Abstract
Background:
IgG anti-nuclear antibodies (ANA) are a feature of several autoimmune diseases. These antibodies arise through defects in central or peripheral tolerance checkpoints. The specific checkpoints breached in autoimmune disease are not fully understood.
Objectives:
To study whether autoreactive plasma cells in lupus models and SLE patients arise as a consequence of defective antigen-specific selection or a global enhancement of IgG PC differentiation.
Methods and Results:
We optimized and validated a novel technique to detect naturally occurring ANA+ B cells and PC. We observed a major checkpoint for generation of ANA+ IgG+ PC in both non-autoimmune mice and healthy human subjects. Interestingly, we observed increased numbers of ANA+ IgG+ PC despite normal tolerance checkpoints in immature and naïve B cells in lupus-prone MRL/lpr and NZB/W mice as well as patients with systemic lupus erythematosus (SLE). This increase was due to increased numbers of total IgG+ PC rather than lack of selection against ANA+ PC.
Conclusion:
Using a method that permits quick and accurate quantification of autoreactive B cells and PC in vivo within a native B cell repertoire in mice and humans, we demonstrate the importance of a checkpoint that restricts the generation of IgG plasma cells and protects against IgG ANA. Our observations suggest a fundamentally revised understanding of SLE: that it is a disease of aberrant B cell differentiation rather than a defect in antigen-specific B cell tolerance.
Clinical implication:
Therapies for SLE might need to be targeted at IgG plasma cell differentiation rather than antigen-specific tolerance.
Keywords: plasma cells, tolerance, autoimmunity, systemic lupus erythematosus
Graphical Abstract

Capsule Summary:
Using a novel assay for ANA+ plasma cells, we show the presence of a strong tolerance checkpoint. Loss of tolerance in lupus results from expansion of IgG+ plasma cells rather than aberrant antigen-specific selection.
Introduction
Anti-nuclear antibodies (ANA) encompass a spectrum of nuclear specificities. The presence of IgG-ANA is a diagnostic feature for systemic lupus erythematosus (SLE) and other systemic autoimmune diseases, and these antibodies have an important role in disease pathogenesis.1 In contrast, IgM-ANA can be present in healthy individuals and assist in the non-inflammatory clearance of cellular debris. IgM-ANA are thought to protect against autoimmunity as they inhibit proinflammatory responses induced by IgG-ANA.2
Most of our knowledge of immune tolerance to nuclear antigens, and the presence of ANA in SLE patients, is derived from studies with B cell receptor (BCR) -transgenic mice and single cell studies in humans. In both human and mouse, self-reactivity is usually censored in developing B cells prior to their achieving immunocompetence, either through receptor editing or through deletion in the central compartment.3-5 Autoreactive B cells that escape these mechanisms often become anergic or are excluded from entering the B cell follicles in secondary lymphoid organs,6-9 processes that mitigate against these cells giving rise to high affinity ANA IgG-producing plasma cells. Some studies suggest that SLE patients and lupus-prone mice have defects in central tolerance towards nuclear antigens; other studies have shown defective B cell anergy and follicular exclusion in ANA− or DNA-reactive B cells in SLE patients and lupus-prone mice;8, 10-17 and yet other studies suggest that lupus is a disease of polyclonal activation rather than aberrant selection.18
The distinction between enhanced polyclonal activation and a selection defect is difficult to assess in BCR-transgenic models, as either mechanism will lead to increased serum titers of autoantibodies. In addition, B cell tolerance mechanisms depend on antigen and BCR affinity for antigen,19-21 explaining in part discrepancies among different BCR-transgenes. Most importantly, plasma cells have not been widely studied, in particular not in patients.
As we wished to understand whether autoreactive plasma cells arise in lupus models and SLE patients due to skewed B cell differentiation or is due to aberrant selection, we developed and validated a simple and quick flow cytometry-based method to examine naturally occurring ANA+ B cells and ANA+ plasma cells in mice and humans. Our results support the notion that lupus is a disease of increased IgG class switching and plasma cell differentiation rather than a failure of tolerance to nuclear antigens.
Methods
Mice and human subjects
The following female mice were purchased from The Jackson Laboratory: Balb/c, C57BL/6, NZB/W F1, and MRL/lpr mice.
Blood from 15 SLE patients and 9 age-matched healthy subjects was collected in heparinized tubes. SLE diagnosis was based on 1997 revised ACR criteria.22 SLE patients receiving Rituximab, Belimumab or Cyclophosphamide in the preceding 12 months were excluded from the study. Patient characteristics are shown in Table S1. All subjects gave written informed consent.
The studies were approved by the the Institutional Animal Care and Use Committee and the Northwell Health Institutional Review Board.
Flow cytometry for ANA+ B cells
Nuclear extract from HeLa cells was obtained as described.8 Briefly, nuclei from HeLa cells were isolated using the Nuclei EZ lysis kit (Sigma), fragmented by vortexing with 0.5 mm cell disruption glass beads (Scientific Industries) and biotinylated using EZ-Link-Sulfo-NHS-LC-biotin (Thermo Scientific). Staining with nuclear extract was done in 1.5% non-fat dry milk (LabScientific) in Hank’s Balanced Salt Solution (HBSS) or Permeabilization buffer (for intracellular staining). After thorough washing, cells were stained in HBSS + 2% Fetal Bovine Serum (FBS) containing 1 μg/mL streptavidin-APC (Life Technologies) Dead cells were excluded using eFluor 506 labeled fixable viability dye (FVD) (eBioscience) or 4′,6-diamidino-2-phenylindole (DAPI) (Thermo Fisher). For intracellular staining, cells were fixed and permeabilized with the Transcription Factor Staining Buffer Set (eBioscience). To obtain the percentage of ANA+ cells, the percentage of APC+ cells in the streptavidin control was subtracted for each B cell population. Antibodies used for flow cytometry are shown in supplemental Methods.
Statistics
P values of <0.05 were considered statistically significant. Each experiment was performed at least twice to ensure reproducibility. For comparison of ANA+ and ANA− cells within the same individual or mouse, paired-samples T-test or Analysis Of Variance (ANOVA) with Bonferroni posthoc test was performed, except for ELISpot, as these data were not normally distributed and the Mann Whitney U test was used instead. For comparison of groups of mice or subjects, the Mann Whitney U test was used. For comparison of lupus mouse strains to non-autoimmune mice only those differences that were significant compared to both Balb/c and C57BL/6 mouse strains are shown. To compare frequencies, Chi-square test and Fisher exact test were used as indicated. Linear regression was used to study correlations. Statistical analysis was performed using GraphPad Prism 5.
