishida KT, Klann E. Sources and targets of reactive oxygen species in synaptic plasticity and memory. Antioxid Redox Signal. 2007. February;9(2):233–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knez D, Coquelle N, Pišlar A, Žakelj S, Jukič M, Sova M, Mravljak J, Nachon F, Brazzolotto X, Kos J, Colletier JP, Gobec S. Multi-target-directed ligands for treating Alzheimer’s disease: Butyrylcholinesterase inhibitors displaying antioxidant and neuroprotective activities. Eur J Med Chem. 2018. August 5;156:598–617. doi: 10.1016/j.ejmech.2018.07.033. [DOI] [PubMed] [Google Scholar]
- Korkia P, Stimson GV. Indications of prevalence, practice and effects of anabolic steroid use in Great Britain. Int J Sports Med. 1997. October;18(7):557–62. [DOI] [PubMed] [Google Scholar]
- Kouvelas D, Pourzitaki C, Papazisis G, Dagklis T, Dimou K, Kraus MM. Nandrolone abuse decreases anxiety and impairs memory in rats via central androgenic receptors. Int J Neuropsychopharmacol. 2008. November;11(7):925–34. doi: 10.1017/S1461145708008754. [DOI] [PubMed] [Google Scholar]
- Kovacs GG, Horvath MC, Majtenyi K, Lutz MI, Hurd YL, Keller E. Heroin abuse exaggerates age-related deposition of hyperphosphorylated tau and p62-positive inclusions. Neurobiol Aging 2015. November;36(11):3100–3107. doi: 10.1016/j.neurobiolaging.2015.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovatsi L, Njau S, Nikolaou K, Tsolakidou A, Karamouzis I, Thisiadou K. Isoprostane as a marker of oxidative stress in chronic heroin users: correlation with duration of heroin use or concomitant hepatitis C infection. Am J Drug Alcohol Abuse 2010a. January;36(1):13–7. doi: 10.3109/00952990903544794. [DOI] [PubMed] [Google Scholar]
- Kovatsi L, Njau S, Nikolaou K, Topouridou K, Papamitsou T, Koliakos G. Evaluation of prooxidantantioxidant balance in chronic heroin users in a single assay: an identification criterion for antioxidant supplementation. Am J Drug Alcohol Abuse 2010b. July;36(4):228–32. doi: 10.3109/00952990.2010.495438. [DOI] [PubMed] [Google Scholar]
- Kowalczyk-Pachel D, Iciek M, Wydra K, Nowak E, Górny M, Filip M, Włodek L, Lorenc-Koci E. Cysteine Metabolism and Oxidative Processes in the Rat Liver and Kidney after Acute and Repeated Cocaine Treatment. PLoS One 2016. January 25;11(1):e0147238. doi: 10.1371/journal.pone.0147238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnankutty A, Kimura T, Saito T, Aoyagi K, Asada A, Takahashi SI, Ando K, Ohara-Imaizumi M, Ishiguro K, Hisanaga SI. In vivo regulation of glycogen synthase kinase 3β activity in neurons and brains. Sci Rep. 2017. August 17;7(1):8602. doi: 10.1038/s41598-017-09239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kröncke KD, Klotz LO. Zinc fingers as biologic redox switches? Antioxid Redox Signal. 2009. May;11(5):1015–27. doi: 10.1089/ARS.2008.2269. [DOI] [PubMed] [Google Scholar]
- Kumar A, Yegla B, Foster TC. Redox Signaling in Neurotransmission and Cognition During Aging. Antioxid Redox Signal. 2018a. June 20;28(18):1724–1745. doi: 10.1089/ars.2017.7111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M, Sandhir R. Neuroprotective Effect of Hydrogen Sulfide in Hyperhomocysteinemia Is Mediated Through Antioxidant Action Involving Nrf2. Neuromolecular Med. 2018b. December;20(4):475–490. doi: 10.1007/s12017-018-8505-y. [DOI] [PubMed] [Google Scholar]
- Kurochkin IV, Goto S. Alzheimer’s beta-amyloid peptide specifically interacts with and is degraded by insulin degrading enzyme. FEBS Lett. 1994. May 23;345(1):33–7. [DOI] [PubMed] [Google Scholar]
- Kwon KJ, Lee EJ, Cho KS, Cho DH, Shin CY, Han SH. Ginkgo biloba extract (Egb761) attenuates zinc-induced tau phosphorylation at Ser262 by regulating GSK3β activity in rat primary cortical neurons. Food Funct. 2015. June;6(6):2058–67. doi: 10.1039/c5fo00219b. [DOI] [PubMed] [Google Scholar]
- Lai AY, Lan CP, Hasan S, Brown ME, McLaurin J. scyllo-Inositol promotes robust mutant Huntingtin protein degradation. J Biol Chem. 2014. February 7;289(6):3666–76. doi: 10.1074/jbc.M113.501635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauro F, Giancotti LA, Ilari S, Dagostino C, Gliozzi M, Morabito C, Malafoglia V, Raffaeli W, Muraca M, Goffredo BM, Mollace V, Muscoli C. Inhibition of Spinal Oxidative Stress by Bergamot Polyphenolic Fraction Attenuates the Development of Morphine Induced Tolerance and Hyperalgesia in Mice. PLoS One. 2016. May 26;11(5):e0156039. doi: 10.1371/journal.pone.0156039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecomte E, Herbeth B, Pirollet P, Chancerelle Y, Arnaud J, Musse N, Paille F, Siest G, Artur Y. Effect of alcohol consumption on blood antioxidant nutrients and oxidative stress indicators. Am J Clin Nutr. 1994. August;60(2):255–61. [DOI] [PubMed] [Google Scholar]
- Lee D, Lee WS, Lim S, Kim YK, Jung HY, Das S, Lee J, Luo W, Kim KT, Chung SK. A guanidine-appended scyllo-inositol derivative AAD-66 enhances brain delivery and ameliorates Alzheimer’s phenotypes. Sci Rep. 2017. October 26;7(1):14125. doi: 10.1038/s41598-017-14559-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Choi BR, Kim J, LaFerla FM, Park JHY, Han JS, Lee KW, Kim J. Sulforaphane Upregulates the Heat Shock Protein Co-Chaperone CHIP and Clears Amyloid-β and Tau in a Mouse Model of Alzheimer’s Disease. Mol Nutr Food Res. 2018. June;62(12):e1800240. doi: 10.1002/mnfr.201800240. [DOI] [PubMed] [Google Scholar]
- Leroy K, Yilmaz Z, Brion JP. Increased level of active GSK-3beta in Alzheimer’s disease and accumulation in argyrophilic grains and in neurones at different stages of neurofibrillary degeneration. Neuropathol Appl Neurobiol. 2007. February;33(1):43–55. [DOI] [PubMed] [Google Scholar]
- Li MD, Kane JK, Matta SG, Blaner WS, Sharp BM. Nicotine enhances the biosynthesis and secretion of transthyretin from the choroid plexus in rats: implications for beta-amyloid formation. J Neurosci. 2000. February 15;20(4):1318–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Pomès R. Binding mechanism of inositol stereoisomers to monomers and aggregates of Aβ(16–22). J Phys Chem B. 2013. June 6;117(22):6603–13. doi: 10.1021/jp311350r. [DOI] [PubMed] [Google Scholar]
- Li X, Zhang X, Ladiwala AR, Du D, Yadav JK, Tessier PM, Wright PE, Kelly JW, Buxbaum JN. Mechanisms of transthyretin inhibition of β-amyloid aggregation in vitro. J Neurosci. 2013a. December 11;33(50):19423–33. doi: 10.1523/JNEUROSCI.2561-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Liang T, He Q, Guo C, Xu L, Zhang K, Duan X. Puerarin, isolated from Kudzu root (Willd.), attenuates hepatocellular cytotoxicity and regulates the GSK-3β/NF-κB pathway for exerting the hepatoprotection against chronic alcohol-induced liver injury in rats. Int Immunopharmacol. 2013b. September;17(1):71–8. doi: 10.1016/j.intimp.2013.05.023. [DOI] [PubMed] [Google Scholar]
- Li YZ, Tang XH, Wang CY, Hu N, Xie KL, Wang HY, Yu YH, Wang GL. Glycogen synthase kinase-3β inhibition prevents remifentanil-induced postoperative hyperalgesia via regulating the expression and function of AMPA receptors. Anesth Analg. 2014. October;119(4):978–87. doi: 10.1213/ANE.0000000000000365. [DOI] [PubMed] [Google Scholar]
- Li L, Chen J, Sun S, Zhao J, Dong X, Wang J. Effects of Estradiol on Autophagy and Nrf-2/ARE Signals after Cerebral Ischemia. Cell Physiol Biochem. 2017;41(5):2027–2036. doi: 10.1159/000475433. [DOI] [PubMed] [Google Scholar]
- Liang B, Li YH, Kong H. Serum paraoxonase, arylesterase activities and oxidative status in patients with insomnia. Eur Rev Med Pharmacol Sci. 2013a. September;17(18):2517–22. [PubMed] [Google Scholar]
- Liang E, Garzone P, Cedarbaum JM, Koller M, Tran T, Xu V, Ross B, Jhee SS, Ereshefsky L, Pastrak A, Abushakra S. Pharmacokinetic Profile of Orally Administered Scyllo-Inositol (Elnd005) in Plasma, Cerebrospinal Fluid and Brain, and Corresponding Effect on Amyloid-Beta in Healthy Subjects. Clin Pharmacol Drug Dev. 2013b. April;2(2):186–94. doi: 10.1002/cpdd.14. [DOI] [PubMed] [Google Scholar]
- Lin JF, Lin YH, Liao PC, Lin YC, Tsai TF, Chou KY, Chen HE, Tsai SC, Hwang TI. Induction of testicular damage by daily methamphetamine administration in rats. Chin J Physiol. 2014. February 28;57(1):19–30. doi: 10.4077/CJP.2014.BAB155. [DOI] [PubMed] [Google Scholar]
- Lin CH, Hsieh YS, Wu YR, Hsu CJ, Chen HC, Huang WH, Chang KH, Hsieh-Li HM, Su MT, Sun YC, Lee GC, Lee-Chen GJ. Identifying GSK-3β kinase inhibitors of Alzheimer’s disease: Virtual screening, enzyme, and cell assays. Eur J Pharm Sci. 2016. June 30;89:11–9. doi: 10.1016/j.ejps.2016.04.012. [DOI] [PubMed] [Google Scholar]
- Lindqvist AS, Moberg T, Eriksson BO, Ehrnborg C, Rosén T, Fahlke C. A retrospective 30-year follow-up study of former Swedish-elite male athletes in power sports with a past anabolic androgenic steroids use: a focus on mental health. Br J Sports Med. 2013. October;47(15):965–9. doi: 10.1136/bjsports-2012-091340. [DOI] [PubMed] [Google Scholar]
- Liu PY, Yee B, Wishart SM, Jimenez M, Jung DG, Grunstein RR, Handelsman DJ. The short-term effects of high-dose testosterone on sleep, breathing, and function in older men. J Clin Endocrinol Metab. 2003a. August;88(8):3605–13. [DOI] [PubMed] [Google Scholar]
- Liu SJ, Fang ZY, Yang Y, Deng HM, Wang JZ. Alzheimer-like phosphorylation of tau and neurofilament induced by cocaine in vivo. Acta Pharmacol Sin. 2003b. June;24(6):512–8. [PubMed] [Google Scholar]
- Liu L, Yu J, Li L, Zhang B, Liu L, Wu CH, Jong A, Mao DA, Huang SH. Alpha7 nicotinic acetylcholine receptor is required for amyloid pathology in brain endothelial cells induced by Glycoprotein 120, methamphetamine and nicotine. Sci Rep. 2017a. January 11;7:40467. doi: 10.1038/srep40467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CC, Hu J, Zhao N, Wang J, Wang N, Cirrito JR, Kanekiyo T, Holtzman DM, Bu G. Astrocytic LRP1 Mediates Brain Aβ Clearance and Impacts Amyloid Deposition. J Neurosci. 2017b. April 12;37(15):4023–4031. doi: 10.1523/JNEUROSCI.3442-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Jevtic S, Markham-Coultes K, Ellens NPK, O’Reilly MA, Hynynen K, Aubert I, McLaurin J. Investigating the efficacy of a combination Aβ-targeted treatment in a mouse model of Alzheimer’s disease. Brain Res. 2018. January 1;1678:138–145. doi: 10.1016/j.brainres.2017.10.015. [DOI] [PubMed] [Google Scholar]
- Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, Ballard C, Banerjee S, Burns A, Cohen-Mansfield J, Cooper C, Fox N, Gitlin LN, Howard R, Kales HC, Larson EB, Ritchie K, Rockwood K, Sampson EL, Samus Q, Schneider LS, Selbæk G, Teri L, Mukadam N. Dementia prevention, intervention, and care. Lancet 2017. December 16;390(10113):2673–2734. doi: 10.1016/S0140-6736(17)31363-6. [DOI] [PubMed] [Google Scholar]
