Abstract
Frontal fibrosing alopecia (FFA) is a relatively new scarring alopecia that is considered a variant of lichen planopilaris (LPP) with no recognized promising treatments. In this study, we tried to clarify the underlying signaling pathways and their roles in the pathogenesis and progression of FFA. Because of several differences in clinical manifestations, response to treatments, and pathological findings, these two conditions could be differentiated from each other. Taking into account the already discussed signaling pathways and involved players such as T cells, mast cells, and sebaceous glands, different possible therapeutic options could be suggested. In addition to treatments supported by clinical evidence, such as 5 alpha-reductase inhibitors, topical calcineurin inhibitors, hydroxychloroquine, peroxisome proliferator-activated receptor gamma agonists, and oral retinoid agents, various other treatment strategies and drugs, such as phototherapy, Janus kinase inhibitors, dehydroepiandrosterone, sirolimus, cetirizine, and rituximab, could be suggested to mitigate disease progression. Of course, such lines of treatment need further evaluation in clinical trials.
Keywords: Autoimmunity, scarring alopecia, frontal fibrosing alopecia, lichen planopilaris, immune response, treatment
Introduction
Frontal fibrosing alopecia (FFA) is a relatively newly described scarring alopecia known as a clinical variant of lichen planopilaris (LPP; Kossard et al., 1997). FFA is characterized by slowly progressive scarring alopecia on the hairline and affects explicitly postmenopausal women. Recently, an FFA severity score was suggested to be effectively used for categorizing patients in clinical practice and in research studies (Saceda-Corralo et al., 2018). Inflammation around the hair follicles is suggested as the etiology of hair loss in primary scarring alopecia, but there is not enough detail about the pathogenesis of LPP and FFA.
Recently, a worldwide increase in the incidence of FFA has been observed (MacDonald et al., 2012). Despite the global acceptance of a close association between FFA and LPP, there is limited evidence to support or reject this concept. Unfortunately, currently available treatments are not very effective to stop hair loss, although the progression of the diseases can be slowed with 5 alpha-reductase inhibitors (5αRI; eg, dutasteride and finasteride), hydroxychloroquine (HCQ), and oral retinoid agents (MacDonald et al., 2012). Conversely, topical and systemic corticosteroids and immunosuppressive drugs, such as mycophenolate mofetil, do not halt the progression of FFA (Kossard et al., 1997, Tosti et al., 2005), which may be due to the difference in predominant immune responses and the involvement of different immune pathways in these diseases. Moreover, the differences in the targeted hair types (ie, vellus, intermediate, and terminal) need more discussion.
During this study, we attempted to discuss the probable pathogenesis of FFA, risk factors, and the role of the immune system and to suggest novel therapeutic options. We searched the PubMed database using the keywords “frontal fibrosing alopecia” OR “FFA” OR “lichen planopilaris” OR “LPP” to access the latest findings related to these conditions. Subsequently, the most recent studies, those that consisted of novel findings, and made the most sense in this review in terms of quality of the article and current understanding of the disease were selected. Moreover, according to the literature and the authors’ experiences, some speculations have been added.
Similarities and differences between lichen planopilaris and frontal fibrosing alopecia
Both LPP and FFA are categorized as cicatricial alopecia with lichenoid infiltration around the bulge area of the hair follicles (Vano-Galvan et al., 2014). Nonetheless, some major differences in demography, clinical manifestations, pathology, and response to treatment exist between these two entities (Table 1).
Table 1.
Clinical, pathological and treatment differences between FFA and LPP.
| Frontal fibrosing alopecia (FFA) | Lichen planopilaris (LPP) | |
|---|---|---|
| -Clinical manifestation | - Involvement of vellus, intermediate hairs, and just hairline terminal hairs. - Hairline recession. -Often affects postmenopausal women - Yellowish facial papules and pigmented skin patches (Hu et al., 2012, Vano-Galvan et al., 2014) -Highly affects eyebrows. -Special manifestations: lonely hair sign (Ladizinski et al., 2013). Eyelash loss (Vano-Galvan et al., 2014), Red dots (Martinez-Perez and Churruca-Grijelmo, 2015), depression of frontal veins (Martinez-Perez and Churruca-Grijelmo, 2015), pigmented facial macules (Martinez-Perez and Churruca-Grijelmo, 2015), Limb hair loss (Ladizinski et al., 2013), Side beard hair loss in males, Hypopigmentation in wood lamp (Martinez-Perez and Churruca-Grijelmo, 2015), -Rarely associated with other cutaneous LP variants. -Associated with androgen deficiency. | -Terminal hair involvement. -Patchy diffused alopecia. -Usually affects the middle-aged population. -Pigmented skin patches. -Rarely involve eyebrows. -Involvement of the skin, mucosal, and nail. -Associated with androgen excess. |
| -Response to treatment | -5α-reductase inhibitors (5αRI) (Vano-Galvan et al., 2014). -Hydroxychloroquine (HCQ) (Chiang et al., 2010, Samrao et al., 2010). -Oral retinoids (Pedrosa et al., 2017, Pirmez et al., 2017, Rakowska et al., 2017). -Poor response to topical and systemic steroids and other immunosuppressives. | Responds well to topical or intralesional injections of corticosteroids and immunosuppressive drugs such as mycophenolate mofetil, cyclosporine. |
| -Pathology | -Much more apoptosis and less inflammatory in compare with LPP. -FFA may have inflammation extending below the isthmus in comparison with LPP (Wong and Goldberg, 2017). -Hypertrophic sebaceous glands with no associated vellus hair follicles (Pedrosa et al., 2017). | -Presence of a peri-vascular infiltrates in the dermis and colloid bodies. -Affection of interfollicular epidermis. |
Demography
FFA specifically affects postmenopausal women, although recently the disease has been reported in rare instances in young men and women. On the other hand, LPP is observed in the middle-aged population and its prevalence seems slightly higher in women than men (Dlova et al., 2013, Babahosseini et al., 2018).
Clinical manifestation
Unlike LPP, in which fundamentally terminal hairs are affected, FFA does not affect most of the terminal pigmented hairs on the scalp except those at the hairline, and both miniaturized terminal hairs (intermediate hairs) and vellus hairs are the primary victims (Holmes, 2016). Another important characteristic of FFA is the involvement of the eyebrows, which has been reported in up to 95% of cases, but occurs rarely with LPP (Bolduc et al., 2016). Although eyebrow loss was reported in both men and women, women may be affected more frequently than men with FFA (Bolduc et al., 2016). Moreover, eyebrow loss as the initial clinical presentation was associated with mild forms of FFA (Bolduc et al., 2016). Interestingly, involvement of the eyebrows precedes a frontal recession without any sign of clinical inflammation (Ladizinski et al., 2013). Moreover, both limb and flexural hairs could be affected in 25% of patients with FFA (Ladizinski et al., 2013).
