Abstract
Tick-borne encephalitis virus (TBEV), a member of the genus Flavivirus within the family Flaviviridae, causes fatal encephalitis with severe sequelae in humans. TBEV is prevalent over a wide area of the Eurasian continent including Europe, Russia, Far-Eastern Asia, and Japan. While it was previously thought that TBEV was not endemic in Japan, the first confirmed case of serologically diagnosed TBE was reported in 1993 in the southern area of Hokkaido Prefecture, Japan. In addition, TBEV has been isolated from dogs, wild rodents and ticks in the area. Our epizootiological survey indicated that endemic foci of TBEV were maintained in Hokkaido and other areas of Honshu. TBEV can be divided into three subtypes based on phylogenetic analyses. The Japanese isolates were classified as the Far Eastern subtype, which causes severe neural disorders with a higher mortality rate up to 30%. However, how viral replication and pathogenicity contribute to the neurological manifestations remains unclear. Recent studies have revealed distinctive mechanisms of TBEV pathogenicity and viral genetic factors associated with virulence. This review discusses the recent findings regarding the epidemiology and pathogenesis of TBEV.
Keywords: epidemiology, flavivirus, pathogenicity, tick-borne encephalitis virus
Tick-borne encephalitis virus (TBEV), belonging to the Flavivirus genus of the Flaviviridae family, causes mild or moderate febrile illness and fatal encephalitis with sequelae in humans. TBEV is divided into European, Siberian, and Far-Eastern subtypes. The Far-Eastern subtype is the most lethal. The virus is endemic in many parts of Europe and Asia [27, 39], and >10,000 cases of the disease are reported annually. TBEV is maintained in transmission cycles between ixodid ticks and wild mammalian hosts, particularly rodents. Furthermore, transovarial and transstadial transmission have been reported in tick vectors [6, 35]. TBEV infects a wide range of animal species via bites from infected ticks. Alimentary transmission has been also reported via the consumption of unpasteurized milk and milk products from infected livestock [4].
Tick bites often remain unnoticed, and the incubation period of TBE is usually 7–14 days (range, 2–28 days) [19, 28]. The symptoms of TBEV infection can be categorized in two phases. The European subtype produces biphasic febrile illness, whereas the other two subtypes display a monophasic course. Flu-like symptoms are observed during the initial viremic phase of the illness, including fever, fatigue, general malaise, and headache and muscular pains; these symptoms last 2–7 days (range, 1–10 days) [12, 19]. No signs or symptoms of meningoencephalitis are usually seen during this phase. The asymptomatic interval before the second phase lasts up to 7 days (range 1–21 days). Illness caused by the Far-Eastern and Siberian subtypes usually progress without this asymptomatic phase. The second phase presents with symptoms and signs ranging from mild meningitis to severe encephalitis. The main clinical neurological syndromes associated with TBE are febrile headache, aseptic meningitis, meningoencephalitis, meningomyeloencephalitis, and postencephalitic syndrome. The chronic form of TBE is often associated with the Siberian subtype [33]. Chronic disease begins with or without the typical acute-phase symptoms. In many cases, it can take years to develop neurological symptoms after the tick bite.
As no specific antiviral treatments are available for TBE, prophylaxis is important for its control. Active immunization with a vaccine is effective for the prevention of TBE. Immunogenicity is mostly associated with virions, particularly with the viral envelope protein. Formaldehyde-inactivated and purified whole virus is used as a TBE vaccine, which is currently produced by five manufacturers. The European TBE vaccines have a well-established safety record [2]. In Austria, the FSME-immune vaccine is used extensively, and mass vaccination has reduced the number of reported cases [14]. However, no TBE vaccines are currently licensed in Japan.
In Japan, patients infected with a virus within the TBE serocomplex had encephalitis during an epidemic of Japanese encephalitis (JE) in 1948 in the Tokyo area. The isolated virus, named Negishi virus, was retrospectively identified as a member of the louping ill virus through antigenic and phylogenetic analyses conducted decades later [32, 42]. No subsequent cases of TBE in Japan were reported until 1993, when a case of viral encephalitis in southern Hokkaido was diagnosed as TBE [29]. TBEV was isolated from dogs and Ixodes ovatus in the area where the patient lived, and the virus strains were identified as the Far-Eastern subtype [40, 41]. In 2016 and 2017, three more cases of TBE were identified in Hokkaido [43, 45]. To control TBEV infection and to design an effective prevention and vaccination plan, requisite specific targeting was performed to define TBEV-endemic areas and to identify the characteristics of endemic TBEV.
