Abstract
Aims:
We assessed whether fasting glucose (FG) and insulin resistance (IR) trajectories during young adulthood are associated with changes in cardiac structure and function.
Methods:
We used data from the Coronary Artery Risk Development in Young Adults (CARDIA) Study (baseline: 1985-1986). Echocardiography was performed after 25 (Y25) and 30 years of follow-up. Group-based modeling was used to determine 25-year trajectories in FG and IR. We assessed differences at Y25 and 5-year longitudinal change in cardiac structure and function after adjustment for demographics, cumulative exposure to traditional cardiovascular risk factors, and baseline FG or IR.
Results:
We identified five FG trajectory groups among 2414 individuals and three IR trajectory groups among 2358 individuals. Moderate-increasing FG trajectory was associated with lower lateral E’ velocity (difference: −0.9 cm/sec, 95%CI: −0.3, −1.5) and with greater left ventricular (LV) mass index (difference: 2.7 g/m2.7, 95%CI: 0.7, 4.7) at Y30 compared to low-stable FG. High-increasing IR trajectory was associated with lower lateral E’ velocity and septal E’ velocity at Y30 compared to low-decreasing IR trajectory.
Conclusions:
Trajectories in FG and IR over 25 years before the development of diabetes are associated with unfavorable differences in LV structure and diastolic function beyond single values of FG and IR.
Keywords: epidemiology, fasting glucose, insulin resistance, trajectories, cardiac structure and function
1. Introduction
Individuals with diabetes in adulthood are at greater risk for heart failure compared to those without diabetes.1 Hyperglycemia, hyperinsulinemia, and insulin resistance (IR) are modifiable risk factors for incident heart failure.2–5 The majority of prior studies have used single measures of fasting glucose (FG) and IR that do not characterize the natural progression in these parameters over time, which may provide additional information for future cardiac risk. The few available prospective studies in this area have demonstrated that a longer duration of diabetes and higher levels of FG in young adulthood are associated with left ventricular (LV) remodeling and function in middle age.6–8 In the absence of diabetes, high IR trajectory paired with obesity during young adulthood is associated with differences in cardiac structure and function later in middle age.6 While IR and hyperglycemia are correlated, the occurrence of either metabolic marker is not contingent upon the other and may confer differential associations with cardiovascular outcomes.9 It remains unknown whether FG and IR trajectories during young adulthood are associated with subsequent differential change in cardiac structure and function during middle age. If adverse FG and IR trajectories are associated with early changes in cardiac structure and function also associated with future heart failure, these data will support efforts to prevent and manage obesity as well as other sources of dysglycemia and insulin resistance, particularly in high-risk populations.
Our objective was to assess whether trajectories of FG and IR below the threshold of diabetes during young adulthood are associated with changes to cardiac structure and function by middle age using data from the Coronary Artery Risk Development in Young Adults (CARDIA) study. We hypothesized that high and increasing patterns of FG and IR over 25 years would be associated with adverse changes in cardiac structure and function.
2. Methods
2.1. Study design and participants
CARDIA is a prospective observational cohort designed to investigate the development of coronary artery disease risk factors. A thorough description of the CARDIA study design and methods has been published previously.10 In brief, 5115 black and white men and women who were 18-30 years of age in 1985-1986 were recruited from four US metropolitan communities: Birmingham, AL; Chicago, IL; Minneapolis, MN; and Oakland, CA. CARDIA participants have undergone in-person examinations at baseline (Year 0: Y0) and at Y2, Y5, Y7, Y10, Y15, Y20, Y25, and Y30. Retention rates among surviving participants at each in-person examination were 91%, 86%, 81%, 79%, 74%, 72%, 72%, and 71%, respectively. Contact is maintained with participants via telephone, mail, or email every 6 months, with annual interim medical history ascertainment. Over the last 5 years, >90% of the surviving cohort members have been directly contacted, and follow up for vital status is virtually complete through related contacts and intermittent National Death Index searches.
Participants were asked to fast for 12 hours and to forgo strenuous exercise or smoking for 2 hours prior to the examination. Detailed demographic, behavioral and lifestyle, medical history and medication use, and clinical data have been collected on participants at each examination using structured questionnaires and standard laboratory procedures.10 Blood was drawn by venipuncture and serum separation was performed before aliquots were stored at −70°C and shipped on dry ice to central laboratories. Seated blood pressure (BP) was measured in triplicate after 5 minutes of rest using a random-zero sphygmomanometer at baseline and with an automated oscillometric BP monitor (Omron HEM-907XL; Online Fitness, Santa Monica, CA) at examination years 25 and 30, and BP values were standardized across exams to the sphygmomanometric measures.11 The average of the last two BP measurements was used for this analysis. Body weight was measured with a calibrated balance beam scale with participants in light clothing and without shoes, and height was measured with a vertical ruler. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Self-reported physical activity was assessed as frequency of participation over the previous 12 months for 8 vigorous-intensity and 5 moderate-intensity sports-related activities, with high validity and reliability.12 Educational attainment was categorized as any up to high school graduate, any college, or more than 4 years of college. Smoking status was determined as never, former, or current smoker. Regular alcohol intake was categorized according to no regular, moderate consumption, or heavy consumption based on quantity and type of alcohol consumed, in ml/day13
2.2. Fasting glucose (FG) and insulin resistance (IR)
Glucose and insulin were measured in fasting non-pregnant individuals at baseline and 7, 10, 15, 20, 25, and 30 years after baseline. Serum glucose was measured at baseline using the hexokinase ultraviolet method by American Bio-Science Laboratories (VanNuys,CA) and at subsequent examinations using hexokinase coupled to glucose-6-phosphate dehydrogenase by Linco Research (St. Louis, MO). Serum insulin was determined by radioimmunoassay (Linco Research, St. Charles, Missouri) prior to Y25 and by an Elecsys sandwich immunoassay (Roche Diagnostics, Rotkreuz, Switzerland) at Y25 and Y30. CARDIA investigators performed a recalibration study in order to harmonize glucose and insulin values across CARDIA exams.14 Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated by dividing the product of fasting insulin (microunits per milliliter) and FG (millimoles per liter) by 22.5.15 The natural logarithmic transformation of HOMA-IR was used for these analyses to normalize the distribution.
