Abstract
We compared the temporomandibular joint structure between species of the order Carnivora and investigated its variation among family lineages. We also investigated the effect of the masticatory muscle physiological cross-sectional area (PCSA) on temporomandibular joint structure. The masticatory muscle is composed of multiple muscles, which contract in different directions and exert pressure on the temporomandibular joint. We investigated the effect of the ratio of each muscle’s PCSA—an indicator of muscle force—and muscle size relative to body size on temporomandibular joint structure. The temporalis PCSA relative to body size showed the highest correlation with temporomandibular joint structure. When the temporalis PCSA is large relative to body size, the preglenoid projects caudally, the postglenoid projects rostrally and the pre-postglenoid angle interval is small, indicating that the condyle is locked in the fossa to reinforce the temporomandibular joint. Most Carnivora use blade-like carnassial teeth when slicing food. However, dislocation occurs when the carnassial teeth are used by the temporalis muscle. Our results suggest that the temporomandibular joint is reinforced to prevent dislocation caused by the temporalis muscle. In Mustelidae, the temporomandibular joint with a rostrally projecting postglenoid is suitable for carnassial biting using the temporalis muscle. In Felidae, the force of the masseter onto the carnassial teeth is diverted to the canine by tightening the temporomandibular joint. In Canidae, the masticatory muscle arrangement is well-balanced, enabling combined action. Hence, reinforcement of the temporomandibular joint by bone structure is unnecessary.
Keywords: Carnivora, masseter, masticatory muscle, temporalis, temporomandibular joint
Mastication, which enables efficient energy extraction, is a process unique to mammals. During mastication, the masticatory muscles, the structure of the temporomandibular joint and the shape of the teeth play important roles. Carnivora are an ecologically diverse group with a wide habitat range and are found in all habitats, both terrestrial and aquatic. Their diet consists of not only meat, but also various foods such as fruit and fish [12, 28]. The masticatory morphology varies among lineages. Carnivorans have blade-like molars with laterally flattened cusps [12], which are called carnassial teeth. The cusps of the carnassial teeth in species belonging to the Felidae are especially sharp and are appropriate for slashing flesh [36, 37, 49, 51].
The masticatory force is exerted by multiple muscles, which contract in different directions. The masticatory muscles include the temporalis muscle, the masseter muscle, the medial pterygoid muscle and the lateral pterygoid muscle. Among these, the masseter muscle can be separated into three layers [48, 56]. The arrangement of the masticatory muscles also varies among lineages. Mustelidae have a large temporalis muscle, whereas Felidae have a large masseter muscle [24, 34, 35].
The temporomandibular joint is comprised of the mandibular condyle, the glenoid fossa, which lies in the zygomatic process of the temporal bone, the articular disc and multiple ligaments. A prominent postglenoid process marks the posteromedial boundary of the fossa and a bony ridge continues laterally to form its posterior margin. The preglenoid, which is another bony ridge that forms the anterior margin of the joint, is developed slightly at its anterolateral border. These bony limits restrict the condylar movements in Carnivora [15]. The carnivoran temporomandibular joint is generally a hinge structure with a mediolaterally narrow and cylindrical condyle locked in the mandibular fossa [23]. Although limited because of canines and carnassials, a transverse movement is also possible [15, 37]. For this reason, we suspected possible variations in the structure of the temporomandibular joint in carnivorans. Although the temporomandibular joint structure has been investigated in some carnivoran species, variations in the joint’s traits among lineages are not fully understood [4, 8, 16, 25].
Furthermore, the temporomandibular joint of carnivorans is suitable for pulling the mandible vertically using the temporalis muscle [5, 26, 48]. However, when carnivorans slice the meat with their carnassial teeth using the temporalis muscle, the load on the temporomandibular joint, which is the fulcrum, increases and results in dislocation. When carnivorans bite with their canine teeth using the masseter muscle, the temporomandibular joint load increases and also results in dislocation [38]. To prevent dislocation of the temporomandibular joint, the combined action of the temporalis muscle and the masseter muscle is necessary [5, 48]. Turnbull [48] illustrated this process in a refined diagram based on Davis [5], including the force of the medial pterygoid muscle, which acts synergistically with the masseter muscle. In addition, Davis remarked that together with the combined action of the temporalis muscle and the masseter muscle, the development of the postglenoid also prevents dislocation of the temporomandibular joint. The force performed by the masticatory muscle moves the mandible and is transferred to the teeth to destruct the prey. This force is also transferred to the temporomandibular joint. To date, the effect of the masticatory muscle on temporomandibular joint structure has been studied in certain species [5, 7, 8, 17, 38, 48]. However, there has been no study on a wide range of different carnivoran species. For this reason, the general pattern of the relationship between the masticatory muscle and the temporomandibular joint in carnivorans is not clearly understood. This is primarily because of the difficulty in collecting masticatory data from carnivorans, as many species are threatened. Collecting in vivo data on each masticatory muscle using electromyography in all carnivoran species is nearly impossible because many species are rare and even when specimens are available, they are often difficult to manipulate experimentally. Thus, it is more realistic to measure the muscle physiological cross-sectional area (PCSA), which is an indicator of muscle force, from dead carnivorans. There are many studies that focus on masticatory muscle PCSA measurements [1,2,3, 6, 9,10,11, 13, 19,20,21,22, 27, 29,30,31,32,33, 39,40,41,42,43,44,45,46,47, 54, 55]. Nonetheless, none of the previous studies focused on the relationship between the masticatory muscle PCSA and the temporomandibular joint structure. By comparing the carnivoran masticatory muscle PCSA and the temporomandibular joint structure, we are able to understand the relationship between the change in the arrangement of masticatory muscles and the change in the temporomandibular joint structure. This study investigated the morphological pattern of the carnivoran temporomandibular joint by focusing on the relationship between the masticatory muscle PCSA and the temporomandibular joint structure.