Additional methods are provided in supplemental Methods.
Results
ANA production by B cells stained with nuclear extract in mice
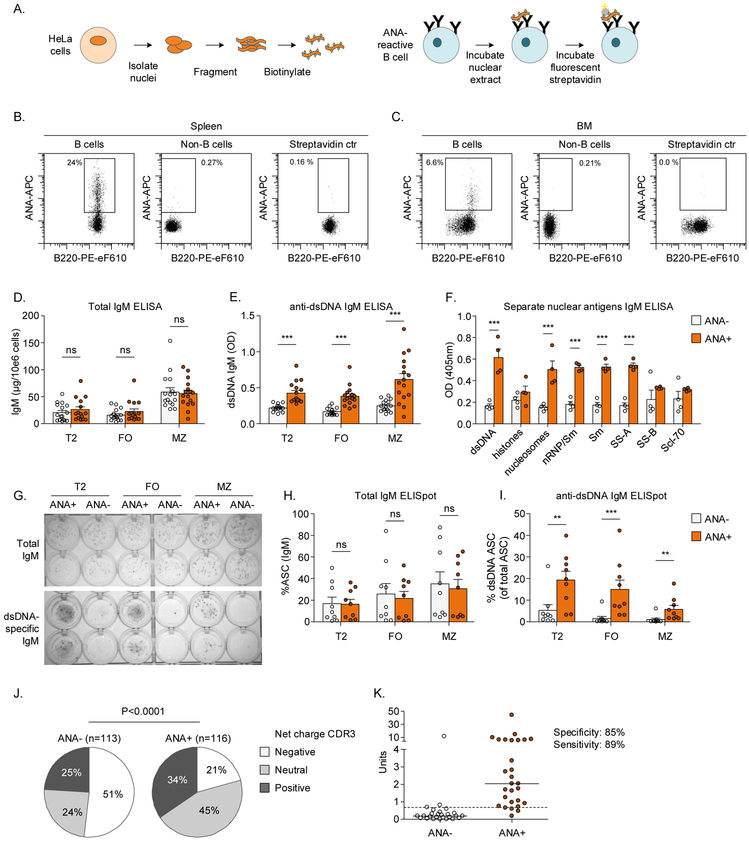
In order to characterize the population of naturally occurring ANA+ B cells, we developed a flow cytometry-based assay for their detection using biotinylated nuclear extract in humans (Figure 1A-C).8 Here we aimed to confirm the sensitivity and specificity of this method in mice. Analyzed by enzyme-linked immunosorbent assay (ELISA) and enzyme-linked immunospot (ELISpot), we found that ANA+ B cells from both C57BL/6 and Balb/c mice contained a higher percentage of anti-dsDNA antibody secreting cells (Figure 1D-I). An ELISA for distinct ANA antigens revealed that supernatants from ANA+ B cells contained a collection of ANA antibodies with reactivity to dsDNA, nucleosomes, nRNP/Sm, Sm, and SS-A (Figure 1F). These results confirm that B cells stained with the nuclear extract are ANA-reactive.
Figure 1: Validation of flow cytometric assay fo ANA+ B cells in mice.
A) An overview of the generation of biotinylated nuclear extract (left) and staining B cells with the nuclear extract (right) as described in Methods. B,C) Representative examples of ANA staining.
D-I) ANA+ B cells and ANA− B cells from the spleen were sorted and subsequently cultured for 4 days to promote differentiation into antibody secreting cells (ASC), after which antibody secretion was analyzed by ELISA (D-F) and ELISpot (G-H).
J,K) Single ANA+ B cells and ANA− B cells from 3 female Balb/c mice were sorted and monoclonal antibodies were cloned and expressed. J) Net charge of heavy chain CDR3 is shown as relative frequency. K) ANA reactivity of monoclonal antibodies obtained from ANA− (n=26) and ANA+ (n=28) B cells by ELISA.
Bars show mean ± SEM. Asterisks indicate significant differences (*** p<0.001; ** p<0.01) analyzed using Two-way ANOVA and Bonferroni posthoc test (D-F), Mann-Whitney test (H,I) or Chi-Square test (J).
ANA: Anti-nuclear antibody; ASC: Antibody secreting cell; BM: Bone marrow; FO: Follicular B cell; FVD: Fixable viability dye; MZ: Marginal zone B cell; ns: not significant; T2: T2 transitional B cell.
Several characteristics that have been described for DNA-binding B cells, such as positive and neutral charge in Complementarity-Determining Region 3 (CDR3) of heavy chains, lambda light chain expression and light chain allelic inclusion,23-26 were increased in ANA+ B cells (Figure 1J, Figure S2).
We next established the specificity and sensitivity of the staining on the single cell level, analyzing cloned and expressed BCRs from single B cells. Nuclear reactivity was tested by ELISA and was observed in 3/26 ANA− and 24/28 ANA+ cells, yielding a specificity of 85% and a sensitivity of 89% (Figure 1K). Together, these results validate the flow cytometric assay for ANA+ B cells in mice.