- Llorens-Martín M, Jurado J, Hernández F, Avila J. GSK-3β, a pivotal kinase in Alzheimer disease. Front Mol Neurosci. 2014. May 21;7:46. doi: 10.3389/fnmol.2014.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locklear MN, Kritzer MF. Assessment of the effects of sex and sex hormones on spatial cognition in adult rats using the Barnes maze. Horm Behav. 2014. July;66(2):298–308. doi: 10.1016/j.yhbeh.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh SY, Giribabu N, Salleh N. Effects of gonadectomy and testosterone treatment on aquaporin expression in the kidney of normotensive and hypertensive rats. Exp Biol Med (Maywood) 2017. July;242(13):1376–1386. doi: 10.1177/1535370217703360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Pedrajas R, Ramírez-Lamelas DT, Muriach B, Sánchez-Villarejo MV, Almansa I, Vidal-Gil L, Romero FJ, Barcia JM, Muriach M. Cocaine promotes oxidative stress and microglial-macrophage activation in rat cerebellum. Front Cell Neurosci. 2015. July 28;9:279. doi: 10.3389/fncel.2015.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord KC, Shenouda SK, McIlwain E, Charalampidis D, Lucchesi PA, Varner KJ. Oxidative stress contributes to methamphetamine-induced left ventricular dysfunction. Cardiovasc Res. 2010. July 1;87(1):111–8. doi: 10.1093/cvr/cvq043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell MA, Robertson JD, Teesdale WJ, Campbell JL, Markesbery WR. Copper, iron and zinc in Alzheimer’s disease senile plaques. J Neurol Sci. 1998. June 11;158(1):47–52. [DOI] [PubMed] [Google Scholar]
- Luijkx T, Velthuis BK, Backx FJ, Buckens CF, Prakken NH, Rienks R, Mali WP, Cramer MJ. Anabolic androgenic steroid use is associated with ventricular dysfunction on cardiac MRI in strength trained athletes. Int J Cardiol. 2013. August 10;167(3):664–8. doi: 10.1016/j.ijcard.2012.03.072. [DOI] [PubMed] [Google Scholar]
- Luo Y, Zhou X, Yang X, Wang J. Homocysteine induces tau hyperphosphorylation in rats. Neuroreport 2007. December 3;18(18):2005–8. [DOI] [PubMed] [Google Scholar]
- McAllister C, Long J, Bowers A, Walker A, Cao P, Honda S, Harada N, Staufenbiel M, Shen Y, Li R. Genetic targeting aromatase in male amyloid precursor protein transgenic mice down-regulates beta-secretase (BACE1) and prevents Alzheimer-like pathology and cognitive impairment. J Neurosci. 2010. May 26;30(21):7326–34. doi: 10.1523/JNEUROSCI.1180-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaddon A, Davies G, Hudson P, Tandy S, Cattell H. Total serum homocysteine in senile dementia of Alzheimer type. Int J Geriatr Psychiatry 1998. April;13(4):235–9. [DOI] [PubMed] [Google Scholar]
- McConnell SE, Alla J, Wheat E, Romeo RD, McEwen B, Thornton JE. The role of testicular hormones and luteinizing hormone in spatial memory in adult male rats. Horm Behav. 2012. April;61(4):479–86. doi: 10.1016/j.yhbeh.2012.01.003. [DOI] [PubMed] [Google Scholar]
- McKelvey W, Gwynn RC, Jeffery N, Kass D, Thorpe LE, Garg RK, Palmer CD, Parsons PJ. A biomonitoring study of lead, cadmium, and mercury in the blood of New York city adults. Environ Health Perspect. 2007. October;115(10):1435–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaurin J, Golomb R, Jurewicz A, Antel JP, Fraser PE. Inositol stereoisomers stabilize an oligomeric aggregate of Alzheimer amyloid beta peptide and inhibit abeta -induced toxicity. J Biol Chem. 2000. June 16;275(24):18495–502. [DOI] [PubMed] [Google Scholar]
- Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. 2013;53:401–26. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma F, Liu D. 17β-trenbolone, an anabolic-androgenic steroid as well as an environmental hormone, contributes to neurodegeneration. Toxicol Appl Pharmacol. 2015. January 1;282(1):68–76. doi: 10.1016/j.taap.2014.11.007. [DOI] [PubMed] [Google Scholar]
- Ma J, Bao YP, Wang RJ, Su MF, Liu MX, Li JQ, Degenhardt L, Farrell M, Blow FC, Ilgen M, Shi J, Lu L. Effects of medication-assisted treatment on mortality among opioids users: a systematic review and meta-analysis. Mol Psychiatry 2018. June 22 10.1038/s41380-018-0094-5. [DOI] [PubMed] [Google Scholar]
- Magnusson K, Hånell A, Bazov I, Clausen F, Zhou Q, Nyberg F. Nandrolone decanoate administration elevates hippocampal prodynorphin mRNA expression and impairs Morris water maze performance in male rats. Neurosci Lett. 2009. December 31;467(3):189–93. doi: 10.1016/j.neulet.2009.09.041. [DOI] [PubMed] [Google Scholar]
- Malone DA Jr, Dimeff RJ, Lombardo JA, Sample RH. Psychiatric effects and psychoactive substance use in anabolic-androgenic steroid users. Clin J Sport Med. 1995;5(1):25–31. [DOI] [PubMed] [Google Scholar]
- Mansouri MT, Naghizadeh B, Ghorbanzadeh B, Alboghobeish S, Amirgholami N, Houshmand G, Cauli O. Venlafaxine prevents morphine antinociceptive tolerance: The role of neuroinflammation and the larginine-nitric oxide pathway. Exp Neurol. 2018. May;303:134–141. doi: 10.1016/j.expneurol.2018.02.009. [DOI] [PubMed] [Google Scholar]
- Martikainen H, Alén M, Rahkila P, Vihko R. Testicular responsiveness to human chorionic gonadotrophin during transient hypogonadotrophic hypogonadism induced by androgenic/anabolic steroids in power athletes. J Steroid Biochem. 1986. July;25(1):109–12. [DOI] [PubMed] [Google Scholar]
- Massaad CA, Klann E. Reactive oxygen species in the regulation of synaptic plasticity and memory. Antioxid Redox Signal. 2011. May 15;14(10):2013–54. doi: 10.1089/ars.2010.3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masvekar RR, El-Hage N, Hauser KF, Knapp PE. GSK3β-activation is a point of convergence for HIV-1 and opiate-mediated interactive neurotoxicity. Mol Cell Neurosci. 2015. March;65:11–20. doi: 10.1016/j.mcn.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matousek RH, Sherwin BB. Sex steroid hormones and cognitive functioning in healthy, older men. Horm Behav. 2010. March;57(3):352–9. doi: 10.1016/j.yhbeh.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer U, Preiss F, Brauns-Schubert P, Schlicher L, Charvet C. GSK-3 - at the crossroads of cell death and survival. J Cell Sci. 2014. April 1;127(Pt 7):1369–78. doi: 10.1242/jcs.138057. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Teoh SK. Neuroendocrine consequences of alcohol abuse in women. Ann N Y Acad Sci. 1989;562:211–40. [DOI] [PubMed] [Google Scholar]
- Melo P, Zanon-Moreno V, Alves CJ, Magalhães A, Tavares MA, Pinazo-Duran MD, Moradas-Ferreira P. Oxidative stress response in the adult rat retina and plasma after repeated administration of methamphetamine. Neurochem Int. 2010. February;56(3):431–6. doi: 10.1016/j.neuint.2009.11.017. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Mello NK. Alcohol, aggression and androgens. Res Publ Assoc Res Nerv Ment Dis. 1974;52:225–47. [PubMed] [Google Scholar]
- Mendelson JH, Mendelson JE, Patch VD. Plasma testosterone levels in heroin addiction and during methadone maintenance. J Pharmacol Exp Ther. 1975. January;192(1):211–17. [PubMed] [Google Scholar]
- Mendelson JH, Ellingboe J, Mello NK, Kuehnle J. Effects of alcohol on plasma testosterone and luteinizing hormone levels. Alcohol Clin Exp Res. 1978. July;2(3):255–8. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Teoh SK, Lange U, Mello NK, Weiss R, Skupny A, Ellingboe J. Anterior pituitary, adrenal, and gonadal hormones during cocaine withdrawal. Am J Psychiatry 1988. September;145(9):1094–8. [DOI] [PubMed] [Google Scholar]
- Meng P, Yoshida H, Matsumiya T, Imaizumi T, Tanji K, Xing F, Hayakari R, Dempoya J, Tatsuta T, Aizawa-Yashiro T, Mimura J, Kosaka K, Itoh K, Satoh K. Carnosic acid suppresses the production of amyloid-β 1–42 by inducing the metalloprotease gene TACE/ADAM17 in SH-SY5Y human neuroblastoma cells. Neurosci Res. 2013. February;75(2):94–102. doi: 10.1016/j.neures.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Meydan S, Kus I, Tas U, Ogeturk M, Sancakdar E, Dabak DO, Zararsız I, Sarsılmaz M. Effects of testosterone on orchiectomy-induced oxidative damage in the rat hippocampus. J Chem Neuroanat. 2010. December;40(4):281–5. doi: 10.1016/j.jchemneu.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Miller JS, Tallarida RJ, Unterwald EM. Cocaine-induced hyperactivity and sensitization are dependent on GSK3. Neuropharmacology 2009. June;56(8):1116–23. doi: 10.1016/j.neuropharm.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirecki A, Fitzmaurice P, Ang L, Kalasinsky KS, Peretti FJ, Aiken SS, Wickham DJ, Sherwin A, Nobrega JN, Forman HJ, Kish SJ. Brain antioxidant systems in human methamphetamine users. J Neurochem. 2004. June;89(6):1396–408. [DOI] [PubMed] [Google Scholar]
- Mishra S, Blazey TM, Holtzman DM, Cruchaga C, Su Y, Morris JC, Benzinger TLS, Gordon BA. Longitudinal brain imaging in preclinical Alzheimer disease: impact of APOE ε4 genotype. Brain. 2018. June 1;141(6):1828–1839. doi: 10.1093/brain/awy103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modlinski R, Fields KB. The effect of anabolic steroids on the gastrointestinal system, kidneys, and adrenal glands. Curr Sports Med Rep. 2006. April;5(2):104–9. [DOI] [PubMed] [Google Scholar]
- Moffat SD, Zonderman AB, Metter EJ, Blackman MR, Harman SM, Resnick SM. Longitudinal assessment of serum free testosterone concentration predicts memory performance and cognitive status in elderly men. J Clin Endocrinol Metab. 2002. November;87(11):5001–7. [DOI] [PubMed] [Google Scholar]
- Montine TJ, Huang DY, Valentine WM, Amarnath V, Saunders A, Weisgraber KH, Graham DG, Strittmatter WJ. Crosslinking of apolipoprotein E by products of lipid peroxidation. J Neuropathol Exp Neurol. 1996. February;55(2):202–10. [DOI] [PubMed] [Google Scholar]
- Montoliu C, Vallés S, Renau-Piqueras J, Guerri C. Ethanol-induced oxygen radical formation and lipid peroxidation in rat brain: effect of chronic alcohol consumption. J Neurochem. 1994. November;63(5):1855–62. [DOI] [PubMed] [Google Scholar]
- Moreno-Gonzalez I, Estrada LD, Sanchez-Mejias E, Soto C. Smoking exacerbates amyloid pathology in a mouse model of Alzheimer’s disease. Nat Commun. 2013;4:1495. doi: 10.1038/ncomms2494. [DOI] [PubMed] [Google Scholar]
- Morrone CD, Thomason LA, Brown ME, Aubert I, McLaurin J. Effects of Neurotrophic Support and Amyloid-Targeted Combined Therapy on Adult Hippocampal Neurogenesis in a Transgenic Model of Alzheimer’s Disease. PLoS One 2016. October 21;11(10):e0165393. doi: 10.1371/journal.pone.0165393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motaghinejad M, Karimian M, Motaghinejad O, Shabab B, Yazdani I, Fatima S. Protective effects of various dosage of Curcumin against morphine induced apoptosis and oxidative stress in rat isolated hippocampus. Pharmacol Rep. 2015. April;67(2):230–5. doi: 10.1016/j.pharep.2014.09.006. [DOI] [PubMed] [Google Scholar]
- Muller M, den Tonkelaar I, Thijssen JH, Grobbee DE, van der Schouw YT. Endogenous sex hormones in men aged 40–80 years. Eur J Endocrinol. 2003. December;149(6):583–9. [DOI] [PubMed] [Google Scholar]
