Abstract
Purpose
The purpose of this study was to assess, in a large sample of healthy young adults, sex differences in the binding potential of [11C]ABP688, a positron emission tomography (PET) tracer selective for the metabotropic glutamate type 5 (mGlu5) receptor.
Methods
High resolution [11C]ABP688 PET scans were acquired in 74 healthy volunteers (25 male, 49 female, mean age 20 ± 3.0). Mean binding potential (BPND = fND * (Bavail / KD)) values were calculated in the prefrontal cortex, striatum, and limbic regions using the simplified reference tissue model with cerebellar grey matter as the reference region.
Results
[11C]ABP688 BPND was significantly higher in men compared to women in the prefrontal cortex (p < 0.01), striatum (p < 0.001), and hippocampus (p < 0.05). Whole-brain BPND was 17% higher in men. BPND was not related to menstrual phase in women.
Conclusions
Binding availability of mGlu5 receptors as measured by PET [11C]ABP688 is higher in healthy men than women. This likely represents a source of variability in [11C]ABP688 studies and could have relevance for sex differences in cognitive-behavioral functions and neuropsychiatric disorders.
Keywords: Metabotropic glutamate receptors, mGluR5, PET, Gender
Introduction
ABP688 (3-((6-methylpyridin-2-yl)ethynyl)cyclo-hex-2-en-1-one-O-methyloxime) is a selective allosteric ligand of the metabotropic glutamate type 5 (mGlu5) receptor. Positron emission tomography (PET) studies with 11C labeled ABP688 have identified replicable group differences [1–4], but variability in the tracer’s binding in humans has proven to be unexpectedly high [5, 6]. Some sources of variability have been identified, including circadian changes in receptor availability [6, 7] and tracer (E)-isomer content [8]. However, variability remains high when these factors are accounted for [5, 8], suggesting that further sources remain unknown.
One potential source of variability is biological sex. Lower PET [11C]ABP688 binding in healthy women compared to men was seen in one study [1], but this was not found in a more recent comparison [9]. In clinical populations, the majority of scans have been conducted in men, but some evidence has emerged of sex-specific disease effects. In people with schizophrenia, regional [11C]ABP688 BPND was higher in female patients but lower in male patients relative to sex-matched healthy controls [10]. Pre-clinical and post-mortem research also suggests that sex differences exist in the role of mGlu5 receptors in substance use disorders, depression, and responses to stress [11–14].
Given the high variability in tracer binding measures, the relatively small sample sizes in previous studies coupled with the possibility of menstrual-cycle associated changes might have limited the ability to detect sex differences. Thus, the current study’s objective was to assess sex differences in [11C]ABP688 BPND in a large sample of healthy young adults.
Methods
Seventy-four healthy volunteers were included in this study (25 men and 49 women, mean age 20 ± 3.0 years). Five participants were current cigarette smokers (4 women, 1 man); none of the participants had any Axis I psychiatric disorders. Participants were recruited from community advertisements (n = 25) or from one of three longitudinal cohorts (Quebec Study of Newborn Twins, n = 5, and two cohorts from the Quebec Longitudinal Study of Child Development, n = 44). In the case of twins, only a single volunteer per twin pair was included. The study was carried out in accordance with the Declaration of Helsinki and approved by the Research Ethics Board of the Montreal Neurological Institute, McGill University, the ethics committee of the CHU Sainte-Justine Research Center, and the ethics committe of the Institut de la Statistique du Quebec. All participants provided written informed consent.
For female participants, menstrual phase at the time of the scan was determined based on the date of last menstrual period and length of cycle (self-report). Serum levels of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) were measured in a subset of participants (n = 10) to confirm this. Of women not using hormonal contraception (n = 29), the majority (n = 21) were tested during the follicular phase, five during the luteal phase, and three during ovulation.
