Abstract
Objective
The aim of this meta-analysis of randomized placebo-controlled trials was to examine whether ursodeoxycholic acid treatment is an effective lipid-lowering agent.
Methods
PubMed-Medline, SCOPUS, Web of Science and Google Scholar databases were searched in order to find randomized controlled trials evaluating the effect of ursodeoxycholic acid on lipid profile. A random-effect model and the generic inverse variance weighting method were used for quantitative data synthesis. Sensitivity analysis was conducted using the leave-one-out method. A random-effects meta-regression model was performed to explore the association between potential confounders and the estimated effect size on plasma lipid concentrations.
Results
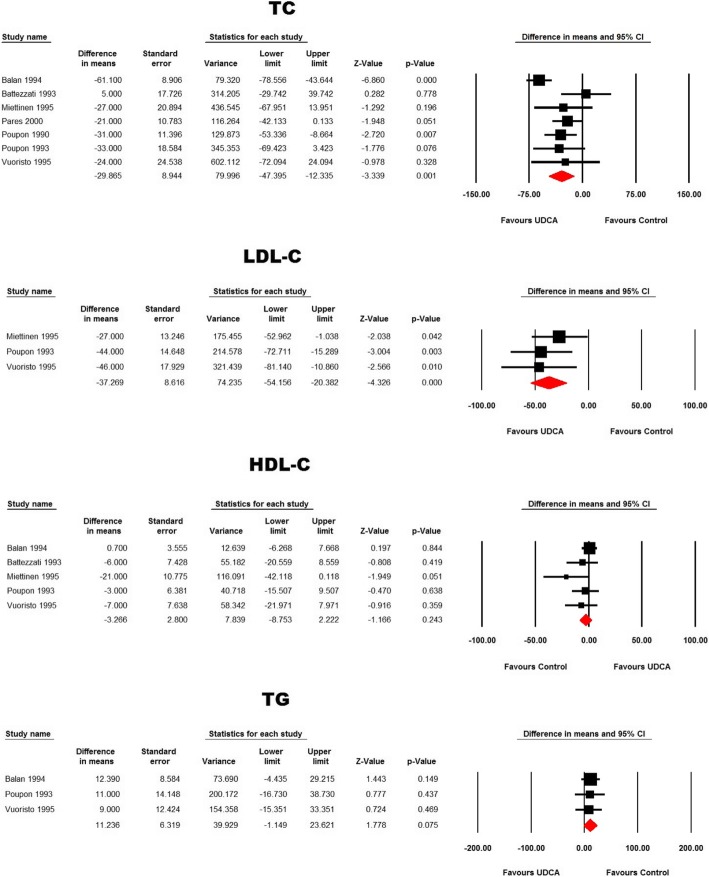
Meta-analysis of 20 treatment arms revealed a significant reduction of total cholesterol following ursodeoxycholic acid treatment (WMD: − 13.85 mg/dL, 95% CI: -21.45, − 6.25, p < 0.001). Nonetheless, LDL-C (WMD: -6.66 mg/dL, 95% CI: -13.99, 0.67, p = 0.075), triglycerides (WMD: − 1.42 mg/dL, 95% CI: -7.51, 4.67, p = 0.648) and HDL-C (WMD: -0.18 mg/dL, 95% CI: -5.23, 4.87, p = 0.944) were not found to be significantly altered by ursodeoxycholic acid administration. In the subgroup of patients with primary biliary cirrhosis, ursodeoxycholic acid reduced total cholesterol (WMD: − 29.86 mg/dL, 95% CI: -47.39, − 12.33, p = 0.001) and LDL-C (WMD: -37.27 mg/dL, 95% CI: -54.16, − 20.38, p < 0.001) concentrations without affecting TG and HDL-C.
Conclusion
This meta-analysis suggests that ursodeoxycholic acid therapy might be associated with significant total cholesterol lowering particularly in patients with primary biliary cirrhosis.
Keywords: Ursodeoxycholic acid, Lipid profile, Total cholesterol, Triglycerides, LDL, HDL, Meta-analysis
Introduction
The global prevalence of hypercholesterolemia among adults is still increased [1]. Abnormal lipid levels, frequently accompanied by central obesity, high blood pressure and type 2 diabetes, have been clearly identified as a major risk factor for cardiovascular disease [2]. Moreover, the high prevalence of overweight and obesity have led to the increase in lipid disorders [3]. Given that pharmacological treatment may be insufficient to achieve the recommended goals for lipid concentrations, alternative lipid-lowering therapies are needed to reduce the risk of atherosclerotic cardiovascular disease [4–12].
Ursodeoxycholic acid is a primary bile acid formed in the human liver [13, 14]. This hydrophilic molecule has a low toxicity and is usually used at a pharmacological dose of 10–15 mg/kg/day [14, 15]. Ursodeoxycholic acid is widely prescribed in the treatment of several cholestatic liver diseases such as cholesterol-gallstone dissolution, primary biliary cirrhosis and cholestasis of pregnancy [16, 17]. Evidence suggests that the therapeutic effects of ursodeoxycholic acid are explained by an increased hydrophilicity index of the bile acid pool, stimulation of hepatocellular and ductular secretions, cytoprotection against bile acid and cytokine-induced injury, immunomodulation and anti-inflammatory effects [17]. Additionally, some clinical trials have observed a significant decrease in total cholesterol levels after ursodeoxycholic acid treatment [18–20]; however, other studies found no beneficial effect of this bile acid on lipid metabolism [21–23]. Thus, the lipid-lowering activity of ursodeoxycholic acid is currently uncertain and remains to be elucidated. Therefore, the present meta-analysis of randomized placebo-controlled trials aimed to examine whether ursodeoxycholic acid treatment is an effective lipid-lowering agent.
Materials and methods
Search strategy
This study was designed according to the guidelines of the 2009 preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement [24]. In order to find randomized controlled trials evaluating the effect of ursodeoxycholic acid on lipid profile, PubMed-Medline, SCOPUS, Web of Science and Google Scholar databases were searched using the following search terms within titles and abstracts (also in combination with MESH terms): (ursodeoxycholic acid) AND (cholesterol OR “low-density lipoprotein” OR LDL OR LDL-C OR LDL-cholesterol OR “high-density lipoprotein” OR HDL-cholesterol OR HDL-C OR triglyceride OR hyperlipidemia OR hyperlipidemic OR dyslipidemia OR dyslipidemic OR lipid OR lipoprotein). The wild-card term “*” was used to increase the sensitivity of the search strategy. The search was limited to articles published in English language. The literature was searched from inception to June 06, 2018.
Study selection
Original studies were included if they met the following inclusion criteria: (1) being a randomized placebo-controlled trial with either parallel or cross-over design, (2) evaluating the effect of ursodeoxycholic acid on plasma/serum concentrations of lipids, and, (3) presentation of sufficient information on lipid concentrations at baseline and at the end of follow-up in each group or providing the net change values. Exclusion criteria were: (1) non-interventional trials, (2) lack of a placebo group for ursodeoxycholic acid treatment, (3) observational studies with case-control, cross-sectional or cohort design, and (4) lack of sufficient information on baseline or follow-up (or net change) lipid concentrations.
Data extraction
Eligible studies were reviewed and the following data were abstracted: 1) first author’s name; 2) year of publication; 3) study design; 4) number of participants in the intervention and placebo groups; 5) dose and duration of treatment with ursodeoxycholic acid; 6) age, gender and body mass index (BMI) of study participants; and 7) circulating concentrations of lipids.
