Abstract
Aim
To evaluate whether history of pregnancy complications [pre-eclampsia, gestational hypertension, preterm delivery, or small for gestational age (SGA)] improves risk prediction for cardiovascular disease (CVD).
Methods and results
This population-based, prospective cohort study linked data from the HUNT Study, Medical Birth Registry of Norway, validated hospital records, and Norwegian Cause of Death Registry. Using an established CVD risk prediction model (NORRISK 2), we predicted 10-year risk of CVD (non-fatal myocardial infarction, fatal coronary heart disease, and non-fatal or fatal stroke) based on established risk factors (age, systolic blood pressure, total and HDL-cholesterol, smoking, anti-hypertensives, and family history of myocardial infarction). We evaluated whether adding pregnancy complication history improved model fit, calibration, discrimination, and reclassification. Among 18 231 women who were parous, ≥40 years of age, and CVD-free at start of follow-up, 39% had any pregnancy complication history and 5% experienced a CVD event during a median follow-up of 8.2 years. While pre-eclampsia and SGA were associated with CVD in unadjusted models (HR 1.96, 95% CI 1.44–2.65 for pre-eclampsia and HR 1.46, 95% CI 1.18–1.81 for SGA), only pre-eclampsia remained associated with CVD after adjusting for established risk factors (HR 1.60, 95% CI 1.16–2.17). Adding pregnancy complication history to the established prediction model led to small improvements in discrimination (C-index difference 0.004, 95% CI 0.002–0.006) and reclassification (net reclassification improvement 0.02, 95% CI 0.002–0.05).
Conclusion
Pre-eclampsia independently predicted CVD after controlling for established risk factors; however, adding pre-eclampsia, gestational hypertension, preterm delivery, and SGA made only small improvements to CVD prediction among this representative sample of parous Norwegian women.
Keywords: Prediction, Stroke, Coronary heart disease, Pregnancy, Women’s health
See page 1121 for the editorial comment on this article (doi: 10.1093/eurheartj/ehz021)
Introduction
Cardiovascular disease (CVD) is the leading cause of death in women1 and is underdiagnosed in women compared to men.2,3 Cardiovascular disease risk prediction tools are commonly used to identify individuals at higher risk, allowing physicians to target interventions to patients who would benefit the most. However, clinical guidelines have noted that current tools identify few high risk women prior to age 70 and have called for research to improve sensitivity for younger women.4,5
Pregnancy may provide an ideal ‘window’ to predict future cardiovascular health in young women.6,7 Emerging evidence suggests that pregnancy complications, including pre-eclampsia,8,9 gestational hypertension,10 preterm delivery,11,12 and delivery of an infant small for gestational age (SGA),13–15 are associated with future CVD. Associations between pregnancy complications and CVD are likely due to shared etiologic pathways7 (e.g. metabolic syndrome, vascular dysfunction, and inflammation). Current guidelines recommend screening women for pregnancy complications and monitoring cardiovascular risk factors postpartum.2,16,17 However, it is unclear whether pregnancy complications also have utility in predicting CVD above-and-beyond established risk factors already included in prediction models. There is some evidence that pregnancy complications do not lead to large improvements in CVD risk prediction.18,19 However, only one previous study examined the benefit of adding pregnancy complications to existing CVD prediction models in a clinical setting,18 and no published studies have examined the benefit of adding history of preterm delivery or SGA to existing models.
Using linked data from the Nord-Trøndelag Health Study (the HUNT Study), the Medical Birth Registry of Norway (MBRN), validated hospital records, and the Norwegian Cause of Death Registry, we evaluated whether adding history of pregnancy complications to the NORRISK 2 risk prediction model,20 the model currently recommended for use in clinical practice in Norway,21 improved prediction performance in parous women.
Methods
Study population
The HUNT Study is an ongoing population-based cohort study of residents in Norway’s Nord-Trøndelag county. Approximately every decade, all county residents 20 years of age and older are invited to participate in an extensive health assessment, including a clinical examination and questionnaires.22 Three surveys were completed by the time of this analysis, HUNT1 (1984–86),23 HUNT2 (1995–97),24 and HUNT3 (2006–08).22 Using the national identification number assigned to Norwegian citizens, we linked HUNT data to the MBRN25 to capture information about all deliveries that occurred after the birth registry began in 1967.
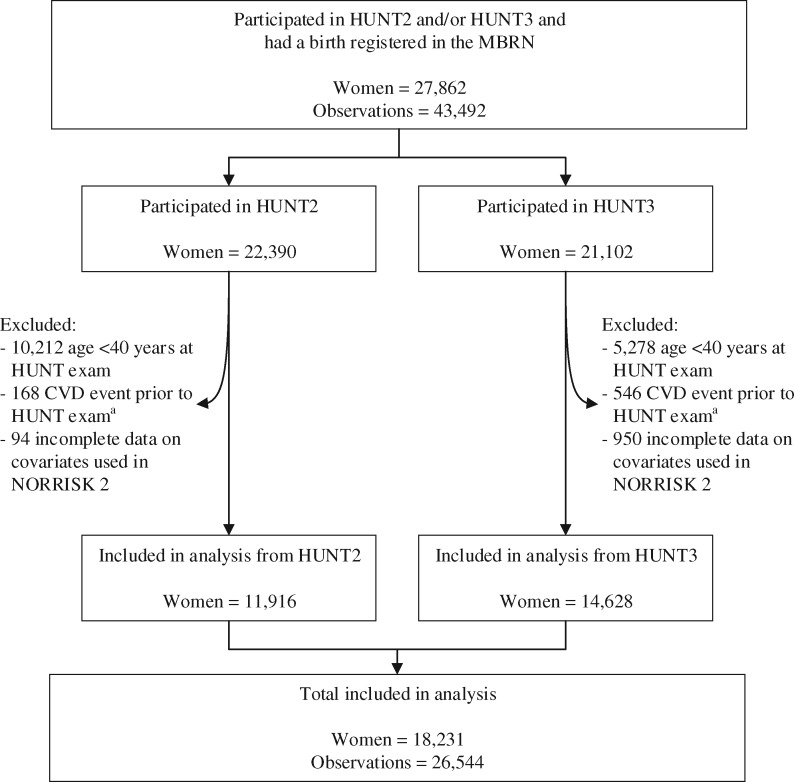
We restricted this analysis to participants in the HUNT2 and HUNT3 surveys, during which serum samples were collected for all participants, to enable inclusion of lipids in CVD risk prediction models. We identified a total of 27 862 parous women who participated in HUNT2 and/or HUNT3 and had a birth registered in the MBRN (Figure 1). As the NORRISK 2 CVD risk prediction model20 included only adults ages 40 years and older, we excluded women younger than 40 years at the time of the HUNT exam (n = 9056). We also excluded women with a history of CVD prior to the HUNT exam (n = 292). History of CVD was identified through either (i) self-report of myocardial infarction (MI) or stroke via questionnaire during the HUNT exam or any previous HUNT exam (including HUNT1) or (ii) validated record of hospitalization for MI or stroke from the start of record collection in 1987 through the date of the HUNT exam. After additionally excluding women with incomplete data on covariates used in the NORRISK 2 prediction model20 (n = 283), the final study population included 18 231 women. All HUNT study participants signed an informed consent form permitting use of their data and samples for research. The Central Norway Regional Committee for Medical and Health Research Ethics approved this project, and the Harvard T. H. Chan School of Public Health exempted this project from IRB review.
