Abstract
A pentapeptide macrocyclic ligand, KYCAR (lysyl-tyrosyl-cystyl-alanyl-arginine), has been designed as a potential chelating ligand for SPECT imaging and therapeutic in vivo agents. This study shows the synthesis and characterization of KYCAR complexes containing nonradioactive rhenium, 99mTc, or 188Re. The metal complexes were also biologically evaluated to determine in vivo distribution in healthy mice. The overall goals of this project were (1) to synthesize the Tc/Re pentapeptide complexes, (2) to identify spectroscopic methods for characterization of syn versus anti rhenium peptide complexes, (3) to analyze the ex vivo stability, and (4) to assess the biological properties of the [99mTc]TcO-KYCAR and [188Re]ReO-KYCAR complexes in vivo. Details on these efforts are provided below.
Methods
NatRe/99mTc/188ReO-KYCAR complexes were synthesized, and macroscopic species were characterized via HPLC, IR, NMR, and CD. These characterization data were compared to the crystallographic data of ReO-KYC to assist in the assignment of diastereomers and to aid in the determination of the structure of the complex.
Results
The radiometal complexes were synthesized with high purity (>95%). HPLC, IR, NMR and CD data on the macroscopic natReO-KYCAR complexes confirm the successful complexation as well as the presence of two diastereomers in syn and anti conformations. Tracer level complexes show favorable stabilities ex vivo for 2+ hours.
Conclusion
Macroscopic metal complexes form diastereomers with the KYCAR ligand; however, this phenomenon is not readily observed on the tracer level due to the rapid interconversion. It was determined through pKa measurements that the macroscopic natReO-KYCAR complex is 0 at physiological pH. The [99mTc]TcO-KYCAR is stable in vitro while the [188Re]ReO-KYCAR shows 50% decomposition in PBS and serum. Biologically, the tracer level complexes clear through the hepatobiliary pathway. Some decomposition of both tracers is evident by uptake in the thyroid and stomach.
Keywords: KYCAR, 99mTc, 188Re, Metal peptide Complex, SPECT, Radiometals
1. Introduction
Central to molecular imaging is the development of novel imaging probes; advances in radiometal technology and novel biological targeting vectors have provided the motivation and opportunities for new diagnostic and therapeutic agents [1–4]. Radioactive isotopes of technetium (Tc) and rhenium (Re) have utility in both diagnostic imaging and radiotherapy, due to their associated decay schemes and physical half-life properties. 99mTc, a pure γ-emitter (γ: 142 keV; t1/2: 6.02 h), is an isotope that is used in over 80% of nuclear imaging [5] due to its excellent physical properties, convenience, and minimal radiation burden to the patient[6, 7]. Rhenium, the third-row transition metal congener of Tc, exhibits similar complexation chemistry to technetium, and its non-radioactive isotope is often used as a surrogate for the characterization of 99mTc radiopharmaceuticals. The radioactive isotope 188Re has exceptional potential as a radiotherapeutic isotope. 188Re possesses a half-life of 16 h, emits a high-energy β– (Emax= 2.11 MeV) that is effective for cell killing, and also generates an imageable γ-ray of 155 keV [8–10]. The availability of 188Re from the 188W/188Re generator renders 188Re as a convenient, inexpensive radiotherapeutic isotope. Both 99mTc and 188Re form stable peptide complexes with amine and amide nitrogens, carboxylate oxygens, and thiolate sulfurs [11, 12].
Short peptide sequences labeled with 99mTc and 188Re can be useful as chelators in targeted radiopharmaceuticals. In some agents, a portion of the peptide serves as a chelator to the metal and a separate portion serves as a target for a specific receptor [13–19]. Generally, Tc peptide complexes have been investigated due to their favorable pharmacokinetics and their receptor targeting [20, 21]. Due to their small size, peptides generally exhibit rapid pharmacokinetics and are able to penetrate tumors very efficiently [22, 23]. They also exhibit fast blood clearance and lower kidney retention. It is possible to engineer peptides to exhibit desired pharmacokinetic characteristics by changing the peptide sequence during solid-phase synthesis or by grafting additional elements such as polyethylene glycol (PEG) linkers to the chelate region. The coordination chemistry of the metal-peptide complex will determine the geometry, stability of the radiopeptide construct, and has the potential to influence receptor affinity and/or pharmacokinetics in vivo [24].
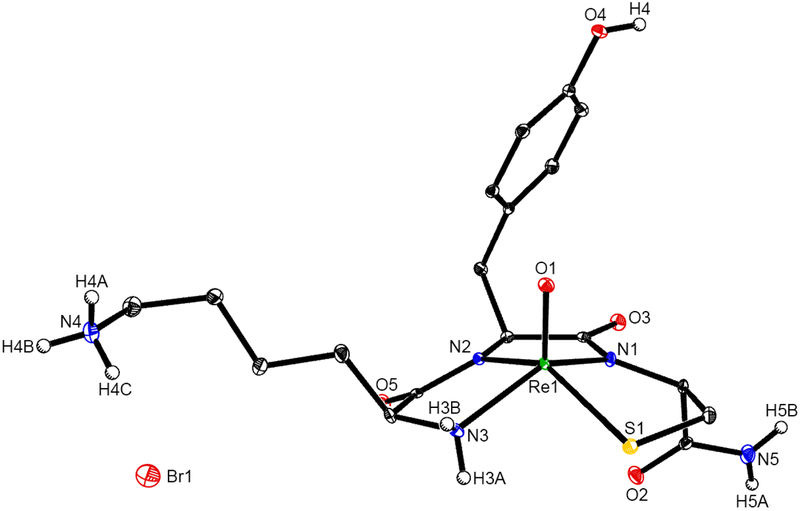
In this work, structures of 99mTc, 188Re, and macroscopic natRe complexes of the KYCAR (lysyl-tyrosylcystyl-alanyl-arginine) peptide sequence are prepared, characterized, diastereomers determined, chemistry of the complexes investigated, and charge of the chelator region determined. The KYCAR ligand was chosen because the [99mTc]TcO-KYCAR was reported to exhibit tumor uptake [25, 26]. The in vitro and in vivo stabilities and biodistribution studies are reported. Chelation about the radiometal includes three nitrogens (one amine of Lys side chain, two amide nitrogens of Tyr and Cys) and one sulfur (thiol sulfur of Cys) to give a N3S donor set of atoms (Figure 1).
Figure 1.
KYCAR Ligand.
The thiol group has a high affinity for “soft” transition metals, a property convenient for labeling cysteine-based proteins with 99mTcV=O and 188Rev=O moieties [11, 27, 28]. When peptide-based chelators are comprised of amino acids containing side chains, chiral centers are incorporated into the coordination environment, and diastereomeric complexes are known to form where the M=O group (M= Tc, Re) is either syn or anti to the substituents[29, 30]. In this work, it was found that two main products are formed when KYCAR is radiolabeled with rhenium, and these are purported to be the syn and anti diastereomers of ReO-KYCAR. The two diastereomers are referred to as syn or anti with regard to the position of the side chain of Tyr relative to the M=O bond (Figure 2).
Figure 2.
Anti and syn Diastereomers of ReO-KYCAR
To this end, we have (1) synthesized the 99mTc/188Re/natRe pentapeptide complexes, (2) identified spectroscopic methods for their characterization, (3) investigated the chemical reactivity of the residues of the metal peptide complexes; (4) identified the charge of the radiometal chelator region under physiological conditions, (5) analyzed the in vitro stability, and (6) assessed the biological properties of the [99mTc]TcO-KYCAR and [188Re]ReO-KYCAR complex in vivo. Importantly, this study allows a direct comparison of the 188Re to 99mTc products with respect to radiolabeling, yields, stability and biodistribution.
2. Materials and Methods
2.1. Reagents
99mTc was obtained via Memorial Sloan Kettering Cancer Center, New York, New York, as Na99mTcO4, eluted from a Technelite 99Mo/99mTc Generator from Lantheus, Billerica, Massachusetts, USA. The isotope 188Re was obtained as Na188ReO4 was eluted from an 188W/188 Re generator with saline (0.9% NaCl solution). The 188W/188Re generator was purchased from RadioMedix, Houston, Texas, USA. Activity measurements were obtained from an Atomic Products Corporation Atomlab 100 Dose Calibrator. The ligands, KYCAR and KYC-NH2, were purchased from United Peptide, Herndon, Virginia, USA. Methanol (MeOH), dimethyl sulfoxide (DMSO), HPLC-grade acetonitrile, triflouroacetic acid (TFA), and 0.9% NaCl solution were purchased from Fisher Scientific. Tin (II) tartrate 99%, tin (II) chloride dehydrate, d-gluconic acid and silica gel TLC plates were purchased from Sigma Aldrich. Deionized water (18 MΩ) was obtained from a 0.22 μm Millipore filtration system. All in vivo experiments were performed according to protocols approved by the Memorial Sloan Kettering Institutional Animal Care and Use Committee. The rhenium starting material, (Bu4N)[ReOBr4(H2O)]•H2O, was prepared following the method of Rose et. al. [31].