MATERIALS AND METHODS
Samples and CT scanning
Twenty-eight species of carnivorans from ten families were analyzed (Table 1). Specimens used in this study were obtained from zoos. All of the animals died either accidentally or because of diseases. They were donated to The University Museum of The University of Tokyo. Roadkill animals were also included in the study. The skulls of all samples were CT scanned (Asteion PREMIUM 4 EDITION, Toshiba Medical Systems, Tokyo, Japan) to measure preglenoid angle and postglenoid angle. Pixel resolution ranged from 0.19 to 0.60 mm and the thickness of the slices ranged from 0.3 to 0.5 mm, depending on the size of the skull. The current and voltage were 120 kV and 100 mA, respectively. The 3D visualization was conducted using Avizo 6.1 (Visualization Sciences Group, Burlington, MA, U.S.A.).
Table 1. Descriptive data of specimens.
| Family | Species | Common name | n | Body mass (kg) |
Skull length (mm) |
Sex | Specimen number | Reference |
|---|---|---|---|---|---|---|---|---|
| Felidae | Puma concolor | Puma | 1 | - | 192.80 | Male | UMUT14195 | [24] |
| Felis catus | Cat | 2 | - | 85.33 | - | UMUT14193, UMUT14199 | [24] | |
| Leopardus pardalis | Ocelot | 1 | - | 129.30 | Male | UMUT18001 | This study | |
| Neofelis nebulosa | Clouded leopard | 1 | - | 134.80 | Female | UMUT18005 | This study | |
| Panthera leo | Asiatic lion | 1 | - | 284.63 | Female | UMUT18003 | This study | |
| Panthera pardus | Leopard | 1 | - | 164.63 | Female | UMUT15090 | This study | |
| Panthera tigris | Siberian Tiger | 1 | - | 278.23 | Female | UMUT18002 | This study | |
| Panthera uncia | Snow leopard | 1 | - | 171.14 | Female | UMUT14201 | [24] | |
| Viverridae | Paguma larvata | Masked palm civet | 2 | - | 106.47 | - | UMUT14186, UMUT14192 | [24] |
| Ursidae | Ursus maritimus | Polar bear | 1 | - | 348.44 | Male | UMUT15121 | This study |
| Ursus thibetanus | Asian black bear | 1 | - | 226.29 | Female | UMUT18004 | This study | |
| Helarctos malayanus | Sun bear | 1 | - | 217.34 | Male | UMUT15122 | This study | |
| Ailuridae | Ailurus fulgens | Red panda | 1 | - | 117.87 | Male | UMUT18007 | This study |
| Mustelidae | Aonyx capensis | Cape clawless otter | 1 | 9.20 | 123.16 | Female | UMUT14189 | [24] |
| Aonyx cinerea | Asian short clawed otter | 1 | 3.10 | 81.04 | Female | UMUT14183 | [24] | |
| Enhydra lutris | Sea otter | 1 | - | 136.48 | Male | UMUT08392 | [24] | |
| Neovison vison | American mink | 2 | 1.26 | 70.52 | - | UMUT11026, UMUT11028 | [24] | |
| Mustela itatsi | Japanese weasel | 2 | - | 55.48 | - | UMUT13044, UMUT14200 | [24] | |
| Martes melampus | Japanese marten | 1 | 1.30 | 85.65 | Male | UMUT14194 | [24] | |
| Meles anakuma | Japanese badger | 1 | 3.40 | 101.56 | Male | UMUT14198 | [24] | |
| Procyonidae | Nasua nasua | South American coati | 1 | - | 126.85 | - | UMUT14187 | [24] |
| Procyon lotor | Raccoon | 1 | - | 117.57 | - | UMUT14181 | [24] | |
| Potos flavus | Kinkajou | 1 | - | 80.23 | - | UMUT8375 | [24] | |
| Canidae | Canis familiaris | Dog (beagle) | 1 | 5.90 | 135.02 | Female | UMUT14182 | [24] |
| Cuon alpinus | Dhole | 1 | - | 170.99 | Female | UMUT15113 | This study | |
| Speothos venaticus | Bush dog | 1 | - | 141.80 | Female | UMUT18006 | This study | |
| Nyctereutes procyonoides | Raccoon dog | 2 | - | 111.02 | - | UMUT14188, UMUT14191 | [24] | |
| Vulpes vulpes | Red fox | 1 | 4.00 | 141.01 | - | UMUT14190 | [24] | |
UMUT, University Museum of University of Tokyo. The values of each masticatory muscle are a mean in Felis catus, Paguma larvata, Neovison vison, Mustela itatsi and Nyctereutes procyonoides.
Measurement
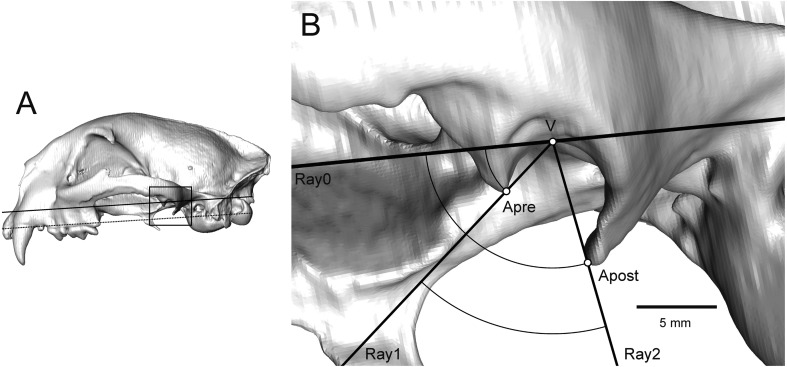
Preglenoid and postglenoid angles and pre-postglenoid angle interval were measured from the orthographic view, using the software Avizo 6.1. The vertex of the angle is the point of tangent of the mandibular fossa on the line Ray0, which is parallel to the line drawn from prosthion to the aboral border of the occipital condyles in a lateral view [53]. Ray1 is a line passing through the vertex and the preglenoid apex. Ray2 is a line passing through the vertex and the postglenoid apex. The preglenoid angle was measured between Ray0 and Ray1. In addition, the postglenoid angle was measured between Ray0 and Ray2. The pre-postglenoid angle interval is the difference between the preglenoid angle and postglenoid angle (Fig. 1).