Figure 1.
Flow chart of study population. aIncludes self-reported history of myocardial infarction or stroke at HUNT exam and hospitalizations for myocardial infarction or stroke recorded from 1987 through the date of HUNT exam. CVD, cardiovascular disease; HUNT, the Nord-Trøndelag Health Study; MBRN, Medical Birth Registry of Norway.
Established cardiovascular risk factors
Established risk factors [including systolic blood pressure, total cholesterol, high density lipoprotein cholesterol (HDL-C), smoking, anti-hypertensive use, and family history of premature MI] were chosen to match the NORRISK 220 model to the extent possible. Trained HUNT study staff measured systolic blood pressure and collected non-fasting serum samples to quantify total cholesterol and HDL-C. We defined low HDL-C as <1.3 mmol/L. Supplementary material online, Table S1 includes additional measurement details. From the HUNT questionnaires, we identified current daily smoking and current anti-hypertensive use at the time of the HUNT exam as well as family history of premature MI, defined as having a first-degree family member who suffered a MI before the age of 60 years.
Pregnancy complications
We identified pregnancy complication history from the MBRN, including all pregnancies from 1967 to the time of the HUNT exam. Diagnoses of pre-eclampsia or gestational hypertension used internationally recommended criteria,26,27 with gestational hypertension generally defined as de novo hypertension (≥140 mmHg systolic and/or ≥90 mmHg diastolic) after 20 weeks of gestation, and pre-eclampsia also requiring proteinuria (300 mg/24 h or ≥1+ on the dipstick test). We identified gestation length based on ultrasound dating where available (2% of deliveries) or last menstrual period and defined preterm delivery as <37 weeks gestation. Multiple gestational pregnancies delivered <37 weeks were excluded from the preterm delivery definition. Small for gestational age was defined as the lowest 10% of birthweights by gestational age and sex observed in the MBRN,28 with percentiles calculated separately for multiple vs. single gestational pregnancies. Validation studies within HUNT26,29 found positive predictive values (PPV) of 88% for pre-eclampsia and 93% for preterm delivery. Gestational hypertension’s PPV was 68%, due in part to mislabeling of pre-eclampsia cases as gestational hypertension. Among women classified as gestational hypertensives, 88% had a hypertensive disorder of pregnancy (HDP). Although SGA was not included in validation studies, the PPV for low birth weight (<2500 g) in the HUNT study was 100%. Pregnancy complications were analysed using separate variables indicating whether the women had a history of the complication of interest across any pregnancy.
Cardiovascular endpoints
Consistent with NORRISK 2, we predicted hard CVD endpoints (non-fatal MI, fatal CHD, or non-fatal or fatal stroke). CVD events were captured from the two primary hospitals in Nord-Trøndelag county: Levanger Hospital and Namsos Hospital (Nord-Trøndelag Hospital Trust) from 1, September 1987 (the beginning of electronic recording) through 24 April 2015. All study participants with at least one cardiovascular diagnosis had their medical records reviewed by one of the two experienced cardiologists (B.K. and H.D.) to determine the first validated occurrence of MI or stroke. We diagnosed MI using Joint ESC/ACCF/AHA/WHF Task Force criteria30 and stroke based on typical symptoms and signs combined with radiological evidence from CT or MRI scans. Supplementary material online, Text S1 includes additional validation process details. In addition, we identified fatal CVD events using ICD diagnostic codes from the national Cause of Death Registry, which has had mandatory reporting since 1951 (Supplementary material online, Table S2).
Statistical analysis
We sought to compare an established CVD risk factor model to that same model additionally including history of pregnancy complications. For each of these models, we used the same statistical methods as NORRISK 2.20 We estimated the 10-year risk of CVD using Fine and Gray competing risk models,31 accounting for deaths from other causes as competing events. Women contributed person-time to the analysis from the index HUNT exam through first CVD event, death from other causes, or censoring at either the end of data collection on 24 April 2015 or emigration from Nord-Trøndelag county. Women who participated in both the HUNT2 and HUNT3 exams (n = 8313) were allowed to contribute two independent (non-overlapping) observations to the analysis as long as they met eligibility criteria at the start of follow-up. These women could contribute an observation with follow-up (i.e. at the time of the exam) from HUNT2 to HUNT3 using risk factor information assessed at the time of HUNT2 and an observation with follow-up from HUNT3 using risk factor information assessed during HUNT3. Although women could have contributed more than 10 years of follow-up to the analysis based on the timing of exams, we predicted CVD risk at 10 years of follow-up. To account for the correlation in measurements among women who participated in HUNT2 and HUNT3, we used variance estimates which account for repeated measures32 (Supplementary material online, Text S2). Some pregnancies were missing from the registry due to women giving birth before the start of the birth registry in 1967 (n = 4387 women), and some pregnancies had missing values for gestation length and/or birthweight or pregnancies with Z-scores for birthweight by gestation length that were >4 or <−4, suggesting error during data entry (n = 2042 women). We used multiple imputation to impute pregnancy complications for these pregnancies missing from the registry or with incomplete information in the registry. Supplementary material online, Text S2 provides details about multiple imputation methods used.