Quality assessment
A systematic assessment of bias in the included randomized placebo-controlled clinical trials was performed using the Cochrane criteria [25]. The items used for the assessment of each study were as follows: adequacy of random sequence generation, allocation concealment, blinding of participants, personnel, outcome assessment, not addressing dropouts (incomplete outcome data), selective outcome reporting, and other potential sources of bias. According to the recommendations of the Cochrane Handbook, a judgment of “yes” indicated low risk of bias, while “no” indicated high risk of bias. Labeling an item as “unclear” indicated an unclear or unknown risk of bias.
Quantitative data synthesis
Meta-analysis was conducted using Comprehensive Meta-Analysis (CMA) V2 software (Biostat, NJ) [26]. Effect size was calculated as: (measure at the end of follow-up in the treatment group − measure at baseline in the treatment group) − (measure at the end of follow-up in the control group − measure at baseline in the control group). A random-effect model (using DerSimonian-Laird method) and the generic inverse variance weighting method were used to compensate for the heterogeneity of studies in terms of study design, treatment duration, and the characteristics of populations being studied [27]. All units were collated as mg/dL. Standard deviations (SDs) of the mean difference were calculated using the following formula: SD = square root [(SDpre-treatment)2 + (SDpost-treatment)2 – (2R × SDpre-treatment × SDpost-treatment)], assuming a correlation coefficient (R) = 0.5. Effect sizes were expressed as weighted mean difference (WMD) and 95% confidence interval (CI). Inter-study heterogeneity was quantitatively assessed using the I2 index. In order to evaluate the influence of each study on the overall effect size, a sensitivity analysis was conducted using the leave-one-out method (i.e., removing one study each time and repeating the analysis) [28–30].
Meta-regression
As a potential confounder of treatment response, treatment duration was entered into a random-effects meta-regression model to explore their association with the estimated effect size on plasma lipid concentrations.
Publication bias
Evaluation of funnel plot, Begg’s rank correlation and Egger’s weighted regression tests were employed to assess the presence of publication bias in the meta-analysis. When there was an evidence of funnel plot asymmetry, potentially missing studies were imputed using the “trim and fill” method [31].
Results
Flow of study selection
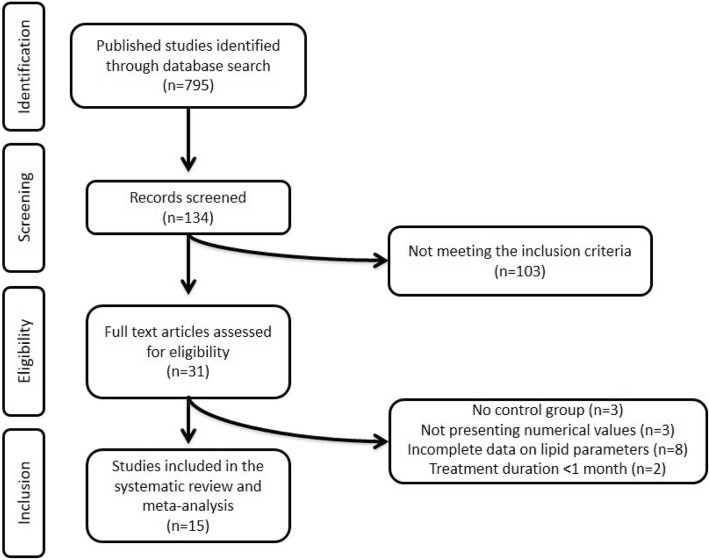
Our initial search identified 795 published trials. After screening of titles and abstracts, 661 studies were excluded. Of these, 103 studies excluded for not meeting the inclusion criteria. Subsequently, 31 full-text articles were carefully reviewed for eligibility and 16 clinical trials were excluded for having no control group (n = 3), not presenting numerical values (n = 3), incomplete data on lipid parameters (n = 8), and treatment duration < 1 month (n = 2). Finally, 15 studies were selected and included in the present meta-analysis. The detailed study selection process is presented in Fig. 1.
Fig. 1.
Flow chart of the number of studies identified and included in this meta-analysis
Characteristics of included studies
Data were pooled from 15 randomized placebo-controlled trials comprising a total 1370 subjects, including 735 and 635 participants in the intervention and placebo arms, respectively. Included studies were published between 1977 and 2013. The clinical trials used different doses of ursodeoxycholic acid. The range of treatment duration was from 1 month [32, 33] to 2 years [18, 20, 23, 34–36]. Study design of included trials was parallel and cross-over. Selected studies enrolled subjects with primary biliary cirrhosis [18, 20, 21, 34–38], primary hypercholesterolemia [22], hypertriglyceridemia [32], gallstones [23, 37], nonalcoholic fatty liver disease (NAFLD) [19, 39], nonalcoholic steatohepatitis (NASH) [40], and healthy volunteers [33]. Characteristics of the included clinical trials are shown in Table 1.
Table 1.
Demographic characteristics of the included studies
| Author | Study design | Target Population | Treatment duration | n | Study groups | Age, years | Female (n, %) | BMI, (kg/m2) | Total cholesterol (mg/dl) | LDL cholesterol (mg/dl) | HDL cholesterol (mg/dl) | Triglycerides (mg/dl) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Balan et al. (1994) [18] | Randomized, double-blind, placebo-controlled | Primary biliary cirrhosis | 2 years | 89 | Placebo | 52 ± 9.4 | 77 (87) | ND | 277.5 ± 106.0 | ND | 60.9 ± 20.3 | 117.2 ± 70.7 |
| 88 | UDCA 13–15 mg/kg/day | 54 ± 9.3 | 80 (91) | ND | 288.3 ± 121.7 | ND | 63.1 ± 23.6 | 102.0 ± 50.4 | ||||
| Battezzati et al. (1993) [21] | Randomized, double-blind, placebo-controlled | Primary biliary cirrhosis | 6 months | 44 | UDCA 500 mg/day | 54 ± 2 | 37 (84) | ND | 263 ± 12 | ND | 78 ± 6 | ND |
| 44 | Placebo | 55 ± 2 | 41 (93) | ND | 266 ± 13 | ND | 61 ± 5 | ND | ||||
| Braga et al. (2009) [22] | Randomized, double-blind, placebo-controlled | Primary hypercholeste-rolemia | 6 months | 57 | UDCA 13–15 mg/kg/day | ND | ND | ND | 241.1 ± 30 | 160.2 ± 23 | 47.7 ± 12 | 166.0 ± 70 |
| 68 | Placebo | ND | ND | ND | 244.7 ± 29 | 160.6 ± 19 | 48.0 ± 12 | 180.8 ± 96 | ||||
| Carulli et al. (1981) [32] | Randomized, double-blind, placebo-controlled | Hypertriglyce-ridemia | 1 month | 8 | UDCA 600 mg/day | 40.4a | 1 (12) | ND | 266a | ND | 38a | 405a |
| 8 | Placebo | 40.2a | 2 (25) | ND | 254a | ND | 39a | 249a | ||||
| Fromm et al. (1983) [23] | Randomized, double-blind, placebo-controlled | Patients with gallstones | 2 years | 12 | Placebo | 55 ± 10.3 | 10 (82) | ND | 201 ± 7.6 | ND | ND | 162 ± 30.8 |