2.3. Echocardiography
The scanning protocol for Y30 was standardized across all field centers and consistent with the protocol designed to acquire echocardiographic views at CARDIA years 5 and 25 and the current recommendations from the American Society of Echocardiography.16–18 Experienced sonographers performed echocardiography after receiving training in the procedure prior to each examination cycle and underwent continual monitoring for image quality and protocol adherence during the entire period of participant examinations. Sonographers used Artida cardiac ultrasound machines (Toshiba Medical Systems, Otawara, Japan) to acquire echocardiographic images which were digitized and read at the Johns Hopkins University reading center.18 We assessed several measures of cardiac structure and function. Left ventricular (LV) dimensions were measured with two-dimensional (2D) guided M-mode echocardiography from the optimized parasternal short-axis view, acquiring LV internal diameter in diastole, interventricular septal and posterior wall thicknesses in diastole. The Devereux formula was used to calculate LV mass.19 We calculated stroke volume as the difference between LV end-systolic volume and LV end-diastolic volume. Ejection fraction was calculated as a percentage: the ratio of stroke volume to diastolic volume multiplied by 100. We calculated relative wall thickness (RWT) by dividing the sum of the posterior wall and interventricular septal thickness by the internal LV diameter. Pulsed-wave Doppler was used to measure early and late mitral annular diastolic filling velocities. Peak early (E) and late (A) diastolic filling velocity and peak early diastolic mitral annular velocity (MV) (e’) were measured and used to calculate LV filling pressures, and E/e’ ratio.
2.4. Statistical Analysis
In order to examine trajectories in FG and HOMA-IR below the threshold of diabetes we excluded individuals who developed diabetes (FG >7.0 mmol/L, post-challenge [75gram] glucose >11.1 mmol/L, hemoglobin A1c >6.5%, or use of diabetes medications at any examination) by examination Y25. Using data from baseline and examination years 7, 10, 15, 20, and 25, we modeled trajectories among 3577 participants with FG measured at 3 or more examinations and among 3418 participants with both FG and insulin measured at 3 or more examinations (for HOMA-IR). Trajectory groups were determined using latent class analysis, fitting models using SAS® Proc Traj to identify distinct sub-groups of CARDIA participants sharing an underlying trajectory for FG and HOMA-IR, separately, with age at examination used for the time scale.20–22 This modeling approach assumes the sample population is comprised of multiple trajectory groups and simultaneously estimates each individual’s probability of membership in each trajectory group. We used Bayesian Information Criterion (BIC) to assess model fit and examined models with different number of trajectory groups, all in quadratic form, comparing the BIC when 5, 4, 3, 2, and 1 group were specified. We selected a final model for FG with five trajectory groups and for HOMA-IR with three trajectory groups, all quadratic order, based on BIC, qualitative assessment, and prior findings.6,22–24 Individuals were assigned trajectory group membership for which they had the greatest posterior predicted probability. To account for uncertainty in group assignment we generated 10 multiple imputations of trajectory membership using the posterior probabilities.25 Of the individuals used to determine trajectories, 2600 assigned a FG trajectory and 2535 a HOMA-IR trajectory were present at examination Y25 and Y30. Of these sub-samples, we included individuals for analysis who had complete data for each respective echocardiographic measure.
We calculated cumulative exposure to smoking, alcohol intake, BMI, physical activity, SBP, and BP-lowering medications using data from each exam, as the product of the variable level and duration between exams. Less than 5% of each exam sample was missing data for any one covariate. We used fully conditional specification multiple imputation methods to generate 10 data sets of imputed values for missing covariates.26,27 We fit a linear regression model for each of the 10 data sets by regressing each echocardiographic measure on FG and HOMA-IR trajectory group membership, separately, and combining results across the multiple imputations. For FG and HOMA-IR each, the lowest trajectory group was considered the reference group comparison for all other trajectory groups. Model adjustments included baseline age, sex, race, field center, educational attainment, and cumulative measures for number of years as a current smoker, milliliters of daily alcohol intake, BMI, physical activity, SBP, years of BP-lowering medication use, and heart rate at the time of imaging. We also included adjustment for baseline data on FG or HOMA-IR, to determine whether trajectory group membership was informative beyond a single measurement. We assessed the sensitivity of our estimates after including adjustment for BMI measured at the time of echocardiography, as concurrent measures of adiposity may be more strongly associated with LV mass, EDV, and LA dimensions.28 We assessed for effect modification of each association by sex and race, respectively. A two-sided alpha=0.05 was considered statistically significant and SAS statistical software version 9.4 (SAS Institute Inc., Cary, NC) was used for statistical analysis.
3. Results
3.1. FG and HOMA-IR trajectory groups
The five FG trajectory groups are illustrated in Figure 1A. These groups included low FG throughout follow-up (termed the low-stable group), moderate FG levels throughout follow-up (moderate-stable), moderate initial FG levels with a steady increase in FG (moderate-increase), high initial FG and remained steady (high-stable), and high initial FG with an increase in FG (high-increase). The three HOMA-IR trajectory groups are illustrated in Figure1B and included low HOMA-IR level over follow-up (low-decreasing), moderate HOMA-IR levels throughout follow-up (moderate-stable), and high initial HOMA-IR levels and increased over follow-up (high-increasing). The largest overlap in trajectory groups was between the moderate-stable FG trajectory group with low-decreasing (19.5% of sample) and moderate-stable (18.8% of sample) HOMA-IR trajectory groups (Supplemental Figure 1).
Figure 1.