Fig. 1.
Puma concolor temporomandibular joint. (A) Isosurface of the left lateral view. Dotted line indicates the line from Prosthion to the aboral border of the occipital condyles. A solid line indicates a parallel line of the dotted line, with the point of tangent on the mandibular fossa. (B) Same view showing the angle measurements. Apre is the preglenoid apex; Apost is the postglenoid apex, Ray0 is a line parallel to the line drawn from Prosthion to the aboral border of the occipital condyles; Ray1 is a line passing through vertex and preglenoid apex; Ray2, is a line passing through vertex and the postglenoid apex; V is the vertex of the angle.
The masticatory muscles concerning with closing of the mandible are comprised of the temporalis muscle, medial pterygoid muscle, lateral pterygoid muscle and the masseter muscle, which is composed of the superficial, intermediate and deep masseter muscle layers. PCSA was measured as it is an indicator of muscle force. The masticatory muscle data are based on Ito and Endo [24]. Measuring the fascicle length of the lateral pterygoid muscle was difficult in some species as the bundle was thin and brittle. As this muscle barely contributes to the total muscle mass (less than 3% of total masticatory muscle mass), we decided to provide only basic descriptive data of the lateral pterygoid muscle and did not include it in the following comparative analyses and relevant discussions. Thus, we focused on PCSA analyses of the temporalis, the whole masseter, the medial pterygoid and the three layers of masseter muscles. The measurement method of PCSA in Ito and Endo [24] is problematic for two reasons. First, the muscles were soaked in water prior to weighing as some materials were severely dried. However, the tissue may have experienced a certain degree of bloating or the soaking may have caused the tissue to degrade. Thus, soaking may have affected the weight and subsequently, the calculated PCSA. Second, the fascicles were cut apart by eye to obtain the average fibre length. As reported by Hartstone-Rose and colleagues [19], dissecting the muscles with a surgical knife may result in shorter fascicle measurements than those obtained using other methods.
Three samples were used in Ito and Endo [24] for Nyctereutes procyonoides; however, unfortunately, one specimen (UMUT14184) was lost from the collection. Thus, we only used the morphometric data of two specimens (UMUT14188, UMUT14191). The average PCSA and the average ratio of each muscle’s PCSA (%) of the two specimens of N. procyonoides are 9.42 cm2 (46.23%), 2.76 cm2 (13.56%), 8.19 cm2 (40.21%), 4.08 cm2 (46.23%), 1.84 cm2 (9.04%) and 2.27 cm2 (11.14%) for temporalis muscle, medial pterygoid muscle, whole masseter muscle, superficial masseter muscle layer, intermediate masseter muscle layer and deep masseter muscle layer, respectively. In addition, since pinnipeds swallow food, we excluded Phoca largha used in Ito and Endo (2016) from this study.
Statistical analyses
To investigate the phylogenetic pattern, the preglenoid and postglenoid angles and pre-postglenoid angle interval of each species were compared between families. The Mann-Whitney U test was used to test for significant differences between the families, including eight Felidae, three Ursidae, seven Mustelidae, three Procyonidae and three Canidae species. Because of the small number of sample species, Viverridae (to which Paguma larvata belongs) and Ailuridae (to which Ailurus fulgens belongs) were excluded from this statistical analysis. In the present study, P<0.05 was regarded as statistically significant. To investigate the correlation between each masticatory muscle and the preglenoid and postglenoid angles and pre-postglenoid angle interval, Spearman’s rank correlation coefficient test was used. In addition, each angle was measured from the same skulls used to measure the PCSAs. The correlation between the ratio of each masticatory muscle to the total muscle PCSA and each angle was tested. The correlation between the residuals for each muscle PCSA after adjusting for skull length and each angle was also tested.
We calculated the ratio of the PCSA of each muscle and layer against the PCSA of the total masticatory muscles excluding the lateral pterygoid and the ratio of the PCSA of each layer of the masseter muscle against the PCSA of the total muscles excluding the lateral pterygoid. Because necropsy was performed by veterinarians on the samples provided from zoos, some portion of the internal organs was extracted prior to the donation. For this reason, we were not able to use body mass as an indicator of body size. Following Van Valkenburgh [50], which states that skull length is strongly correlated with body mass, we measured the skull length using software, Avizo 6.1 and utilized it as an indicator of body size. To eliminate the effect of body size, we performed a reduced major axis (RMA) regression of the PCSA of each masticatory muscle against skull length and calculated the residuals from each allometry. Then, we plotted the residuals from the first regression versus each angle and fitted an RMA line to the data. RMA regressions were conducted in PAST v. 2.17c [18].
RESULTS
To investigate the phylogenetic pattern of the preglenoid and postglenoid angles and pre-postglenoid angle interval among families, each angle was compared between species (Table 2). The results of the statistical analysis by using the Mann-Whitney U test indicate that the preglenoid angle of the felids is significantly larger compared to that of the canids. Also, mustelids have a significantly smaller postglenoid angle compared to that of the canids. In terms of the pre-postglenoid interval angle, felids and mustelids have a significantly smaller value compared to that of the canids (Table 3).
Table 2. Temporomandibular joint angles.