Tolerance checkpoints for ANA+ B cells in mice
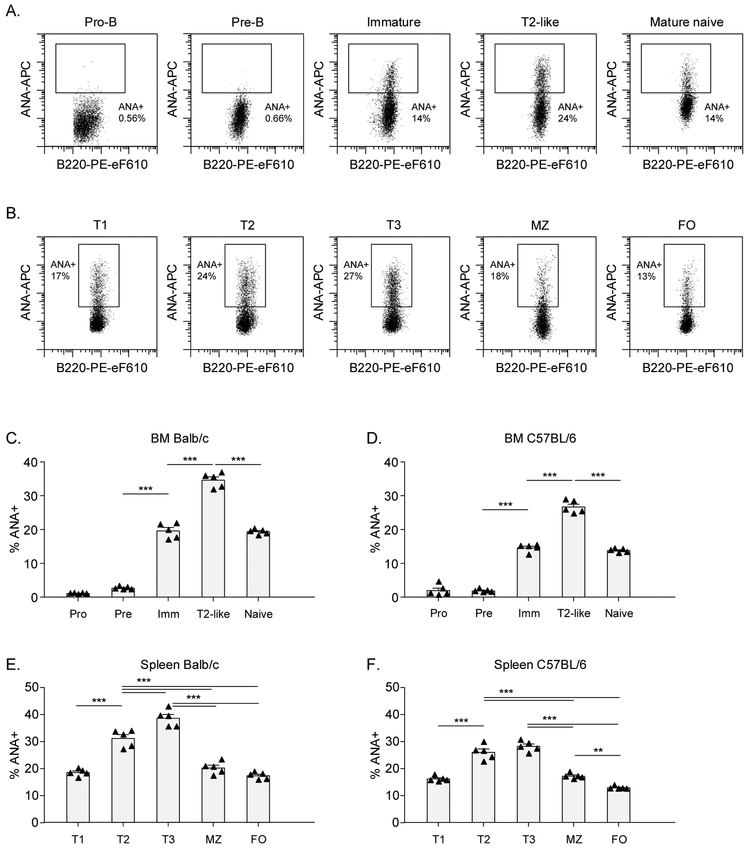
Several tolerance checkpoints have been identified in pre-immune B cell development, prior to acquisition of immunocompetence (the naïve stage), in both bone marrow (BM) and spleen. These tolerance checkpoints include the pre-B to immature B cells, where clonal deletion and receptor editing occur, and the transitional stage where additional checkpoints can lead to activation-induced apoptosis or anergy of autoreactive cells. To confirm these findings, we analyzed the percentage of ANA+ cells throughout B cell development in non-autoimmune Balb/c and C57BL/6 mice. Gating strategies are shown in Figure S1. As expected, BM pro- and pre-B cells, which do not express surface IgM, did not bind the nuclear extract, and therefore this staining does not allow us to study the pre-B cell to immature B cell tolerance checkpoint. Interestingly, the frequency of nuclear reactivity increased as B cells progressed from immature to T2-like B cells in the BM and, likewise, and from T1 transitional B cells to T2 and T3 subsets in the spleen (Figure 2C-F). The frequency of nuclear reactivity was highest in T2-like cells in the BM and T2 and T3 cells in the spleen, consistent with studies suggesting that positive selection can occur in the T2 stage.27 After the transitional stage and prior to achieving immunocompetence, tolerance checkpoints from transitional to naïve B cells were identified in the spleen: from T2/T3 to marginal zone B cells and from T2/T3 to follicular B cells. Furthermore, a lower frequency of ANA+ B cells in follicular compared to marginal zone B cells was observed. These data confirm tolerance checkpoints that have been described for autoreactive B cells.
Figure 2: Pre-immune tolerance checkpoints for ANA+ B cells in non-autoimmune mice.
BM cells and splenocytes from 3-month old Balb/c and C57BL/6 were stained with nuclear extract to analyze the percentage of ANA+ B cells. A,B) Representative flow cytometry plots for ANA staining in C57BL/6 mice are shown for each B cell subset in BM and spleen, respectively. 4500 cells are shown for each subset. C-F) Percentage of ANA+ B cells in each B cell subset in 5 Balb/c and 5 C57BL/6 mice.
Each symbol represents an individual mouse and bars indicate the mean ± SEM. The data shown are from a representative experiment that was replicated in at least 3 independent experiments for each mouse strain. Asterisks indicate significant differences (*** p<0.001; ** p<0.01; * p<0.05) analyzed using repeated measures ANOVA with Bonferroni posthoc test (C-F).
ANA: Anti-nuclear antibody; BM: Bone marrow; FO: Follicular B cell; Imm: Immature B cell; MZ: Marginal Zone B cell.
Largely intact pre-immune tolerance in MRL/lpr and NZB/W mice
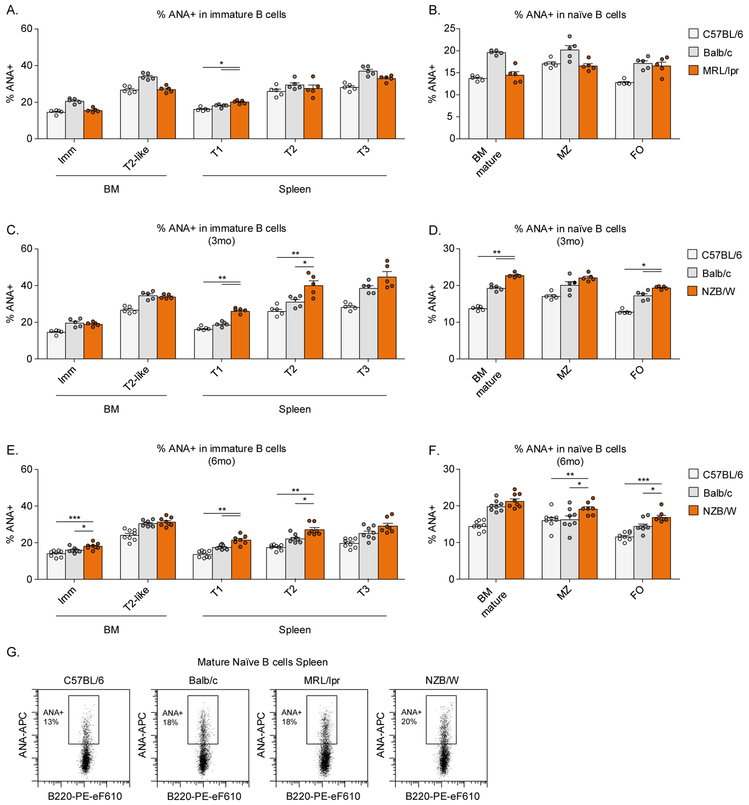
To address which tolerance checkpoints might be defective in lupus-prone MRL/lpr and NZB/W mice, we analyzed the percentage and number of ANA+ B cells in BM and spleen of these strains compared to the non-autoimmune Balb/c and C57BL/6 mice. NZB/W developed autoantibodies at a later timepoint than MRL/lpr mice (Figure S3E-J), we, therefore, analyzed these mice before (age 3 months) and after (age 6 months) seroconversion to determine whether a break of tolerance would be present prior to the development of serum autoantibodies.
The frequency of ANA+ B cells in all B cell subsets in BM and spleen of MRL/lpr mice were within the range of non-autoimmune strains: comparable to C57BL/6 mice, and lower than Balb/c mice (Figure 3A,B,G). In contrast to MRL/lpr mice, we observed an increased percentage of ANA+ mature naïve B cells in BM and spleens of young and old NZB/W mice compared to non-autoimmune mice, in particular in transitional and mature populations (Figure 3C-G), suggesting that NZB/W mice have a modest but significant defect in pre-immune tolerance.
Figure 3: Pre-immune tolerance in MRL/lpr and NZB/W mice.
BM cells and splenocytes from C57BL/6, Balb/c, MRL/lpr, and NZB/W mice were stained with nuclear extract to analyze the percentage of ANA+ B cells. A-F) Summary of the percentage of nuclear reactivity within B cell subsets in BM and spleen. G) Representative examples of the percentage of ANA+ mature naïve B cells in spleens.
