Abstract
Fabry disease is an X-linked lysosomal storage disease caused by loss of alpha galactosidase A (α-Gal A) activity and is characterized by progressive accumulation of globotriaosylceramide and its analogs in all cells and tissues. Although enzyme replacement therapy (ERT) is considered standard of care, the long-term effects of ERT on renal and cardiac manifestations remain uncertain and thus novel therapies are desirable. We herein report preclinical studies evaluating systemic messenger RNA (mRNA) encoding human α-Gal A in wild-type (WT) mice, α-Gal A-deficient mice, and WT non-human primates (NHPs). The pharmacokinetics and distribution of h-α-Gal A mRNA encoded protein in WT mice demonstrated prolonged half-lives of α-Gal A in tissues and plasma. Single intravenous administration of h-α-Gal A mRNA to Gla-deficient mice showed dose-dependent protein activity and substrate reduction. Moreover, long duration (up to 6 weeks) of substrate reductions in tissues and plasma were observed after a single injection. Furthermore, repeat i.v. administration of h-α-Gal A mRNA showed a sustained pharmacodynamic response and efficacy in Fabry mice model. Lastly, multiple administrations to non-human primates confirmed safety and translatability. Taken together, these studies across species demonstrate preclinical proof-of-concept of systemic mRNA therapy for the treatment of Fabry disease and this approach may be useful for other lysosomal storage disorders.
Keywords: α-Gal A, mRNA, Fabry, Gb3, lyso-Gb3, enzyme replacement therapy, ERT, gene therapy, lipid nanoparticles, LNPs
Introduction
Fabry disease (MIM: 301500) is an X-linked lysosomal storage disorder caused by mutations in GLA (MIM: 300644) which encodes the enzyme alpha galactosidase A (α-Gal A). Deficiency of α-Gal A results in the accumulation of glycosphingolipids, particularly globotriaosylceramide (Gb3) and the deacylated Gb3 analog globotriaosylsphingosine (lyso-Gb3) in most cell types.1, 2 Recently, lyso-Gb3 has been reported to be a useful biomarker of Fabry disease.3, 4, 5, 6, 7 It is markedly elevated in both classic male and symptomatic female Fabry-affected individuals relative to the level of Gb3 in affected tissues. Over time, accumulation of glycosphingolipids triggers cellular dysfunction and progressive damage in affected tissues such as kidney, heart, and skin.8, 9, 10, 11 Individuals with Fabry disease present clinically with a spectrum of disease severity that directly correlates with the level of the residual enzyme activity. Individuals with the early-onset or classic phenotype during childhood or adolescence typically have less than 1% of residual α-Gal A activity and often present with symptoms such as angiokeratoma, acroparesthesias, corneal and lenticular opacities, and hypohidrosis.10, 11
Fabry disease is the most common lysosomal storage disease with reported prevalence of 1:40,000 to 1:117,000.9, 12, 13 The cardiac variant of Fabry disease affects 1:50,000 individuals.8 However, newborn screening assessments have found that the screen positive rate for mutations in the GLA ranges from 1:3,000 to 1:10,000, indicating that Fabry disease might be significantly underdiagnosed.14, 15, 16 Currently there are several commercially available treatments for Fabry disease including enzyme replacement therapies (ERTs) such as Fabrazyme (Genzyme Sanofi) and Replagal (Shire) as well as a small molecule chaperone therapy (CT) such as Galafold (Amicus Therapeutics). While availability of these treatments depends on geographical location, most individuals have access to one or more of these therapies. Aside from currently available therapies there are several treatment modalities currently in preclinical or clinical development for Fabry disease including ERT, gene therapy (GT), substrate reduction therapy (SRT), and combinatory modalities such as CT or SRT with ERT therapy. The standard of care for Fabry disease is ERT. ERT has been shown to have clinical benefit in Fabry-affected individuals, alleviating clinical symptoms such as neuropathic pain and gastrointestinal symptoms as well as normalizing plasma Gb3 levels and improving renal and cardiac manifestations in some individuals.12, 17 However, long-term evaluation of Fabry-affected individuals receiving ERT treatment demonstrates that despite clinical improvement some individuals still lose renal function at greater than normal rates and develop cardiac manifestations and experience infusion-associated reactions, which can be severe in some patients; thus an unmet medical need still exists for these individuals.18, 19, 20, 21
Messenger RNA treatment is emerging as a treatment modality that can treat a variety of diseases. Intravenous administration of mRNA formulated in lipid nanoparticles (LNPs) can produce therapeutic proteins to replace mutated or missing proteins within target cells such as hepatocytes. Therapeutic proteins made from exogenously administered mRNA in the body may mimic the endogenous target protein more closely than recombinant proteins, such as ERTs, which are manufactured from CHO, human, or plant cell lines. mRNA treatment produces transient protein levels, while avoiding genomic integration and the off-target risks of gene therapy or gene editing therapy.22, 23 Multiple mRNA-based cancer immunotherapies and vaccines are currently in clinical trials.24 Preclinical studies have evaluated mRNA-based therapy for various rare diseases. Recently we have reported preclinical proof-of-concept studies of mRNA therapy for liver diseases methylmalonic acidemia (MMA [MIM: 251000]) and acute intermittent porphyria (AIP [MIM: 176000]). These studies demonstrated restoration of functional human methylmalonyl-CoA mutase (hMUT) and human porphobilinogen deaminase (hPBGD) in the liver by systemic mRNA treatment with corresponding amelioration of disease in animal models of MMA and AIP, respectively.25, 26
We herein report preclinical studies in different species evaluating a systemically delivered mRNA encoding human alpha galactosidase A (h-α-Gal A) for treatment of lysosomal storage disease-Fabry disease. Pharmacokinetics and biodistribution of h-α-Gal A were characterized after a single administration of LNP formulated mRNA in wild-type CD1 mice. We observed a prolonged half-life of h-α-Gal A in plasma, liver, kidney, and heart. A single intravenous (i.v.) administration of h-α-Gal A mRNA in Gla-deficient mice showed a dose-dependent restoration of h-α-Gal A activity with concomitant depletion of lyso-Gb3 in plasma and tissues (liver, kidney, heart, spleen). Moreover, lyso-Gb3 concentrations in plasma and tissues were significantly reduced 6 weeks after a single i.v. injection of h-α-Gal A mRNA in Fabry mice. Repeat i.v. administration (every other week and monthly) of h-α-Gal A mRNA in Fabry mice demonstrated significant substrate reduction (lyso-Gb3 and Gb3) in a dose-dependent manner. Administration of h-α-Gal A mRNA every other week in WT NHPs demonstrated sustained functional α-Gal A protein in plasma after each dose, with the absence of an immune responses to the protein encoded by the mRNA. Taken together, these preclinical proof-of-concept studies indicate that systemic mRNA therapy could be a potential treatment for Fabry disease.
Material and Methods
Animals
Wild-type CD1 mice were purchased from Charles River Laboratories. Fabry mice (B6;129-Glatm1Kul/J Stock 003535),27 were purchased from the Jackson Laboratory. All studies were conducted with ∼5-month-old male mice weighing between 20 and 40 g. Mice were provided standard mouse chow and water, ad libitum, and were housed in a facility providing 12 h light/dark cycles. Protocols for animal studies were reviewed and approved by the Moderna Institutional Animal Care and Use Committee (IACUC).
NHP studies were performed at Charles River Laboratories. The study and procedures involving the care and use of animals was reviewed and approved by Charles River Laboratory IACUC. The care and use of animals were conducted in accordance with the guidelines of the USA National Research Council and the Canadian Council on Animal Care.
mRNA and Lipid Nanoparticle Synthesis and Formulation
mRNA encoding eGFP and h-α-Gal A were synthesized in vitro by T7 RNA polymerase-mediated transcription where uridine was fully replaced with 5-methoxyuridine.25, 28, 29 LNP formulations were prepared by ethanol-drop nanoprecipitation as previously described.30 Briefly, lipids dissolved in ethanol at a molar ratio of 50:10:38.5:1.5 (ionizable:helper:structural:PEG) were mixed with acidified mRNA at a ratio of 3:1 (lipid:mRNA). Formulations were dialyzed against phosphate-buffered saline (pH 7.4) in dialysis cassettes for at least 18 h, concentrated using Amicon ultra centrifugal filters (EMD Millipore), passed through a 0.22-μm filter, and stored at 4°C until use. All formulations were tested for particle size, RNA encapsulation, and endotoxin and were found to be between 80 nm and 100 nm in size, with greater than 90% encapsulation and <10 EU/mL endotoxin.
α-Gal A Activity Assay
α-Gal A activity was determined as described previously.31 Briefly, animal tissue lysates or plasma were mixed with 2 mM 4-Methylum-belliferyl-α-D-galactopyranoside (or 4MU-α-Gal) and 50 mM N-acetyl galactosamine in 100 mM sodium citrate buffer (pH 4.6). The reaction mixture was incubated in 37°C for half an hour. The reaction was stopped by adding Glycine-NaOH (500 mM) buffer (pH 10.5). α-Gal A activity was expressed as nmol/h/mg total protein or nmol/h/mL plasma.