- Muriach M, López-Pedrajas R, Barcia JM, Sanchez-Villarejo MV, Almansa I, Romero FJ. Cocaine causes memory and learning impairments in rats: involvement of nuclear factor kappa B and oxidative stress, and prevention by topiramate. J Neurochem. 2010. August;114(3):675–84. doi: 10.1111/j.1471-4159.2010.06794.x. [DOI] [PubMed] [Google Scholar]
- Naha N, Gandhi DN, Gautam AK, Prakash JR. Nicotine and cigarette smoke modulate Nrf2-BDNF-dopaminergic signal and neurobehavioral disorders in adult rat cerebral cortex(). Hum Exp Toxicol. 2018. May;37(5):540–556. doi: 10.1177/0960327117698543. [DOI] [PubMed] [Google Scholar]
- Najafi K, Ahmadi S, Rahpeyma M, Khazaie H, Vaisi-Raygani A, Moini A, Kiani A. Study of Serum Malondialdehyde Level in Opioid and Methamphetamine Dependent Patients. Acta Med Iran. 2017. October;55(10):616–620. [PubMed] [Google Scholar]
- Ndengele MM, Cuzzocrea S, Masini E, Vinci MC, Esposito E, Muscoli C, Petrusca DN, Mollace V, Mazzon E, Li D, Petrache I, Matuschak GM, Salvemini D. Spinal ceramide modulates the development of morphine antinociceptive tolerance via peroxynitrite-mediated nitroxidative stress and neuroimmune activation. J Pharmacol Exp Ther. 2009. April;329(1):64–75. doi: 10.1124/jpet.108.146290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nead KT, Gaskin G, Chester C, Swisher-McClure S, Dudley JT, Leeper NJ, Shah NH. Androgen Deprivation Therapy and Future Alzheimer’s Disease Risk. J Clin Oncol. 2016. February 20;34(6):566–71. doi: 10.1200/JCO.2015.63.6266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo Sock ET, Chapados NA, Lavoie JM. LDL receptor and Pcsk9 transcripts are decreased in liver of ovariectomized rats: effects of exercise training. Horm Metab Res. 2014. July;46(8):550–5. doi: 10.1055/s-0034-1370910. [DOI] [PubMed] [Google Scholar]
- Nikolic T, Zivkovic V, Jevdjevic M, Djuric M, Srejovic I, Djuric D, Jeremic N, Djuric D, Bolevich S, Jakovljevic V. The effects of chronic administration of nandrolone decanoate on redox status in exercised rats. Mol Cell Biochem. 2016. January;411(1–2):95–105. doi: 10.1007/s11010-015-2571-3. [DOI] [PubMed] [Google Scholar]
- Niu H, Qu Y, Li Z, Wang R, Li L, Li M, Lv X, Gao C, Song Y, Li B. Smoking and Risk for Alzheimer Disease: A Meta-Analysis Based on Both Case-Control and Cohort Study. J Nerv Ment Dis. 2018. September;206(9):680–685. doi: 10.1097/NMD.0000000000000859. [DOI] [PubMed] [Google Scholar]
- Nunes MA, Viel TA, Buck HS. Microdose lithium treatment stabilized cognitive impairment in patients with Alzheimer’s disease. Curr Alzheimer Res. 2013. January;10(1):104–7. [DOI] [PubMed] [Google Scholar]
- Obregon D, Hou H, Deng J, Giunta B, Tian J, Darlington D, Shahaduzzaman M, Zhu Y, Mori T, Mattson MP, Tan J. Soluble amyloid precursor protein-α modulates β-secretase activity and amyloid-β generation. Nat Commun. 2012. April 10;3:777. doi: 10.1038/ncomms1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda A, Tamaoka A, Araki W. Oxidative stress up-regulates presenilin 1 in lipid rafts in neuronal cells. J Neurosci Res. 2010. April;88(5):1137–45. doi: 10.1002/jnr.22271. [DOI] [PubMed] [Google Scholar]
- Oliver CN, Ahn BW, Moerman EJ, Goldstein S, Stadtman ER. Age-related changes in oxidized proteins. J Biol Chem. 1987. April 25;262(12):5488–91. [PubMed] [Google Scholar]
- Oset-Gasque MJ, Marco-Contelles J. Alzheimer’s Disease, the “One-Molecule, One-Target” Paradigm, and the Multitarget Directed Ligand Approach. ACS Chem Neurosci. 2018. March 21;9(3):401–403. doi: 10.1021/acschemneuro.8b00069. [DOI] [PubMed] [Google Scholar]
- Owen JB, Sultana R, Aluise CD, Erickson MA, Price TO, Bu G, Banks WA, Butterfield DA. Oxidative modification to LDL receptor-related protein 1 in hippocampus from subjects with Alzheimer disease: implications for Aβ accumulation in AD brain. Free Radic Biol Med. 2010. December 1;49(11):1798–803. doi: 10.1016/j.freeradbiomed.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaita A, Puighermanal E, Maldonado R. Regulation of PI3K/Akt/GSK-3 pathway by cannabinoids in the brain. J Neurochem. 2007. August;102(4):1105–14. [DOI] [PubMed] [Google Scholar]
- Pan Y, Short JL, Newman SA, Choy KHC, Tiwari D, Yap C, Senyschyn D, Banks WA, Nicolazzo JA. Cognitive benefits of lithium chloride in APP/PS1 mice are associated with enhanced brain clearance of β-amyloid. Brain Behav Immun. 2018. May;70:36–47. doi: 10.1016/j.bbi.2018.03.007. 12. [DOI] [PubMed] [Google Scholar]
- Pang T, Wang YJ, Gao YX, Xu Y, Li Q, Zhou YB, Xu L, Huang ZJ, Liao H, Zhang LY, Gao JR, Ye Q, Li J. A novel GSK-3β inhibitor YQ138 prevents neuronal injury induced by glutamate and brain ischemia through activation of the Nrf2 signaling pathway. Acta Pharmacol Sin. 2016. June;37(6):741–52. doi: 10.1038/aps.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panizzon MS, Hauger R, Dale AM, Eaves LJ, Eyler LT, Fischl B, Fennema-Notestine C, Franz CE, Grant MD, Jak AJ, Jacobson KC, Lyons MJ, Mendoza SP, Neale MC, Prom-Wormley EC, Seidman LJ, Tsuang MT, Xian H, Kremen WS. Testosterone modifies the effect of APOE genotype on hippocampal volume in middle-aged men. Neurology 2010. September 7;75(10):874–80. doi: 10.1212/WNL.0b013e3181f11deb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panizzon MS, Hauger R, Xian H, Vuoksimaa E, Spoon KM, Mendoza SP, Jacobson KC, Vasilopoulos T, Rana BK, McKenzie R, McCaffery JM, Lyons MJ, Kremen WS, Franz CE. Interaction of APOE genotype and testosterone on episodic memory in middle-aged men. Neurobiol Aging 2014. July;35(7):1778.e1–8. doi: 10.1016/j.neurobiolaging.2013.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papasozomenos SC. The heat shock-induced hyperphosphorylation of tau is estrogen-independent and prevented by androgens: implications for Alzheimer disease. Proc Natl Acad Sci U S A 1997. June 24;94(13):6612–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papasozomenos SCh, Shanavas A. Testosterone prevents the heat shock-induced overactivation of glycogen synthase kinase-3 beta but not of cyclin-dependent kinase 5 and c-Jun NH2-terminal kinase and concomitantly abolishes hyperphosphorylation of tau: implications for Alzheimer’s disease. Proc Natl Acad Sci U S A. 2002. February 5;99(3):1140–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson AB, Evans NA. Anabolic androgenic steroids: a survey of 500 users. Med Sci Sports Exerc. 2006. April;38(4):644–51. [DOI] [PubMed] [Google Scholar]
- Parkitna JR, Obara I, Wawrzczak-Bargiela A, Makuch W, Przewlocka B, Przewlocki R. Effects of glycogen synthase kinase 3beta and cyclin-dependent kinase 5 inhibitors on morphine-induced analgesia and tolerance in rats. J Pharmacol Exp Ther. 2006. November;319(2):832–9. [DOI] [PubMed] [Google Scholar]
- Patterson BW, Elbert DL, Mawuenyega KG, Kasten T, Ovod V, Ma S, Xiong C, Chott R, Yarasheski K, Sigurdson W, Zhang L, Goate A, Benzinger T, Morris JC, Holtzman D, Bateman RJ. Age and amyloid effects on human central nervous system amyloid-beta kinetics. Ann Neurol. 2015. September;78(3):439–53. doi: 10.1002/ana.24454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereska Z, Dejanova B, Bozinovska C, Petkovska L. Prooxidative/antioxidative homeostasis in heroin addiction and detoxification. Bratisl Lek Listy. 2007;108(9):393–8. [PubMed] [Google Scholar]
- Pérez-Casanova A, Husain K, Noel RJ Jr, Rivera-Amill V, Kumar A. Interaction of SIV/SHIV infection and morphine on plasma oxidant/antioxidant balance in macaque. Mol Cell Biochem. 2008. January;308(1–2):169–75. [DOI] [PubMed] [Google Scholar]
- Perianes-Cachero A, Canelles S, Aguado-Llera D, Frago LM, Toledo-Lobo MV, Carrera I, Cacabelos R, Chowen JA, Argente J, Arilla-Ferreiro E, Barrios V. Reduction in Aβ-induced cell death in the hippocampus of 17β-estradiol-treated female rats is associated with an increase in IGF-I signaling and somatostatinergic tone. J Neurochem. 2015. December;135(6):1257–71. doi: 10.1111/jnc.13381. [DOI] [PubMed] [Google Scholar]
- Perrine SA, Miller JS, Unterwald EM. Cocaine regulates protein kinase B and glycogen synthase kinase-3 activity in selective regions of rat brain. J Neurochem. 2008. October;107(2):570–7. doi: 10.1111/j.1471-4159.2008.05632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persky H, O’Brien CP, Fine E, Howard WJ, Khan MA, Beck RW. The effect of alcohol and smoking on testosterone function and aggression in chronic alcoholics. Am J Psychiatry 1977. June;134(6):621–5. [DOI] [PubMed] [Google Scholar]
- Pey A, Saborido A, Blázquez I, Delgado J, Megías A. Effects of prolonged stanozolol treatment on antioxidant enzyme activities, oxidative stress markers, and heat shock protein HSP72 levels in rat liver. J Steroid Biochem Mol Biol. 2003. December;87(4–5):269–77. [DOI] [PubMed] [Google Scholar]
- Pieretti S, Mastriota M, Tucci P, Battaglia G, Trabace L, Nicoletti F, Scaccianoce S. Brain nerve growth factor unbalance induced by anabolic androgenic steroids in rats. Med Sci Sports Exerc. 2013. January;45(1):29–35. doi: 10.1249/MSS.0b013e31826c60ea. [DOI] [PubMed] [Google Scholar]
- Pike CJ. Testosterone attenuates beta-amyloid toxicity in cultured hippocampal neurons. Brain Res. 2001. November 16;919(1):160–5. [DOI] [PubMed] [Google Scholar]
- Pintana H, Pongkan W, Pratchayasakul W, Chattipakorn N, Chattipakorn SC. Testosterone replacement attenuates cognitive decline in testosterone-deprived lean rats, but not in obese rats, by mitigating brain oxidative stress. Age (Dordr). 2015. October;37(5):84. doi: 10.1007/s11357-015-9827-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomierny-Chamioło L, Moniczewski A, Wydra K, Suder A, Filip M. Oxidative stress biomarkers in some rat brain structures and peripheral organs underwent cocaine. Neurotox Res. 2013. January;23(1):92–102. doi: 10.1007/s12640-012-9335-6. Erratum in: Neurotox Res. 2013 Jan;23(1):103–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope HG Jr, Katz DL. Psychiatric and medical effects of anabolic-androgenic steroid use. A controlled study of 160 athletes. Arch Gen Psychiatry 1994. May;51(5):375–82. [DOI] [PubMed] [Google Scholar]
- Pope HG Jr, Kouri EM, Hudson JI. Effects of supraphysiologic doses of testosterone on mood and aggression in normal men: a randomized controlled trial. Arch Gen Psychiatry 2000. February;57(2):133–40. [DOI] [PubMed] [Google Scholar]
- Pope HG Jr, Wood RI, Rogol A, Nyberg F, Bowers L, Bhasin S. Adverse health consequences of performance-enhancing drugs: an Endocrine Society scientific statement. Endocr Rev. 2014a. June;35(3):341–75. doi: 10.1210/er.2013-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope HG Jr, Kanayama G, Athey A, Ryan E, Hudson JI, Baggish A. The lifetime prevalence of anabolic-androgenic steroid use and dependence in Americans: current best estimates. Am J Addict. 2014b. Jul-Aug;23(4):371–7. doi: 10.1111/j.1521-0391.2013.12118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen K, Bahl JM, Simonsen AH, Hasselbalch SG, Heegaard NH. Distinct transthyretin oxidation isoform profile in spinal fluid from patients with Alzheimer’s disease and mild cognitive impairment. Clin Proteomics 2014. March 29;11(1):12. doi: 10.1186/1559-0275-11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryor WA, Stone K. Oxidants in cigarette smoke. Radicals, hydrogen peroxide, peroxynitrate, and peroxynitrite. Ann N Y Acad Sci. 1993. May 28;686:12–27; discussion 27–8. Review. [DOI] [PubMed] [Google Scholar]