2.2. Synthesis of ReO-KYCAR
The KYCAR ligand (0.0403 mmol) was dissolved in 500 μL CH3OH. The above synthesized (Bu4N)[ReOBr4(H2O)]•H2O (0.0403 mmol) was dissolved in 500 μL CH3OH and added dropwise to the ligand. The resulting reddish-brown solution was allowed to stir at 200 rpm for 10 min. Following stirring, the solution was allowed to sit at room temperature for ten days to reach equilibrium. For semi preparative collections, the methanol solvent used to synthesize macroscopic rhenium complexes was evaporated under a stream of N2(g) for about 4 h. The dry complex was then reconstituted in a 95%/5% (solvent A/ solvent B) mixture of the mobile phase for HPLC analysis. The ReO-KYCAR product was purified via HPLC; the diastereomers were separately collected and fractions were lyophilized to a powder. The lyophilized powders ranged in color depending on the diastereomer ranging from light peach (peak 1, anti) to a darker pinkish/peach (peak 2, syn). Yields were 23.2% and 16.8% for the anti and syn diastereomers respectively, for a total yield of 40.0%. LCMS calculated for C27H42N9O8ReS ([M + H]+), 839.2435, found 840.2493. HPLC retention times: Intermediate (INT): 12.5 min; anti diastereomer, peak 1: 14.1 min; syn diastereomer, peak 2: 16.5 min.
1H NMR (600 MHz, 0.01 M HCl/D2O 50:50 (v:v)) peak 1: δ 8.45–8.44 (d, 1H), 8.26–8.25 (d, 1H), 7.51 (br, 1H), 7.10–6.98 (m, 1H), 6.84–6.83 (d, 1H), 6.58–6.57 (d, 1H), 4.89–4.87 (m, 1H), 4.78–4–74 (m, 1H), 4.18–4.17 (t, 1H), 4.12–4.11 (d, 1H), 3.98–3.96 (t, 1H), 3.80–3.78 (d, 2H), 3.34–3.32 (t, 2H), 3.04–3.02 (d, 1H), 2.93–2.91 (t, 1H), 1.88–1.86 (t, 1H), 1.83–1.81 (m, 1H), 1.76–1.74 (m, 1H), 1.66–1.55 (m, 3H), 1.54–1.40 (m, 1H), 1.31–1.21 (m, 1H).
1H NMR (600 MHz, 0.01 M HCl/D2O 50:50 (v:v)) peak 2: δ 8.23–8.19 (m, 1H), 7.75–7.74 (d, 1H), 7.46 (br, 1H), 7.10–6.95 (m, 1H), 6.48–6.47 (d, 1H), 6.42–6.41 (d, 1H), 5.09–5.08 (m, 1H), 5.04–5.03 (m, 1H), 4.10–4.04 (m, 1H), 3.96–3.95 (m, 1H), 3.86–3.85 (m, 1H), 3.34–3.26 (m, 2H), 2.93–2.91 (m, 2H), 2.85–2.84 (d, 1H), 2.62–2.55 (m, 1H), 2.15–2.06 (m, 1H), 1.74–1.73 (m, 1H), 1.63–1.61 (m, 1H), 1.50–1.48 (m, 2H), 1.26–1.25 (m, 1H), 1.16–1.15 (m, 1H).
2.3. Synthesis of ReO-KYC-NH2 (ReO-KYC)
The KYC-NH2 ligand (0.0403 mmol) was dissolved in 500 μL CH3OH. (Bu4N)[ReOBr4(H2O)]•H2O (0.0403 mmol) was dissolved in 500 μL CH3OH and added dropwise to the ligand. The resulting reddish-brown solution was allowed to stir with a stir bar at 200 rpm for 10 min. Subsequently, the solution was allowed to sit at room temperature for 40 min. For semi preparative HPLC purification of the diastereomers, the methanol solvent was evaporated under a stream of N2(g) for about 4 h. The dry complex was then reconstituted in a 95%/5% (solvent A/ solvent B) mixture of the mobile phase for preparative HPLC purification. Two fractions were collected and lyophilized to powders that were pinkish in color. The yield of the ReO-KYC was 12.5% and 10.5% for the anti and syn diastereomers, respectively, for a total yield of 23.0%. X-ray quality crystals of ReO-KYC were obtained by supersaturation in methanol over 1 week at room temperature. HPLC retention times: anti diastereomer, peak 1: 12.1 min; syn diastereomer, peak 2: 14.5 min.
2.4. Synthesis of [99mTc]TcO-KYCAR
To a saline solution of 99mTcO4− (370 MBq; 10 mCi), KYCAR ligand (200 μL, 0.00234 mmol solution) in saline was added. To that mixture tin(II) tartrate (40 μL, 0.0221 mmol solution) in water was added and the mixture was vortexed. The reaction mixture was then kept at 90 °C for 10 min followed by incubating at room temperature for 30 min. The solution was then filtered through a 0.1 μm syringe filter and analyzed or purified via HPLC. Radiochemical Purity determined by iTLC was 99.9%. HPLC retention times: anti diastereomer, peak 1: 9.1 min; syn diastereomer, Peak 2: 10.1 min. Radiochemical Yield 62.2%.
For in vitro and in vivo studies, the [99mTc]TcO-KYCAR complex (syn) was purified by injecting on to a 4.6 × 150 mm Water Atlantis dc18 3 μm 100 Å column. The collected fraction was reduced in volume using vacuum and reconstituted in the appropriate solvent.
2.5. Synthesis of [188Re]ReO-KYCAR
To a saline solution of 188ReO4− (370 MBq; 10 mCi), tin (II) chloride (75 μL, 0.105 mmol solution) in water and d-gluconic acid (150 μL, 0.102 mmol solution) in water were added. The reaction mixture was vortexed and kept at 37 °C for 30 min. To this solution the KYCAR ligand (200 μL, 0.00313 mmol solution) in saline was added, the reaction mixture was vortexed and kept at 37 °C for 30 min. The solution was then filtered through a 0.1 μm syringe filter and analyzed or purified via HPLC. Radiochemical Purity determined by iTLC was 99.9%. HPLC retention time: anti diastereomer, peak 1: 9.1 min. Radiochemical Yield 84.2%.
For in vitro and in vivo studies, the [188Re]ReO-KYCAR complex (syn) was purified by injecting on to a 4.6 × 150 mm Water Atlantis dc18 3 μm 100 Å column. The collected fraction was reduced in volume using vacuum and reconstituted in the appropriate solvent.
2.6. HPLC Analyses and Purifications
Complexes were analyzed using a Waters Symmetry 4.6 × 150 mm C18 3.5 μm 100 Å analytical column. Complexes were purified using a semi-preparative Waters Nova Pak HR 19 × 300 mm C18 6 μm 60 Å column. All non-radioactive Re HPLC experiments were conducted on a Rainin HPXL solvent delivery system with a Prostar 325 UV-vis Detector. Radioactive 99mTc, 188Re HPLC experiments were completed using a Varian Pro Star HPLC system. The radioactive complexes were analyzed and purified using a Waters Atlantis 4.6 × 150 mm dc18 3 μm 100 Å column. The γ emissions were monitored using a Bicron 2m2/2 NaI detector and Tennelec Minibin components. The software used for all HPLC systems was the ProStar WorkStation. The solvents used were ultra-pure deionized water with 0.1% trifluoroacetic acid (solvent A), and HPLC-grade acetonitrile with 0.1% trifluoroacetic acid (solvent B). Method 1 was used for all non-radioactive analytical and preparative natRe HPLC experiments: 5–25% B over 26 min. Method 2 was used for all radioactive HPLC experiments: 5–25% B over 20 min. The flow rate for analytical analyses was 1.0 mL/min, and for semi-preparative separations 16.7 mL/min. For all HPLC analyses the UV detection was monitored at 280 nm.
2.7. Mass Spectrometry Analyses
For macroscopic, cold rhenium compounds, mass spectral data were obtained on a LCMS system comprised of an Agilent 1200 LC system coupled to an Agilent 6340 ion trap mass spectrometer. Samples were injected onto an Agilent Zorbax column (SB-C8, 5 μM, 2.1 × 50 mm) using a linear gradient of 5–95% acetonitrile in water (0.5% formic acid) over 10 min. The samples were prepared by dissolving the lyophilized ReO-KYCAR samples in water:acetonitrile solution (v:v, 450:50 μL).
2.8. NMR Analyses
The 1D proton and 2D TOCSY (total correlation spectroscopy) NMR spectra were obtained from a Bruker Avance III 600 MHz with a TCI cryoprobe NMR spectrometer with chemical shift referenced to H2O, at T=296 K. ReO-KYCAR samples (peak 1, anti, and peak 2, syn) (5–10 mg) were dissolved in an 800 μL solution of 0.01 M HCl/D2O 50:50 (v:v). The solution was then transferred to a 5 mm U-Thin NMR tube. 1D proton NMR spectra were collected at 8 scans. 2D COSY scans were collected at 2 scans, 256 increments.
pKa determination of the amine nitrogen of lysine in the ReO-KYCAR complex is described by Cantorias et. al. [30] Briefly 10 mg of the macroscopic rhenium complex was dissolved in 700 μL 0.01 M DCl in D2O. The pH was recorded, the solution was transferred to a 5 mm U-Thin NMR tube, and 1D proton and 2D COSY (correlation spectroscopy) NMR spectra were obtained. The sample was then titrated with 0.05 M NaOD until the pH changed from 0.5 to 1 intervals. The volume and pH were recorded, and NMR spectra were obtained at each interval. We recognize that measuring the pD with a pH electrode impacts the pKa values. General pKD versus pKH correlations have been reported for a series of different acids and for macrocyclic ligands [32]. For Brønsted acids the correlation is:
For macrocycles the correlation is:
Applying these correlations to convert the pKaD values measured in our NMR will result in pKa values that are 0.4 to 1.0 pH units lower than our measured values. Since these correlations are for acids and macrocycles, not metal peptide complexes, we note that the actual pKa values may be lower than our measured value.