We calculated measures of model fit, calibration, discrimination, and reclassification because no single measure captures all of the information needed to assess improvement in model performance after adding pregnancy complications.33 Prior to building models, we compared the established risk factors between those with and without a history of pregnancy complications and tested whether there were significant differences using either χ2 tests for categorical measures or Student’s t-tests for continuous measures. We then estimated an unadjusted model including only pregnancy complications to confirm their ability to predict CVD endpoints in our population. Next, we identified whether the addition of pregnancy complications to the established risk factor model improved model fit using a Wald test. We assessed model calibration (the equivalence between observed CVD risk and model-predicted CVD risk) for models using the Greenwood–Nam–D’Agostino test for censored survival data.34 We measured model discrimination (the ability to distinguish between CVD cases and non-cases) by obtaining the C-index for each model and comparing the difference between them.35 The C-index is an extension of the concept of the area under the receiver operating characteristic (ROC) curve but modified to be appropriate for the survival setting.
A key indicator of improved model performance is if, after adding pregnancy complications, women who went on to have a CVD event were reclassified into higher risk categories while women who did not have an event were reclassified into lower risk categories.36 This information is summarized in the net reclassification improvement (NRI) which we calculated overall and separately among women who did and did not go on to have a CVD event, using an extension of the formula for survival data.37 To calculate these measures, we stratified women into clinically relevant categories based on their 10-year risk of CVD: low (<5%), intermediate (5 to <10%), and high (≥10%). We also calculated the integrated discrimination improvement (IDI),36 which is a related measure of model improvement that does not rely on cut-points to categorize risk. Confidence intervals for discrimination and reclassification measures were calculated using 1000 bootstraps. All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) and used publically available macros to assess prediction performance.38 See Supplementary material online, Text S2 for additional details about prediction performance measures.
Additional analyses
In post hoc analyses suggested by reviewers, we included additional terms for recurrent pregnancy complications (i.e. for each pregnancy complication, having experienced the complication during two or more pregnancies) and interaction terms between the pregnancy complications to test alternative parameterizations for modelling pregnancy complication history. As a sensitivity analysis, we also tested the degree of model overfitting to our dataset by adjusted estimates of discrimination and reclassification for model optimism using 1000 bootstrap samples.39
Results
Among 18 231 parous women, 965 (5%) had an incident CVD event during follow-up (median 8.2 years, interquartile range 7.4–11.1 years) while 295 (2%) died from other causes. Non-fatal events comprised the majority of incident CVD events (97%) with 5% dying on the day of or within 30 days of the event. Thirty percent of women experienced at least one pregnancy complication based on birth registry records. After imputing pregnancy complications for births not captured in the registry, 39% of women had a history of pregnancy complications in any pregnancy. In age-standardized comparisons, women with a history of pregnancy complications based on birth registry records had higher systolic blood pressure (P < 0.001) and were more likely to smoke (P < 0.001), take anti-hypertensives (P < 0.001), have low HDL (P = 0.006), and have a family history of premature MI (P < 0.001) compared to women with no pregnancy complications (Table 1).
Table 1.
Descriptive statistics of parous HUNT2 and HUNT3 participants by history of pregnancy complications at start of follow-up
| All study participants (n = 26 544a) | Did not experience a pregnancy complicationb(n = 18 608a) | Experienced at least one pregnancy complicationb(n = 7936a) | |
|---|---|---|---|
| Age at HUNT exam in years, median (IQR) | 52 (46–59) | 52 (46–60) | 51 (46–58) |
| Age-standardizedc risk factors from NORRISK 2 model | |||
| Systolic blood pressure in mmHg, median (IQR) | 128 (117–142) | 127 (116–141) | 130 (118–144) |
| Serum total cholesterol in mmol/L, median (IQR) | 5.8 (5.1–6.6) | 5.8 (5.1–6.6) | 5.8 (5.1–6.6) |
| Current daily smoking | 31% | 29% | 35% |
| Current anti-hypertensive use | 13% | 12% | 18% |
| Low HDL-Cd | 37% | 37% | 39% |
| Family history of premature MIe | 17% | 16% | 19% |
| Reproductive history | |||
| Number of births | |||
| 1 | 8% | 8% | 7% |
| 2 | 38% | 39% | 37% |
| 3 | 34% | 34% | 35% |
| 4+ | 19% | 19% | 21% |
| Pre-eclampsia in any pregnancy | 5% | 0% | 17% |
| Gestational hypertension in any pregnancy | 4% | 0% | 12% |
| Any preterm delivery | 8% | 0% | 28% |
| Any small for gestational age delivery | 18% | 0% | 62% |
HUNT, the Nord-Trøndelag Health Study; HDL-C, high density lipoprotein cholesterol; IQR, interquartile range; MI, myocardial infarction.
Women who participated in only HUNT2 (n = 3603) or HUNT3 (n = 6315) contributed one observation while women who participated in both HUNT2 and HUNT3 (n = 8313) contributed two.
Pregnancy complications include pre-eclampsia, gestational hypertension, preterm delivery, and small for gestational age delivery.
Risk factors standardized to the age distribution of the study population.
Low HDL-C <1.3 mmol/L.
First degree family member suffered MI before the age of 60 years.
Model fit
The associations between established risk factors and CVD were similar to those reported in the original NORRISK 2 publication by Selmer et al.20 (Supplementary material online, Table S3). In unadjusted models including all pregnancy complications, pre-eclampsia, and SGA were independently associated with CVD (HR 1.96, 95% CI 1.44–2.65 for pre-eclampsia and HR 1.46, 95% CI 1.18–1.81 for SGA) (Table 2). After adjusting for the established risk factors only pre-eclampsia was associated with an increased rate of CVD (HR 1.60, 95% CI 1.16–2.17). Model fit was improved with the addition of pregnancy complications (Wald test P = 0.04).
Table 2.
