Abstract
Background:
Serotonin modulates many processes through a family of seven serotonin receptors. However, no studies have screened for interactions between general anesthetics currently in clinical use and serotonergic G-protein-coupled receptors (GPCRs). Given that both intravenous and inhalational anesthetics have been shown to target other classes of GPCRs, we hypothesized that general anesthetics might interact directly with some serotonin receptors and thus modify their function.
Methods:
Radioligand binding assays were performed to screen serotonin receptors for interactions with propofol and isoflurane as well as for affinity determinations. Docking calculations using the crystal structure of 5-HT2B were performed to computationally confirm the binding assay results and locate anesthetic binding sites.
Results:
The 5-HT2B class of receptors interacted significantly with both propofol and isoflurane in the primary screen. The affinities for isoflurane and propofol were determined to be 7.78 and .95 μM, respectively, which were at or below the clinical concentrations for both anesthetics. The estimated free energy derived from docking calculations for propofol (−6.70 kcal/mol) and isoflurane (−5.10 kcal/mol) correlated with affinities from the binding assay. The anesthetics were predicted to dock at a pharmacologically relevant binding site of 5HT2B.
Conclusions:
The molecular interactions between propofol and isoflurane with the 5-HT2B class of receptors were discovered and characterized. This finding implicates the serotonergic GPCRs as potential anesthetic targets.
Keywords: isoflurane, propofol, anesthetics, serotonin receptor
Introduction
Serotonin (5-hydroxytryptamine; 5-HT) is an endogenous monoamine neurotransmitter involved in many central nervous system (CNS) processes, including the modulation of behavior, mood, aggression, anxiety, and nociception – an area of natural interest for anesthesiology (Bradley et al., 1986; Hamon et al., 1989; Kesim et al., 2005; Roth, Ciaranello, & Meltzer, 1992). Serotonin mediates its actions through a family of receptors divided into seven different classes and located both in the CNS and the periphery. Though six of these classes are composed of G-protein-coupled receptors (GPCRs), past research on the interactions between anesthetic agents and the serotonergic receptors has primarily focused on the 5-HT3 class, a group comprising ligand-gated ion channel receptors. These receptors structurally and functionally resemble the heavily studied neuronal nicotinic acetylcholine receptors, GABA receptors, and glycine receptors (Joshi et al., 2006). This focus has resulted in numerous electrophysiological studies that have examined the modulatory effects of many general anesthetics vis-à-vis the 5-HT3 class of receptors (Barann et al., 2000; Barann, Linden, Witten, & Urban, 2008; Jenkins, Franks, & Lieb, 1996; Machu & Harris, 1994; Suzuki, Koyama, Sugimoto, Uchida, & Mashimo, 2002; Zhang, Oz, Stewart, Peoples, & Weight, 1997).
Despite the focus on the 5-HT3 receptors, anesthetic-related research outside of this class has been comparatively sparse and focused on the effects of anesthetics on the downstream physiological endpoints modulated by the serotonergic system. Previous studies have demonstrated that anesthetics at millimolar concentrations interact with two types of serotonin receptors (5-HT2A and 5-HT3) (Barann et al., 2000; Minami, Minami, & Harris, 1997). Despite these early findings and mounting evidence demonstrating that both intravenous and volatile anesthetics directly interact with GPCRs, no studies have systematically determined whether any of the other 5-HT receptors interact with general anesthetic agents at pharmacologically relevant concentrations (Hollmann, Strumper, Herroeder, & Durieux, 2005; Ishizawa, Pidikiti, Liebman, & Eckenhoff, 2002; Peterlin, Ishizawa, Araneda, Eckenhoff, & Firestein, 2005).
Given that GPCRs are anesthetic targets and that some of the physiological endpoints mediated by the serotonergic receptors overlap with side effects from anesthetic treatments, in the present study, we searched for possible interactions between the 5-HT receptors and two different anesthetics commonly utilized in surgical procedures – propofol (intravenous) and isoflurane (inhalational). We hypothesized that general anesthetics might interact directly with some serotonin receptors and thus modify their function through either agonist or antagonist modes of action. We screened available serotonin receptors with these two common clinical general anesthetics and the candidate receptor was further investigated through the use of high-resolution crystal structure data.
Materials and methods
Isoflurane and propofol were obtained from Halocarbon Laboratories (Liberty Corner, NJ). All other chemicals were of reagent grade or better and obtained from Sigma–Aldrich (St. Louis, MO).
Radioligand binding assays
A primary binding assay with 11 different 5-HT receptors was performed to determine the class of receptors for which the anesthetics displayed potential interaction. Evidence for interaction was based on the inhibition of the reference ligand-binding signal. Propofol, isoflurane, and the reference ligands for each receptor were diluted in standard binding buffer (50 mM Tris-HCl, 10 mM MgCl2, .1 mM EDTA, and pH 7.4) to a final concentration of 10 μM. The radioligands used were [3H] Way100635 at a final concentration of .4nM for 5-HT1A; [3H] 5-CT at final concentrations in the range of 1.5–2.0 nM for 5-HT1B and 5-HT1D; [3H] 5-HT at a final concentration of 3.5 nM for 5-HT1E; [3H] Ketanserin at a final concentration of 1.5 nM for 5-HT2A; [3H] LSD at final concentrations in the range of 1–5 nM for 5-HT2B, 5-HT5A, 5-HT6, and 5-HT7A; [3H] Mesulergine at a final concentration of 3 nM for 5-HT2C; and [3H] GR65630 at a final concentration of 1 nM for 5-HT3. Fifty microliter aliquots of the radioligand were added to wells of a 96-well plate, which contained 25 μl aliquots of references or test ligands. Crude membrane fractions containing the receptors were suspended in standard binding buffer and were added to each well. Detailed information about membrane preparation can be obtained from the protocol online (http://pdsp.med.unc.edu/PDSP%20Protocols%20II%202013-03-28.pdf, pp. 18–24, last date accessed: 15 October 2013). The reactions were incubated at room temperature for 1.5 h to allow radioligand binding equilibration. Bound radioactivity was harvested by rapid filtration onto a .3% polyethyleneimine-treated 96-well filter mat using a 96-well filtermate harvester. The dried filters were treated with melted scintillant and a Microbeta scintillation counter was used to measure the radioactivity retained on the filter. Quadruplicate samples corresponding to every ligand concentration point were counted.