DEVELOPMENT OF NEW SEROLOGICAL DIAGNOSTIC METHOD AND ITS APPLICATION TO EPIDEMIOLOGICAL RESEARCH FOR TBE IN JAPAN
In the Prevention of Infectious Diseases and Medical Care for Infectious Patients Act that became legislation in Japan in 1999, TBE was classified as a reportable infectious disease and TBEV was classified as a Class 3 select agent handled under bio-safety level (BSL) 3 conditions. Therefore, laboratory examinations are available only in limited facilities due to restrictions in handling TBEV as a BSL 3 agent, leading to the difficulties in conducting epidemiological survey of TBE in Japan. Several diagnostic tests are available to detect TBEV infection. The neutralization test (NT) is useful for areas where two or more flaviviruses are endemic, as it has high specificity for each virus. However, performing the NT is time-consuming, and high-biosafety-level facilities are required to handle the live virus. The enzyme-linked immunosorbent assay (ELISA) is a relatively safe diagnostic method for detecting antibodies in infected individuals because it utilizes inactivated TBE virions. However, the commercially available TBE-ELISA kit shows cross-reactivity against antibodies to other flaviviruses [7, 16, 30], and these kits are applicable only to infection in humans, not in other animals. Therefore, it is critical to develop a safe and simple serological diagnostic method that can be applied to a wide range of animal species.
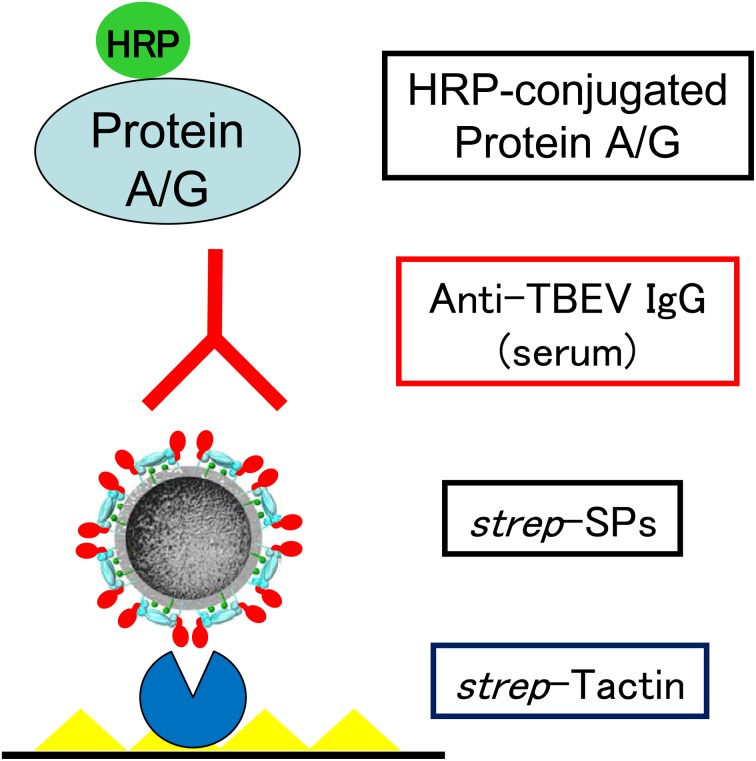
The entire premembrane (prM) and envelope (E) gene sequences are expressed and the products are secreted as membrane-bound subviral particles (SPs) [1]. SPs consist of a viral envelope without a nucleocapsid or genomic RNA. Because SPs maintain antigenicity similar to that of authentic virions, they have been applied as substitutes for infectious virions in serological diagnoses [8, 17, 31]. In a previous study, we constructed SPs of TBEV by fusing a small peptide tag (strep-tag), which has a high binding affinity for strep-tactin, to the N-terminus of the E protein. The SPs with strep-tag (strep-SPs) were used in an IgG-ELISA to detect reactive antibodies in a broad range of mammal species (Fig. 1) [18]. Compared to NT results, the ELISA showed high sensitivity and specificity (>95%) (Table 1), showing no cross-reactivity with antibodies to Japanese encephalitis virus.
Fig. 1.