In contrast, patchy permanent scalp hair loss occurs with rare involvement of the eyebrows and body hair in cases of LPP. With LPP, the involvement of the skin, mucosal, and nails of classic lichen planus can be seen, but this is very rare in FFA (Ladizinski et al., 2013). Moreover, the most common skin lesions are characteristic yellowish facial papules during FFA, which are not usually seen in LPP patients. Another difference between the clinical findings is the association between FFA and androgenetic alopecia (AGA), which is not common in LPP (Vano-Galvan et al., 2014). Recently, Ranasinghe et al. (2017) found that LPP is associated with androgen excess, but FFA is related to androgen deficiency.
Response to treatment
There are major differences related to the response to treatment by patients with FFA and LPP. LPP responds well to topical or intralesional injection of corticosteroid agents as well as some immunosuppressive therapies, but these treatments were not found effective for FFA (Lajevardi et al., 2015). On the other hand, there is growing evidence that FFA progression can be halted with 5αRI (eg, dutasteride and finasteride), oral retinoid agents, and peroxisome proliferator-activated receptor gamma (PPAR-γ) agonists (Chiang et al., 2010, Pedrosa et al., 2017, Pirmez et al., 2017, Rakowska et al., 2017, Samrao et al., 2010, Vano-Galvan et al., 2014). Of note, some treatments, such as HCQ, appear effective not only for FFA, but also in decreasing symptoms and signs of LPP (Chiang et al., 2010).
Pathology
From the pathologic point of view, Miteva and Tosti (2012) proposed a follicular triad (ie, involvement of vellus, intermediate, and terminal hairs) as the pathologic clue to the diagnosis of early frontal FFA. Poblet et al. (2006) studied eight patients with FFA and eight patients with LPP, and found differences in the pathology of these two conditions. Some of the reported differences include the presence of a perivascular infiltrate in the dermis and an affected interfollicular epidermis, the presence of colloid bodies, and fibrosis in the papillary dermis in LPP, which are absent with FFA, but a milder tissue lichenoid reaction as well as more apoptotic cells in FFA.
Moreover, after evaluation of 11 paraffin-embedded tissue samples from patients with a clinical and histopathologic diagnosis of FFA, cluster of differentiation (CD) 8-biased T-cell infiltration with increased numbers of Langerhans cells in the infundibulum isthmus region was suggested (Ma et al., 2017). Recently, a study of 66 patients showed that FFA can be associated with inflammation that extends below the isthmus compared with LPP (92% vs 63%; P = .002; Wong and Goldberg, 2017). Based on the results from their study, Pedrosa et al. (2017) also proposed that hypertrophic sebaceous glands with no associated vellus hair follicles could be another distinguishing finding of FFA.
Risk factors
Genetic and familial background
Familial cases of FFA have been reported, and a positive family history has been related to approximately 8% of published case reports (Dlova et al., 2013, Vano-Galvan et al., 2014). In one study that analyzed four families, eight cases of mothers and daughters with FFA were perused. All mothers had postmenopausal FFA, and the daughters developed the disease before menopause, suggesting that the antecedent relatives of the disease could be associated with an earlier onset of FFA (Misiak-Galazka et al., 2016).
At the present time, the genetic loci associated with FFA are not characterized. Human leukocyte antigen-D related 1 (HLA-DR1) positivity has already been linked to some cases of lichen planus and Lassueur-Graham–Little-Piccardi syndrome in two family members (Viglizzo et al., 2004), but Chan et al. (2014) reported two cases of familial FFA with negative HLA-DR1 status. The inheritance of both LPP and FFA suggests the critical role of genetics , in combination with exposure to external factor, for the development of these diseases (Misiak-Galazka et al., 2016, Vano-Galvan et al., 2014).
Environmental factors
The recent onset and apparent increasing incidence of FFA can suggest the probable role of environmental factors in the FFA etiology (MacDonald et al., 2012). For instance, Aldoori et al. (2016) detected a higher frequency of sunscreen usage and positive patch tests to sunscreen ingredients in women with FFA, although the study required systematic and methodologic revisions (Seegobin et al., 2016). In the meantime, in a cohort study among men, Kidambi et al. (2017) found a significant relation between FFA and leave-on facial products, such as moisturizing creams and sunscreens.
On the other hand, there is growing evidence that neurogenic inflammation can play a role with FFA. For example, substance P (a stress-associated neuropeptide) can induce hair follicle immune privilege (HFIP) collapse in human organ cultures through the upregulation of major histocompatibility complex I and beta-2-microglobulin (Peters et al., 2004, Peters et al., 2005, Peters et al., 2007). Clinical evidence in support of this hypothesis includes increased sweating of the scalp (mediated by neurogenic mediators), which has been reported in a series of patients with FFA (Harries and Paus, 2010). Interestingly, botulinum toxin injections in two separate patients have improved both excess sweating and FFA signs and symptoms. In addition, a recent cross-sectional study of 72 women diagnosed with FFA found that chronic tobacco exposure may play a protective role in the development of FFA (Fonda-Pascual et al., 2017).
Proposed theoretical mechanisms in lichen planopilaris and frontal fibrosing alopecia
FFA is well known to develop as the consequence of HFIP collapse (Harries et al., 2013). Currently, there is no explicit theory to justify the specific pattern of hairline involvement in FFA. One reason could be the formation of an aberrant immune response against some components of miniaturized-vellus hairs of scalp hairline follicles (Miteva and Tosti, 2012). This speculated aberrant immune response could be related to some neo autoantigen formation during the hair follicular miniaturization process.
On the other hand, considering that hairline terminal hair can also concurrently be affected during FFA, Dawn et al. (2003) have suggested that hair follicles of the frontotemporal scalp might be differently programmed for apoptosis, which may be promoted with postmenopausal changes.
Innate and adaptive immune responses
Since the discovery of FFA and LPP, there have been very few studies on cytokine profile evaluations or T cell populations in these diseases. Harries et al. (2013) pointed out the significant role of T CD8 + attack and gamma interferon (IFN-γ)-induced HFIP collapse in the pathogenesis of LPP and FFA. The researchers analyzed the biopsy tissues of lesional and non-lesional scalp skins of 42 patients with LPP or FFA and found an increase in the expression of major histocompatibility complex Classes I and II, beta-2-microglobulin, and IFN-γ-inducible chemokines (CXCL9/10/11) in both LPP and FFA lesions (Harries et al., 2013). Additionally, a reduced expression of transforming growth factor-beta 2 and CD200 in lesional LPP hair follicles was shown. CD200 is an immuno-inhibitory no-danger molecule for hair follicles, which, lack of its expression can intensify the HFIP collapse. The researchers also suggested that a chronic exposure of hair follicles to IFN-γ could lead to hair follicle stem cell exhustion (Harries et al., 2013).
On the other hand, although LLP is classified in the lymphocytic group of cicatricial alopecia, initial aberrant innate immune responses could be the initiating event during FFA and LPP. Indeed, lymphocytes may not be necessarily involved in the primary stages of FFA and LPP, but they might act during the chronic phase of the disease. Since the innate immune system is an active participant in response to trauma, the development of LPP and FFA after trauma (such as hair transplantation or facelifts) might imply the critical role of innate immune responses in the LPP and FFA pathogenesis (Chiang et al., 2012). Almodovar-Real et al. (2015) also reported on a case of FFA in the absence of lymphocytic infiltrate and an increase in the number of the mast cells.