Immunohistochemistry (IHC)
Immunohistochemistry was performed on Formalin-Fixed Paraffin Embedded (FFPE) sections using the Leica Bond RX auto-stainer (Leica Microsystems). Sections were baked and deparaffinized on the instrument, followed by an epitope retrieval for 30 min using Leica Epitope Retrieval Buffer 2. Dako serum-free protein block (X090930-2, Agilent Dako) was incubated on the slides for 15 min at room temperature. Anti-Galactosidase alpha (α-Gal A) antibody (ab168341, Abcam plc) was used at 2.5 μg/mL at room temperature for 60 min. Secondary antibody detection was done using the Bond Polymer Refine Detection Kit (cat no. DS9800, Leica Microsystems). Bond DAB Enhancer (AR9432, Leica Microsystems) and bluing reagent (3802918, Leica Microsystems) were used to enhance the color.
All images were captured at 20× magnification with the Pannoramic 250 Flash II (3DHISTECH) digital slide scanner.
Image analysis was done using Halo software (Indica Labs).
Biomarker Analysis
Globotriaosylceramide (Gb3) and globotriaosylsphingosine (lyso-Gb3) were analyzed by LC-MS/MS as described.3, 6, 32 Gb3 concentration in tissues (area of Gb3/area of internal standard) was expressed as the total ion count of six Gb3 isoform signals (C16:0, C16:1, C18:0, C22:0, C24:1, C24:0) and expressed in nM in plasma. Globotriaosylsphingosine (lyso-Gb3) and analogs with m/z 786, m/z 784 (−2), m/z 802 (+16), m/z 804 (+18) were measured and expressed as pmol/g of tissue or nM in plasma.
The data from groups dosed with h-α-Gal A mRNA was normalized to that in age-matched control Fabry mice (represented 100% substrate load) and expressed as percentage of substrate (Gb3 or lyso-Gb3) of untreated. The control mice were dosed with either eGFP mRNA or PBS but without h-α-Gal A mRNA treatment (untreated control).
Anti-α-Gal A Assay
To quantify antibodies against α-Gal A protein, we coated Nunc Immuno Maxisorp plates (Thermo Scientific, #442404) with 20 ng recombinant human alphα-Galactosidase A-his protein (Sino Biological Inc, #12078-H08H) in 50 mM Na2CO3 and blocked overnight at 4°C. Mouse serum samples at a 1:50 dilution or cynomolgus serum samples at a 1:200 dilution were incubated 1 h at room temperature. Rabbit anti-human α-Gal A IgG (LifeSpan BioSciences, #LS-B11102) at 0 to 400 ng was used as a standard curve. We used goat anti-rabbit IgG-HRP (Abcam, #ab6721) diluted to 1:100,000, goat anti-mouse IgG H+L-HRP (Fitzgerald Laboratories, #43-GM30) diluted to 1:100,000, and goat anti-monkey IgG H+L-HRP (Fitzgerald Laboratories, #43C-CB1603) diluted to 1:50,000 as secondary antibodies. Secondary antibodies were incubated at room temperature for 1 h prior to development with TMB substrate (Cell Signaling Technology, #7004S).
Quantification of Protein by LC-MS/MS
Absolute quantification of h-α-Gal A protein was measured by LC-MS/MS (Thermo Easy 1200 nano-LC and Thermo Q Exactive Plus Hybrid Quadrupole-Orbitrap mass spectrometer). Human α-Gal A-specific peptide FDGCYCDSLENLADGYK was selected as the signature peptide for quantification of h-α-Gal A. Briefly, tissues were homogenized in a buffer containing 100 mM ammonium bicarbonate and 8 M urea. Each sample was spiked with isotopically labeled signature peptide (FDGCYCDSLENLADGYK∗, natural C and N atoms on lysine∗ are fully replaced by 13C and 15N isotopes, respectively) as internal standard. Protein in samples with internal standard peptide were reduced, alkylated, and trypsinized. Samples were cleaned up before loading to LC-MS/MS for analysis.
Statistical Analysis
Data are expressed as means ± SD or means ± SEM as specified in the figure legend. Means were compared by unpaired t test for comparing two groups or one-way ANOVA for several group comparisons. Non-compartmental analysis was used to estimate the half-life of mRNA encoded h-α-Gal A in tissues. p values < 0.05 were considered statistically significant. Statistical analysis was performed using Prism 7 (GraphPad) software.
Results
Pharmacokinetics and Biodistribution of h-α-Gal A Protein Encoded by h-α-Gal A mRNA in Wild-Type Mice
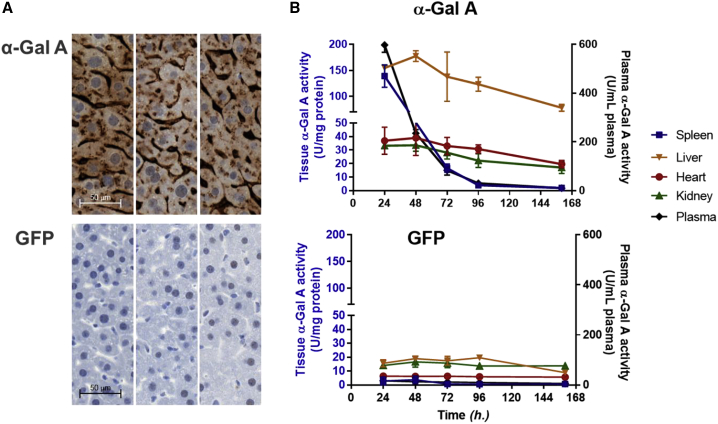
Functional h-α-Gal A protein encoded by exogenously administered mRNA was tested in WT mice. Up to 20 different codon-optimized h-α-Gal A mRNA constructs as well as unoptimized constructs encapsulated in lipid nanoparticles (LNPs) were administrated in CD1 mice (n = 3) for selection of lead mRNA constructs (Figure S1). A single mRNA construct was selected for further characterization based on α-Gal A activity level in liver at 24 h after dose (Figure S1, α-Gal A mRNA#17). Pharmacokinetic characterization of h-α-Gal A protein were also performed in CD1 mice (n = 3/time point). A single dose of 0.5 mg/kg h-α-Gal A mRNA or a control mRNA (enhanced green fluorescent protein, eGFP) encapsulated in LNPs were intravenously administrated to CD1 mice. Mice were sacrificed at 24, 48, 72, 96, and 160 h after mRNA-LNP administration. Tissues were collected and evaluated for h-α-Gal A localization and activity. Immunohistochemistry (IHC) staining of the liver samples collected at 48 h after dose showed strong h-α-Gal A protein in livers of mice treated with h-α-Gal A mRNA compared to those treated with eGFP mRNA (Figure 1A). The IHC analysis indicated that h-α-Gal A protein was in hepatocytes and sinusoidal endothelial cells. The tissue distribution of h-α-Gal A was determined by h-α-Gal A activity (Figure 1B). Activity analysis of α-Gal A demonstrated that the protein distributed to four tested tissues: liver, spleen, heart, and kidney. The Tmax of h-α-Gal A was 24–48 h after i.v. in liver, heart, and kidney. The half-lives of h-α-Gal A in these tissues were more than 100 h (Table S1). Relative to the control group, the highest h-α-Gal A activity was observed in the liver, spleen, and plasma which increased more than 8-fold while heart and kidney enzymatic activity increases were in the range of 2- to 3-fold at 24 h after dose of h-α-Gal A mRNA (Figure 1B). Additionally, persistent h-α-Gal A activity was observed throughout the study (160 h after dose) in the liver, heart, and kidney tissues.
Figure 1.
Expression, Pharmacokinetics, and Distribution of h-α-Gal A Protein in CD1 Mice after a Single 0.5 mg/kg Intravenous Administration of mRNAs Encapsulated in LNPs
The mice (n = 3 per time point) received h-α-Gal A mRNA or eGFP mRNA as a vehicle control. Mice were sacrificed at 24, 48, 72, 96, and 160 h after injection.
(A) Representative images of immunohistochemistry (IHC) staining of h-α-Gal A protein in liver samples from mice injected with 0.5 mg/kg h-α-Gal A mRNA (top, n = 3) and eGFP mRNA (bottom, n = 3) 48 h after dose (scale 20× images).
(B) Kinetics and distribution of h-α-Gal A in plasma and tissues (liver, spleen, kidney, and heart) were measured by α-Gal A activity. Top: plasma and tissue α-Gal A activity from mice injected with 0.5 mg/kg h-α-Gal A mRNA (n = 3 per group) and 0.5 mg/kg eGFP mRNA (bottom, n = 3 per group). Tissue α-Gal A activity was expressed as U/mg of protein and plasma α-Gal A activity as U/mL of plasma, U = nmol/h. Results were plotted as mean ± SD.