The secondary binding assay was performed only when a test compound had the signal inhibition greater than 50%. A secondary binding assay was performed to determine the binding affinity for each respective anesthetic. Propofol, isoflurane, and methysergide (reference ligand) were prepared in the standard binding buffer and serially diluted to the following concentrations (5× of final concentrations): 0 nM, .05 nM, .5 nM, 1.5 nM, 5 nM, 15 nM, 50 nM, 150 nM, 500 nM, 1.5 μM, 5 μM, and 50 μM.
50 μL aliquots of [3H] LSD (5nM) were added to wells of a 96-well plate, which contained 25 μl aliquots of the reference or test ligands. Fifty microliter of a crude membrane fraction of cells expressing the 5-HT2B were added to each well. Reaction incubation, harvesting, and radioactivity measurement procedures from the primary assay were repeated. Triplicate samples corresponding to every ligand concentration point were counted.
Docking
Structures of isoflurane (CID: 3763), propofol (CID: 4943), dopamine (CID: 681), serotonin (CID: 5202), DOI (4-iodo-2,5-dimethoxyphenylisopropylamine, CID: 1229), LSD (lysergic acid diethylamide, CID: 5761), methysergide (CID: 9681), and ketanserin (CID: 3822) were obtained from the PubChem compound database. These structures were appropriately protonated and energy minimized using the PRODRG (Schüttelkopf & van Aalten, 2004) server. Then the ligand input files for docking were prepared using AutoDock Tools by removing non-polar hydrogen and setting up active torsions. The same tool was used to prepare the receptor input file by adding polar hydrogen to the crystal structure of 5-HT2B (PDB: 4IB4 (Wacker et al., 2013)).
AutoDock Vina (Trott & Olson, 2009) was used to perform docking of the above eight ligands into 5-HT2B. In all cases, the side chains of the receptor were considered rigid. The search volume was selected to cover the extracellular oriented “half” of 5-HT2B with dimensions 25 Å × 25 Å × 25 Å. The global search consisted of the default eight independent runs starting from distinct random positions of the ligand relative to the receptor. For each ligand, only the top-scored binding pose was considered. Due to the non-deterministic nature of the search algorithm, 100 dockings were repetitively performed with different initial random seeds. Then the most popular binding mode was selected, and the estimated binding affinities for structures with this mode were averaged for each ligand.
Data analysis
The data are presented as mean ± SE from quadruplicate results. The results were analyzed using GraphPad Prism (version 5.02 Windows version, GraphPad Software Inco, La Jolla, CA).
Results
Primary binding assay
Among the 11 different receptors tested in the primary binding assay, only the 5-HT2B subclass of serotonin receptors demonstrated significant signal inhibition in the presence of 10 μM of propofol and isoflurane (Table 1). Propofol but not isoflurane treatment significantly inhibited the binding signal in 5-HT5A. Further investigation on this receptor was not pursued because the secondary binding assay confirmed that the affinity would be higher than 10 μM if there is any specific interaction.
Table 1.
Signal inhibition of serotonin receptors by propofol and isoflurane.
| Receptor | Isoflurane signal inhibition (%) |
Propofol signal inhibition (%) |
|---|---|---|
| 5-HT1A | 26.4 ± 4.9 | 17.4 ± 6.6 |
| 5-HT1B | 5.9 ± 2.3 | 12.7 ± 4.0 |
| 5-HT1D | −.3 ± 3.5 | −6.1 ± 3.9 |
| 5-HT1E | −4.9 ± 2.0 | −3.8 ± 3.0 |
| 5-HT2A | 4.4 ± 7.4 | 7.2 ± 7.6 |
| 5-HT2B | 61.7 ± 2.8 | 86.9 ± 1.1 |
| 5-HT2C | 11.1 ± 5.8 | 48.8 ± 1.7 |
| 5-HT3 | 14.1 ± 6.2 | 28.4 ± 3.9 |
| 5-HT5A | 54.2 ± 1.4 | 43.2 ± 2.0 |
| 5-HT6 | 30.8 ± 1.7 | 23 ± 2.2 |
| 5-HT7 | 42.6 ± .9 | 35.0 ± 2.6 |
Note: Data are presented as mean ± SE from four replicates. Both anesthetics had a final concentration of 10 μM when incubated with the receptors. Secondary binding assay for affinity determination was performed only when signal inhibition was greater than 50% for a given anesthetic.
Affinity determination
In the secondary assay, binding by both anesthetics occurred in a concentration-dependent manner comparable to the 5-HT2B antagonist methysergide (Figure 1). The values of (Ki) for isoflurane (7.78 μM) and propofol (.95 μM) were substantially higher than that of methysergide (19 nM), indicating lower affinity.