| Species | Preglenoid angle (°) | Postglenoid angle (°) | Pre-postglenoid angle interval (°) |
|---|---|---|---|
| Puma concolor | 46.9 | 95.6 | 48.7 |
| Felis catus | 22.8 | 116.3 | 93.6 |
| Leopardus pardalis | 38.7 | 114.1 | 75.4 |
| Neofelis nebulosa | 40.5 | 120.4 | 79.9 |
| Panthera leo | 33.1 | 88.4 | 55.3 |
| Panthera pardus | 23.6 | 108.7 | 85.1 |
| Panthera tigris | 42.1 | 98.7 | 56.6 |
| Panthera uncia | 40.6 | 111.1 | 70.5 |
| Paguma larvata | 30.6 | 134.7 | 104.1 |
| Ursus maritimus | 0.0 | 108.8 | 108.8 |
| Ursus thibetanus | 16.6 | 92.5 | 75.9 |
| Helarctos malayanus | 24.7 | 83.7 | 59.0 |
| Ailurus fulgens | 42.6 | 102.9 | 60.3 |
| Aonyx capensis | 52.0 | 80.7 | 28.7 |
| Aonyx cinerea | 68.7 | 80.1 | 11.4 |
| Enhydra lutris | 52.7 | 94.6 | 41.9 |
| Neovison vison | 28.4 | 104.5 | 76.1 |
| Mustela itatsi | 7.5 | 91.4 | 83.9 |
| Martes melampus | −21.2 | 119.4 | 140.6 |
| Meles anakuma | 45.3 | 71.7 | 26.4 |
| Nasua nasua | 30.2 | 113.8 | 83.6 |
| Procyon lotor | 29.7 | 101.5 | 71.8 |
| Potos flavus | 7.7 | 106.9 | 99.2 |
| Canis familiaris | −10.8 | 134.6 | 145.4 |
| Cuon alpinus | −11.6 | 108.1 | 119.7 |
| Speothos venaticus | 18.3 | 110.2 | 91.9 |
| Nyctereutes procyonoides | −6.7 | 105.4 | 112.1 |
| Vulpes vulpes | −6.8 | 104.6 | 111.4 |
The values of each masticatory muscle are a mean in Felis catus, Paguma larvata, Neovison vison, Mustela itatsi and Nyctereutes procyonoides.
Table 3. Statistical analysis based on Mann–Whitney U test between families.
| Felidae | Ursidae | Mustelidae | Procyonidae | Canidae | |
|---|---|---|---|---|---|
| Preglenoid angle | 36.03 ± 8.80a) | 13.77 ± 12.59 | 33.34 ± 31.05 | 22.53 ± 22.53 | −3.52 ± 12.40a) |
| Postglnoid angle | 106.66 ± 11.21 | 95.00 ± 12.74 | 91.76 ± 16.30b) | 107.40 ± 6.17 | 112.57 ± 12.52b) |
| Pre-postglenoid angle interval | 70.63 ± 15.84c) | 81.23 ± 25.32 | 58.42 ± 44.92d) | 84.87 ± 13.74 | 112.57 ± 12.52c,d) |
Unit of the values are in degree. Mean values ± standard deviations are arranged. Values with superscript a, b, c and d have P value <0.05.
The correlation between each masticatory muscle’s PCSA and the preglenoid and postglenoid angles and pre-postglenoid angle interval were analyzed using the Spearman’s rank correlation coefficient (Tables 5 and 6). The correlation between the ratio of the PCSA of each masticatory muscle to the total muscle PCSA and each angle showed different results than the correlation between the size of each masticatory muscle’s PCSA after adjustment for body size and each angle. In terms of the correlation between the ratio of each masticatory muscle’s PCSA to the total muscle PCSA against each angle, the postglenoid angle correlated negatively with the temporalis muscle and positively with the masseter muscle (Table 4). All angles strongly correlated with the temporalis muscle PCSA after the elimination of the effect of body size (Table 5). For the elimination of the effect of body size, RMA regression for each masticatory muscle against skull length that produced the residuals are given in Table 6. The adjusted PCSA of the temporalis muscle correlated positively with the preglenoid and negatively with the postglenoid and the pre-postglenoid angle interval.
Table 5. Spearman’s rank correlation coefficient for the size of masticatory muscle PCSA relative to body size against each angle.
| Preglenoid angle |
Postglenoid angle |
Pre-postglenoid angle interval |
||||
|---|---|---|---|---|---|---|
| r | P | r | P | r | P | |
| Temporalis | 0.69 | 0.00 | −0.70 | 0.00 | −0.75 | 0.00 |
| Medial pterygoid | 0.11 | 0.67 | −0.04 | 0.87 | −0.11 | 0.68 |
| Whole masseter | 0.13 | 0.62 | 0.00 | 0.99 | −0.10 | 0.70 |
| Superficial masseter | 0.25 | 0.32 | −0.10 | 0.71 | −0.23 | 0.37 |
| Intermediate masseter | 0.08 | 0.76 | −0.27 | 0.29 | −0.23 | 0.38 |
| Deep masseter | 0.01 | 0.96 | 0.07 | 0.79 | 0.03 | 0.90 |
P values lower than 0.05 are in bold, as are r values above 0.6 and below −0.6.
Table 6. RMA regressions of the masticatory muscle PCSA against skull length.
| Variable | RMA slope | y-intercept | r |
|---|---|---|---|
| Log10 PCSA of temporalis vs. Log10 SL2 | 1.08 | −1.23 | 0.91 |
| Log10 PCSA of medial pterygoid vs. Log10 SL2 | 1.42 | −2.61 | 0.93 |
| Log10 PCSA of whole masseter vs. Log10 SL2 | 1.36 | −2.00 | 0.93 |
| Log10 PCSA of superficial masseter vs. Log10 SL2 | 1.52 | −2.68 | 0.90 |
| Log10 PCSA of intermidiate masseter vs. Log10 SL2 | 1.17 | −2.22 | 0.87 |
| Log10 PCSA of deep masseter vs. Log10 SL2 | 1.50 | −2.83 | 0.90 |
SL indicates skull length.
Table 4. Spearman’s rank correlation coefficient for the ratio of the masticatory muscle PCSA against each angle.
| Preglenoid angle |
Postglenoid angle |
Pre-postglenoid angle interval |
||||
|---|---|---|---|---|---|---|
| r | P | r | P | r | P | |
| Temporalis | 0.20 | 0.43 | −0.56 | 0.02 | −0.36 | 0.15 |
| Medial pterygoid | −0.05 | 0.85 | 0.36 | 0.16 | 0.18 | 0.49 |
| Whole masseter | −0.20 | 0.44 | 0.58 | 0.02 | 0.36 | 0.16 |
| Superficial masseter | 0.05 | 0.84 | 0.42 | 0.10 | 0.12 | 0.65 |
| Intermediate masseter | −0.33 | 0.19 | −0.07 | 0.78 | 0.13 | 0.61 |
| Deep masseter | −0.06 | 0.82 | 0.29 | 0.25 | 0.18 | 0.48 |
P values lower than 0.05 are in bold, as are r values above 0.5 and below −0.5.