IgG-ELISA using strep-tagged subviral particles of TBEV. Strep-tagged subviral particles (strep-SPs) of TBEV are captured by strep-tactin coated onto a plate, and anti-TBEV IgG antibodies in serum samples are reacted. The IgG antibodies are detected by enzyme-conjugated Protein A/G.
Table 1. Comparison of the results obtains by neutralization and IgG-ELISA using strep-SPs (SPs ELISA) in human serum (From Inagaki et al. [18]).
| Neutralization test | SPs ELISA |
Total | |
|---|---|---|---|
| Positive | Negative | ||
| Positive | 78 | 4 | 82 |
| Negative | 1 | 24 | 25 |
Our IgG-ELISA using strep-SPs was introduced to prefectural institute of public health for serological diagnoses of TBE-suspected human cases, which led to the detection of patients with TBE in 2017 [43]. In addition, the IgG-ELISA was employed in sero-epizootiological surveys in wild animals, which revealed that the endemic foci of TBEV are widely distributed in Hokkaido, and that TBEV or TBE–serocomplex viruses may be broadly distributed in western Japan.
VIRAL GENETIC DETERMINANT OF TBEV PATHOGENICITY
The flavivirus genome consists of a positive-polarity, single-stranded RNA of approximately 11 kb, which encodes three structural proteins: (the core [C], prM, and E proteins) and seven non-structural (NS) proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5) within a single open reading frame [5]. The 5′ and 3′ UTRs predict secondary structures that are implicated in viral replication, translation, and packaging of the genome [11, 34].
Reverse genetics of viruses allows the generation and manipulation of viral genomes to investigate the direct effects of changes on virus biology and pathogenesis. Infectious cDNA clones of flaviviruses, including TBEV, generated using reverse genetics systems [10, 13, 24] have provided useful platforms on which to investigate the genetic determinants of flavivirus virulence.
Several viral determinants of TBEV pathogenicity have been identified using reverse genetics (Table 2). For example, the important role of the E protein in pathogenicity has been well characterized. Mutations of specific amino acid residues in the upper lateral surface of domain III in the E protein affect neurovirulence in mice [23]. Mutations that increase glycosaminoglycan (GAG)-binding affinity, located on the outer surface of the E protein, usually occur near existing positive clusters [26]. Increased GAG-binding affinity appears to be a major mechanism of attenuation of neuroinvasiveness via the E proteins. In the majority of TBEV strains, as in other flaviviruses, the E protein contains a conserved N-linked glycosylation site. In mammalian cells, loss of glycosylation affects the conformation of protein E during secretion, reducing the infectivity of secreted virions and affecting TBEV pathogenicity in mice [46].
Table 2. Viral genetic determinant of TBEV pathogenicity identified by using reverse genetics.
| Viral gene | Functions in TBEV pathogenicity | Reference |
|---|---|---|
| 5′ UTR | Genomic RNA cyclization and replication | [9, 20] |
| Viral RNA transport in dendrites | [15] | |
| E | Receptor-binding and neurovirulence | [23] |
| Glycosaminoglycan-binding affinity in neuroinvasiveness | [26] | |
| Maturation of the E protein in mammalian cells | [46] | |
| NS5 | Interferon antagonism | [22] |
| Attenuation of neurite outgrowth | [44] | |
| 3′ UTR | Genomic RNA cyclization and replication | [9, 20] |
| Adaptation or selection in mammalian cells | [37, 38] |
The viral NS5 protein consists of two principle domains: the methyltransferase (MTase) domain located on the N terminal side of the protein and the RNA-dependent RNA polymerase (RdRp) domain on the C terminal side [21, 36]. NS5 in several flaviviruses has interferon (IFN) antagonist activity [3]. Tick-borne flavivirus infection downregulates the cellular expression of IFN receptor subunit, IFNAR1, through an interaction between NS5 and the host protein prolidase [22]. A TBEV variant with a NS5 mutation lacking IFN-I antagonism shows reduced virulence in mice. While the majority of TBE complex viruses cause encephalitis, three tick-borne flaviviruses cause hemorrhagic disease, including Omsk hemorrhagic fever virus (OHFV). A recent study that compared TBEV and OHFV revealed that a 4 amino acid region near the C-terminus of NS5 is critical for the neuropathogenesis of TBEV [44]. This region is involved in the attenuation of neurite outgrowth, resulting in the neurological disease phenotype in mice.