Figure 1.
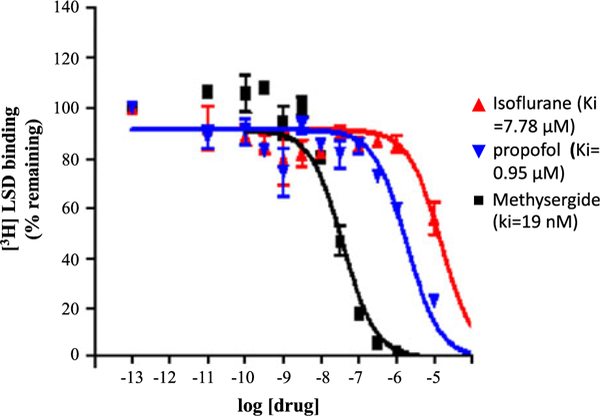
Isoflurane and propofol binding affinity. Propofol exhibited higher affinity to 5-HT2B compared to isoflurane; both values were lower than that of methysergide (5-HT2B antagonist).
Docking
The affinities estimated from the docking experiments correlated closely to the experimentally derived affinities (Figure 2). The calculated affinities for propofol (−6.7 kcal/mol) and isoflurane (−5.10kcal/mol) were comparable to those of the endogenous neurotransmitters dopamine (−5.90kcal/mol) and serotonin (−6.50 kcal/mol) as well as the agonist 2,5-dimethoxy-4-iodoamphetamine (DOI, −6.30 kcal/mol). The estimated affinities for the anesthetics were substantially weaker than those of the agonist lysergic acid diethylamide (LSD, −10.20 kcal/mol) and the antagonist ketanserin (−10.50 kcal/mol).
Figure 2.
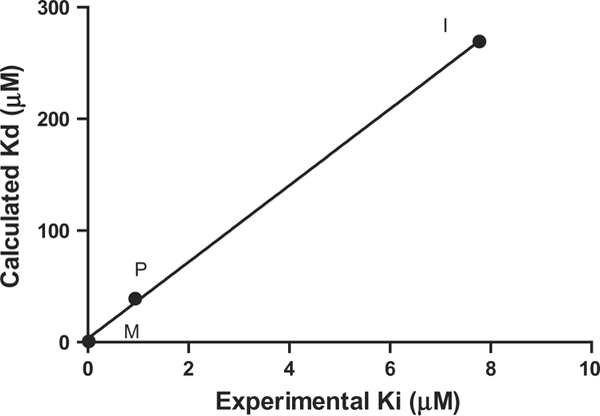
Comparison between docking and experimental binding constants of isoflurane (I), propofol (P), and methysergide (M) to 5-HT2B.
Based on the docking results, the putative binding pocket was mainly defined by residues from transmembrane 3 (TM3), TM5, TM6, and TM7. Asp3.32 hydrogen bonds with Tyr7.43 (both residues are strictly conserved among all serotonin receptors, opioid receptors, adrenergic receptors, and histamine receptors). The disruption of this hydrogen bond by a charge–charge interaction between Asp3.32 and the amine moiety of the agonists is believed to trigger the subsequent conformation change of the helices bundle from an inactive state to active state (Nichols & Nichols, 2008). The van der Waals volumes of ligands were compatible with the 5-HT2B binding pocket (Figure 3(a) and (b)). Among them, the antagonist ketanserin is the largest ligand and occupies the entire putative binding pocket.
Figure 3.
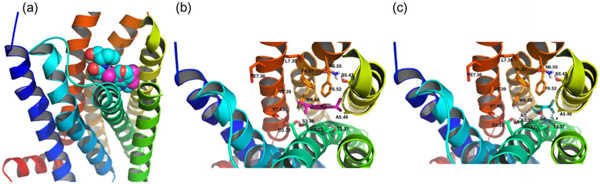
(a) The van der Waals shapes of the ligands are compatible with the binding pocket. Propofol (magenta) docking within the pocket overlaps well with that of methysergide (cyan). (b) Stick representation of key residues in the binding pocket of 5-HT2B involved in propofol binding. (c) Isoflurane.
Asp3.32 is a highly conserved residue amongst the serotonergic receptors and it is believed to act as a counter-ion for the protonated amine of various ligands (Kristiansen et al., 2000; Wang, Gallaher, & Shin, 1993). The interaction between Asp3.32 and the amine group of methysergide, serotonin, dopamine, DOI, LSD, and ketanserin was also observed in the docking results. Among them, the distance between the amine hydrogen and the carboxylic oxygen of Asp3.32 was 2–3 Å for methysergide, serotonin, DOI, LSD, and dopamine.
Another residue, Ser3.36, provides a second interaction site for the protonated amine of serotonin but not of LSD or bufotenin (Almaula, Ebersole, Zhang, Weinstein, & Sealfon, 1996). This interaction was also observed in our docking results. The distance between the amine hydrogen and the hydroxyl oxygen of Ser3.36 was 2.6 Å for both DOI and dopamine, respectively. However, this interaction distance is much larger for methysergide and LSD: 4.2–5.0 Å.