DISCUSSION
The pre-postglenoid interval, which shows the size of the gape, ranged widely from 11.4° in Aonyx cinerea to 145.4° in Canis familiaris (Table 2). Therefore, the fossa opening, which is the distance between the preglenoid and the postglenoid apices, also ranged widely among the carnivorans. Although all carnivorans are considered to have a hinge joint [12, 23], we found variation among species. Some groups had a loose temporomandibular joint with a wide fossa opening, while others had a tight temporomandibular joint. Previous studies of teeth and jaw movements show that some carnivoran groups have a great ability to grind food [14, 36, 49]. We suspect that these groups have the ability to grind food due to their loose temporomandibular joint.
A tight temporomandibular joint is a counterplan to dislocation, which is caused by the force of the temporalis muscle applied to it [5, 48]. By having a tight temporomandibular joint and preventing dislocation, the force can be transmitted from the balancing side, which is the side on which biting does not occur, to the working side where the biting occurs [4, 8]. As a great amount of the contracting force of the masticatory muscles can be transmitted, the bite force can be increased. As a result, species with a tight temporomandibular joint are able to exert a high bite force [8].
With PCSA as an indicator of muscle force, the correlation between each masticatory muscle PCSA and the angles of the preglenoid, postglenoid and the pre-postglenoid angle interval were investigated. When the correlation between the ratio of each masticatory muscle PCSA and each angle was examined, the postglenoid angle and the temporalis muscle showed a negative correlation, while the postglenoid angle and the masseter muscle showed a positive correlation (Table 4). When the ratio of the PCSA of the temporalis muscle is large, the postglenoid angle is small. However, when the ratio of the PCSA of the masseter muscle is large, the postglenoid angle is large. According to Davis [5], when the force of the temporalis muscle pulls the coronoid process dorsally, pressure is applied against the rear of the mandibular fossa. Since this leads to a dislocation of the temporomandibular joint, the postglenoid process in Carnivora is developed to prevent this dislocation [5]. When the PCSA of the temporalis muscle becomes large, the postglenoid angle becomes small. This small angle means that the apex of the postglenoid projects anteriorly. In fact, this is the “development of the glenoid process” that Davis [5] mentions in his report on the biomechanics of the temporomandibular joint. In addition, Davis remarks that by the combined action of the temporalis muscle and the masseter muscle, the force applied against the temporomandibular joint, which is the fulcrum, is reduced. Turnbull [48] illustrates in a refined diagram based on Davis [5] how the force of the medial pterygoid muscle acts synergistically with the masseter muscle. Our results indicate that as the ratio of the masseter muscle PCSA increases, the postglenoid angle becomes large, meaning that the apex of the postglenoid does not develop prominently. However, the ratio of the medial pterygoid PCSA does not influence the postglenoid angle. In other words, when the ratio of the masseter muscle PCSA becomes large, it restores balance with the temporalis muscle. As a result, the load on the temporomandibular joint is reduced. For this reason, postglenoid development, which can be interpreted from the small postglenoid angle, becomes unnecessary when there is a high ratio of the whole masseter muscle PCSA. In addition, when the postglenoid angle is large, the advantage is that the temporomandibular joint is free from restriction. Since the masseter muscle is effective during carnassial biting [38], presumably the loose temporomandibular joint enables the grinding at carnassial teeth using the masseter muscle.
The residuals of the masticatory muscle PCSA against skull lengths (an indicator of body size) and each angle have higher correlations compared to those between the ratio of the masticatory muscle PCSA and each angle. The correlations between the temporalis muscle relative to body size and all angles are high (Table 5). Carnivorans with large temporalis muscle PCSA relative to body size have a preglenoid with a caudally projecting apex and a postglenoid with a rostrally projecting apex. As a result, the angle between the apices is reduced, forming a tight temporomandibular joint. Our results indicate that the carnivoran group in which the temporalis muscle exerts a large force during mastication has a tight temporomandibular joint. We believe that this is related to the use of the canine and carnassial teeth. According to Smith and Savage [38], the temporomandibular joint experiences little stress during canine biting action using the temporalis muscle. Thus, there is no dislocation. However, the authors also remarked that dislocation is induced when only the temporalis muscle is used during carnassial biting. Therefore, species producing large forces with their temporalis muscle experience a risk of dislocating their temporomandibular joint when utilizing their carnassial teeth. Our results suggest that dislocation is prevented by reinforcing the joint with bone structures such as the preglenoid and postglenoid. The carnivorans with a large temporalis muscle PCSA relative to skull size (as an indicator of body size) have a caudally projecting preglenoid apex and a rostrally projecting postglenoid apex, forming a small pre-post glenoid interval. However, the preglenoid apex and the postglenoid apex do not develop in carnivorans with a small temporalis muscle PCSA relative to skull size. Consequently, the temporomandibular joint is loose. Dislocation does not occur during carnassial biting in this case because of the small temporalis muscle PCSA. Carnassial biting using only the temporalis muscle is most likely difficult in these species. As the masseter muscle and the medial pterygoid muscle are suitable for carnassial biting [48], the force lacking from the temporalis to utilize the carnassial teeth is presumably supplemented by the masseter muscle and the medial pterygoid muscle.
When the angles of preglenoid, postglenoid and pre-postglenoid angle interval were compared among families, phylogenetic constraint was observed. Mustelidae show a small postglenoid angle and a small pre-postglenoid angle interval (Table 3). This means that mustelids have a small fossa opening with a rostrally projecting postglenoid apex and a small pre-postglenoid angle interval. Consequently, the condyle of mustelids is locked in the mandibular fossa. This indicates that mustelids have a tight temporomandibular joint. As Mustelidae have a large temporalis muscle [24, 34, 35], a tight temporomandibular joint is necessary to utilize the carnassial teeth.
