Abstract
[Purpose] Determining the thickness of the intercostal muscle with ultrasound imaging would be a useful parameter in evaluating respiratory muscle activity in patients with tetraplegia and neuromuscular weakness. However, it has not been clarified whether ultrasound imaging can measure changes in intercostal muscle thickness during breathing. This study aimed to measure contractions of the human intercostal muscle in the anterior, lateral, and posterior parts with ultrasound imaging during maximal breathing. [Participants and Methods] The participants were 12 healthy males. Intercostal muscle thickness was measured using ultrasound at rest and at maximal breathing. The measurement sites were the anterior, lateral, and posterior portions of the right intercostal spaces. Statistical analysis was performed using a paired t-test comparing intercostal muscle thickness at rest and maximal breathing. [Results] The thickness of the intercostal muscle showed significant increases in the first, second, third, fourth, and sixth intercostal spaces of the anterior portions. There were no significant differences in the lateral or posterior portions between rest and maximal breathing. [Conclusion] Human intercostal muscle thickness can be measured with ultrasound and increases only in the anterior portions during maximal breathing.
Key words: Intercostal muscle thickness, Ultrasound imaging, Maximal breathing
INTRODUCTION
Intercostal muscles contribute to the important work of respiratory movement by expanding the thorax. In patients with cervical spinal cord injuries without injury to the descending pathway to the diaphragm, vital capacity decreases because of intercostal muscle paralysis. In participants with ventilator-dependent tetraplegia1), it has been reported that maximum inspired volumes can be augmented by adding intercostal pacing more so than with diaphragmatic pacing alone. These data suggest that intercostal muscles play important roles in respiratory function; therefore, it is important to evaluate intercostal muscle activity with respiratory movement.
Intercostal muscles are divided into two parts: external intercostal muscles at the superficial layer and internal intercostal muscles in the deep layer. The external intercostal muscle functions during the inspiration phase and the internal intercostal muscle functions during the expiration phase2). In basic experiments, it has been reported that activity of the intercostal muscle varies across the anterior, lateral, and the posterior portions of the rib cage3). Nevertheless, this phenomenon has not been investigated in detail in human intercostal muscles.
Needle electromyograms are used to measure muscle activity of the intercostal muscle4); however, they pose the risk of pneumothorax, therefore they cannot be easily used as part of a clinical evaluation. In recent years, ultrasound images have been used to evaluate muscle activity. There is a correlation between the muscle activity measured by electromyogram and muscle thickness measured by ultrasound image. Ultrasound is also useful to evaluate muscle activity5). This suggests that ultrasound can measure changes in intercostal muscle thickness during respiration.
It was hypothesized that intercostal muscles acting as inspiratory muscles increase their thickness during maximal breathing. The purpose of this study was to clarify whether ultrasound can measure changes in intercostal muscle thickness during respiration, and to determine whether human intercostal muscle activity is based on changes in intercostal muscle thickness during maximal breathing. Increasing muscle thickness of the intercostal muscle would be a useful parameter in evaluating respiratory muscle activity in patients with tetraplegia patients.
PARTICIPANTS AND METHODS
The participants were 12 healthy men (mean age 23.9 ± 3.6 years old; mean height 172.8 ± 4.8 cm; mean weight 61.9 ± 6.3 kg, mean %vital capacity 104.1 ± 12.5%) who had no smoking history. Participants with previous respiratory disease were excluded. This study was approved by the Ibaraki Prefectural University of Health Sciences Ethics Review Committee (approval number, 839). All participants gave informed consent and the procedures were approved by the institutional ethics committee.
Intercostal thickness were measured by ultrasound (GE, LOGIQ iM, Tokyo, Japan). The ultrasound imaging was set up in B mode at 12 Hz using a linear probe (11 l). The measurement site was the right side of the thorax, in the intercostal spaces of the anterior, lateral, and posterior portions. The measurement of anterior part was the 1st−6th intercostal spaces and 25–30 mm outside from the right edge of the sternum. The measurement of lateral part was the 3rd, 6th, and 9th intercostal space, at the line connecting the axillary anterior border with the anterior superior iliac spine. The measurement of posterior part was the 3rd, 6th, and 9th intercostal spaces, and it was 50–60 mm lateral to the thoracic spinous process. For the measurements of anterior intercostal spaces, participants were in the supine position. For measurements of lateral intercostal and posterior intercostal spaces, participants were in the left side-lying position. Thicknesses of intercostal muscles were measured in the center of parallel parts of the upper and lower fascia and perpendicular to the muscle. Intercostal muscle thickness was analyzed in units of 0.01 mm. Intra-rater reliability was calculated for the 2nd, 3rd, and 4th intercostal space of the anterior intercostal space.