Fibrosis pathway: Epithelial-to-mesenchymal transition
There is a growing body of evidence that fibrotic pathways and specifically epithelial-to-mesenchymal transition (EMT) are deeply involved in FFA. EMT is a physiologic feature during embryogenesis and wound healing, but it also occurs during fibrotic diseases, malignant transformation of epithelial cells, and metastasis (Nieto et al., 2016). During EMT, epithelial cells gradually represent fibroblast-like morphology (such as vimentin and fibronectin upregulation) as a result of E-cadherin suppression via E-box binding factors, such as SNAI1, SNAI2, and TWIST (Nieto et al., 2016).
Ito et al. (2005) first cited the concept of EMT involvement in FFA after they showed that the pure elimination of epithelial stem cell (eSCs) in mice results in alopecia with no scar formation. Therefore, they concluded that something more than the elimination of eSCs is needed to establish scarring alopecia. Simultaneously, abnormal expression of SNAI1 was identified in the fibrotic dermis of FFA (Nakamura and Tokura, 2010). Recently, Imanishi et al. (2018) investigated the involvement of EMT in the scalp of patients with LPP and FFA with prodigious results. The researchers found E-cadherin protein expression reduction, SNAI1 and SNAI2 increases in the bulge epithelium, and Vimentin +, fibronectin + cells in the bulge of lesional LPP, which all imply EMT activation within the eSCs niche. The researchers also found K15 +/Vimentin + cells, which points to eSCs converting to fibroblast cells. As another innovation, they induced EMT in full-length human anagen VI hair follicles within 3 days by using an EMT-promoting cocktail (composed of epidermal growth factor, transforming growth factor-beta-1 [TGF-ß1], IFN-γ, and the selective E-cadherin inhibiting peptide SWELYYPLRANL [peptide A]), and concluded that TGF-ß1, epidermal growth factor, and IFN-γ are vital molecular signals that are sufficient to induce EMT in primary human eSCs in situ. The researchers also showed that adding pioglitazone to the culture medium containing the EMT cocktail could prevent EMT by downregulating TGF-ß1, SMAD2, and SMAD3, especially if used within the first 3 days of adding the EMT cocktail. They also showed that a topical PPAR-γ modulator (N-Acetyl-GED) can prevent and even reserve EMT in the mentioned culture medium.
Not surprisingly, a high load of IFN-γ can lead to EMT induction. From a therapeutic point of view, EMT inhibitors can potentially halt FFA progression. In addition to PPAR-γ agonists, which have documented efficacy in hampering EMT (Burgess et al., 2005, Zafiriou et al., 2005), retinoid agents are well known to have proven inhibitory effects against fibrosis pathways and can act as an agonist of PPAR-γ (Rankin et al., 2013). Furthermore, new evidence supports the inhibitory effect of 5αRIs in the TGF-β1-fibrosis pathway for the treatment of various types of alopecia (Inui and Itami, 2011, Yoo et al., 2006). Also, there is evidence for the role of androgens, especially dehydroepiandrosterone (DHEA), in suppressing TGF-β1 and fibrosis.
Peroxisome proliferator-activated receptor gamma pathway and sebaceous gland involvement
PPAR-γ and its agonists have recently been of interest in the pathogenesis and treatment of LPP and FFA. Karnik et al. (2009) reported a link between PPAR-γ deficiency, deregulated lipid metabolism, and scarring alopecia. The researchers reported downregulation of the PPAR-γ gene expression in bulge region stem cells of both lesional and uninvolved samples from patients with LPP and assume this is the beginning step of scarring alopecia. However, Harries et al. (2013) conducted a study with a higher number of biopsy samples from the scalp and observed that bulge PPAR-γ transcription was unaltered compared with non-lesional LPP hair follicles.
In recent years, PPAR-γ agonist therapy was introduced as a novel and effective treatment of patients with LPP (Spring et al., 2013). Pioglitazone, a PPAR-γ agonist, can decrease the proliferation and activation of effector CD4 + T cells, but it increases the proliferation and function of regulatory T cells (Tregs; Zhao et al., 2013). Moreover, pioglitazone has been demonstrated to cause a decrease in plasma levels of tumor necrosis factor-alpha (TNF-α) and interleukin-1-beta (IL-1β; Gupta et al., 2015). PPAR-γ has been suggested to cause an inhibition of IL-12 production, IL-12 signaling, and T-helper 1 (Th1) cell differentiation in experimental allergic encephalomyelitis (Gupta et al., 2015). Meanwhile, the loss of PPAR-γ resulted in overproduction of IFN-γ in response to IL-12 in the mouse model (Gupta et al., 2015). The consequence of lower Th1 cells is fewer IFN-γ concentration, which could slow the HFIP collapse and fibrosis.
In addition to effector responses, PPAR-γ leads to induction of regulatory responses, which can downregulate proinflammatory responses (Gupta et al., 2015). PPAR-γ is also required for the formation of sebaceous glands that seem to be involved in LPP and FFA, and can modulate the expression of numerous genes involved in lipid metabolisms, such as adipocyte protein 2, acyl-CoA synthase, and lipoprotein lipase (Schoonjans et al., 1996). These alterations could be the possible reason for lipid toxicity or the lack of production of anti-inflammatory components. Indeed, PPAR-γ contributes to the prevention of lipotoxicity by controlling adipose tissue expandability and peripheral lipid metabolism (Medina-Gomez et al., 2007). These findings could explain the effectiveness of PPAR-γ agonist therapy. Lastly, PPARγ dysfunction may promote fibrosis, and it has known inhibitory effects on the EMT and fibrosis pathway.
Hormonal factors: Steroidal agents and thyroid hormones
Evidence and controversies exist with regard to the role of steroids in the FFA pathogenesis. The high prevalence of FFA in postmenopausal women is the first evidence of this role, although several cases of FFA in young adult women and men have been reported recently (Kossard et al., 1997, Tosti et al., 2005). Some theories can be contemplated to explain these controversies.
At first glance, considering the high prevalence of FFA in postmenopausal women, estrogen deficiency could be considered as a triggering factor of FFA initiation. Estrogen has many known effects on different parts of the immune system, and it has regulatory effects on the function and number of neutrophils and macrophages (Hsieh et al., 2009, Kovats, 2015). Moreover, high levels of estrogen (eg, during pregnancy) can shift the immune response from Th1 to Th2 cells (Sabahi et al., 1995).
Simultaneously, estrogen receptor-alpha (ERα)-mediated signaling upregulates the expression of FoxP3, programmed cell death protein 1, and CTLA-4. Thus, estrogen seems capable of promoting the expansion of Tregs, which are critical players in the downregulation of immune responses (Polanczyk et al., 2005). However, the serum levels of estrogen have been reported as normal in previous reports, and FFA has been reported in a man who received estrogen as part of neoadjuvant hormonal therapy for prostate cancer. These observations question the protective role of estrogen in FFA (Banka et al., 2014).