| 12 | UDCA 400 mg/day | 56 ± 16.9 | 8 (67) | ND | 227 ± 16.5 | ND | ND | 162 ± 20.6 | ||||
| 12 | UDCA 800 mg/day | 55 ± 16.9 | 6 (50) | ND | 248 ± 17.7 | ND | ND | 180 ± 32.4 | ||||
| Gianturco et al. (2013) [39] | Randomized, double-blind, placebo-controlled | NAFLD | 1 year | 53 | ALA 400 mg/day + UDCA 300 mg/day | 65 ± 5 | 23 (43) | 30 ± 2.1 | 203 ± 8 | 133 ± 9 | 45 ± 5 | 123 ± 11 |
| 54 | ALA 400 mg/day | 60 ± 4 | 25 (46) | 29.5 ± 2 | 208 ± 9 | 133 ± 10 | 49 ± 6 | 128 ± 15 | ||||
| 46 | UDCA 300 mg/day | 62 ± 6 | 21 (45) | 29.7 ± 1.6 | 209 ± 10 | 136 ± 11 | 47 ± 7 | 127 ± 11 | ||||
| 47 | Placebo | 61 ± 4 | 23 (48) | 29.3 ± 1.3 | 207 ± 7 | 138 ± 12 | 43 ± 8 | 129 ± 9 | ||||
| Leuschner et al. (2010) [40] | Randomized, double-blind, placebo-controlled | NASH | 18 months | 95 | UDCA 23–28 mg/kg/day | 41.4 (18–71)a | 32 (33) | ND | 148 ± 102 | ND | ND | 208 ± 111 |
| 91 | Placebo | 45.0 (18–73)a | 28 (30) | ND | 162 ± 94 | ND | ND | 202 ± 111 | ||||
| Lindenthal et al. (2002) [33] | Randomized, placebo-controlled, cross-over | Healthy volunteers | 1 month | 20 | Overall | 19–38b | 5 (25) | ND | ||||
| 20 | UDCA 750 mg/day | 186 ± 24 | ND | ND | ND | |||||||
| 20 | Placebo | 186 ± 19 | ND | ND | ND | |||||||
| Méndez-Sánchez et al. (2004) [19] | Randomized, double-blind, placebo-controlled | NAFLD | 6 weeks | 14 | UDCA 1200 mg/day | 39.7 ± 8 | 14 (100) | 34.2 ± 4.2 | 196.1 ± 36.7 | ND | ND | ND |
| 13 | Placebo | 37.8 ± 8 | 13 (100) | 33.3 ± 1.6 | 177.7 ± 29.3 | ND | ND | ND | ||||
| Miettinen et al. (1995) [20] | Randomized, double-blind, placebo-controlled | Primary biliary cirrhosis | 2 years | 23 | UDCA 12–15 mg/kg/day | 50 ± 9 | 18 (78) | 24.1 ± 2.8 | 250 ± 122 | 138 ± 39 | 65 ± 19 | ND |
| 22 | Placebo | 57 ± 14 | 21 (95) | 24.8 ± 3.7 | 226 ± 76 | 131 ± 21 | 51 ± 26 | ND | ||||
| Nakagawa et al. (1977) [37] | Randomized, double-blind, placebo-controlled | Patients with gallstones | 6 months | 13 | Placebo | ND | ND | ND | 196 ± 30 | ND | ND | 120 ± 33 |
| 16 | UDCA 150 mg/day | ND | ND | ND | 192 ± 41 | ND | ND | 110 ± 37 | ||||
| 15 | UDCA 600 mg/day | ND | ND | ND | 193 ± 29 | ND | ND | 143 ± 59 | ||||
| Parés et al. (2000) [34] | Randomized, double-blind, placebo-controlled | Primary biliary cirrhosis | 2 years | 99 | UDCA 14–16 mg/kg/day | 57.4 ± 8.9 | 92 (92) | ND | 276 ± 89 | ND | ND | ND |
| 93 | Placebo | 53.5 ± 9.6 | 87 (93) | ND | 276 ± 86 | ND | ND | ND | ||||
| Poupon et al. (1990) [35] | Randomized, double-blind, placebo-controlled | Primary biliary cirrhosis | 6 months | 70 | UDCA 13–15 mg/kg/day | 55 ± 11 | 66 (94) | ND | 282 ± 73 | ND | ND | ND |
| 68 | Placebo | 58 ± 9 | 60 (89) | ND | 266 ± 65 | ND | ND | ND | ||||
| Poupon et al. (1993) [38] | Randomized, double-blind, placebo-controlled | Primary biliary cirrhosis | 2 years | 17 | UDCA 13–15 mg/kg/day | 55 ± 12 | ND | ND | 289 ± 66 | 155 ± 52 | 44 ± 17 | 93 ± 32 |
| 16 | Placebo | 58 ± 8 | ND | ND | 273 ± 36 | 134 ± 38 | 41 ± 14 | 102 ± 49 | ||||
| Vuoristo et al. (1995) [36] | Randomized, double-blind, placebo-controlled | Primary biliary cirrhosis | 2 years | 31 | Placebo | 57a | 27 (87) | 24a | 278 ± 105 | 189 ± 84 | 61 ± 21 | 106 ± 48 |
| 30 | UDCA 12–15 mg/kg/day | 52a | 22 (73) | 24a | 251 ± 86 | 158 ± 64 | 58 ± 42 | 115 ± 49 |
Values are expressed as mean ± SD
Abbreviations: ND no data, BMI body mass index, IQR interquartile range
aMean only
bRange
Risk of bias assessment
According to the Cochrane criteria, most of included studies showed insufficient information about random sequence generation and one study had a high risk of bias [38]. With respect to allocation concealment, several trials exhibited limited information. Regarding blinding of participants, personnel and outcome assessors, several studies revealed lack of information and one trial presented high risk of bias [33]. Finally, all the evaluated trials had low risk of bias for incomplete outcome data and selective outcome reporting. Details for the risk of bias assessment is presented in Table 2.
Table 2.
Quality of bias assessment of the included studies according to the Cochrane guidelines
| Study | Sequence generation | Allocation concealment | Blinding of participants, personnel and outcome assessors | Incomplete outcome data | Selective outcome reporting | Other sources of bias |
|---|---|---|---|---|---|---|
| Balan et al. (1994) [18] | U | U | L | L | L | U |
| Battezzati et al. (1993) [21] | L | L | L | L | L | L |
| Braga et al. (2009) [22] | U | U | U | L | L | U |
| Carulli et al. (1981) [32] | U | U | U | L | L | U |
| Fromm et al. (1983) [23] | U | U | U | L | L | U |
| Gianturco et al. (2013) [39] | L | L | U | L | L | U |
| Leuschner et al. (2010) [40] | U | U | U | L | L | U |
| Lindenthal et al. (2002) [33] | U | U | H | L | L | U |
| Méndez-Sánchez et al. (2004) [19] | L | L | U | L | L | U |
| Miettinen et al. (1995) [20] | U | U | U | L | L | U |
| Nakagawa et al. (1977) [37] | U | L | L | L | L | U |
| Parés et al. (2000) [34] | U | L | L | L | L | U |
| Poupon et al. (1990) [35] | U | U | U | L | L | U |
| Poupon et al. (1993) [38] | H | U | U | L | L | U |
| Vuoristo et al. (1995) [36] | U | U | U | L | L | U |
L low risk of bias, H high risk of bias, U unclear risk of bias
Effect of ursodeoxycholic acid on lipids
Meta-analysis of 20 treatment arms revealed a significant reduction of total cholesterol following ursodeoxycholic acid treatment (WMD: − 13.85 mg/dL, 95% CI: -21.45, − 6.25, p < 0.001). This effect size was robust in the sensitivity analysis (Figs. 2 and 3). Nonetheless, other lipid indices including LDL-C (WMD: -6.66 mg/dL, 95% CI: -13.99, 0.67, p = 0.075), TG (WMD: -1.42 mg/dL, 95% CI: -7.51, 4.67, p = 0.648) and HDL-C (WMD: -0.18 mg/dL, 95% CI: -5.23, 4.87, p = 0.944) were not found to be significantly altered by ursodeoxycholic acid administration (Figs. 2 and 3).
