Abstract
Malignant tension hydrothorax is rare. Here we present a malignant tension hydrothorax secondary to small cell lung cancer (SCLC). To our knowledge, this is the first report of this entity. Briefly, a 53‐year‐old male smoker presented in acute distress after months of fatigue, shortness of breath, and weight loss. On examination his heart rate was 122, respiratory rate 31, blood pressure 102/60, oxygen saturation 92%, and he had tracheal deviation to the left with absent breath sounds on the right. Work‐up revealed hyponatraemia and a large pleural effusion causing tracheal deviation, mediastinal shift and compression of abdominal contents. There were associated masses in the right lung upper lobe and tail of the pancreas. Urgent, interval drainage of the effusion over several hours with tube thoracostomy relieved his symptoms while also avoiding life‐threatening re‐expansion pulmonary oedema. Subsequent thoracoscopic pleural biopsies revealed extensive SCLC. He was advised to begin urgent chemotherapy.
Keywords: Malignant pleural effusion, re‐expansion pulmonary oedema, small cell lung cancer, tension hydrothorax
Introduction
Tension hydrothorax is an uncommon clinical entity and malignant aetiology is rare 1. The physiology of tension pneumothorax and hydrothorax are similar; however, their management strategies have notable differences. Here we present a case of malignant tension hydrothorax secondary to small cell lung cancer (SCLC). To our knowledge, this is the first report of this entity.
Case Report
A 53‐year‐old male with no known prior medical or surgical history presented in acute distress after several months of shortness of breath, fatigue, anorexia, and weight loss. He was a current 35 pack‐year smoker with a history of heavy alcohol use. He was afebrile, heart rate was 122, respiratory rate was 31, blood pressure was 102/60, and oxygen saturation was 92% on room air. He was unable to lie flat or sit reclined. Physical examination revealed a patent airway, tracheal deviation to the left, and distended neck veins. Breath sounds were audible over the left chest and absent on the right with dullness to percussion. A chest X‐ray was notable for complete opacification of the right hemithorax with evidence of mediastinal shift. Computed tomography imaging revealed a large pancreatic tail mass; intra‐abdominal and mediastinal adenopathy; a large right upper lobe mass extending into the mediastinum that was compressing the superior vena cava, right main pulmonary artery, and the right mainstem bronchus; and a large right pleural effusion causing tracheal deviation to the left, mediastinal shift and compression of the abdominal contents (Fig. 1).
Figure 1.

(A) Chest radiograph on presentation shows complete opacification of right hemithorax with tracheal deviation and mediastinal shift. (B) Contrast‐enhanced coronal computed tomography image at same time shows a right mediastinal mass compressing the superior vena cava (SVC), right main pulmonary artery, and right mainstem bronchus with an associated large right pleural effusion causing compression of abdominal contents.
An urgent 14‐Fr tube thoracostomy was performed, which resulted in return of serous fluid under pressure. Interval drainage of 1 L of fluid at a time followed by catheter clamping for 45 min was employed to avoid re‐expansion pulmonary oedema (RPE). At 4 h, the patient had resolution of symptoms. Repeat imaging revealed re‐expansion of the right middle and lower lobes with resolution of compression but persistence of a right upper lobe lesion. Laboratory findings were significant for severe hyponatraemia (115 mmol/L), a protein level of 6.2 g/dL, an albumin level of 3.5 g/dL, and an elevated serum lactate dehydrogenase (LDH, 311 unit/L). Hepatic function panel was within normal limits. Amylase and lipase levels were mildly elevated (107/156 unit/L, respectively). Pleural fluid biochemistry revealed a protein level of 4.0 g/dL, an albumin level of 2.6 g/dL, an LDH level of 387 unit/L, and a cholesterol level of 68 mg/dL. Pleural cytology was significant for abnormal cells with suspicion of malignancy. Over the next 48 h, his chest tube output continued to be high with a total accumulation prior to surgery of 5.8 L. He underwent further staging work‐up with simultaneous diagnostic bronchoscopy and right video‐assisted thoracic surgery with placement of a tunnelled intra‐pleural catheter. Intra‐operative findings demonstrated complete obliteration of the right upper lobe bronchus and narrowing of the bronchus intermedius. There was also concern for carcinomatosis of the right pleural space. Pathology of endobronchial and pleural biopsies revealed SCLC (Fig. 2). Pleural cytology from the initial drainage also ultimately demonstrated findings consistent with SCLC. The patient was recommended to begin urgent chemotherapy.
Figure 2.