2.9. Infrared Spectroscopy Analyses
Infrared spectra were obtained using a Perkin-Elmer Spectrum Two IR Spectrometer with attached UATR in the range of 500–4000 nm. Briefly a small amount of lyophilized sample was placed on the UATR and the appropriate pressure was applied.
2.10. Circular Dichroism Analyses
Circular Dichroism spectra were collected on an Aviv Biomedical Circular Dichroism spectrometer of approximately 1 nanomol solutions of Re-KYCAR in 1 mL methanol. The samples were scanned from 200 to 680 nm. The natReO-KYCAR samples analyzed were peak 1 and peak 2 from the HPLC prep purification.
2.11. X-ray Structure Determination
X-ray diffraction data were collected on a Bruker X8 Kappa Apex II diffractometer using Mo Kα radiation. The structure was solved using direct methods and standard difference map techniques, and was refined by a full-matrix least-squares procedures on F2 with SHELXTL (version 2014/7)[33, 34]. All hydrogen atoms bound to carbon were placed in calculated positions and refined with a riding model [Uiso(H) = 1.2–1.5Ueq(C)], while hydrogen atoms bound to nitrogen and oxygen were located on the difference map before refinement with a riding model.
2.12. Instant Thin Layer Chromatography
To assess the radiochemical purity of the HPLC purified radiometal complexes, instant thin layer chromatography (iTLC) was completed post HPLC purification. Briefly 1 μL of the product was placed on a silica gel TLC plate with glass backing. The TLC strip was then placed in a falcon tube containing 70% ethanol and allowed to develop. Using this solvent system 99mTc and 188ReO-KYCAR complexes remains at the origin (Rf= 0.0–0.1), while 99mTcO4 −188 and ReO4− run with the solvent front (Rf= 0.9–1.0). TLC strips were analyzed on an Eckert & Ziegler Bioscanner to determine percent of activity at the origin versus at the solvent front.
2.13. Log D Studies
To determine the distribution coefficients the radioactive metal (99mTc, 188Re) complexes were HPLC purified, dried via high vacuum system, and reconstituted in PBS and placed into a micro centrifuge tube with an equal amount of 1 octanol. The samples were vortexed followed by centrifugation at 10,000 rpm for 5 min. Layers were separated and aliquots counted on an Atomic Products Corporation Atomlab 100 Dose Calibrator.
2.14. Biodistribution Studies
All animals were treated according to the guidelines set by the Institutional Animal Care and Use Committee. The HPLC purified 99mTc and 188Re labelled KYCAR complexes were evaluated for their biological distribution in healthy athymic nude female mice 6–8 weeks old (Charles River Labs). 3.7 – 7.4 MBq (100 μCi to 200 μCi) of the radiolabeled complex was injected intravenously into the mice via the tail vein. Mice were sacrificed (CO2 (g) asphyxiation) 30 min, 1, 2 and 4 h post injection; 5 mice per time point. The following tissues were removed, rinsed in water, and dried in air; blood, heart, lungs, liver, spleen, stomach, small intestines, large intestines, kidneys, muscle, bone, skin, thyroid, and gall bladder. The tissues were then counted on a Perkin Elmer Automatic Wizard γ-counter calibrated for either 99mTc or 188Re. Counts were converted to activity using a calibration curve generated from known standards. Count data were background- and decay-corrected to the time of injection, and the percent injected dose per gram (%ID/g) were calculated and reported.
3. Results and Discussion
3.1. Synthesis and Characterization of natRe KYCAR Complexes
Synthesis of nat Re KYCAR
The reaction of ReVOBr4− with the KYCAR pentapeptide in methanol resulted in two major [ReVO] complexes after incubation at room temperature for 10 days. The HPLC data are shown on Figure 3. Four hours after mixing, three peaks are observed. These are an intermediate peak, denoted INT, peak 1 and peak 2. The intermediate complex disappears over time. Peaks 1 and 2 are the two major ReO-KYCAR peaks. Peak 1 converts into peak 2 over time; after 2 days the peak 1/peak 2 ratio was approximately 2.1:1. After 3 days, the ratio of peaks 1/peak 2 was approximately 1:1. The two major complexes reached equilibrium after 6 days, where peak 2 was the slightly dominant peak at a ratio of approximately 0.8:1. The ratios do not change after 6 days and the two complexes represented by peaks 1 and 2 were HPLC purified after 10 days.
Figure 3.
Synthesis of ReO-KYCAR over a 6 day period in methanol. Immediately post synthesis there are three peaks present (INT, peak 1 and peak 2). Peak INT (short-lived intermediate complex) converts to peak 1, “anti” diastereomer and peak 2, “syn” diastereomer. Peak 1 converts to peak 2 over time. The two diastereomers were isolated at day 10 by preparative HPLC, lyophilized and characterized. See text.
These peaks (1 and 2) represent ReO-KYCAR diastereomers: the anti complex is represented by the earlier eluting peak, 1, and the syn complex is represented by the later eluting peak, 2. These assignments are based on previous work ([16, 30]and were confirmed in this study, specifically by direct comparison to the ReO-KYC syn diastereomer (peak 2) crystal structure as well as circular dichroism experiments, vide infra.
To fully characterize all species, peaks 1 and 2 were collected separately after 10 days and the intermediate peak (INT) was collected after one day by preparative HPLC, Method 1. The fractions were lyophilized to powders. After lyophilization, the powders from both the intermediate peak (INT) and peak 1 products exhibited a peach color, whereas the powder from peak 2 was darker in relation to peak 1. Peak 1 revealed a molecular ion [M + H]+ peak at m/z 840, which is consistent with the exact mass of the ReO-KYCAR complex (mass= 839.24 g/mol) (Figure S1).
Peak 2 revealed a molecular ion [M + H]+ peak at m/z 854, which is 14 dalton above the exact mass of ReO-KYCAR. This represents a methyl ester adduct (Figure S2) wherein an acid-catalyzed esterification between methanol and the carboxylic acid (of the arginine) occurred during the synthesis. This is expected for a free carboxylate in a methanolic synthesis. The intermediate peak contained a mixture of both complexes, showing m/z 840 and 854. Two lines of evidence support the methyl ester adduct assignment. First, conducting the reaction in methanol for two hours instead of ten days and collecting peak 2 results in mass spectral data showing both m/z 840 and m/z 854 demonstrating that a rapid conversion from the carboxylate to the methyl ester occurred. The second experiment was to run the reaction in acetonitrile instead of methanol (supporting material, Figure S3A and S3B). For this acetonitrile reaction the mass spectral data demonstrated that both peak 1 and peak 2 displayed signals at m/z 840 with no mass observed at m/z 854.
Unfortunately, the yield of peak 2 was extremely low when the reaction was performed in acetonitrile. Moreover, unlike the reactions conducted in methanol, reactions conducted in acetonitrile showed no interconversion of peak 1 to peak 2 (Figure S3A) thus further minimizing access to peak 2. Therefore, all reactions to isolate peak 2 were conducted in methanol. However, the data discussed below, specifically the crystal structure of the ReO-KYC (peak 2) and the circular dichroism studies confirm the assignments of peak 1: anti diastereomer and peak 2: syn diastereomer, vide infra.
3.2. Crystallography
Due to the unsuccessful attempts crystallizing ReO-KYCAR, described in the Supporting Information (Tables S1 and S2), ReO-KYC-NH2 (ReO-KYC) (Figure S4) crystallization was attempted to investigate the structure around the complexation region. An amide capped peptide was used to prevent any potential methyl esterification from occurring. Crystals of similar ReO and 99TcO tripeptide complexes bearing cysteine at the carboxylate terminus have been previously obtained and reported in the literature [30]. ReO-KYC was synthesized and an aliquot from the reaction mixture was analyzed via reverse phase HPLC (Figure S5). The HPLC trace shows that there are two major peaks present corresponding to two diastereomers. Orange crystals were obtained by the supersaturation of the remaining reaction mixture in methanol over 1 week. The crystal and structure refinement data are found in Supporting Information (Table S3). Bond lengths and bond angles are tabulated in Table 1, and the structure is shown in Figure 4 and Figure S6.
Table 1.
Bond lengths and bond angles for ReO-KYC peak 2 (Syn).
| [(KYC-H)ReO][Br] | Bond Length (Å) |
|---|---|
| Re-O | 1.676(3) |
| Re-N1 | 1.983(3) |
| Re-N2 | 1.984(3) |
| Re-N3 | 2.117(3) |
| Re-S | 2.2630(9) |
| N3-C6 | 1.497(5) |
| Bond Angle (deg) | |
| N2-Re-N3 | 79.06(12) |
| N3-Re-S | 91.22(9) |
| N1-Re-N2 | 78.72(12) |
| N1-Re-S | 82.66(9) |
| O-Re-N3 | 107.62(12) |
| O-Re-N2 | 113.23(12) |
| O-Re-N1 | 111.94(13) |
| O-Re-S | 109.80(10) |
Figure 4.