See Table S1 for unpaired t test analysis to compare p values of α-Gal A-treated group with eGFP-treated group.
mRNA Expression, after a Single i.v. Administration, Results in a Dose-Dependent Production of h-α-Gal A Protein Levels, Activity, and Efficacy in Fabry Mice
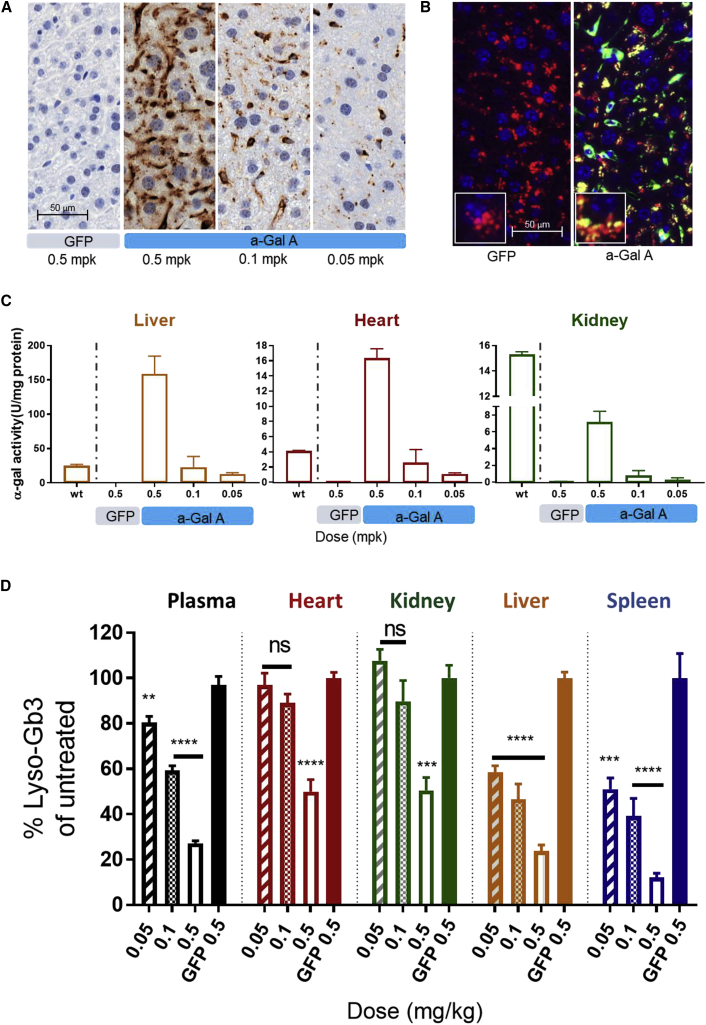
Evaluation of dose-responsive efficacy after systemic administration of h-α-Gal A mRNA was performed in Gla−/− mice (Fabry mice). Adult Gla−/− male mice have been used as a Fabry disease model for ERTs and gene therapies27, 33, 34, 35, 36, 37, 38, 39 to evaluate efficacy of potential treatments as they accumulate Gb3 and lyso-Gb3 in both plasma and tissues in a similar manner to Fabry disease-affected individuals. A single i.v. bolus dose of LNP encapsulated h-α-Gal A mRNA was injected into Fabry mice (n = 5, 20 weeks old, male) at three different dosages, 0.5, 0.1, and 0.05 mg/kg. Plasma was collected at pre-dose and multiple time points post dose. Various tissues were collected 72 h after injection. Human α-Gal A level in liver samples was dose responsive with the largest amount of protein observed in tissues from animals who received 0.5 mg/kg h-α-Gal A (Figure 2A). Cellular localization of h-α-Gal A protein was performed and as expected was found to localize in liver tissue lysosomes of the Fabry mice determined by co-localization h-α-Gal A and lysosomal marker Lamp2A immunofluorescence (Figure 2B). Consistent with h-α-Gal A protein level, h-α-Gal A activity also was dose responsive in liver, kidney, heart, spleen, and plasma (Figures 2C and S2). At 0.5 mg/kg, the activity of h-α-Gal A was significantly increased in the liver and in the heart of Fabry mice 72 h after injection compared to WT mouse α-Gal A activity. By contrast, the activity of h-α-Gal A in kidney 72 h after dose was 50% of WT mouse activity levels. Not surprisingly, the h-α-Gal A activity in plasma was more than 500-fold higher than levels in WT mice, 6 h after dose (Figure S2A). Activity increases were proportional with increases in protein levels as corresponding lyso-Gb3 depletion in tissues and plasma (Figure 2D). Lyso-Gb3 levels were reduced by 80% in liver and spleen isolated from animals that received 0.5 mg/kg h-α-Gal A mRNA and reductions in kidney and heart were greater than 50% compared to untreated control animals (eGFP group). Plasma, liver, and spleen isolated from Fabry mice that received mid and low doses (0.1 mg/kg and 0.05 mg/kg) of h-α-Gal A mRNA also had measurable reductions in lyso-Gb3 compared to control. However, kidney and heart isolated from low and mid dose group mice did not have significant reduction in substrate compared to untreated control (eGFP group).
Figure 2.
Expression, Lysosomal Co-localization, and Dose-Dependent Restoration of h-α-Gal A Activity and Depletion of Substrate in Plasma and Tissues from Fabry Mice after a Single 0.5, 0.1, or 0.05 mg/kg i.v. Administration of mRNA Encapsulated in LNPs
A control group of Fabry mice was administered one dosage of eGFP at 0.5 mg/kg. Fabry mice (n = 3–5 per group) were sacrificed 72 h after dose.
(A) Representative IHC images h-α-Gal A protein in liver of mice injected with 0.5 mg/kg eGFP mRNA (panel 1) and 0.5, 0.1, and 0.05 mg/kg h-α-Gal A mRNA (panels 2–4); scale 20× images.
(B) Co-localization of lysosomal marker Lamp2A with h-α-Gal A in liver sections from Fabry mice treated with 0.5 mg/kg of eGFP (left) or 0.5 mg/kg h-α-Gal A (right). Nuclei (Hoeschts) stains blue, h-α-Gal A green (Alexa 488), and Lamp2A red (Alexa 594), scale 20× main image with 40× inset image.
(C) h-α-Gal A activity in liver (left), heart (middle), and kidney (right) from Fabry mice dosed with either eGFP or h-α-Gal A mRNA at different dosages. Enzyme levels in tissue from Fabry mice were compared to tissues collected from WT mice.
(D) Lyso-Gb3 levels in tissues harvested from Fabry mice 3 days after single i.v. administration of mRNA. The data from groups dosed with h-α-Gal A mRNA was normalized to that in age-matched Fabry mice dosed with control eGFP mRNA (represented 100% substrate load) and expressed as lyso-Gb3% of untreated control level. Results were plotted as mean ± SD. p values were obtained from one-way ANOVA of multiple comparison with untreated control eGFP group. ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.0001.
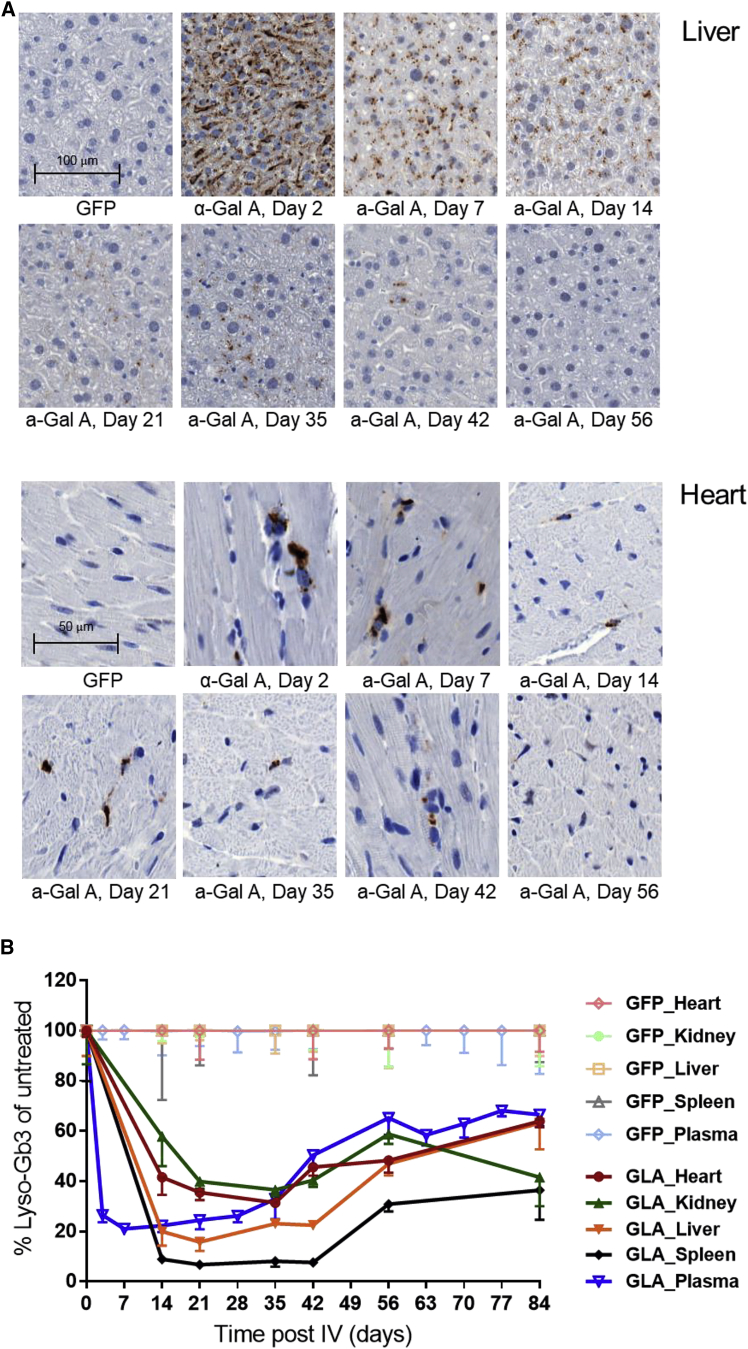
Sustained Duration of Action in Fabry Mice after a Single i.v. Administration of mRNA
To determine the duration of action of h-α-Gal A protein encoded by exogenously administered h-α-Gal A mRNA, a single 0.5 mg/kg dose of h-α-Gal A mRNA or eGFP mRNA encapsulated in LNPs were intravenously injected into Fabry mice. Plasma was collected at pre-dose and different time points after dose for substrate lyso-Gb3 analysis. Mice were taken down at different time points (from 2 week to 12 weeks) after a single i.v. injection of mRNA and tissues were collected for IHC and lyso-Gb3 analysis. IHC analysis of heart and liver samples from h-α-Gal A mRNA-treated Fabry mice demonstrated that while h-α-Gal A staining decreases over time it remains detectable 42 days after a single administration (Figure 3A). Enzyme h-α-Gal A activity was detectable in plasma, heart, and liver tissues at day 28 after dose in a pilot duration study (Figures S3A and S3B). Lyso-Gb3 depletion and re-accumulation rates were tissue specific. When compared with the lyso-Gb3 level in control eGFP group, plasma lyso-Gb3 was reduced by 80%, 7 days after dose, and began to reaccumulate at day 35 after dose (Figures 3B and S3C). Maximum depletion of lyso-Gb3 was observed in liver (∼80%) and spleen (∼90%) 14 days after dose and was maintained through day 42 after dose before beginning to increase (Figure 3B). Heart and kidney lyso-Gb3 decreased by approximately 60%–70% by 28–35 days after dose (Figures 3B and S3D) and began to reaccumulate after 35 days after dose (Figure 3B). While re-accumulation occurred over time, it is interesting to note that lyso-Gb3 levels did not return to baseline levels (as seen in eGFP-treated mice) by completion of the study (84 days or 12 weeks after dose).