Despite the lack of evidence and effective treatment for FFA, 5αRIs are among few evidence-based treatments that can halt FFA progression (Babahosseini et al., 2018). The reported effectiveness of 5αRIs in FFA management can be justified with the microenvironmental estrogen deficiency theory. Aromatase is the responsible enzyme for the conversion of androgens (specifically testosterone) into estrogen, and it is found in many tissues including hair follicles. Both finasteride and dutasteride inhibit the conversion of testosterone into dihydrotestosterone, leading to higher levels of testosterone in the scalp. Subsequently, this accumulated testosterone is converted to estrogen by activated aromatase. Consequently, 5αRIs increase the estrogen level in the scalp microenvironment, which could implement many anti-inflammatory effects. However, considering the high prevalence of AGA in patients with FFA and the well-known effects of 5αRIs in the treatment of AGA. The efficacy of 5αRIs in FFA patients could probably conditioned by its effect on improving AGA rather than FFA. (Vano-Galvan et al., 2014).
Second, a new growing concept is built on the basis of the low androgen level theory, which has been discussed very recently in the literature. Ranasinghe et al. (2017) reported that androgen deficiency (especially DHEA and DHEA sulphate) has been identified in many patients with FFA. DHEA and DHEA sulphate are the most abundant circulating steroid hormones in humans. Their production in women reaches the highest levels between the age of 25 and 30 years and starts decreasing at age 60 years, with only 10% to 20% of peak levels (Mendoza-Milla et al., 2013).
DHEA is well known to have many regulatory effects on the immune system, fat metabolism, and even fibrosis pathways by its effects on the PPAR γ pathway (Hazeldine et al., 2010). There is some evidence that DHEA has inhibitory effects on the TGF-β1-fibrosis pathway. For instance, a reduction of DHEA levels was reported in idiopathic pulmonary fibrosis (Mendoza-Milla et al., 2013). Surprisingly, adding DHEA in in vitro trials has prevented EMT (Xu et al., 2014). In view of the biochemical effects, DHEA exhibits affinity to the androgen receptor (AR) and estrogen receptors (ERs) with a preference for ER-β (Chen et al., 2005).
Surprisingly, depending on the circulating testosterone and dihydrotestosterone levels, DHEA has been shown to behave as a partial agonist of AR, and therefore probably has an anti-androgen effect. On the other hand, DHEA is a full agonist of ERβ with similar or slightly greater effect than estradiol. As such, DHEA has been proposed to be an important and potentially major endogenous estrogen in the body (Webb et al., 2006). Thus, considering the anti-inflammatory, anti-fibrosis, anti-androgen, and estrogen-like effects, DHEA can be a potentially effective treatment for FFA. Of note, despite these theories on the role of DHEA, since FFA is occurs in postmenopausal women in > 90% of cases, the significantly lower serum levels of DHEA in the study by Ranasinghe et al. (2017) could be due to the higher mean age of the patients with FFA, and not necessarily a triggering factor for FFA.
Lastly, the association of FFA with hypothyroidism and the effect of thyroid hormones on K15, CD200, and deiodinase 2 in cultured human K15-GFP + cells suggest a potential role of thyroid hormones in maintaining the stem cell niche/bulge immune privilege in FFA (Tiede et al., 2010).
Potential therapeutic options
To date, there is evidence of the relative effectiveness of different treatment options for patients with FFA, including 5αRIs (dutasteride and finasteride), topical calcineurin inhibitors (tacrolimus and pimecrolimus), and HCQ (Fertig and Tosti, 2016). As discussed, new evidence has shed light on the possible mechanisms of PPAR-γ agonists in FFA management. In addition to the approved PPAR-γ agonists (eg, pioglitazone and N-Acetyl-GED), there is evidence of the activation of PPAR-γ by nonsteroidal anti-inflammatory drugs, angiotensin II receptor antagonists, and oral retinoid agents (Gupta et al., 2015). Likewise, there is growing data in support of oral retinoid efficacy in the management of FFA. Oral retinoid agents are PPAR-γ agonists, and there is substantial interaction between PPARs, retinoic acid receptors, and retinoid X receptors (Gupta et al., 2015).
Furthermore, oral retinoid agents may act through the promotion of Tregs (Tavakolpour et al., 2016). Lastly, retinoid has many known inhibitory effects on the TGF-β1–fibrosis pathway (Marcellus et al., 1999, Xiao et al., 2011). At least two prospective studies and two retrospective studies have revealed a high percentage of efficacies for oral retinoid agents in the treatment of FFA facial papules and hair regression (Babahosseini et al., 2018, Pedrosa et al., 2017, Pirmez et al., 2017, Rakowska et al., 2017).
In addition to these treatments, which were shown to be effective in clinics, there are other options that probably could be employed to treat patients with FFA. Ultraviolet B phototherapy or excimer laser, which are possible inhibitors of inflammatory responses, are potentially effective therapeutic options for patients with FFA and LPP (Navarini et al., 2011). Ultraviolet B irradiation could induce IL-10 in human keratinocytes as well as the promotion of FoxP3 transcription, while decreased levels of several cytokines are involved in the differentiation of Th1 and Th17 cells, including IL-12, IL-17, IL-20, IL-22, IL-23, and TNF-α (Zhang et al., 2016, Coimbra et al., 2010).
Interfering with the JAK-STAT signaling pathway may be another novel approach to suppress aberrant immune responses in patients with FFA and LPP. The increased expression of IFN-γ-inducible chemokines (CXCL9, CXC10, and CXC11) and cognate receptor 3 (CXR3) in lesional LPP and FFA bulge epithelium suggest that the CXCL9/10/11-CXCR3 signaling system plays a major role in attracting the inflammatory cell infiltrate in LPP. Therefore, JAK inhibitors, such as tofacitinib and ruxolitinib, with noticeable well-known inhibitory effects on CXCL9/10/11-CXCR3 and STAT signaling pathways may be a promising modality (Boyle et al., 2015, Fenwick et al., 2015).
JAK inhibitors were also suggested for the treatment of other skin and hair disorders, such as psoriasis, atopic dermatitis, alopecia areata/universalis, and vitiligo (Samadi et al., 2017), and have been very recently proposed as a potent treatment for refractory pemphigus (Tavakolpour, 2018). Interestingly, Yang et al. (2018) have very recently found that treatment with tofacitinib could result in a clinically measurable improvement of patients with LPP.
According to the theoretical effects in suppressing EMT and considering the anti-androgen and estrogen-like effects, DHEA and its derivatives can also have a potentially effective treatment for FFA, but needs to be researched in clinical studies. Sirolimus is another potential treatment for FFA, because suppresses T-lymphocyte activation, proliferation, and antibody production. On the other hand, sirolimus inhibits the TGF-β1-induced fibrogenesis in many different conditions (Hillel and Gelbard, 2015). Considering the supposed role of the immune system and EMT in the pathogenesis of FFA, sirolimus can be named as a potentially promising treatment for FFA.
Due to the possible role of mast cells in LPP and the capacity of cetirizine to reduce the number of tryptase-positive mast cells (Pestelli et al., 2001), combined therapy with cetirizine at a dosage of 30 mg/day and a topical steroid agent was suggested as a valid option for the treatment of LPP (d'Ovidio et al., 2010), and can ameliorate pruritus in these patients, which is a common finding.