Structure for ReO-KYC (syn).
The crystal structure determined that the coordinating nitrogen on the lysine (N3) is an amine nitrogen and the epsilon residue (N4) is protonated, thus the overall charge of the molecule is +1. This is balanced by the presence of a bromide counterion. With respect to the geometry at the Re center, Addison et. al. describes a τ5 parameter as a range from 0 (C4v square pyramid) to 1 (D3h trigonal bipyramid). If we examine the τ5 value of the ReO-KYC crystal (0.04) we see that this indicates square pyramidal geometry [35]. The crystallization in a chiral space group confirms that there is only one enantiomer present.
Examination of the bond angles about N2 and N1 demonstrate that these are amidate nitrogen atoms, i.e. that a proton is lost upon metal complexation. The angles range from 116.3(3) degrees to 125.4(2) degrees, encompassing 120 degrees would be expected for a sp2 hybridized nitrogen atom. In contrast the C6-N3-Re angle is 111.4(2) degrees which is closer to 104.5 degrees representative of a sp3 hybridized nitrogen atom [30].
The crystals were dissolved and reinjected into the HPLC to confirm the presence of one peak at 14.50 min that corresponds to “peak 2” (Figure S7). Since these crystals of ReO-KYC were the syn diastereomer, it can be inferred that peak 2 of the ReO-KYCAR is also the syn diastereomer. This is consistent with the Circular Dichroism and NMR data as well as our previous work in which we demonstrated that for Tc and Re tripeptides, peak 1 corresponds to the anti diastereomer and peak 2 corresponds to the syn diastereomer [30].
3.3. Spectroscopic Characteristics
NMR
The 2-Dimensional 1H NMR data were collected and the chemical shifts from the 1H NMR spectra of the macroscopic ReO-KYCAR diastereomers were compared to the free KYCAR ligand (Table 2). The 2-Dimensional TOCSY NMR data for ReO-KYCAR peak 1 (anti) and peak 2 (syn) complexes are shown in Figures S8, S9, S10, and S11. The 1-Dimensional 1H NMR data are shown in figure S12. The2-Dimensional data allow assignment of the protons of the diastereomers and reveal information regarding the coordination of the chelate about the rhenium. The discussion below is facilitated by referring to Figures 1–2, S13-S14.
Table 2.
1H NMR of KYCAR, ReO-KYCAR peak 1, ReO-KYCAR peak 2, and the change (Δ) in ppm between peak 1 and peak 2.
| Residue | Free Ligand |
Peak 1 | Peak 2 | Δ ppm | |
|---|---|---|---|---|---|
| K | HN | 7.42 | - | - | - |
| Hα | 3.84 | 3.97 | 3.95 | 0.02 | |
| Hβ | 1.73 | 1.88 | 2.10 | 0.22 | |
| - | 1.82 | 1.82 | |||
| Hγ | 1.24 | 1.5 | 1.48 | 0.02 | |
| Hδ | 1.53 | 1.66 | 1.65 | 0.01 | |
| Hε | 2.85 | 2.92 | 2.92 | 0.00 | |
| Hζ | - | 7.51 | 7.46 | 0.05 | |
| Y | HN | 8.69 | - | - | - |
| Hα | 4.41 | 4.88 | 5.09 | 0.21 | |
| Hβ | 2.91 | 3.79 | 3.30 | 0.49 | |
| 2.75 | 3.32 | 2.91 | 0.41 | ||
| Hδ | 6.96 | 6.84 | 6.48 | 0.36 | |
| Hε | 6.66 | 6.58 | 6.42 | 0.16 | |
| C | HN | 8.07 | - | - | - |
| Hα | 4.20 | 4.88 | 5.03 | 0.15 | |
| Hβ | 2.60 | 3.79 | 3.28 | 0.51 | |
| - | 3.32 | 2.64 | 0.68 | ||
| A | HN | 8.19 | 8.45 | 7.75 | 0.70 |
| Hα | 4.04 | 4.11 | 4.09 | 0.02 | |
| Hβ | 1.25 | 1.27 | 1.15 | 0.12 | |
| R | HN | 8.24 | 8.27 | 8.21 | 0.06 |
| Hα | 4.19 | 4.20 | 3.85 | 0.35 | |
| Hβ | 1.78 | 1.79 | 1.72 | 0.07 | |
| 1.64 | 1.62 | 1.26 | 0.36 | ||
| Hγ | 1.49 | 1.47 | 1.53 | 0.06 | |
| Hδ | 3.05 | 3.07 | 2.85 | 0.22 | |
| Hε | 7.03 | 7.03 | 7.03 | 0.00 |
All of the protons can be assigned in the NMR under the acidic, aqueous conditions used in the experiments except for the amine protons on the lysine (K) that are not observed. This is due to exchange of the amine protons in aqueous solution, which is a common occurrence known to be dependent on factors such as pH and concentration. The protons of the amide groups of the tyrosine (Y) and cysteine (C) amino acids were not observed, that is consistent with deprotonation of the amide nitrogen atoms to form amidate-Re bonds. Amidate-Re bonds for tyrosine and cysteine are found in the crystal structure, vida supra, thus the NMR data confirms coordination of these amino acids to the Reoxo core in aqueous solution. The β-protons of the cysteine are split into two resonances in each complex that also is consistent with complexation of the thiol sulfur to the metal. The NMR data are similar to those reported for 99TcO and ReO tripeptide complexes [30] and demonstrate that the ReO-KYCAR maintains its structure in solution. Typically, technetium(V)-oxo and rhenium(V)-oxo-complexes carry a square pyramidal structure with the metal oxo group in the apical position when complexed to a tetradentate ligand [36–39] such as a tripeptide chelate. Crystal structure data, vide supra, and circular dichroism, vide infra, are better indicators of the absolute configuration of the diastereomers.
Circular Dichroism
To observe structural signatures of each of the isolated peaks, peaks 1 (anti) and peak 2 (syn), CD spectra were obtained and are shown below (Figure S15). The areas that provide the most information lie between 280 and 340 nm. Within this region are transitions, which include ligand to metal charge transfer. According to previous studies on rhenium-Schiff base complexes, this region correlates to the oxygen to rhenium charge transfer transitions [39, 40]. The strong positive Cotton effect, or characteristic change in optical rotatory, around 280 nm is assigned to the Re-oxo core in the anti position. Conversely, we see a negative Cotton effect of the Re-oxo core in the syn position. The postulated anti and syn circular dichroism spectra can be confirmed by comparing this CD data to previously reported data showing anti and syn complexes with crystallographically characterized Re- and Tc peptides (Figure S16) [30].
Infrared Spectroscopy
The infrared spectra for the anti and syn peaks, B and C, show a Re=O stretching frequency at 991 cm−1 and 990 cm−1, respectively. These values are slightly shifted to higher energies from 984 cm−1, which was observed from (Bu4N)[ReOBr4(H2O)]•H2O, indicating that the Re=O bond from peaks B and C exhibit slightly more double bond character. The diastereomers also exhibit a broad peak at 2800–3500 cm−1, characteristic of the phenol and carboxylic acid O-H and amine N-H. Both peaks exhibit a strong intensity stretching frequency at around 1649 cm−1 (anti) and 1654 cm−1 (syn), indicative of the C=O secondary amide bond, specifically representing an amide I band. New medium intensity stretching frequencies at 1515 cm−1 (anti) and 1514 cm−1 (syn) are characteristic of a secondary amide as well, specifically corresponding to an amide II band. The last new major peak that appears in ReO-KYCAR exhibits a strong intensity stretching frequency at 1199 cm−1 (anti) and 1198 cm−1 (syn), corresponding to the secondary amide C=O amide III band. For the syn peak specifically, a stretching frequency characteristic of a C=O methyl ester band overlaps with the amide carbonyl stretch at 1654 cm−1. The infrared spectra of the syn peak reveals that there are actually 2 peaks at around 1654 cm−1, whereas the infrared spectra of the anti isomer exhibit only 1 peak at 1649 cm−1. This double peak indicates that the ester and amide carbonyl stretches are overlapping (Figure S17).
pKa Measurements
The charge on the chelate is very important to assess/understand biological behavior. We attempt to identify the charge on the chelate and the charge on the overall complex.