Propofol and isoflurane lack this amine moiety and therefore bind to 5-HT2B in different ways. Phe6.52 and Trp6.48 are believed to be involved in anchoring the aromatic moiety of the ligands (Roth, Shoham, Choudhary, & Khan, 1997b). The binding poses in the docking showed that most ligands (except isoflurane) placed their aromatic rings close to these aromatic residues. Accordingly, propofol bound with 5HT2B through van der Waals interactions between its phenyl ring and the aromatic residues Trp6.48, Phe6.51, and Phe6.52 in TM6 of 5HT2B (Figure 3(b)).
Isoflurane does not possess an aromatic group, and the docking results showed that hydrogen bonding plays an important role in its interaction with 5HT2B (Figure 3(c)). The main determinant of binding of isoflurane to 5HT2B appeared to be hydrogen bonding between its ethanol oxygen and Thr3.37 (with a O–O distance of 3.1 Å). A fluorine atom in the trifluoromethyl group is poised to hydrogen bond with this same residue (with a F–O distance of 3.4 Å). A fluorine atom in the difluoro methyl moiety on the other side of the ligand was poised to form a hydrogen bond with Ser3.36 (with a F–O distance of 4.3 Å).
Discussion
A radioligand binding screen on the serotonin receptors identified interactions between general anesthetics with the 5HT2B subclass of receptors. Secondary binding assays confirmed the interactions and determined that the affinities were below commonly used clinical concentrations. The results from the docking calculations on the crystal structure of 5HT2B (PDB: 4IB4) are consistent with the experimental data and provide insights into how anesthetics interact with 5HT2B receptors.
GPCRs are an important area of research for anesthesiology as the signaling cascades for many members of these membrane proteins modulate nociception both in the CNS and the periphery. Various agents commonly used in the perioperative period such as opiates, α2-adrenergic agonists, adenosine, and other derivative compounds have long been recognized as direct molecular targets for GPCRs (Hollmann et al., 2005). Moreover, in recent years, mounting evidence has suggested that inhalational anesthetics such as halothane and isoflurane either directly target or indirectly modify the functionality of these targets (Icaza et al., 2009; Ishizawa et al., 2002; Peterlin et al., 2005).
Studies exploring and characterizing potential interactions between general anesthetics and all the serotonergic GPCRs had previously been lacking, but the primary binding assay demonstrated that both propofol (intravenous) and isoflurane (inhalational) exhibited significant interactions with 5-HT2B due to the large signal inhibition (Table 1). Propofol but not isoflurane exhibited potential interaction with 5-HT5A, a GPCR whose physiological functions are relatively obscure though some data implicate it in the control of circadian rhythms (Thomas, 2006). However, a secondary binding study failed to demonstrate any specific interactions. Both isoflurane and propofol failed to demonstrate significant binding with the ligand-gated ion channel 5-HT3 receptor in the primary assay. Though other studies have reported the potentiation of this receptor following isoflurane treatment, the lack of significant binding signal demonstrated here may be explained by the low concentration utilized in our study (10 μM). The concentrations of isoflurane used in these previously studies were several orders of magnitude higher than that used in the current study (Barann et al., 2000; Jenkins et al., 1996; Suzuki et al., 2002). The receptor’s lack of interaction with propofol correlated with previous electrophysiological studies which demonstrated that it had no effect on channel potentiation (Machu & Harris, 1994; Zhang et al., 1997).
Results from the secondary binding assay determined that the binding affinity (Ki) of isoflurane and propofol for 5-HT2B are 7.78 and .95 μM, respectively. While the affinity constants for the anesthetics deviate from the classical values for specific interactions, these results remain relevant as both affinity constants represent values that are at or below the clinically effective concentrations for both compounds. Because propofol binds to soluble human blood proteins, thereby leaving 1–3% of the compound free for potential interactions with receptors, its clinically effective concentration is generally considered to be ~1 μM (Franks & Lieb, 1994; Servin, Farinotti, Haberer, & Desmonts, 1993). The free aqueous concentration of isoflurane needed for anesthesia is often reported to be 100–300 μM (Franks, 2008; Franks & Lieb, 1993, 1998).
The docking position and pose of methysergide overlaps with that of ergotamine, which is present in the crystal structure (Figure 4(a)) (PDB: 4IB4). The protonated amines of methysergide and ergotamine align very well and they both interact with the carboxylic oxygen of Asp3.32 (H–O distances 2.1 and 1.8 Å, respectively). Almaula et al. found that mesulergine (selective for 5-HT2C) and ergoline (selective for 5-HT2A) reversed their relative affinity with mutations A5.46S for 5-HT2C and S5.46A for 5HT2A (Almaula, Ebersole, Ballesteros, Weinstein, & Sealfon, 1996). Thus, they proposed that a hydrogen-bonding interaction existed between Ser5.46 of 5-HT2A and the N1 hydrogen of N1-unsubstituted ergolines. At the same position, Ala5.46 of 5-HT2C facilitates the interaction with N1 methyl group of N1-substituted ergolines. Our docking results were consistent with this, showing a distance of 3.0 Å (C–C distance) between the N1 methyl of methysergide and Ala5.46 of 5-HT2B. LSD, which has the same binding pose as methysergide but contains N1 hydrogen instead, had a slightly lower binding affinity to 5-HT2B (.1 kcal/mol difference). The docking results showed that the hydroxyl group of serotonin is in proximity to and poised for hydrogen bond with Ser5.43 with a H–O distance of 2.1 Å, which might explain previous observations (Johnson, Wainscott, Lucaites, Baez, & Nelson, 1997) for 5-HT2A where serotonin showed over a 10-fold decrease of affinity with the mutation S5.43A. This interaction with Ser5.43 was also observed for one hydroxyl group of dopamine in our docking results. Docking calculations for isoflurane, propofol, and methysergide yielded binding affinities that correlated with the experimental results (Figure 2).