UTRs are important for many functions in flavivirus multiplication. The complementary sequences in the 5′ and 3′ UTRs cyclize the viral genome, which is essential for viral genome replication [9, 20]. A recent study showed that the 5′ UTR of TBEV functions in RNA transport in neuron dendrites. Using this function, TBEV hijacks the transport system of host mRNA in dendrites and affects neuronal functions, such as neurogenesis and the plasticity of synaptic communication [15].
The 3′ UTR can be divided into two regions: the variable region, which varies among TBEV strains, and the core element, which has a highly conserved sequence [11]. The core element shows a high degree of sequence conservation among TBEV strains and contains sequences necessary for viral genome replication, such as those involved in cyclization [20]; deletions in the core element cause virus attenuation in mice [25]. Recent studies have identified deletions and insertions of poly (A) sequences in the variable region, which cause severe pathological changes associated with the Far Eastern subtype of TBEV [37, 38]. A deletion in the 3′ UTR occurs during passage in mammalian cell cultures or in mice; however, strains freshly isolated from ticks and wild rodents have no deletion in the variable region. Therefore, this region is considered essential for the natural transmission cycle of TBEV. These data suggest that the deletion caused by adaptation or selection in mammalian cells may be associated with the increased virulence in mammals.
Important advances in TBEV research have been made in recent years. Modern diagnostic methods are being used to investigate TBEV in an increasing number of laboratories, which will lead to further understanding of TBEV and raise greater awareness of TBE. Understanding the pathogenic mechanism of TBEV will contribute to the future development of vaccines and therapies to treat TBE.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
Acknowledgments
This work was supported by JSPS KAKENHI (Grant Number 17H03910) and AMED (grant numbers 16fk0108117j0001 and 18fk0108036h0002).
REFERENCES
- 1.Allison S. L., Stadler K., Mandl C. W., Kunz C., Heinz F. X.1995. Synthesis and secretion of recombinant tick-borne encephalitis virus protein E in soluble and particulate form. J. Virol. 69: 5816–5820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anonymous Vaccines against tick-borne encephalitis: WHO position paper. Wkly. Epidemiol. Rec. 86: 241–256. [PubMed] [Google Scholar]
- 3.Best S. M., Morris K. L., Shannon J. G., Robertson S. J., Mitzel D. N., Park G. S., Boer E., Wolfinbarger J. B., Bloom M. E.2005. Inhibition of interferon-stimulated JAK-STAT signaling by a tick-borne flavivirus and identification of NS5 as an interferon antagonist. J. Virol. 79: 12828–12839. doi: 10.1128/JVI.79.20.12828-12839.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bogovic P., Strle F.2015. Tick-borne encephalitis: A review of epidemiology, clinical characteristics, and management. World J. Clin. Cases 3: 430–441. doi: 10.12998/wjcc.v3.i5.430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chambers T. J., Hahn C. S., Galler R., Rice C. M.1990. Flavivirus genome organization, expression, and replication. Annu. Rev. Microbiol. 44: 649–688. doi: 10.1146/annurev.mi.44.100190.003245 [DOI] [PubMed] [Google Scholar]
- 6.Danielová V., Daniel M., Schwarzová L., Materna J., Rudenko N., Golovchenko M., Holubová J., Grubhoffer L., Kilián P.2010. Integration of a tick-borne encephalitis virus and Borrelia burgdorferi sensu lato into mountain ecosystems, following a shift in the altitudinal limit of distribution of their vector, Ixodes ricinus (Krkonose mountains, Czech Republic). Vector Borne Zoonotic Dis. 10: 223–230. doi: 10.1089/vbz.2009.0020 [DOI] [PubMed] [Google Scholar]