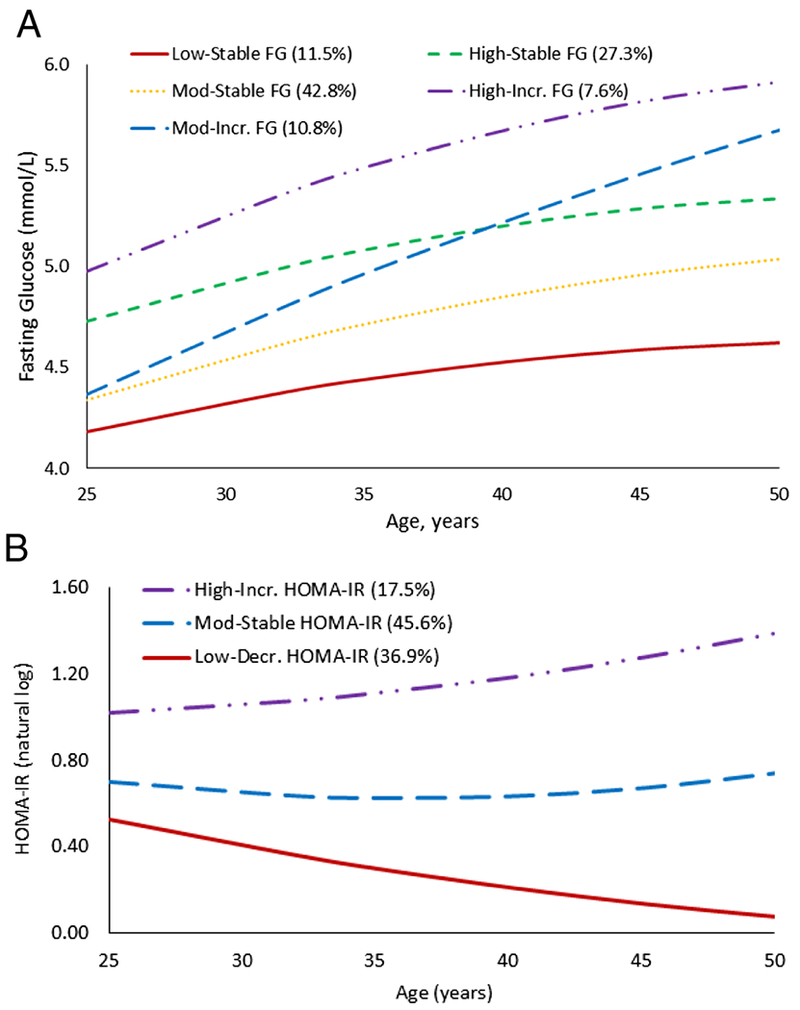
Trajectory group membership for (A) fasting glucose and (B) homeostasis model assessment of insulin resistance (natural logarithm).
We present descriptive statistics according to FG (n=2414) and HOMA-IR (n=2358) trajectories. Greater baseline levels for BMI, SBP, DBP, insulin, HOMA-IR, physical activity, male sex, and white race were associated with increasing FG trajectory (Table 1). These associations were similar across increasing HOMA-IR trajectory, with the exception of physical activity, which was lower with increasing HOMA-IR trajectory (Supplemental Table 1). For both FG and HOMA-IR, age was inversely associated with trajectory group and incidence of diabetes from Y25 to Y30 was greatest for the high-increasing trajectory groups.
Table 1.
Participant characteristics according to group membership of 25-year trajectory of fasting glucose.
| Fasting Glucose (FG) trajectory group | |||||
|---|---|---|---|---|---|
| Characteristic | Low-Stable | Moderate-Stable | Moderate-Increasing | High-Stable | High-Increasing |
| N (% total sample) | 290 (12%) | 1058 (44%) | 247 (10%) | 645 (27%) | 174 (7%) |
| Women, n (%) | 235 (83%) | 721 (68%) | 106 (44%) | 270 (42%) | 41 (24%) |
| Black, n (%) | 143 (50%) | 432 (40%) | 113 (46%) | 265 (41%) | 74 (43%) |
| Age at year 0 (Y 0), years | 25.5 ± 3.4 | 25.4 ± 3.5 | 25.7 ± 3.4 | 24.6 ± 3.6 | 23.8 ± 3.8 |
| Education attained by Y25 >4 years of college, n (%) | 85 (30%) | 325 (30%) | 57 (23%) | 160 (25%) | 33 (19%) |
| Current smoker at Y0, n (%) | 70 (24%) | 230 (22%) | 63 (25%) | 163 (25%) | 47 (27%) |
| Current smoker at Y25, n (%) | 47 (16%) | 143 (13%) | 33 (13%) | 98 (15%) | 33 (19%) |
| Heavy weekly alcohol consumption at Y0, n (%)* | 38 (13%) | 132 (12%) | 36 (15%) | 86 (13%) | 20 (12%) |
| Heavy weekly alcohol consumption at Y25, n (%)* | 41 (14%) | 170 (16%) | 34 (14%) | 92 (14%) | 27 (16%) |
| Physical activity at Y 0, exercise units | 381 ± 261 | 419 ± 296 | 463 ± 304 | 437 ± 294 | 502 ± 350 |
| Physical activity at Y25, exercise units | 329 ± 264 | 346 ± 274 | 347 ± 282 | 363 ± 280 | 384 ± 294 |
| Body mass index at Y 0, kg/m2 | 22.7 ± 4.2 | 23.5 ± 4.2 | 24.3 ± 4.4 | 24.4 ± 4.3 | 25.5 ± 4.4 |
| Body mass index at Y25, kg/m2 | 26.6 ± 6.0 | 28.6 ± 6.4 | 30.7 ± 6.4 | 30.0 ± 6.5 | 31.6 ± 5.9 |
| Systolic blood pressure at Y 0, mm Hg | 106 ± 10 | 108 ± 10 | 113 ± 11 | 111 ± 10 | 115 ± 9 |
| Systolic blood pressure at Y25, mm Hg | 114 ± 15 | 116 ± 15 | 121 ± 15 | 118 ± 14 | 123 ± 16 |
| Diastolic blood pressure at Y0, mm Hg | 67 ± 9 | 68 ± 9 | 69 ± 10 | 69 ± 9 | 69 ± 10 |
| Diastolic blood pressure at Y25, mm Hg | 70 ± 11 | 72 ± 11 | 76 ± 11 | 74 ± 11 | 77 ± 11 |
| Blood pressure-lowering medication use at Y0, n (%) | 1 (0%) | 16 (2%) | 5 (2%) | 8 (1%) | 2 (1%) |
| Blood pressure-lowering medication use at Y25, n (%) | 42 (15%) | 204 (19%) | 74 (30%) | 138 (21%) | 46 (27%) |
| Fasting glucose at Y0, mmol/L | 4.23 ± 0.37 | 4.38 ± 0.35 | 4.53 ± 0.41 | 4.73 ± 0.37 | 89.8 ± 0.43 |
| Fasting glucose at Y25, mmol/L | 4.6 ± 0.35 | 5.00 ± 0.36 | 5.74 ± 0.43 | 5.28 ± 0.36 | 105.6 ± 0.43 |
| Fasting insulin at Y0, uU/mL | 9.9 ± 4.7 | 10.4 ± 4.4 | 10.8 ± 4.0 | 11.3 ± 4.5 | 11.8 ± 4.4 |
| Fasting insulin at Y25, uU/mL | 6.7 ± 4.9 | 9.0 ± 6.4 | 13.4 ± 8.3 | 10.6 ± 7.1 | 13.4 ± 7.8 |
| Natural logarithmic HOMA-IR at Y0 | 0.53 ± 0.39 | 0.63 ± 0.36 | 0.69 ± 0.35 | 0.79 ± 0.37 | 0.89 ± 0.34 |
