Abstract
Male infertility is a major concern affecting human reproductive health. Asthenoteratospermia can cause male infertility through reduced motility and abnormal morphology of spermatozoa. Several genes, including DNAH1 and some CFAP family members, are involved in multiple morphological abnormalities of the sperm flagella (MMAF). However, these known genes only account for approximately 60% of human MMAF cases. Here, we conducted further genetic analyses by using whole-exome sequencing in a cohort of 65 Han Chinese men with MMAF. Intriguingly, bi-allelic mutations of TTC21A (tetratricopeptide repeat domain 21A) were identified in three (5%) unrelated, MMAF-affected men, including two with homozygous stop-gain mutations and one with compound heterozygous mutations of TTC21A. Notably, these men consistently presented with MMAF and additional abnormalities of sperm head-tail conjunction. Furthermore, a homozygous TTC21A splicing mutation was identified in two Tunisian cases from an independent MMAF cohort. TTC21A is preferentially expressed in the testis and encodes an intraflagellar transport (IFT)-associated protein that possesses several tetratricopeptide repeat domains that perform functions crucial for ciliary function. To further investigate the potential roles of TTC21A in spermatogenesis, we generated Ttc21a mutant mice by using CRISPR-Cas9 technology and revealed sperm structural defects of the flagella and the connecting piece. Our consistent observations across human populations and in the mouse model strongly support the notion that bi-allelic mutations in TTC21A can induce asthenoteratospermia with defects of the sperm flagella and head-tail conjunction.
Keywords: flagella, male infertility, sequencing, sperm, TTC21A, CRISPR, exome, MMAF
Main Text
Human infertility is a worldwide health concern and affects in excess of 186 million people.1 Male infertility is often caused by asthenoteratospermia, characterized by obviously decreased sperm motility and multiple morphological abnormalities of the flagella (MMAF).2 Intraflagellar transport (IFT) is known to be essential for the development and maintenance of flagella; thus, it plays a very important role in spermatogenesis and male fertility.3, 4 IFT was first characterized in the green algae Chlamydomonas reinhardtii and was shown to rely on a motor-driven trafficking system that transports flagellar precursors to the site of assembly.3, 5, 6 To date, many studies on model organisms have shown that IFT defects cause a range of cilia-dependent disorders and male infertility. For example, a deletion mutation affecting Chlamydomonas IFT52 prevents flagellar formation.7 Furthermore, germ-cell-specific Ift140-knockout mice are completely infertile because of sperm flagellar malformation and dramatic reductions in sperm count.8 Notably, all of the Ift20-, Ift25-, and Ift27-knockout mutants in mice result in male infertility.9, 10, 11 Despite numerous examples highlighting the relationship between IFT and flagellar formation in Chlamydomonas and animal models, the influence of IFT in human male infertility has not been reported.
Previous genetic studies on human MMAF-affected subjects have revealed several genes responsible for sperm flagellar defects. For example, AK7 (MIM: 615364), AKAP4 (MIM: 300185), ARMC2, CFAP43 (MIM: 617558), CFAP44 (MIM: 617559), CFAP69 (MIM: 617949), DNAH1 (MIM: 603332), FSIP2 (MIM: 618153), QRICH2 (MIM: 618304), and WDR66 (also known as CFAP251; MIM: 618146).12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26 However, the known MMAF-associated genes only account for approximately 60% of human MMAF cases. These findings indicate both that MMAF has a strong genetic heterogeneity and that, potentially, unknown genes involved in human MMAF exist.
In this study, 65 unrelated Han Chinese men with MMAF were enrolled from the First Affiliated Hospital of Anhui Medical University and the Affiliated Suzhou Hospital of Nanjing Medical University in China. All 65 men presented with primary infertility, and none had obvious primary-ciliary-dyskinesia-related symptoms, such as bronchitis, sinusitis, otitis media, and pneumonia. Nine individuals were from consanguineous families. The cytogenetic karyotype analysis was performed in all of these cases, and it indicated normal male chromosomal karyotypes (46; XY) and no large-scale deletions in the human Y chromosome. This study was approved by the institutional review boards at Fudan University, the First Affiliated Hospital of Anhui Medical University, and the Affiliated Suzhou Hospital of Nanjing Medical University. Informed consent was obtained from each individual.
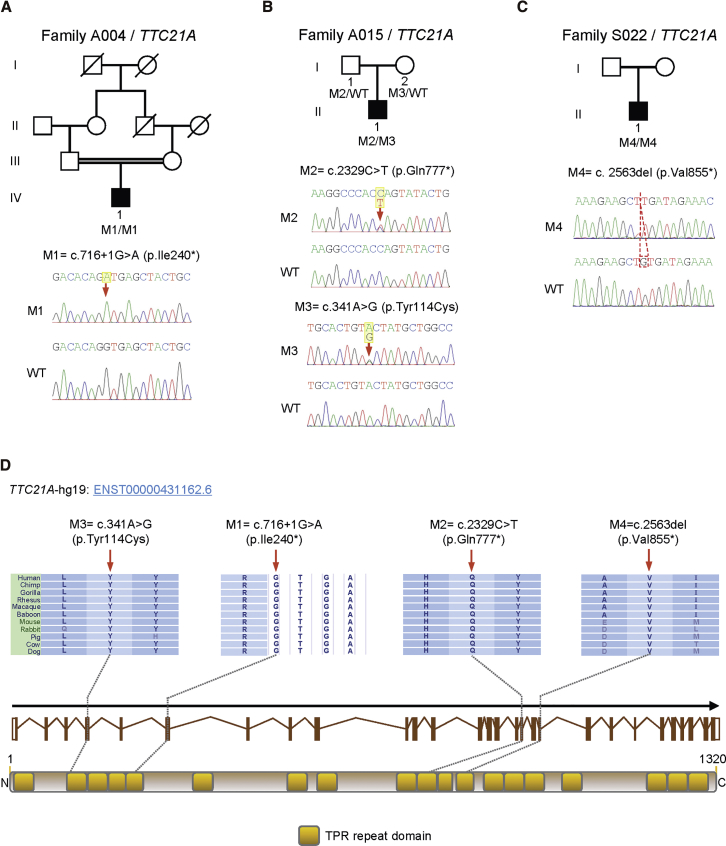
To investigate the unknown genetic factors involved in human MMAF, we carried out whole-exome sequencing (WES) and bioinformatic analyses on all 65 MMAF-affected men (see Supplemental Material and Methods). Intriguingly, bi-allelic mutations of TTC21A (tetratricopeptide repeat domain 21A, also known as IFT139A; MIM: 611430) were identified in one consanguineous family and two simplex individuals (Figure 1 and Table 1) who do not have bi-allelic pathogenic mutations in any known MMAF-associated genes. All the TTC21A mutations in MMAF-affected, Chinese men and their family members were verified by Sanger sequencing (Figure 1 and Table S1). TTC21A contains several tetratricopeptide repeat (TPR) domains that frequently exist in IFT proteins and seem important for ciliary function.27, 28 Notably, TTC21A is highly and specifically expressed in the human testis according to the databases of the Encyclopedia of DNA Elements (ENCODE), the Functional Annotation of the Mammalian Genome (FANTOM), and the Genotype-Tissue Expression (GTEx) project.