- 7.Dobler G., Treib J., Kiessig S. T., Blohn W. V., Frösner G., Haass A.1996. Diagnosis of tick-borne encephalitis: evaluation of sera with borderline titers with the TBE-ELISA. Infection 24: 405–406. doi: 10.1007/BF01716097 [DOI] [PubMed] [Google Scholar]
- 8.Ferlenghi I., Clarke M., Ruttan T., Allison S. L., Schalich J., Heinz F. X., Harrison S. C., Rey F. A., Fuller S. D.2001. Molecular organization of a recombinant subviral particle from tick-borne encephalitis virus. Mol. Cell 7: 593–602. doi: 10.1016/S1097-2765(01)00206-4 [DOI] [PubMed] [Google Scholar]
- 9.Gould E. A., Solomon T.2008. Pathogenic flaviviruses. Lancet 371: 500–509. doi: 10.1016/S0140-6736(08)60238-X [DOI] [PubMed] [Google Scholar]
- 10.Gritsun T. S., Gould E. A.1995. Infectious transcripts of tick-borne encephalitis virus, generated in days by RT-PCR. Virology 214: 611–618. doi: 10.1006/viro.1995.0072 [DOI] [PubMed] [Google Scholar]
- 11.Gritsun T. S., Venugopal K., Zanotto P. M., Mikhailov M. V., Sall A. A., Holmes E. C., Polkinghorne I., Frolova T. V., Pogodina V. V., Lashkevich V. A., Gould E. A.1997. Complete sequence of two tick-borne flaviviruses isolated from Siberia and the UK: analysis and significance of the 5′ and 3′-UTRs. Virus Res. 49: 27–39. doi: 10.1016/S0168-1702(97)01451-2 [DOI] [PubMed] [Google Scholar]
- 12.Haglund M., Günther G.2003. Tick-borne encephalitis—pathogenesis, clinical course and long-term follow-up. Vaccine 21 Suppl 1: S11–S18. doi: 10.1016/S0264-410X(02)00811-3 [DOI] [PubMed] [Google Scholar]
- 13.Hayasaka D., Gritsun T. S., Yoshii K., Ueki T., Goto A., Mizutani T., Kariwa H., Iwasaki T., Gould E. A., Takashima I.2004. Amino acid changes responsible for attenuation of virus neurovirulence in an infectious cDNA clone of the Oshima strain of tick-borne encephalitis virus. J. Gen. Virol. 85: 1007–1018. doi: 10.1099/vir.0.19668-0 [DOI] [PubMed] [Google Scholar]
- 14.Heinz F. X., Holzmann H., Essl A., Kundi M.2007. Field effectiveness of vaccination against tick-borne encephalitis. Vaccine 25: 7559–7567. doi: 10.1016/j.vaccine.2007.08.024 [DOI] [PubMed] [Google Scholar]
- 15.Hirano M., Muto M., Sakai M., Kondo H., Kobayashi S., Kariwa H., Yoshii K.2017. Dendritic transport of tick-borne flavivirus RNA by neuronal granules affects development of neurological disease. Proc. Natl. Acad. Sci. U.S.A. 114: 9960–9965. doi: 10.1073/pnas.1704454114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holzmann H., Kundi M., Stiasny K., Clement J., McKenna P., Kunz C., Heinz F. X.1996. Correlation between ELISA, hemagglutination inhibition, and neutralization tests after vaccination against tick-borne encephalitis. J. Med. Virol. 48: 102–107. doi: [DOI] [PubMed] [Google Scholar]
- 17.Ikawa-Yoshida A., Yoshii K., Kuwahara K., Obara M., Kariwa H., Takashima I.2011. Development of an ELISA system for tick-borne encephalitis virus infection in rodents. Microbiol. Immunol. 55: 100–107. doi: 10.1111/j.1348-0421.2010.00296.x [DOI] [PubMed] [Google Scholar]
- 18.Inagaki E., Sakai M., Hirano M., Muto M., Kobayashi S., Kariwa H., Yoshii K.2016. Development of a serodiagnostic multi-species ELISA against tick-borne encephalitis virus using subviral particles. Ticks Tick Borne Dis. 7: 723–729. doi: 10.1016/j.ttbdis.2016.03.002 [DOI] [PubMed] [Google Scholar]
- 19.Kaiser R.2008. Tick-borne encephalitis. Infect. Dis. Clin. North Am. 22: 561–575, x x. doi: 10.1016/j.idc.2008.03.013 [DOI] [PubMed] [Google Scholar]