| Natural logarithmic HOMA-IR at Y25 | 0.10 ± 0.66 | 0.48 ± 0.67 | 1.05 ± 0.63 | 0.72 ± 0.64 | 1.09 ± 0.58 |
| Develop diabetes between Y25 and Y30, n (%) | 0 (0%) | 5 (0.5%) | 14 (5.7%) | 11 (1.7%) | 16 (9.2%) |
Mean ± standard deviation for continuous characteristics and n (column percentage) for categorical characteristics
Heavy weekly alcohol consumption: greater than 1 drink daily for women and greater than 2 drinks daily for men
3.2. FG and HOMA-IR trajectories and cross-sectional echocardiographic measures
We observed differences in Y25 and Y30 adjusted means for multiple measures of LV structure and diastolic function when comparing the increasing FG and HOMA-IR trajectory groups to the reference trajectory group (FG: low-stable and HOMA-IR: low-decreasing) (Table 2). The moderate-increasing FG trajectory was associated with lower lateral E’ velocity at Y25 (difference: −0.9 cm/sec, 95% CI: −0.3, −1.5) and Y30 (difference: −0.9 cm/sec, 95% CI: −0.3, −1.5) and also associated with greater LV mass index (difference: 2.7 g/m2.7, 95% CI: 0.7, 4.7) at Y30 compared to low-stable FG after adjustment for CVD risk factors and baseline FG. High-increasing HOMA-IR trajectory was associated with greater RWT and lower lateral E’ velocity and septal E’ velocity at Y25 compared to low-decreasing HOMA-IR trajectory after adjustment for CVD risk factors and baseline HOMA-IR. These associations remained for lateral E’ velocity and septal E’ velocity at Y30 and we observed a linear trend across increasing trajectory group in change for each of these echocardiographic parameters.
Table 2.
Multivariable adjusted means (95% confidence interval) for echocardiographic parameters according to fasting glucose trajectory group membership
| Fasting Glucose (FG) trajectory group | |||||
|---|---|---|---|---|---|
| Low-Stable | Moderate-Stable | Moderate-Increasing | High-Stable | High-Increasing | |
| Left Ventricular Structure | |||||
| Left ventricular mass index, g/m2.7 | |||||
| Year 25 | 31.8 (30.7, 32.9) | 32.5 (32.0, 33.1) | 33.3 (31.9, 34.7) | 32.7 (32.0, 33.4) | 32.8 (31.1, 34.6) |
| Year 30 | 36.8 (35.5, 38.0) | 38.0 (37.3, 38.7) | 39.5 (38.0, 40.9)* | 38.8 (37.9, 39.7)* | 38.2 (36.5, 39.9) |
| 5-year change | 4.8 (3.6, 6.0) | 5.5 (4.9, 6.1) | 6.4 (5.1, 7.6) | 6.2 (5.5, 6.9) | 5.5 (3.7, 7.2) |
| Relative Wall Thickness | |||||
| Year 25 | 0.32 (0.31, 0.32) | 0.31 (0.31, 0.32) | 0.31 (0.30, 0.32) | 0.31 (0.30, 0.32) | 0.32 (0.30, 0.33) |
| Year 30 | 0.39 (0.38, 0.40) | 0.39 (0.38, 0.40) | 0.39 (0.38, 0.40) | 0.39 (0.38, 0.40) | 0.38 (0.36, 0.40) |
| 5-year change | 0.08 (0.06, 0.09) | 0.08 (0.07, 0.08) | 0.08 (0.07, 0.09) | 0.08 (0.07, 0.08) | 0.07 (0.05, 0.08) |
| Left Ventricular Function | |||||
| Ejection fraction, % | |||||
| Year 25 | 62.2 (61.2, 63.3) | 61.9 (61.3, 62.4) | 62.33 (61.0, 63.6) | 61.7 (61.0, 62.5) | 61.2 (59.8, 62.6) |
| Year 30 | 59.9 (59.1, 60.8) | 60.1 (59.7, 60.6) | 59.3 (58.3, 60.3) | 59.9 (59.2, 60.6) | 59.6 (58.5, 60.8) |
| 5-year change | −2.0 (−2.8, −1.2) | −1.7 (−2.1, −1.3) | −2.7 (−3.7, −1.7) | −1.9 (−2.6, −1.3) | −2.1 (−3.2, −1.0) |
| E/e’ ratio | |||||
| Year 25 | 6.90 (6.60, 7.19) | 6.98 (6.83, 7.13) | 7.09 (6.69, 7.49) | 7.07 (6.85, 7.30) | 7.06 (6.63, 7.49) |
| Year 30 | 6.84 (6.57, 7.11) | 7.08 (6.94, 7.23) | 7.18 (6.79, 7.58) | 7.17 (6.98, 7.37) | 7.18 (6.71, 7.65) |
| 5-year change | −0.06 (−0.30, 0.18) | 0.14 (0.01, 0.28) | 0.19 (−0.11, 0.49) | 0.19 (0.02, 0.36) | 0.21 (−0.22, 0.63) |