Each dot indicates an individual mouse (n=5-8 for each group) and the bars represent the mean ± SEM. Asterisks indicate significant differences (*** p<0.001; ** p<0.01; * p<0.05) analyzed using Mann Whitney U test. Asterisks are only shown when the lupus-prone mice were significantly different from both non-autoimmune strains.
ANA: Anti-nuclear antibody; BM: Bone marrow; FO: Follicular B cell; MZ: Marginal Zone B cell.
A tolerance checkpoint for ANA+ IgG+ plasma cells
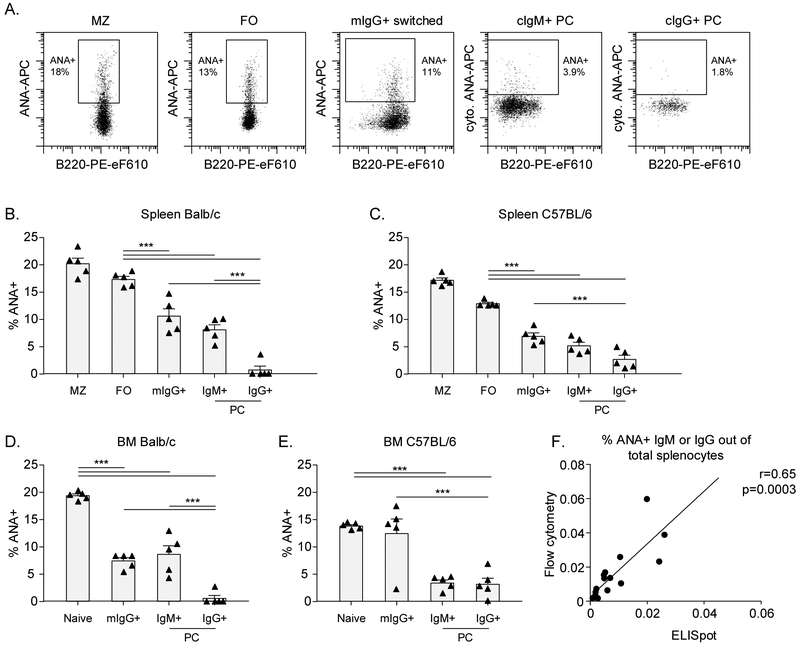
As we were surprised to find that there was no major breach in tolerance checkpoints for ANA+ antigen-naïve B cells in lupus mice, we decided that it would be important to analyse antigen-experienced IgG switched B cells and plasma cells. As most plasma cells, in particular of the IgG isotype, do not express surface immunoglobulin, we adapted our assay to detect ANA together with immunoglobulin isotype in cytoplasm of plasma cells (Figure 4A and Figure S1 for gating strategies). The number of plasma cells positive for ANA by flow cytometry correlated well with ELISpot, although ELISpot identified fewer cells (~1.5-fold) than flow cytometric staining (Figure 4F).
Figure 4: Tolerance checkpoints for antigen-experienced ANA+ B cells in non-autoimmune mice.
BM cells and splenocytes from 3-month old Balb/c and C57BL/6 were stained with nuclear extract to analyze the percentage of ANA+ B cells. A) Representative flow cytometry plots for ANA staining in splenocytes from C57BL/6 mice are shown for each B cell subset. Gates for cytoplasmic ANA in plasma cells were based on a cytoplasmic streptavidin control (not shown). B-E) Percentage of ANA+ B cells in each B cell subset in 5 Balb/c and C57BL/6 mice. F) Correlation of %ANA by ELISpot versus our flow cytometry assay.
Each symbol represents an individual mouse and bars indicate the mean ± SEM. The data shown are from a representative experiment that was replicated in at least 3 independent experiments for each mouse strain. Asterisks indicate significant differences (*** p<0.001; ** p<0.01; * p<0.05) analyzed using repeated measures ANOVA with Bonferroni posthoc test (B-E). Linear regression was used to calculate correlation (F).
ANA: Anti-nuclear antibody; BM: Bone marrow; cIg: Cytoplasmic Ig; FO: Follicular B cell;; mIg: Membrane Ig; MZ: Marginal Zone B cell; PC: Plasma cell.
In non-autoimmune mice, we observed tolerance checkpoints after the naïve stage, with a sequential decrease in ANA reactivity from naïve to IgG+ switched B cells (defined as surface IgG+) and then to plasma cells (Figure 4B-E). The profound reduction in IgG+ plasma cell reactivity against nuclear antigens compared to IgG+ switched B cells and IgM+ plasma cells points to the cardinal importance of this tolerance checkpoint.
Expansion of IgG+ plasma cells in lupus-prone mice
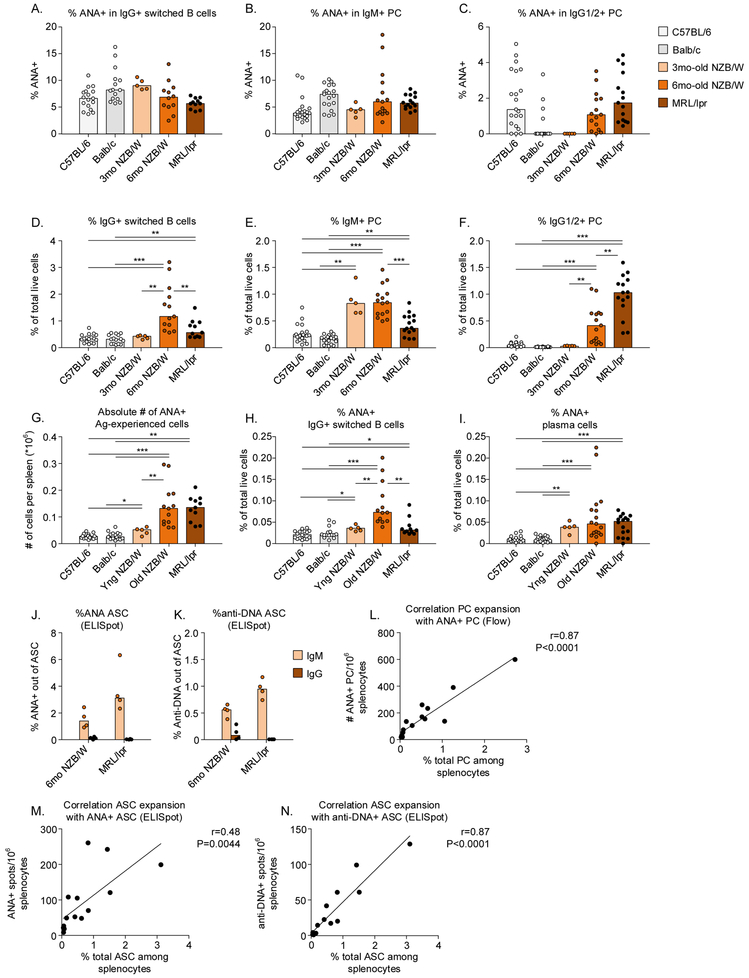
We also examined whether tolerance in antigen-experienced B cells was compromised in NZB/W and MRL/lpr mice. In both strains of lupus-prone mice, ANA reactivity in antigen-experienced B cells was comparable to what we observed in non-autoimmune mice, such that the percentage of ANA+ cells was never higher than in both of the non-autoimmune strains (Figure 5A-C). While there was no increase in the percentage of ANA+ cells in each of the antigen-experienced subsets, these was an expansion of these subsets (Figure 5D-F) consistent with previous data on generalized B cell hyperresponsiveness in SLE.28 This led to an overall increased absolute number and percentage of ANA+ antigen-experienced cells among total splenocytes in both lupus-prone mouse strains (Figure 5G-I).