Figure 1.
Bi-allelic TTC21A Mutations Identified in Chinese Men with MMAF-Associated Asthenoteratospermia
(A) A splice-site mutation, c.716+1G>A, of TTC21A was identified in the consanguineous family A004. The proband (IV-1) was homozygous for this mutation. The amino acid alteration was predicted according to the verified alteration of cDNA.
(B) Compound heterozygous mutations of TTC21A were identified in the proband (II-1) from family A015. The bi-allelic mutations were confirmed to be inherited from his parental, heterozygous carriers.
(C) A 1 bp deletion of c.2563del resulted in a TTC21A nonsense mutation in family S022. The proband (II-1) was homozygous for this mutation.
(D) These four TTC21A mutations (M1–M4) are located at the conserved sites in TPR domains.
Yellow squares stand for TPR repeat domains as described by the Uniprot server. All mutations were verified by Sanger sequencing. The mutation positions are indicated by red arrows and a dashed box. Mutations are annotated in accordance with the HGVS’s recommendations. Abbreviations are as follows: WT = wild-type.
Table 1.
Bi-allelic TTC21A Mutations Identified in Chinese Men with Asthenoteratospermia
| Gene |
Subject |
|||
|---|---|---|---|---|
|
A004 IV-1 |
A015 II-1 |
S022 II-1 |
||
| TTC21A | TTC21A | TTC21A | TTC21A | |
| DNA changea | c.716+1G>A (homozygous) | c.341A>G (allele 1) | c.2329C>T (allele 2) | c.2563del (homozygous) |
| Amino acid alterationb | p.Ile240∗ | p.Tyr114Cys | p.Gln777∗ | p.Val855∗ |
| Mutation type | splice site | missense | nonsense | nonsense |
| Allele Frequency in Human Populations | ||||
| East Asians in ExAC | 0 | 1.2 × 10−4 | 0 | 0 |
| Han Chinese in 1000 Genomes Project | 0 | 0 | 0 | 0 |
| Han Chinese controlsc | 0 | 0 | 0 | 0 |
| Conservationd | ||||
| PhyloP | 5.292 | 4.669 | 0.481 | 2.439 |
| PhastCons | 1.000 | 1.000 | 1.000 | 0.116 |
| Functional Prediction | ||||
| SIFT | N/A | damaging | N/A | N/A |
| PolyPhen-2 | N/A | probably damaging | N/A | N/A |
| MutationTaster | N/A | disease causing | disease causing | N/A |
Abbreviations are as follows: N/A = not applicable.
The accession number of human TTC21A is GenBank: NM_145755.2.
Full-length TTC21A has 1,320 amino acids.
The Han Chinese controls consist of 300 fertile individuals and 668 individuals affected by non-reproductive disorders.
The PhastCons value is close to 1 when a nucleotide is conserved, and the predicted conserved sites are assigned positive scores by PhyloP.
In the consanguineous family A004, a homozygous splice-site TTC21A mutation, c.716+1G>A, that alters a consensus splice donor site in intron 6 was identified in proband IV-1 (Figure 1A). To further investigate the consequence of splicing, we carried out reverse-transcription PCR (RT-PCR) and cDNA sequencing (Figure S1 and Table S2). The RT-PCR product obtained from proband A004 IV-1 was longer than that of a control male subject (Figure S1A). Sanger sequencing revealed a 1 bp substitution (c.716+1G>A) at the splice donor site and partial retention of intron 6 (Figure S1B). Explaining this aberration, the utilization of a downstream, cryptic splice donor site in intron 6 instead of the normal splice donor site leads to an immediate premature stop codon (p.Ile240∗; Figure S1C).
In family A015, a stop-gain mutation, c.2329C>T (p.Gln777∗), and a missense mutation, c.341A>G (p.Tyr114Cys), of TTC21A were identified in proband II-1 (Figure 1B). Notably, this missense mutation was predicted to be potentially deleterious by all three bioinformatic tools: SIFT, PolyPhen-2, and MutationTaster (Table 1). The variant p.Tyr114Cys was located at a conserved, cilia-related TPR domain of the TTC21A protein (Figure 1D). The bi-allelic mutations in subject A015 II-1 were confirmed to be inherited from parental heterozygous carriers. In family S022, proband II-1 was homozygous for the TTC21A stop-gain mutation c.2563del (p.Val855∗) (Figure 1C).
To further estimate the allele frequencies of these candidate pathogenic mutations in TTC21A, we investigated 968 Han Chinese control individuals and the thousands of individuals archived in the 1000 Genomes Project and ExAC databases (Table 1). Our Han Chinese controls consisted of 300 healthy individuals and 668 individuals affected by non-reproductive disorders. The TTC21A missense mutation in subject A015 II-1 was extremely rare in the human population datasets (the allele frequency in East Asians of the ExAC database is 1.2 × 10−4), and it was absent from the 968 Han Chinese individuals of the control group (Table 1). The other three TTC21A mutations in the MMAF-affected, Chinese individuals in this study were absent from the human population datasets (Table 1). These observations are consistent with the autosomal-recessive inheritance of MMAF pathogenesis.