Figure 4.
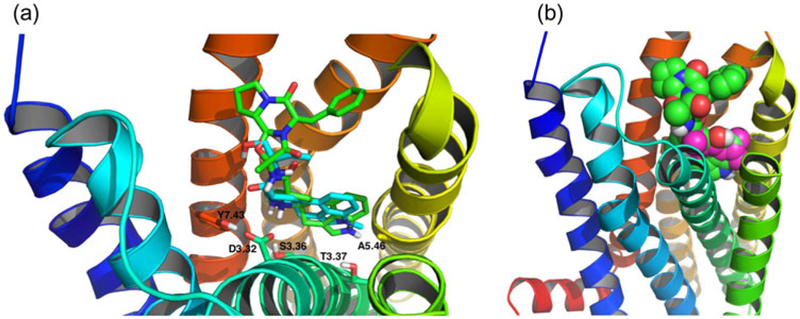
(a) Stick representation of overlapping between methysergide (cyan and docking result) and ergotamine (green, crystal structure). (b) Propofol (magenta) docking within the pocket overlaps well with that of ergotamine (green).
The docking position of propofol also overlaps with that of ergotamine in the crystal structure (Figure 4(b)) (PDB: 4IB4). Propofol binding was partly caused by interactions between the molecule’s phenyl ring and the aromatic residues Trp6.48, Phe6.51, and Phe6.52. Interestingly, this aromatic cluster in helix VI is conserved between the three 5HT2 receptor subtypes. Previous work with 5HT2C models proposed that Trp6.48 and Phe6.52 were involved in the binding of serotonin through the formation of hydrophobic interactions with the phenyl rings of these ligands (Kristiansen & Dahl, 1996). Mutagenesis studies with 5HT2A further confirmed the importance of interactions with these residues for agonist, but not antagonist, binding (Choudhary, Craigo, & Roth, 1993; Roth, Shoham, Choudhary, & Khan, 1997a). Furthermore, Phe6.52 has been reported to play a role in agonist activation of the 5HT2A receptor (Braden, Parrish, Naylor, & Nichols, 2006).
Studies on the contributions of Phe6.51 interaction, however, have provided conflicting results. Early work indicated that the residue did not participate in agonist binding but rather contributed to antagonist stabilization (Choudhary et al., 1993; Roth et al., 1997a). However, recent evidence has suggested that it is involved in agonist binding (Braden et al., 2006). Based on these previous findings and our results, the mode of action of propofol on the 5HT2B receptor cannot be extrapolated and further testing will be needed to determine this. However, our results nonetheless suggest that propofol docks at a binding site with pharmacological activity as the interacting aromatic residues on helix VI mediate G-protein activation and second-messenger production via direct coupling to the third intracellular loop (Suel, Lockless, Wall, & Ranganathan, 2003; Wess, Brann, & Bonner, 1989).
Our results demonstrated that hydrogen bonding interactions with Ser3.36 and Thr3.37 were important for isoflurane binding with the receptor. Much like propofol, isoflurane’s mode of action on the receptor cannot be extrapolated, but its interactions occur at or near a pharmacologically relevant site as it overlaps with the one for the natural endogenous ligand serotonin.
The 5HT2B receptors have been implicated in mediating several downstream physiological endpoints. In the CNS, the receptors are responsible for modulating mood, behavior, migraines, and nociception. In the periphery, the receptor has been found in arterial endothelium and the characterization of its cardiovascular effects has been a major area of research (Kaumann & Levy, 2006; Tanaka, Ludwig, Kersten, Pagel, & Warltier, 2004). In particular, the subclinical value of the affinity constant coupled with our computational studies of ligand–receptor interactions could further elucidate the mechanisms underlying the side effects of propofol treatment.
Decreases in systemic arterial pressure commonly occur following anesthetic administration (Boer, Bovill, Ros, & van Ommen, 1991; Claeys, Gepts, & Camu, 1988; Rouby et al., 1991; Turner et al., 1998). Multiple mechanisms have been proposed to explain this phenomenon. Some studies have pointed to an inhibition of sympathetic vasoconstrictive nerve activity (Hoka, Yamaura, Takenaka, & Takahashi, 1998; Robinson, Ebert, O’Brien, Colinco, & Muzi, 1997). Others have implicated myocardial depression (Pagel & Warltier, 1993) while considerable evidence has suggested a nitric oxide-mediated mechanism and therefore a direct anesthetic action on smooth muscle relaxation (Gragasin & Davidge, 2009; Muzi, Berens, Kampine, & Ebert, 1992; Petros, Bogle, & Pearson, 1993; Rouby et al., 1991). Nitric oxide (NO) produced and released from the vascular endothelium plays a key role in vascular dilatation (Palmer, Ashton, & Moncada, 1988). It mediates systemic, coronary, and pulmonary vascular tone through a guanylyl cyclase-dependent mechanism in vascular smooth muscle cells (Levin, 1995). Evidence has implicated the activation of 5HT2B in the production of NO in the endothelial cells of the human coronary artery and consequent arterial relaxation (Ishida, Kawashima, Hirata, & Yokoyama, 1998). Further evidence has also demonstrated that 5HT2B is the receptor responsible for modulating endothelial-dependent vasodilation in porcine pulmonary arteries (Glusa & Pertz, 2000; Glusa & Roos, 1996). Given the fact that 5HT2B exists throughout the systemic vascular system (Choi & Maroteaux, 1996), the interaction between 5HT2B and propofol may explain the decreased systemic arterial pressure seen in patients during propofol and isoflurane administration. However, more in vitro and in vivo studies are needed to confirm such a hypothesis.