From crystallography of the ReO-KYC, previous crystallographic and NMR studies [16, 17, 30, 38, 41–46], and the present NMR characterization, it is evident that the tyrosine and cysteine amide protons are lost to form amidate-Re bonds on complexation and that the cysteine thiol loses the proton to form a thiolate-Re bond (Figure 2, S13-S14). This phenomenon is common for rhenium and technetium peptide complexes or Re/Tc peptide-like complexes that contain amide bonds [16, 17, 30, 38, 41–46]. In our previous studies [30], we observed by crystallography and by potentiometric and NMR titrations that the first amino acid of a tripeptide possessing lysine in the second position deprotonates to form an amide bond to the Re. In fact the pKa of the phenylalanine amine in the Re-tripeptide complex ReO-FKC, reported in reference [30], was 5.63. This was determined by NMR titrations monitoring the chemical shifts of the β proton of the phenylalanine. The lysine residue appeared to profoundly increase the acidity of the phenylalanine amine proton in ReO-FKC compared to another Re-tripeptide, ReO-FGC, complex that did not contain a lysine. Titration studies revealed a further protonation equilibrium at pKa= 11.7 for ReO-FKC which was assigned to the ε-NH3 of the lysine side chain. Considering the influence of deuterium, we measured the pKaD. Applying the equations mentioned in the experimental to correlate pKD to pKH for macrocycles and acids, vide supra, the pKaH values for all of our measured pKa would be lower by 0.4 to 1.0 pH units. Since the pKD:pKH correlations were for macrocycles and acids they may not exactly apply to our studies on rhenium peptide complexes. We note here that the true pKa values may be lower than our measured pKa values reported below. However, the trends in pKa values discussed below are still valid.
In this study, we performed similar NMR titration studies to determine the pKa of the lysine (K) amine proton bound to the Re in the ReO-KYCAR (anti). For this study, it was decided to monitor the Hα found on the lysine (Figure S14, S18-S19). The Hα of the lysine residue is adjacent to the amine moiety and thus, should reflect the environment of the amine and allow calculation of the amine pKa. We performed the titration on the free KYCAR to validate our method. We were not able to obtain suitable data on the ε lysine proton to determine the pKa of the ε lysine proton without significant errors. In the future we plan to use the lysine as a conjugation point to targeting vectors and did not pursue the pKa measurement. However, from our previous studies, the pKa of the ε lysine proton was around 11.7 for ReO-FKC.
An NMR titration procedure described by Popov et. al[47] was employed to determine the pKa values of the lysine amine in the KYCAR ligand and ReO-KYCAR (anti diastereomer). Briefly, the values were determined by plotting pH vs. 100[(σobs-σHL)/ (σL-σHL)]. Here σobs is the chemical shift at a specific pH; σHL is the chemical shift of the highest pH; σL is the chemical shift of the lowest pH. Tables summarizing the calculations and figures highlighting the plots and the pKa values can be found in Supplementary Information (Tables S4–S5, and Figures S20-S23). In the free KYCAR ligand, it was determined that the pKa values of the alpha proton on the lysine was 7.48; this value is reasonable for N-terminal amino acids, and serves to validate our method. For the ReO-KYCAR (anti diastereomer), the pKa of the amine proton of the lysine was 6.00. See supporting information for experiments and graphs including a graphical representation of the potentiometric titration.
The pKa of 6.00 for the amine proton on the lysine of ReO-KYCAR is consistent with our reported findings of the pKa of 5.6 for the amine proton of the Re-tripeptide complex, ReO-FKC [30]. Our previous work also demonstrated that Re-tripeptide complexes that did not contain lysine, such as ReO-FGC, demonstrated a pKa value of 6.80 that was considerably higher than for the ReO-FKC. A study by Marzilli [48] indicated that a negatively charged carboxylate pendant group in the proximity of a secondary amine is responsible for a lower pKa and deprotonation of the amine. Further studies by this group showed that pKa’s of amines bound to ReV=O are in the physiological range when pendant groups, such as carboxylate moieties, can bind to form a six-coordinate metal. They suggest that the acidity of amines is influenced by pendant groups, rigidity of chelates and the position of the C=O group with the chelate framework. In previous studies [30] and here, the pendant lysine group seems to serve in a similar capacity, perhaps interacting with the Re=O core or C=O of the amide functionality of tyrosine or cysteine and increasing the acidity of the lysine amine.
With pKa information from this study and others, we can determine the charge on the chelator region, ReO-KYC, and the entire ReO-KYCAR molecule. The pKa measurement of 6.00 suggests that at physiological pH (7.4), deprotonation of the lysine NH2 terminus would occur creating a Re-Namide bond for the lysine N-terminus of the peptide. The Re-Namide bond would then carry a negative charge. Also, under physiological pH, the lysine epsilon amine is positively charged. Therefore, under physiological pH, the charge on the chelator region only in 0 due to the “zwitterionic effect”, where the lysine epsilon amine is positively charged, and the amide bound to the rhenium is negatively charged. However, considering the entire molecule (ReO-KYCAR) at physiological pH, the charge on the entire molecule would be 0, again due to a “zwitterionic effect” (a positive arginine and a negative carboxylate). Scheme 1 shows the deprotonations of the lysine amine and the epsilon amine of the chelator region only (ReO-KYC) at low pH, physiological pH, and high pH. At low pH the charge on the chelator region only is +1 due to the positive lysine epsilon amine and the neutral amine donating to the rhenium core. At physiological pH (discussed above), the charge on the chelator region is zero. At high pH the charge on the chelator region is −1 due to the neutral lysine epsilon amine and the negatively charged amide bound to the rhenium.
Scheme 1.
Deprotonation of the lysine epsilon amine and formation of the metal amide (showing the chelator region only) of ReO-KYCAR (anti) at varying pH. R= Ala Arg (AR).
3.4. Synthesis and Characterization of 99mTc and 188Re KYCAR
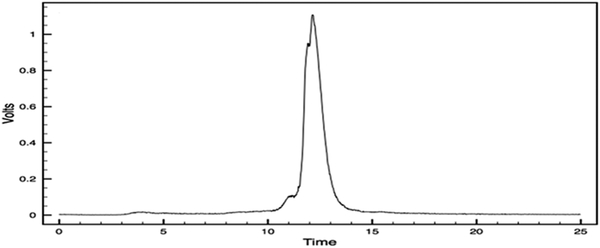
The reaction of the KYCAR ligand with 99mTcO4− reduced with tin(II) tartrate results in one major complex. Figure 5 shows the immediate post synthesis reverse phase HPLC trace. It is hypothesized that this single species occurs due to the extended heating/ reaction time during the synthesis. Instant thin layer chromatography shows that the purity post HPLC purification is 99.9%.
Figure 5.
HPLC Trace [99mTc]TcO-KYCAR.
The purified [99mTc]TcO-KYCAR was then co-injected with the macroscopic ReO-KYCAR lyophilized peak 1 (anti) and the coelution is shown in Figure 6. This HPLC (Figure 6) shows the macroscopic rhenium complex eluting between 9.0 and 12.0 min and the tracer technetium complex eluting between 10.0 and 12.5 min. Due to the location and sequence of the UV and radio detectors in this HPLC system, UV active species should elute before radio detection. We note that the shoulder appears in the radiotrace at 10.0 min; this co-elutes with the ReO-KYCAR (anti) and is assigned to the anti 99mTc diastereomer. The majority of the [99mTc]TcO-KYCAR is assigned to the syn diastereomer. The first radiotrace peak (anti diastereomer) likely occurs due to the orders of magnitude of macroscopic, carrier ReO-KYCAR (anti). The coelution of [99mTc]TcO-KYCAR and the syn diastereomer of the macroscopic ReO-KYCAR (Figure S24) was also completed using the ReO-KYCAR methyl ester complex. This coelution shows the first macroscopic rhenium peak (anti diastereomer) eluting at 10.2 min. The radiotrace peak, [99mTc]TcO-KYCAR (syn diasteromer), elutes at 11.3 min while the ReO-KYCAR syn diastereomer elutes at 12.0 min as expected.
Figure 6.
Coelution of [99mTc]TcO-KYCAR (peaks 1 and 2, anti and syn) with ReO-KYCAR Peak 1(anti).
The reaction of 188ReO4− reduced with tin (II) chloride in the presence of d-gluconic acid forms the putative [188Re]ReO-gluconic acid derivative that can be displaced by subsequent reaction with the KYCAR ligand. In this reaction d-gluconic acid is an exchange ligand; this reaction also results in a major complex. Figure 7 shows the immediate post synthesis reverse phase HPLC trace. It is observed that there is only one peak present due to the heated incubation, which allows for complete conversion to the syn product (retention time: 11.1 min with HPLC Method 1). Instant thin layer chromatography demonstrates that the purity post HPLC purification is 99.9%. The purified [188Re]ReO-KYCAR was then coeluted with the macroscopic ReO-KYCAR lyophilized peak 1 (anti) (Figure 8). This shows the macroscopic rhenium complex eluting between 7.0 and 11.0 min and the tracer rhenium complex eluting between 8.0 and 12.0 min. The retention time for the [188Re]ReO-KYCAR is 9.1 min under HPLC Method 1, and this corresponds to the anti disastereomer. The conversion of the [188Re]ReO-KYCAR (syn) to the [188Re]ReO-KYCAR (anti) diaseteromer can be facilitated with the use of the macroscopic ReO-KYCAR anti diastereomer in a “carrier effect”, as well as the instability of 188Re complexes compared to the 99mTc analogs. This instability is also demonstrated by some decomposition to 188ReO4−. Conversion of syn to anti diastereomers as well as formation of 188ReO4− was observed in a [188Re]ReO-tripeptide study (data not shown). There appears to be small amounts of other decomposition products as seen by the radiopeak at 4.5 min and 14.5 min.
Figure 7.
HPLC Trace of [188Re]ReO-KYCAR syn diastereomer.
Figure 8.