| Lateral E’ velocity, cm/sec | |||||
| Year 25 | 12.4 (11.9, 12.8) | 12.0 (11.8, 12.2) | 11.5 (11.1, 11.9)*† | 11.8 (11.5, 12.1) | 11.8 (11.2, 12.3) |
| Year 30 | 12.0 (11.5, 12.4) | 11.5 (11.3, 11.7) | 11.1 (10.6, 11.6)* | 11.4 (11.1, 11.7)* | 11.4 (10.9, 12.0) |
| 5-year change | −0.2 (−0.6, 0.1) | −0.5 (−0.7, −0.3) | −0.7 (−1.0, −0.3) | −0.5 (−0.7, −0.3) | −0.5 (−0.9, 0.01) |
| Septal E’ velocity, cm/sec | |||||
| Year 25 | 9.6 (9.2, 10.0) | 9.4 (9.3, 9.6) | 9.3 (9.0, 9.7) | 9.4 (9.1, 9.6) | 9.2 (8.8, 9.7) |
| Year 30 | 9.1 (8.7, 9.4) | 9.0 (8.8, 9.2) | 8.8 (8.4, 9.2) | 9.1 (8.9, 9.3) | 8.9 (8.5, 9.4) |
| 5-year change | −0.5 (−0.8, −0.2) | −0.5 (−0.7, −0.4) | −0.7 (−1.1, −0.3) | −0.4 (−0.6, −0.2) | −0.5 (−1.0, −0.1) |
| Mitral valve Peak A velocity, cm/sec | |||||
| Year 25 | 61.2 (59.2, 63.1) | 62.5 (61.5, 63.5) | 63.0 (60.7, 65.3) | 62.2 (60.7, 63.7) | 61.9 (59.2, 64.6) |
| Year 30 | 65.1 (62.9, 67.4) | 68.2 (67.1, 69.3)* | 68.3 (66.1, 70.5) | 68.7 (67.2, 70.2)* | 68.2 (65.0, 71.4) |
| 5-year change | 3.7 (1.6, 5.7) | 5.9 (5.0, 6.9) | 5.7 (3.8, 7.6) | 6.6 (5.4, 7.9)*† | 6.3 (3.7, 8.9) |
Model adjustments included age, sex, race, field center, educational attainment, and cumulative measures for each of the following: number of years as a current smoker, milliliters of daily alcohol intake, body mass index, physical activity, systolic blood pressure, years of blood pressure-lowering medication use, heart rate, and baseline fasting glucose (and 5-year change models adjusted for year 25 echocardiographic measurement)
P<0.05 compared to Low-Stable fasting glucose trajectory group
P<0.05 compared to Low-Stable fasting glucose trajectory group after adjusted for the covariates in the model above and body mass index at time of echocardiography
3.3. FG and HOMA-IR trajectories with 5-year change in echocardiographic measures
After adjustment for baseline demographic factors, we observed each increasing trajectory in FG was associated with greater 5-year change in stroke volume and MV peak A velocity compared to the low-stable FG trajectory. After further adjustment for cumulative risk factors and baseline FG level, only high-stable FG trajectory remained associated with 5-year change in MV peak A velocity compared to low-stable FG trajectory (Table 2).
Moderate-stable and high-increasing HOMA-IR trajectories were associated (p<0.05) with 5-year change in LV mass index, RWT, E/e’, septal E’ velocity, and MV peak A velocity compared to the low-decreasing HOMA-IR trajectory after adjustment for demographics. After adjustment for cumulative exposure to cardiovascular risk factors and baseline HOMA-IR level, high-increasing HOMA-IR remained associated with 5-year change in RWT (Table 3). We observed a linear trend across increasing trajectory group in change for RWT. In sensitivity analyses for both the FG and HOMA-IR trajectories, none of the 5-year change associations persisted after adjustment for BMI at the time of echocardiography. For all analyses, we observed stronger associations when using non-imputed data (data not shown). We did not find evidence for effect modification by sex or race for any association (all p>0.15). However, because we did observe differences in trajectory group membership by race and sex, we present results stratified by sex and race in the supplement (Supplementary Tables 1-9).
Table 3.