Fig. 2.
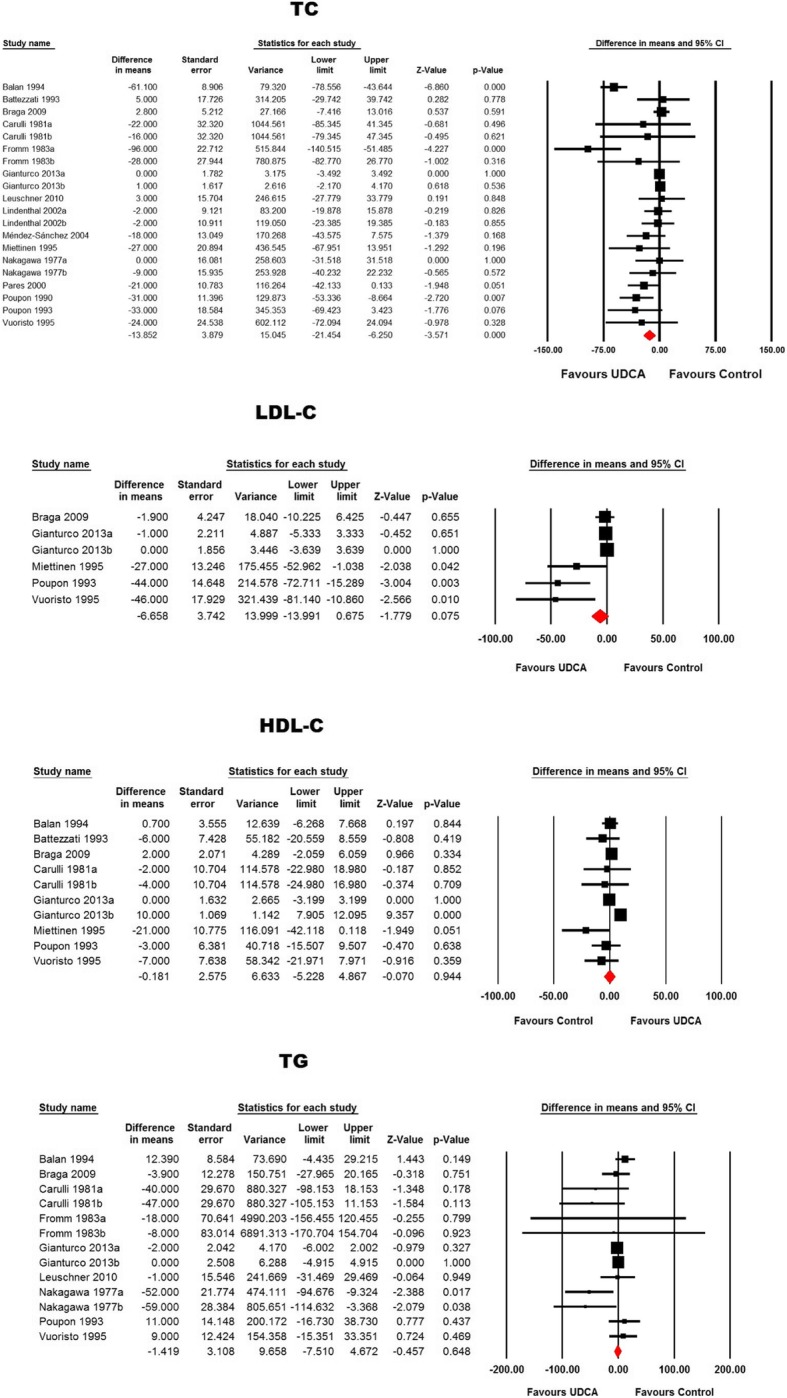
Forest plot displaying the weighted mean difference and 95% confidence intervals for the impact of treatment with UDCA on lipid indices
Fig. 3.
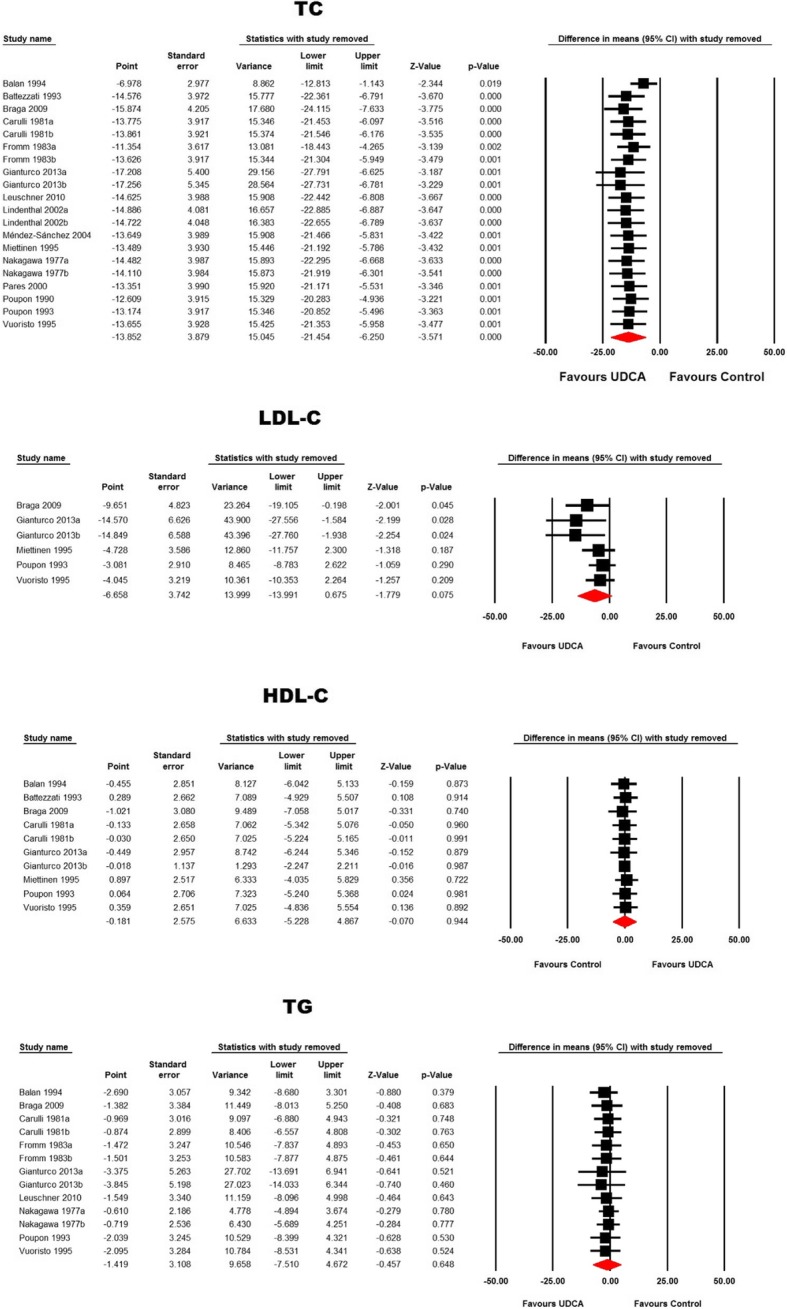
Leave-one-out sensitivity analysis for the meta-analysis of UDCA’s effects on plasma lipid indices
In patients with primary biliary cirrhosis, ursodeoxycholic acid reduced total cholesterol (WMD: − 29.86 mg/dL, 95% CI: -47.39, − 12.33, p = 0.001) and LDL-C (WMD: -37.27 mg/dL, 95% CI: -54.16, − 20.38, p < 0.001) concentrations without affecting TG (WMD: 11.24 mg/dL, 95% CI: -1.15, 23.62, p = 0.075) and HDL-C (WMD: -3.27 mg/dL, 95% CI: -8.75, 2.22, p = 0.243) levels (Fig. 4).