- Qian W, Shi J, Yin X, Iqbal K, Grundke-Iqbal I, Gong CX, Liu F. PP2A regulates tau phosphorylation directly and also indirectly via activating GSK-3beta. J Alzheimers Dis. 2010;19(4):1221–9. doi: 10.3233/JAD-2010-1317. [DOI] [PubMed] [Google Scholar]
- Qiusheng Z, Yuntao Z, Rongliang Z, Dean G, Changling L. Effects of verbascoside and luteolin on oxidative damage in brain of heroin treated mice. Pharmazie 2005. July;60(7):539–43. [PubMed] [Google Scholar]
- Quinn TJ, Gallacher J, Deary IJ, Lowe GD, Fenton C, Stott DJ. Association between circulating hemostatic measures and dementia or cognitive impairment: systematic review and meta-analyzes. J Thromb Haemost. 2011. August;9(8):1475–82. doi: 10.1111/j.1538-7836.2011.04403.x. [DOI] [PubMed] [Google Scholar]
- Quintanilla ME, Morales P, Ezquer F, Ezquer M, Herrera-Marschitz M, Israel Y. Commonality of Ethanol and Nicotine Reinforcement and Relapse in Wistar-Derived UChB Rats: Inhibition by N-Acetylcysteine. Alcohol Clin Exp Res. 2018. October;42(10):1988–1999. doi: 10.1111/acer.13842. [DOI] [PubMed] [Google Scholar]
- Rada P, Rojo AI, Evrard-Todeschi N, Innamorato NG, Cotte A, Jaworski T, Tobón-Velasco JC, Devijver H, García-Mayoral MF, Van Leuven F, Hayes JD, Bertho G, Cuadrado A. Structural and functional characterization of Nrf2 degradation by the glycogen synthase kinase 3/β-TrCP axis. Mol Cell Biol. 2012. September;32(17):3486–99. doi: 10.1128/MCB.00180-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghuraman G, Rai V, Peng YJ, Prabhakar NR, Kumar GK. Pattern-specific sustained activation of tyrosine hydroxylase by intermittent hypoxia: role of reactive oxygen species-dependent downregulation of protein phosphatase 2A and upregulation of protein kinases. Antioxid Redox Signal. 2009. August;11(8):1777–89. doi: 10.1089/ARS.2008.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raheem OA, Patel SH, Sisul D, Furnish TJ, Hsieh TC. The Role of Testosterone Supplemental Therapy in Opioid-Induced Hypogonadism: A Retrospective Pilot Analysis. Am J Mens Health. 2017. July;11(4):1208–1213. doi: 10.1177/1557988316672396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimi HR, Dehpour AR, Mehr SE, Sharifzadeh M, Ghahremani MH, Razmi A, Ostad SN. Lithium attenuates cannabinoid-induced dependence in the animal model: involvement of phosphorylated ERK1/2 and GSK-3β signaling pathways. Acta Med Iran 2014;52(9):656–63. [PubMed] [Google Scholar]
- Ramage SN, Anthony IC, Carnie FW, Busuttil A, Robertson R, Bell JE. Hyperphosphorylated tau and amyloid precursor protein deposition is increased in the brains of young drug abusers. Neuropathol Appl Neurobiol. 2005. August;31(4):439–48. [DOI] [PubMed] [Google Scholar]
- Ramanathan L, Gulyani S, Nienhuis R, Siegel JM. Sleep deprivation decreases superoxide dismutase activity in rat hippocampus and brainstem. Neuroreport 2002. August 7;13(11):1387–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsden M, Nyborg AC, Murphy MP, Chang L, Stanczyk FZ, Golde TE, Pike CJ. Androgens modulate beta-amyloid levels in male rat brain. J Neurochem. 2003. November;87(4):1052–5. [DOI] [PubMed] [Google Scholar]
- Rasmussen JJ, Selmer C, Østergren PB, Pedersen KB, Schou M, Gustafsson F, Faber J, Juul A, Kistorp C. Former Abusers of Anabolic Androgenic Steroids Exhibit Decreased Testosterone Levels and Hypogonadal Symptoms Years after Cessation: A Case-Control Study. PLoS One. 2016. August 17;11(8):e0161208. doi: 10.1371/journal.pone.0161208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen JJ, Schou M, Madsen PL, Selmer C, Johansen ML, Ulriksen PS, Dreyer T, Kümler T, Plesner LL, Faber J, Gustafsson F, Kistorp C. Cardiac systolic dysfunction in past illicit users of anabolic androgenic steroids. Am Heart J. 2018a. September;203:49–56. doi: 10.1016/j.ahj.2018.06.010. [DOI] [PubMed] [Google Scholar]
- Rasmussen JJ, Schou M, Madsen PL, Selmer C, Johansen ML, Hovind P, Ulriksen PS, Faber J, Gustafsson F, Kistorp C. Increased blood pressure and aortic stiffness among abusers of anabolic androgenic steroids: potential effect of suppressed natriuretic peptides in plasma? J Hypertens. 2018b. February;36(2):277–285. doi: 10.1097/HJH.0000000000001546. [DOI] [PubMed] [Google Scholar]
- Reis J, Cagide F, Valencia ME, Teixeira J, Bagetta D, Pérez C, Uriarte E, Oliveira PJ, Ortuso F, Alcaro S, Rodríguez-Franco MI, Borges F. Multi-target-directed ligands for Alzheimer’s disease: Discovery of chromone-based monoamine oxidase/cholinesterase inhibitors. Eur J Med Chem. 2018. October 5;158:781–800. doi: 10.1016/j.ejmech.2018.07.056. [DOI] [PubMed] [Google Scholar]
- Resko JA, Pereyra-Martinez AC, Stadelman HL, Roselli CE. Cellular observations and hormonal correlates of feedback control of luteinizing hormone secretion by testosterone in long-term castrated male rhesus monkeys. Biol Reprod. 2000. September;63(3):872–8. [DOI] [PubMed] [Google Scholar]
- Reuter AM, Kennes F. Influence of castration and of testosterone on prealbumin in mouse serum. Experientia 1966. December 15;22(12):840–1. [DOI] [PubMed] [Google Scholar]
- Riezzo I, Turillazzi E, Bello S, Cantatore S, Cerretani D, Di Paolo M, Fiaschi AI, Frati P, Neri M, Pedretti M, Fineschi V. Chronic nandrolone administration promotes oxidative stress, induction of pro-inflammatory cytokine and TNF-α mediated apoptosis in the kidneys of CD1 treated mice. Toxicol Appl Pharmacol. 2014. October 1;280(1):97–106. doi: 10.1016/j.taap.2014.06.031. [DOI] [PubMed] [Google Scholar]
- Riisøen H. Reduced prealbumin (transthyretin) in CSF of severely demented patients with Alzheimer’s disease. Acta Neurol Scand. 1988. December;78(6):455–9. [DOI] [PubMed] [Google Scholar]
- Rojo AI, Sagarra MR, Cuadrado A. GSK-3beta down-regulates the transcription factor Nrf2 after oxidant damage: relevance to exposure of neuronal cells to oxidative stress. J Neurochem. 2008. April;105(1):192–202. Epub 2007 Nov 13. [DOI] [PubMed] [Google Scholar]
- Rojo AI, Pajares M, Rada P, Nuñez A, Nevado-Holgado AJ, Killik R, Van Leuven F, Ribe E, Lovestone S, Yamamoto M, Cuadrado A. NRF2 deficiency replicates transcriptomic changes in Alzheimer’s patients and worsens APP and TAU pathology. Redox Biol. 2017. October;13:444–451. doi: 10.1016/j.redox.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario ER, Chang L, Stanczyk FZ, Pike CJ. Age-related testosterone depletion and the development of Alzheimer disease. JAMA 2004. September 22;292(12):1431–2. [DOI] [PubMed] [Google Scholar]
- Rosario ER, Carroll JC, Oddo S, LaFerla FM, Pike CJ. Androgens regulate the development of neuropathology in a triple transgenic mouse model of Alzheimer’s disease. J Neurosci. 2006. December 20;26(51):13384–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario ER, Chang L, Beckett TL, Carroll JC, Paul Murphy M, Stanczyk FZ, Pike CJ. Age-related changes in serum and brain levels of androgens in male Brown Norway rats. Neuroreport 2009. November 25;20(17):1534–7. doi: 10.1097/WNR.0b013e328331f968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario ER, Carroll J, Pike CJ. Testosterone regulation of Alzheimer-like neuropathology in male 3xTg-AD mice involves both estrogen and androgen pathways. Brain Res. 2010. November 4;1359:281–90. doi: 10.1016/j.brainres.2010.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario ER, Chang L, Head EH, Stanczyk FZ, Pike CJ. Brain levels of sex steroid hormones in men and women during normal aging and in Alzheimer’s disease. Neurobiol Aging 2011. April;32(4):604–13. doi: 10.1016/j.neurobiolaging.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario ER, Carroll JC, Pike CJ. Evaluation of the effects of testosterone and luteinizing hormone on regulation of β-amyloid in male 3xTg-AD mice. Brain Res. 2012. July 23;1466:137–45. doi: 10.1016/j.brainres.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein AL, Carpenter DM. Association Between Commonly Prescribed Opioids and Androgen Deficiency in Men: A Retrospective Cohort Analysis. Pain Med. 2017. April 1;18(4):637–644. doi: 10.1093/pm/pnw182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder J, Su Y, Liu F, Li B, Zhou Y, Ni B. Divergent roles of GSK3 and CDK5 in APP processing. Biochem Biophys Res Commun. 2003. December 26;312(4):922–9. [DOI] [PubMed] [Google Scholar]
- Ryou MG, Mallet RT, Metzger DB, Jung ME. Intermittent hypoxia training blunts cerebrocortical presenilin 1 overexpression and amyloid-β accumulation in ethanol-withdrawn rats. Am J Physiol Regul Integr Comp Physiol. 2017. July 1;313(1):R10–R18. doi: 10.1152/ajpregu.00050.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowska-Krępa E, Kłapcińska B, Jagsz S, Nowara A, Szołtysek-Bołdys I, Chalimoniuk M, Langfort J, Chrapusta SJ. High-dose testosterone enanthate supplementation boosts oxidative stress, but exerts little effect on the antioxidant barrier in sedentary adolescent male rat liver. Pharmacol Rep. 2017. August;69(4):673–678. doi: 10.1016/j.pharep.2017.02.023. [DOI] [PubMed] [Google Scholar]
- Sagare A, Deane R, Bell RD, Johnson B, Hamm K, Pendu R, Marky A, Lenting PJ, Wu Z, Zarcone T, Goate A, Mayo K, Perlmutter D, Coma M, Zhong Z, Zlokovic BV. Clearance of amyloid-beta by circulating lipoprotein receptors. Nat Med. 2007. September;13(9):1029–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagoe D, Molde H, Andreassen CS, Torsheim T, Pallesen S. The global epidemiology of anabolicandrogenic steroid use: a meta-analysis and meta-regression analysis. Ann Epidemiol. 2014. May;24(5):383–98. doi: 10.1016/j.annepidem.2014.01.009. [DOI] [PubMed] [Google Scholar]
- Sagoe D, McVeigh J, Bjørnebekk A, Essilfie MS, Andreassen CS, Pallesen S. Polypharmacy among anabolic-androgenic steroid users: a descriptive metasynthesis. Subst Abuse Treat Prev Policy. 2015. March 15;10:12. doi: 10.1186/s13011-015-0006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar M, Rojo AI, Velasco D, de Sagarra RM, Cuadrado A. Glycogen synthase kinase-3beta inhibits the xenobiotic and antioxidant cell response by direct phosphorylation and nuclear exclusion of the transcription factor Nrf2. J Biol Chem. 2006. May 26;281(21):14841–51. [DOI] [PubMed] [Google Scholar]