In addition, WES and data analyses were also performed on an additional cohort of 167 men with MMAF. In this cohort, 83 individuals who originated from North Africa were enrolled from the Clinique des Jasmin in Tunis. 52 individuals with a Middle Eastern origin (Iran) were treated in Tehran at the Royan Institute. 32 individuals were recruited in France: 25 at the Cochin Institute, 3 in Rouen, 2 in Grenoble, and 1 in Lille and Caen. All of these affected individuals had primary infertility with a typical MMAF phenotype. The details of the cohort and of the performed analysis were previously reported.25 Interestingly, a homozygous variant (c.3116+5G>T; Figure S2A) close to the TTC21A exon 23 splice donor site was identified in two unrelated Tunisian men (F001 and F002) from this cohort. This variant was also rare in the human population datasets and had an allelic frequency of 0.00058 in the Genome Aggregation Database (gnomAD). The TTC21A c.3116+5G>T variant was predicted to affect splicing by the Human Splicing Finder. RT-PCR was performed on sperm mRNA extracted from these two Tunisian subjects (F001 and F002) and two control subjects. The presence of cDNA was validated in both the controls and subjects by successful amplification of a control gene (PRM1). Amplification of TTC21A cDNA yielded a good signal in controls, but no amplification was obtained from the two TTC21A-mutated subjects, suggesting mRNA decay in these two subjects and thus validating the deleterious effect of variant c.3116+5G>T (Table S3 and Figure S2B).
The detailed phenotypes of the three Chinese, TTC21A-mutated men were examined through semen analysis and light microscopy (see Supplemental Material and Methods). Notably, the spermatozoa of subjects A004 IV-1 and S022 II-1 presented with very low motility and no progressive motility (Table 2). Severe abnormalities in sperm motility were also observed in subject A015 II-1. No obvious reductions in semen volume and sperm concentration were observed in the TTC21A-mutated men according to the standard of the World Health Organization (WHO) guidelines.
Table 2.
Semen Characteristics and Sperm Morphology in Chinese Men with Bi-allelic TTC21A Mutations
| Gene |
Subject |
|||
|---|---|---|---|---|
|
A004 IV-1 |
A015 II-1 |
S022 II-1 |
||
| TTC21A | TTC21A | TTC21A | ||
| Semen Parameters | ||||
| Semen volume (mL) | 4.6 | 3.4 | 3.8 | |
| Sperm concentration (106/mL) | 27.8 | 13.9 | 9.4 | |
| Motility (%) | 0.8 | 6.7 | 1.0 | |
| Progressive motility (%) | 0.0 | 1.7 | 0.0 | |
| Sperm Morphology | ||||
| Normal spermatozoa (%) | 0.0 | 0.0 | 0.0 | |
| Abnormal head-tail conjunction | Abnormal neck (%) | 46.5 | 23.0 | 45.9 |
| Absent head (%) | 0.5 | 0.5 | 1.3 | |
| Abnormal flagella | Short flagella (%) | 63.0 | 82.0 | 89.0 |
| Absent flagella (%) | 23.5 | 0.5 | 9.4 | |
| Coiled flagella (%) | 1.5 | 5.0 | 1.4 | |
| Angulation (%) | 2.5 | 1.0 | 0.0 | |
| Irregular caliber (%) | 5.5 | 2.0 | 0.0 | |
Semen samples were stained according to the modified Papanicolaou staining protocol for morphological evaluation. At least 200 spermatozoa were examined under a light microscope (Figures 2A–E). All three Chinese men with bi-allelic TTC21A mutations consistently presented with sperm neck defects, such as abnormal and/or broken necks (Table 2). Two major categories of sperm morphological abnormalities were investigated: abnormal head-tail conjunction and abnormal flagella (Table 2). One spermatozoon might present morphological abnormalities simultaneously in the sperm neck and flagellum. There were five sub-categories of abnormal flagella: (1) short flagella, (2) absent flagella, (3) coiled flagella, (4) angulation of flagella, and (5) irregular caliber.13 Each spermatozoon was classified to only one morphological sub-category of abnormal flagella according to its major flagellar abnormality.29 Remarkably, no normal spermatozoa were apparent in any of the TTC21A-mutated men. Abnormal necks and short flagella were the most frequently observed defects in these cases (Table 2).
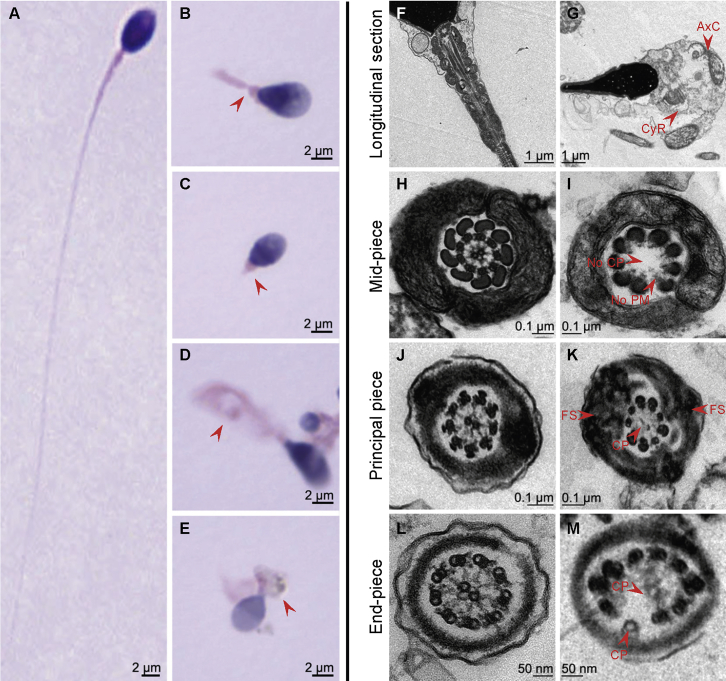
Figure 2.
Sperm Morphology and Ultra-Structures
(A) Normal morphology of a spermatozoon from a healthy control man.
(B–E) Most spermatozoa of A015 II-1 presented with multiple morphological abnormalities (red arrows), such as abnormal sperm necks, abnormal flagella (e.g., absent, coiled, irregular-caliber, or short flagella), and cytoplasm residue.
(F) The longitudinal section of a normal spermatozoon from a healthy control man showed regular axonemal structures that had a normal connecting piece and a mitochondrial sheath in the mid-piece of the sperm flagellum.
(G) In A015 II-1, most spermatozoa presented cytoplasm residue and scattered and disorganized axonemal components in sperm necks and flagella.