Hazard ratios for 10-year cardiovascular disease risk from Fine and Gray competing risk models comparing models with and without pregnancy complication history
| Covariates | Unadjusted model for pregnancy complication history |
Established risk factor model |
Established risk factor model + pregnancy complication historyd |
|||
|---|---|---|---|---|---|---|
| HRc | 95% CI | HRc | 95% CI | HRc | 95% CI | |
| Age (per 1 year) | 1.09 | 1.06–1.13 | 1.10 | 1.06–1.13 | ||
| Age squared (per 1 year) | 1.00 | 1.00–1.00 | 1.00 | 1.00–1.00 | ||
| Systolic blood pressure (per 10 mmHg) | 1.19 | 1.11–1.28 | 1.19 | 1.10–1.28 | ||
| Serum total cholesterol (per 1 mmol/L) | 1.12 | 1.06–1.18 | 1.12 | 1.06–1.18 | ||
| Daily smoking (yes/no) | 3.22 | 2.37–4.37 | 3.24 | 2.38–4.41 | ||
| Systolic blood pressure × age | 1.00 | 1.00–1.00 | 1.00 | 1.00–1.00 | ||
| Daily smoking × age | 0.98 | 0.97–1.00 | 0.98 | 0.97–1.00 | ||
| Anti-hypertensives (yes/no) | 1.41 | 1.21–1.66 | 1.38 | 1.16–1.64 | ||
| Low HDL-cholesterol (yes/no)a | 1.65 | 1.45–1.87 | 1.65 | 1.45–1.87 | ||
| Family history of premature MIb | 1.53 | 1.31–1.77 | 1.52 | 1.30–1.76 | ||
| Pre-eclampsia in any pregnancy (yes/no) | 1.96 | 1.44–2.65 | 1.60 | 1.16–2.17 | ||
| Gestational HTN in any pregnancy (yes/no) | 1.16 | 0.70–1.89 | 0.73 | 0.46–1.15 | ||
| Any preterm delivery (yes/no) | 1.13 | 0.85–1.51 | 0.98 | 0.73–1.31 | ||
| Any SGA delivery (yes/no) | 1.46 | 1.18–1.81 | 1.06 | 0.85–1.32 | ||
CI, confidence interval; HDL-C, high density lipoprotein cholesterol; HR, hazard ratio; HTN, hypertension; MI, myocardial infarction; SGA, small for gestational age.
Low HDL-cholesterol: < 1.3 mmol/L.
First degree family member suffered MI before the age of 60 years.
CVD-specific hazard ratio from Fine and Gray competing risk model.
Wald test for joint significance of all four pregnancy complications: P = 0.04.
Calibration
The established risk factor model was well-calibrated both before (P = 0.23 for the null hypothesis of equal observed and predicted risk) and after (P = 0.26) the addition of pregnancy complications. A visual depiction of model calibration is provided in Supplementary material online, Figure S1.
Discrimination
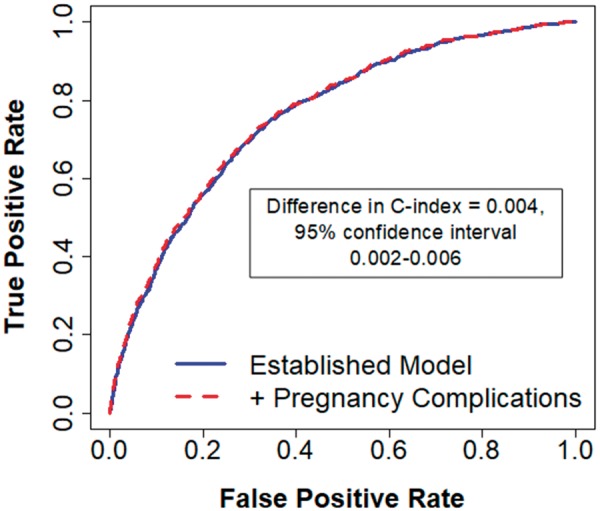
Inclusion of pregnancy complications in the established risk factor model led to a statistically significant but small 0.004 increase in the C-index (95% CI 0.002–0.006) (Take home figure). This small improvement in discrimination was driven mostly by the inclusion of pre-eclampsia (Table 3).
Take home figure.

Pregnancy complications did not substantially improve 10-year cardiovascular disease risk prediction compared with an established model.
Table 3.
Discrimination statistics comparing models with and without pregnancy complication history
| Model | C-index | C-index difference from established CVD risk factor model | 95% CI for C-index difference |
|---|---|---|---|
| Established CVD risk factors | 0.789 | ||
| Established CVD risk factors + pre-eclampsia in any pregnancy | 0.792 | 0.003 | (0.001–0.005) |
| Established CVD risk factors + gestational HTN in any pregnancy | 0.790 | 0.0006 | (−0.0004 to 0.001) |
| Established CVD risk factors + any preterm delivery | 0.789 | 0.0002 | (0.0001–0.0002) |
| Established CVD risk factors + any SGA delivery | 0.790 | 0.0003 | (−0.0001 to 0.0008) |
| Established CVD risk factors + all pregnancy complications | 0.793 | 0.004 | (0.002–0.006) |
CI, confidence interval; CVD, cardiovascular disease; HTN, hypertension; SGA, small-for-gestational age.
Reclassification of clinical risk categories
Among the 965 women with an incident CVD event during follow-up, 38 were correctly reclassified into a higher risk category after the inclusion of pregnancy complication history while 17 were incorrectly reclassified into a lower risk category (Table 4). This lead to a marginal improvement in reclassification as measured by the NRI for events (0.02, 95% CI −0.002 to 0.04). Among the 25579 observations without a CVD event, 274 were incorrectly reclassified into a higher risk category while 375 were correctly reclassified into a lower risk category, which similarly resulted in a small improvement in reclassification [NRI for non-events 0.004 (95% CI 0.002–0.006)]. Overall, there was a small improvement in reclassification 0.02 (95% CI 0.002–0.05). No obvious improvements in reclassification were apparent when examining continuous predicted probabilities (Supplementary material online, Figure S2) and the IDI [−0.0002 (95% CI −0.001 to 0.0007)] did not indicate overall improvement on a continuous scale. Similar small improvements in reclassification were observed when comparing the established risk factor model to a model including pre-eclampsia history only (Supplementary material online, Table S4) and no improvement in reclassification was seen for the other pregnancy complications examined individually.
Table 4.
Reclassification of cardiovascular disease risk category after including pregnancy complication historya,b
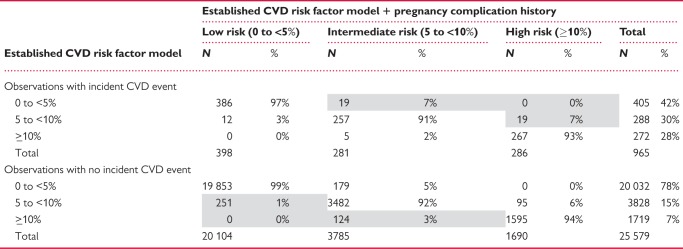 |
Net reclassification improvement (NRI) = 0.02 (95% CI 0.002 to 0.05), P = 0.04.