Felidae shows large preglenoid angle and small pre-postglenoid angle interval (Table 3). This indicates that felids have a small opening with a caudally projecting preglenoid apex and a small pre-postglenoid angle interval. Consequently, the condyle of felids is locked in the mandibular fossa. This indicates that felids have a tight temporomandibular joint similar to that in mustelids. However, contrary to the situation in mustelids, the ratio of the whole masseter muscle and superficial masseter muscle layer PCSA is large in felids. In addition, the whole masseter muscle and superficial masseter muscle layer PCSA are large relative to their body size. Felids use the masseter muscle and utilize their developed carnassial teeth effectively [24]. The results of the present study suggest that the force generated by the masseter muscle onto the carnassial teeth is diverted to the canine teeth by tightening the temporomandibular joint. Felidae and Mustelidae are considered to a have high bite force among carnivorans [34, 35]. The temporomandibular joint structure of these groups allows them to exert a high bite force.
The pre-postglenoid angle interval is large, the preglenoid angle is small and the postglenoid angle is large in Canidae (Table 3). These results show that canids have a large fossa opening with undeveloped preglenoid and postglenoid apices and a large pre-postglenoid angle interval. Consequently, the temporomandibular joint is loose and the condyle is not locked within the mandibular fossa. Although canids, compared to mustelids and felids, have a small bite force [34, 35, 52], ecological evidence confirms that they are able to exert sufficient bite force during prey capture. Presumably, the masticatory muscle arrangement in canids is well balanced and it enables the combined action achieved by the temporalis muscle, the masseter muscle and the medial pterygoid muscle, as noted by Davis [5] and Turnbull [48]. Hence, enforcement of the temporomandibular joint by bone structure is unnecessary.
We investigated the correlation of each masticatory muscle PCSA against each angle, as measured from preglenoid and postglenoid apices. Our results show that as the ratio of the temporalis muscle PCSA increases, the postglenoid angle decreases. In contrast, as the ratio of the masseter muscle PCSA increases, the postglenoid angle increases (Table 4). Moreover, the variation in the structure of the temporomandibular joint caused by the change in the postglenoid is affected by the ratio of the temporalis and the masseter muscle PCSAs. The size of the masticatory muscle PCSA relative to body size and angles showed higher correlations compared to the correlations between the ratio of the masticatory muscle PCSA and angles (Table 5). High correlations were observed between the temporalis muscle PCSA relative to body size and all angles. This could be attributed to the reinforcement of the temporomandibular joint with a small pre-postglenoid angle interval, as this allows carnassial biting using the strong temporalis muscle. Our results showed such generality among carnivorans. However, the quantitative data analysis of the line of action of each masticatory muscle that was not carried out in this study is also important to discuss the structure of the temporomandibular joint. Phylogenetic constraints are seen in the structure of the temporomandibular joint. Mustelids have a tight temporomandibular joint because of the rostrally projecting postglenoid apex and felids have a tight temporomandibular joint because of the caudally projecting preglenoid apex. Canids have a loose temporomandibular joint with undeveloped preglenoid and postglenoid apices. Our data suggest that these traits of the temporomandibular joint are suitable to perform the function of the masticatory muscles, canines and carnassial teeth.
Acknowledgments
We are grateful to the staff of the Chiba Zoological Park, Futami Sea Paradise, Hamamatsu Zoological Garden, Hirakawa Zoological Park, Oita Marine Park and Yokohama Zoological Gardens for donating the carcasses of Carnivora specimens. We thank Dr. Daisuke Koyabu (Musashino Art University) for giving us the information about the masticatory muscles. This study was supported by JSPS (KAKENHI grant number 25304005, 16K15057 and 18J14168).
REFERENCES
- 1.Anapol F., Shahnoor N., Ross C. F.2008. Scaling of reduced physiologic cross-sectional area in primate muscles of mastication. pp. 201–216. In: Primate Craniofacial Function and Biology (Vinyard, C., Ravosa, M.J. and Wall, C. eds.), Springer US, New York. [Google Scholar]
- 2.Antón S. C.1999. Macaque masseter muscle: internal architecture, fiber length and cross-sectional area. Int. J. Primatol. 20: 441–462. doi: 10.1023/A:1020509006259 [DOI] [Google Scholar]
- 3.Bouvier M., Tsang S. M.1990. Comparison of muscle weight and force ratios in New and Old World monkeys. Am. J. Phys. Anthropol. 82: 509–515. doi: 10.1002/ajpa.1330820410 [DOI] [PubMed] [Google Scholar]
- 4.Clausen P., Wroe S., McHenry C., Moreno K., Bourke J.2008. The vector of jaw muscle force as determined by computer-generated three dimensional simulation: a test of Greaves’ model. J. Biomech. 41: 3184–3188. doi: 10.1016/j.jbiomech.2008.08.019 [DOI] [PubMed] [Google Scholar]
- 5.Davis D.1955. Masticatory apparatus in the spectacled bear, Tremarctos ornatus. Fieldiana Zool. 37: 25–46. [Google Scholar]