Measurements were taken at rest and at maximal breathing. First, intercostal muscle thickness was measured at the resting expiratory level. Next, intercostal muscle thickness was measured at the maximal inspiratory level using an inspiratory resistance loading device (CHEST, HI-801, Fukuoka, Japan).
The same examiner performed ultrasound imaging for measurement of muscle thickness twice. The reliability of the study was assessed with intraclass correlation coefficients (ICCs).
Statistical analysis was performed using the paired t-test for intercostal muscle thickness at rest and at maximal breathing. The data were analyzed using IBM SPSS Statistics version 22.0. All values were expressed as mean (mm), and the p-value for statistical significance was set at 0.05.
RESULTS
A typical example of an ultrasound image is shown in Fig. 1. The results of the intra-rater reliability are shown in Table 1. The ICC was high in the 2nd, 3rd, and 4th intercostal spaces during both phases. The mean value of each measurement and the results of the paired t-tests are shown in Table 2. The intercostal muscle thickness was significantly greater in the 1st, 2nd, 3rd, 4th, and 6th intercostal spaces in the anterior part during maximal breathing. There were no significant differences in values of the lateral and the posterior portions between rest and maximal breathing.
Fig. 1.
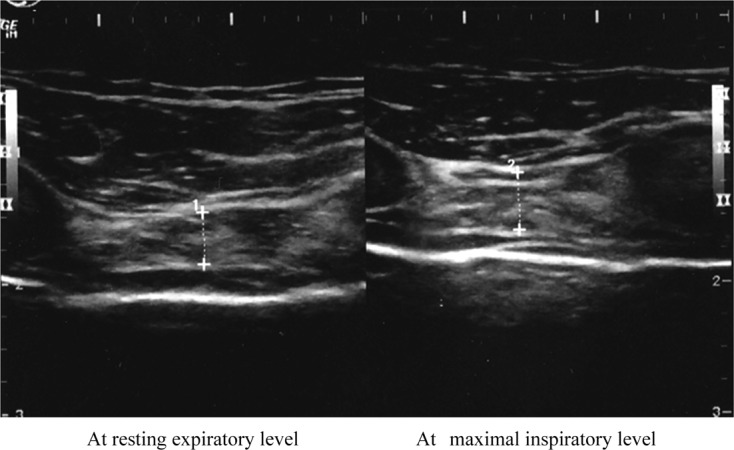
Ultrasound image of the intercostal muscle.
Typical ultrasound image of the intercostal muscle in 2nd intercostal space of the anterior at rest (left panel) and maximal breathing (right panel). Muscle thickness increased during maximal breathing.
Table 1. Intra-rater reliability ICC (1.1) for intercostal muscle thickness.
| Intercostal space of anterior portions | Rest breathing | Maximal breathing |
| 2nd | 0.649 | 0.660 |
| 3rd | 0.790 | 0.836 |
| 4th | 0.815 | 0.801 |
Table 2. Average value of three portions of intercostal muscle thickness during breathing.
| Intercostal space | Rest breathing | Maximal breathing | p value | Effect size (r) | |
| Anterior part | 1st | 1.97 ± 0.66 | 2.51 ± 0.94 | 0.015* | 0.61 |
| 2nd | 2.17 ± 0.83 | 2.62 ± 0.95 | 0.001* | 0.75 | |
| 3rd | 2.65 ± 1.13 | 3.19 ± 1.24 | 0.000* | 0.82 | |
| 4th | 2.79 ± 0.86 | 3.55 ± 1.02 | 0.000* | 0.87 | |
| 5th | 2.31 ± 0.89 | 2.78 ± 1.00 | 0.050 | 0.48 | |
| 6th | 2.52 ± 0.52 | 2.80 ± 0.66 | 0.027* | 0.56 | |
| Lateral part | 3rd | 3.68 ± 1.45 | 3.64 ± 1.46 | 0.304 | 0.15 |
| 6th | 3.03 ± 0.77 | 3.43 ± 1.00 | 0.087 | 0.42 | |
| 9th | 3.18 ± 1.10 | 3.33 ± 1.13 | 0.226 | 0.23 | |
| Posterior part | 3rd | 4.85 ± 1.60 | 5.11 ± 1.90 | 0.177 | 0.32 |
| 6th | 4.25 ± 1.52 | 4.33 ± 1.34 | 0.369 | 0.09 | |
| 9th | 4.27 ± 1.56 | 4.22 ± 1.44 | 0.372 | 0.12 | |
Mean ± SD, mm.
