Abstract
[Purpose] To investigate the characteristics and distributions of the myofascial trigger point (TrP) and pressure pain threshold (PPT) of the active TrP in individuals with chronic tension-type headache (CTTH). [Participants and Methods] Fifty-three CTTH patients and 53 age and gender-matched individuals without CTTH (CON) were recruited. The TrPs and tenderness points were first identified by manual palpation, and the PPTs of the active TrPs were determined by using a manual algometer. [Results] The active TrP, latent TrP and tenderness point totals per person in the head, neck, shoulder and upper back in CTTH were 4.3 ± 2.1, 0.6 ± 1.0 and 1.9 ± 1.8, respectively, while those in CON were 0, 0.7 ± 1.5 and 1.9 ± 1.8, respectively. The PPT levels of the active TrPs were 0.7 ± 0.2 to 1.2 ± 0.6 kg/cm2 in the muscles of the head, neck, shoulder and upper back. A larger number of active TrPs and lower PPT levels of the active TrPs were found in the head, neck and shoulder regions than in the upper back region. [Conclusion] Lower PPTs of the active TrPs in the head, neck and shoulder regions could influence the individuals with CTTH.
Key words: Head and neck pain, Myofascial tenderness, Pressure pain threshold
INTRODUCTION
A headache is one of the most commonly reported complaints in the general adult population1). The most common headache types are tension-type headaches (TTH) and migraines. TTHs can lead to impairments in functional, emotional, and social activities2, 3); therefore, they are known as psychogenic, stress, psychomyogenic and idiopathic headaches. The prevalence of TTH peaks between the ages of 30 and 39 years old, and it decreases slightly with an increasing age4). TTHs are generally related to emotional conflicts and psychosocial stress; however, the primary cause of TTH remains unknown. TTH can be categorized into episodic tension-type headaches (ETTH) or chronic tension-type headaches (CTTH). Myofascial tenderness and muscle hardness are often observed in CTTH; therefore, these headaches might be due to an excess of nociceptive inputs from peripheral factors5). However, whether CTTH pain originates from the myofascial tissues or from central mechanisms in the brain is still a matter for debate.
The headaches in individuals suffering from CTTH are associated with the trigger point (TrP) in the head and neck muscles6). A TrP was defined as a hyperirritable spot within a taut band of skeletal muscle7). A TrP is often misclassified as a tenderness point, which hurts when pressed but does not refer the pain elsewhere8). TrPs are hypersensitive to mechanical pressure, and they responds with referred pain in TTHs. An active TrP can be distinguished from a latent TrP; a latent TrP is not a source of clinical pain complaints, but mechanical stimulation may elicit referred pain or a muscle contraction7). Marcus et al. reported that individuals suffering from CTTH exhibit a greater number of either active or latent TrPs than healthy individuals; however, they did not specify the painful area in which active TrPs are frequently found9). No studies have investigated the characteristics and painful areas of myofascial TrPs in individuals with CTTH. Therefore, the objective of this study was to determine the characteristics and distributions of the active TrPs and pressure pain threshold (PPT) of the active TrPs in individuals with CTTH and compare them with those of healthy individuals. This study could provide scientific evidence for TrPs in the head, neck and shoulder and upper back muscles as well as a guideline for the diagnosis of pain and tension in individuals with CTTH.
PARTICIPANTS AND METHODS
Participants with CTTH were recruited from the general community using bulletin boards and verbal requests to volunteer to take part in this study. The Ethics Committee of Khon Kaen University approved the research protocol. A total of 130 participants responded to the recruitment advertisements, and they were screened for eligibility. The participants were between 20 and 50 years old with the presence of a headache over at least the last 3 months. After the participants completed the screening procedures which used questionnaires and physical examinations, they provided their informed consent. Then, their baseline data was collected, and they were assigned into either the CTTH or control (CON) groups. The CTTH inclusion criteria were those participants who fulfilled the International Headache Society CTTH criteria as follows: 1) Headaches occurring on 15 or more days per month, on average, for more than 3 months (180 or more days per year); 2) A headache that lasts hours or that may be continuous; 3) At least three of the following pain characteristics: bilateral location, pressing or tightening (non-pulsating), neither moderate nor severe nausea nor vomiting and not aggravated by routine physical activity, such as walking or climbing stairs; 4) No nausea, vomiting (anorexia can occur), photophobia or phonophobia. The CTTH exclusion criteria were examined and confirmed by neurologist, the presence of more than one type of headache in addition to the TTH, such as a migraine, or having a history of one of the following diseases or disorders: 1) Cervical disorder, such as cervical spondylosis, spondylolysis, spondylolisthesis or a herniated disc; 2) Neurological deficit, such as hemiplegia/paresis; 3) Skin disease: smallpox, chickenpox, herpes zoster, herpes or hives; 4) Uncontrolled hypertension, blood pressure >170/90 mmHg; 5) Thrombophlebitis; 6) Hypermobile joints; 7) Unable to follow instructions or poor communication skills; 8) Medication-overuse headache, as defined by the International Headache Society 200410); 9) Taking analgesics or muscle relaxants 24 hr prior to the examination; 10) Menstruation or a high body temperature (fever of more than 38.5 °C) on the day of the examination; and 11) Taking anticoagulant medications, such as aspirin, clopidogrel, prasugrel or ticlopidine. The healthy CON participants were selected by matching the same gender and age range within plus or minus 5 years. They had no headaches present or any experiences of infrequent episodic TTH (ETTH) for more than 2 months prior to being entered into the study. The inclusion criteria were that the participants fulfilled the International Headache Society ETTH infrequency criteria as follows: 1) At least 10 episodes that occurred less than 1 day per month (less than 12 days per year); 2) A headache lasting from 30 min to 7 days; and 3) At least three of the following pain characteristics: bilateral location, pressing or tightening (non-pulsating), mild or moderate intensity, not aggravated by routine physical activity (such as walking or climbing stairs), no nausea, vomiting (anorexia can occur), photophobia or phonophobia. The sample size estimation was based on a previous study by Fernández-de-Las-Peñas et al., and the TrP proportions presenting in the CTTH and CON groups were 76% and 48%, respectively. The sample size was calculated for two-sample comparison of proportions with a power of 80% and at 5% significance was allowed for estimating the final sample size data using the STATA Version 10 (StataCorp LP, Texas, USA). According to these criteria, 53 CTTH participants (age: 38.2 ± 8.7 years; BMI: 22.9 ± 3.2 kg/m2, mean ± SD) met the inclusion criteria and 53 age and gender-matched CON participants (age: 37.6 ± 9.4 years; BMI: 23.5 ± 4.2 kg/m2, mean ± SD) were selected.