- 6.Davis J. L., Santana S. E., Dumont E. R., Grosse I. R.2010. Predicting bite force in mammals: two-dimensional versus three-dimensional lever models. J. Exp. Biol. 213: 1844–1851. doi: 10.1242/jeb.041129 [DOI] [PubMed] [Google Scholar]
- 7.Dessem D.1989. Interactions between jaw-muscle recruitment and jaw-joint forces in Canis familiaris. J. Anat. 164: 101–121. [PMC free article] [PubMed] [Google Scholar]
- 8.Dessem D., Druzinsky R. E.1992. Jaw-muscle activity in ferrets, Mustela putorius furo. J. Morphol. 213: 275–286. doi: 10.1002/jmor.1052130211 [DOI] [PubMed] [Google Scholar]
- 9.Dickinson E., Stark H., Kupczik K.2018. Non-destructive determination of muscle architectural variables through the use of DiceCT. Anat. Rec. (Hoboken) 301: 363–377. doi: 10.1002/ar.23716 [DOI] [PubMed] [Google Scholar]
- 10.Druzinsky R. E.2010. Functional anatomy of incisal biting in Aplodontia rufa and sciuromorph rodents - part 2: sciuromorphy is efficacious for production of force at the incisors. Cells Tissues Organs (Print) 192: 50–63. doi: 10.1159/000284930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eng C. M., Ward S. R., Vinyard C. J., Taylor A. B.2009. The morphology of the masticatory apparatus facilitates muscle force production at wide jaw gapes in tree-gouging common marmosets (Callithrix jacchus). J. Exp. Biol. 212: 4040–4055. doi: 10.1242/jeb.029983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ewer R. F.1998. The Carnivores. Cornell University, New York. [Google Scholar]
- 13.Furuuchi K., Koyabu D., Mori K., Endo H.2013. Physiological cross-sectional area of the masticatory muscles in the giraffe physiological cross-sectional area of the masticatory muscles in the giraffe (Giraffa camelopardalis). Mammal Study 38: 67–71. doi: 10.3106/041.038.0109 [DOI] [Google Scholar]
- 14.Goillot C., Blondel C., Peigné S.2009. Relationships between dental microwear and diet in Carnivora (Mammalia)—Implications for the reconstruction of the diet of extinct taxa. Palaeogeogr. Palaeoclimatol. Palaeoecol. 271: 13–23. doi: 10.1016/j.palaeo.2008.09.004 [DOI] [Google Scholar]
- 15.Gorniak G. C., Gans C.1980. Quantitative assay of electromyograms during mastication in domestic cats (Felis catus). J. Morphol. 163: 253–281. doi: 10.1002/jmor.1051630304 [DOI] [PubMed] [Google Scholar]
- 16.Greaves W. S.1985. The generalized carnivore jaw. Zool. J. Linn. Soc. 85: 267–274. doi: 10.1111/j.1096-3642.1985.tb01506.x [DOI] [Google Scholar]
- 17.Greaves W. S.2000. Location of the vector of jaw muscle force in mammals. J. Morphol. 243: 293–299. doi: [DOI] [PubMed] [Google Scholar]
- 18.Hammer Ø., Harper D. A. T., Ryan P. D.2001. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electronica 4: 1–9. [Google Scholar]
- 19.Hartstone-Rose A., Deutsch A. R., Leischner C. L., Pastor F.2018. Dietary correlates of primate masticatory muscle fiber architecture. Anat. Rec. (Hoboken) 301: 311–324. doi: 10.1002/ar.23715 [DOI] [PubMed] [Google Scholar]
- 20.Hartstone-Rose A., Perry J. M. G., Morrow C. J.2012. Bite force estimation and the fiber architecture of felid masticatory muscles. Anat. Rec. (Hoboken) 295: 1336–1351. doi: 10.1002/ar.22518 [DOI] [PubMed] [Google Scholar]
- 21.Herrel A., De Smet A., Aguirre L. F., Aerts P.2008. Morphological and mechanical determinants of bite force in bats: do muscles matter? J. Exp. Biol. 211: 86–91. doi: 10.1242/jeb.012211 [DOI] [PubMed] [Google Scholar]
- 22.Herring S. W.1980. Functional design of cranial muscles : Comparative and physiological studies in pigs. Am. Zool. 20: 283–293. doi: 10.1093/icb/20.1.283 [DOI] [Google Scholar]
- 23.Herring S. W., Popowics T. E.2006. Teeth, jaws and muscles in mammalian mastication. pp. 61–83. In: Feeding in Domestic Vertebrates: From Structure to Behaviour (Bels, V. L. ed.), CAB International, Wallingford. [Google Scholar]
- 24.Ito K., Endo H.2016. Compartive study of physiological cross-sectional area of masticatory muscles among species of carnivora. Mammal Study 41: 181–190. doi: 10.3106/041.041.0403 [DOI] [Google Scholar]
- 25.Long C. A.1965. Functional aspects of the jaw-articulation in the North American badger, with comments on adaptiveness of tooth-wear. Trans. Kans. Acad. Sci. 68: 156–162. doi: 10.2307/3626358 [DOI] [PubMed] [Google Scholar]
- 26.Moore W. J.1981. Mammalian Skull, Cambridge University Press, New York. [Google Scholar]
- 27.Naples V. L.1985. Form and function of the masticatory musculature in the tree sloths, Bradypus and Choloepus. J. Morphol. 183: 25–50. doi: 10.1002/jmor.1051830104 [DOI] [PubMed] [Google Scholar]
- 28.Nowak R.1999. Walker’s Mammals of the World, Johns Hopkins University, Maryland. [Google Scholar]
- 29.Perry J. M. G.2018. Inferring the diets of extinct giant lemurs from osteological correlates of muscle dimensions. Anat. Rec. (Hoboken) 301: 343–362. doi: 10.1002/ar.23719 [DOI] [PubMed] [Google Scholar]
- 30.Perry J. M. G., Hartstone-Rose A., Logan R. L.2011. The jaw adductor resultant and estimated bite force in primates. Anat. Res. Int. 2011: 929848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perry J. M. G., Hartstone-Rose A., Wall C. E.2011. The jaw adductors of strepsirrhines in relation to body size, diet, and ingested food size. Anat. Rec. (Hoboken) 294: 712–728. doi: 10.1002/ar.21354 [DOI] [PubMed] [Google Scholar]