Fig. 4.
Forest plot displaying the weighted mean difference and 95% confidence intervals for the impact of treatment with UDCA on lipid indices in patients with primary biliary cirrhosis
Meta-regression
Meta-regression analysis revealed that the effects of ursodeoxycholic acid on total cholesterol (slope: − 1.51; p < 0.001), LDL-C (slope: − 1.97; p = 0.001) and TG (slope: 1.38; p = 0.004) but not HDL-C (slope: − 0.23; p = 0.482) concentrations were associated with treatment duration (Fig. 5).
Fig. 5.
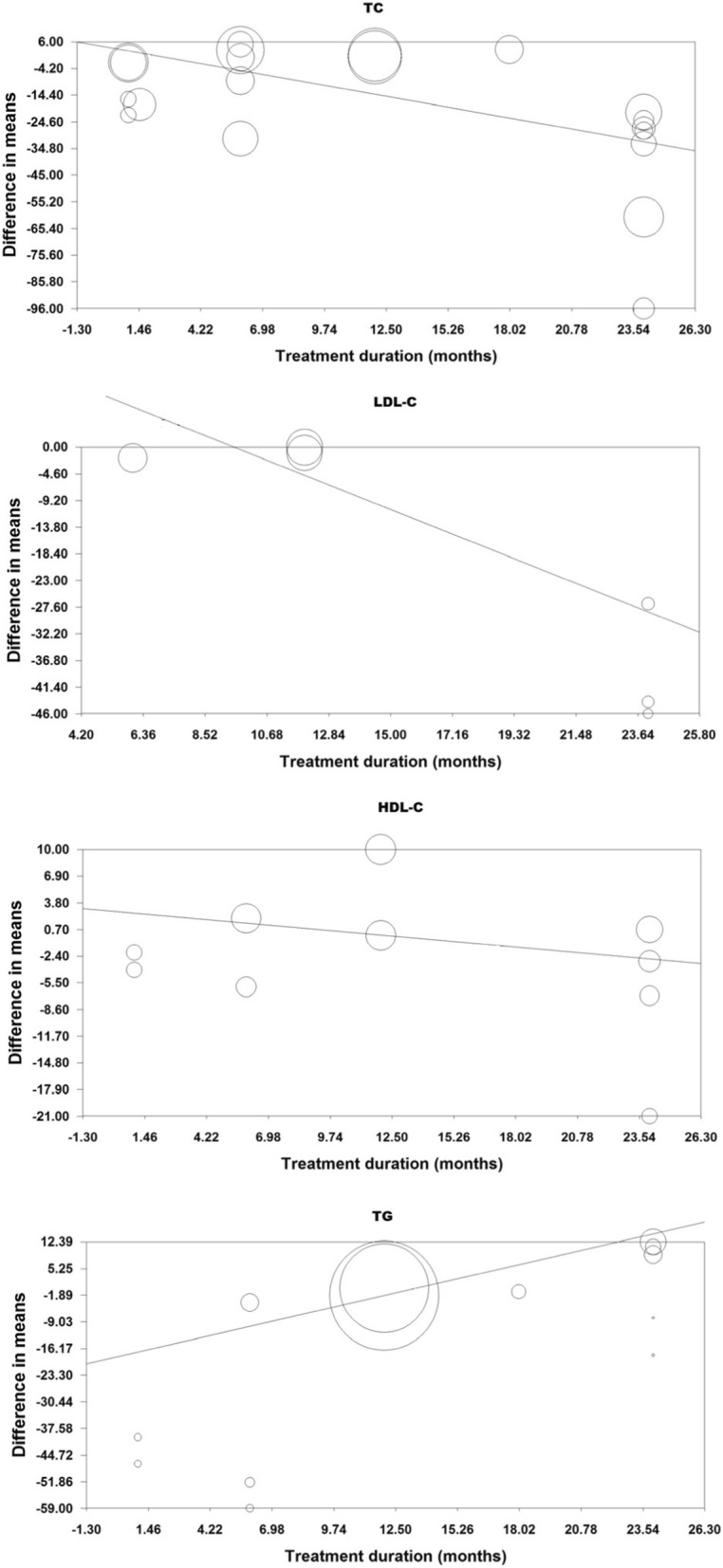
Meta-regression bubble plot of the association between mean changes in plasma lipids concentrations following UDCA supplementation with the duration of supplementation. The size of each circle is inversely proportional to the variance of change
Publication bias
Publication bias assessment revealed asymmetric funnel plots and evidence suggestive of bias. This asymmetry was corrected by imputing potentially missing studies using “trim and fill” method (Fig. 6). Egger’s regression test suggested the presence of publication bias in the meta-analyses of total cholesterol (p = 0.008), LDL-C (p = 0.003) and HDL-C (p = 0.026). Begg’s rank correlation test suggested the presence of publication bias only in the meta-analysis of LDL-C (p = 0.024).
Fig. 6.
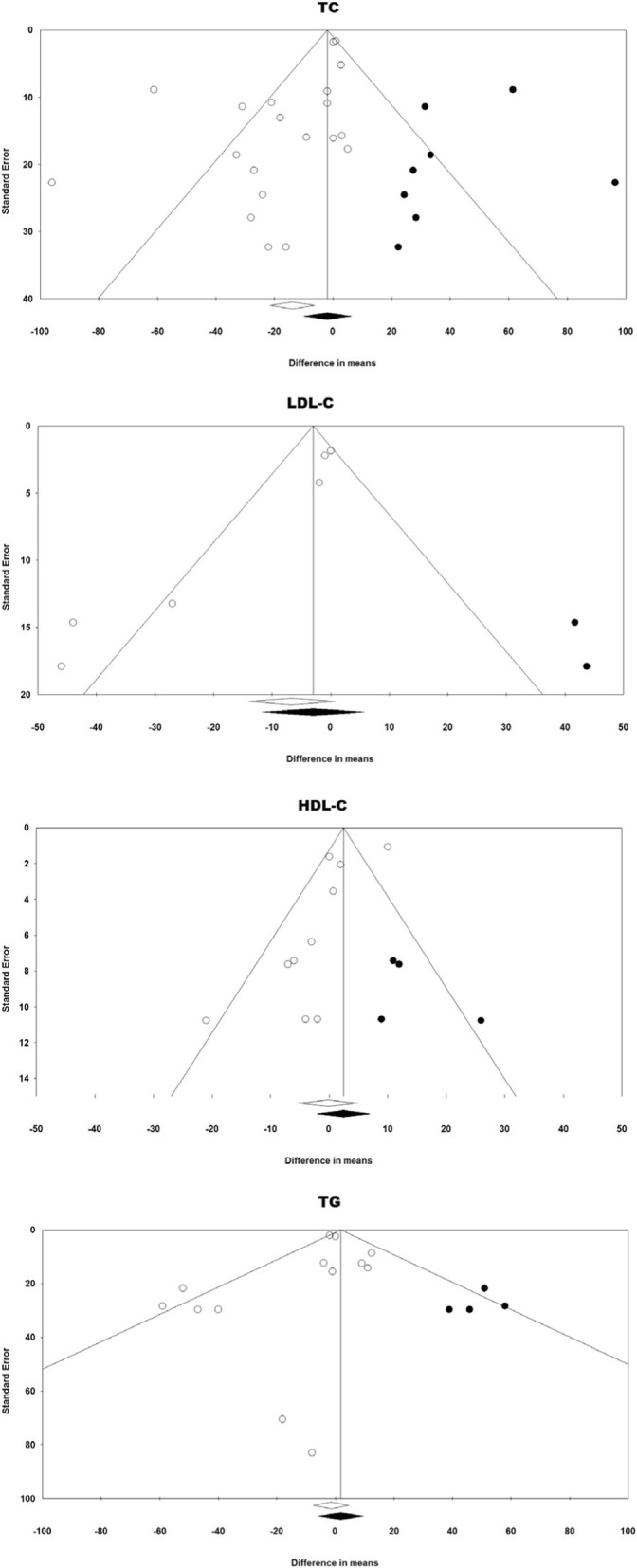
Funnel plot detailing publication bias in the studies reporting the impact of UDCA on lipid indices. Open circles represent observed published studies while closed circles represent imputed unpublished studies using trim and fill method
Discussion
The present meta-analysis of randomized placebo-controlled trials examined whether ursodeoxycholic acid treatment might be an effective lipid-lowering agent. Indeed, this meta-analysis revealed a significant reduction in total cholesterol levels following ursodeoxycholic acid therapy (− 13.85 mg/dL), but the rest of parameters of lipid profile were not significantly changed.