Figure 3.
Duration of Action of h-α-Gal A in Fabry Mice
Fabry mice (n = 3–5 per group at 22 weeks old) were injected i.v. with either 0.5 mg/kg h-α-Gal A mRNA or 0.5 mg/kg eGFP mRNA encapsulated in LNPs. Mice were sacrificed at different time points after injection (A) Representative images of IHC staining of h-α-Gal A protein in liver (top) and heart (bottom) from Fabry mice treated with single dose of h-α-Gal A mRNA or eGFP mRNA. Mice were sacrificed at day 14, 21, 35, 42, 56, and 84. Scale, liver 10× images, heart 20× images.
(B) Lyso-Gb3 depletion following a single i.v. injection of h-α-Gal A mRNA. Data from groups dosed with h-α-Gal A mRNA was normalized to age-matched Fabry mice dosed with control eGFP mRNA and expressed as percentage of lyso-Gb3 of untreated control (eGFP group). Results were plotted as mean ± SEM. Statistical analysis of lyso-Gb3 duration curve (AUC) was performed using unpaired t test (see Table S2). Note: lyso-Gb3 data from tissues of one mouse in 12-week group dosed with h-α-Gal A were excluded due to tissue collection error.
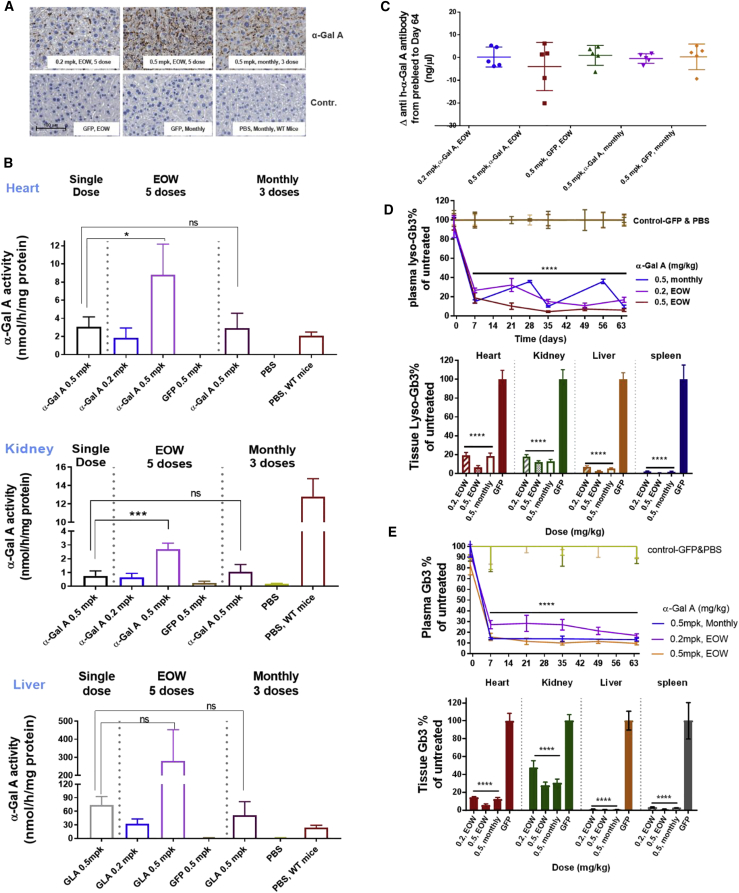
Repeat i.v. Administration of h-α-Gal A mRNA in Fabry Mice Restored α-Gal A Activity in Tissues and Reduced Substrates in a Dose-Dependent Manner
We conducted repeat dose experiments to evaluate the therapeutic effect of h-α-Gal A mRNA in Fabry mice to determine its efficacy and tolerability as a potential treatment. We administered 0.2 mg/kg or 0.5 mg/kg h-α-Gal A mRNA every other week (EOW) or 0.5 mg/kg h-α-Gal A mRNA every month for 3 months. Control groups were comprised of mice administered 0.5 mg/kg eGFP or PBS every other week or monthly. Plasma samples were collected throughout the study and heart, kidney, liver, and spleen were collected at the end of the study, 7 days after the last dose. IHC analysis of liver samples demonstrated a dose-responsive increase in h-α-Gal A by protein staining in the liver with a rank order of 0.5 mg/kg EOW > 0.5 mg/kg monthly > 0.2 mg/kg EOW (Figure 4A). These data are consistent with h-α-Gal A activity data from heart, kidney, and liver. The h-α-Gal A activity was greatest in groups that received 0.5 mg/kg EOW among the three h-α-Gal A mRNA dose groups. In this group, the activity of h-a-Gal A has been significantly restored in the liver and in the heart of Fabry mice, amounting to 8-fold and 4-fold increase at 7 days post i.v. comparing with WT mouse a-Gal A activity, respectively. By contrast, the activity of h-a-Gal A in kidney of this group kept at ∼25% of WT mouse a-Gal A activity level 7 days after i.v. dose (Figure 4B).
Figure 4.
Therapeutic Efficacy of h-α-Gal A in Fabry Mice after Repeat i.v. Administration of h-α-Gal A mRNA at Different Dose Frequencies
h-α-Gal A mRNA was intravenously injected into Fabry mice (n = 5) every other week (EOW) at 0.2 or 0.5 mg/kg for 5 doses or 3 monthly doses of 0.5 mg/kg. As controls, 0.5 mg/kg eGFP mRNA or PBS were injected every other week or monthly into mice. Plasma from Fabry mice in EOW groups was collected before the first dose and 7 days after each dose, and in the monthly group, plasma was collected before each dose and 7 days after each dose. Fabry mice were sacrificed 7 days after the last administration.
(A) Representative image of a dose-dependent expression of h-α-Gal A protein in liver of Fabry mice by IHC staining. Liver staining from Fabry mice after multiple injections of h-α-Gal A mRNA (top) and liver staining for groups receiving eGFP mRNA or PBS (bottom). Scale, 10× images.
(B) The restoration of h-α-Gal A activity in heart (top), kidney (middle), and liver (bottom) of Fabry mice administered eGFP or h-α-Gal A every other week or monthly. The h-α-Gal A activity in heart and kidney in Fabry mice received multi doses was compared to that in Fabry mice received single dose of h-α-Gal A mRNA via one-way ANOVA of multiple comparison.
(C) Plasma anti-h-α-Gal A antibody was measured before the first dose and after the last dose and expressed as antibody level changes from before to after.
(D and E) Lyso-Gb3 depletion (D) in plasma (top) and tissues (bottom) and Gb3 depletion (E) in plasma (top) and tissues (bottom) after repeat i.v. injection of h-α-Gal A mRNA. These data from groups dosed with h-α-Gal A mRNA were normalized to that with eGFP mRNA and PBS and expressed as percentage of lyso-Gb3 of untreated control. All results were plotted as mean ± SD. p values were obtained using one-way ANOVA of multiple comparison of h-α-Gal A mRNA-treated groups with eGFP control group. p < 0.05 was considered as statistically significant, ∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. Note: one mouse from h-α-Gal A mRNA monthly group died before the end of study due to fighting and the heart of one mouse in h-α-Gal A mRNA EOW group was for biomarkers and staining assays but not enough for activity assay.