PET scans were acquired between 10 am and 3 pm using a high-resolution research tomograph (HRRT, CTI/Siemens). Prior to injection of the ligand, a 6-min transmission scan was performed with 137Cs to correct for tissue attenuation. Subsequently, a 60-min dynamic scan was initiated concurrent with the beginning of a one-minute bolus injection of 370 MBq [11C]ABP688. Dynamic data were collected with the scanner in list mode and reconstructed using an ordered subset maximization algorithm including motion correction to the transmission scan. High-resolution (1 mm3) T1-weighted anatomical magnetic resonance imaging (MRI) scans were acquired using a 1.5 T Siemens Sonata scanner (gradient echo pulse sequence, repetition time = 9.7 ms, echo time = 4 ms, flip angle = 12°, field of view = 250 mm and matrix = 256 × 256) or a 3 T Siemens Trio TIM scanner (MPRAGE sequence, repetition time = 2300 ms, echo time = 3.42 ms, flip angle = 9°, field of view = 256 mm and matrix = 256 × 256).
Regions of interest (ROIs) were defined using standard masks on the MNI152 template then registered to individual PET images. The ROIs included three prefrontal cortex subregions (orbitofrontal, dorsolateral, and medial), three functional striatum subregions (associative, sensorimotor and ventral), insula, hippocampus, and amygdala. Regional non-displaceable binding potential values (BPND) were extracted from each ROI using the simplified reference tissue model with cerebellar grey matter as the reference tissue. Scan start times were compared between men and women using independent samples t tests. Percent (E)-isomer content and injected tracer mass were compared using the Wilcoxon rank sum test due to their non-normal distribution. The effect of sex on BPND values was analyzed using repeated measures analysis of covariance (ANCOVA) with region as a repeated measure, sex as a between subject factor, and tracer (Z)-isomer content and smoking status as covariates. Post-hoc independent samples t-tests were then performed within each ROI. Whole brain voxel-wise analyses of BPND were compared using SPM12 (Wellcome Functional Imaging Laboratory). Summary BPND values were computed as the unweighted mean of all examined regions. One-way ANOVA was used to assess the effect of menstrual phase on BPND. In exploratory analyses, correlations between summary BPND and serum LH or FSH levels were assessed using Pearson’s r.
Results
Sample and PET scan characteristics are summarized in Table 1. Scans performed on men and women did not differ in start time (t = −0.001, p = 1.0) or % (E)-isomer in tracer batch (Wilcoxon rank sum test W = 708, p = 0.28). Tracer injected mass was higher in scans performed on women than men (W = 437, p = 0.045). Injected mass was not correlated with BPND in any region (ps > 0.06, uncorrected) and was therefore not included in subsequent analyses [15].
Table 1.
Participant and scan characteristics
| Characteristic | Men | Women | p-value |
|---|---|---|---|
| Age (mean ± SD) | 20.7 ± 4.2 | 19.6 ± 2.2 | 0.13 |
| Smokers (n) | 1 | 4 | 0.66 |
| Recruitment method (n) | 9 CA, 16 QCS | 16 CA, 33 QCS | 0.98 |
| Scan start time, minutes from 10:00 (mean ± SD) | 102 ± 56.2 | 102 ± 58.1 | 1.0 |
| % (E)-isomer (mean ± SD) | 91.6 ± 4.9 | 91.8 ± 3.2 | 0.23 |
| Mass tracer injected, μg (mean ± SD) | 7.07 ± 6.1 | 10.2 ± 6.6 | 0.045 |
CA, community advertisement; QCS, Quebec cohort studies (Quebec Longitudinal Study of Child Development, n = 44 or the Quebec Study of Newborn Twins, n = 5)
p values from Fisher’s exact test (smokers), Chi-squared test (recruitment method), independent samples t-tests (age and start time), or Wilcoxon rank sum tests in the case of non-normality (isomer content and tracer mass)
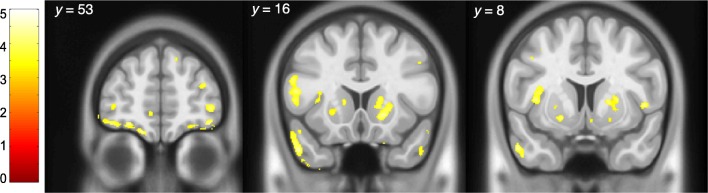
In the ROI analysis of BPND, the ANCOVA yielded main effects of sex (F1,68 = 12.8, p = 0.001) and region (F8,544 = 3.6, p < 0.001) and a sex × region interaction (F8,624 = 6.0, p < 0.001). In post hoc comparisons of each ROI, BPND was significantly higher in men compared to women in all regions (ps < 0.042) apart from the amygdala (p = 0.062) (Table 2). Comparison of the magnitude of sex differences in each region suggests that the sex × region interaction emerges from greater difference between sexes in prefrontal cortex subregions than in subcortical limbic structures. The largest magnitude of difference was in the orbitofrontal cortex where mean BPND was 22% greater in men compared to women, and the smallest in the amygdala, where BPND was 11% higher in men. Voxel-wise analyses were consistent with these findings, with clusters of higher BPND in men emerging in the prefrontal cortex, striatum, and insula (Fig. 1), as well as in the temporal, parietal, and occipital cortices (cluster-level ps < 0.05, familywise-error-corrected).