(H, J, and L) The ultra-structures seen in a cross section of a normal spermatozoa from a healthy control man. The typical “9 + 2” microtubule structure is shown, consisting of nine peripheral microtubule doublets and the central pair of microtubules. The organized mitochondrial sheath, outer dense fibrous sheath, and fibrous sheath were all observed.
(I, K, and M) The cross sections in the spermatozoa from A015 II-1 revealed multiple ultra-structural abnormalities, such as missing or disordered central-pair microtubules, the absence or disorders of the nine peripheral microtubule doublets, or hyperplasia of the fibrous sheaths.
These sperm morphological abnormalities were confirmed in the other TTC21A mutant men.
Abbreviations are as follows: AxC = axonemal components; CyR = cytoplasm residue; CP = central-pair microtubules; FS = fibrous sheath; and PM = peripheral microtubule doublets.
We conducted transmission electron microscopy (TEM) analyses to further investigate the ultra-structures of spermatozoa in the men with bi-allelic TTC21A mutations (see Supplemental Material and Methods). In contrast with the spermatozoa of the healthy male individual, spermatozoa of the TTC21A-mutated men had multiple ultra-structural abnormalities (Figures 2F–M). For example, cytoplasm residue and scattered and disorganized axonemal components were observed in the sperm necks and flagella (Figure 2G). Some cross sections showed absent or misplaced central-pair microtubules, absent or misplaced peripheral microtubule doublets, or hyperplasia of the fibrous sheaths (Figures 2I, 2K, and 2M).
To further investigate the biological consequences of TTC21A mutations in MMAF-affected men and the roles of TTC21A in sperm flagellar formation, we employed CRISPR-Cas9 technology to generate C57BL/6 mutant mice harboring a Ttc21a frameshift mutation.30, 31 The single-guide RNA (sgRNA) that was used in this study was specifically designed against chr9:119958998–119959075 (GRCm38/mm10) according to the position of the TTC21A stop-gain variant p.Val855∗, which is closer to the TTC21A C terminus and might permit the translation of a longer truncated protein than those that are expected from the other two stop-gain variants (p.Ile240∗ and p.Gln777∗) of this study. All experiments involving mice were performed according to the guideline for the care and use of laboratory animals of the US National Institutes of Health. This study was approved by the animal ethics committee at the School of Life Science, Fudan University.
A frameshift mutation (c.2534del) was identified in the founder mouse, and the presence of the variant in offspring was confirmed by Sanger sequencing (the primer information is provided in Table S4). This Ttc21a mutation (p.Lys845Argfs∗5) was predicted to cause premature translational termination (Figure S3). Real-time quantitative PCR (RT-qPCR) assays that used RNA from mouse testes were performed to compare the relative expression of Ttc21a transcripts between wild-type (WT) and Ttc21a-mutated (Ttc21amut/mut) male mice. The primer sequences and RT-qPCR conditions are indicated in Table S5. Notably, the relative mRNA expression level of Ttc21a in the mutated mice was significantly reduced by approximately 26% compared to that in WT mice (p < 0.001) (Figure S4). This indicates partial nonsense-mediated mRNA decay triggered by premature translational termination. The commercially available antibody anti-TTC21A (Abcam, UK), which targets the C terminus of human TTC21A, failed to react with mouse TTC21A.
Pubescent male mice with heterozygous and homozygous Ttc21a mutations were mated with WT females, and the numbers of pups per litter were counted to evaluate fertility. Compared with the litters of the WT male mice, litters of homozygous Ttc21a-mutated male mice (Ttc21amut/mut) were significantly smaller (Figure 3E). Notably, approximately 78% of Ttc21amut/mut male mice were infertile. No obvious difference of reproductive phenotypes was observed between WT and heterozygous Ttc21a-mutated carriers (Ttc21a+/mut), further suggesting the autosomal-recessive inheritance for Ttc21a-associated asthenoteratospermia.
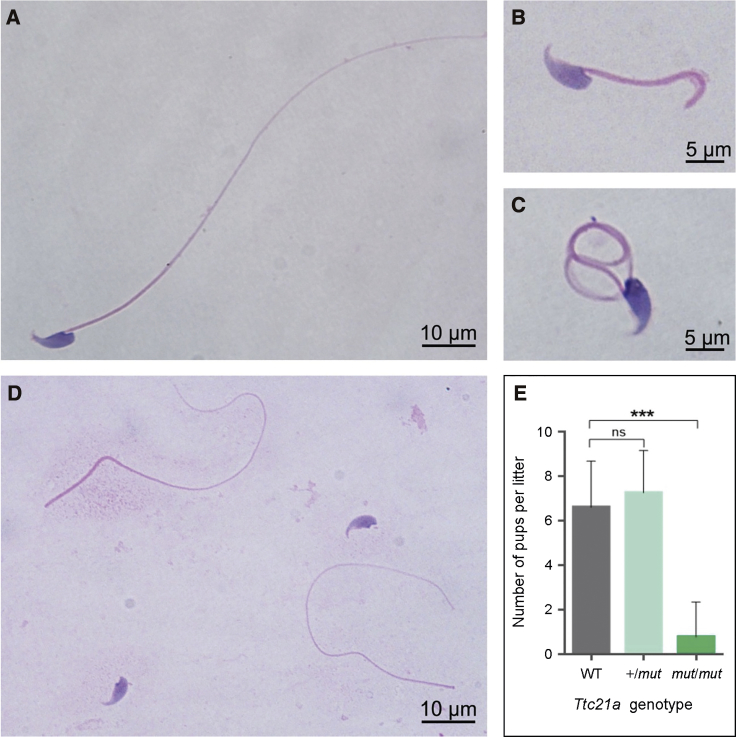
Figure 3.
Sperm Morphology and Fertility of the Ttc21a Mutant Male Mice
(A) A spermatozoon from a wild-type (WT) male mouse.
(B–D) Multiple morphological abnormalities, including short, coiled flagella and/or tailless spermatozoa (abnormal head-tail conjunction), in the spermatozoa from the Ttc21a mutant (Ttc21amut/mut) male mice, were observed under light microscopy.
(E) Fertility of heterozygous (Ttc21a+/mut) and homozygous (Ttc21amut/mut) male mice. Heterozygous and homozygous males were mated with WT females, and the numbers of pups per litter were counted. No significant difference in fertility was observed between the heterozygous carriers and the WT male mice. However, the litter sizes of homozygous mutated male mice were significantly reduced, and approximately 78% of these males were infertile.