Repeat administrations of human proteins to mice pose significant challenges due in part to hypersensitivity responses which can impact pharmacokinetics and pharmacodynamics of therapeutic proteins. To evaluate whether repeat i.v. administration of h-α-Gal A mRNA generates neutralizing anti-h-α-Gal A antibodies, we dosed mice either 3 or 5 times and measured anti-α-Gal A antibody levels both prior to the first dose and 7 days after each dose. We did not detect a notable change in anti-h-α-Gal A antibody levels (Figure 4C), suggesting that our treatment did not induce immune responses in these mice. Importantly, significant h-α-Gal A activity accumulation was observed in EOW group dosed with 0.5 mg/kg of h-α-Gal A mRNA, when compared to the group receiving a single dose of h-α-Gal A mRNA (Figure 4B). The accumulation of h-α-Gal A activity in the multiple dose group further demonstrate that there was no neutralizing anti-α-Gal A antibody development.
In this study, we measured both lyso-Gb3 and Gb3 reductions and analyzed correlations between depletion of these two substrates (Figures 4D and 4E, Tables S3 and S4). Lyso-Gb3 reduction was observed in plasma, heart, kidney, liver, and spleen. Tissue levels of lyso-Gb3 were reduced by greater than 80% in animals that received 0.2 mg/kg, 0.5 mg/kg EOW, or 0.5 mg/kg monthly (Figure 4D). Plasma lyso-Gb3 correlated well with tissue lyso-Gb3 depletion (Figure S4 and Table S4). Similar Gb3 reduction was observed in plasma and tissues (Figure 4E). In addition, plasma Gb3 and lyso-Gb3 reductions showed a significant correlation at all time points and all tested dose levels (Figure S4A). This observation was not specific to plasma Gb3 levels as good correlations were also observed between tissue lyso-Gb3 and tissue Gb3 (Figures S4B–S4D and Table S4). Taken together these data suggest that efficacy in Fabry mice receiving h-α-Gal A mRNA EOW is equivalent to monthly dosing at 0.5 mg/kg irrespective of the substrate evaluated (i.e., Gb3 or lyso-Gb3).
Pharmacokinetics, Distribution, and Safety of h-α-Gal A mRNA in Non-human Primates (NHPs)
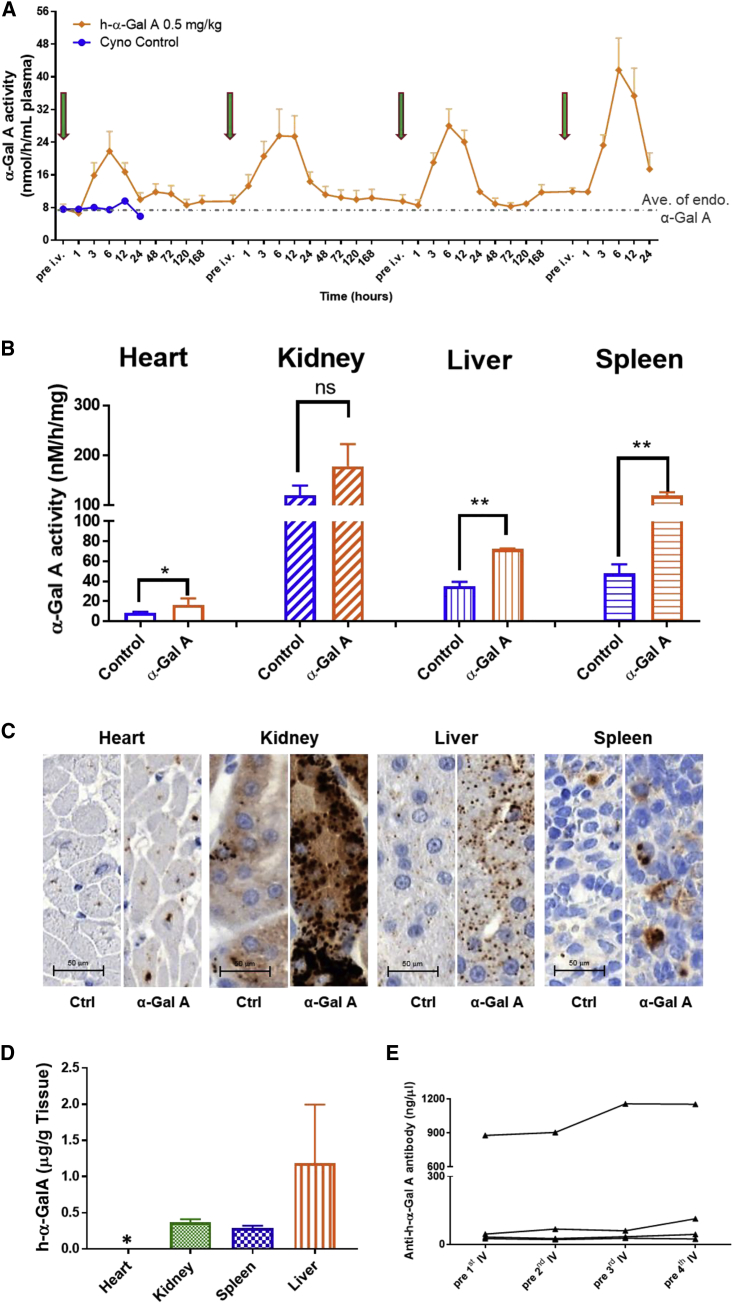
Humanα-Gal A mRNA was further evaluated in WT NHPs to characterize the pharmacokinetics and distribution of secreted h-α-Gal A in a larger mammalian species. h-α-Gal A mRNA encapsulated in LNPs at 0.5 mg/kg was given to NHP (n = 4) via a 60-min intravenous infusion every other week for 4 doses. Plasma was collected prior to each dose and at multiple time points post dose. Two of the four monkeys in the h-α-Gal A mRNA group were sacrificed 24 h after the 4th injection. Liver, spleen, kidney, and heart were collected and assayed for α-Gal A activity and evaluated for protein by IHC. Similar to data generated in mice, α-Gal A activity in plasma increased significantly after each dose as showed in Figure 5A. The Cmax of plasma α-Gal A activity is 3- to 5-fold above endogenous α-Gal A levels. Tmax was achieved between 6 and 12 h after dose. On average tissue α-Gal A activity increased in NHPs that received h-α-Gal A mRNA compared to control as shown in Figure 5B. Liver tissue IHC staining showed an increase in α-Gal A protein levels (Figure 5C) in animals that received h-α-Gal A mRNA, which correlated well with the activity data. The antibody used for IHC staining of α-Gal A is cross reactive against NHP α-Gal A and human α-Gal A. To determine whether h-α-Gal A protein encoded by h-α-Gal A mRNA was expressed in NHPs, we developed a method using a human α-Gal A-specific peptide for h-α-Gal A protein quantitation by LC-MS/MS (Figure 5D). Human α-Gal A was quantified in all tested tissues except heart in which the amount of h-α-Gal A was below the quantification limit. It confirmed that the h-α-Gal A protein was expressed in NHPs.
Figure 5.
α-Gal A Activity in Plasma and Tissues of Wild-Type Non-human Primates (NHPs) after Biweekly i.v. Administrations of h-α-Gal A mRNA
Four doses of h-α-Gal A mRNA at 0.5 mg/kg were intravenously injected in cynomolgus monkey (n = 4) with naive cyno as control (n = 1). Plasma (n = 4) was collected prior to each dose, 1, 3, 6, 12, 24, 48, 72, 120, and 168 h after the first three doses, and 1, 3, 6, 12, 24 h after last dose. Two cynos were sacrificed at 24 h after last dose.
(A) α-Gal A activity in plasma with the peak activity between 6 and 12 h after i.v. injection of each dose.
(B) α-Gal A activity in tissues of cynos. 6 cyno monkeys dosed with unrelated mRNA from reported studies26 were used as controls. All results were plotted as mean ± SD. p values were obtained via unpaired t test to compare the activity in h-α-Gal A mRNA-dosed cynos with that in control cynos. p < 0.5 was considered as statistically significant. ∗p < 0.1, ∗∗p < 0.01.
(C) IHC images showing increases in α-Gal A protein staining in group administered h-α-Gal A mRNA over the endogenous levels of α-Gal A protein staining seen in untreated control group; scale, 20× images.
(D) h-α-Gal A protein was detected by LC-MS/MS in kidney, liver, and spleen of NHP that received h-α-Gal A mRNA. ∗stands for out of LLOQ.
(E) Anti-h-α-Gal A levels after repeat dose of h-α-Gal A mRNA in NHP. Lines connect the same individual NHP tested. All results were plotted as mean ± SD.
As with any liver-directed therapy, liver safety is paramount. Therefore, we evaluated markers of liver toxicity after repeat doses administration in NHPs. Serum albumin, AST, ALP, ALT, GGT, TBIL, and TPROT levels were measured at baseline and 24 h after the 1st and 4th administration of 0.5 mg/kg h-α-Gal A mRNA (Table 1). Liver enzymes remained within normal reference ranges. No significant changes in the parameters of clinical chemistry were observed after one or four consecutive i.v. administrations of h-α-Gal A mRNA. As mentioned previously, introduction of foreign proteins into animals can cause an immune stimulatory response. In this study we didn’t observe an increase trend in serum anti-α-Gal A antibody levels after each dose (Figure 5E). This suggests that administration of h-α-Gal A mRNA did not induce an immune response in NHPs.
Table 1.