Figure 5: Expansion of ANA+ plasma cells in MRL/lpr and NZB/W mice.
Splenocytes from C57BL/6, Balb/c, MRL/lpr, and NZB/W mice were stained with nuclear extract to analyze the percentage of ANA+ B cells. A-C) Nuclear reactivity within IgG+ switched B cells, IgM+ plasma cells, and IgG+ plasma cells in spleen. D-F) Percentage of antigen-experienced subsets in spleen, as a percentage of total live cells. G) Absolute number of ANA+ antigen-experienced cells in the spleen. H,I) Percentage of ANA+ antigen-experienced cells in the spleen out of total live cells. J,K) Percentage of ANA+ or anti-DNA+ IgM and IgG spots among total ASC in spleens from NZB/W and MRL/lpr mice. L-N) Correlation of IgM+ IgG PC/ASC with the number of ANA+ or anti-DNA+ PC/ASC determined by flow cytometry (L) or ELISpot (M,N).
Each dot indicates an individual mouse (n=5-17 for each group) and the bars represent the median. Asterisks indicate significant differences (*** p<0.001; ** p<0.01; * p<0.05) analyzed using Mann Whitney U test and are only shown if they were observed compared to both C57BL/6 and Balb/c mice.. Linear regression was used to calculate correlation (L-N).
ANA: Anti-nuclear antibody; cIg: Cytoplasmic Ig; mIg: Membrane Ig; PC: Plasma cell.
The difference in spontaneous secretion of ANA IgM and IgG and the low frequency of ANA+ IgG plasma cells in lupus-prone mice was confirmed by ELISpot (Figure 5J). To address reactivity to a dominant nuclear antigen, we analysed anti-DNA binding by ELISpot, and observed very similar findings as with total ANA, with even lower percentages of IgM and IgG spots observed, as expected when analyzing a single antigen compared to multiple nuclear antigens together (Figure 5K). A strong correlation between the number of ANA+ or DNA+ plasma cells among total splenocytes and the number of total IgM and IgG secreting cells was found (Figure 5L-N), suggesting that the expansion of the total plasma cell compartment is responsible for increased numbers of autoreactive plasma cells.
Similar findings were observed in BM, with no increase in the percentage of ANA+ cells within each antigen-experienced population, but expansion of some of the total populations in lupus-prone mice (Figure S4).
Together, these results reveal an increased percentage of antigen-experienced B cells suggesting enhanced activation of B cells. The increase in ANA+ IgG+ plasma cells in both lupus-prone mouse strains results from the expansion of this subset and likely arises from increased B cell activation and differentiation to IgG plasma cells without a major impairment in tolerance for ANA+ or DNA+ cells.
Expansion of ANA+ IgG+ plasmablasts in SLE patients
We next wondered how these findings translate to humans. We analyzed the percentage of ANA+ B cells and plasma cells in 9 healthy subjects and 15 SLE patients (Figure 6, Figure S5, Table S1). In healthy subjects, we observed a significant decrease in ANA+ B cells from naïve to IgG+ memory B cells and from IgG+ memory to plasmablasts, suggesting that the tolerance checkpoint for ANA+ IgG+ plasma cells that we observed in non-autoimmune mice also exists in healthy non-autoimmune humans (Figure 6A,B, Figure S5A,B).
Figure 6: Expansion of ANA+ IgG+ plasma cells in SLE patients.
PBMCs from healthy subjects and SLE patients were stained with nuclear extract to analyze the percentage of ANA+ B cells and plasma cells. A) Representative flow cytometry plots. Gates for cytoplasmic ANA in plasma cells were based on cytoplasmic streptavidin control (not shown). B) Percentage of ANA+ B cells in each B cell subset in PBMCs from healthy subjects. C-E) Nuclear reactivity within antigen-experienced cell subsets. F,G) Representative flow cytometry plots for plasma cell percentage and isotype in PBMCs from a healthy individual and an SLE patient. H-J) Percentage of antigen-experienced subsets, out of total B cells. K) Number of ANA+ IgG+ plasma cells in 1*10e6 total B cells. L) Correlation of IgG PC with the number of ANA+ IgG PC.
Each dot indicates an individual (healthy: n=9; SLE: n=15) and the bars represent the mean ± SEM (B) or median (C-K). Asterisks indicate significant differences (*** p<0.001; ** p<0.01; * p<0.05) analyzed using repeated measures ANOVA with Bonferroni posthoc test (B) or Mann Whitney U test (C-M). Linear regression was used to calculate correlation (L).
ANA: Anti-nuclear antibody; cIg: Cytoplasmic Ig; mIg: Membrane Ig; PC: Plasma cell.
Next, we analyzed the frequency of ANA+ B cells and plasmablasts in SLE patients compared to the healthy subjects. We confirmed our previous data that the percentage of ANA+ B cells in transitional and naïve subsets is not altered in SLE patients compared to healthy subjects, except for 3 of 15 patients who showed an increase of ANA+ cells in the naïve subset (Figure S5C,D). However, all SLE patients, including these three had a low percentage of ANA within plasmablasts, confirming the presence of this checkpoint. The percentage of nuclear reactivity within IgG+ memory B cells was comparable between healthy subjects and SLE patients (Figure 6C). Most importantly, the percentage of nuclear reactivity within plasmablasts was comparable between healthy subjects and SLE patients (Figure 6D,E). These results suggest that, like in lupus-prone mice, only a small proportion of IgG+ plasmablasts in SLE patients react with nuclear antigens.