Coelution of [188Re]ReO-KYCAR anti with natReO-KYCAR anti diastereomer. The red trace shows the UV detection of the natReO-KYCAR; the black trace monitors the gamma radioactivity. This study shows co-elution of anti diastereomers with slight decomposition to 188ReO4−.
3.3. In vitro and in vivo evaluations
The stability of [99mTc]TcO-KYCAR and [188Re]ReO-KYCAR in phosphate buffered saline (PBS) (Figure S25 and S27) and human serum (Figure S26) were evaluated in vitro via HPLC analysis. The [99mTc]TcO]-KYCAR remained stable with less than 3% 99mTcO4− growing in up to 2 h. Decomposition of the complex to 99mTcO4− would result in a peak with an elution time of 5.0 min. The in vitro incubation of [99mTc]TcO-KYCAR in human serum results in an immediate 5–10% decomposition of the complex to 99mTcO4− and another polar species. There is no further decomposition up to 2 h.
The biological distribution of [99mTc]TcO-KYCAR were examined in healthy mice and the data is summarized as the percent of the injected dose to each selected organ of the mouse (%ID/g) (Table 3; Figure 9). [99mTc]TcO-KYCAR shows high uptake in the liver after 30 min (22.10 %ID/g). After 4 h, the radioactivity in the liver decreased to 1.8 %ID/g indicating efficient clearance and low amounts of accumulation over time in the liver. Concurrently, [99mTc]TcO-KYCAR exhibited rapid accumulation in the small intestine 30 min post injection (22.8% ID/g), with subsequent depletion 4 h post injection (3.6 %ID/g). As expected from this trend, levels of the radiotracer in the large intestine increased from 0.3 %ID/g at 30 min to 36.8 %ID/g at 4 h. Extremely high uptake is observed in the gall bladder that decreases with increasing uptake in the small and large intestines. The majority of the radioactivity is excreted via the hepatobiliary system despite the hydrophilic nature of our radiotracer (log D =−2.631).
Table 3.
Biodistribution results of [99mTc]TcO-KYCAR(syn) in healthy mice (n=5). The results are reported in %ID/g. Shows that the activity travels through the gastrointestinal track. Also shows some decomposition to potential 99mTcO4− products as seen with thyroid accumulation.
| [99mTcO]-KYCAR(syn), %ID/g ± S.D. | ||||
|---|---|---|---|---|
| Organ | 0.5 h | 1 h | 2 h | 4 h |
| Blood | 1.77 ± 0.37 | 1.37 ± 0.14 | 1.08 ± 0.20 | 0.91 ± 0.04 |
| Heart | 0.66 ± 0.60 | 0.42 ± 0.05 | 0.58 ± 0.22 | 0.33 ± 0.06 |
| Lungs | 1.39 ±0.23 | 1.13 ± 0.18 | 1.52 ± 0.98 | 0.99 ± 0.38 |
| Liver | 22.10 ± 4.80 | 13.35 ± 1.85 | 4.37 ± 1.55 | 1.83 ± 0.19 |
| Spleen | 0.82 ± 0.25 | 0.69 ± 0.13 | 0.57 ± 0.18 | 0.44 ± 0.17 |
| Stomach | 4.57 ±0.86 | 5.06 ± 1.33 | 5.83 ± 2.10 | 4.07 ± 1.03 |
| Small Intestine | 22.82 ± 3.66 | 30.49 ± 10.05 | 21.18 ±10.14 | 3.62 ± 1.28 |
| Large Intestine | 0.31 ± 0.07 | 0.30 ± 0.04 | 1.82 ± 1.81 | 36.77 ± 10.76 |
| Kidneys | 5.95 ± 0.60 | 5.58 ± 0.85 | 5.09 ± 0.50 | 4.87 ± 0.54 |
| Muscle | 0.25 ±0.11 | 0.24 ± 0.10 | 0.19 ± 0.01 | 0.24 ± 0.12 |
| Bone | 0.71 ± 0.15 | 0.59 ± 0.16 | 0.53 ± 0.32 | 0.50 ± 0.18 |
| Skin | 1.12 ± 0.36 | 0.57 ± 0.06 | 0.49 ± 0.11 | 0.47 ± 0.07 |
| Thyroid | 38.44 ± 11.07 | 118.75 ± 15.13 | 116.71 ± 23.20 | 146.44 ± 42.20 |
| Gall Bladder | 590.36 ± 274.09 | 840.72 ± 523.07 | 206.78 ± 104.24 | 164.07 ± 136.89 |
Figure 9.
Biodistribution of [99mTc]TcO-KYCAR(syn) in healthy mice (n=5). The results are reported in %ID/g. Shows that the activity travels through the gastrointestinal track. Also shows some decomposition to potential 99mTcO4− products as seen with thyroid accumulation.
Uptake and retention of [99mTc]TcO-KYCAR is also seen in moderate amounts in the kidneys (5.9 %ID/g at 30 min to 4.9 %ID/g at 4 h) indicating some excretion through the renal system. These findings are contradictory to the hypothesis that relatively high hydrophilicity (log D: −2.631) would favor renal excretion over hepatobiliary excretion; however, it is possible that the large molecular weight of the radiolabeled peptide complex favors this method of excretion. The high uptake and retention observed in the thyroid indicates potential decomposition of the [99mTc]TcO-KYCAR complex. When compared to the thyroid uptake of the previous tripeptide complexes reported by Cantorias et. al.[30], this high uptake supports the instability of the [99mTc]TcO-KYCAR in vivo. Significantly, low uptake and retention of [99mTc]TcO-KYCAR was observed at 4 h in the heart (0.3 %ID/g), lungs (0.9 %ID/g), spleen (0.4 %ID/g) and muscle (0.2 %ID/g). The low level of radioactivity in the blood at 30 min (1.8 %ID/g) indicates favorable and rapid blood clearance (decreases to 0.9 %ID/g at 4 h).
The stability of [188Re]ReO-KYCAR in PBS over time can be found in Supporting Information (Figure S25). This complex shows an immediate 50% decomposition with observation of three peaks in PBS. The first peak which elutes at around 5.0 min corresponds to 188ReO4−, and the second peak that elutes at 6.0 min is possibly a [188Re]ReO-KYCAR fragment or decomposition product. The third peak that elutes at 13.3 min represents the [188Re]ReO-KYCAR syn complex. Though the initial contact with PBS results in a relatively large amount of decomposition, the subsequent HPLC traces show a small increase (ca 10%) of the perrhenate peak. The stability of [188Re]ReO-KYCAR in serum is particularly interesting in that the complex does not appear to be very stable. After contacting the rhenium complex with human serum for 30 min there is only 188ReO4− present in the supernatant. In general, rhenium complexes tend to oxidize more easily than their Tc analogs and this trend appears to be observed in this study.
Despite the in vitro results, we analyzed the behavior of [188Re]ReO-KYCAR in vivo and the data is shown in Table 4 and Figure 10. Similar trends are observed with the [188Re]ReO-KYCAR construct compared to [99mTc]TcO-KYCAR. Its log D value of −2.280 reveals that it is slightly more hydrophobic than the [99mTc]TcO-KYCAR construct. The complex clears mainly through the hepatobiliary pathway. At 30 min, radioactivity in the liver is 17.6 %ID/g, and similar to the technetium-labeled pentapeptide, after 4 h, the radioactivity decreases to 0.9 %ID/g. As with the Tc analog, the gall bladder exhibits high uptake that decreases with time possibly emptying into the intestines. At 30 min, the radioactivity in the small intestine is 33.2 %ID/g, while the radioactivity in the large intestine is low at 0.4 %ID/g. After 4 h, the radioactivity in the small intestine decreases to 0.9 %ID/g, while the radioactivity in the large intestine increases to 31.9 %ID/g. Although there is uptake in the kidneys (3.8 %ID/g at 30 min), this is less than for [99mTc]TcO-KYCAR, consistent with the more positive log D values for the 188Re analog, and confirming that the rhenium construct is even more inclined to be excreted through the hepatobiliary system. Similar to the technetium construct, the radioactivity accumulation in the stomach for [188Re]ReO-KYCAR decreases with time, showing 2.5 %ID/g retained 4 h post injection, as compared to 6.2 %ID/g at 30 min. However, the thyroid is consistently high supporting decomposition to perrhenate that was observed in the in vitro serum stability studies. The uptake value for all other collected organs is <1.0%ID/g after 4 h.
Table 4.