- 20.Kofler R. M., Hoenninger V. M., Thurner C., Mandl C. W.2006. Functional analysis of the tick-borne encephalitis virus cyclization elements indicates major differences between mosquito-borne and tick-borne flaviviruses. J. Virol. 80: 4099–4113. doi: 10.1128/JVI.80.8.4099-4113.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koonin E. V.1993. Computer-assisted identification of a putative methyltransferase domain in NS5 protein of flaviviruses and lambda 2 protein of reovirus. J. Gen. Virol. 74: 733–740. doi: 10.1099/0022-1317-74-4-733 [DOI] [PubMed] [Google Scholar]
- 22.Lubick K. J., Robertson S. J., McNally K. L., Freedman B. A., Rasmussen A. L., Taylor R. T., Walts A. D., Tsuruda S., Sakai M., Ishizuka M., Boer E. F., Foster E. C., Chiramel A. I., Addison C. B., Green R., Kastner D. L., Katze M. G., Holland S. M., Forlino A., Freeman A. F., Boehm M., Yoshii K., Best S. M.2015. Flavivirus antagonism of type I interferon signaling reveals prolidase as a regulator of IFNAR1 surface expression. Cell Host Microbe 18: 61–74. doi: 10.1016/j.chom.2015.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mandl C. W., Allison S. L., Holzmann H., Meixner T., Heinz F. X.2000. Attenuation of tick-borne encephalitis virus by structure-based site-specific mutagenesis of a putative flavivirus receptor binding site. J. Virol. 74: 9601–9609. doi: 10.1128/JVI.74.20.9601-9609.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mandl C. W., Ecker M., Holzmann H., Kunz C., Heinz F. X.1997. Infectious cDNA clones of tick-borne encephalitis virus European subtype prototypic strain Neudoerfl and high virulence strain Hypr. J. Gen. Virol. 78: 1049–1057. doi: 10.1099/0022-1317-78-5-1049 [DOI] [PubMed] [Google Scholar]
- 25.Mandl C. W., Holzmann H., Meixner T., Rauscher S., Stadler P. F., Allison S. L., Heinz F. X.1998. Spontaneous and engineered deletions in the 3′ noncoding region of tick-borne encephalitis virus: construction of highly attenuated mutants of a flavivirus. J. Virol. 72: 2132–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mandl C. W., Kroschewski H., Allison S. L., Kofler R., Holzmann H., Meixner T., Heinz F. X.2001. Adaptation of tick-borne encephalitis virus to BHK-21 cells results in the formation of multiple heparan sulfate binding sites in the envelope protein and attenuation in vivo. J. Virol. 75: 5627–5637. doi: 10.1128/JVI.75.12.5627-5637.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mansfield K. L., Johnson N., Phipps L. P., Stephenson J. R., Fooks A. R., Solomon T.2009. Tick-borne encephalitis virus - a review of an emerging zoonosis. J. Gen. Virol. 90: 1781–1794. doi: 10.1099/vir.0.011437-0 [DOI] [PubMed] [Google Scholar]
- 28.Mickiene A., Laiskonis A., Günther G., Vene S., Lundkvist A., Lindquist L.2002. Tickborne encephalitis in an area of high endemicity in lithuania: disease severity and long-term prognosis. Clin. Infect. Dis. 35: 650–658. doi: 10.1086/342059 [DOI] [PubMed] [Google Scholar]
- 29.Morita K., Igarashi A., Sato T., Takezawa T.1994. A suspected case of tick-borne encephalitis in Hokkaido. Infectious Agents Surveillance Report 15: 273–274. [Google Scholar]
- 30.Niedrig M., Vaisviliene D., Teichmann A., Klockmann U., Biel S. S.2001. Comparison of six different commercial IgG-ELISA kits for the detection of TBEV-antibodies. J. Clin. Virol. 20: 179–182. doi: 10.1016/S1386-6532(00)00178-5 [DOI] [PubMed] [Google Scholar]
- 31.Obara M., Yoshii K., Kawata T., Hayasaka D., Goto A., Mizutani T., Kariwa H., Takashima I.2006. Development of an enzyme-linked immunosorbent assay for serological diagnosis of tick-borne encephalitis using subviral particles. J. Virol. Methods 134: 55–60. doi: 10.1016/j.jviromet.2005.11.018 [DOI] [PubMed] [Google Scholar]
- 32.Okuno T., Oya A., Ito T.1961. The identification of Negishi virus, a presumably new member of Russian spring-summer encephalitis virus family isolated in Japan. Jpn. J. Med. Sci. Biol. 14: 51–59. doi: 10.7883/yoken1952.14.51 [DOI] [PubMed] [Google Scholar]