In consistency with our findings, several clinical trials have found a significant reduction in total cholesterol concentrations after ursodeoxycholic acid administration [18, 19, 39, 40]; however, the potential mechanisms involved in the cholesterol-lowering effects of this bile acid have not been clarified. In this regard, it has been proposed that ursodeoxycholic acid may decrease the cholesterol biosynthesis by reducing the activity of hydroxymethylglutaryl-coenzime A reductase [41, 42]. Also, ursodeoxycholic acid decreases the dietary cholesterol absorption lowering serum cholesterol levels [43]. Additionally, it has been proven that the administration of ursodeoxycholic improves hepatic function through increasing the synthesis of bille acid, cholesterol and steatosis, and decreasing the activity of farnesoid X receptor (FXR) [44].
Experimental data suggested that ursodeoxycholic acid has also the ability to protect the cholangiocytes against hydrophobic bile acids by simultaneous decrease of the concentration of hydrophobic bile and reduction of the bile acid cytotoxicity [45]. Besides, it has been reported that this pharmacological agent increases hepatic LDL uptake through a direct interaction with the LDL receptor [46]. Furthermore, ursodeoxycholic acid was reported to be able to change the hydrophobicity index of the bile acid pool [47, 48]. Ursodeoxycholic acid may improve the cell resistance to reactive oxygen species, to decrease the permeability of the mitochondrial membrane and to inhibit release of hydrolytic enzymes from damaged hepatocytes [49, 50]. Moreover, some important genes involved in lipid uptake (Cd36 and Ldlr) and hepatic lipid synthesis (PPARG, Chrebp-a/−b, Acaca, Fasn, Me1, and Scd1) seems to be modulated by ursodeoxycholic acid, as mecanisms of protection against hepatic fat accumulation [51]. Ursodeoxycholic acid may also influence the adipose tissue through increasing triglyceride levels, and increasing the esterification and desaturation of fatty acids [52].
Of particular interest is the clinically relevant decrease in TC and LDL-C specifically observed in primary biliary cirrhosis patients. This could be of particular interest given the increased coronary artery disease risk observed in patients affected by this condition [53].
There are some limitations of this meta-analysis that deserve to be mentioned. First, the lipid-lowering action of ursodeoxycholic acid was not the primary outcome in almost all selected studies; hence, further clinical trials are needed in order to corroborate the hypolipidemic effect of this acid bile as primary endpoint. Second, several studies included in this meta-analysis presented insufficient information with respect to the quality of bias assessment suggesting caution in the overall quality. Third, although the selected studies were heterogeneous in terms of target population and characteristics, we tried to minimize the inter-study heterogeneity using a random-effects model. Finally, most of the trials assessed were performed on small sample sizes resulting in a limited pooled population in the overall analysis.
Conclusion
This meta-analysis suggests that ursodeoxycholic acid therapy might be associated with significant total cholesterol lowering. Nonetheless, these results could have been influenced by the variability, the sample size, and the quality of the studies included. Fuurther investigation is required to elucidate if observed lipid-lowering effects of ursodeoxycholic acid in patients with primary biliary cirrhosis can contribute to the prevention of cardiovascular events and whether there is any added value of using ursodeoxycholic acid as an adjunct or alternative to current or novel lipid-modifyinga gents [54, 55]
Acknowledgements
None.
Funding
None.
Availability of data and materials
Data from this analysis are available through collaboration under a data usage agreement with the corresponding author.
Authors’ contributions
LE-M, MS-M and AS contributed to the conception of the study. LES-M, MS-M and AS-G carried out the literature search and data abstraction. AS performed the statistical analysis. LES, MS and AS wrote the manuscript. M-CS, MB, AFGC revised the draft and helped with interpretations. All authors approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Dr. Banach has served on speaker’s bureau and as an advisory board member for Amgen, Sanofi-Aventis and Lilly. Other authors have no conflict of interests to disclose.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Investigators. EFHSCEFHSCF Overview of the current status of familial hypercholesterolaemia care in over 60 countries - the EAS familial Hypercholesterolaemia studies collaboration (FHSC) Atherosclerosis. 2018;277:234–255. doi: 10.1016/j.atherosclerosis.2018.08.051. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin EJVS, Callaway CW, et al. Heart disease and stroke Statistics-2018 update: a report from the American Heart Association. Circulation. 2018;137:e67–e492. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 3.Franssen RMH, Stroes ES, Kastelein JJ. Obesity and dyslipidemia. Med Clin North Am. 2011;95:893–902. doi: 10.1016/j.mcna.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Cicero AFG, Colletti A, Bajraktari G, Descamps O, Djuric DM, Ezhov M, Fras Z, Katsiki N, Langlois M, Latkovskis G, et al. Lipid lowering nutraceuticals in clinical practice: position paper from an international lipid expert panel. Arch Med Sci. 2017;13:965–1005. doi: 10.5114/aoms.2017.69326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banach M, Patti AM, Giglio RV, Cicero AFG, Atanasov AG, Bajraktari G, Bruckert E, Descamps O, Djuric DM, Ezhov M, et al. The role of nutraceuticals in statin intolerant patients. J Am Coll Cardiol. 2018;72:96–118. doi: 10.1016/j.jacc.2018.04.040. [DOI] [PubMed] [Google Scholar]