NRI for events = 0.02 (95% CI −0.002 to 0.04), P = 0.08.
NRI for non-events = 0.004 (95% CI 0.002 to 0.006), P < 0.001.
Integrated discrimination improvement (IDI) = −0.0002 (95% CI −0.001 to 0.0007), P = 0.65.
Shaded areas represent improvements in reclassification after the addition of pregnancy complication history.
This table includes observations with censoring prior to 10 years or follow-up longer than 10 years (median = 8.2 years of follow-up). Reclassification metrics explicitly take into account follow-up time (see Supplementary material online, Text S2) and cannot be directly calculated using numbers in this table.
Additional analyses
Including additional variables for recurrent pregnancy complications and interactions between pregnancy complications did not lead to substantial improvements in prediction performance compared to models including only a single variable for history of each pregnancy complication (Supplementary material online, Table S5). Estimates were similar after adjusting for optimism (Supplementary material online, Table S6).
Discussion
Addition of pregnancy complication history led to very modest to no improvements in an established cardiovascular risk prediction model recommended for clinical practice in Norway. We observed improvements in model discrimination and reclassification; however, differences were small in magnitude and unlikely to be clinically meaningful. Although pre-eclampsia and SGA were significant predictors of atherosclerotic CVD events in unadjusted models, only pre-eclampsia predicted an increased risk of CVD after adjusting for established risk factors.
Comparison with current literature
Previous studies have examined the association between pregnancy complications and CVD8–15; however, significant associations do not necessarily translate into improved prediction.40 Only one previous study based on clinical data assessed prediction model performance after adding pregnancy complications and similarly found very modest to no improvement in CVD risk prediction.18 Our study expands on this work using a larger general population sample, covering a wider age range, using validated CVD events, and including preterm and SGA history in addition to hypertensive disorders of pregnancy. Our findings are also supported by a recent study based on self-reported data in female nurses, in which previous pre-eclampsia did not improve CVD risk prediction.19
The goal of this study was to examine the ability of pregnancy complications to predict CVD rather than their associations; however, it is worth noting that the magnitude of observed associations between pregnancy complications and CVD in our study were lower than those reported in previous studies, particularly for preterm, gestational hypertension, and SGA.10–15 This was true even compared to some Norwegian studies,41–44 although one study found similarly small estimates for preterm and SGA.44 Many previous studies were conducted using death registries and associations with pregnancy complications tend to be lower for non-fatal events8,11 which comprised the majority of events included in this study. Most previous studies also began follow-up time immediately after an index birth and there is some evidence that associations between pregnancy complications and CVD are substantially stronger at younger ages.45,46 In contrast, we started follow-up for CVD events after age 40 (mean = 52 years) to mimic clinical settings in which NORRISK 2 is used, which could also contribute to the modest associations seen in our study. In addition, few previous studies have adjusted for established risk factors included in prediction models.8,12 There is evidence that the association between HDP and CVD is mediated to a substantial degree by CVD risk factors,47 which may explain why including pre-eclampsia in the NORRISK 2 model did not substantially improve discrimination or reclassification.
Prediction modelling choices
We chose the NORRISK 2 as our established risk factor model because it is currently recommended for clinical practice in Norway and was built using Norwegian data, including HUNT2 data among other sources. All decisions about how to model variables were made a priori, aligning with NORRISK 2 wherever possible, thus the risk of overfitting the prediction model to our dataset was low. We also confirmed that estimates were similar after adjusting for model optimism. We used identical definitions of model variables, CVD outcomes, and modelling techniques as the NORRISK 2 model with only one exception. NORRISK 2 includes separate variables for one compared to two or more family members with premature MI; however, this level of detail was not collected in HUNT3 so we used a single variable to summarize any family history of premature MI. The NORRISK 2 model is used exclusively in Norway and pregnancy complications may improve model fit more or less using other established risk prediction models. However, there is substantial overlap in the variables and methods used in NORRISK 2 compared to other commonly used CVD prediction models such as European SCORE,48 Framingham,49 or Pooled Cohort Risk Equations.4
Although there are many ways to model pregnancy complication history, we chose a priori to include a single variable indicating history of each pregnancy complication because it would be more feasible to implement a similar model in the clinical setting and aid in the interpretation of results. Still, models with alternative parameterizations of pregnancy complications yielded similar results, indicating little benefit to increasing model complexity.
Limitations
While our study location in Nord-Trøndelag county is fairly representative of Norway,24 findings may not be generalizable to non-Nordic populations. For example, although smoking rates seen in our study were similar to the female Norwegian average during our study period,50 smoking prevalence is greater in Norway than most higher-income countries. Our study was also limited to parous women who made up about 90% of the population of women during the time period of this study.51 We would expect similar findings after including nulliparous women, although small improvements may be seen due to the association between parity and CVD.52,53 Another limitation of this study was the lack of data on gestational diabetes, which was likely underdiagnosed in the MBRN before 1988.54 The proportion of women who had ever experienced a pregnancy complication in this population (an estimated 39%) was higher than estimates from the United States (29%)6 but similar to a study from the United Kingdom (36%)55 One explanation for our high proportion of women with pregnancy complication history in this study is the relatively high fertility rate in Norway compared to other high-income countries.56 Another explanation is that the reference population used to identify birthweight percentiles by gestation length used a more recent sample of births in the MBRN. An advantage of using an external reference population is that our definition of SGA could be more easily recreated in other populations; however, increases in birthweights over time may have led to an overestimation of SGA deliveries.
Strengths
Strengths of our study include the use of a general population sample of parous women and assessment of CVD risk factors during exams that reflect a realistic clinical scenario. In addition, 97% of the events used in this study were validated by study staff. Our linkage project provided a unique combination of rich clinical data, reproductive history, and follow-up for non-fatal and fatal events ideal for examining this research question.
Clinical relevance and future directions
After adding pregnancy complications to the model, an additional 0.4% of women without events were correctly reclassified into lower risk categories while 2% of women with events were correctly reclassified into higher risk categories. In Norway, risk thresholds to initiative treatment to prevent CVD are age-specific but roughly align with thresholds used in this article,21 thus we would expect to see small improvements in appropriate treatment if pregnancy complications to CVD risk prediction models in clinical practice. While these benefits are small in magnitude, subsequent studies should evaluate whether greater improvements are seen for other populations, especially among younger women. Pregnancy complications occur early in life and may be useful for primary and primordial prevention in younger populations. Findings should also be validated in other populations, including non-Nordic countries.