DISCUSSION
In this study, the hypothesis was that human intercostal muscle thickness increased during maximal breathing and that ultrasound could capture slight changes in intercostal muscle thickness during breathing. Ultrasound images revealed increases in muscle thickness only in the anterior portion of the intercostal space during maximal breathing, and there were no significant differences in the lateral and posterior portions. The same examiner measured twice so that the intra-rather reliability could be calculated. According to the criteria of Landis et al.6), perfect reliability is 0.81–1.00, substantial is 0.61–0.80, moderate is 0.41–0.60, fair is 0.21–0.40, and slight is 0.0–0.21. In ultrasound imaging data, intra-rater reliability was substantial in the 2nd intercostal space; the 3rd intercostal space and the 4th intercostal space were almost perfect−substantial, and the reliability was considered high.
Intercostal muscle thickness showed significant increases during maximal breathing in the 1st, 2nd, 3rd, 4th, and 6th intercostal spaces in the anterior portions. Measurement of the anterior portion ranged from the right edge of the sternum to the outside 25–30 mm, and this portion is anatomically located on the superficial layer of the parasternal intercostal muscle. The parasternal intercostal muscle is the costal cartilage portion of the internal intercostal muscle, and this part is a monolayer that overlaps with other intercostal muscles. The parasternal intercostal muscle was found to be active during inspiration according to an electromyogram7). In a previous study using ultrasound, Simon et al. reported that muscle thickness increased in more than 20% of maximal voluntary contraction in skeletal muscles such as the tibialis anterior4). Our experimental data also showed that parasternal intercostal muscle activity increased during maximal breathing.
Conversely, in the lateral and posterior portions of the intercostal space, intercostal muscle thickness did not change during maximal breathing. Using ultrasound, Diab et al. measured the area of human intercostal muscle at the 5th–11th intercostal space on the posterior surface during maximal expiration and maximal inspiration8). They found significant differences in the 7th–8th intercostal spaces, but no significant difference in the other intercostal spaces. Anatomically, the external intercostal muscle and internal intercostal muscle overlap, covering the rib cage. In the lateral and posterior part of the intercostal space, internal intercostal muscles and external intercostal muscles overlap, and ultrasound cannot separate both muscle layers. Therefore, it can not be observed that increasing muscle thickness of the external intercostal muscle that works in the lateral and posterior intercostal spaces, under the conditions of maximal inspiratory breathing task alone, and the condition of a simple transversely ultrasound imaging without considering the muscle fiber orientation in both internal intercostal muscles and external intercostal muscles.
It was found that intercostal muscle thickness at the anterior portion increases with maximal breathing. The experiment can make a significant contribution to the literature because muscle thickness of the intercostal muscle with ultrasound imaging would be a useful parameter for assessing the respiratory muscle activity in patients with tetraplegia and neuromuscular weakness. However, intercostal muscle thickness did not increase in the lateral and posterior intercostal spaces. In the future, it would be necessary to modify the protocol in order to separate the muscle thickness of the internal and external intercostal muscles.
Conflict of interest
None.
Acknowledgments
We thank Prof. Kouichi Iwai, Ibaraki Prefectural University of Health Sciences, for making this study possible. All authors have declared that there are no additional relationships or activities that may appear to have influenced the submitted work.
REFERENCES
- 1.DiMarco AF, Takaoka Y, Kowalski KE: Combined intercostal and diaphragm pacing to provide artificial ventilation in patients with tetraplegia. Arch Phys Med Rehabil, 2005, 86: 1200–1207. [DOI] [PubMed] [Google Scholar]
- 2.Nigel P, Derek F, Roger WS: The trunk and neck in anatomy and human movement. Nigel P, Derek F, Roger WS, Ed. London: Gutenberg Press, 2002, pp 480–481. [Google Scholar]
- 3.Duron B, Rose D: The intercostal muscles in neural control of the respiratory muscles. Miller AD, Bianchi AL, Bishop BP, Ed. Florida: CRC Press, 1997, pp 29–30. [Google Scholar]
- 4.Gandevia SC, Gorman RB, McKenzie DK, et al. : Effects of increased ventilatory drive on motor unit firing rates in human inspiratory muscles. Am J Respir Crit Care Med, 1999, 160: 1598–1603. [DOI] [PubMed] [Google Scholar]
- 5.Hodges PW, Pengel LH, Herbert RD, et al. : Measurement of muscle contraction with ultrasound imaging. Muscle Nerve, 2003, 27: 682–692. [DOI] [PubMed] [Google Scholar]
- 6.Landis JR, Koch GG: The measurement of observer agreement for categorical data. Biometrics, 1977, 33: 159–174. [PubMed] [Google Scholar]
- 7.Taylor A: The contribution of the intercostal muscles to the effort of respiration in man. J Physiol, 1960, 151: 390–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diab KM, Shalabi A, Sevastik JA, et al. : A method for morphometric study of the intercostal muscles by high-resolution ultrasound. Eur Spine J, 1998, 7: 224–228. [DOI] [PMC free article] [PubMed] [Google Scholar]