Biodistribution results for [188Re]ReO-KYCAR (syn) in healthy mice (n=5). The results are reported in %ID/g. Shows that the activity travels through the gastrointestinal track. Also shows some decomposition to potential 188ReO4− products as seen with thyroid and stomach accumulation.
| [188ReO]-KYCAR(syn), %ID/g ± S.D. | ||||
|---|---|---|---|---|
| Organ | 0.5 h | 1 h | 2 h | 4 h |
| Blood | 1.29 ± 0.43 | 0.94 ± 0.12 | 0.71 ± 0.18 | 0.38 ± 0.07 |
| Heart | 0.45 ± 0.15 | 0.54 ± 0.28 | 0.34 ± 0.23 | 0.14 ± 0.02 |
| Lungs | 0.97 ± 0.31 | 2.13 ± 1.50 | 0.87 ± 0.59 | 0.29 ± 0.07 |
| Liver | 17.61 ± 4.30 | 9.93 ± 1.53 | 3.07 ± 0.68 | 0.99 ± 0.14 |
| Spleen | 0.65 ±0.06 | 3.07 ± 5.50 | 0.38 ± 0.05 | 0.24 ± 0.03 |
| Stomach | 6.24 ± 2.49 | 7.76 ± 5.12 | 7.32 ± 3.80 | 2.50 ± 0.79 |
| Small Intestine | 33.15 ± 14.41 | 48.77 ± 12.68 | 20.68 ± 22.60 | 0.95 ± 0.76 |
| Large Intestine | 0.38 ± 0.15 | 0.65 ± 0.44 | 4.03 ± 4.47 | 31.99 ± 9.13 |
| Kidneys | 3.81 ±1.26 | 3.26 ± 1.47 | 2.33 ± 0.07 | 1.33 ± 0.22 |
| Muscle | 0.49 ± 0.53 | 0.38 ± 0.08 | 0.35 ± 0.23 | 0.18 ± 0.10 |
| Bone | 0.58 ± 0.05 | 0.55 ± 0.10 | 0.41 ± 0.07 | 0.32 ± 0.05 |
| Skin | 0.74 ± 0.35 | 0.49 ± 0.09 | 0.36 ± 0.09 | 0.24 ± 0.04 |
| Thyroid | 57.21 ± 23.57 | 113.99 ± 67.52 | 118.34 ± 49.75 | 84.39 ± 27.31 |
| Gall Bladder | 1085.49 ± 1084.29 | 826.44 ± 1105.72 | 365.84 ± 287.48 | 168.99 ± 196.65 |
Figure 10.
[188Re]ReO-KYCAR (syn) biodistribution in healthy mice (n=5). The results are reported in %ID/g. Shows that the activity travels through the gastrointestinal track. Also shows some decomposition to potential 188ReO4− products as seen with thyroid and stomach accumulation.
4. Conclusion
We have isolated two diastereomers of ReO-KYCAR and identified the anti and syn diastereomers that elute as peak 1 and peak 2 on reverse phase HPLC, respectively. These complexes have been thoroughly characterized by Infrared Spectroscopy, proton NMR spectroscopy, circular dichroism and by comparison to a crystal structure of a related analog. The proton NMR spectroscopy reveals the coordination environment about the ReVO core, supporting the amide nitrogen coordination of the tyrosine and cysteine residues as well as the deprotonation and coordination of the thiol. The 1H NMR data also show shifts in the ligand residues resulting from a change in the position of the Re-oxo core, further suggesting the disposition of the anti and syn diastereomers as peaks 1 and 2, respectively on RP-HPLC. The identity of the syn diastereomer is supported by crystallography of a ReO-KYC analog as well as by comparison to previous Tc tripeptide complexes. Circular dichroism data compared to previous work on rhenium tripeptides provides compelling information on the structures of the diastereomers of ReO-KYCAR: the first eluted complex, the anti diastereomer, follows the trend of having a large positive Cotton effect about the region that correlates to the oxygen-rhenium charge transfer transitions. Furthermore, the second eluted complex, the syn diastereomer, follows the trend of having a negative Cotton effect about the same region.
We also identified the pKa of the amine nitrogen, which determines the charge of the chelate in solution, through pKa measurements. We found that at biological pH, 7.35 −7.45, the amine nitrogen of the lysine is likely deprotonated and bears a negative charge, while the epsilon nitrogen of the lysine residue is likely protonated and bears a positive charge. As a result, the ReO-KYCAR is expected to be neutral overall. Moreover, when we compare our pKa measurements to previous metal-peptide work we find that having lysine in the chelator region appears to modulate the pKa of the amine protons, thus influencing the charge on the chelator.
Biologically, the 99mTc, tracer level complex is stable under in vitro conditions in PBS and human serum up to 2 h. The 188Re tracer complex appears to undergo 50% dissociation to perrhenate and an unidentified 188Re peptide complex in PBS and decomposition to perrhenate in serum studies. [99mTc]TcO-KYCAR shows high uptake in the liver, gallbladder, small intestines and large intestines up to 4 h post injection. The biodistribution data demonstrates that the complex is excreted via the hepatobiliary system despite the relatively hydrophilic nature of our radiotracer. There is considerable uptake in the thyroid, which would indicate in vivo decomposition products. Further studies, including blocking studies, should be completed to determine whether thyroid uptake is due to the localization of decomposition of the metal complexes or if the thyroid is targeted by the metal peptide. The [188Re]ReO-KYCAR complex shows very similar organ uptake to [99mTc]TcO-KYCAR. One noticeable difference is the increased uptake in the stomach, likely due to decomposition of the 188ReO4− complex, as shown in the in vitro data. It appears the localization of the complexes in the gastrointestinal region, particularly the gallbladder, is facilitated by the KYCAR ligand. 99mTcO4− and 188ReO4− independently evaluated in vivo show high accumulation in the thyroid and quick excretion via the renal track. The gastrointestinal localization could be circumvented through the use of a targeting vector.
Supplementary Material
Acknowledgments:
We thank Dr. Jason Lewis (MSKCC) for reading and commenting on this manuscript as well as providing the resources for work undertaken at MSKCC.
Funding Sources: The authors gratefully acknowledge the MSKCC Small Animal Imaging Core Facility, which was supported in part by NIH grant P30 CA08748. The authors also thank the NSF (NSF-DGE-0965983) and gratefully acknowledge Mr. William H. and Mrs. Alice Goodwin and the Commonwealth Foundation for Cancer Research and The Center for Experimental Therapeutics of Memorial Sloan Kettering Cancer Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hoffman JM and Gambhir SS, Molecular imaging: the vision and opportunity for radiology in the future. Radiology, 2007. 244(1): p. 39. [DOI] [PubMed] [Google Scholar]
- 2.Lee S, Xie J, and Chen X, Peptide-based probes for targeted molecular imaging. Biochemistry, 2010. 49(7): p. 1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Massoud TF and Gambhir SS, Molecular imaging in living subjects: seeing fundamental biological processes in a new light. Genes & development, 2003. 17(5): p. 545. [DOI] [PubMed] [Google Scholar]
- 4.Weissleder R and Mahmood U, Molecular imaging. Radiology, 2001. 219(2): p. 316. [DOI] [PubMed] [Google Scholar]
- 5.Banerjee S, Pillai MR, and Ramamoorthy N, Evolution of Tc-99m in diagnostic radiopharmaceuticals. Seminars in nuclear medicine, 2001. 31(4): p. 260. [DOI] [PubMed] [Google Scholar]
- 6.Jurisson S, et al. , Coordination compounds in nuclear medicine. Chemical reviews, 1993. 93(3): p. 1137. [Google Scholar]
- 7.Maresca KP, et al. , Novel polar single amino acid chelates for technetium-99m tricarbonyl-based radiopharmaceuticals with enhanced renal clearance: application to octreotide. Bioconjugate chemistry, 2010. 21(6): p. 1032. [DOI] [PubMed] [Google Scholar]
- 8.Jeong JM and Chung JK, Therapy with 188Re-labeled radiopharmaceuticals: an overview of promising results from initial clinical trials. Cancer biotherapy & radiopharmaceuticals, 2003. 18(5): p. 707. [DOI] [PubMed] [Google Scholar]
- 9.Knapp FF Jr., et al. , Availability of rhenium-188 from the alumina-based tungsten-188/rhenium-188 generator for preparation of rhenium-188-labeled radiopharmaceuticals for cancer treatment. Anticancer Research, 1997. 17(3B): p. 1783. [PubMed] [Google Scholar]
- 10.Ercan MT and Caglar M, Therapeutic radiopharmaceuticals. Current pharmaceutical design, 2000. 6(11): p. 1085. [DOI] [PubMed] [Google Scholar]
- 11.Vanbilloen HP, et al. , Complexes of technetium-99m with tetrapeptides, a new class of 99mTc-labelled agents. Nuclear medicine and biology, 1995. 22(3): p. 325. [DOI] [PubMed] [Google Scholar]
- 12.Giblin MF, et al. , Design and characterization of alpha-melanotropin peptide analogs cyclized through rhenium and technetium metal coordination. Proceedings of the National Academy of Sciences of the United States of America, 1998. 95(22): p. 12814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pearson DA, et al. , Thrombus Imaging Using Technetium-99m-Labeled High-Potency GPIIb/IIIa Receptor Antagonists. Chemistry and Initial Biological Studies. Journal of Medicinal Chemistry, 1996. 39(7): p. 1372–1382. [DOI] [PubMed] [Google Scholar]