Error bars represent the standard error of the mean. ∗∗∗ p < 0.001 (Student’s t test). Abbreviations are as follows: ns = no significance.
The publicly available data from the mouse ENCODE transcriptome and FANTOM5 project indicate that the expression of Ttc21a is predominant in adult mouse testes. Thus, we carefully assessed semen characteristics, sperm morphology, and the sperm ultra-structure of the Ttc21a mutant male mice (see Supplemental Material and Methods). Notably, the motility and progressive motility of spermatozoa were significantly reduced in the Ttc21amut/mut male mice (Table S6).
Semen samples from mice were stained with hematoxylin and eosin. Morphological abnormalities of spermatozoa of the Ttc21amut/mut male mice included short, coiled flagella and/or tailless spermatozoa (abnormal head-tail conjunction) (Figures 3B–D). The numbers of morphologically abnormal spermatozoa in the Ttc21amut/mut male mice were carefully counted (Table S6). As observed in humans, the most frequently observed abnormality in the Ttc21amut/mut male mice was abnormal head-tail conjunction in spermatozoa (Table S6).
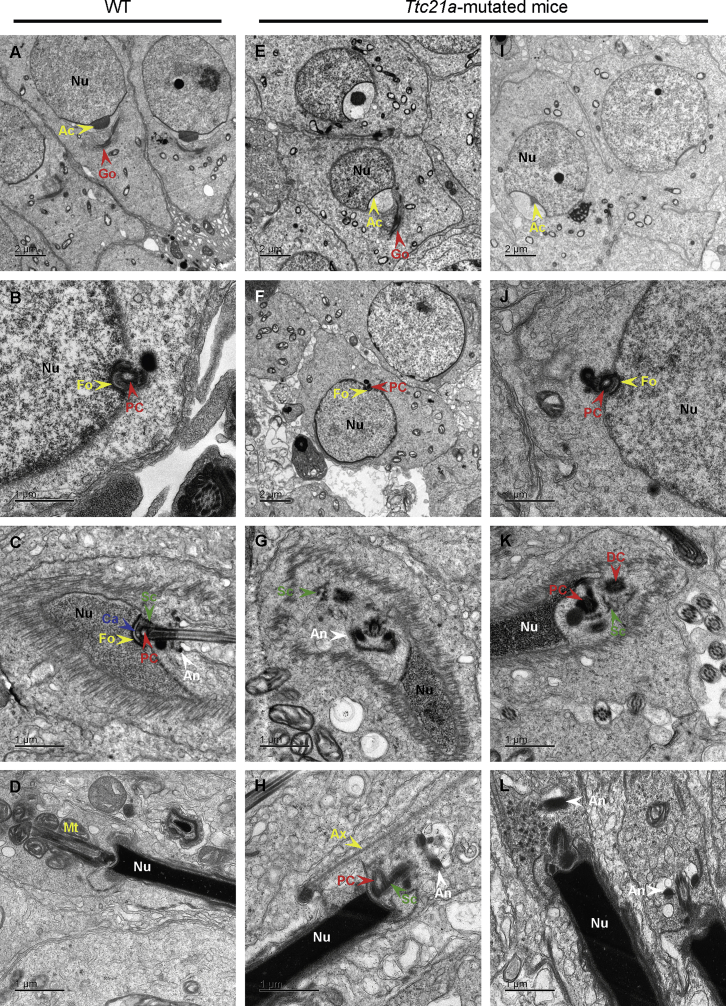
TEM observations were also performed on the spermatozoa of the testes and the cauda epididymes from the Ttc21amut/mut male mice, revealing the structural abnormalities of the connecting piece during spermiogenesis and multiple structural defects of the flagella (Figures 4 and S5). In round spermatids of the Ttc21amut/mut mice, the Golgi apparatuses were forming acrosomes (Figures 4E and 4I), and the proximal centrioles were implanted normally into nuclear fossa (Figures 4F and 4J). During nuclear condensation and the late elongated stage, the connecting pieces were not formed or maintained successfully in spermatids of the Ttc21amut/mut mice (Figures 4G, 4H, 4K, and 4L). The segmented columns and annulus were scattered. The mid-piece could not be formed without being surrounded by the mitochondria and having the annulus relocated, leading to an abnormal junction between the nucleus and the axoneme. These observations were consistent with the morphological abnormality of head-tail decapitation observed under light microscopy (Figure 3D). Additional axonemal structure abnormalities such as abnormal bulges, extra peripheral microtubule doublets, lack of central-pair microtubules, absent dynein arms, and abnormal arrangement of the nine peripheral microtubule doublets were also frequent (Figure S5).
Figure 4.
TEM Analyses of Testes from the Ttc21a Mutant Male Mice Reveal Structural Defects of the Connecting Piece During Spermiogenesis
(A–D) Representative ultra-structures of testicular spermatids from wild-type (WT) male mice during spermiogenesis. Golgi apparatuses were forming acrosomes (A), and the proximal centriole was transplanted into the nuclear fossa (B) in round spermatids. The connecting piece was formed by the assembly of the capitulum, proximal centriole, and segmented columns, and it was attached to the basal plates at the implantation fossa during nuclear condensation (C). In the late, elongated spermatid, the axoneme was surrounded by mitochondria and the forming mid-piece (D).
(E–L) Ultra-structures of testicular spermatids from the Ttc21amut/mut male mice during spermiogenesis. No obvious abnormality was observed in the early deformation stage of the round spermatid (E–J). However, during nuclear condensation and the late deformation stage, abnormal formations of the connecting pieces were observed. The structures indicated by green arrows were columnar, which was consistent with the previously reported structural characteristics of segmented columns.33 Several similar columnar structures appeared nearby. These columnar structures and annulus were scattered (G and K). The mid-pieces could not be formed without being surrounded by mitochondria and without relocation of the annulus (H and L). The misalignment between nucleus and axoneme was observed as well (H and L).
Abbreviations are as follows: Ac = acrosome; An = annulus; Ax = axoneme; Ca = capitilum; Dc = distal centriole; Fo = fossa; Go = Golgi apparatus; Mt = mitochondrion; Nu = nucleus; Pc = proximal centriole; and Sc = segmented column.