Summary of Clinical Chemistry in NHPs Dosed Repeatedly with h-α-Gal A mRNA
| Parameter (unit) | 1 Week before Dose | 24 h after 1st Dose | 24 h after 4th Dose |
|---|---|---|---|
| AST (U/L) | 53.8 ± 22.9 | 56.3 ± 8.5 | 51.3 ± 9.5 |
| ALT (U/L) | 50.0 ± 12.0 | 50.0 ± 4.5 | 45.0 ± 9.5 |
| ALP (U/L) | 579.0 ± 216.2 | 619.3.0 ± 241.8 | 576.0 ± 211.7 |
| GGT (U/L) | 81.0 ± 16.4 | 85.3 ± 18.7 | 79.0 ± 13.4 |
| ALB (g/dL) | 4.35 ± 0.19 | 4.28 ± 0.19 | 4.23 ± 0.17 |
| TBIL (mg/dL) | 0.138 ± 0.021 | 0.243 ± 0.081 | 0.120 ± 0.042 |
| TPROT (g/dL) | 6.98 ± 0.39 | 7.10 ± 0.50 | 6.88 ± 0.41 |
Data are mean ± SD (n = 4).
Discussion
There have been remarkable developments for the treatment of Fabry disease including ERTs, small molecule therapy (CT and SRT), and gene therapy. Despite advances in standard of care, unmet medical needs still exist with respect to renal and to a lesser extent cardiac decline. In addition, administration by infusion every other week often becomes burdensome for individuals and their families.35 Therefore, novel therapies which improve efficacy and convenience are desirable.
In this paper we have described that exogenously administered mRNA can result in increased target protein levels and decrease substrate burden in the Fabry disease mouse model. Our approach is to package mRNA into bio-degradable LNPs that once administered intravenously are taken up by the liver and translated into the therapeutically active protein. In the case of h-α-Gal A, the protein is produced within liver, then secreted into circulation, internalized systemically, and then targeted to the lysosomes via endocytosis.
Following administration of mRNA, h-α-Gal A protein is transiently secreted into the circulation of mice and NHP as early as 3 h after i.v. injection and reaches Cmax peak time at 6–12 h. The protein is detectable in circulation for approximately 96 and 48 h in mice and NHP, respectively, largely due to mRNA stability providing continuous translation. We believe the extended time in circulation relative to agalsidase beta (Fabrazyme) and agalsidase alfa (Replagal), allows for better tissue distribution.34, 35, 40 Once the enzyme reaches the lysosomal compartment of tissues, the protein has a long tissue half-life, at least in rodents, as evident by substrate reduction which was ∼42 days in tissue and approximately 4 weeks in plasma. Hearts and kidneys of Fabry-affected individuals are generally affected as cardiac lesions and eGFR decline, which are clinical hallmarks of the disease.41, 42, 43 Our data demonstrate that h-α-Gal A produced in liver from administered mRNA distributes to heart, kidney, and spleen, as confirmed by a number of distinct techniques such as tissue enzyme activity, immunohistochemistry, and LC-MS/MS.
The α-Gal A-deficient Fabry mouse model was utilized to evaluate the efficacy of mRNA encapsulated in LNPs since wild-type animals do not accumulate appreciable levels of substrate. To that end, single administration of 0.5 mg/kg mRNA in LNPs was given to Fabry mice. Substrate lyso-Gb3 was measured in plasma, spleen, liver, kidney, and heart. In the Fabry mouse model, lyso-Gb3 was maximally reduced for 4–6 weeks after dose. Additionally, it remained well below baseline/control mRNA-treated levels through the end of the study, 12 weeks after dose, in all tissues and plasma. Since Fabry mice accumulate lyso-Gb3 (and Gb-3) relatively slowly, the animals must reach approximately 20 weeks of age prior to the start of efficacy studies.27 Therefore, the observation that substrate remained below baseline may be due to the slow rate of accumulation of substrate once enzyme is degraded.
Given that current approved/marketed ERTs are administered every other week to individuals, a study was conducted to evaluate the efficacy of every other week administration compared to monthly administration of LNP encapsulated mRNA. In this study Fabry mice received 0.2 and 0.5 mg/kg every other week and 0.5 mg/kg monthly for a total of 5 doses (every other week regimen) or 3 doses (monthly regimen). Not surprisingly, the enzyme level in tissues evaluated correlated with dose level and frequency of dose. The groups that received 5 doses at 0.5 mg/kg had the most enzyme in tissues and Fabry mice that received 0.2 mg/kg every other week or 0.5 mg/kg monthly both had similar levels of detectable enzyme in tissues. Despite obvious difference in h-α-Gal A levels in tissues in groups of animals that received 5 doses of 0.5 mg/kg, substrate reduction was only slightly better than in animals that received either 5 doses of 0.2 mg/kg or 3 monthly doses of 0.5 mg/kg mRNA in all tissues examined. These data add support to the single-dose data suggesting that h-α-Gal A produced from exogenously delivered mRNA has a long duration of action in Fabry mice. Of note, the most severely affected Fabry individuals and have ≤1% of residual enzyme. Therefore, as little as >1% of enzyme within a tissue is thought to be sufficient to slow accumulation in individuals, which may explain why there is such a prolonged effect following a single dose of h-α-Gal A mRNA to Fabry mice. Initial enzyme levels in tissue are highest after mRNA administration and decrease over time, consistent with α-Gal A half-life. While the initial concentrations of enzyme are necessary to deplete accumulated substrate, low levels of active enzyme are sufficient to keep substrate levels from accumulating. Once the enzyme is gone, the substrate then reaccumulates within the tissue. One interesting observation is that treatment with increasing doses up to 0.5 mg/kg of h-α-Gal A mRNA does not reduce substrates to zero, especially in kidney. This could be due to accumulation of substrate outside of the lysosome in compartments that may not be accessible to protein. Conversely it could also represent substrate accumulation in cells that are inaccessible to the enzyme or to damaged proper uptake. The other possibility may be that the analogs of substrates Gb3 and lyso-Gb3 in Fabry mice are slightly different from those in human and cannot be completely cleared by h-α-Gal A protein.44
As mentioned previously, parenteral administration of human proteins to other species has been known to cause hypersensitivity and induce formation of anti-drug antibodies.45 In fact, studies published by Garman et al.46 demonstrate that antidrug antibodies develop to recombinant human α-Gal A approximately 2 weeks after the first dose in multiple dose studies in the Fabry mouse model. An interesting observation is that repeated mRNA administration to Fabry mice or NHP did not result in anti h-α-Gal A antibody production, indicating improved tolerability to the human protein produced in the murine liver. In addition, the sustained PD effect after each dose in Fabry mice or NHPs, and accumulation of h-α-Gal A activity in liver, kidney, and heart in Fabry mice after repeat administrations suggest that neutralizing antibodies may not be present.
In our studies, h-α-Gal A encoded by exogenously administrated h-α-Gal A mRNA has a prolonged plasma and tissue half-life. which results in greater clearance of heart and kidney substrates than published data using ERT, combination therapy of ERT/CT, and some gene therapy.31, 34, 35, 36, 37, 47, 48 Preclinical published data from Protalix has demonstrated that pegunigalsidase alfa (PRX-102) has an extended circulating half-life and greater Gb3 reductions in tissues such as kidney and heart compared to commercial ERTs. In their studies the reduction of Gb3 analyzed by high-performance thin layer chromatography (HPTLC) was 36% in kidney and 77% in heart following biweekly administrations of 2 mg/kg for 4 doses.35 Our data from liquid chromatography-mass spectrometry (LC-MS) analysis demonstrated >70% reduction in kidney Gb3 and ∼90% reduction in heart Gb3 levels after 3 monthly or 5 biweekly doses of 0.5 mg/kg h-α-Gal A mRNA. The reason why mRNA approach can achieve such a great therapeutic effect is unknown but may in part be the result of a consistently glycosylated protein when produced endogenously rather than in cell production systems49 and/or a prolonged circulation time of the enzyme allowing a better tissue distribution. Self-protein production and glycosylation alone cannot be the sole driver of enhanced efficacy as published work has demonstrated. AAV-based gene therapy studies by Sangamo Therapeutics33 have demonstrated that significant amounts of h-α-Gal A produced in the liver can be taken up by other tissues such as the heart in Fabry mice. However, despite having h-α-Gal A levels 100-fold of WT α-Gal A level of Fabry mice after 2 months AAV-based gene treatment, substrate such as Gb3 and lyso-Gb3 in heart remains at ∼5% and >10% of untreated control level, respectively, which does not correlate well with the amount of enzyme and indicates that continuous overexpression of h-α-Gal A may not be able to achieve maximum efficacy or are not targeting necessary cell types. By contrast, our data demonstrated a larger therapeutic effect achieving greater Gb3 (5.7% of untreated) and lyso-Gb3 (6.7% of untreated) reductions in heart after repeat administrations (0.5 mpk, biweekly, 5 doses) compared to AAV-based gene treatment.