Similar to our results in mice, we observed an increase in total plasmablasts and specifically IgG+ plasmablasts among total B cells (Figure 6F-J), correlating with an increased ratio of IgG to IgM plasmablasts (Figure S5E-G). This expansion of the IgG+ plasma cell compartment therefore led to an increase in the percentage of ANA+ IgG+ plasmablasts among total B cells (Figure 6K). Similar to the lupus mice, the numbers of ANA+ plasmablasts among total B cells correlated most strongly with the number of total plasmablasts (Figure 6L) and not with the frequency of ANA within the plasmablast compartment (data not shown).
These results confirm the findings in mice and demonstrate that SLE patients exhibit increased numbers of autoreactive plasma cells due to a generalized expansion of the plasma cell compartment, while preserving tolerance checkpoints.
Discussion
In this study we assessed immune tolerance to nuclear antigens and its breakdown in lupus by analyzing naturally occurring ANA+ B cells and plasma cells using flow cytometry. Our major findings were (1) lupus-prone mice had only minor defects, if any, in pre-immune tolerance, (2) reactivity towards nuclear antigens is more stringently regulated in IgG+ plasma cells compared to IgG+ switched/memory B cells and IgM+ plasma cells, and (3) an overall expansion of IgG+ plasma cells underlies the increase in ANA+ IgG+ plasma cells in lupus-prone mice and SLE patients.
Using our technique, we identified various tolerance checkpoints leading to a decrease in the frequency of ANA+ B cells as B cells mature. Interestingly, our results suggest that T2 and T3 subsets in the spleen have the highest frequency of nuclear reactivity. This is in line with positive selection of autoreactive B cells in late transitional cells that has been reported,27 and may reflect BAFF-dependent proliferation in these cells.29 We found that ANA+ cells are present among IgG+ switched B cells and IgM-producing plasma cells (although at a lower frequency than among naïve cells), but are precluded from becoming IgG+ plasma cells in non-autoimmune mice and healthy human subjects. The different regulation of autoreactive IgG+ plasma cells and IgG+ memory cells may reflect the importance of cell-fate decisions in the germinal center. Whereas plasma cell differentiation is specifically initiated in high-affinity germinal center B cells, memory B cells are generated from a more mixed population of low- and high-affinity clones.30 The differential selection of germinal center B cells into these compartments relates, in part, to the kinetics of the germinal center response, with memory B cells emerging from the germinal center earlier than plasma cells.31, 32 Several mechanisms can reduce autoreactivity in the germinal center, such as mutation away from self-reactivity or elimination of high-affinity self-reactive germinal center B cells33, 34; it is not clear whether or how these mechanisms affect tolerance more stringently in IgG+ plasma cells than in memory B cells.
The most important finding we obtained is that the expansion of ANA+ plasma cells in lupus-prone mice and SLE patients seems to occur through a generalized expansion of IgG+ plasma cells, rather than antigen-specific tolerance defects. In line with other studies,18, 35 the majority of the plasma cell expansion that we observed was not reactive with nuclear antigens. Studies from the 1970s and 1980s suggested that autoantibodies in lupus mice result from polyclonal activation.18, 36-38 In some of these, ELISpot assays for several antigens, including dsDNA, were performed in mice with an unmanipulated repertoire, and results showed that the frequency of self-reactivity among total Ig secreting plasma cells was not increased in MRL/lpr or NZB/W mice compared to wildtype mice, but rather that the plasma cell compartment as a whole was increased18, 38, in accordance with our findings. Similarly, we previously showed that healthy individuals and SLE patients have a similar frequency of ANA+ B cells in the transitional and naïve B cell compartments, but SLE patients have a lower percent of B cells that have undergone anergy induction.8 This loss of anergy affected both ANA+ and ANA− cells.
Some studies using anti-DNA BCR transgenic mice have shown an increased frequency of DNA binding among hybridomas in lupus-prone strains carrying an anti-DNA BCR transgene compared to wildtype mice carrying the same transgene,11, 12 or a loss of anergy or follicular exclusion for anti-DNA B cells in lupus-prone mice.14, 15, 39, 40 These observations are consistent with either antigen-specific tolerance defects or increased B cell activation. Most studies using BCR-transgenic mice have evaluated loss of tolerance in plasma cells by analyzing serum antibodies or analyzing the frequency of autoreactive plasma cells among total splenocytes.14, 41 This would not reveal whether the tolerance defect is antigen-specific or a result of increased numbers of total plasma cells. In addition, discrepancies can also arise through the nature of the BCR transgene that may lead to different regulation of autoreactive cells compared to a native polyclonal repertoire that we studied. In anti-DNA transgenic non-autoimmune mice, depending on the affinity of the BCR, many of the DNA-binding B cells are censored in the central compartment through clonal deletion or receptor editing, or in the peripheral compartment through anergy induction and follicular exclusion.6, 7, 26, 42-44 In the native repertoire that we studied here, some ANA+ B cells are allowed to mature into the naïve follicular B cell compartment, and a proportion of these, as determined by ELISpot, bind DNA (~5-10% of ANA+ cells).
There are some differences between our method and the studies performed using monoclonal antibodies derived from single B cells in humans. In particular, using monoclonal antibodies, the percentage of ANA+ cells within the IgG+ memory cells was increased compared to naïve B cells in healthy individuals.45 We observed a decrease in the frequency of ANA+ IgG memory cells in both mice and humans. This difference may reflect the fact that both IgM+ naïve and IgG memory BCRs were expressed as monomeric IgG when monoclonal antibodies were generated. IgM that binds self-antigen as a pentamer might not bind self-antigen as a monomer, thereby lowering the sensitivity to detect autoreactivity in naïve B cells in those studies. However, in line with our findings, there was a strong tolerance checkpoint between IgG+ memory and IgG+ plasma cells using single cell-derived monoclonal antibodies.46 Also in line with the studies using monoclonal antibodies derived from single cells10, 47 and our previous study,8 there was no difference in the frequency of ANA+ B cells in transitional or naïve B cells between healthy controls and SLE patients. Although polyreactive antibodies and antibodies reactive to cytoplasmic antigens were more common among naïve B cells from SLE patients, the binding to nuclear antigens was similar to healthy controls.10 Using this assay, we do not assess fine specificity and affinity, and therefore we cannot exclude that there may be some differences between non-autoimmune mice and or humans and those with a lupus-like pathology or SLE. However, we previously showed that monoclonal ANA-reactive antibodies derived from B cells from healthy individuals and SLE patients bound antigen at the same concentration, suggesting similar affinity of ANA-reactive B cells.8 Studies of monoclonal antibodies obtained from memory IgG+ B cells found no difference in the frequency of ssDNA- or dsDNA-reactivity on the level of antigen binding between SLE patients and healthy individuals (median and range SLE patients: 17% (10-26%); and healthy individuals: 19.5% (11-23%))45, 47, similar to our study. In addition, we showed a similar frequency of DNA-specific plasma cells in lupus-prone and non-autoimmune mice, confirming that even for one of the major nuclear antigens in lupus, dsDNA, no evidence of an antigen-specific loss of tolerance is present in SLE patients.