As mentioned above, we identified six TTC21A mutant alleles in Chinese subjects with MMAF. The majority (5/6) of these TTC21A alleles contain stop-gain mutations (Figure S1). Furthermore, we compared the distributions of TTC21A loss-of-function (LoF) variants in the ExAC database and in this study (Table S7). Our observations (p = 7.01 × 10−7, two tailed Fisher’s exact test) suggest that TTC21A LoF variants are associated with male infertility. Therefore, the MMAF phenotypes in these human subjects are preferentially explained by the bi-allelic mutations in TTC21A.
TTC21A encodes a member of the TPR family. Some proteins of the TPR family have been associated with ciliary function in non-human model organisms. For example, TTC10 (also known as IFT88) was previously reported to be involved in cilium biogenesis.27 The mutations in mouse Ttc10 can cause polycystic kidneys.27 Notably, some proteins of the TPR family (including TTC21A) are highly or preferentially expressed in the human testis according to the ENCODE, FANTOM, and GTEx databases. However, none of these TPR proteins had been directly linked to human male infertility. Our identification of bi-allelic TTC21A mutations in MMAF-affected men establishes the association between TRP proteins and human infertility.
TTC21A is also termed IFT139A. Although the molecular functions of TTC21A remains unclear, the data from functional protein association networks (STRING) interestingly indicated that the vast majority of predicted functional partners of both human TTC21A and mouse TTC21A were IFT components. The IFT particles contain two distinct protein complexes, A and B. As we mentioned above, IFT140 is a component of the IFT complex A, which is required for retrograde ciliary transport.8 IFT20 and IFT52 are components of the IFT complex B, which also plays important roles in the development and maintenance of the cilia.7, 9 Therefore, we carried out co-immunoprecipitation (Co-IP) experiments using the antibodies (Proteintech) for these three IFT components (IFT140, IFT20, and IFT52), respectively. Total protein was extracted from semen samples of control human subjects. As shown in Figure S6, the interactions between TTC21A and these IFT components were revealed, suggesting that TTC21A is very likely an IFT component interacting with IFT proteins.
The assembly of the connecting piece, such as the segmented columns between the sperm head and tail, is organized by the proximal centrioles, whereas both flagellar axoneme and outer dense fibers (ODFs) originate from the distal centrioles.32 The caudal ends of the nine segmented columns in the connecting piece fuse with the nine ODFs in the flagellar mid-piece during the late flagellar developmental stage.32 Therefore, the assemblies of the connecting piece and flagellar axoneme seem to be independent of each other. However, previous studies in mouse Spata6 strongly indicated that these two processes are, in fact, interrelated.33 Spata6 encodes a protein involved in myosin-based microfilament transport. This protein is required for the formation of the segmented columns and capitulum (parts essential for linking the developing flagellum to the head during late spermiogenesis), and its expression is restricted to the segmented columns and capitulum.33 Intriguingly, TEM analysis of the Spata6-knockout mice evidenced malformations of both the segmented columns and of the axoneme;33 these findings are quite similar to the observations made regarding the Ttc21amut/mut mice of our study.
TTC21B (MIM: 612014), the paralog of TTC21A, encodes an intraflagellar transport-A component protein. The TTC21A and TTC21B proteins are approximately 50% identical in amino acid sequences and have similar TRP domains. Mutations in TTC21B were reported to be associated with various ciliopathies, but the TTC21B mutations were not described to induce male infertility.34, 35 According to the expression data from the Human Protein Atlas, TTC21B has ubiquitous expression in various tissues, including male tissues and female tissues, whereas the expression of TTC21A is high and specific to the testes. Moreover, the immunohistochemical signal of TTC21B can be detected in both cells of the seminiferous ducts and in Leydig cells. Specific TTC21A staining (Figure S7) is detected in preleptotene spermatocytes, pachytene spermatocytes, round spermatids, and elongated spermatids. In addition, our ultra-structural analyses of testes from the Ttc21amut/mut male mice revealed some severe abnormalities of the connecting piece during nuclear condensation and the late elongated stage. The differential expressions between human TTC21A and TTC21B, together with our experimental observations made with TEM, strongly suggest that TTC21A has specific IFT functions in spermatogenesis.
In summary, our findings from both human subjects and the mouse model demonstrate that bi-allelic mutations in TTC21A can induce asthenoteratospermia characterized by reduced sperm motility and multiple sperm malformations. The TTC21A-associated morphological abnormalities affect sperm flagella and head-tail conjunction. The detailed molecular contributions of TTC21A to sperm flagellar formation and intraflagellar transport need to be further investigated in future studies. Our findings also suggest that other TPR-related genes or IFT transport genes could be involved in human asthenoteratospermia and other related ciliopathies.36, 37
Declaration of Interests
The authors declare no competing interests.
Acknowledgments
We would like to thank the Center of Cryo-Electron Microscopy at Zhejiang University for technical support. This work was supported by the National Natural Science Foundation of China (31625015 and 31521003), the Foundation of the Department of Science and Technology of Anhui Province (2017070802D150), the Foundation of the Education Department of Anhui Province (KJ2016A370), the Shanghai Medical Center of Key Programs for Female Reproductive Diseases (2017ZZ01016), and the Shanghai Municipal Science and Technology Major Project (2017SHZDZX01).
Published: March 28, 2019
Footnotes
Supplemental Data can be found online at https://doi.org/10.1016/j.ajhg.2019.02.020.
Contributor Information
Yunxia Cao, Email: caoyunxia6@126.com.
Feng Zhang, Email: zhangfeng@fudan.edu.cn.