The secretion, stability of secreted protein, and efficiency of endocytosis of α-Gal A are the key factors for effective protein or gene-based therapies and directly affect the efficacy of treatment. It was known that lysosomal proteins including α-Gal A can be selectively secreted and taken up by cells via endocytosis. However, the mechanism of secretion and endocytosis have not been fully understood.50, 51 Conventional wisdom was that mannose-6-phosphate (M6P) receptor-mediated endocytosis was the key mechanism for h-α-Gal A uptake by different cells, but recent studies have shown other receptors such as mannose receptor also contribute to the process of the endocytosis of α-Gal A.34 In addition, it has been reported that the stability of the recombinant α-Gal A protein greatly affects the therapeutic efficacy.35, 36 Taken together, one additional possibility is that the production of h-α-Gal A in vivo encoded by exogenously administrated mRNA mimics the endogenous processes, including translation and post-translational modifications, increasing the secretion, stability, and efficiency of endocytosis, as compared to that of parenterally administered protein. However, several questions remain to be addressed in order to fully understand the characteristics of α-Gal A and improve treatment strategies for Fabry disease. Further in vivo mechanistic studies of secretion and endocytosis of α-Gal A are needed such as characterization and comparison of the therapeutic recombinant α-Gal A, endogenous h-α-Gal A, and the enzyme encoded by h-α-Gal A mRNA.
In summary, our data in WT and Fabry mice as well as NHP demonstrates that mRNA administration results in the production of functional h-α-Gal A in liver, which then is secreted into the circulation. Secreted h-α-Gal A is taken up by distal tissues such as kidney, heart, and spleen and is targeted to the lysosomes via endocytosis. As mentioned above, α-Gal A activity was significantly increased in rodents and NHPs. In the disease model, h-α-Gal A produced from a single dose of 0.5 mg/kg mRNA attenuates substrate accumulation in target tissues for at least 12 weeks. If this duration of action were to translate to the clinic, individuals may be able to receive less frequent dosing than is currently required for ERT therapy, which is administered every other week. Lastly, repeated administration in mice and NHP did not induce anti h-α-Gal A antibodies, which is commonly observed with ERT and which could have an impact on efficacy in some individuals. Taken together these data demonstrate compelling preclinical proof-of-concept supporting the systemic administration of h-α-Gal A mRNA as a therapeutic modality for protein restoration and substrate depletion.
Declaration of Interests
All authors are employees and shareholders of Moderna Inc. Some of the authors have been named as inventors on a patent application related to this work.
Acknowledgments
This work was funded by Moderna, Inc. We thank Dr. Ed Miracco for his careful review of the manuscript.
Published: March 14, 2019
Footnotes
Supplemental Data can be found with this article online at https://doi.org/10.1016/j.ajhg.2019.02.003.
Web Resources
OMIM, http://www.omim.org/
Supplemental Data
References
- 1.Murray G.J., Anver M.R., Kennedy M.A., Quirk J.M., Schiffmann R. Cellular and tissue distribution of intravenously administered agalsidase alfa. Mol. Genet. Metab. 2007;90:307–312. doi: 10.1016/j.ymgme.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brady R.O., Schiffmann R. Clinical features of and recent advances in therapy for Fabry disease. JAMA. 2000;284:2771–2775. doi: 10.1001/jama.284.21.2771. [DOI] [PubMed] [Google Scholar]
- 3.Auray-Blais C., Lavoie P., Boutin M., Ntwari A., Hsu T.-R., Huang C.-K., Niu D.-M. Biomarkers associated with clinical manifestations in Fabry disease patients with a late-onset cardiac variant mutation. Clin. Chim. Acta. 2017;466:185–193. doi: 10.1016/j.cca.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 4.Sueoka H., Aoki M., Tsukimura T., Togawa T., Sakuraba H. Distributions of globotriaosylceramide isoforms, and globotriaosylsphingosine and its analogues in an α-galactosidase A knockout mouse, a model of Fabry disease. PLoS ONE. 2015;10:e0144958. doi: 10.1371/journal.pone.0144958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sueoka H., Ichihara J., Tsukimura T., Togawa T., Sakuraba H. Nano-LC-MS/MS for quantification of Lyso-Gb3 and its analogues reveals a useful biomarker for Fabry disease. PLoS ONE. 2015;10:e0127048. doi: 10.1371/journal.pone.0127048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boutin M., Gagnon R., Lavoie P., Auray-Blais C. LC-MS/MS analysis of plasma lyso-Gb3 in Fabry disease. Clin. Chim. Acta. 2012;414:273–280. doi: 10.1016/j.cca.2012.09.026. [DOI] [PubMed] [Google Scholar]
- 7.Krämer J., Lenders M., Canaan-Kühl S., Nordbeck P., Üçeyler N., Blaschke D., Duning T., Reiermann S., Stypmann J., Brand S.-M.M. Fabry disease under enzyme replacement therapy-new insights in efficacy of different dosages. Nephrol. Dial. Transplant. 2017;33:1362–1372. doi: 10.1093/ndt/gfx319. [DOI] [PubMed] [Google Scholar]
- 8.Tadevosyan A. Fabry disease: A fundamental genetic modifier of cardiac function. Curr. Res. Transl. Med. 2017;65:10–14. doi: 10.1016/j.retram.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Yuasa T., Takenaka T., Higuchi K., Uchiyama N., Horizoe Y., Cyaen H., Mizukami N., Takasaki K., Kisanuki A., Miyata M., Ohishi M. Fabry disease. J. Echocardiogr. 2017;15:151–157. doi: 10.1007/s12574-017-0340-x. [DOI] [PubMed] [Google Scholar]
- 10.Beck M. Treatment strategies for lysosomal storage disorders. Dev. Med. Child Neurol. 2018;60:13–18. doi: 10.1111/dmcn.13600. [DOI] [PubMed] [Google Scholar]
- 11.Motabar O., Sidransky E., Goldin E., Zheng W. Fabry disease - current treatment and new drug development. Curr. Chem. Genomics. 2010;4:50–56. doi: 10.2174/1875397301004010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kubo T. Fabry disease and its cardiac involvement. J. Gen. Fam. Med. 2017;18:225–229. doi: 10.1002/jgf2.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meikle P.J., Hopwood J.J., Clague A.E., Carey W.F. Prevalence of lysosomal storage disorders. JAMA. 1999;281:249–254. doi: 10.1001/jama.281.3.249. [DOI] [PubMed] [Google Scholar]
- 14.Miller J.J., Aoki K., Moehring F., Murphy C.A., O’Hara C.L., Tiemeyer M., Stucky C.L., Dahms N.M. Neuropathic pain in a Fabry disease rat model. JCI Insight. 2018;3:e99171. doi: 10.1172/jci.insight.99171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elliott S., Buroker N., Cournoyer J.J., Potier A.M., Trometer J.D., Elbin C., Schermer M.J., Kantola J., Boyce A., Turecek F. Pilot study of newborn screening for six lysosomal storage diseases using Tandem Mass Spectrometry. Mol. Genet. Metab. 2016;118:304–309. doi: 10.1016/j.ymgme.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spada M., Pagliardini S., Yasuda M., Tukel T., Thiagarajan G., Sakuraba H., Ponzone A., Desnick R.J. High incidence of later-onset fabry disease revealed by newborn screening. Am. J. Hum. Genet. 2006;79:31–40. doi: 10.1086/504601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Germain D.P., Charrow J., Desnick R.J., Guffon N., Kempf J., Lachmann R.H., Lemay R., Linthorst G.E., Packman S., Scott C.R. Ten-year outcome of enzyme replacement therapy with agalsidase beta in patients with Fabry disease. J. Med. Genet. 2015;52:353–358. doi: 10.1136/jmedgenet-2014-102797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hebert A., Lacbawan L., Taber T., Goker-Alpan O. Evaluation of long-term enzyme replacement therapy for children with Fabry disease. Mol. Genet. Metab. 2013;108:S47. [Google Scholar]
- 19.Komori M., Sakurai Y., Kojima H., Ohashi T., Moriyama H. Long-term effect of enzyme replacement therapy with fabry disease. Int. J. Otolaryngol. 2013;2013:282487. doi: 10.1155/2013/282487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Messalli G., Imbriaco M., Avitabile G., Russo R., Iodice D., Spinelli L., Dellegrottaglie S., Cademartiri F., Salvatore M., Pisani A. Role of cardiac MRI in evaluating patients with Anderson-Fabry disease: assessing cardiac effects of long-term enzyme replacement therapy. Radiol. Med. (Torino) 2012;117:19–28. doi: 10.1007/s11547-011-0710-9. [DOI] [PubMed] [Google Scholar]
- 21.Weidemann F., Niemann M., Breunig F., Herrmann S., Beer M., Störk S., Voelker W., Ertl G., Wanner C., Strotmann J. Long-term effects of enzyme replacement therapy on fabry cardiomyopathy: evidence for a better outcome with early treatment. Circulation. 2009;119:524–529. doi: 10.1161/CIRCULATIONAHA.108.794529. [DOI] [PubMed] [Google Scholar]