Multivariable adjusted means (95% confidence interval) for echocardiographic parameters according to homeostasis model assessment of insulin resistance trajectory group membership
| Homeostasis model assessment of insulin resistance (HOMA-IR) trajectory group | ||||
|---|---|---|---|---|
| Low-Decreasing | Moderate-Stable | High-Increasing | P-trend‡ | |
| Left Ventricular Structure | ||||
| Left ventricular mass, g/m2.7 | ||||
| Year 25 | 32.4 (31.8, 33.0) | 32.6 (32.1, 33.1) | 32.9 (31.8, 34.0) | 0.43 |
| Year 30 | 38.4 (37.7, 39.2) | 38.2 (37.5, 39.0) | 38.5 (37.1, 39.9) | 0.96 |
| 5-year change | 5.5 (4.9, 6.1) | 5.7 (5.2, 6.3) | 6.2 (5.2, 7.2) | 0.27 |
| Relative Wall Thickness | ||||
| Year 25 | 0.31 (0.30, 0.31) | 0.31 (0.31, 0.32) | 0.32 (0.31, 0.33)*† | <0.005 |
| Year 30 | 0.38 (0.38, 0.39) | 0.39 (0.38, 0.39) | 0.39 (0.38, 0.40) | 0.04 |
| 5-year change | 0.07 (0.07, 0.08) | 0.08 (0.08, 0.08) | 0.08 (0.08, 0.09)* | 0.03 |
| Left Ventricular Function | ||||
| Ejection fraction, % | ||||
| Year 25 | 62.0 (61.4, 62.5) | 62.0 (61.5, 62.5) | 61.0 (60.0, 62.0) | 0.29 |
| Year 30 | 60.0 (59.5, 60.5) | 59.9 (59.5, 60.3) | 59.7 (58.8, 60.5) | 0.54 |
| 5-year change | −1.9 (−2.4, −1.5) | −1.9 (−2.3, −1.6) | −1.9 (−2.6, −1.1) | 0.92 |
| E/e’ ratio | ||||
| Year 25 | 6.89 (6.72, 7.06) | 7.04 (6.90, 7.19) | 7.22 (6.95, 7.49) | 0.06 |
| Year 30 | 7.10 (6.92, 7.28) | 7.09 (6.94, 7.24) | 7.23 (6.92, 7.54) | 0.62 |
| 5-year change | 0.15 (0.00, 0.29) | 0.09 (−0.03, 0.22) | 0.21 (−0.05, 0.48) | 0.86 |
| Lateral E’ velocity, cm/sec | ||||
| Year 25 | 12.3 (12.1, 12.5) | 11.8 (11.6, 11.9)*† | 11.3 (10.9, 11.7)*† | <0.00001 |
| Year 30 | 11.8 (11.5, 12.0) | 11.4 (11.3, 11.6)* | 11.1 (10.7, 11.5)*,† | <0.005 |
| 5-year change | −0.4 (−0.6, −0.2) | −0.5 (−0.6, −0.3) | −0.7 (−1.0, −0.4) | 0.27 |
| Septal E’ velocity, cm/sec | ||||
| Year 25 | 9.7 (9.6, 9.9) | 9.3 (9.1, 9.4)*† | 8.8 (8.5, 9.2)*† | <0.00001 |
| Year 30 | 9.3 (9.1, 9.4) | 8.9 (8.8, 9.1)*† | 8.7 (8.4, 9.0)*† | <0.005 |
| 5-year change | −0.4 (−0.6, −0.2) | −0.6 (−0.7, −0.4) | −0.6 (−0.9, −0.4) | 0.12 |
| Mitral valve Peak A velocity, cm/sec | ||||
| Year 25 | 62.1 (60.8, 63.4) | 62.8 (61.7, 63.8) | 61.1 (59.1, 63.2) | 0.74 |
| Year 30 | 68.3 (66.8, 69.7) | 68.7 (67.4, 69.9) | 66.7 (64.3, 69.1) | 0.50 |
| 5-year change | 5.6 (4.4, 6.7) | 6.1 (5.2, 7.0) | 5.6 (3.8, 7.4) | 0.81 |
Model adjustments included age, sex, race, field center, educational attainment, and cumulative measures for each of the following: number of years as a current smoker, milliliters of daily alcohol intake, body mass index, physical activity, systolic blood pressure, years of blood pressure-lowering medication use, heart rate, and baseline HOMA-IR (and 5-year change models adjusted for year 25 echocardiographic measurement)
P<0.05 compared to Low-decreasing HOMA-IR trajectory group
P<0.05 compared to Low-decreasing HOMA-IR trajectory group after adjusted for the covariates in the model above and body mass index at time of echocardiography
p trend is a test for linear association across the increasing HOMA-IR trajectory group
4. Discussion
We identified unique trajectories in FG and HOMA-IR over a 25-year span during young adulthood and middle age. We observed that moderate-increasing and high-stable FG trajectory group membership was associated with differences in LV diastolic function in mid-life and with differential 5-year rate of change in parameters of LV structure. These differences remained significant after adjustment for traditional CVD risk factors. Individuals who developed diabetes during the 25-year trajectory period were excluded and a majority of the study sample had FG levels below the threshold for prediabetes by the end of follow-up, suggesting that a rapid increasing FG trajectory below the threshold for diabetes is adversely associated with cardiac structure and function. More broadly, this suggests that the rate and timing of FG and HOMA-IR change may be as important to cardiac risk as singular levels of each for sub-groups of individuals. These high risk groups cannot be identified in traditional analyses without trajectory modeling and speaks to the clinical utility of this data.
Our findings for trajectory modeling of HOMA-IR are consistent with prior studies in CARDIA.6,23 FG and HbA1c trajectories over 12 years were assessed in an Australian cohort of adults aged 25-85 (stratified by age: <60 and ≥60).29 Investigators observed three trajectory groups for both FG and HbA1c among individuals without diabetes: low-stable, low-increasing, and high-stable.29 Differences in our trajectory group numbers may be the result of the younger population with more frequent assessment and longer follow-up in CARDIA.
Prior work in this area has not assessed FG and IR trajectories in relation to subclinical longitudinal changes in cardiac structure and function. Novel observations in this current work include that steeper IR trajectory was associated with changes in LV structure (RWT) over 5-years. Our observation of an association between FG trajectory and early differences in diastolic function extends previous findings in CARDIA showing an association between development of diabetes and trajectory of HOMA-IR with early differences in cardiac structure and function.6 Another original finding of ours is that we observed that a moderate-increasing and high-stable FG trajectory before the onset of diabetes was associated with differences in LV mass index at year 30 (moderate-increasing: 2.7 grams/m2.7 and high-stable: 2.0 grams/m2.7), compared to low-stable FG. In a cohort of older adults, a 1 g/m2.7 increment in LV mass index was associated with 3% increase in risk for incident heart failure.30 The early differences in LV mass coupled with the differential 5-year change in MV peak A velocity according to FG trajectory in our current study suggest that individuals with the most rapid increasing FG trajectory but without diabetes are already at greater risk for future heart failure by this point in middle age.