Web Resources
1000 Genomes Project, http://www.internationalgenome.org
ENCODE, https://www.encodeproject.org
ExAC Browser, http://exac.broadinstitute.org
FANTOM, http://fantom.gsc.riken.jp
Human Protein Atlas, https://www.proteinatlas.org
Human Splicing Finder, http://www.umd.be/HSF3/
OMIM, http://www.omim.org
PolyPhen-2, http://genetics.bwh.harvard.edu/pph2/
SIFT, http://sift.jcvi.org
STRING, https://string-db.org
UCSC Genome Browser, http://genome.ucsc.edu
UniProt, https://www.uniprot.org
Supplemental Data
References
- 1.Inhorn M.C., Patrizio P. Infertility around the globe: New thinking on gender, reproductive technologies and global movements in the 21st century. Hum. Reprod. Update. 2015;21:411–426. doi: 10.1093/humupd/dmv016. [DOI] [PubMed] [Google Scholar]
- 2.Coutton C., Escoffier J., Martinez G., Arnoult C., Ray P.F. Teratozoospermia: Spotlight on the main genetic actors in the human. Hum. Reprod. Update. 2015;21:455–485. doi: 10.1093/humupd/dmv020. [DOI] [PubMed] [Google Scholar]
- 3.Pedersen L.B., Rosenbaum J.L. Intraflagellar transport (IFT) role in ciliary assembly, resorption and signalling. Curr. Top. Dev. Biol. 2008;85:23–61. doi: 10.1016/S0070-2153(08)00802-8. [DOI] [PubMed] [Google Scholar]
- 4.Buisson J., Chenouard N., Lagache T., Blisnick T., Olivo-Marin J.C., Bastin P. Intraflagellar transport proteins cycle between the flagellum and its base. J. Cell Sci. 2013;126:327–338. doi: 10.1242/jcs.117069. [DOI] [PubMed] [Google Scholar]
- 5.Piperno G., Mead K. Transport of a novel complex in the cytoplasmic matrix of Chlamydomonas flagella. Proc. Natl. Acad. Sci. USA. 1997;94:4457–4462. doi: 10.1073/pnas.94.9.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ishikawa H., Marshall W.F. Ciliogenesis: Building the cell’s antenna. Nat. Rev. Mol. Cell Biol. 2011;12:222–234. doi: 10.1038/nrm3085. [DOI] [PubMed] [Google Scholar]
- 7.Deane J.A., Cole D.G., Seeley E.S., Diener D.R., Rosenbaum J.L. Localization of intraflagellar transport protein IFT52 identifies basal body transitional fibers as the docking site for IFT particles. Curr. Biol. 2001;11:1586–1590. doi: 10.1016/s0960-9822(01)00484-5. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y., Liu H., Li W., Zhang Z., Zhang S., Teves M.E., Stevens C., Foster J.A., Campbell G.E., Windle J.J. Intraflagellar transporter protein 140 (IFT140), a component of IFT-A complex, is essential for male fertility and spermiogenesis in mice. Cytoskeleton (Hoboken) 2018;75:70–84. doi: 10.1002/cm.21427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Z., Li W., Zhang Y., Zhang L., Teves M.E., Liu H., Strauss J.F., 3rd, Pazour G.J., Foster J.A., Hess R.A., Zhang Z. Intraflagellar transport protein IFT20 is essential for male fertility and spermiogenesis in mice. Mol. Biol. Cell. 2016;27:3705–3716. doi: 10.1091/mbc.E16-05-0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu H., Li W., Zhang Y., Zhang Z., Shang X., Zhang L., Zhang S., Li Y., Somoza A.V., Delpi B. IFT25, an intraflagellar transporter protein dispensable for ciliogenesis in somatic cells, is essential for sperm flagella formation. Biol. Reprod. 2017;96:993–1006. doi: 10.1093/biolre/iox029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y., Liu H., Li W., Zhang Z., Shang X., Zhang D., Li Y., Zhang S., Liu J., Hess R.A. Intraflagellar transporter protein (IFT27), an IFT25 binding partner, is essential for male fertility and spermiogenesis in mice. Dev. Biol. 2017;432:125–139. doi: 10.1016/j.ydbio.2017.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baccetti B., Collodel G., Estenoz M., Manca D., Moretti E., Piomboni P. Gene deletions in an infertile man with sperm fibrous sheath dysplasia. Hum. Reprod. 2005;20:2790–2794. doi: 10.1093/humrep/dei126. [DOI] [PubMed] [Google Scholar]
- 13.Ben Khelifa M., Coutton C., Zouari R., Karaouzène T., Rendu J., Bidart M., Yassine S., Pierre V., Delaroche J., Hennebicq S. Mutations in DNAH1, which encodes an inner arm heavy chain dynein, lead to male infertility from multiple morphological abnormalities of the sperm flagella. Am. J. Hum. Genet. 2014;94:95–104. doi: 10.1016/j.ajhg.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang S., Wang X., Li W., Yang X., Li Z., Liu W., Li C., Zhu Z., Wang L., Wang J. Biallelic mutations in CFAP43 and CFAP44 cause male infertility with multiple morphological abnormalities of the sperm flagella. Am. J. Hum. Genet. 2017;100:854–864. doi: 10.1016/j.ajhg.2017.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coutton C., Vargas A.S., Amiri-Yekta A., Kherraf Z.E., Ben Mustapha S.F., Le Tanno P., Wambergue-Legrand C., Karaouzène T., Martinez G., Crouzy S. Mutations in CFAP43 and CFAP44 cause male infertility and flagellum defects in Trypanosoma and human. Nat. Commun. 2018;9:686. doi: 10.1038/s41467-017-02792-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sha Y.W., Wang X., Su Z.Y., Mei L.B., Ji Z.Y., Bao H., Li P. Patients with multiple morphological abnormalities of the sperm flagella harbouring CFAP44 or CFAP43 mutations have a good pregnancy outcome following intracytoplasmic sperm injection. Andrologia. 2019;51:e13151. doi: 10.1111/and.13151. [DOI] [PubMed] [Google Scholar]
- 17.Lorès P., Coutton C., El Khouri E., Stouvenel L., Givelet M., Thomas L., Rode B., Schmitt A., Louis B., Sakheli Z. Homozygous missense mutation L673P in adenylate kinase 7 (AK7) leads to primary male infertility and multiple morphological anomalies of the flagella but not to primary ciliary dyskinesia. Hum. Mol. Genet. 2018;27:1196–1211. doi: 10.1093/hmg/ddy034. [DOI] [PubMed] [Google Scholar]