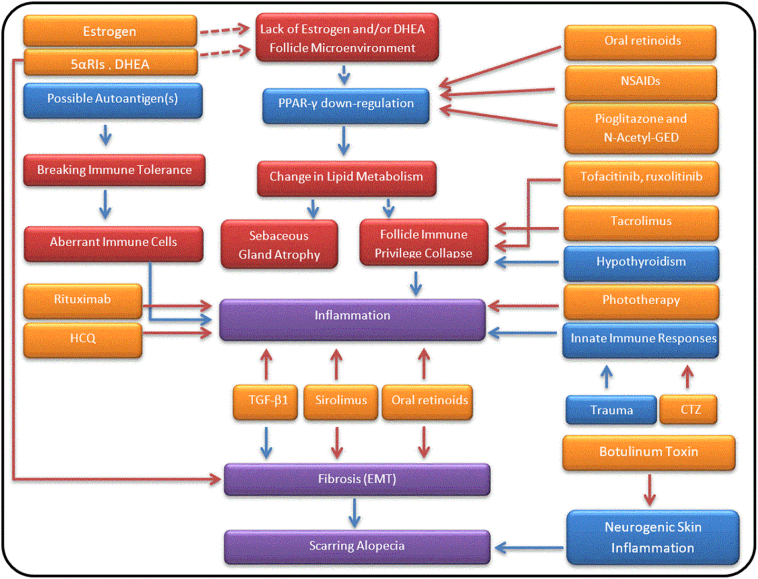
Anti-CD20 agent (rituximab) may improve LPP by inhibiting production of proinflammatory cytokines, such as IL-6, IFN-γ, and TNF-α, and probably restoring regulatory responses (eg, promotion of Tregs and B10 cells), which make rituximab a potential alternative treatment for patients with FFA (Lund, 2008, Sfikakis et al., 2007). Failure of LPP to respond to ustekinumab can show that Th1 and Th17 are at least not the primary players in LPP. The list of these potential therapeutic options as well as the possible signaling pathways associated with the etiology and pathogenesis of FFA is summarized in Figure 1.
Fig. 1.
Possible signaling pathways and potential therapeutic options in frontal fibrosing alopecia and lichen planopilaris. Red arrows mean inhibition and blue ones induction. The orange color is representative of drugs; blue and red boxes of triggers and their outcomes, respectively. Purple boxes are related to final outcomes, which are directly involved in disease development. Abbreviations: 5αRIs = 5α-reductase inhibitors; CTZ = cetirizine; DHEA = dehydroepiandrosterone; EMT = epithelial-to-mesenchymal transition; HCQ = hydroxychloroquine; NSAID = non-steroidal anti-inflammatory drug; PPAR-γ = peroxisome proliferator-activated receptor gamma; TGF-β1 = transforming growth factor-β1.
Conclusions
There are many unknowns with regard the pathogenesis and treatment of both LPP and FFA. Further immunologic and clinical analyses are needed to specify the exact pathogenesis and treatments for these diseases. Accordingly, in addition to previous effective treatments, including 5αRIs, topical calcineurin inhibitors (tacrolimus and pimecrolimus), HCQ, PPAR-γ agonists, and oral retinoid agents, various other treatment strategies and drugs, such as phototherapy, JAK inhibitors, DHEA, sirolimus, cetirizine, and rituximab, could be suggested.
In fact, these treatments appear effective in theory, but the clinical application may be associated with different side effects and limitations. Moreover, because the majority of the conducted studies did not distinguish between treatment for FFA and LPP and because of a lack of clinical trials and studies with high-level evidence, comparisons of mentioned approaches may not be possible. Therefore, further clinical trials and hypothesis studies are needed to clarify the most effective approaches for the treatment of FFA and LPP.
References
- Aldoori N., Dobson K., Holden C.R., McDonagh A.J., Harries M., Messenger A.G. Frontal fibrosing alopecia: Possible association with leave-on facial skin care products and sunscreens; a questionnaire study. Br J Dermatol. 2016;175:762–767. doi: 10.1111/bjd.14535. [DOI] [PubMed] [Google Scholar]
- Almodovar-Real A., Diaz-Martinez M.A., Ruiz-Villaverde R., Naranjo-Sintes R. Mast cells and scarring alopecia: Is there a clear pathophysiological relationship? Actas Dermosifiliogr. 2015;106:854–857. doi: 10.1016/j.ad.2015.03.021. [DOI] [PubMed] [Google Scholar]
- Babahosseini H., Tavakolpour S., Mahmoudi H. Lichen planopilaris: retrospective study on the characteristics and treatment of 291 patients. Journal of Dermatological Treatment. 2018:1–7. doi: 10.1080/09546634.2018.1542480. [DOI] [PubMed] [Google Scholar]
- Banka N., Mubki T., Bunagan M.J., McElwee K., Shapiro J. Frontal fibrosing alopecia: A retrospective clinical review of 62 patients with treatment outcome and long-term follow-up. Int J Dermatol. 2014;53:1324–1330. doi: 10.1111/ijd.12479. [DOI] [PubMed] [Google Scholar]
- Bolduc C., Sperling L.C., Shapiro J. Primary cicatricial alopecia: Other lymphocytic primary cicatricial alopecias and neutrophilic and mixed primary cicatricial alopecias. J Am Acad Dermatol. 2016;75:1101–1117. doi: 10.1016/j.jaad.2015.01.056. [DOI] [PubMed] [Google Scholar]
- Boyle D.L., Soma K., Hodge J., Kavanaugh A., Mandel D., Mease P. The JAK inhibitor tofacitinib suppresses synovial JAK1-STAT signalling in rheumatoid arthritis. Ann Rheum Dis. 2015;74:1311–1316. doi: 10.1136/annrheumdis-2014-206028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess H.A., Daugherty L.E., Thatcher T.H., Lakatos H.F., Ray D.M., Redonnet M. PPARgamma agonists inhibit TGF-beta induced pulmonary myofibroblast differentiation and collagen production: Implications for therapy of lung fibrosis. Am J Physiol Lung Cell Mol Physiol. 2005;288:L1146–L1153. doi: 10.1152/ajplung.00383.2004. [DOI] [PubMed] [Google Scholar]
- Chan D.V., Kartono F., Ziegler R., Abdulwahab N., DiPaola N., Flynn J. Absence of HLA-DR1 positivity in 2 familial cases of frontal fibrosing alopecia. J Am Acad Dermatol. 2014;71:e208–e210. doi: 10.1016/j.jaad.2014.05.064. [DOI] [PubMed] [Google Scholar]
- Chen F., Knecht K., Birzin E., Fisher J., Wilkinson H., Mojena M. Direct agonist/antagonist functions of dehydroepiandrosterone. Endocrinology. 2005;146:4568–4576. doi: 10.1210/en.2005-0368. [DOI] [PubMed] [Google Scholar]
- Chiang C., Sah D., Cho B.K., Ochoa B.E., Price V.H. Hydroxychloroquine and lichen planopilaris: efficacy and introduction of Lichen Planopilaris Activity Index scoring system. J Am Acad Dermatol. 2010;62:387–392. doi: 10.1016/j.jaad.2009.08.054. [DOI] [PubMed] [Google Scholar]
- Chiang Y.Z., Tosti A., Chaudhry I.H., Lyne L., Farjo B., Farjo N. Lichen planopilaris following hair transplantation and face-lift surgery. Br J Dermatol. 2012;166:666–670. doi: 10.1111/j.1365-2133.2011.10692.x. [DOI] [PubMed] [Google Scholar]
- Coimbra S., Oliveira H., Reis F., Belo L., Rocha S., Quintanilha A. Interleukin (IL)-22, IL-17, IL-23, IL-8, vascular endothelial growth factor and tumour necrosis factor-alpha levels in patients with psoriasis before, during and after psoralen-ultraviolet A and narrowband ultraviolet B therapy. Br J Dermatol. 2010;163:1282–1290. doi: 10.1111/j.1365-2133.2010.09992.x. [DOI] [PubMed] [Google Scholar]