Together, our results suggest that a greatly increased plasma cell output, even if only a small number of them produce autoantibodies, can lead to autoimmunity. Based on our results we think it is now time to reconsider that autoantibody production in lupus is a consequence of IgG plasma cell expansion rather than aberrant B cell selection.
Supplementary Material
Acknowledgements
We thank Heriberto Borrero and Chris Colon (flow cytometry core facility, The Feinstein Institute for Medical Research) for their support in flow assisted cell sorting. JS received financial support from Mallinckrodt Pharmaceuticals and American Autoimmune Related Disease Association. MYW was supported by the Newham US Committee Travel Bursary. This work was further supported by NIH 1P01 AI073693 and the Lupus Research Institute.
Abbreviations:
- ANA
Anti-nuclear antibodies
- ANOVA
Analysis of variance
- ASC
Antibody secreting cell
- BCR
B cell receptor
- BM
Bone marrow
- CDR3
Complementarity-determining region 3
- cIg
Cytoplasmic Ig
- DAPI
4′,6-diamidino-2-phenylindole
- ELISA
Enzyme-linked immunosorbent assay
- ELISpot
Enzyme-linked immunospot
- FBS
Fetal bovine serum
- FO
Follicular B cell
- FVD
Fixable viability dye
- HBSS
Hank’s balanced salt solution
- Imm
Immature B cell
- mIg
Membrane Ig
- MZ
Marginal zone B cell
- ns
not significant
- PC
Plasma cell
- SLE
Systemic lupus erythematosus
- T2
T2 transitional B cell
Footnotes
Conflict of interest
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Suurmond J, Diamond B. Autoantibodies in systemic autoimmune diseases: specificity and pathogenicity. J Clin Invest 2015;125(6):2194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vas J, Gronwall C, Marshak-Rothstein A, Silverman GJ. Natural antibody to apoptotic cell membranes inhibits the proinflammatory properties of lupus autoantibody immune complexes. Arthritis Rheum 2012;64(10):3388–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goodnow CC, Crosbie J, Adelstein S, Lavoie TB, Smith-Gill SJ, Brink RA, et al. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature 1988;334(6184):676–82. [DOI] [PubMed] [Google Scholar]
- 4.Chen C, Prak EL, Weigert M. Editing disease-associated autoantibodies. Immunity 1997;6(1):97–105. [DOI] [PubMed] [Google Scholar]
- 5.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science 2003;301(5638): 1374–7. [DOI] [PubMed] [Google Scholar]
- 6.Erikson J, Radic MZ, Camper SA, Hardy RR, Carmack C, Weigert M. Expression of anti-DNA immunoglobulin transgenes in non-autoimmune mice. Nature 1991;349(6307):331–4. [DOI] [PubMed] [Google Scholar]
- 7.Mandik-Nayak L, Bui A, Noorchashm H, Eaton A, Erikson J. Regulation of anti-double-stranded DNA B cells in nonautoimmune mice: localization to the T-B interface of the splenic follicle. J Exp Med 1997;186(8): 1257–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malkiel S, Jeganathan V, Wolfson S, Manjarrez Orduno N, Marasco E, Aranow C, et al. Checkpoints for Autoreactive B Cells in the Peripheral Blood of Lupus Patients Assessed by Flow Cytometry. Arthritis Rheumatol 2016;68(9):2210–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pugh-Bernard AE, Silverman GJ, Cappione AJ, Villano ME, Ryan DH, Insel RA, et al. Regulation of inherently autoreactive VH4-34 B cells in the maintenance of human B cell tolerance. J Clin Invest 2001;108(7):1061–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yurasov S, Wardemann H, Hammersen J, Tsuiji M, Meffre E, Pascual V, et al. Defective B cell tolerance checkpoints in systemic lupus erythematosus. J Exp Med 2005;201(5):703–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y, Li H, Ni D, Weigert M. Anti-DNA B cells in MRL/lpr mice show altered differentiation and editing pattern. J Exp Med 2002;196(12):1543–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roark JH, Kuntz CL, Nguyen KA, Caton AJ, Erikson J. Breakdown of B cell tolerance in a mouse model of systemic lupus erythematosus. J Exp Med 1995;181(3):1157–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cappione A 3rd, Anolik JH, Pugh-Bernard A, Barnard J, Dutcher P, Silverman G, et al. Germinal center exclusion of autoreactive B cells is defective in human systemic lupus erythematosus. J Clin Invest 2005; 115(11):3205–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mandik-Nayak L, Seo SJ, Sokol C, Potts KM, Bui A, Erikson J. MRL-lpr/lpr mice exhibit a defect in maintaining developmental arrest and follicular exclusion of anti-double-stranded DNA B cells. J Exp Med 1999;189(11):1799–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen C, Li H, Tian Q, Beardall M, Xu Y, Casanova N, et al. Selection of anti-double-stranded DNA B cells in autoimmune MRL-lpr/lpr mice. J Immunol 2006;176(9):5183–90. [DOI] [PubMed] [Google Scholar]
- 16.Wellmann U, Werner A, Winkler TH. Altered selection processes of B lymphocytes in autoimmune NZB/W mice, despite intact central tolerance against DNA. Eur J Immunol 2001;31(9):2800–10. [DOI] [PubMed] [Google Scholar]
- 17.Kench JA, Russell DM, Nemazee D. Efficient peripheral clonal elimination of B lymphocytes in MRL/lpr mice bearing autoantibody transgenes. J Exp Med 1998;188(5):909–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klinman DM, Steinberg AD. Systemic autoimmune disease arises from polyclonal B cell activation. J Exp Med 1987;165(6): 1755–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hartley SB, Crosbie J, Brink R, Kantor AB, Basten A, Goodnow CC. Elimination from peripheral lymphoid tissues of self-reactive B lymphocytes recognizing membrane-bound antigens. Nature 1991;353(6346):765–9. [DOI] [PubMed] [Google Scholar]
- 20.Sandel PC, Monroe JG. Negative selection of immature B cells by receptor editing or deletion is determined by site of antigen encounter. Immunity 1999;10(3):289–99. [DOI] [PubMed] [Google Scholar]