In conclusion, the molecular interactions between propofol and isoflurane with the 5-HT2B class of receptors were discovered and characterized. This finding implicates the serotonergic GPCR as potential anesthetic targets.
Acknowledgments
RYL acknowledges support from the Department of Anesthesiology and Critical Care at the University of Pennsylvania and support from Roderic G. Eckenhoff, MD and Jin Xi, MS, at the Department of Anesthesiology and Critical Care at the University of Pennsylvania. JGS acknowledges infrastructural support from the Penn Nano/Bio Interface Center (National Science Foundation NSEC-32802).
Funding
This research was supported by departmental funding from the Department of Anesthesiology and Critical Care at University of Pennsylvania (PI RL), funding from the Foundation for Anesthesia Education and Research (PI, RL), NIH [grant number K08-GM-093115–01] (PI:RL). Jeffery G. Saven acknowledges support from NIH [grant number P01GM055876] and NSF [grant number DMR-1120901]. This work is also supported by the National Institute of Mental Health’s Psychoactive Drug Screening Program, Contract # HHSN-271–2008-00025-C (NIMH PDSP), which is directed by Bryan L. Roth MD, PhD at the University of North Carolina at Chapel Hill and Project Officer Jamie Driscol at NIMH, Bethesda MD, USA.
References
- Almaula N, Ebersole BJ, Ballesteros JA, Weinstein H, & Sealfon SC (1996). Contribution of a helix 5 locus to selectivity of hallucinogenic and nonhallucinogenic ligands for the human 5-hydroxytryptamine(2A) and 5-hydroxytryptamine(2C) receptors: direct and indirect effects on ligand affinity mediated by the same locus. Molecular Pharmacology, 50, 34–42. [PubMed] [Google Scholar]
- Almaula N, Ebersole BJ, Zhang DQ, Weinstein H, & Sealfon SC (1996). Mapping the binding site pocket of the serotonin 5-hydroxytryptamine(2A) receptor – Ser(3.36 (159)) provides a second interaction site for the protonated amine of serotonin but not of lysergic acid diethylamide or bufotenin. Journal of Biological Chemistry, 271, 14672–14675. [DOI] [PubMed] [Google Scholar]
- Barann M, Dilger JP, Bönisch H, Göthert M, Dybek A, & Urban BW (2000). Inhibition of 5-HT3 receptors by propofol: Equilibrium and kinetic measurements. Neuropharmacology, 39, 1064–1074. [DOI] [PubMed] [Google Scholar]
- Barann M, Linden I, Witten S, & Urban BW (2008). Molecular actions of propofol on human 5-HT3A receptors: Enhancement as well as inhibition by closely related phenol derivatives. Anesthesia & Analgesia, 106, 846–857, table of contents. [DOI] [PubMed] [Google Scholar]
- Boer F, Bovill JG, Ros P, & van Ommen H (1991). Effect of thiopentone, etomidate and propofol on systemic vascular resistance during cardiopulmonary bypass. BJA: British Journal of Anaesthesia, 67, 69–72. [DOI] [PubMed] [Google Scholar]
- Braden MR, Parrish JC, Naylor JC, & Nichols DE (2006). Molecular interaction of serotonin 5-HT2A receptor residues Phe339(6.51) and Phe340(6.52) with superpotent N-benzyl phenethylamine agonists. Molecular Pharmacology, 70, 1956–1964. [DOI] [PubMed] [Google Scholar]
- Bradley PB, Engel G, Feniuk W, Fozard JR, Humphrey PP, Middlemiss DN, ... Saxena PR. (1986). Proposals for the classification and nomenclature of functional receptors for 5-hydroxytryptamine. Neuropharmacology, 25, 563–576. [DOI] [PubMed] [Google Scholar]
- Choi DS, & Maroteaux L (1996). Immunohistochemical localisation of the serotonin 5-HT2B receptor in mouse gut, cardiovascular system, and brain. FEBS Letters, 391, 45–51. [DOI] [PubMed] [Google Scholar]
- Choudhary MS, Craigo S, & Roth BL (1993). A single point mutation (Phe340→Leu340) of a conserved phenylalanine abolishes 4-[125I]iodo-(2,5-dimethoxy)phenylisopropylamine and [3H]mesulergine but not [3H]ketanserin binding to 5-hydroxytryptamine2 receptors. Molecular Pharmacology, 43, 755–761. [PubMed] [Google Scholar]
- Claeys MA, Gepts E, & Camu F (1988). Haemodynamic changes during anaesthesia induced and maintained with propofol. BJA: British Journal of Anaesthesia, 60, 3–9. [DOI] [PubMed] [Google Scholar]
- Franks NP (2008). General anaesthesia: From molecular targets to neuronal pathways of sleep and arousal. Nature Reviews Neuroscience, 9, 370–386. [DOI] [PubMed] [Google Scholar]
- Franks NP, & Lieb WR (1993). Selective actions of volatile general anaesthetics at molecular and cellular levels. BJA: British Journal of Anaesthesia, 71, 65–76. [DOI] [PubMed] [Google Scholar]
- Franks NP, & Lieb WR (1994). Molecular and cellular mechanisms of general anaesthesia. Nature, 367, 607–614. [DOI] [PubMed] [Google Scholar]
- Franks NP, & Lieb WR (1998). Which molecular targets are most relevant to general anaesthesia? Toxicology Letters, 100–101, 1–8. [DOI] [PubMed] [Google Scholar]