- Dawn G., Holmes S.C., Moffat D., Munro C.S. Post-menopausal frontal fibrosing alopecia. Clin Exp Dermatol. 2003;28:43–45. doi: 10.1046/j.1365-2230.2003.01140.x. [DOI] [PubMed] [Google Scholar]
- Dlova N., Goh C.L., Tosti A. Familial frontal fibrosing alopecia. Br J Dermatol. 2013;168:220–222. doi: 10.1111/j.1365-2133.2012.11101.x. [DOI] [PubMed] [Google Scholar]
- d'Ovidio R., Rossi A., Di Prima T.M. Therapeutic hotline. Effectiveness of the association of cetirizine and topical steroids in lichen planus pilaris--an open-label clinical trial. Dermatol Ther. 2010;23:547–552. doi: 10.1111/j.1529-8019.2010.01359.x. [DOI] [PubMed] [Google Scholar]
- Fenwick P.S., Macedo P., Kilty I.C., Barnes P.J., Donnelly L.E. Effect of JAK inhibitors on release of CXCL9, CXCL10 and CXCL11 from human airway epithelial cells. PLoS One. 2015;10 doi: 10.1371/journal.pone.0128757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fertig R., Tosti A. Frontal fibrosing alopecia treatment options. Intractable Rare Dis Res. 2016;5:314–315. doi: 10.5582/irdr.2016.01065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonda-Pascual P., Saceda-Corralo D., Moreno-Arrones O.M., Alegre-Sanchez A., Vano-Galvan S. Frontal fibrosing alopecia and environment: May tobacco be protective? J Eur Acad Dermatol Venereol. 2017;31:e98–e99. doi: 10.1111/jdv.13817. [DOI] [PubMed] [Google Scholar]
- Gupta M., Mahajan V.K., Mehta K.S., Chauhan P.S., Rawat R. Peroxisome proliferator-activated receptors (PPARs) and PPAR agonists: The 'future' in dermatology therapeutics? Arch Dermatol Res. 2015;307:767–780. doi: 10.1007/s00403-015-1571-1. [DOI] [PubMed] [Google Scholar]
- Harries M.J., Meyer K., Chaudhry I., E Kloepper J., Poblet E., Griffiths C.E. Lichen planopilaris is characterized by immune privilege collapse of the hair follicle's epithelial stem cell niche. J Pathol. 2013;231:236–247. doi: 10.1002/path.4233. [DOI] [PubMed] [Google Scholar]
- Harries M.J., Paus R. The pathogenesis of primary cicatricial alopecias. Am J Pathol. 2010;177:2152–2162. doi: 10.2353/ajpath.2010.100454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazeldine J., Arlt W., Lord J.M. Dehydroepiandrosterone as a regulator of immune cell function. J Steroid Biochem Mol Biol. 2010;120:127–136. doi: 10.1016/j.jsbmb.2009.12.016. [DOI] [PubMed] [Google Scholar]
- Hillel A.T., Gelbard A. Unleashing rapamycin in fibrosis. Oncotarget. 2015;6:15722–15723. doi: 10.18632/oncotarget.4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes S. Frontal fibrosing alopecia. Skin Ther Lett. 2016;21:5–7. [PubMed] [Google Scholar]
- Hsieh C.H., Nickel E.A., Chen J., Schwacha M.G., Choudhry M.A., Bland K.I. Mechanism of the salutary effects of estrogen on kupffer cell phagocytic capacity following trauma-hemorrhage: pivotal role of Akt activation. J Immunol. 2009;182:4406–4414. doi: 10.4049/jimmunol.0803423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H.M., Zhang S.B., Lei X.H., Deng Z.L., Guo W.X., Qiu Z.F. Estrogen leads to reversible hair cycle retardation through inducing premature catagen and maintaining telogen. PLoS One. 2012;7(7) doi: 10.1371/journal.pone.0040124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imanishi H., Ansell D.M., Cheret J., Harries M., Bertolini M., Sepp N. Epithelial-to-mesenchymal stem cell transition in a human organ: Lessons from lichen planopilaris. J Invest Dermatol. 2018;138:511–519. doi: 10.1016/j.jid.2017.09.047. [DOI] [PubMed] [Google Scholar]
- Inui S., Itami S. Androgen receptor transactivity is potentiated by TGF-beta1 through Smad3 but checked by its coactivator Hic-5/ARA55 in balding dermal papilla cells. J Dermatol Sci. 2011;64:149–151. doi: 10.1016/j.jdermsci.2011.08.010. [DOI] [PubMed] [Google Scholar]
- Ito M., Liu Y., Yang Z., Nguyen J., Liang F., Morris R.J. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med. 2005;11:1351–1354. doi: 10.1038/nm1328. [DOI] [PubMed] [Google Scholar]
- Karnik P., Tekeste Z., McCormick T.S., Gilliam A.C., Price V.H., Cooper K.D. Hair follicle stem cell-specific PPARgamma deletion causes scarring alopecia. J Invest Dermatol. 2009;129:1243–1257. doi: 10.1038/jid.2008.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kossard S., Lee M.S., Wilkinson B. Postmenopausal frontal fibrosing alopecia: A frontal variant of lichen planopilaris. J Am Acad Dermatol. 1997;36:59–66. doi: 10.1016/s0190-9622(97)70326-8. [DOI] [PubMed] [Google Scholar]
- Kovats S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol. 2015;294:63–69. doi: 10.1016/j.cellimm.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladizinski B., Bazakas A., Selim M.A., Olsen E.A. Frontal fibrosing alopecia: A retrospective review of 19 patients seen at Duke University. J Am Acad Dermatol. 2013;68:749–755. doi: 10.1016/j.jaad.2012.09.043. [DOI] [PubMed] [Google Scholar]
- Lajevardi V., Ghodsi S.Z., Goodarzi A., Hejazi P., Azizpour A., Beygi S. Comparison of systemic mycophenolate mofetil with topical clobetasol in lichen planopilaris: A parallel-group, assessor- and analyst-blinded, randomized controlled trial. Am J Clin Dermatol. 2015;16:303–311. doi: 10.1007/s40257-015-0122-z. [DOI] [PubMed] [Google Scholar]
- Lund F.E. Cytokine-producing B lymphocytes-key regulators of immunity. Curr Opin Immunol. 2008;20:332–338. doi: 10.1016/j.coi.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S.A., Imadojemu S., Beer K., Seykora J.T. Inflammatory features of frontal fibrosing alopecia. J Cutan Pathol. 2017;44:672–676. doi: 10.1111/cup.12955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald A., Clark C., Holmes S. Frontal fibrosing alopecia: A review of 60 cases. J Am Acad Dermatol. 2012;67:955–961. doi: 10.1016/j.jaad.2011.12.038. [DOI] [PubMed] [Google Scholar]