Conclusion
In this population-based, prospective cohort study, pregnancy complications (pre-eclampsia, gestational hypertension, preterm delivery, and SGA) led to only small improvements in 10-year CVD risk prediction for parous women, as measured by changes in model discrimination and reclassification. Although overall prevalence of at least one pregnancy complication was high in this population, pregnancy complications were not strong enough independent predictors of CVD after controlling for established risk factors to substantially improve prediction performance.
Supplementary Material
Acknowledgements
The Nord-Trøndelag Health Study (The HUNT Study) is a collaboration between HUNT Research Centre (Faculty of Medicine and Health Sciences, NTNU, Norwegian University of Science and Technology), Nord-Trøndelag County Council, Central Norway Regional Health Authority, and the Norwegian Institute of Public Health.
Funding
American Heart Association (16PRE29690006 to A.M.); Research Council of Norway (231149/F20 to B.O.Å., J.H., and E.B.H); National Institute of Child Health and Human Development, National Institutes of Health Training Grant in Reproductive, Perinatal and Pediatric Epidemiology (T32HD060454 to A.M.); Liaison Committee for education, research and innovation in Central Norway (to B.O.Å.); St. Olavs Hospital and the Faculty of Medicine and Health Sciences, NTNU (to B.O.Å.); Fulbright Program (to B.O.Å.); National Heart, Lung, and Blood Institute, National Institutes of Health Ruth L. Kirchstein National Research Service Award (F31 HL131222 to L.J.T); National Heart, Lung, and Blood Institute, National Institutes of Health Training Grant (T32HL098048 to J.J.S and L.J.T); UK MRC personal fellowship (MR/M009351/1 to A.F.); UK MRC funding (MC_UU_12013/5 and MC_UU_12013/9 to A.F.'s unit); Swedish Research Council International Postdoc Grant (2014-6790 to S.T.); Bundy Academy, Lund, Sweden (to S.T.).
Conflict of interest: none declared.
References
- 1.World Health Organization. Women’s Health. http://www.who.int/mediacentre/factsheets/fs334/en/ (21 October 2016).
- 2. Mosca L, Benjamin EJ, Berra K, Bezanson JL, Dolor RJ, Lloyd-Jones DM, Newby LK, Piña IL, Roger VL, Shaw LJ, Zhao D, Beckie TM, Bushnell C, D'Armiento J, Kris-Etherton PM, Fang J, Ganiats TG, Gomes AS, Gracia CR, Haan CK, Jackson EA, Judelson DR, Kelepouris E, Lavie CJ, Moore A, Nussmeier NA, Ofili E, Oparil S, Ouyang P, Pinn VW, Sherif K, Smith SC, Sopko G, Chandra-Strobos N, Urbina EM, Vaccarino V, Wenger NK.. Effectiveness-based guidelines for the prevention of cardiovascular disease in women—2011 update: a guideline from the American Heart Association. Circulation 2011;123:1243–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shaw LJ, Bairey Merz CN, Pepine CJ, Reis SE, Bittner V, Kelsey SF, Olson M, Johnson BD, Mankad S, Sharaf BL, Rogers WJ, Wessel TR, Arant CB, Pohost GM, Lerman A, Quyyumi AA, Sopko G; WISE Investigators. Insights from the NHLBI-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study: Part I: Gender differences in traditional and novel risk factors, symptom evaluation, and gender-optimized diagnostic strategies. J Am Coll Cardiol 2006;47:S4–S20. [DOI] [PubMed] [Google Scholar]
- 4. Goff DC, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC, Sorlie P, Stone NJ, Wilson PWF; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2935–2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, Foster E, Hlatky MA, Hodgson JM, Kushner FG, Lauer MS, Shaw LJ, Smith SC, Taylor AJ, Weintraub WS, Wenger NK.. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2010;56:e50–103. [DOI] [PubMed] [Google Scholar]
- 6. Rich-Edwards JW, Fraser A, Lawlor DA, Catov JM.. Pregnancy characteristics and women’s future cardiovascular health: an underused opportunity to improve women’s health? Epidemiol Rev 2014;36:57–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sattar N. Do pregnancy complications and CVD share common antecedents? Atheroscler Suppl 2004;5:3–7. [DOI] [PubMed] [Google Scholar]
- 8. Bellamy L, Casas J-P, Hingorani AD, Williams DJ.. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ 2007;335:974.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McDonald SD, Malinowski A, Zhou Q, Yusuf S, Devereaux PJ.. Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. Am Heart J 2008;156:918–930. [DOI] [PubMed] [Google Scholar]
- 10. Tooher J, Thornton C, Makris A, Ogle R, Korda A, Hennessy A.. All hypertensive disorders of pregnancy increase the risk of future cardiovascular disease. Hypertension 2017;70:798–803. [DOI] [PubMed] [Google Scholar]
- 11. Robbins CL, Hutchings Y, Dietz PM, Kuklina EV, Callaghan WM.. History of preterm birth and subsequent cardiovascular disease: a systematic review. Am J Obstet Gynecol 2014;210:285–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wu P, Gulati M, Kwok CS, Wong CW, Narain A, O’Brien S, Chew‐Graham CA, Verma G, Kadam UT, Mamas MA.. Preterm delivery and future risk of maternal cardiovascular disease: a systematic review and meta‐analysis. J Am Heart Assoc 2018;7:e007809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Morken N-H, Halland F, DeRoo L, Wilcox A, Skjærven R. Offspring birthweight by gestational age and parental cardiovascular mortality: a population-based cohort study. BJOG: Int J Obstet Gy 2017;125:336–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lykke JA, Paidas MJ, Triche EW, Langhoff-Roos J.. Fetal growth and later maternal death, cardiovascular disease and diabetes. Acta Obstet Gynecol Scand 2012;91:503–510. [DOI] [PubMed] [Google Scholar]
- 15. Li C-Y, Chen H-F, Sung F-C, Chen C-C, Lu T-H, Yang C-H, Ko M-C.. Offspring birth weight and parental cardiovascular mortality. Int J Epidemiol 2010;39:1082–1090. [DOI] [PubMed] [Google Scholar]