- 21.Kouskoff V, Famiglietti S, Lacaud G, Lang P, Rider JE, Kay BK, et al. Antigens varying in affinity for the B cell receptor induce differential B lymphocyte responses. J Exp Med 1998;188(8): 1453–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40(9):1725. [DOI] [PubMed] [Google Scholar]
- 23.Barbas SM, Ditzel HJ, Salonen EM, Yang WP, Silverman GJ, Burton DR. Human autoantibody recognition of DNA. Proc Natl Acad Sci U S A 1995;92(7):2529–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tiller T, Kofer J, Kreschel C, Busse CE, Riebel S, Wickert S, et al. Development of self-reactive germinal center B cells and plasma cells in autoimmune Fc gammaRIIB-deficient mice. J Exp Med 2010;207(12):2767–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liang Z, Xie C, Chen C, Kreska D, Hsu K, Li L, et al. Pathogenic profiles and molecular signatures of antinuclear autoantibodies rescued from NZM2410 lupus mice. J Exp Med 2004;199(3):381–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H, Jiang Y, Prak EL, Radic M, Weigert M. Editors and editing of anti-DNA receptors. Immunity 2001;15(6):947–57. [DOI] [PubMed] [Google Scholar]
- 27.Julien S, Soulas P, Garaud JC, Martin T, Pasquali JL. B cell positive selection by soluble self-antigen. J Immunol 2002;169(8):4198–204. [DOI] [PubMed] [Google Scholar]
- 28.Dorner T, Giesecke C, Lipsky PE. Mechanisms of B cell autoimmunity in SLE. Arthritis Res Ther 2011;13(5):243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meyer-Bahlburg A, Andrews SF, Yu KO, Porcelli SA, Rawlings DJ. Characterization of a late transitional B cell population highly sensitive to BAFF-mediated homeostatic proliferation. J Exp Med 2008;205(1):155–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phan TG, Paus D, Chan TD, Turner ML, Nutt SL, Basten A, et al. High affinity germinal center B cells are actively selected into the plasma cell compartment. J Exp Med 2006;203(11):2419–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shinnakasu R, Inoue T, Kometani K, Moriyama S, Adachi Y, Nakayama M, et al. Regulated selection of germinal-center cells into the memory B cell compartment. Nat Immunol 2016;17(7):861–9. [DOI] [PubMed] [Google Scholar]
- 32.Weisel FJ, Zuccarino-Catania GV, Chikina M, Shlomchik MJ. A Temporal Switch in the Germinal Center Determines Differential Output of Memory B and Plasma Cells. Immunity 2016;44(1): 116–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sabouri Z, Schofield P, Horikawa K, Spierings E, Kipling D, Randall KL, et al. Redemption of autoantibodies on anergic B cells by variable-region glycosylation and mutation away from self-reactivity. Proc Natl Acad Sci U S A 2014;111(25):E2567–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan TD, Wood K, Hermes JR, Butt D, Jolly CJ, Basten A, et al. Elimination of germinal-center-derived self-reactive B cells is governed by the location and concentration of self-antigen. Immunity 2012;37(5):893–904. [DOI] [PubMed] [Google Scholar]
- 35.Tipton CM, Fucile CF, Darce J, Chida A, Ichikawa T, Gregoretti I, et al. Diversity, cellular origin and autoreactivity of antibody-secreting cell population expansions in acute systemic lupus erythematosus. Nat Immunol 2015;16(7):755–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Izui S, McConahey PJ, Dixon FJ. Increased spontaneous polyclonal activation of B lymphocytes in mice with spontaneous autoimmune disease. J Immunol 1978;121 (6): 2213–9. [PubMed] [Google Scholar]
- 37.Starobinski M, Lacour M, Reininger L, Izui S. Autoantibody repertoire analysis in normal and lupus-prone mice. J Autoimmun 1989;2(5):657–74. [DOI] [PubMed] [Google Scholar]
- 38.Klinman DM. Polyclonal B cell activation in lupus-prone mice precedes and predicts the development of autoimmune disease. J Clin Invest 1990;86(4):1249–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steeves MA, Marion TN. Tolerance to DNA in (NZB x NZW)F1 mice that inherit an anti-DNA V(H) as a conventional micro H chain transgene but not as a V(H) knock-in transgene. J Immunol 2004;172(11):6568–77. [DOI] [PubMed] [Google Scholar]
- 40.Yachimovich-Cohen N, Fischel R, Bachar N, Yarkoni Y, Eilat D. Autoimmune NZB/NZW F1 mice utilize B cell receptor editing for generating high-affinity anti-dsDNA autoantibodies from low-affinity precursors. Eur J Immunol 2003;33(9):2469–78. [DOI] [PubMed] [Google Scholar]
- 41.Sweet RA, Christensen SR, Harris ML, Shupe J, Sutherland JL, Shlomchik MJ. A new site-directed transgenic rheumatoid factor mouse model demonstrates extrafollicular class switch and plasmablast formation. Autoimmunity 2010;43(8):607–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pewzner-Jung Y, Friedmann D, Sonoda E, Jung S, Rajewsky K, Eilat D. B cell deletion, anergy, and receptor editing in "knock in" mice targeted with a germline-encoded or somatically mutated anti-DNA heavy chain. J Immunol 1998;161(9):4634–45. [PubMed] [Google Scholar]
- 43.Bynoe MS, Spatz L, Diamond B. Characterization of anti-DNA B cells that escape negative selection. Eur J Immunol 1999;29(4):1304–13. [DOI] [PubMed] [Google Scholar]
- 44.Chen C, Nagy Z, Radic MZ, Hardy RR, Huszar D, Camper SA, et al. The site and stage of anti-DNA B-cell deletion. Nature 1995;373(6511):252–5. [DOI] [PubMed] [Google Scholar]
- 45.Tiller T, Tsuiji M, Yurasov S, Velinzon K, Nussenzweig MC, Wardemann H. Autoreactivity in human IgG+ memory B cells. Immunity 2007;26(2):205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scheid JF, Mouquet H, Kofer J, Yurasov S, Nussenzweig MC, Wardemann H. Differential regulation of self-reactivity discriminates between IgG+ human circulating memory B cells and bone marrow plasma cells. Proc Natl Acad Sci U S A 2011;108(44):18044–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mietzner B, Tsuiji M, Scheid J, Velinzon K, Tiller T, Abraham K, et al. Autoreactive IgG memory antibodies in patients with systemic lupus erythematosus arise from nonreactive and polyreactive precursors. Proc Natl Acad Sci U S A 2008;105(28):9727–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.