Table 2.
Mean [11C]ABP688 BPND is higher in men than in women across the brain
| Region | Men, mean ± SD BPND |
Women, mean ± SD BPND |
% Difference (men > women) |
p-value |
|---|---|---|---|---|
| mPFC | 1.1 ± 0.26 | 0.94 ± 0.17 | 16% | 0.0097 |
| dlPFC | 1.0 ± 0.25 | 0.83 ± 0.16 | 20% | 0.0045 |
| OFC | 0.95 ± 0.22 | 0.78 ± 0.14 | 22% | 0.00093 |
| Associative striatum | 1.3 ± 0.22 | 1.1 ± 0.18 | 16% | 0.00068 |
| Sensorimotor striatum | 1.0 ± 0.18 | 0.86 ± 0.14 | 17% | 0.00016 |
| Ventral striatum | 1.4 ± 0.24 | 1.2 ± 0.18 | 17% | 0.00029 |
| Insula | 1.3 ± 0.21 | 1.1 ± 0.17 | 17% | 0.000081 |
| Hippocampus | 0.72 ± 0.18 | 0.64 ± 0.14 | 13% | 0.041 |
| Amygdala | 0.76 ± 0.18 | 0.69 ± 0.15 | 11% | 0.062 |
| Summary BP ND | 1.0 ± 0.20 | 0.89 ± 0.15 | 17% | 0.00037 |
mPFC, medial prefrontal cortex; dlPFC, dorsolateral prefrontal cortex; OFC, orbitofrontal cortex; p values from two-tailed t-tests. Summary BPND was computed as the unweighted mean of all regions
Fig. 1.
Voxel-wise t-map showing higher [11C]ABP688 BPND in men compared to women (threshold t = 3.21)
In women, BPND values did not differ across menstrual phase groups (ps > 0.05 in each region) (Fig. 2). BPND was not statistically related to LH (r = −0.33, p = 0.35) or FSH (r = −0.043, p = 0.91) levels in a subset of ten women.
Fig. 2.
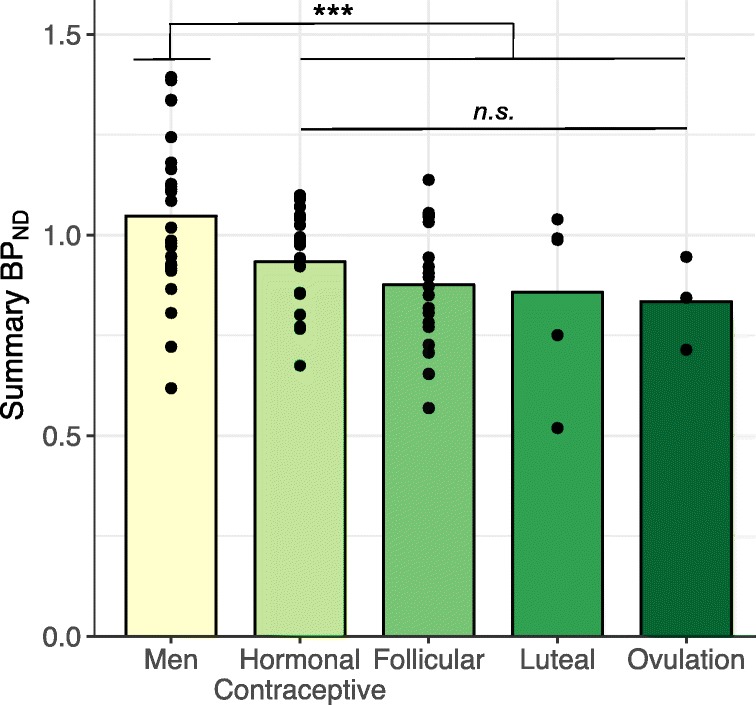
Mean BPND values across regions are higher in men compared to women but did not differ across menstrual phase in women (F1,47 = 2.4, p = 0.13); *** indicates p < 0.005
Discussion
In this pooled analysis of healthy young adults comprising the largest sample reported to date, brain [11C]ABP688 BPND values were significantly higher in men compared to women.