- 33.Poponnikova T. V.2006. Specific clinical and epidemiological features of tick-borne encephalitis in Western Siberia. Int. J. Med. Microbiol. 296 Suppl 40: 59–62. doi: 10.1016/j.ijmm.2006.01.023 [DOI] [PubMed] [Google Scholar]
- 34.Proutski V., Gould E. A., Holmes E. C.1997. Secondary structure of the 3′ untranslated region of flaviviruses: similarities and differences. Nucleic Acids Res. 25: 1194–1202. doi: 10.1093/nar/25.6.1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Radda A., Hofmann H., Pretzmann G.1969. Threshold of viraemia in Apodemus flavicollis for infection of Ixodes ricinus with tick-borne encephalitis virus. Acta Virol. 13: 74–77. [PubMed] [Google Scholar]
- 36.Rice C. M., Lenches E. M., Eddy S. R., Shin S. J., Sheets R. L., Strauss J. H.1985. Nucleotide sequence of yellow fever virus: implications for flavivirus gene expression and evolution. Science 229: 726–733. doi: 10.1126/science.4023707 [DOI] [PubMed] [Google Scholar]
- 37.Sakai M., Muto M., Hirano M., Kariwa H., Yoshii K.2015. Virulence of tick-borne encephalitis virus is associated with intact conformational viral RNA structures in the variable region of the 3′-UTR. Virus Res. 203: 36–40. doi: 10.1016/j.virusres.2015.03.006 [DOI] [PubMed] [Google Scholar]
- 38.Sakai M., Yoshii K., Sunden Y., Yokozawa K., Hirano M., Kariwa H.2014. Variable region of the 3′ UTR is a critical virulence factor in the Far-Eastern subtype of tick-borne encephalitis virus in a mouse model. J. Gen. Virol. 95: 823–835. doi: 10.1099/vir.0.060046-0 [DOI] [PubMed] [Google Scholar]
- 39.Suss J.2008. Tick-borne encephalitis in Europe and beyond—the epidemiological situation as of 2007. Euro Surveill. 13: 13. [PubMed] [Google Scholar]
- 40.Takashima I., Morita K., Chiba M., Hayasaka D., Sato T., Takezawa C., Igarashi A., Kariwa H., Yoshimatsu K., Arikawa J., Hashimoto N.1997. A case of tick-borne encephalitis in Japan and isolation of the the virus. J. Clin. Microbiol. 35: 1943–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takeda T., Ito T., Chiba M., Takahashi K., Niioka T., Takashima I.1998. Isolation of tick-borne encephalitis virus from Ixodes ovatus (Acari: Ixodidae) in Japan. J. Med. Entomol. 35: 227–231. doi: 10.1093/jmedent/35.3.227 [DOI] [PubMed] [Google Scholar]
- 42.Venugopal K., Buckley A., Reid H. W., Gould E. A.1992. Nucleotide sequence of the envelope glycoprotein of Negishi virus shows very close homology to louping ill virus. Virology 190: 515–521. doi: 10.1016/0042-6822(92)91245-P [DOI] [PubMed] [Google Scholar]
- 43.Yamaguchi H., Komagome R., Miyoshi M., Ishida S., Nagano H., Okano M., Shimada K., Fukami M., Tanaka K., Takeuchi N., Yoshii K., Kobayashi S., Kariwa H.2018. tick-borne encephalitis in Hokkaido in 2017. Infectious Agents Surveillance Report 39: 46–47. [Google Scholar]
- 44.Yoshii K., Sunden Y., Yokozawa K., Igarashi M., Kariwa H., Holbrook M. R., Takashima I.2014. A critical determinant of neurological disease associated with highly pathogenic tick-borne flavivirus in mice. J. Virol. 88: 5406–5420. doi: 10.1128/JVI.00421-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoshii K., Tajima Y., Bando K., Moriuchi R.2016. A confirmed case of tick-borne encephalitis in Hokkaido in 2016. Infectious Agents Surveillance Report 38: 126. [Google Scholar]
- 46.Yoshii K., Yanagihara N., Ishizuka M., Sakai M., Kariwa H.2013. N-linked glycan in tick-borne encephalitis virus envelope protein affects viral secretion in mammalian cells, but not in tick cells. J. Gen. Virol. 94: 2249–2258. doi: 10.1099/vir.0.055269-0 [DOI] [PubMed] [Google Scholar]