- 16. Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, Blomström-Lundqvist C, Cífková R, De Bonis M, Iung B, Johnson MR, Kintscher U, Kranke P, Lang IM, Morais J, Pieper PG, Presbitero P, Price S, Rosano GMC, Seeland U, Simoncini T, Swan L, Warnes CA, Deaton C, Simpson IA, Aboyans V, Agewall S, Barbato E, Calda P, Coca A, Coman IM, De Backer J, Delgado V, Di Salvo G, Fitzsimmons S, Fitzsimons D, Garbi M, Gevaert S, Hindricks G, Jondeau G, Kluin J, Lionis C, McDonagh TA, Meier P, Moons P, Pantazis A, Piepoli MF, Rocca B, Roffi M, Rosenkranz S, Sarkozy A, Shlyakhto E, Silversides CK, Sliwa K, Sousa-Uva M, Tamargo J, Thorne S, Van de Velde M, Williams B, Zamorano JL, Windecker S, Aboyans V, Agewall S, Barbato E, Bueno H, Coca A, Collet J-P, Coman IM, Dean V, Delgado V, Fitzsimons D, Gaemperli O, Hindricks G, Iung B, Jüni P, Katus HA, Knuuti J, Lancellotti P, Leclercq C, McDonagh TA, Piepoli MF, Ponikowski P, Richter DJ, Roffi M, Shlyakhto E, Simpson IA, Sousa-Uva M, Zamorano JL, Hammoudi N, Piruzyan A, Mascherbauer J, Samadov F, Prystrom A, Pasquet A, Caluk J, Gotcheva N, Skoric B, Heracleous H, Vejlstrup N, Maser M, Kaaja RJ, Srbinovska-Kostovska E, Mounier-Vehier C, Vakhtangadze T, Rybak K, Giannakoulas G, Kiss RG, Thrainsdottir IS, Erwin RJ, Porter A, Geraci G, Ibrahimi P, Lunegova O, Mintale I, Kadri Z, Benlamin H, Barysiene J, Banu CA, Caruana M, Gratii C, Haddour L, Bouma BJ, Estensen M-E, Hoffman P, Petris AO, Moiseeva O, Bertelli L, Tesic BV, Dubrava J, Koželj M, Prieto-Arévalo R, Furenäs E, Schwerzmann M, Mourali MS, Ozer N, Mitchenko O, Nelson-Piercy C; ESC Scientific Document Group. 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J 2018;39:3165–3241. [DOI] [PubMed] [Google Scholar]
- 17. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney M-T, Corrà U, Cosyns B, Deaton C, Graham I, Hall MS, Hobbs FDR, Løchen M-L, Löllgen H, Marques-Vidal P, Perk J, Prescott E, Redon J, Richter DJ, Sattar N, Smulders Y, Tiberi M, van der Worp HB, van Dis I, Verschuren WMM.. 2016 European Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J 2016;37:2315–2381.27222591 [Google Scholar]
- 18. Timpka S, Fraser A, Schyman T, Stuart JJ, Åsvold BO, Mogren I, Franks PW, Rich-Edwards JW.. The value of pregnancy complication history for 10-year cardiovascular disease risk prediction in middle-aged women. Eur J Epidemiol 2018;33:1003–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stuart JJ, Tanz LJ, Cook NR, Spiegelman D, Missmer SA, Rimm EB, Rexrode KM, Mukamal KJ, Rich-Edwards JW.. Hypertensive disorders of pregnancy and 10-year cardiovascular risk prediction. J Am Coll Cardiol 2018;72:1252–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Selmer R, Igland J, Ariansen I, Tverdal A, Njølstad I, Furu K, Tell GS, Klemsdal TO.. NORRISK 2: a Norwegian risk model for acute cerebral stroke and myocardial infarction. Eur J Prev Cardiol 2017;24:773–782. [DOI] [PubMed] [Google Scholar]
- 21.Nasjonal faglig retningslinje for forebygging av hjerte- og karsykdom. Helsedirektoratet.no. https://helsedirektoratet.no/retningslinjer/forebygging-av-hjerte-og-karsykdom (17 May 2018).
- 22. Krokstad S, Langhammer A, Hveem K, Holmen TL, Midthjell K, Stene TR, Bratberg G, Heggland J, Holmen J.. Cohort profile: the HUNT Study, Norway. Int J Epidemiol 2013;42:968–977. [DOI] [PubMed] [Google Scholar]
- 23. Holmen J, Midthjell K, Bjartveit K, Hjort PF, Lund-Larsen PG, The Nord-Trøndelag health Survey 1984-1986. Purpose, background and methods. Participation, non-participation and frequency distributions, p1–257. Report No. 4. Verdal: Senter for samfunnsmedisinsk forskning, Statens Institutt for folkehelse (SIFF), Helsetjenesteforskning; 1990.
- 24. Holmen J, Midthjell K, Krüger Ø, Langhammer A, Holmen TL, Bratberg GH, Vatten L, Lund-Larsen PG.. The Nord-Trøndelag Health Study 1995-97 (HUNT 2): objectives, contents, methods and participation. Norsk Epidemiol 2003;13:19–32. [Google Scholar]
- 25. Irgens LM. The Medical Birth Registry of Norway. Epidemiological research and surveillance throughout 30 years. Acta Obstet Gynecol Scand 2000;79:435–439. [PubMed] [Google Scholar]
- 26. Thomsen LCV, Klungsøyr K, Roten LT, Tappert C, Araya E, Baerheim G, Tollaksen K, Fenstad MH, Macsali F, Austgulen R, Bjørge L.. Validity of the diagnosis of pre-eclampsia in the Medical Birth Registry of Norway. Acta Obstet Gynecol Scand 2013;92:943–950. [DOI] [PubMed] [Google Scholar]
- 27.American College of Obstetricians and Gynecologists, Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol 2013;122:1122–1131. [DOI] [PubMed] [Google Scholar]
- 28. Skjaerven R, Gjessing HK, Bakketeig LS.. Birthweight by gestational age in Norway. Acta Obstet Gynecol Scand 2000;79:440–449. [PubMed] [Google Scholar]
- 29. Moth FN, Sebastian TR, Horn J, Rich-Edwards J, Romundstad PR, Åsvold BO.. Validity of a selection of pregnancy complications in the Medical Birth Registry of Norway. Acta Obstet Gynecol Scand 2016;95:519–527. [DOI] [PubMed] [Google Scholar]
- 30. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Katus HA, Lindahl B, Morrow DA, Clemmensen PM, Johanson P, Hod H, Underwood R, Bax JJ, Bonow RO, Pinto F, Gibbons RJ, Fox KA, Atar D, Newby LK, Galvani M, Hamm CW, Uretsky BF, Steg PG, Wijns W, Bassand J-P, Menasché P, Ravkilde J, Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Simoons ML, Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G, Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D, Smith SC, Hu D, Lopez-Sendon J-L, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ, Mendis S.. Third universal definition of myocardial infarction. Circulation 2012;126:2020–2035. [DOI] [PubMed] [Google Scholar]
- 31. Fine JP, Gray RJ.. A Proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509. [Google Scholar]
- 32. Lin DY, Wei LJ.. The robust inference for the Cox proportional hazards model. J Am Stat Assoc 1989;84:1074–1078. [Google Scholar]
- 33. Hlatky MA, Greenland P, Arnett DK, Ballantyne CM, Criqui MH, Elkind MSV, Go AS, Harrell FE, Hong Y, Howard BV, Howard VJ, Hsue PY, Kramer CM, McConnell JP, Normand S-LT, O'Donnell CJ, Smith SC, Wilson PWF; American Heart Association Expert Panel on Subclinical Atherosclerotic Diseases and Emerging Risk Factors and the Stroke Council . Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. Circulation 2009;119:2408–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Demler OV, Paynter NP, Cook NR.. Tests of calibration and goodness-of-fit in the survival setting. Stat Med 2015;34:1659–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pencina MJ, D'Agostino RB.. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med 2004;23:2109–2123. [DOI] [PubMed] [Google Scholar]
- 36. Pencina MJ, D' Agostino RB, D'Agostino RB, Vasan RS.. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 2008;27:157–172; discussion 207–212. [DOI] [PubMed] [Google Scholar]
- 37. Pencina MJ, D'Agostino RB, Steyerberg EW.. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med 2011;30:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.SAS Macros. http://ncook.bwh.harvard.edu/sas-macros.html (19 May 2018).