- 32.Perry J. M. G., Macneill K. E., Heckler A. L., Rakotoarisoa G., Hartstone-Rose A.2014. Anatomy and adaptations of the chewing muscles in Daubentonia (Lemuriformes). Anat. Rec. (Hoboken) 297: 308–316. doi: 10.1002/ar.22844 [DOI] [PubMed] [Google Scholar]
- 33.Perry J. M. G., St Clair E. M., Hartstone-Rose A.2015. Craniomandibular signals of diet in adapids. Am. J. Phys. Anthropol. 158: 646–662. doi: 10.1002/ajpa.22811 [DOI] [PubMed] [Google Scholar]
- 34.Radinsky L. B.1981. Evolution of skull shape in carnivores: 1. Representative modern carnivores. Biol. J. Linn. Soc. Lond. 15: 369–388. doi: 10.1111/j.1095-8312.1981.tb00770.x [DOI] [Google Scholar]
- 35.Radinsky L. B.1981. Evolution of skull shape in carnivores: 2. Additional modern carnivores. Biol. J. Linn. Soc. Lond. 16: 337–355. doi: 10.1111/j.1095-8312.1981.tb01657.x [DOI] [Google Scholar]
- 36.Scapino R.1981. Morphological investigation into functions of the jaw symphysis in carnivorans. J. Morphol. 167: 339–375. doi: 10.1002/jmor.1051670308 [DOI] [PubMed] [Google Scholar]
- 37.Simpson C. D.1978. Comparative mammalian mastication. Angle Orthod. 48: 93–105. [DOI] [PubMed] [Google Scholar]
- 38.Smith J. M., Savage R.1959. The mechanics of mammalian jaws. Sch. Sci. Rev. 141: 289–301. [Google Scholar]
- 39.St Clair E. M., Reback N., Perry J. M. G.2018. Craniomandibular variation in phalangeriform marsupials: Functional comparisons with primates. Anat. Rec. (Hoboken) 301: 227–255. doi: 10.1002/ar.23717 [DOI] [PubMed] [Google Scholar]
- 40.Taylor A. B., Eng C. M., Anapol F. C., Vinyard C. J.2009. The functional correlates of jaw-muscle fiber architecture in tree-gouging and nongouging callitrichid monkeys. Am. J. Phys. Anthropol. 139: 353–367. doi: 10.1002/ajpa.20991 [DOI] [PubMed] [Google Scholar]
- 41.Taylor A. B., Jones K. E., Kunwar R., Ravosa M. J.2006. Dietary consistency and plasticity of masseter fiber architecture in postweaning rabbits. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 288: 1105–1111. doi: 10.1002/ar.a.20382 [DOI] [PubMed] [Google Scholar]
- 42.Taylor A. B., Terhune C. E., Toler M., Holmes M., Ross C. F., Vinyard C. J.2018. Jaw-muscle fiber architecture and leverage in the hard-object feeding sooty mangabey are not structured to facilitate relatively large bite forces compared to other Papionins. Anat. Rec. (Hoboken) 301: 325–342. doi: 10.1002/ar.23718 [DOI] [PubMed] [Google Scholar]
- 43.Taylor A. B., Vinyard C. J.2004. Comparative analysis of masseter fiber architecture in tree-gouging (Callithrix jacchus) and nongouging (Saguinus oedipus) callitrichids. J. Morphol. 261: 276–285. doi: 10.1002/jmor.10249 [DOI] [PubMed] [Google Scholar]
- 44.Taylor A. B., Vinyard C. J.2008. The relationship between jaw-muscle architecture and feeding behavior in primates: tree-gouging and nongouging gummivorous callitrichids as a natural experiment. pp. 241–262. In: Primate Craniofacial Function and Biology (Vinyard, C., Ravosa, M. J. and Wall, C. eds.), Springer US, New York. [Google Scholar]
- 45.Taylor A. B., Vinyard C. J.2009. Jaw-muscle fiber architecture in tufted capuchins favors generating relatively large muscle forces without compromising jaw gape. J. Hum. Evol. 57: 710–720. doi: 10.1016/j.jhevol.2009.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taylor A. B., Vinyard C. J.2013. The relationships among jaw-muscle fiber architecture, jaw morphology, and feeding behavior in extant apes and modern humans. Am. J. Phys. Anthropol. 151: 120–134. doi: 10.1002/ajpa.22260 [DOI] [PubMed] [Google Scholar]
- 47.Terhune C. E., Hylander W. L., Vinyard C. J., Taylor A. B.2015. Jaw-muscle architecture and mandibular morphology influence relative maximum jaw gapes in the sexually dimorphic Macaca fascicularis. J. Hum. Evol. 82: 145–158. doi: 10.1016/j.jhevol.2015.02.006 [DOI] [PubMed] [Google Scholar]
- 48.Turnbull W. D.1970. Mammalian masticatory apparatus. Fieldiana Geol. 18: 149–356. [Google Scholar]
- 49.van Valkenburgh B.1989. Carnivore dental adaptations and diet: a study of trophic diversity within guilds. pp. 410–436. In: Carnivore Behavior, Ecology, and Evolution (Gittleman, J. L. ed.), Springer US, New York. [Google Scholar]
- 50.van Valkenburgh B.1990. Skeletal and dental predictors of body mass in carnivores. pp. 181–206. In: Body Size in Mammalian Paleobiology: Estimation and Biological Implications (Damuth, J. and MacFadden, B. J. eds.) Cambridge University Press, Cambridge. [Google Scholar]
- 51.Van Valkenburgh B.2007. Deja vu: the evolution of feeding morphologies in the Carnivora. Integr. Comp. Biol. 47: 147–163. doi: 10.1093/icb/icm016 [DOI] [PubMed] [Google Scholar]
- 52.van Valkenburgh B., Ruff B.1987. Canine tooth strength and killing behaviour in large carnivores. J. Zool. (Lond.) 212: 379–397. doi: 10.1111/j.1469-7998.1987.tb02910.x [DOI] [Google Scholar]
- 53.von den Driesch A.1976. A guide to the measurement of animal bones from archaeological sites. Peabody Mus. Bull. 1: 1–137. [Google Scholar]
- 54.Weijs W. A., Dantuma R.1975. Electromyography and mechanics of mastication in the albino rat. J. Morphol. 146: 1–33. doi: 10.1002/jmor.1051460102 [DOI] [PubMed] [Google Scholar]
- 55.Weijs W. A., Hillen B.1985. Physiological cross-section of the human jaw muscles. Acta Anat. (Basel) 121: 31–35. doi: 10.1159/000145938 [DOI] [PubMed] [Google Scholar]
- 56.Yoshikawa T., Suzuki T.1965. The comparative anatomical study of the masseter of the mammal (II). Okajimas Folia Anat. Jpn. 40: 339–363. doi: 10.2535/ofaj1936.40.4-6_339 [DOI] [PubMed] [Google Scholar]