These results are in agreement with a previous study finding lower [11C]ABP688 BPND in female nonsmokers compared to male nonsmokers [1]. In comparison, several studies with an even sex split or greater numbers of female participants found no effect of sex on tracer binding [2, 9, 16]. Given the high variability in [11C]ABP688 binding estimates, sex differences may have been masked in previous studies with smaller sample sizes.
The observed sex differences might reflect an influence of gonadal hormones. Estrogen receptors are functionally coupled to mGlu5 receptors in striatal medium spiny neurons, and treatment with a negative allosteric modulator for mGlu5 abolishes estradiol enhancement of stimulant sensitization [12, 17]. The present work suggests that sex differences are present in healthy humans and can be identified using PET [11C]ABP688 imaging. Future studies should account for these differences both as a possible source of variability in binding measures and as a biological factor potentially contributing to sex differences in neurocognitive function and neuropsychiatric disorders.
Acknowledgments
This work was supported by the Canadian Institutes for Health Research, grants MOP-133537 (ML, JRS, CB, and MB), 119509 (ML and CB), 44072 (JRS), and 97910 (JRS); the Fonds du Recherche en Santé du Québec (FRQS), grants 981055 and 991027 (JRS); a grant from FRQS ERA-NET (ML); the Social Sciences and Humanities Research Council of Canada, grants 839-2000-1008 and 410-99-1048 (JRS and MB); and the Fonds de recherche du Québec Société et culture, grants 2002-RS-79238 and 2009-RG-124779 (JRS and MB).
Compliance with ethical standards
The authors declare that they have no conflict of interest. All procedures performed in studies involving human participants were in accordance with the ethical standards of the Research Ethics Board of the Montreal Neurological Institute, McGill University, the ethics committee of the CHU Sainte-Justine Research Center, the ethics committee of the Institut de la Statistique du Quebec, and the 1964 Helsinki declaration and its later amendments. Informed consent was obtained from all individual participants included in the study. This article does not contain any studies with non-human animals performed by any of the authors.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
K. Smart and S. M. L. Cox contributed equally to this work.
References
- 1.Akkus F, Ametamey SM, Treyer V, Burger C, Johayem A, Umbricht D, et al. Marked global reduction in mGluR5 receptor binding in smokers and ex-smokers determined by [11C]ABP688 positron emission tomography. Proc Natl Acad Sci USA. 2013;110:737–742. doi: 10.1073/pnas.1210984110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akkus F, Treyer V, Johayem A, Ametamey SM, Mancilla BG, Sovago J, et al. Association of Long-Term Nicotine Abstinence with Normal Metabotropic Glutamate Receptor-5 binding. Biol Psychiatry. 2016;79:474–480. doi: 10.1016/j.biopsych.2015.02.027. [DOI] [PubMed] [Google Scholar]
- 3.Milella MS, Marengo L, Larcher K, Fotros A, Dagher A, Rosa-Neto P, et al. Limbic system mGluR5 availability in cocaine dependent subjects: a high-resolution PET [(11)C]ABP688 study. NeuroImage. 2014;98:195–202. doi: 10.1016/j.neuroimage.2014.04.061. [DOI] [PubMed] [Google Scholar]
- 4.Martinez D, Slifstein M, Nabulsi N, Grassetti A, Urban NBL, Perez A, et al. Imaging glutamate homeostasis in cocaine addiction with the metabotropic glutamate receptor 5 positron emission tomography radiotracer [(11)C]ABP688 and magnetic resonance spectroscopy. Biol Psychiatry. 2014;75:165–171. doi: 10.1016/j.biopsych.2013.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smart K, Cox SML, Nagano-Saito A, Rosa-Neto P, Leyton M, Benkelfat C. Test-retest variability of [11C]ABP688 estimates of metabotropic glutamate receptor subtype 5 availability in humans. Synapse. 2018;72:e22041. doi: 10.1002/syn.22041. [DOI] [PubMed] [Google Scholar]