- 39. Harrell FE. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York: Springer; 2010. [Google Scholar]
- 40. Pepe MS, Janes H, Longton G, Leisenring W, Newcomb P.. Limitations of the odds ratio in gauging the performance of a diagnostic, prognostic, or screening marker. Am J Epidemiol 2004;159:882–890. [DOI] [PubMed] [Google Scholar]
- 41. Rich-Edwards JW, Klungsoyr K, Wilcox AJ, Skjaerven R.. Duration of pregnancy, even at term, predicts long-term risk of coronary heart disease and stroke mortality in women: a population-based study. Am J Obstet Gynecol 2015;213:518.e1–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Skjaerven R, Wilcox AJ, Klungsøyr K, Irgens LM, Vikse BE, Vatten LJ, Lie RT.. Cardiovascular mortality after pre-eclampsia in one child mothers: prospective, population based cohort study. BMJ 2012;345:e7677.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Riise HKR, Sulo G, Tell GS, Igland J, Nygård O, Vollset SE, Iversen A-C, Austgulen R, Daltveit AK.. Incident coronary heart disease after preeclampsia: role of reduced fetal growth, preterm delivery, and parity. J Am Heart Assoc 2017;6:e004158.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Riise HKR, Sulo G, Tell GS, Igland J, Nygård O, Iversen A-C, Daltveit AK.. Association between gestational hypertension and risk of cardiovascular disease among 617 589 Norwegian women. J Am Heart Assoc 2018;7:e008337.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cirillo PM, Cohn BA.. Pregnancy complications and cardiovascular disease death: fifty-year follow-up of the child health and development studies pregnancy cohort. Circulation 2015;132:1234–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Smith GD, Sterne J, Tynelius P, Lawlor DA, Rasmussen F.. Birth weight of offspring and subsequent cardiovascular mortality of the parents. Epidemiology 2005;16:563–569. [DOI] [PubMed] [Google Scholar]
- 47. Stuart J, Tanz L, Rimm E, Spiegleman D, Missmer S, Mukamal K, Rich-Edwards J.. Hypertensive Disorders in First Pregnancy and Maternal Cardiovascular Disease: Mediation by Postpartum Cardiovascular Risk Factors. Seattle, Washington; 2017. [Google Scholar]
- 48. Conroy RM, Pyörälä K, Fitzgerald AP, Sans S, Menotti A, De BG, De BD, Ducimetière P, Jousilahti P, Keil U, Njølstad I, Oganov RG, Thomsen T, Tunstall PH, Tverdal A, Wedel H, Whincup P, Wilhelmsen L, Graham IM.. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J 2003;24:987–1003. [DOI] [PubMed] [Google Scholar]
- 49. D’Agostino RB, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB.. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 2008;117:743–753. [DOI] [PubMed] [Google Scholar]
- 50. Lund I, Lund KE.. Lifetime smoking habits among Norwegian men and women born between 1890 and 1994: a cohort analysis using cross-sectional data. BMJ Open 2014;4:e005539.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Decline in fertility. ssb.no. http://www.ssb.no/en/befolkning/statistikker/fodte/aar/2017-03-09 (15 February 2018).
- 52. Parikh NI, Jeppson RP, Berger JS, Eaton CB, Kroenke CH, LeBlanc ES, Lewis CE, Loucks EB, Parker DR, Rillamas-Sun E, Ryckman KK, Waring ME, Schenken RS, Johnson KC, Edstedt-Bonamy A-K, Allison MA, Howard BV.. Reproductive risk factors and coronary heart disease in the Women’s Health Initiative Observational Study. Circulation 2016;133:2149–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lv H, Wu H, Yin J, Qian J, Ge J.. Parity and cardiovascular disease mortality: a dose-response meta-analysis of cohort studies. Sci Rep 2015;5:13411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Stene LC, Eidem I, Vangen S, Joner G, Irgens LM, Moe N.. The validity of the diabetes mellitus diagnosis in the Medical Birth Registry of Norway. Nor Epidemiol 2007;17:165–174. [Google Scholar]
- 55. Fraser A, Nelson SM, Macdonald-Wallis C, Cherry L, Butler E, Sattar N, Lawlor DA.. Associations of pregnancy complications with calculated cardiovascular disease risk and cardiovascular risk factors in middle age clinical perspective. Circulation 2012;125:1367–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kravdal Ø. Not so low fertility in Norway—a result of affluence, liberal values, gender-equality ideals, and the welfare state, p13–47 In: Rindfuss R, Choe MK., eds. Low Fertility, Institutions, and Their Policies. Cham: Springer International Publishing AG; 2016. pp. 13–47. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.