- Marcellus D.C., Altomonte V.L., Farmer E.R., Horn T.D., Freemer C.S., Grant J. Etretinate therapy for refractory sclerodermatous chronic graft-versus-host disease. Blood. 1999;93:66–70. [PubMed] [Google Scholar]
- Martinez-Perez M., Churruca-Grijelmo M. Frontal Fibrosing Alopecia: An Update on Epidemiology and Treatment. Actas Dermosifiliogr. 2015;106(9):757–758. doi: 10.1016/j.ad.2014.12.002. [DOI] [PubMed] [Google Scholar]
- Medina-Gomez G., Gray S.L., Yetukuri L., Shimomura K., Virtue S., Campbell M. PPAR gamma 2 prevents lipotoxicity by controlling adipose tissue expandability and peripheral lipid metabolism. PLoS Genet. 2007;3:e64. doi: 10.1371/journal.pgen.0030064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza-Milla C., Valero Jimenez A., Rangel C., Lozano A., Morales V., Becerril C. Dehydroepiandrosterone has strong antifibrotic effects and is decreased in idiopathic pulmonary fibrosis. Eur Respir J. 2013;42:1309–1321. doi: 10.1183/09031936.00027412. [DOI] [PubMed] [Google Scholar]
- Misiak-Galazka M., Olszewska M., Rudnicka L. Lichen planopilaris in three generations: grandmother, mother, and daughter - a genetic link? Int J Dermatol. 2016;55:913–915. doi: 10.1111/ijd.13146. [DOI] [PubMed] [Google Scholar]
- Miteva M., Tosti A. The follicular triad: A pathological clue to the diagnosis of early frontal fibrosing alopecia. Br J Dermatol. 2012;166:440–442. doi: 10.1111/j.1365-2133.2011.10533.x. [DOI] [PubMed] [Google Scholar]
- Nakamura M., Tokura Y. Expression of Snail1 in the fibrotic dermis of postmenopausal frontal fibrosing alopecia: Possible involvement of an epithelial-mesenchymal transition and a review of the Japanese patients. Br J Dermatol. 2010;162:1152–1154. doi: 10.1111/j.1365-2133.2010.09682.x. [DOI] [PubMed] [Google Scholar]
- Navarini A.A., Kolios A.G., Prinz-Vavricka B.M., Haug S., Trueb R.M. Low-dose excimer 308-nm laser for treatment of lichen planopilaris. Arch Dermatol. 2011;147:1325–1326. doi: 10.1001/archdermatol.2011.335. [DOI] [PubMed] [Google Scholar]
- Nieto M.A., Huang R.Y., Jackson R.A., Thiery J.P. EMT: 2016. Cell. 2016;166:21–45. doi: 10.1016/j.cell.2016.06.028. [DOI] [PubMed] [Google Scholar]
- Pedrosa A.F., Duarte A.F., Haneke E., Correia O. Yellow facial papules associated with frontal fibrosing alopecia: A distinct histologic pattern and response to isotretinoin. J Am Acad Dermatol. 2017;77:764–766. doi: 10.1016/j.jaad.2017.04.1118. [DOI] [PubMed] [Google Scholar]
- Pestelli E., Caproni M., Giomi B. Cetirizine reduces the number of tryptase-positive mast cells in psoriatic patients: a double-blind controlled study. Int J Tissue React. 2001;23(3):97–103. [PubMed] [Google Scholar]
- Peters E.M., Handjiski B., Kuhlmei A., Hagen E., Bielas H., Braun A. Neurogenic inflammation in stress-induced termination of murine hair growth is promoted by nerve growth factor. Am J Pathol. 2004;165:259–271. doi: 10.1016/S0002-9440(10)63294-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters E.M., Kuhlmei A., Tobin D.J., Muller-Rover S., Klapp B.F., Arck P.C. Stress exposure modulates peptidergic innervation and degranulates mast cells in murine skin. Brain Behav Immun. 2005;19:252–262. doi: 10.1016/j.bbi.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Peters E.M., Liotiri S., Bodo E., Hagen E., Bíró T., Arck P.C. Probing the effects of stress mediators on the human hair follicle: Substance P holds central position. Am J Pathol. 2007;171:1872–1886. doi: 10.2353/ajpath.2007.061206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirmez R., Duque-Estrada B., Barreto T., Quintella D.C., Cuzzi T. Successful treatment of facial papules in frontal fibrosing alopecia with oral isotretinoin. Skin Appendage Disord. 2017;3:111–113. doi: 10.1159/000464334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poblet E., Jimenez F., Pascual A., Pique E. Frontal fibrosing alopecia versus lichen planopilaris: a clinicopathological study. Int J Dermatol. 2006;45:375–380. doi: 10.1111/j.1365-4632.2006.02507.x. [DOI] [PubMed] [Google Scholar]
- Polanczyk M.J., Hopke C., Huan J., Vandenbark A.A., Offner H. Enhanced FoxP3 expression and Treg cell function in pregnant and estrogen-treated mice. J Neuroimmunol. 2005;170:85–92. doi: 10.1016/j.jneuroim.2005.08.023. [DOI] [PubMed] [Google Scholar]
- Rakowska A., Gradzinska A., Olszewska M., Rudnicka L. Efficacy of isotretinoin and acitretin in treatment of frontal fibrosing alopecia: Retrospective analysis of 54 cases. J Drugs Dermatol. 2017;16:988–992. [PubMed] [Google Scholar]
- Ranasinghe G.C., Piliang M.P., Bergfeld W.F. Prevalence of hormonal and endocrine dysfunction in patients with lichen planopilaris (LPP): A retrospective data analysis of 168 patients. J Am Acad Dermatol. 2017;76:314–320. doi: 10.1016/j.jaad.2016.05.038. [DOI] [PubMed] [Google Scholar]
- Rankin A.C., Hendry B.M., Corcoran J.P., Xu Q. An in vitro model for the pro-fibrotic effects of retinoids: Mechanisms of action. Br J Pharmacol. 2013;170:1177–1189. doi: 10.1111/bph.12348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabahi F., Rola-Plesczcynski M., O'Connell S., Frenkel L.D. Qualitative and quantitative analysis of T lymphocytes during normal human pregnancy. Am J Reprod Immunol. 1995;33:381–393. doi: 10.1111/j.1600-0897.1995.tb00907.x. [DOI] [PubMed] [Google Scholar]
- Saceda-Corralo D., Moreno-Arrones O.M., Fonda-Pascual P., Pindado-Ortega C., Buendía-Castaño D., Alegre-Sánchez A. Development and validation of the frontal fibrosing alopecia severity score. J Am Acad Dermatol. 2018;78:522–529. doi: 10.1016/j.jaad.2017.09.034. [DOI] [PubMed] [Google Scholar]