- 22.Lux C.T., Scharenberg A.M. Therapeutic gene editing safety and specificity. Hematol. Oncol. Clin. North Am. 2017;31:787–795. doi: 10.1016/j.hoc.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yee J.K. Off-target effects of engineered nucleases. FEBS J. 2016;283:3239–3248. doi: 10.1111/febs.13760. [DOI] [PubMed] [Google Scholar]
- 24.Sahin U., Karikó K., Türeci Ö. mRNA-based therapeutics--developing a new class of drugs. Nat. Rev. Drug Discov. 2014;13:759–780. doi: 10.1038/nrd4278. [DOI] [PubMed] [Google Scholar]
- 25.An D., Schneller J.L., Frassetto A., Liang S., Zhu X., Park J.-S., Theisen M., Hong S.-J., Zhou J., Rajendran R. Systemic messenger RNA therapy as a treatment for methylmalonic acidemia. Cell Rep. 2017;21:3548–3558. doi: 10.1016/j.celrep.2017.11.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang L., Berraondo P., Jericó D., Guey L.T., Sampedro A., Frassetto A., Benenato K.E., Burke K., Santamaría E., Alegre M. Systemic messenger RNA as an etiological treatment for acute intermittent porphyria. Nat. Med. 2018;24:1899–1909. doi: 10.1038/s41591-018-0199-z. [DOI] [PubMed] [Google Scholar]
- 27.Bangari D.S., Ashe K.M., Desnick R.J., Maloney C., Lydon J., Piepenhagen P., Budman E., Leonard J.P., Cheng S.H., Marshall J., Thurberg B.L. α-Galactosidase A knockout mice: progressive organ pathology resembles the type 2 later-onset phenotype of Fabry disease. Am. J. Pathol. 2015;185:651–665. doi: 10.1016/j.ajpath.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 28.Richner J.M., Jagger B.W., Shan C., Fontes C.R., Dowd K.A., Cao B., Himansu S., Caine E.A., Nunes B.T.D., Medeiros D.B.A. Vaccine mediated protection against Zika virus-induced congenital disease. Cell. 2017;170:273–283.e12. doi: 10.1016/j.cell.2017.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richner J.M., Himansu S., Dowd K.A., Butler S.L., Salazar V., Fox J.M., Julander J.G., Tang W.W., Shresta S., Pierson T.C. Modified mRNA vaccines protect against Zika virus infection. Cell. 2017;168:1114–1125.e10. doi: 10.1016/j.cell.2017.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sabnis S., Kumarasinghe E.S., Salerno T., Mihai C., Ketova T., Senn J.J., Lynn A., Bulychev A., McFadyen I., Chan J. A novel amino lipid series for mRNA delivery: improved endosomal escape and sustained pharmacology and safety in non-human primates. Mol. Ther. 2018;26:1509–1519. doi: 10.1016/j.ymthe.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ishii S., Chang H.-H., Yoshioka H., Shimada T., Mannen K., Higuchi Y., Taguchi A., Fan J.-Q. Preclinical efficacy and safety of 1-deoxygalactonojirimycin in mice for Fabry disease. J. Pharmacol. Exp. Ther. 2009;328:723–731. doi: 10.1124/jpet.108.149054. [DOI] [PubMed] [Google Scholar]
- 32.Auray-Blais C., Boutin M., Gagnon R., Dupont F.O., Lavoie P., Clarke J.T. Urinary globotriaosylsphingosine-related biomarkers for Fabry disease targeted by metabolomics. Anal. Chem. 2012;84:2745–2753. doi: 10.1021/ac203433e. [DOI] [PubMed] [Google Scholar]
- 33.Huston M., Yasuda M., Pagant S., St. Martin S., Sproul S., Santiago Y., DeKelver R., Holmes M.C., Desnick R., Weschler T. Liver-based expression of the human alpha-galactosidase A gene (GLA) in a murine Fabry model results in continuous supra-physiological enzyme activity and effective substrate reduction. Mol. Genet. Metab. 2017;120:S69. [Google Scholar]
- 34.Shen J.-S., Busch A., Day T.S., Meng X.-L., Yu C.I., Dabrowska-Schlepp P., Fode B., Niederkrüger H., Forni S., Chen S. Mannose receptor-mediated delivery of moss-made α-galactosidase A efficiently corrects enzyme deficiency in Fabry mice. J. Inherit. Metab. Dis. 2016;39:293–303. doi: 10.1007/s10545-015-9886-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kizhner T., Azulay Y., Hainrichson M., Tekoah Y., Arvatz G., Shulman A., Ruderfer I., Aviezer D., Shaaltiel Y. Characterization of a chemically modified plant cell culture expressed human α-Galactosidase-A enzyme for treatment of Fabry disease. Mol. Genet. Metab. 2015;114:259–267. doi: 10.1016/j.ymgme.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 36.Xu S., Lun Y., Brignol N., Hamler R., Schilling A., Frascella M., Sullivan S., Boyd R.E., Chang K., Soska R. Coformulation of a novel human α-Galactosidase A with the pharmacological chaperone AT1001 leads to improved substrate reduction in Fabry mice. Mol. Ther. 2015;23:1169–1181. doi: 10.1038/mt.2015.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benjamin E.R., Khanna R., Schilling A., Flanagan J.J., Pellegrino L.J., Brignol N., Lun Y., Guillen D., Ranes B.E., Frascella M. Co-administration with the pharmacological chaperone AT1001 increases recombinant human α-galactosidase A tissue uptake and improves substrate reduction in Fabry mice. Mol. Ther. 2012;20:717–726. doi: 10.1038/mt.2011.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ziegler R.J., Lonning S.M., Armentano D., Li C., Souza D.W., Cherry M., Ford C., Barbon C.M., Desnick R.J., Gao G. AAV2 vector harboring a liver-restricted promoter facilitates sustained expression of therapeutic levels of α-galactosidase A and the induction of immune tolerance in Fabry mice. Mol. Ther. 2004;9:231–240. doi: 10.1016/j.ymthe.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 39.Ziegler R.J., Yew N.S., Li C., Cherry M., Berthelette P., Romanczuk H., Ioannou Y.A., Zeidner K.M., Desnick R.J., Cheng S.H. Correction of enzymatic and lysosomal storage defects in Fabry mice by adenovirus-mediated gene transfer. Hum. Gene Ther. 1999;10:1667–1682. doi: 10.1089/10430349950017671. [DOI] [PubMed] [Google Scholar]
- 40.Sakuraba H., Murata-Ohsawa M., Kawashima I., Tajima Y., Kotani M., Ohshima T., Chiba Y., Takashiba M., Jigami Y., Fukushige T. Comparison of the effects of agalsidase alfa and agalsidase beta on cultured human Fabry fibroblasts and Fabry mice. J. Hum. Genet. 2006;51:180–188. doi: 10.1007/s10038-005-0342-9. [DOI] [PubMed] [Google Scholar]
- 41.Seydelmann N., Wanner C., Störk S., Ertl G., Weidemann F. Fabry disease and the heart. Best Pract. Res. Clin. Endocrinol. Metab. 2014;29:195–204. doi: 10.1016/j.beem.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 42.Pisani A., Visciano B., Imbriaco M., Di Nuzzi A., Mancini A., Marchetiello C., Riccio E. The kidney in Fabry’s disease. Clin. Genet. 2014;86:301–309. doi: 10.1111/cge.12386. [DOI] [PubMed] [Google Scholar]
- 43.Trimarchi H. The kidney in Fabry disease: more than mere sphingolipids overload. J. Inborn Errors Metab. 2016;Screening 4 Published online May 13, 2016. [Google Scholar]
- 44.Ioannou Y.A., Zeidner K.M., Gordon R.E., Desnick R.J. Fabry disease: preclinical studies demonstrate the effectiveness of alpha-galactosidase A replacement in enzyme-deficient mice. Am. J. Hum. Genet. 2001;68:14–25. doi: 10.1086/316953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Warnock D., Alon S., Chertkoff R., Brill-Almon E. Progression of nephropathy in Fabry patients receiving enzyme replacement therapy (ERT); relation to anti-drug antibody (ADA) status and proteinuria. Nephrol. Dial. Transplant. 2018;33:i634–i635. [Google Scholar]
- 46.Garman R.D., Munroe K., Richards S.M. Methotrexate reduces antibody responses to recombinant human α-galactosidase A therapy in a mouse model of Fabry disease. Clin. Exp. Immunol. 2004;137:496–502. doi: 10.1111/j.1365-2249.2004.02567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pacienza N., Yoshimitsu M., Mizue N., Au B.C., Wang J.C., Fan X., Takenaka T., Medin J.A. Lentivector transduction improves outcomes over transplantation of human HSCs alone in NOD/SCID/Fabry mice. Mol. Ther. 2012;20:1454–1461. doi: 10.1038/mt.2012.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Higuchi K., Yoshimitsu M., Fan X., Guo X., Rasaiah V.I., Yen J., Tei C., Takenaka T., Medin J.A. Alpha-galactosidase A-Tat fusion enhances storage reduction in hearts and kidneys of Fabry mice. Mol. Med. 2010;16:216–221. doi: 10.2119/molmed.2009.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee K., Jin X., Zhang K., Copertino L., Andrews L., Baker-Malcolm J., Geagan L., Qiu H., Seiger K., Barngrover D. A biochemical and pharmacological comparison of enzyme replacement therapies for the glycolipid storage disorder Fabry disease. Glycobiology. 2003;13:305–313. doi: 10.1093/glycob/cwg034. [DOI] [PubMed] [Google Scholar]
- 50.Ioannou Y.A., Bishop D.F., Desnick R.J. Overexpression of human alpha-galactosidase A results in its intracellular aggregation, crystallization in lysosomes, and selective secretion. J. Cell Biol. 1992;119:1137–1150. doi: 10.1083/jcb.119.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hohman T.C., Bowers B. Hydrolase secretion is a consequence of membrane recycling. J. Cell Biol. 1984;98:246–252. doi: 10.1083/jcb.98.1.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.