- 18.Dong F.N., Amiri-Yekta A., Martinez G., Saut A., Tek J., Stouvenel L., Lorès P., Karaouzène T., Thierry-Mieg N., Satre V. Absence of CFAP69 causes male infertility due to multiple morphological abnormalities of the flagella in human and mouse. Am. J. Hum. Genet. 2018;102:636–648. doi: 10.1016/j.ajhg.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He X., Li W., Wu H., Lv M., Liu W., Liu C., Zhu F., Li C., Fang Y., Yang C. Novel homozygous CFAP69 mutations in humans and mice cause severe asthenoteratospermia with multiple morphological abnormalities of the sperm flagella. J. Med. Genet. 2019;56:96–103. doi: 10.1136/jmedgenet-2018-105486. [DOI] [PubMed] [Google Scholar]
- 20.Kherraf Z.E., Amiri-Yekta A., Dacheux D., Karaouzène T., Coutton C., Christou-Kent M., Martinez G., Landrein N., Le Tanno P., Fourati Ben Mustapha S. A homozygous ancestral SVA-insertion-mediated deletion in WDR66 induces multiple morphological abnormalities of the sperm flagellum and male infertility. Am. J. Hum. Genet. 2018;103:400–412. doi: 10.1016/j.ajhg.2018.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Auguste Y., Delague V., Desvignes J.P., Longepied G., Gnisci A., Besnier P., Levy N., Beroud C., Megarbane A., Metzler-Guillemain C., Mitchell M.J. Loss of calmodulin- and radial-spoke-associated complex protein CFAP251 leads to immotile spermatozoa lacking mitochondria and infertility in men. Am. J. Hum. Genet. 2018;103:413–420. doi: 10.1016/j.ajhg.2018.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li W., He X., Yang S., Liu C., Wu H., Liu W., Lv M., Tang D., Tan J., Tang S. Biallelic mutations of CFAP251 cause sperm flagellar defects and human male infertility. J. Hum. Genet. 2019;64:49–54. doi: 10.1038/s10038-018-0520-1. [DOI] [PubMed] [Google Scholar]
- 23.Martinez G., Kherraf Z.E., Zouari R., Fourati Ben Mustapha S., Saut A., Pernet-Gallay K., Bertrand A., Bidart M., Hograindleur J.P., Amiri-Yekta A. Whole-exome sequencing identifies mutations in FSIP2 as a recurrent cause of multiple morphological abnormalities of the sperm flagella. Hum. Reprod. 2018;33:1973–1984. doi: 10.1093/humrep/dey264. [DOI] [PubMed] [Google Scholar]
- 24.Liu W., Wu H., Wang L., Yang X., Liu C., He X., Li W., Wang J., Chen Y., Wang H. Homozygous loss-of-function mutations in FSIP2 cause male infertility with asthenoteratospermia. J. Genet. Genomics. 2018;46 doi: 10.1016/j.jgg.2018.09.006. S1673-8527(18)30204-2. [DOI] [PubMed] [Google Scholar]
- 25.Coutton C., Martinez G., Kherraf Z.E., Amiri-Yekta A., Boguenet M., Saut A., He X., Zhang F., Cristou-Kent M., Escoffier J. Bi-allelic mutations in ARMC2 lead to severe astheno-teratozoospermia due to sperm flagellum malformations in humans and mice. Am. J. Hum. Genet. 2019;104:331–340. doi: 10.1016/j.ajhg.2018.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen Y., Zhang F., Li F., Jiang X., Yang Y., Li X., Li W., Wang X., Cheng J., Liu M. Loss-of-function mutations in QRICH2 cause male infertility with multiple morphological abnormalities of the sperm flagella. Nat. Commun. 2019;10:433. doi: 10.1038/s41467-018-08182-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pazour G.J., Dickert B.L., Vucica Y., Seeley E.S., Rosenbaum J.L., Witman G.B., Cole D.G. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J. Cell Biol. 2000;151:709–718. doi: 10.1083/jcb.151.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Dam T.J., Townsend M.J., Turk M., Schlessinger A., Sali A., Field M.C., Huynen M.A. Evolution of modular intraflagellar transport from a coatomer-like progenitor. Proc. Natl. Acad. Sci. USA. 2013;110:6943–6948. doi: 10.1073/pnas.1221011110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang S.M., Li H.B., Wang J.X., Shi Y.C., Cheng H.B., Wang W., Li H., Hou J.Q., Wen D.G. Morphological characteristics and initial genetic study of multiple morphological anomalies of the flagella in China. Asian J. Androl. 2015;17:513–515. doi: 10.4103/1008-682X.146100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ran F.A., Hsu P.D., Wright J., Agarwala V., Scott D.A., Zhang F. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 2013;8:2281–2308. doi: 10.1038/nprot.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abbasi F., Miyata H., Ikawa M. Revolutionizing male fertility factor research in mice by using the genome editing tool CRISPR/Cas9. Reprod. Med. Biol. 2017;17:3–10. doi: 10.1002/rmb2.12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chemes H.E. Sperm Centrioles and Their Dual Role in Flagellogenesis and Cell Cycle of the Zygote. In: Schatten H., editor. The Centrosome: Cell and Molecular Mechanisms of Functions and Dysfunctions in Disease. Humana Press; Totowa, NJ: 2012. pp. 33–48. [Google Scholar]
- 33.Yuan S., Stratton C.J., Bao J., Zheng H., Bhetwal B.P., Yanagimachi R., Yan W. Spata6 is required for normal assembly of the sperm connecting piece and tight head-tail conjunction. Proc. Natl. Acad. Sci. USA. 2015;112:E430–E439. doi: 10.1073/pnas.1424648112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tran P.V., Haycraft C.J., Besschetnova T.Y., Turbe-Doan A., Stottmann R.W., Herron B.J., Chesebro A.L., Qiu H., Scherz P.J., Shah J.V. THM1 negatively modulates mouse sonic hedgehog signal transduction and affects retrograde intraflagellar transport in cilia. Nat. Genet. 2008;40:403–410. doi: 10.1038/ng.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davis E.E., Zhang Q., Liu Q., Diplas B.H., Davey L.M., Hartley J., Stoetzel C., Szymanska K., Ramaswami G., Logan C.V., NISC Comparative Sequencing Program TTC21B contributes both causal and modifying alleles across the ciliopathy spectrum. Nat. Genet. 2011;43:189–196. doi: 10.1038/ng.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Badano J.L., Mitsuma N., Beales P.L., Katsanis N. The ciliopathies: An emerging class of human genetic disorders. Annu. Rev. Genomics Hum. Genet. 2006;7:125–148. doi: 10.1146/annurev.genom.7.080505.115610. [DOI] [PubMed] [Google Scholar]
- 37.Fliegauf M., Benzing T., Omran H. When cilia go bad: Cilia defects and ciliopathies. Nat. Rev. Mol. Cell Biol. 2007;8:880–893. doi: 10.1038/nrm2278. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.