- 6.DeLorenzo C, Kumar JSD, Mann JJ, Parsey RV. In vivo variation in metabotropic glutamate receptor subtype 5 binding using positron emission tomography and [11C]ABP688. J Cereb Blood Flow Metab. 2011;31:2169–2180. doi: 10.1038/jcbfm.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elmenhorst D, Mertens K, Kroll T, Oskamp A, Ermert J, Elmenhorst E-M, et al. Circadian variation of metabotropic glutamate receptor 5 availability in the rat brain. J Sleep Res. 2016;25:754–761. doi: 10.1111/jsr.12432. [DOI] [PubMed] [Google Scholar]
- 8.Smart K, Cox SML, Kostikov A, Shalai A, Scala SG, Tippler M, et al. Effect of (Z)-isomer content on [11C]ABP688 binding potential in humans. Eur J Nucl Med Mol Imaging. 2019. 10.1007/s00259-018-4237-3. [DOI] [PubMed]
- 9.DuBois JM, Rousset OG, Rowley J, Porras-Betancourt M, Reader AJ, Labbe A, et al. Characterization of age/sex and the regional distribution of mGluR5 availability in the healthy human brain measured by high-resolution [(11)C]ABP688 PET. Eur J Nucl Med Mol Imaging. 2016;43:152–162. doi: 10.1007/s00259-015-3167-6. [DOI] [PubMed] [Google Scholar]
- 10.Akkus F, Treyer V, Ametamey SM, Johayem A, Buck A, Hasler G. Metabotropic glutamate receptor 5 neuroimaging in schizophrenia. Schizophr Res. 2017;183:95–101. doi: 10.1016/j.schres.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Ma Y, Hu J, Cheng W, Jiang H, Zhang X, et al. Prenatal chronic mild stress induces depression-like behavior and sex-specific changes in regional glutamate receptor expression patterns in adult rats. Neuroscience. 2015;301:363–374. doi: 10.1016/j.neuroscience.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 12.Grove-Strawser D, Boulware MI, Mermelstein PG. Membrane estrogen receptors activate the metabotropic glutamate receptors mGluR5 and mGluR3 to bidirectionally regulate CREB phosphorylation in female rat striatal neurons. Neuroscience. 2010;170:1045–1055. doi: 10.1016/j.neuroscience.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cozzoli DK, Kaufman MN, Nipper MA, Hashimoto JG, Wiren KM, Finn DA. Functional regulation of PI3K-associated signaling in the accumbens by binge alcohol drinking in male but not female mice. Neuropharmacology. 2016;105:164–174. doi: 10.1016/j.neuropharm.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gray AL, Hyde TM, Deep-Soboslay A, Kleinman JE, Sodhi MS. Sex differences in glutamate receptor gene expression in major depression and suicide. Mol Psychiatry. 2015;20:1057–1068. doi: 10.1038/mp.2015.91. [DOI] [PubMed] [Google Scholar]
- 15.Kraemer HC. A source of false findings in published research studies: adjusting for covariates. JAMA Psychiatry. 2015;72:961. doi: 10.1001/jamapsychiatry.2015.1178. [DOI] [PubMed] [Google Scholar]
- 16.Esterlis I, DellaGioia N, Pietrzak RH, Matuskey D, Nabulsi N, Abdallah CG, et al. Ketamine-induced reduction in mGluR5 availability is associated with an antidepressant response: an [11C]ABP688 and PET imaging study in depression. Mol Psychiatry. 2018;23:824–832. doi: 10.1038/mp.2017.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinez LA, Peterson BM, Meisel RL, Mermelstein PG. Estradiol facilitation of cocaine-induced locomotor sensitization in female rats requires activation of mGluR5. Behav Brain Res. 2014;271:39–42. doi: 10.1016/j.bbr.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]