- Salloway S, Sperling R, Keren R, Porsteinsson AP, van Dyck CH, Tariot PN, Gilman S, Arnold D, Abushakra S, Hernandez C, Crans G, Liang E, Quinn G, Bairu M, Pastrak A, Cedarbaum JM; ELND005-AD201 Investigators. A phase 2 randomized trial of ELND005, scyllo-inositol, in mild to moderate Alzheimer disease. Neurology 2011. September 27;77(13):1253–62. doi: 10.1212/WNL.0b013e3182309fa5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen EK, Portin RI, Koskinen AI, Helenius HY, Nurmi MJ. Estradiol and cognition during androgen deprivation in men with prostate carcinoma. Cancer 2005. April 1;103(7):1381–7. [DOI] [PubMed] [Google Scholar]
- Samarghandian S, Azimi-Nezhad M, Afshari R, Farkhondeh T, Karimnezhad F. Effects of buprenorphine on balance of oxidant/antioxidant system in the different ages of male rat liver. J Biochem Mol Toxicol. 2015. June;29(6):249–53. doi: 10.1002/jbt.21691. [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ, Kim JH, Wasserman MA. Testosterone modulates performance on a spatial working memory task in male rats. Horm Behav. 2006. June;50(1):18–26. [DOI] [PubMed] [Google Scholar]
- Sandhya CV, Sreekumari RG, Vidhukumar K. Higher oxidative stress in current alcoholics compared to abstinent alcoholics and normal controls: a cross-sectional study. J. Evol. Med. Dental Sci. 2016;5(37):2221–5. [Google Scholar]
- Santora LJ, Marin J, Vangrow J, Minegar C, Robinson M, Mora J, Friede G. Coronary calcification in body builders using anabolic steroids. Prev Cardiol. 2006. Fall;9(4):198–201. [DOI] [PubMed] [Google Scholar]
- Sarma MK, Nagarajan R, Macey PM, Kumar R, Villablanca JP, Furuyama J, Thomas MA. Accelerated echo-planar J-resolved spectroscopic imaging in the human brain using compressed sensing: a pilot validation in obstructive sleep apnea. AJNR Am J Neuroradiol. 2014. June;35(6 Suppl):S81–9. doi: 10.3174/ajnr.A3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnyai Z, Dhabhar FS, McEwen BS, Kreek MJ. Neuroendocrine-related effects of long-term, ‘binge’ cocaine administration: diminished individual differences in stress-induced corticosterone response. Neuroendocrinology 1998. November;68(5):334–44. [DOI] [PubMed] [Google Scholar]
- Schneider MP, Delles C, Schmidt BM, Oehmer S, Schwarz TK, Schmieder RE, John S. Superoxide scavenging effects of N-acetylcysteine and vitamin C in subjects with essential hypertension. Am J Hypertens. 2005. August;18(8):1111–7. [DOI] [PubMed] [Google Scholar]
- Schwarzinger M, Pollock BG, Hasan OSM, Dufouil C, Rehm J; QalyDays Study Group. Contribution of alcohol use disorders to the burden of dementia in France 2008–13: a nationwide retrospective cohort study. Lancet Public Health 2018. March;3(3):e124–e132. doi: 10.1016/S2468-2667(18)30022-7. [DOI] [PubMed] [Google Scholar]
- Schwarzman AL, Gregori L, Vitek MP, Lyubski S, Strittmatter WJ, Enghilde JJ, Bhasin R, Silverman J, Weisgraber KH, Coyle PK, et al. Transthyretin sequesters amyloid beta protein and prevents amyloid formation. Proc Natl Acad Sci U S A 1994. August 30;91(18):8368–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepulcre J, Sabuncu MR, Becker A, Sperling R, Johnson KA. In vivo characterization of the early states of the amyloid-beta network. Brain 2013. July;136(Pt 7):2239–52. doi: 10.1093/brain/awt146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepulcre J, Grothe MJ, d’Oleire Uquillas F, Ortiz-Terán L, Diez I, Yang HS, Jacobs HIL, Hanseeuw BJ, Li Q, El-Fakhri G, Sperling RA, Johnson KA. Neurogenetic contributions to amyloid beta and tau spreading in the human cortex. Nat Med. 2018. December;24(12):1910–1918. doi: 10.1038/s41591-018-0206-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshadri S, Beiser A, Selhub J, Jacques PF, Rosenberg IH, D’Agostino RB, Wilson PW, Wolf PA. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N Engl J Med. 2002. February 14;346(7):476–83. [DOI] [PubMed] [Google Scholar]
- Shang G, Tang X, Gao P, Guo F, Liu H, Zhao Z, Chen Q, Jiang T, Zhang N, Li H. Sulforaphane attenuation of experimental diabetic nephropathy involves GSK-3 beta/Fyn/Nrf2 signaling pathway. J Nutr Biochem. 2015. June;26(6):596–606. doi: 10.1016/j.jnutbio.2014.12.008. [DOI] [PubMed] [Google Scholar]
- Shao H, Breitner JC, Whitmer RA, Wang J, Hayden K, Wengreen H, Corcoran C, Tschanz J, Norton M, Munger R, Welsh-Bohmer K, Zandi PP; Cache County Investigators. Hormone therapy and Alzheimer disease dementia: new findings from the Cache County Study. Neurology. 2012. October 30;79(18):1846–52. doi: 10.1212/WNL.0b013e318271f823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma RA, Varga AW, Bubu OM, Pirraglia E, Kam K, Parekh A, Wohlleber M, Miller MD, Andrade A, Lewis C, Tweardy S, Buj M, Yau PL, Sadda R, Mosconi L, Li Y, Butler T, Glodzik L, Fieremans E, Babb JS, Blennow K, Zetterberg H, Lu SE, Badia SG, Romero S, Rosenzweig I, Gosselin N, Jean-Louis G, Rapoport DM, de Leon MJ, Ayappa I, Osorio RS. Obstructive Sleep Apnea Severity Affects Amyloid Burden in Cognitively Normal Elderly. A Longitudinal Study. Am J Respir Crit Care Med. 2018. April 1;197(7):933–943. doi: 10.1164/rccm.201704-0704OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen WW, Zhang YS, Li LH, Liu Y, Huang XN, Chen LH, Zhou W. Long-term use of methamphetamine disrupts the menstrual cycles and hypothalamic-pituitary-ovarian axis. J Addict Med. 2014. May-Jun;8(3):183–8. doi: 10.1097/ADM.0000000000000021. [DOI] [PubMed] [Google Scholar]
- Shi X, Miller JS, Harper LJ, Poole RL, Gould TJ, Unterwald EM. Reactivation of cocaine reward memory engages the Akt/GSK3/mTOR signaling pathway and can be disrupted by GSK3 inhibition. Psychopharmacology (Berl). 2014. August;231(16):3109–18. doi: 10.1007/s00213-014-3491-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata M, Yamada S, Kumar SR, Calero M, Bading J, Frangione B, Holtzman DM, Miller CA, Strickland DK, Ghiso J, Zlokovic BV. Clearance of Alzheimer’s amyloid-ss(1–40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J Clin Invest. 2000. December;106(12):1489–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin SY, Chin BR, Lee YH, Kim JH. Involvement of glycogen synthase kinase-3beta in hydrogen peroxide-induced suppression of Tcf/Lef-dependent transcriptional activity. Cell Signal. 2006. May;18(5):601–7. [DOI] [PubMed] [Google Scholar]
- Shinall H, Song ES, Hersh LB. Susceptibility of amyloid beta peptide degrading enzymes to oxidative damage: a potential Alzheimer’s disease spiral. Biochemistry 2005. November 22;44(46):15345–50. [DOI] [PubMed] [Google Scholar]
- Shirafuji N, Hamano T, Yen SH, Kanaan NM, Yoshida H, Hayashi K, Ikawa M, Yamamura O, Kuriyama M, Nakamoto Y. Homocysteine Increases Tau Phosphorylation, Truncation and Oligomerization. Int J Mol Sci. 2018. March 17;19(3). pii: E891. doi: 10.3390/ijms19030891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shokri-Kojori E, Wang GJ, Wiers CE, Demiral SB, Guo M, Kim SW, Lindgren E, Ramirez V, Zehra A, Freeman C, Miller G, Manza P, Srivastava T, De Santi S, Tomasi D, Benveniste H, Volkow ND. β-Amyloid accumulation in the human brain after one night of sleep deprivation. Proc Natl Acad Sci U S A. 2018. April 24;115(17):4483–4488. doi: 10.1073/pnas.1721694115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva RH, Abílio VC, Takatsu AL, Kameda SR, Grassl C, Chehin AB, Medrano WA, Calzavara MB, Registro S, Andersen ML, Machado RB, Carvalho RC, Ribeiro Rde A, Tufik S, Frussa-Filho R. Role of hippocampal oxidative stress in memory deficits induced by sleep deprivation in mice. Neuropharmacology 2004. May;46(6):895–903. [DOI] [PubMed] [Google Scholar]
- Silva CS, Eira J, Ribeiro CA, Oliveira Â, Sousa MM, Cardoso I, Liz MA. Transthyretin neuroprotection in Alzheimer’s disease is dependent on proteolysis. Neurobiol Aging 2017. November;59:10–14. doi: 10.1016/j.neurobiolaging.2017.07.002. [DOI] [PubMed] [Google Scholar]
- Singh R, Kiloung J, Singh S, Sharma D. Effect of paradoxical sleep deprivation on oxidative stress parameters in brain regions of adult and old rats. Biogerontology 2008. June;9(3):153–62. doi: 10.1007/s10522-008-9124-z. [DOI] [PubMed] [Google Scholar]
- Sinha M, Saha A, Basu S, Pal K, Chakrabarti S. Aging and antioxidants modulate rat brain levels of homocysteine and dehydroepiandrosterone sulphate (DHEA-S): implications in the pathogenesis of Alzheimer’s disease. Neurosci Lett. 2010. October 11;483(2):123–6. doi: 10.1016/j.neulet.2010.07.075. [DOI] [PubMed] [Google Scholar]
- Sinha M, Bir A, Banerjee A, Bhowmick P, Chakrabarti S. Multiple mechanisms of age-dependent accumulation of amyloid beta protein in rat brain: Prevention by dietary supplementation with N-acetylcysteine, α-lipoic acid and α-tocopherol. Neurochem Int. 2016. May;95:92–9. doi: 10.1016/j.neuint.2015.10.003. [DOI] [PubMed] [Google Scholar]
- Skarberg K, Nyberg F, Engstrom I. Multisubstance use as a feature of addiction to anabolic-androgenic steroids. Eur Addict Res. 2009;15(2):99–106. doi: 10.1159/000199045. [DOI] [PubMed] [Google Scholar]
- Skogastierna C, Hotzen M, Rane A, Ekström L. A supraphysiological dose of testosterone induces nitric oxide production and oxidative stress. Eur J Prev Cardiol. 2014. August;21(8):1049–54. doi: 10.1177/2047487313481755. [DOI] [PubMed] [Google Scholar]
- Smith CD, Carney JM, Starke-Reed PE, Oliver CN, Stadtman ER, Floyd RA, Markesbery WR. Excess brain protein oxidation and enzyme dysfunction in normal aging and in Alzheimer disease. Proc Natl Acad Sci U S A 1991. December 1;88(23):10540–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth B, Fan J, Hser YI. Life expectancy and productivity loss among narcotics addicts thirty-three years after index treatment. J Addict Dis. 2006;25(4):37–47. [DOI] [PubMed] [Google Scholar]
- Song X, Mitnitski A, Rockwood K. Age-related deficit accumulation and the risk of late-life dementia. Alzheimers Res Ther. 2014. September 18;6(5–8):54. doi: 10.1186/s13195-014-0054-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontag E, Luangpirom A, Hladik C, Mudrak I, Ogris E, Speciale S, White CL 3rd. Altered expression levels of the protein phosphatase 2A ABalphaC enzyme are associated with Alzheimer disease pathology. J Neuropathol Exp Neurol. 2004. April;63(4):287–301. [DOI] [PubMed] [Google Scholar]
- Sontag E, Nunbhakdi-Craig V, Sontag JM, Diaz-Arrastia R, Ogris E, Dayal S, Lentz SR, Arning E, Bottiglieri T. Protein phosphatase 2A methyltransferase links homocysteine metabolism with tau and amyloid precursor protein regulation. J Neurosci. 2007. March 14;27(11):2751–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soykut B, Eken A, Erdem O, Akay C, Aydın A, Çetin MK, Dilbaz N. Oxidative stress enzyme status and frequency of micronuclei in heroin addicts in Turkey. Toxicol Mech Methods 2013. November;23(9):684–8. doi: 10.3109/15376516.2013.843106. [DOI] [PubMed] [Google Scholar]