- 14.Pearson DA, et al. , Somatostatin Receptor-Binding Peptides Labeled with Technetium-99m: Chemistry and Initial Biological Studies. Journal of Medicinal Chemistry, 1996. 39(7): p. 1361–1371. [DOI] [PubMed] [Google Scholar]
- 15.Cyr JE, et al. , Somatostatin Receptor-Binding Peptides Suitable for Tumor Radiotherapy with Re-188 or Re-186. Chemistry and Initial Biological Studies. Journal of Medicinal Chemistry, 2007. 50(6): p. 1354–1364. [DOI] [PubMed] [Google Scholar]
- 16.Cyr JE, et al. , Isolation, Characterization, and Biological Evaluation of Syn and Anti Diastereomers of 99mTc Technetium Depreotide: a Somatostatin Receptor Binding Tumor Imaging Agent. Journal of Medicinal Chemistry, 2007. 50: p. 4295–4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Francesconi LC, et al. , Preparation and Characterization of [99TcO] Apcitide: A Technetium Labeled Peptide. Inorganic Chemistry, 2004. 43(9): p. 2867–2875. [DOI] [PubMed] [Google Scholar]
- 18.Wong E, et al. , Tuftsin Receptor-Binding Peptide Labeled with Technetium: Chemistry and Preliminary in Vitro Receptor-Binding Study. Inorganic Chemistry, 2001. 40(22): p. 5695–5700. [DOI] [PubMed] [Google Scholar]
- 19.Chavatte K, et al. , Rhenium (Re) and technetium (Tc)-99M oxocomplexes of neurotensin(8–13). Journal of Labelled Compounds and Radiopharmaceuticals, 1999. 42(5): p. 415–421. [Google Scholar]
- 20.Guo H, Gallazzi F, and Miao Y, Design and Evaluation of New Tc-99m-Labeled Lactam Bridge-Cyclized Alpha-MSH Peptides for Melanoma Imaging. Molecular pharmaceutics, 2013. 10(4): p. 1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu S, Bifunctional coupling agents for radiolabeling of biomolecules and target-specific delivery of metallic radionuclides. Advanced Drug Delivery Reviews, 2008. 60(12): p. 1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blok D, et al. , Peptide radiopharmaceuticals in nuclear medicine. European journal of nuclear medicine, 1999. 26(11): p. 1511. [DOI] [PubMed] [Google Scholar]
- 23.Okarvi SM, Peptide-based radiopharmaceuticals: future tools for diagnostic imaging of cancers and other diseases. Medicinal research reviews, 2004. 24(3): p. 357. [DOI] [PubMed] [Google Scholar]
- 24.Wadas TJ, et al. , Coordinating radiometals of copper, gallium, indium, yttrium, and zirconium for PET and SPECT imaging of disease. Chemical reviews, 2010. 110(5): p. 2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sato S, et al. , Structure of a novel nitrido technetium complex with the peptide chelate ligand KYCAR. Journal of Radioanalytical and Nuclear Chemistry, 2003. 255(2): p. 315–317. [Google Scholar]
- 26.Takayama T, Keisuke S, and Sekine T und Hiroshi Kudo, Syntheses and structures of technetium(V) and rhenium(V) oxo complexes of peptide having KYC-sequence. Vol. 88 2000. 247–251. [Google Scholar]
- 27.Bormans G, et al. , Synthesis and biological characteristics of the four stereoisomers of 99mTc-N,N’-bis-(mercaptoacetyl)-2,3-diaminopropanoate. International journal of radiation applications and instrumentation.Part B, Nuclear medicine and biology, 1990. 17(5): p. 499. [DOI] [PubMed] [Google Scholar]
- 28.Delmon-Moingeon LI, et al. , Strategies for labeling monoclonal antibodies and antibody-like molecules with technetium-99m. Journal of nuclear biology and medicine (Turin, Italy : 1991), 1991. 35(1): p. 47. [PubMed] [Google Scholar]
- 29.Bormans G, et al. , Synthesis and labelling characteristics of 99mTc-mercaptoacetyltripeptides. Journal of Labelled Compounds and Radiopharmaceuticals, 1993. 33(11): p. 1065. [Google Scholar]
- 30.Cantorias MV, et al. , MO Tripeptide Diastereomers (M) 99/99mTc, Re): Models To Identify the Structure of 99mTc Peptide Targeted Radiopharmaceuticals. Inorganic Chemistry, 2007. 46: p. 7326–7340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rose DJ, et al. , Synthesis and Characterization of Rhenium Thiolate Complexes. Crystal and Molecular Structures of NBu4 ReO(H2O)Br4) ·2H2O, Bu4N ReOBr4(OPPh3), ReO(SC5H4N)3, ReO(SC4H3N2)3 ReO(OH)(SC5H4N-3,6-(SiMe2But)2)2, Re(N2COC6H5)(SC5H4N)Cl(PPh3)2, and Re(PPh3)(SC4H3N2)3. Inorganic chemistry, 1996. 35(12): p. 3548. [Google Scholar]
- 32.Delgado R, et al. , Dissociation constants of Br∅nsted acids in D2O and H2O: studies on polyaza and polyoxa-polyaza macrocycles and a general correlation. Analytica Chimica Acta, 1991. 245: p. 271–282. [Google Scholar]
- 33.Sheldrick GM, SHELXTL, An Integrated System for Solving, Refining, and Displaying Crystal Structures from Diffraction Data. University of Göttingen, Göttingen, Federal Republic of Germany, 1981. [Google Scholar]
- 34.Sheldrick, G. M, Crystal structure refinement with SHELXL. Vol. 71 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Addison AW, et al. , Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen-sulphur donor ligands; the crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-2[prime or minute]-yl)-2,6-dithiaheptane]copper(II) perchlorate. Journal of the Chemical Society, Dalton Transactions, 1984(7): p. 1349–1356. [Google Scholar]
- 36.Mahood A, et al. , Synthesis and Characterization of N-ethyl-diaminedithiol oxotechnetate(V): A Potential Lung Imaging Agent, in Technetium anf Rhenium in Chemistry and Nuclear Medicine. 1990, Cortina-Raven Press: New York: p. 113. [Google Scholar]
- 37.Tisato F, Refosco F, and Bandoli G, Structural survey of technetium complexes. Coordination Chemistry Reviews, 1994. 135: p. 325. [Google Scholar]
- 38.Wong E, et al. , Rhenium(V) and Technetium(V) Oxo Complexes of an N(2)N’S Peptidic Chelator: Evidence of Interconversion between the Syn and Anti Conformations. Inorganic chemistry, 1997. 36(25): p. 5799. [DOI] [PubMed] [Google Scholar]
- 39.Bereau VM, Khan SI, and Abu-Omar M, Synthesis of Enantiopure Oxorhenium(V) and Arylimidorhenium(V) “3 + 2” Schiff Base Complexes. X-ray Diffraction, Cyclic Voltammetry, UV-Vis, and Circular Dichroism Characterizations. Inorganic chemistry, 2001. 40(26): p. 6767. [DOI] [PubMed] [Google Scholar]
- 40.Cheng Y, Yan YB, and Liu J, Spectroscopic characterization of metal bound phytochelatin analogue (Glu-Cys)4-Gly. Journal of inorganic biochemistry, 2005. 99(10): p. 1952. [DOI] [PubMed] [Google Scholar]
- 41.Lipowska M, et al. , New N3S Donor Ligand Small Peptide Analogues of the N-Mercaptoacetyl glycylglycylglycine Ligand in the Clinically Used Tc-99m Renal Imaging Agent: Evidence for Unusual Amide Oxygen Coordination by Two New Ligands. Inorganic Chemistry, 2002. 41(11): p. 3032–3041. [DOI] [PubMed] [Google Scholar]
- 42.Lipowska M, et al. , Synthesis and Characterization of Rhenium(V) Oxo Complexes with a New Thiol−Amide−Thiourea Ligand System. X-ray Crystal Structure of [1-Phenyl-3-[2-((2-thioacetyl)amino)ethyl]thioureato]oxorhenium(V). Inorganic Chemistry, 1996. 35(15): p. 4484–4489. [DOI] [PubMed] [Google Scholar]
- 43.Lipowska M, et al. , Synthesis of new N2S2 ligands and Re(V)O(N2S2) analogues of 99mTc renal imaging agents. Characterization by NMR spectroscopy, molecular mechanics calculations, and X-ray crystallography. Inorganica Chimica Acta, 2002. 339(Complete): p. 327–340. [Google Scholar]
- 44.Canney DJ, et al. , Dicarboxylate diamide dimercaptide (N2S2) technetium-99m complexes: synthesis and biological evaluation as potential renal radiopharmaceuticals. Journal of Medicinal Chemistry, 1993. 36(8): p. 1032–1040. [DOI] [PubMed] [Google Scholar]
- 45.O’Neil JP, Wilson SR, and Katzenellenbogen JA, Preparation and structural characterization of monoamine-monoamide bis(thiol) oxo complexes of technetium(V) and rhenium(V). Inorganic Chemistry, 1994. 33(2): p. 319–323. [Google Scholar]
- 46.Rao TN, et al. , Technetium (V) and rhenium (V) complexes of 2,3-bis(mercaptoacetamido)propanoate. Chelate ring stereochemistry and influence on chemical and biological properties. Journal of the American Chemical Society, 1990. 112(15): p. 5798–5804. [Google Scholar]
- 47.Popov K, Rönkkömäki H, and Lajunen Lauri HJ, Guidelines for NMR measurements for determination of high and low pKa values (IUPAC Technical Report), in Pure and Applied Chemistry. 2006. p. 663. [Google Scholar]
- 48.Hansen L, et al. , Factors Influencing the pKa of Ligated Amines and the Syn/Anti Isomerization in Cysteine-Based Re(V)O(N2S2) Radiopharmaceutical Analogues As Revealed by a Novel Dominant Tautomer in the Solid State. Inorganic Chemistry, 1999. 38(23): p. 5351–5358. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.