- Samadi A., Ahmad Nasrollahi S., Hashemi A., Nassiri Kashani M., Firooz A. Janus kinase (JAK) inhibitors for the treatment of skin and hair disorders: A review of literature. J Dermatolog Treat. 2017;28:476–483. doi: 10.1080/09546634.2016.1277179. [DOI] [PubMed] [Google Scholar]
- Samrao A., Chew A.L., Price V. Frontal fibrosing alopecia: A clinical review of 36 patients. Br J Dermatol. 2010;163:1296–1300. doi: 10.1111/j.1365-2133.2010.09965.x. [DOI] [PubMed] [Google Scholar]
- Schoonjans K., Peinado-Onsurbe J., Lefebvre A.M., Heyman R.A., Briggs M., Deeb S. PPARalpha and PPARgamma activators direct a distinct tissue-specific transcriptional response via a PPRE in the lipoprotein lipase gene. EMBO J. 1996;15:5336–5348. [PMC free article] [PubMed] [Google Scholar]
- Seegobin S.D., Tziotzios C., Stefanato C.M., Bhargava K., Fenton D.A., McGrath J.A. Frontal fibrosing alopecia: There is no statistically significant association with leave-on facial skin care products and sunscreens. Br J Dermatol. 2016;175:1407–1408. doi: 10.1111/bjd.15054. [DOI] [PubMed] [Google Scholar]
- Sfikakis P.P., Souliotis V.L., Fragiadaki K.G., Moutsopoulos H.M., Boletis J.N., Theofilopoulos A.N. Increased expression of the FoxP3 functional marker of regulatory T cells following B cell depletion with rituximab in patients with lupus nephritis. Clin Immunol. 2007;123:66–73. doi: 10.1016/j.clim.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Spring P., Spanou Z., de Viragh P.A. Lichen planopilaris treated by the peroxisome proliferator activated receptor-gamma agonist pioglitazone: Lack of lasting improvement or cure in the majority of patients. J Am Acad Dermatol. 2013;69:830–832. doi: 10.1016/j.jaad.2013.04.066. [DOI] [PubMed] [Google Scholar]
- Tavakolpour S. Tofacitinib as the potent treatment for refractory pemphigus: A possible alternative treatment for pemphigus. Dermatol Ther. 2018:e12696. doi: 10.1111/dth.12696. [DOI] [PubMed] [Google Scholar]
- Tavakolpour S., Daneshpazhooh M., Mahmoudi H.R., Balighi K. The dual nature of retinoic acid in pemphigus and its therapeutic potential: Special focus on all-trans retinoic acid. Int Immunopharmacol. 2016;36:180–186. doi: 10.1016/j.intimp.2016.04.031. [DOI] [PubMed] [Google Scholar]
- Tiede S., Bohm K., Meier N., Funk W., Paus R. Endocrine controls of primary adult human stem cell biology: Thyroid hormones stimulate keratin 15 expression, apoptosis, and differentiation in human hair follicle epithelial stem cells in situ and in vitro. Eur J Cell Biol. 2010;89:769–777. doi: 10.1016/j.ejcb.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Tosti A., Piraccini B.M., Iorizzo M., Misciali C. Frontal fibrosing alopecia in postmenopausal women. J Am Acad Dermatol. 2005;52:55–60. doi: 10.1016/j.jaad.2004.05.014. [DOI] [PubMed] [Google Scholar]
- Vano-Galvan S., Molina-Ruiz A.M., Serrano-Falcon C., Arias-Santiago S., Rodrigues-Barata A.R., Garnacho-Saucedo G. Frontal fibrosing alopecia: A multicenter review of 355 patients. J Am Acad Dermatol. 2014;70:670–678. doi: 10.1016/j.jaad.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Viglizzo G., Verrini A., Rongioletti F. Familial Lassueur-Graham-Little-Piccardi syndrome. Dermatology. 2004;208:142–144. doi: 10.1159/000076489. [DOI] [PubMed] [Google Scholar]
- Webb S.J., Geoghegan T.E., Prough R.A., Michael Miller K.K. The biological actions of dehydroepiandrosterone involves multiple receptors. Drug Metab Rev. 2006;38:89–116. doi: 10.1080/03602530600569877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong D., Goldberg L.J. The depth of inflammation in frontal fibrosing alopecia and lichen planopilaris: A potential distinguishing feature. J Am Acad Dermatol. 2017;76:1183–1184. doi: 10.1016/j.jaad.2016.10.011. [DOI] [PubMed] [Google Scholar]
- Xiao R., Yoshida N., Higashi Y., Lu Q.J., Fukushige T., Kanzaki T. Retinoic acids exhibit anti-fibrotic activity through the inhibition of 5-lipoxygenase expression in scleroderma fibroblasts. J Dermatol. 2011;38:345–353. doi: 10.1111/j.1346-8138.2010.00993.x. [DOI] [PubMed] [Google Scholar]
- Xu L., Xiang X., Ji X., Wang W., Luo M., Luo S. Effects and mechanism of dehydroepiandrosterone on epithelial-mesenchymal transition in bronchial epithelial cells. Exp Lung Res. 2014;40:211–221. doi: 10.3109/01902148.2013.879966. [DOI] [PubMed] [Google Scholar]
- Yang C.C., Khanna T., Sallee B., Christiano A.M., Bordone L.A. Tofacitinib for the treatment of lichen planopilaris: A case series. Dermatol Ther. 2018:e12656. doi: 10.1111/dth.12656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo H.G., Kim J.S., Lee S.R., Pyo H.K., Moon H.I., Lee J.H. Perifollicular fibrosis: Pathogenetic role in androgenetic alopecia. Biol Pharm Bull. 2006;29:1246–1250. doi: 10.1248/bpb.29.1246. [DOI] [PubMed] [Google Scholar]
- Zafiriou S., Stanners S.R., Saad S., Polhill T.S., Poronnik P., Pollock C.A. Pioglitazone inhibits cell growth and reduces matrix production in human kidney fibroblasts. J Am Soc Nephrol. 2005;16:638–645. doi: 10.1681/ASN.2004040278. [DOI] [PubMed] [Google Scholar]
- Zhang D., Chen Y., Chen L., Yang R., Wang L., Liu W. Ultraviolet irradiation promotes FOXP3 transcription via p53 in psoriasis. Exp Dermatol. 2016;25:513–518. doi: 10.1111/exd.12942. [DOI] [PubMed] [Google Scholar]
- Zhao W., Berthier C.C., Lewis E.E., McCune W.J., Kretzler M., Kaplan M.J. The peroxisome-proliferator activated receptor-gamma agonist pioglitazone modulates aberrant T cell responses in systemic lupus erythematosus. Clin Immunol. 2013;149:119–132. doi: 10.1016/j.clim.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]