- Spira AP, Gamaldo AA, An Y, Wu MN, Simonsick EM, Bilgel M, Zhou Y, Wong DF, Ferrucci L, Resnick SM. Self-reported sleep and β-amyloid deposition in community-dwelling older adults. JAMA Neurol. 2013. December;70(12):1537–43. doi: 10.1001/jamaneurol.2013.4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spritzer MD, Daviau ED, Coneeny MK, Engelman SM, Prince WT, Rodriguez-Wisdom KN. Effects of testosterone on spatial learning and memory in adult male rats. Horm Behav. 2011. April;59(4):484–96. doi: 10.1016/j.yhbeh.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkebaum G, Harlan JM. Endothelial cell injury due to copper-catalyzed hydrogen peroxide generation from homocysteine. J Clin Invest. 1986. April;77(4):1370–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter WJ, Weisgraber KH, Huang DY, Dong LM, Salvesen GS, Pericak-Vance M, Schmechel D, Saunders AM, Goldgaber D, Roses AD. Binding of human apolipoprotein E to synthetic amyloid beta peptide: isoform-specific effects and implications for late-onset Alzheimer disease. Proc Natl Acad Sci U S A 1993. September 1;90(17):8098–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su TP, Pagliaro M, Schmidt PJ, Pickar D, Wolkowitz O, Rubinow DR. Neuropsychiatric effects of anabolic steroids in male normal volunteers. JAMA 1993. June 2;269(21):2760–4. [PubMed] [Google Scholar]
- Süer C, Dolu N, Artis AS, Sahin L, Yilmaz A, Cetin A. The effects of long-term sleep deprivation on the long-term potentiation in the dentate gyrus and brain oxidation status in rats. Neurosci Res. 2011. May;70(1):71–7. doi: 10.1016/j.neures.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Suh SW, Jensen KB, Jensen MS, Silva DS, Kesslak PJ, Danscher G, Frederickson CJ. Histochemically-reactive zinc in amyloid plaques, angiopathy, and degenerating neurons of Alzheimer’s diseased brains. Brain Res. 2000. January 10;852(2):274–8. [DOI] [PubMed] [Google Scholar]
- Suh JH, Shenvi SV, Dixon BM, Liu H, Jaiswal AK, Liu RM, Hagen TM. Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proc Natl Acad Sci U S A. 2004. March 9;101(10):3381–6. Epub 2004 Feb 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan ML, Martinez CM, Gennis P, Gallagher EJ. The cardiac toxicity of anabolic steroids. Prog Cardiovasc Dis. 1998. Jul-Aug;41(1):1–15. [DOI] [PubMed] [Google Scholar]
- Sultana R, Banks WA, Butterfield DA. Decreased levels of PSD95 and two associated proteins and increased levels of BCl2 and caspase 3 in hippocampus from subjects with amnestic mild cognitive impairment: Insights into their potential roles for loss of synapses and memory, accumulation of Abeta, and neurodegeneration in a prodromal stage of Alzheimer’s disease. J Neurosci Res.2010. February 15;88(3):469–77. doi: 10.1002/jnr.22227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumathi T, Nathiya VC, Sakthikumar M. Protective Effect of Bacoside-A against Morphine-Induced Oxidative Stress in Rats. Indian J Pharm Sci. 2011. July;73(4):409–15. doi: 10.4103/0250-474X.95624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun AY, Ingelman-Sundberg M, Neve E, Matsumoto H, Nishitani Y, Minowa Y, Fukui Y, Bailey SM, Patel VB, Cunningham CC, Zima T, Fialova L, Mikulikova L, Popov P, Malbohan I, Janebova M, Nespor K, Sun GY. Ethanol and oxidative stress. Alcohol Clin Exp Res. 2001. May;25(5 Suppl ISBRA):237S–243S. [DOI] [PubMed] [Google Scholar]
- Sun XY, Wei YP, Xiong Y, Wang XC, Xie AJ, Wang XL, Yang Y, Wang Q, Lu YM, Liu R, Wang JZ. Synaptic released zinc promotes tau hyperphosphorylation by inhibition of protein phosphatase 2A (PP2A). J Biol Chem. 2012. March 30;287(14):11174–82. doi: 10.1074/jbc.M111.309070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surampudi P, Swerdloff RS, Wang C. An update on male hypogonadism therapy. Expert Opin Pharmacother. 2014. June;15(9):1247–64. doi: 10.1517/14656566.2014.913022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svartberg J, Jorde R. Endogenous testosterone levels and smoking in men. The fifth Tromsø study. Int J Androl. 2007. June;30(3):137–43. [DOI] [PubMed] [Google Scholar]
- Swainson R, Hodges JR, Galton CJ, Semple J, Michael A, Dunn BD, Iddon JL, Robbins TW, Sahakian BJ. Early detection and differential diagnosis of Alzheimer’s disease and depression with neuropsychological tasks. Dement Geriatr Cogn Disord. 2001. Jul-Aug;12(4):265–80. [DOI] [PubMed] [Google Scholar]
- Tahernejad Z, Baghshani H, Rashidlamir A. Blood biochemical and oxidant/antioxidant alterations following stanozolol treatment along with resistance training in rats. Andrologia 2017. March;49(2)e12613. doi: 10.1111/and.12613. [DOI] [PubMed] [Google Scholar]
- Taipale H, Hamina A, Lampela P, Tanskanen A, Tiihonen J, Karttunen N, Tolppanen AM, Hartikainen S. Is Alzheimer’s Disease Associated with Previous Opioid Use? Pain Med. 2017. August 28. doi: 10.1093/pm/pnx210. [DOI] [PubMed] [Google Scholar]
- Tamagno E, Guglielmotto M, Aragno M, Borghi R, Autelli R, Giliberto L, Muraca G, Danni O, Zhu X, Smith MA, Perry G, Jo DG, Mattson MP, Tabaton M. Oxidative stress activates a positive feedback between the gamma- and beta-secretase cleavages of the beta-amyloid precursor protein. J Neurochem. 2008. February;104(3):683–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan RS, Scally MC. Anabolic steroid-induced hypogonadism--towards a unified hypothesis of anabolic steroid action. Med Hypotheses. 2009. June;72(6):723–8. doi: 10.1016/j.mehy.2008.12.042. [DOI] [PubMed] [Google Scholar]
- Tanehkar F, Rashidy-Pour A, Vafaei AA, Sameni HR, Haghighi S, Miladi-Gorji H, Motamedi F, Akhavan MM, Bavarsad K. Voluntary exercise does not ameliorate spatial learning and memory deficits induced by chronic administration of nandrolone decanoate in rats. Horm Behav. 2013. January;63(1):158–65. doi: 10.1016/j.yhbeh.2012.10.003. [DOI] [PubMed] [Google Scholar]
- Tanokashira D, Motoki K, Minegishi S, Hosaka A, Mamada N, Tamaoka A, Okada T, Lakshmana MK, Araki W. LRP1 Downregulates the Alzheimer’s β-Secretase BACE1 by Modulating Its Intraneuronal Trafficking. eNeuro. 2015. April 22;2(2). pii: 10.1523/ENEURO.0006-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayler H, Fraser T, Miners JS, Kehoe PG, Love S. Oxidative balance in Alzheimer’s disease: relationship to APOE, Braak tangle stage, and the concentrations of soluble and insoluble amyloid-β. J Alzheimers Dis. 2010;22(4):1363–73. doi: 10.3233/JAD-2010-101368. [DOI] [PubMed] [Google Scholar]
- Terwel D, Muyllaert D, Dewachter I, Borghgraef P, Croes S, Devijver H, Van Leuven F. Amyloid activates GSK-3beta to aggravate neuronal tauopathy in bigenic mice. Am J Pathol. 2008. March;172(3):786–98. doi: 10.2353/ajpath.2008.070904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiblin I, Garmo H, Garle M, Holmberg L, Byberg L, Michaëlsson K, Gedeborg R. Anabolic steroids and cardiovascular risk: A national population-based cohort study. Drug Alcohol Depend. 2015. July 1;152:87–92. doi: 10.1016/j.drugalcdep.2015.04.013. [DOI] [PubMed] [Google Scholar]
- Tobiansky DJ, Korol AM, Ma C, Hamden JE, Jalabert C, Tomm RJ, Soma KK. Testosterone and Corticosterone in the Mesocorticolimbic System of Male Rats: Effects of Gonadectomy and Caloric Restriction. Endocrinology 2018. January 1;159(1):450–464. doi: 10.1210/en.2017-00704. [DOI] [PubMed] [Google Scholar]
- Tofighi A, Shirpoor M, Ansari MHK, Shirpoor A, Zerehpoosh M. The effect of nandrolone treatment with and without enforced swimming on histological and biochemical changes in the heart and coronary artery of male rats. Anatol J Cardiol. 2017. March;17(3):176–183. doi: 10.14744/AnatolJCardiol.2016.7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tofighi A, Ahmadi S, Seyyedi SM, Shirpoor A, Kheradmand F, Gharalari FH. Nandrolone administration with or without strenuous exercise promotes overexpression of nephrin and podocin genes and induces structural and functional alterations in the kidneys of rats. Toxicol Lett. 2018. January 5;282:147–153. doi: 10.1016/j.toxlet.2017.10.015. [DOI] [PubMed] [Google Scholar]
- Tóthová L, Celec P, Ostatníková D, Okuliarová M, Zeman M, Hodosy J. Effect of exogenous testosterone on oxidative status of the testes in adult male rats. Andrologia 2013. December;45(6):417–23. doi: 10.1111/and.12032. [DOI] [PubMed] [Google Scholar]
- Trivedi MS, Holger D, Bui AT, Craddock TJA, Tartar JL. Short-term sleep deprivation leads to decreased systemic redox metabolites and altered epigenetic status. PLoS One 2017. July 24;12(7):e0181978. doi: 10.1371/journal.pone.0181978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotti D, Rossi D, Gjesdal O, Levy LM, Racagni G, Danbolt NC, Volterra A. Peroxynitrite inhibits glutamate transporter subtypes. J Biol Chem. 1996. March 15;271(11):5976–9. [DOI] [PubMed] [Google Scholar]
- Trotti D, Rizzini BL, Rossi D, Haugeto O, Racagni G, Danbolt NC, Volterra A. Neuronal and glial glutamate transporters possess an SH-based redox regulatory mechanism. Eur J Neurosci. 1997. June;9(6):1236–43. [DOI] [PubMed] [Google Scholar]
- Tugyan K, Ozbal S, Cilaker S, Kiray M, Pekcetin C, Ergur BU, Kumral A. Neuroprotective effect of erythropoietin on nandrolone decanoate-induced brain injury in rats. Neurosci Lett. 2013. January 15;533:28–33. doi: 10.1016/j.neulet.2012.10.004. [DOI] [PubMed] [Google Scholar]
- Turillazzi E, Neri M, Cerretani D, Cantatore S, Frati P, Moltoni L, Busardò FP, Pomara C, Riezzo I, Fineschi V. Lipid peroxidation and apoptotic response in rat brain areas induced by long-term administration of nandrolone: the mutual crosstalk between ROS and NF-kB. J Cell Mol Med. 2016. April;20(4):601–12. doi: 10.1111/jcmm.12748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udrisar DP, Wanderley MI, Porto RC, Cardoso CL, Barbosa MC, Camberos MC, Cresto JC. Androgenand estrogen-dependent regulation of insulin-degrading enzyme in subcellular fractions of rat prostate and uterus. Exp Biol Med (Maywood) 2005. July;230(7):479–86. [DOI] [PubMed] [Google Scholar]
- Ueda K, Takimoto E, Lu Q, Liu P, Fukuma N, Adachi Y, Suzuki R, Chou S, Baur W, Aronovitz MJ, Greenberg AS, Komuro I, Karas RH. Membrane-Initiated Estrogen Receptor Signaling Mediates Metabolic Homeostasis via Central Activation of Protein Phosphatase 2A. Diabetes 2018. August;67(8):1524–1537. doi: 10.2337/db17-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uys JD, Knackstedt L, Hurt P, Tew KD, Manevich Y, Hutchens S, Townsend DM, Kalivas PW. Cocaine-induced adaptations in cellular redox balance contributes to enduring behavioral plasticity. Neuropsychopharmacology. 2011. November;36(12):2551–60. doi: 10.1038/npp.2011.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uys JD, Mulholland PJ, Townsend DM. Glutathione and redox signaling in substance abuse. Biomed Pharmacother. 2014. July;68(6):799–807. doi: 10.1016/j.biopha.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Beld AW, de Jong FH, Grobbee DE, Pols HA, Lamberts SW. Measures of bioavailable serum testosterone and estradiol and their relationships with muscle strength, bone density, and body composition in elderly men. J Clin Endocrinol Metab. 2000. September;85(9):3276–82. [DOI] [PubMed] [Google Scholar]