- 6.Bianconi V, Mannarino MR, Sahebkar A, Cosentino T, Pirro M. Cholesterol-lowering nutraceuticals affecting vascular function and cardiovascular disease risk. Curr Cardiol Rep. 2018;20:53. doi: 10.1007/s11886-018-0994-7. [DOI] [PubMed] [Google Scholar]
- 7.Johnston TP, Korolenko TA, Pirro M, Sahebkar A. Preventing cardiovascular heart disease: promising nutraceutical and non-nutraceutical treatments for cholesterol management. Pharmacol Res. 2017;120:219–225. doi: 10.1016/j.phrs.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 8.Momtazi AA, Banach M, Pirro M, Katsiki N, Sahebkar A. Regulation of PCSK9 by nutraceuticals. Pharmacol Res. 2017;120:157–169. doi: 10.1016/j.phrs.2017.03.023. [DOI] [PubMed] [Google Scholar]
- 9.Pirro M, Francisci D, Bianconi V, Schiaroli E, Mannarino MR, Barsotti F, Spinozzi A, Bagaglia F, Sahebkar A, Baldelli F. NUtraceutical TReatment for hYpercholesterolemia in HIV-infected patients: the NU-TRY(HIV) randomized cross-over trial. Atherosclerosis. 2019;280:51–57. doi: 10.1016/j.atherosclerosis.2018.11.026. [DOI] [PubMed] [Google Scholar]
- 10.Pirro M, Mannarino MR, Bianconi V, Simental-Mendía LE, Bagaglia F, Mannarino E, Sahebkar A. The effects of a nutraceutical combination on plasma lipids and glucose: a systematic review and meta-analysis of randomized controlled trials. Pharmacol Res. 2016;110:76–88. doi: 10.1016/j.phrs.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 11.Sahebkar A, Serban MC, Gluba-Brzózka A, Mikhailidis DP, Cicero AF, Rysz J, Banach M. Lipid-modifying effects of nutraceuticals: an evidence-based approach. Nutrition. 2016;32:1179–1192. doi: 10.1016/j.nut.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 12.Ward N, Sahebkar A, Banach M, Watts G. Recent perspectives on the role of nutraceuticals as cholesterol-lowering agents. Curr Opin Lipidol. 2017;28:495–501. doi: 10.1097/MOL.0000000000000455. [DOI] [PubMed] [Google Scholar]
- 13.Beuers UBJ, Paumgartner G. Ursodeoxycholic acid in cholestasis: potential mechanisms of action and therapeutic applications. Hepatology. 1998;28:1449–1453. doi: 10.1002/hep.510280601. [DOI] [PubMed] [Google Scholar]
- 14.Paumgartner GBU. Ursodeoxycholic acid in cholestatic liver disease: mechanisms of action and therapeutic use revisited. Hepatology. 2002;36:525–531. doi: 10.1053/jhep.2002.36088. [DOI] [PubMed] [Google Scholar]
- 15.Beuers UTM, Jansen P, Poupon R. New paradigms in the treatment of hepatic cholestasis: from UDCA to FXR, PXR and beyond. J Hepatol. 2015;62:S25–S37. doi: 10.1016/j.jhep.2015.02.023. [DOI] [PubMed] [Google Scholar]
- 16.Perez MJ, Briz O. Bile-acid-induced cell injury and protection. World J Gastroenterol. 2009;15:1677–1689. doi: 10.3748/wjg.15.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poupon R. Ursodeoxycholic acid and bile-acid mimetics as therapeutic agents for cholestatic liver diseases: an overview of their mechanisms of action. Clin Res Hepatol Gastroenterol. 2012;36:S3–12. doi: 10.1016/S2210-7401(12)70015-3. [DOI] [PubMed] [Google Scholar]
- 18.Balan V, Dickson ER, Jorgensen RA, Lindor KD. Effect of ursodeoxycholic acid on serum lipids of patients with primary biliary cirrhosis. Mayo Clin Proc. 1994;69:923–929. doi: 10.1016/S0025-6196(12)61815-1. [DOI] [PubMed] [Google Scholar]
- 19.Méndez-Sánchez NGV, Chávez-Tapia N, Ramos MH, Uribe M. Weight reduction and ursodeoxycholic acid in subjects with nonalcoholic fatty liver disease. A double-blind, placebo-controlled trial. Ann Hepatol. 2004;3:108–112. [PubMed] [Google Scholar]
- 20.Miettinen TAFM, Vuoristo M, Karvonen AL, Leino R, Lehtola J, Friman C, Seppälä K, Tuominen J. Serum cholestanol, cholesterol precursors, and plant sterols during placebo-controlled treatment of primary biliary cirrhosis with ursodeoxycholic acid or colchicine. Hepatology. 1995;21:1261–1268. doi: 10.1016/0270-9139(95)90046-2. [DOI] [PubMed] [Google Scholar]
- 21.Battezzati PMPM, Bianchi FB, Naccarato R, Orlandi F, Surrenti C, Pagliaro L, Manenti F. Ursodeoxycholic acid for symptomatic primary biliary cirrhosis. Preliminary analysis of a double-blind multicenter trial. Italian multicenter Group for the Study of UDCA in PBC. J Hepatol. 1993;17:332–338. doi: 10.1016/S0168-8278(05)80214-4. [DOI] [PubMed] [Google Scholar]
- 22.Braga MFGM, Lenis J, Kennedy FP, Teplinsky AL, Roederer G, Palumbo PJ, Colin P, Leiter LA. Efficacy and safety of ursodeoxycholic acid in primary, type IIa or IIb hypercholesterolemia: a multicenter, randomized, double-blind clinical trial. Atherosclerosis. 2009;203:479–482. doi: 10.1016/j.atherosclerosis.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Fromm HRJ, Gonzalez V, Sarva RP, Farivar S. Comparative efficacy and side effects of ursodeoxycholic and chenodeoxycholic acids in dissolving gallstones. A double-blind controlled study. Gastroenterology. 1983;85:1257–1264. [PubMed] [Google Scholar]
- 24.LA Moher D, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins JPT, Green S, eds. : Cochrane Handbook for Systematic Reviews of Interventions. In Version 502 Edited by Collaboration TC. London; 2009.
- 26.Borenstein MHL, Higgins J, Rothstein H. Comprehensive meta-analysis version 2. Englewood; 2005.
- 27.Sutton AJAK, Jones DR, Sheldon TA, Song F. Methods for meta-analysis in medical research. West Sussex; 2000.