The potential molecular mechanisms that link increased FG and IR trajectory with early differences in cardiac structure and function include inflammation, neurohormonal activation, and myocardial remodeling secondary to increased oxidative stress, advanced glycated end products, free fatty acid uptake, and myocardial energy substrate metabolism.31,32 Hyperinsulinemia has trophic effects on the myocardium directly as shown in rat models, leading to increased myocardial mass and lower cardiac output in rats.33 Our data suggest that deleterious differences in cardiac structure and function emerge during the period of increasing FG and HOMA-IR trajectory, before the clinical manifestation of diabetes, and that trajectory of FG is informative to LV mass changes over time beyond single measurement of FG. We observed that higher FG trajectory was associated with worse LV structure and systolic and diastolic function and higher HOMA-IR was associated with worse LV structure and diastolic function.
The potential impact of obesity prevention on these findings should be considered. We observed higher BMI across FG and HOMA-IR trajectories, despite higher concomitant physical activity levels at baseline among the higher trajectory groups. Individuals with the most rapid FG and HOMA-IR trajectories gained more weight during young adulthood and were more likely to be obese at the Y25 exam. Further, adjustment for Y25 adiposity level attenuated all 5-year echocardiographic change associations, whereas adjustment for Y25physical activity did not. This suggests that heart failure prevention efforts should target avoiding a rapid increase in FG and IR during young adulthood even below the threshold of diabetes and may consider weight loss/stabilization or obesity prevention as the behavior or mechanism of focus.
Strengths of this study include characterizing long-term trajectories in fasting glucose and insulin resistance during young adulthood before the onset of diabetes in a large cohort of black and white adults with careful standardization across exams, good retention, and statistical adjustment to disentangle natural trajectory in FG and IR from baseline and concurrent level. Our findings may be generalizable to other communities with similar demographic, socioeconomic, and health behavior profiles. Several considerations should be noted when interpreting our findings. First, in order to examine trajectories in FG and HOMA-IR below the threshold of diabetes, we excluded individuals who developed diabetes during determination of FG and IR trajectory. Individuals with diabetes by middle age are presumptively the most severe of each trajectory group and known to have greater risk for heart failure. However, this exclusion may artificially lower the cardiac risk among the higher trajectory groups and suggests that our findings may underestimate the association between higher FG and IR trajectory with each cardiac outcome assessed. Second, echocardiography was not assessed at baseline and we cannot quantify the long-term change in cardiac outcomes concomitant with FG and IR trajectory. The low incidence of heart failure precluded assessment of clinical events. Lastly, we adjust for cumulative exposure to multiple cardiovascular risk factors, however, multiple tests of association were performed and residual confounding or chance findings may be present.
5. Conclusions
We observed that FG and IR trajectories over 25 years were associated with differences in cardiac structure and function during middle age above and beyond baseline measurement of FG and IR. These cardiac differences occurred during the natural course of FG and IR trajectory and FG trajectory was associated with 5-year rates of change in left ventricular mass. These results suggest heart failure prevention efforts should target obesity prevention to minimize rapid rise in FG and IR during young adulthood in order to prevent early differences in cardiac structure and function.
Supplementary Material
Acknowledgements:
The authors thank the other investigators, the staff, and the participants of the CARDIA study for their valuable contributions.
Funding: MPB was supported by the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health under Award Number T32HL069771 to conduct the current work. The Coronary Artery Risk Development in Young Adults Study (CARDIA) is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with the University of Alabama at Birmingham (HHSN268201300025C & HHSN268201300026C), Northwestern University (HHSN268201300027C), University of Minnesota (HHSN268201300028C), Kaiser Foundation Research Institute (HHSN268201300029C), and Johns Hopkins University School of Medicine (HHSN268200900041C). CARDIA is also partially supported by the Intramural Research Program of the National Institute on Aging (NIA) and an intra-agency agreement between NIA and NHLBI (AG0005).
Abbreviations:
- 2D
two-dimensional
- BIC
Bayesian Information Criteria
- BMI
body mass index
- BP
blood pressure
- CARDIA
Coronary Artery Risk Development in Young Adults
- CI
confidence interval
- FG
fasting glucose
- IR
insulin resistance
- HOMA
homeostasis model assessment
- LV
left ventricular
- MV
mitral annular velocity
- RWT
relative wall thickness
- SD
standard deviation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts/Disclosure: The authors have no conflicts to disclose. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the NHLBI; the National Institutes of Health; or the U.S. Department of Health and Human Services.