- van der Vaart A, Meng X, Bowers MS, Batman AM, Aliev F, Farris SP, Hill JS, Green TA, Dick D; and the COGA Consortium, Wolstenholme JT, Miles MF. Glycogen synthase kinase 3 beta regulates ethanol consumption and is a risk factor for alcohol dependence. Neuropsychopharmacology 2018. December;43(13):2521–2531. doi: 10.1038/s41386-018-0202-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vafai SB, Stock JB. Protein phosphatase 2A methylation: a link between elevated plasma homocysteine and Alzheimer’s Disease. FEBS Lett. 2002. May 8;518(1–3):1–4. [DOI] [PubMed] [Google Scholar]
- Vanderheyden WM, Lim MM, Musiek ES, Gerstner JR. Alzheimer’s Disease and Sleep-Wake Disturbances: Amyloid, Astrocytes, and Animal Models. J Neurosci. 2018. March 21;38(12):2901–2910. doi: 10.1523/JNEUROSCI.1135-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilaki F, Tsitsimpikou C, Tsarouhas K, Germanakis I, Tzardi M, Kavvalakis M, Ozcagli E, Kouretas D, Tsatsakis AM. Cardiotoxicity in rabbits after long-term nandrolone decanoate administration. Toxicol Lett. 2016. January 22;241:143–51. doi: 10.1016/j.toxlet.2015.10.026. [DOI] [PubMed] [Google Scholar]
- Vekrellis K, Xilouri M, Emmanouilidou E, Stefanis L. Inducible over-expression of wild type alpha-synuclein in human neuronal cells leads to caspase-dependent non-apoptotic death. J Neurochem. 2009. June;109(5):1348–62. doi: 10.1111/j.1471-4159.2009.06054.x. [DOI] [PubMed] [Google Scholar]
- Veldhuizen S, Callaghan RC. Cause-specific mortality among people previously hospitalized with opioid-related conditions: a retrospective cohort study. Ann Epidemiol. 2014. August;24(8):620–4. doi: 10.1016/j.annepidem.2014.06.001. [DOI] [PubMed] [Google Scholar]
- Venâncio DP, Tufik S, Garbuio SA, da Nóbrega AC, de Mello MT. Effects of anabolic androgenic steroids on sleep patterns of individuals practicing resistance exercise. Eur J Appl Physiol. 2008. March;102(5):555–60. [DOI] [PubMed] [Google Scholar]
- Venkataraman A, Kalk N, Sewell G, Ritchie CW, Lingford-Hughes A. Alcohol and Alzheimer’s Disease-Does Alcohol Dependence Contribute to Beta-Amyloid Deposition, Neuroinflammation and Neurodegeneration in Alzheimer’s Disease? Alcohol Alcohol. 2017. March 9;52(2):151–158. doi: 10.1093/alcalc/agw092. Review. Erratum in: Alcohol Alcohol. 2017 Mar 9;52(2):158. [DOI] [PubMed] [Google Scholar]
- Verdile G, Laws SM, Henley D, Ames D, Bush AI, Ellis KA, Faux NG, Gupta VB, Li QX, Masters CL, Pike KE, Rowe CC, Szoeke C, Taddei K, Villemagne VL, Martins RN; AIBL Research Group. Associations between gonadotropins, testosterone and β amyloid in men at risk of Alzheimer’s disease. Mol Psychiatry 2014. January;19(1):69–75. doi: 10.1038/mp.2012.147. [DOI] [PubMed] [Google Scholar]
- Verghese PB, Castellano JM, Garai K, Wang Y, Jiang H, Shah A, Bu G, Frieden C, Holtzman DM. ApoE influences amyloid-β (Aβ) clearance despite minimal apoE/Aβ association in physiological conditions. Proc Natl Acad Sci U S A 2013. May 7;110(19):E1807–16. doi: 10.1073/pnas.1220484110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villemagne VL, Burnham S, Bourgeat P, Brown B, Ellis KA, Salvado O, Szoeke C, Macaulay SL, Martins R, Maruff P, Ames D, Rowe CC, Masters CL; Australian Imaging Biomarkers and Lifestyle (AIBL) Research Group. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study. Lancet Neurol. 2013. April;12(4):357–67. doi: 10.1016/S1474-4422(13)70044-9. [DOI] [PubMed] [Google Scholar]
- Vitcheva V, Simeonova R, Kondeva-Burdina M, Mitcheva M. Selective Nitric Oxide Synthase Inhibitor 7-Nitroindazole Protects against Cocaine-Induced Oxidative Stress in Rat Brain. Oxid Med Cell Longev. 2015;2015:157876. doi: 10.1155/2015/157876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voevodskaya O, Sundgren PC, Strandberg O, Zetterberg H, Minthon L, Blennow K, Wahlund LO, Westman E, Hansson O; Swedish BioFINDER study group. Myo-inositol changes precede amyloid pathology and relate to APOE genotype in Alzheimer disease. Neurology 2016. May 10;86(19):1754–61. doi: 10.1212/WNL.0000000000002672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelsberg-Ragaglia V, Schuck T, Trojanowski JQ, Lee VM. PP2A mRNA expression is quantitatively decreased in Alzheimer’s disease hippocampus. Exp Neurol. 2001. April;168(2):402–12. [DOI] [PubMed] [Google Scholar]
- Wahjoepramono EJ, Wijaya LK, Taddei K, Martins G, Howard M, de Ruyck K, Bates K, Dhaliwal SS, Verdile G, Carruthers M, Martins RN. Distinct effects of testosterone on plasma and cerebrospinal fluid amyloid-beta levels. J Alzheimers Dis. 2008. September;15(1):129–37. [DOI] [PubMed] [Google Scholar]
- Walker J, Winhusen T, Storkson JM, Lewis D, Pariza MW, Somoza E, Somoza V. Total antioxidant capacity is significantly lower in cocaine-dependent and methamphetamine-dependent patients relative to normal controls: results from a preliminary study. Hum Psychopharmacol. 2014. November;29(6):537–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker KA, Power MC, Gottesman RF. Defining the Relationship Between Hypertension, Cognitive Decline, and Dementia: a Review. Curr Hypertens Rep. 2017. March;19(3):24. doi: 10.1007/s11906-017-0724-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin KG, Wood RI. Anabolic-androgenic steroids impair set-shifting and reversal learning in male rats. Eur Neuropsychopharmacol. 2015. April;25(4):583–90. doi: 10.1016/j.euroneuro.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin C, Sholts SB, Österlund N, Luo J, Jarvet J, Roos PM, Ilag L, Gräslund A, Wärmländer SKTS. Alzheimer’s disease and cigarette smoke components: effects of nicotine, PAHs, and Cd(II), Cr(III), Pb(II), Pb(IV) ions on amyloid-β peptide aggregation. Sci Rep. 2017. October 31;7(1):14423. doi: 10.1038/s41598-017-13759-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waly MI, Kharbanda KK, Deth RC. Ethanol lowers glutathione in rat liver and brain and inhibits methionine synthase in a cobalamin-dependent manner. Alcohol Clin Exp Res. 2011. February;35(2):277–83. doi: 10.1111/j.1530-0277.2010.01343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SH, Shih YL, Kuo TC, Ko WC, Shih CM. Cadmium toxicity toward autophagy through ROS-activated GSK-3beta in mesangial cells. Toxicol Sci. 2009. March;108(1):124–31. doi: 10.1093/toxsci/kfn266. [DOI] [PubMed] [Google Scholar]
- Wang R, Malter JS, Wang DS. N-acetylcysteine prevents 4-hydroxynonenal- and amyloid-beta-induced modification and inactivation of neprilysin in SH-SY5Y cells. J Alzheimers Dis. 2010;19(1):179–89. doi: 10.3233/JAD-2010-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Liang H, Mao X, Liu W, Li M, Qiu S. Changes of transthyretin and clusterin after androgen ablation therapy and correlation with prostate cancer malignancy. Transl Oncol. 2012. April;5(2):124–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Fang H, Zhen Y, Xu G, Tian J, Zhang Y, Zhang D, Zhang G, Xu J, Zhang Z, Qiu M, Ma Y, Zhang H, Zhang X. Sulforaphane Prevents Neuronal Apoptosis and Memory Impairment in Diabetic Rats. Cell Physiol Biochem. 2016;39(3):901–7. doi: 10.1159/000447799. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhu Y, Wang L, Hou J, Gao Y, Shen L, Zhang J. Effects of chronic alcohol exposure on ischemia-reperfusion-induced acute kidney injury in mice: the role of β-arrestin 2 and glycogen synthase kinase 3. Exp Mol Med. 2017. June 23;49(6):e347. doi: 10.1038/emm.2017.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt G, Karl T. In vivo Evidence for Therapeutic Properties of Cannabidiol (CBD) for Alzheimer’s Disease. Front Pharmacol. 2017. February 3;8:20. doi: 10.3389/fphar.2017.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei M, Zhao B, Huo K, Deng Y, Shang S, Liu J, Li Y, Ma L, Jiang Y, Dang L, Chen C, Wei S, Zhang J, Yang H, Gao F, Qu Q. Sleep Deprivation Induced Plasma Amyloid-β Transport Disturbance in Healthy Young Adults. J Alzheimers Dis. 2017;57(3):899–906. doi: 10.3233/JAD-161213. [DOI] [PubMed] [Google Scholar]
- Welder AA, Robertson JW, Melchert RB. Toxic effects of anabolic-androgenic steroids in primary rat hepatic cell cultures. J Pharmacol Toxicol Methods 1995. August;33(4):187–95. [DOI] [PubMed] [Google Scholar]
- Westerman ME, Charchenko CM, Ziegelmann MJ, Bailey GC, Nippoldt TB, Trost L. Heavy Testosterone Use Among Bodybuilders: An Uncommon Cohort of Illicit Substance Users. Mayo Clin Proc. 2016. February;91(2):175–82. doi: 10.1016/j.mayocp.2015.10.027. [DOI] [PubMed] [Google Scholar]
- Westlye LT, Kaufmann T, Alnæs D, Hullstein IR, Bjørnebekk A. Brain connectivity aberrations in anabolic-androgenic steroid users. Neuroimage Clin. 2016. November 17;13:62–69. doi: 10.1016/j.nicl.2016.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettstein A. Uber die kunstliche Herstellung des Testikelhormonsullivs Testosteron. Schweiz Med Wochenschr 1935;16:912. [Google Scholar]
- Winhusen T, Walker J, Brigham G, Lewis D, Somoza E, Theobald J, Somoza V. Preliminary evaluation of a model of stimulant use, oxidative damage and executive dysfunction. Am J Drug Alcohol Abuse. 2013. July;39(4):227–34. doi: 10.3109/00952990.2013.798663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbourn CC, Metodiewa D. Reactivity of biologically important thiol compounds with superoxide and hydrogen peroxide. Free Radic Biol Med. 1999. August;27(3–4):322–8. [DOI] [PubMed] [Google Scholar]
- Wisniewski AB, Brown TT, John M, Frankowicz JK, Cofranceso J Jr, Golub ET, Ricketts EP, Dobs AS. Hypothalamic-pituitary-gonadal function in men and women using heroin and cocaine, stratified by HIV status. Gend Med. 2007. March;4(1):35–44. [DOI] [PubMed] [Google Scholar]
- Wolff V, Schlagowski AI, Rouyer O, Charles AL, Singh F, Auger C, Schini-Kerth V, Marescaux C, Raul JS, Zoll J, Geny B. Tetrahydrocannabinol induces brain mitochondrial respiratory chain dysfunction and increases oxidative stress: a potential mechanism involved in cannabis-related stroke. Biomed Res Int. 2015;2015:323706. doi: 10.1155/2015/323706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womersley JS, Townsend DM, Kalivas PW, Uys JD. Targeting Redox Regulation to Treat Substance Use Disorder using N-acetylcysteine. Eur J Neurosci. 2018. in press August 24. doi: 10.1111/ejn.14130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock EA, Lundahl LH, Stoltman JJ, Greenwald MK. Progression to regular heroin use: examination of patterns, predictors, and consequences. Addict Behav. 2015. June;45:287–93. doi: 10.1016/j.addbeh.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J Nutr. 2004. March;134(3):489–92. [DOI] [PubMed] [Google Scholar]