- Glusa E, & Pertz HH (2000). Further evidence that 5-HT-induced relaxation of pig pulmonary artery is mediated by endothelial 5-HT 2B receptors. British Journal of Pharmacology, 130, 692–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glusa E, & Roos A (1996). Endothelial 5-HT receptors mediate relaxation of porcine pulmonary arteries in response to ergotamine and dihydroergotamine. British Journal of Pharmacology, 119, 330–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gragasin FS, & Davidge ST (2009). The effects of propofol on vascular function in mesenteric arteries of the aging rat. AJP: Heart and Circulatory Physiology, 297, H466–H474. [DOI] [PubMed] [Google Scholar]
- Hamon M, Gallissot MC, Menard F, Gozlan H, Bourgoin S, & Vergé D (1989). 5-HT3 receptor binding sites are on capsaicin-sensitive fibres in the rat spinal cord. European Journal of Pharmacology, 164, 315–322. [DOI] [PubMed] [Google Scholar]
- Hoka S, Yamaura K, Takenaka T, & Takahashi S (1998). Propofol-induced increase in vascular capacitance is due to inhibition of sympathetic vasoconstrictive activity. Anesthesiology, 89, 1495–1500. [DOI] [PubMed] [Google Scholar]
- Hollmann MW, Strumper D, Herroeder S, & Durieux ME (2005). Receptors, G proteins, and their interactions. Anesthesiology, 103, 1066–1078. [DOI] [PubMed] [Google Scholar]
- Icaza EE, Huang X, Fu Y, Neubig RR, Baghdoyan HA, & Lydic R (2009). Isoflurane-induced changes in righting response and breathing are modulated by RGS proteins. Anesthesia & Analgesia, 109, 1500–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida T, Kawashima S, Hirata K, & Yokoyama M (1998). Nitric oxide is produced via 5-HT1B and 5-HT2B receptor activation in human coronary artery endothelial cells. The Kobe Journal of Medical Sciences, 44, 51–63. [PubMed] [Google Scholar]
- Ishizawa Y, Pidikiti R, Liebman PA, & Eckenhoff RG (2002). G protein-coupled receptors as direct targets of inhaled anesthetics. Molecular Pharmacology, 61, 945–952. [DOI] [PubMed] [Google Scholar]
- Jenkins A, Franks NP, & Lieb WR (1996). Actions of general anaesthetics on 5-HT3 receptors in N1E-115 neuroblastoma cells. British Journal of Pharmacology, 117, 1507–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MP, Wainscott DB, Lucaites VL, Baez M, & Nelson DL (1997). Mutations of transmembrane IV and V serines indicate that all tryptamines do not bind to the rat 5-HT2A receptor in the same manner. Molecular Brain Research, 49, 1–6. [DOI] [PubMed] [Google Scholar]
- Joshi PR, Suryanarayanan A, Hazai E, Schulte MK, Maksay G, & Bikadi Z (2006). Interactions of granisetron with an agonist-free 5-HT3A receptor model. Biochemistry, 45, 1099–1105. [DOI] [PubMed] [Google Scholar]
- Kaumann AJ, & Levy FO (2006). 5-hydroxytryptamine receptors in the human cardiovascular system. Pharmacology & Therapeutics, 111, 674–706. [DOI] [PubMed] [Google Scholar]
- Kesim M, Duman EN, Kadioglu M, Yaris E, Kalyoncu NI, & Erciyes N (2005). The different roles of 5-HT2 and 5-HT3 receptors on antinociceptive effect of paroxetine in chemical stimuli in mice. Journal of Pharmacological Sciences, 97, 61–66. [DOI] [PubMed] [Google Scholar]
- Kristiansen K, & Dahl SG (1996). Molecular modeling of serotonin, ketanserin, ritanserin and their 5-HT2C receptor interactions. European Journal of Pharmacology, 306, 195–210. [DOI] [PubMed] [Google Scholar]
- Kristiansen K, Kroeze WK, Willins DL, Gelber EI, Savage JE, Glennon RA, & Roth BL (2000). A highly conserved aspartic acid (Asp-155) anchors the terminal amine moiety of tryptamines and is involved in membrane targeting of the 5-HT2A serotonin receptor but does not partivipate in activation via a “salt-bridge disruption” mechanism. Journal of Pharmacology and Experimental Therapeutics, 293, 735–746. [PubMed] [Google Scholar]
- Levin ER (1995). Endothelins. The New England Journal of Medicine, 333, 356–363. [DOI] [PubMed] [Google Scholar]
- Machu TK, & Harris RA (1994). Alcohols and anesthetics enhance the function of 5-hydroxytryptamine3 receptors expressed in Xenopus laevis oocytes. The Journal of Pharmacology and Experimental Therapeutics, 271, 898–905. [PubMed] [Google Scholar]
- Minami K, Minami M, & Harris RA (1997). Inhibition of 5-hydroxytryptamine type 2A receptor-induced currents by n-alcohols and anesthetics. The Journal of Pharmacology and Experimental Therapeutics, 281, 1136–1143. [PubMed] [Google Scholar]
- Muzi M, Berens RA, Kampine JP, & Ebert TJ (1992). Venodilation contributes to propofol-mediated hypotension in humans. Anesthesia and Analgesia, 74, 877–883. [DOI] [PubMed] [Google Scholar]
- Nichols DE, & Nichols CD (2008). Serotonin receptors. Chemical Reviews, 108, 1614–1641. [DOI] [PubMed] [Google Scholar]