- 28.Sahebkar A. Does PPARγ<inf>2</inf> gene Pro12Ala polymorphism affect nonalcoholic fatty liver disease risk? Evidence from a meta-analysis. DNA Cell Biol. 2013;32:188–198. doi: 10.1089/dna.2012.1947. [DOI] [PubMed] [Google Scholar]
- 29.Sahebkar A, Kotani K, Serban C, Ursoniu S, Mikhailidis DP, Jones SR, Ray KK, Blaha MJ, Rysz J, Toth PP, et al. Statin therapy reduces plasma endothelin-1 concentrations: a meta-analysis of 15 randomized controlled trials. Atherosclerosis. 2015;241:433–442. doi: 10.1016/j.atherosclerosis.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 30.Sahebkar A, Serban C, Mikhailidis DP, Undas A, Lip GYH, Muntner P, Bittner V, Ray KK, Watts GF, Hovingh GK, et al. Association between statin use and plasma d-dimer levels: a systematic review and meta-analysis of randomised controlled trials. Thromb Haemost. 2015;114:546–557. doi: 10.1160/TH14-11-0937. [DOI] [PubMed] [Google Scholar]
- 31.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341X.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 32.Carulli N, Ponz de Leon M, Podda M, Zuin M, Strata A, Frigerio G, Digrisolo A. Chenodeoxycholic acid and ursodeoxycholic acid effects in endogenous hypertriglyceridemias. A controlled double-blind trial. J Clin Pharmacol. 1981;21:436–442. doi: 10.1002/j.1552-4604.1981.tb01746.x. [DOI] [PubMed] [Google Scholar]
- 33.Lindenthal B, Sudhop T, Schiedermaier P, Agnan M, Sauerbruch T, von Bergmann K. Serum plant sterols and biliary cholesterol secretion in humans: studies with ursodeoxycholic acid. J Lipid Res. 2002;43:1072–1077. doi: 10.1194/jlr.M100438-JLR200. [DOI] [PubMed] [Google Scholar]
- 34.Parés ACL, Rodés J, Bruguera M, Rodrigo L, García-Plaza A, Berenguer J, Rodríguez-Martínez D, Mercader J, Velicia R. Long-term effects of ursodeoxycholic acid in primary biliary cirrhosis: results of a double-blind controlled multicentric trial. UDCA-cooperative group from the Spanish Association for the Study of the Liver. J Hepatol. 2000;32:561–566. doi: 10.1016/S0168-8278(00)80216-0. [DOI] [PubMed] [Google Scholar]
- 35.Poupon RE, Eschwège E, Poupon R. Ursodeoxycholic acid for the treatment of primary biliary cirrhosis. Interim analysis of a double-blind multicentre randomized trial. The UDCA-PBC study group. J Hepatol. 1990;11:16–21. doi: 10.1016/0168-8278(90)90265-S. [DOI] [PubMed] [Google Scholar]
- 36.Vuoristo MFM, Karvonen AL, Leino R, Lehtola J, Mäkinen J, Mattila J, Friman C, Seppälä K, Tuominen J, et al. A placebo-controlled trial of primary biliary cirrhosis treatment with colchicine and ursodeoxycholic acid. Gastroenterology. 1995;108:1470–1478. doi: 10.1016/0016-5085(95)90696-7. [DOI] [PubMed] [Google Scholar]
- 37.Nakagawa S, Makino I, Ishizaki T, Dohi I. Dissolution of cholesterol gallstones by ursodeoxycholic acid. Lancet. 1977;2:367–369. doi: 10.1016/S0140-6736(77)90301-4. [DOI] [PubMed] [Google Scholar]
- 38.Poupon RE, Ouguerram K, Chrétien Y, Verneau C, Eschwège E, Magot T, Poupon R. Cholesterol-lowering effect of ursodeoxycholic acid in patients with primary biliary cirrhosis. Hepatology. 1993;17:577–582. doi: 10.1002/hep.1840170408. [DOI] [PubMed] [Google Scholar]
- 39.Gianturco V, Troisi G, Bellomo A, Bernardini S, D'Ottavio E, Formosa V, Iacono CL, Verrusio W, Marigliano B, Marigliano V. Impact of combined therapy with alpha-lipoic and ursodeoxycolic acid on nonalcoholic fatty liver disease: double-blind, randomized clinical trial of efficacy and safety. Hepatol Int. 2013;7:570–576. doi: 10.1007/s12072-012-9387-y. [DOI] [PubMed] [Google Scholar]
- 40.Leuschner UF, Lindenthal B, Herrmann G, Arnold JC, Rössle M, Cordes HJ, Zeuzem S, Hein J, Berg T, NASH Study Group High-dose ursodeoxycholic acid therapy for nonalcoholic steatohepatitis: a double-blind, randomized, placebo-controlled trial. Hepatology. 2010;52:472–479. doi: 10.1002/hep.23727. [DOI] [PubMed] [Google Scholar]
- 41.Maton PN, Ellis HJ, Higgins MJ, Dowling RH. Hepatic HMGCoA reductase in human cholelithiasis: effects of chenodeoxycholic and ursodeoxycholic acids. Eur J Clin Investig. 1980;10:325–332. doi: 10.1111/j.1365-2362.1980.tb00040.x. [DOI] [PubMed] [Google Scholar]
- 42.Salen G, Colalillo A, Verga D, Bagan E, Tint GS, Shefer S. Effect of high and low doses of ursodeoxycholic acid on gallstone dissolution in humans. Gastroenterology. 1980;78:1412–1418. [PubMed] [Google Scholar]
- 43.Ponz de Leon M, Carulli N, Loria P, Iori R, Zironi F.: Cholesterol absorption during bile acid feeding. Effect of ursodeoxycholic acid (UDCA) administration. Gastroenterology 1980, 78:214–219. [PubMed]
- 44.Arab JP, Karpen SJ, Dawson PA, Arrese M, Trauner M. Bile acids and nonalcoholic fatty liver disease: molecular insights and therapeutic perspectives. Hepatology. 2017;65:350–362. doi: 10.1002/hep.28709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hatano R, Kawaguchi K, Togashi F, Sugata M, Masuda S, Asano S. Ursodeoxycholic acid ameliorates intrahepatic cholestasis independent of biliary bicarbonate secretion in Vil2(kd/kd) mice. Biol Pharm Bull. 2017;40:34–42. doi: 10.1248/bpb.b16-00529. [DOI] [PubMed] [Google Scholar]
- 46.Bouscarel B, Ceryak S, Robins SJ, Fromm H. Studies on the mechanism of the ursodeoxycholic acid-induced increase in hepatic low-density lipoprotein binding. Lipids. 1995;30:607–617. doi: 10.1007/BF02536997. [DOI] [PubMed] [Google Scholar]
- 47.Bachrach WH, Hofmann AF. Ursodeoxycholic acid in the treatment of cholesterol cholelithiasis. Part II. Dig Dis Sci. 1982;27:833–856. doi: 10.1007/BF01391378. [DOI] [PubMed] [Google Scholar]
- 48.Bachrach WH, Hofmann AF. Ursodeoxycholic acid in the treatment of cholesterol cholelithiasis. Part I. Dig Dis Sci. 1982;27:737–761. doi: 10.1007/BF01393771. [DOI] [PubMed] [Google Scholar]
- 49.Lukivskaya O, Patsenker E, Buko VU. Protective effect of ursodeoxycholic acid on liver mitochondrial function in rats with alloxan-induced diabetes: link with oxidative stress. Life Sci. 2007;80:2397–2402. doi: 10.1016/j.lfs.2007.02.042. [DOI] [PubMed] [Google Scholar]
- 50.Rodrigues CM, Fan G, Wong PY, Kren BT, Steer CJ. Ursodeoxycholic acid may inhibit deoxycholic acid-induced apoptosis by modulating mitochondrial transmembrane potential and reactive oxygen species production. Mol Med. 1998;4:165–178. doi: 10.1007/BF03401914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oh AR, Bae JS, Lee J, Shin E, Oh BC, Park SC, Cha JY. Ursodeoxycholic acid decreases age-related adiposity and inflammation in mice. BMB Rep. 2016;49:105–110. doi: 10.5483/BMBRep.2016.49.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wahlström A, Kovatcheva-Datchary P, Ståhlman M, Bäckhed F, Marschall HU. Crosstalk between bile acids and gut microbiota and its impact on Farnesoid X receptor Signalling. Dig Dis. 2017;35:246–250. doi: 10.1159/000450982. [DOI] [PubMed] [Google Scholar]
- 53.Ungprasert P, Wijarnpreecha K, Ahuja W, Spanuchart I, Thongprayoon C. Coronary artery disease in primary biliary cirrhosis: a systematic review and meta-analysis of observational studies. Hepatol Res. 2015;45:1055–1061. doi: 10.1111/hepr.12452. [DOI] [PubMed] [Google Scholar]
- 54.Sahebkar Amirhossein, Watts Gerald F. New Therapies Targeting apoB Metabolism for High-Risk Patients with Inherited Dyslipidaemias: What Can the Clinician Expect? Cardiovascular Drugs and Therapy. 2013;27(6):559–567. doi: 10.1007/s10557-013-6479-4. [DOI] [PubMed] [Google Scholar]
- 55.Banach Maciej, Aronow Wilbert S., Serban Corina, Sahabkar Amirhossein, Rysz Jacek, Voroneanu Luminita, Covic Adrian. Lipids, blood pressure and kidney update 2014. Pharmacological Research. 2015;95-96:111–125. doi: 10.1016/j.phrs.2015.03.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data from this analysis are available through collaboration under a data usage agreement with the corresponding author.