- Wu LT, Woody GE, Yang C, Mannelli P, Blazer DG. Differences in onset and abuse/dependence episodes between prescription opioids and heroin: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Subst Abuse Rehabil. 2011. May;2011(2):77–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Williams D, Walter GA, Thompson WE, Sidell N. Estrogen increases Nrf2 activity through activation of the PI3K pathway in MCF-7 breast cancer cells. Exp Cell Res. 2014. November 1;328(2):351–60. doi: 10.1016/j.yexcr.2014.08.030. [DOI] [PubMed] [Google Scholar]
- Xia M, Li X, Yang L, Ren J, Sun G, Qi S, Verkhratsky A, Li B. The ameliorative effect of fluoxetine on neuroinflammation induced by sleep deprivation. J Neurochem. 2018. December 9; 146(1):63–75. doi: 10.1111/jnc.14272. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Jing XP, Zhou XW, Wang XL, Yang Y, Sun XY, Qiu M, Cao FY, Lu YM, Liu R, Wang JZ. Zinc induces protein phosphatase 2A inactivation and tau hyperphosphorylation through Src dependent PP2A (tyrosine 307) phosphorylation. Neurobiol Aging 2013. March;34(3):745–56. doi: 10.1016/j.neurobiolaging.2012.07.003. [DOI] [PubMed] [Google Scholar]
- Xu B, Wang Z, Li G, Li B, Lin H, Zheng R, Zheng Q. Heroin-administered mice involved in oxidative stress and exogenous antioxidant-alleviated withdrawal syndrome. Basic Clin Pharmacol Toxicol. 2006. August;99(2):153–61. [DOI] [PubMed] [Google Scholar]
- Xu CM, Wang J, Wu P, Zhu WL, Li QQ, Xue YX, Zhai HF, Shi J, Lu L. Glycogen synthase kinase 3beta in the nucleus accumbens core mediates cocaine-induced behavioral sensitization. J Neurochem. 2009. December;111(6):1357–68. doi: 10.1111/j.1471-4159.2009.06414.x. [DOI] [PubMed] [Google Scholar]
- Xu CM, Wang J, Wu P, Xue YX, Zhu WL, Li QQ, Zhai HF, Shi J, Lu L. Glycogen synthase kinase 3β in the nucleus accumbens core is critical for methamphetamine-induced behavioral sensitization. J Neurochem. 2011. July;118(1):126–39. doi: 10.1111/j.1471-4159.2011.07281.x. [DOI] [PubMed] [Google Scholar]
- Xu H, Chen X, Wang J, Yang T, Liu N, Cheng J, Gao R, Liu J, Xiao H. Involvement of insulin signalling pathway in methamphetamine-induced hyperphosphorylation of Tau. Toxicology 2018. July 4;408:88–94. doi: 10.1016/j.tox.2018.07.002. [DOI] [PubMed] [Google Scholar]
- Yan LJ. Positive oxidative stress in aging and aging-related disease tolerance. Redox Biol. 2014;2:165–9. doi: 10.1016/j.redox.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Wu Q, Yuan C, Gao J, Xiao M, Gu M, Ding J, Hu G. Aquaporin-4 mediates astrocyte response to β-amyloid. Mol Cell Neurosci. 2012. April;49(4):406–14. doi: 10.1016/j.mcn.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Yao M, Nguyen TV, Rosario ER, Ramsden M, Pike CJ. Androgens regulate neprilysin expression: role in reducing beta-amyloid levels. J Neurochem. 2008. June 1;105(6):2477–88. doi: 10.1111/j.1471-4159.2008.05341.x. [DOI] [PubMed] [Google Scholar]
- Yao XQ, Li XC, Zhang XX, Yin YY, Liu B, Luo DJ, Wang Q, Wang JZ, Liu GP. Glycogen synthase kinase-3β regulates leucine-309 demethylation of protein phosphatase-2A via PPMT1 and PME-1. FEBS Lett. 2012. July 30;586(16):2522–8. doi: 10.1016/j.febslet.2012.06.018. [DOI] [PubMed] [Google Scholar]
- Yao PL, Zhuo S, Mei H, Chen XF, Li N, Zhu TF, Chen ST, Wang JM, Hou RX, Le YY. Androgen alleviates neurotoxicity of β-amyloid peptide (Aβ) by promoting microglial clearance of Aβ and inhibiting microglial inflammatory response to Aβ. CNS Neurosci Ther. 2017. November;23(11):855–865. doi: 10.1111/cns.12757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda M, Ide H, Furuya K, Yoshii T, Nishio K, Saito K, Isotani S, Kamiyama Y, Muto S, Horie S. Salivary 8-OHdG: a useful biomarker for predicting severe ED and hypogonadism. J Sex Med. 2008. June;5(6):1482–91. doi: 10.1111/j.1743-6109.2008.00821.x. [DOI] [PubMed] [Google Scholar]
- Yatin SM, Varadarajan S, Link CD, Butterfield DA. In vitro and in vivo oxidative stress associated with Alzheimer’s amyloid beta-peptide (1–42). Neurobiol Aging. 1999. May-Jun;20(3):325–30; [DOI] [PubMed] [Google Scholar]
- Yeap BB, Alfonso H, Chubb SA, Handelsman DJ, Hankey GJ, Norman PE, Flicker L. Reference ranges and determinants of testosterone, dihydrotestosterone, and estradiol levels measured using liquid chromatography-tandem mass spectrometry in a population-based cohort of older men. J Clin Endocrinol Metab. 2012. November;97(11):4030–9. doi: 10.1210/jc.2012-2265. [DOI] [PubMed] [Google Scholar]
- Yee A, Loh HS, Danaee M, Riahi S, Ng CG, Sulaiman AH. Plasma Testosterone and Sexual Function in Southeast Asian Men Receiving Methadone and Buprenorphine Maintenance Treatment. J Sex Med. 2018. February;15(2):159–166. doi: 10.1016/j.jsxm.2017.12.004. [DOI] [PubMed] [Google Scholar]
- Yesilova Z, Ozata M, Oktenli C, Sanisoglu SY, Erbil MK, Dagalp K. Effect of supraphysiologic doses of testosterone on fasting plasma total homocysteine concentrations in men with Klinefelter’s syndrome. Fertil Steril. 2004. May;81(5):1278–82. [DOI] [PubMed] [Google Scholar]
- Yi KD, Chung J, Pang P, Simpkins JW. Role of protein phosphatases in estrogen-mediated neuroprotection. J Neurosci. 2005. August 3;25(31):7191–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi KD, Cai ZY, Covey DF, Simpkins JW. Estrogen receptor-independent neuroprotection via protein phosphatase preservation and attenuation of persistent extracellular signal-regulated kinase 1/2 activation. J Pharmacol Exp Ther. 2008. March;324(3):1188–95. [DOI] [PubMed] [Google Scholar]
- Yu JG, Bonnerud P, Eriksson A, Stål PS, Tegner Y, Malm C. Effects of long term supplementation of anabolic androgen steroids on human skeletal muscle. PLoS One 2014. September 10;9(9):e105330. doi: 10.1371/journal.pone.0105330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, Wang JY, Yuan F, Xie KL, Yu YH, Wang GL. Glycogen synthase kinase-3β contributes to remifentanil-induced postoperative hyperalgesia via regulating N-methyl-D-aspartate receptor trafficking. Anesth Analg. 2013. February;116(2):473–81. doi: 10.1213/ANE.0b013e318274e3f1. [DOI] [PubMed] [Google Scholar]
- Yui D, Nishida Y, Nishina T, Mogushi K, Tajiri M, Ishibashi S, Ajioka I, Ishikawa K, Mizusawa H, Murayama S, Yokota T. Enhanced Phospholipase A2 Group 3 Expression by Oxidative Stress Decreases the Insulin-Degrading Enzyme. PLoS One 2015. December 4;10(12):e0143518. doi: 10.1371/journal.pone.0143518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yukutake Y, Hirano Y, Suematsu M, Yasui M. Rapid and reversible inhibition of aquaporin-4 by zinc. Biochemistry 2009. December 29;48(51):12059–61. doi: 10.1021/bi901762y. [DOI] [PubMed] [Google Scholar]
- Yun J, Lee Y, Yun K, Oh S. Bergenin decreases the morphine-induced physical dependence via antioxidative activity in mice. Arch Pharm Res. 2015. June;38(6):1248–54. doi: 10.1007/s12272-014-0534-y. [DOI] [PubMed] [Google Scholar]
- Yun J, Oliynyk S, Lee Y, Kim J, Yun K, Jeon R, Ryu JH, Oh S. Ajoene restored behavioral patterns and liver glutathione level in morphine treated C57BL6 mice. Arch Pharm Res. 2017. January;40(1):106–111. doi: 10.1007/s12272-016-0773-1. [DOI] [PubMed] [Google Scholar]
- Zaparte A, Viola TW, Grassi-Oliveira R, da Silva Morrone M, Moreira JC, Bauer ME. Early abstinence of crack-cocaine is effective to attenuate oxidative stress and to improve antioxidant defences. Psychopharmacology (Berl). 2015. April;232(8):1405–13. doi: 10.1007/s00213-014-3779-8. [DOI] [PubMed] [Google Scholar]
- Zeng T, Zhang CL, Song FY, Zhao XL, Xie KQ. CMZ reversed chronic ethanol-induced disturbance of PPAR-α possibly by suppressing oxidative stress and PGC-1α acetylation, and activating the MAPK and GSK3β pathway. PLoS One 2014. June 3;9(6):e98658. doi: 10.1371/journal.pone.0098658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CE, Tian Q, Wei W, Peng JH, Liu GP, Zhou XW, Wang Q, Wang DW, Wang JZ. Homocysteine induces tau phosphorylation by inactivating protein phosphatase 2A in rat hippocampus. Neurobiol Aging 2008. November;29(11):1654–65. Epub 2007 May 29. [DOI] [PubMed] [Google Scholar]
- Zhang GL, Wang W, Kang YX, Xue Y, Yang H, Zhou CM, Shi GM. Chronic testosterone propionate supplement could activated the Nrf2-ARE pathway in the brain and ameliorated the behaviors of aged rats. Behav Brain Res. 2013. September 1;252:388–95. doi: 10.1016/j.bbr.2013.05.063. [DOI] [PubMed] [Google Scholar]
- Zhang H, Davies KJA, Forman HJ. Oxidative stress response and Nrf2 signaling in aging. Free Radic Biol Med. 2015. November;88(Pt B):314–336. doi: 10.1016/j.freeradbiomed.2015.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Li S, Kang Y, Che J, Cui R, Song S, Cui H, Shi G. Enhancement of dopaminergic activity and region-specific activation of Nrf2-ARE pathway by intranasal supplements of testosterone propionate in aged male rats. Horm Behav. 2016. April;80:103–116. doi: 10.1016/j.yhbeh.2016.02.001. [DOI] [PubMed] [Google Scholar]
- Zhang K, Zhang Q, Jiang H, Du J, Zhou C, Yu S, Hashimoto K, Zhao M. Impact of aerobic exercise on cognitive impairment and oxidative stress markers in methamphetamine-dependent patients. Psychiatry Res. 2018. August;266:328–333. doi: 10.1016/j.psychres.2018.03.032. [DOI] [PubMed] [Google Scholar]
- Zhao J, Moore AN, Clifton GL, Dash PK. Sulforaphane enhances aquaporin-4 expression and decreases cerebral edema following traumatic brain injury. J Neurosci Res. 2005. November 15;82(4):499–506. [DOI] [PubMed] [Google Scholar]
- Zhao L, Buxbaum JN, Reixach N. Age-related oxidative modifications of transthyretin modulate its amyloidogenicity. Biochemistry 2013. March 19;52(11):1913–26. doi: 10.1021/bi301313b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao K, Luan X, Liu Z, Zhu Z, Chen H, Shen H, Cai Y, Qiu H, Wang Q, Gu Y, Zhu L, He J. Low serum uric acid levels in chronic insomnia patients: A case-control study. Neurosci Lett. 2017. September 14;657:102–105. doi: 10.1016/j.neulet.2017.08.015. [DOI] [PubMed] [Google Scholar]
- Zhong G, Wang Y, Zhang Y, Guo JJ, Zhao Y. Smoking is associated with an increased risk of dementia: a meta-analysis of prospective cohort studies with investigation of potential effect modifiers. PLoS One 2015. March 12;10(3):e0118333. doi: 10.1371/journal.pone.0118333. Erratum in: PLoS One2015;10(4):e0126169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou JF, Yan XF, Ruan ZR, Peng FY, Cai D, Yuan H, Sun L, Ding DY, Xu SS. Heroin abuse and nitric oxide, oxidation, peroxidation, lipoperoxidation. Biomed Environ Sci. 2000. June;13(2):131–9. [PubMed] [Google Scholar]
- Zhu X, Lee HG, Casadesus G, Avila J, Drew K, Perry G, Smith MA. Oxidative imbalance in Alzheimer’s disease. Mol Neurobiol. 2005;31(1–3):205–17. [DOI] [PubMed] [Google Scholar]
- Zou Y, Hong B, Fan L, Zhou L, Liu Y, Wu Q, Zhang X, Dong M. Protective effect of puerarin against beta-amyloid-induced oxidative stress in neuronal cultures from rat hippocampus: involvement of the GSK-3β/Nrf2 signaling pathway. Free Radic Res. 2013. January;47(1):55–63. doi: 10.3109/10715762.2012.742518. [DOI] [PubMed] [Google Scholar]