- Pagel PS, & Warltier DC (1993). Negative inotropic effects of propofol as evaluated by the regional preload recruitable stroke work relationship in chronically instrumented dogs. Anesthesiology, 78, 100–108. [DOI] [PubMed] [Google Scholar]
- Palmer RM, Ashton DS, & Moncada S (1988). Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature, 333, 664–666. [DOI] [PubMed] [Google Scholar]
- Peterlin Z, Ishizawa Y, Araneda R, Eckenhoff R, & Firestein S (2005). Selective activation of G-protein coupled receptors by volatile anesthetics. Molecular and Cellular Neuroscience, 30, 506–512. [DOI] [PubMed] [Google Scholar]
- Petros AJ, Bogle RG, & Pearson JD (1993). Propofol stimulates nitric oxide release from cultured porcine aortic endothelial cells. British Journal of Pharmacology, 109, 6–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson BJ, Ebert TJ, O’Brien TJ, Colinco MD, & Muzi M (1997). Mechanisms whereby propofol mediates peripheral vasolidation in humans Anesthesiology, 86, 64–72. [DOI] [PubMed] [Google Scholar]
- Roth BL, Ciaranello RD, & Meltzer HY (1992). Binding of typical and atypical antipsychotic agents to transiently expressed 5-HT1C receptors. The Journal of Pharmacology and Experimental Therapeutics, 260, 1361–1365. [PubMed] [Google Scholar]
- Roth BL, Shoham M, Choudhary MS, & Khan N (1997a). Identification of conserved aromatic residues essential for agonist binding and second messenger production at 5-hydroxytryptamine2A receptors. Molecular Pharmacology, 52, 259–266. [DOI] [PubMed] [Google Scholar]
- Roth BL, Shoham M, Choudhary MS, & Khan N (1997b). Identification of conserved aromatic residues essential for agonist binding and second messenger production at 5-hydroxytryptamine(2A) receptors. Molecular Pharmacology, 52, 259–266. [DOI] [PubMed] [Google Scholar]
- Rouby JJ, Andreev A, Léger P, Arthaud M, Landault C, ... Viars P. (1991). Peripheral vascular effects of thiopental and propofol in humans with artificial hearts. Anesthesiology, 75, 32–42. [DOI] [PubMed] [Google Scholar]
- Schüttelkopf AW, & van Aalten DMF (2004). PRODRG: A tool for high-throughput crystallography of protein-ligand complexes. Acta Crystallographica Section D: Biological Crystallography, 60, 1355–1363. [DOI] [PubMed] [Google Scholar]
- Servin F, Farinotti R, Haberer JP, & Desmonts JM (1993). Propofol infusion for maintenance of anesthesia in morbidly obese patients receiving nitrous oxide. A clinical and pharmacokinetic study. Anesthesiology, 78, 657–665. [DOI] [PubMed] [Google Scholar]
- Süel GM, Lockless SW, Wall MA, & Ranganathan R (2003). Evolutionarily conserved networks of residues mediate allosteric communication in proteins. Nature Structural Biology, 10, 59–69. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Koyama H, Sugimoto M, Uchida I, & Mashimo T (2002). The diverse actions of volatile and gaseous anesthetics on human-cloned 5-hydroxytryptamine3 receptors expressed in Xenopus oocytes. Anesthesiology, 96, 699–704. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Ludwig LM, Kersten JR, Pagel PS, & Warltier DC (2004). Mechanisms of cardioprotection by volatile anesthetics. Anesthesiology, 100, 707–721. [DOI] [PubMed] [Google Scholar]
- Thomas DR (2006). 5-ht5A receptors as a therapeutic target. Pharmacology & Therapeutics, 111, 707–714. [DOI] [PubMed] [Google Scholar]
- Trott O, & Olson AJ (2009). AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. Journal of Computational Chemistry, 31, 455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner RJ, Gatt SP, Kam PC, Ramzan I, & Daley M (1998). Administration of a crystalloid fluid preload does not prevent the decrease in arterial blood pressure after induction of anaesthesia with propofol and fentanyl. British Journal of Anaesthesia, 80, 737–741. [DOI] [PubMed] [Google Scholar]
- Wacker D, Wang C, Katritch V, Han GW, Huang X-P, Vardy E, ... Stevens RC. (2013). Structural features for functional selectivity at serotonin receptors. Science. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CD, Gallaher TK, & Shin JC (1993). Site-directed mutagenesis of the serotonin 5-hydroxytrypamine(2) receptor – identification of amino-acids necessary for ligand-binding and receptor activation. Molecular Pharmacology, 43, 931–940. [PubMed] [Google Scholar]
- Wess J, Brann MR, & Bonner TI (1989). Identification of a small intracellular region of the muscarinic m3 receptor as a determinant of selective coupling to PI turnover. FEBS Letters, 258, 133–136. [DOI] [PubMed] [Google Scholar]
- Zhang L, Oz M, Stewart RR, Peoples RW, & Weight FF (1997). Volatile general anaesthetic actions on recombinant nACh α7, 5-HT 3 and chimeric nACh α7 −5-HT 3 receptors expressed in Xenopus oocytes. British Journal of Pharmacology, 120, 353–355. [DOI] [PMC free article] [PubMed] [Google Scholar]