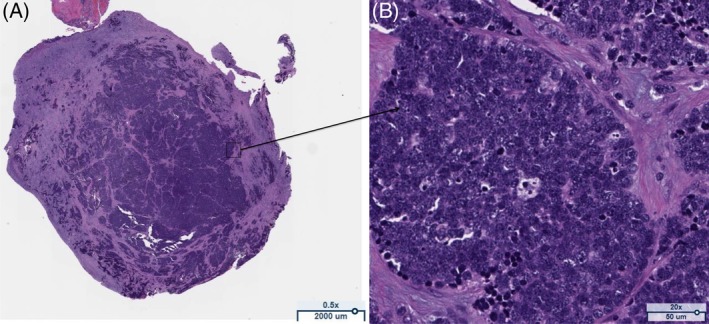
Section from the intra‐operative pleural biopsy. Specimen stained with haematoxylin and eosin (H&E). (A) Low power demonstrating histological appearance of small cell lung cancer (SCLC). (B) High power illustrating dense sheets of small cells with scant cytoplasm and finely granular nuclear chromatin consistent with SCLC.
Discussion
SCLC is a high‐grade neuroendocrine tumour that typically presents in the central airways of smokers and accounts for <15% of lung cancers 2. Serum biochemistry can identify associated paraneoplastic syndromes, such as was seen in our patient with severe hyponatraemia secondary to the syndrome of inappropriate anti‐diuretic hormone secretion. Although up to 70% of exudative pleural effusions are secondary to malignancy, malignant pleural effusion (MPE) from SCLC has only been reported in 4–8% of cases 3, 4. Despite this, MPE in SCLC remains clinically relevant, as it is a metastatic staging variant in the American Joint Committee on Cancer guidelines. Tension hydrothorax is also rare and has limited reports of malignant aetiology 1. To our knowledge, this is the first report of a SCLC MPE causing a tension hydrothorax.
Tension hydrothorax is similar to tension pneumothorax in that both will cause mediastinal compression and subsequent cardiopulmonary collapse. However, tension hydrothorax is due to a massive pleural effusion and not air. The build up of pleural fluid leads to an elevated intrathoracic pressure that eventually compromises diastolic filling and cardiac output. A missed diagnosis can be fatal but so too can an inappropriate intervention strategy. Physical examination and radiographic imaging can quickly differentiate tension hydrothorax and once diagnosed, attention must be given to the possible complications of thoracentesis or tube thoracostomy; pneumothorax, hypotension, bleeding, infection, and RPE 1. To prevent RPE, the British Thoracic Society guidelines suggest only draining 1.5 L of pleural fluid at a time and to avoid suction if possible. If RPE occurs, it can usually be managed with diuretics and supportive care, including oxygen supplementation and positive end expiratory pressure 5. In the case of our patient, his tension hydrothorax was secondary to a MPE from SCLC. Given the intrapleural pressure, we employed an interval drainage regimen of 1 L of fluid followed by clamping for 45 min to allow sufficient equilibration of intra‐pleural and alveolar pressure to prevent the rare, but life‐threatening complication of RPE.
Disclosure Statement
Appropriate written informed consent was obtained for publication of this case report and accompanying images.
Porter, ED , Finley, DJ , Phillips, JD . (2019) Tension hydrothorax secondary to small cell lung cancer. Respirology Case Reports, 7(5), ;e00420. 10.1002/rcr2.420
Associate Editor: Fraser Brims
References
- 1. Putnam JB Jr. 2002. Malignant pleural effusions. Surg. Clin. North Am. 82:867–883. [DOI] [PubMed] [Google Scholar]
- 2. Govindan R, Page N, Morgensztern D, et al. 2006. Changing epidemiology of small‐cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J. Clin. Oncol. 24:4539–4544. [DOI] [PubMed] [Google Scholar]
- 3. Johnston WW. 1985. The malignant pleural effusion. A review of cytopathologic diagnoses of 584 specimens from 472 consecutive patients. Cancer 56:905–909. [DOI] [PubMed] [Google Scholar]
- 4. Porcel JM, Esquerda A, Vives M, et al. 2014. Etiology of pleural effusions: analysis of more than 3,000 consecutive thoracenteses. Arch. Bronconeumol. 50:161–165. [DOI] [PubMed] [Google Scholar]
- 5. Verhagen M, van Buijtenen JM, and Geeraedts LM Jr. 2015. Reexpansion pulmonary edema after chest drainage for pneumothorax: a case report and literature overview. Respir. Med. Case Rep. 14:10–12. [DOI] [PMC free article] [PubMed] [Google Scholar]