References
- 1.Nichols GA, Gullion CM, Koro CE, Ephross SA, Brown JB. The incidence of congestive heart failure in type 2 diabetes: an update. Diabetes care. 2004;27(8):1879–1884. [DOI] [PubMed] [Google Scholar]
- 2.Khan H, Kunutsor SK, Kauhanen J, et al. Fasting plasma glucose and incident heart failure risk: a population-based cohort study and new meta-analysis. Journal of cardiac failure. 2014;20(8):584–592. [DOI] [PubMed] [Google Scholar]
- 3.Banerjee D, Biggs ML, Mercer L, et al. Insulin resistance and risk of incident heart failure: Cardiovascular Health Study. Circulation Heart failure. 2013;6(3):364–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vardeny O, Gupta DK, Claggett B, et al. Insulin resistance and incident heart failure the ARIC study (Atherosclerosis Risk in Communities). JACC Heart failure. 2013;1(6):531–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Held C, Gerstein HC, Yusuf S, et al. Glucose levels predict hospitalization for congestive heart failure in patients at high cardiovascular risk. Circulation. 2007;115(11):1371–1375. [DOI] [PubMed] [Google Scholar]
- 6.Kishi S, Gidding SS, Reis JP, et al. Association of Insulin Resistance and Glycemic Metabolic Abnormalities With LV Structure and Function in Middle Age: The CARDIA Study. JACC Cardiovascular imaging. 2017;10(2):105–114. [DOI] [PubMed] [Google Scholar]
- 7.Desai CS, Ning H, Liu K, et al. Cardiovascular Health in Young Adulthood and Association with Left Ventricular Structure and Function Later in Life: The Coronary Artery Risk Development in Young Adults Study. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2015;28(12):1452–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reis JP, Allen NB, Bancks MP, et al. Duration of Diabetes and Prediabetes During Adulthood and Subclinical Atherosclerosis and Cardiac Dysfunction in Middle Age: The CARDIA Study. Diabetes care. 2018;41(4):731–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840. [DOI] [PubMed] [Google Scholar]
- 10.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41(11):1105–1116. [DOI] [PubMed] [Google Scholar]
- 11.Hozawa A, Jacobs DR Jr., Steffes MW, Gross MD, Steffen LM, Lee DH. Circulating carotenoid concentrations and incident hypertension: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Journal of hypertension. 2009;27(2):237–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobs DJ, Hahn L, Haskell W, Pirie P, Sidney S. Validity and reliability of a short physical activity history: CARDIA and the Minnesota Heart Health Program. J Cardiopulm Rehabil. 1989;9:448–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.US Department of Health Human Services. Dietary guidelines for Americans 2015-2020. Skyhorse Publishing Inc.; 2017. [Google Scholar]
- 14.Park K, Gross M, Lee DH, et al. Oxidative stress and insulin resistance: the coronary artery risk development in young adults study. Diabetes care. 2009;32(7):1302–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonora E, Targher G, Alberiche M, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes care. 2000;23(1):57. [DOI] [PubMed] [Google Scholar]
- 16.Gardin JM, Wong ND, Bommer W, et al. Echocardiographic design of a multicenter investigation of free-living elderly subjects: the Cardiovascular Health Study. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 1992;5(1):63–72. [DOI] [PubMed] [Google Scholar]
- 17.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2015;28(1):1–39.e14. [DOI] [PubMed] [Google Scholar]
- 18.Armstrong AC, Ricketts EP, Cox C, et al. Quality Control and Reproducibility in M-Mode, Two-Dimensional, and Speckle Tracking Echocardiography Acquisition and Analysis: The CARDIA Study, Year 25 Examination Experience. Echocardiography (Mount Kisco, NY). 2015;32(8):1233–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. The American journal of cardiology. 1986;57(6):450–458. [DOI] [PubMed] [Google Scholar]
- 20.Jones BL, Nagin DS, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociological methods & research. 2001;29(3):374–393. [Google Scholar]
- 21.Nagin DS. Analyzing developmental trajectories: a semiparametric, group-based approach. Psychological methods. 1999;4(2):139. [DOI] [PubMed] [Google Scholar]
- 22.Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annual review of clinical psychology. 2010;6:109–138. [DOI] [PubMed] [Google Scholar]
- 23.Van Wagner LB, Ning H, Allen NB, et al. 25-Year Trajectories of Insulin Resistance and Pancreatic beta-Cell Response and Diabetes Risk in Nonalcoholic Fatty Liver Disease. Liver international : official journal of the International Association for the Study of the Liver. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhong VW, Bancks MP, Schreiner PJ, et al. Insulin resistance since early adulthood and appendicular lean mass in middle-aged adults without diabetes: 20years of the CARDIA study. Journal of diabetes and its complications. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siddique J, Chung JY, Brown CH, Miranda J. Comparative effectiveness of medication versus cognitive-behavioral therapy in a randomized controlled trial of low-income young minority women with depression. Journal of consulting and clinical psychology. 2012;80(6):995–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Buuren S Multiple imputation of discrete and continuous data by fully conditional specification. Statistical methods in medical research. 2007;16(3):219–242. [DOI] [PubMed] [Google Scholar]
- 27.Yuan YC. Multiple Imputation for Missing Data: Concepts and New Development (Version 9.0). In. Proceedings of the Twenty-Fifth Annual SAS Users Group International Conference: Cary, NC: SAS Institute; 2000. [Google Scholar]
- 28.Reis JP, Allen N, Gibbs BB, et al. Association of the degree of adiposity and duration of obesity with measures of cardiac structure and function: the CARDIA study. Obesity (Silver Spring). 2014;22(11):2434–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anstey KJ, Sargent-Cox K, Eramudugolla R, Magliano DJ, Shaw JE. Association of cognitive function with glucose tolerance and trajectories of glucose tolerance over 12 years in the AusDiab study. Alzheimer’s research & therapy. 2015;7(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Simone G, Gottdiener JS, Chinali M, Maurer MS. Left ventricular mass predicts heart failure not related to previous myocardial infarction: the Cardiovascular Health Study. European heart journal. 2008;29(6):741–747. [DOI] [PubMed] [Google Scholar]
- 31.Taegtmeyer H, McNulty P, Young ME. Adaptation and maladaptation of the heart in diabetes: Part I: general concepts. Circulation. 2002;105(14):1727–1733. [DOI] [PubMed] [Google Scholar]
- 32.Horwich TB, Fonarow GC. Glucose, obesity, metabolic syndrome, and diabetes relevance to incidence of heart failure. Journal of the American College of Cardiology. 2010;55(4):283–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holmang A, Yoshida N, Jennische E, Waldenstrom A, Bjorntorp P. The effects of hyperinsulinaemia on myocardial mass, blood pressure regulation and central haemodynamics in rats. European journal of clinical investigation. 1996;26(11):973–978. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