After the demographic characteristics and headache histories were recorded, the TrPs and tenderness points were first identified by manual palpation, then the PPTs of the TrPs were determined using a manual algometer (Force Dial FDK/FDN Series Mechanical Force Gage; Wagner Instruments, Greenwich, CT, USA). In order to validate the TrP palpation skills, a comparison of the TrPs in the upper trapezius muscles between two examiners was analysed, and the TrP difference between them was 1.1 ± 0.5 cm, which was acceptable11). The intra-rater reliability and validity of the PPT measurements of the TrPs in the head and neck muscles were established prior to the study using 30 participants. There was high reliability using a two-way mixed effects model (3,1) of the intraclass correlation coefficient [0.97, 95% confidence interval (95% CI)=0.95 to 0.99, p<0.001] and high validity using Pearson’s correlation coefficient (0.9, 95% CI=0.8 to 1.0, p<0.001) when compared with a digital algometer (Digital Hand-held algometer both FPI, Madrid, Spain).
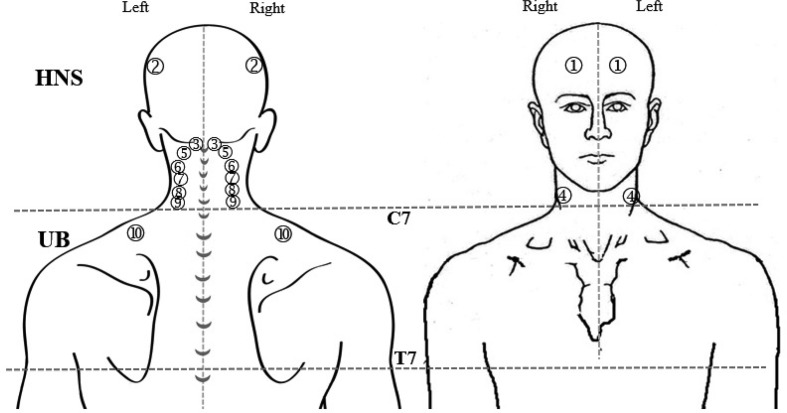
To identify the trigger point (TrP) and the tenderness point, manual palpation was performed on 10 muscles in the head, neck, shoulder and upper back. The palpation areas were divided into in the head, neck and shoulder (HNS) and upper back (UB) on the right and left sides (Fig. 1). The HNS included those areas from the top of the head to the C7 spinous process, and the UB included those areas from C7 to T7. These muscle regions included the occipitofrontalis, temporalis, suboccipital, sternocleidomastoid, splenius capitis, splenius cervicis, semispinalis capitis, semispinalis cervicis and upper and middle trapezius muscles. According to Simons and colleagues, the referred pain pattern of TrP of each muscle was used to specify the accuracy of each muscle and its TrP12). Each TrP location was marked on a piece of transparent with a pre-drawn illustration. During the assessment, the participants were maintained in a prone position. An active TrP was recorded when the participant exhibited spontaneous pain, tenderness in a taut band and referred pain after pressure was applied to the TrP. A latent TrP exhibited characteristics similar to those of an active TrP, except there was no pain unless compressed7). Based on the TrP criteria, the other tenderness point was described as “tenderness” that did not refer pain anywhere.
Fig. 1.
Location of trigger points in the head, neck, shoulder and upper back muscles.
HNS: head, neck, and shoulder area where is from a top of head to C7; UB: upper back area where is from C7 to T7. 1) occipitofrontalis, 2) temporalis, 3) suboccipital, 4) sternocleidomastoid, 5) splenius capitis, 6) splenius cervicis, 7) semispinaliscapitis, 8) semispinaliscervicis, and 9) upper and 10) middle trapezius muscles.
In order to determine the PPT of an active TrP, the compression pressure was gradually increased at a rate of 1 kg/sec. The participant gave a signal when they began feeling any pain or discomfort, at which point the compression was stopped. The pressure ranged from 0 to 11 kg/cm2, with increments of 0.1 kg. The TrP was measured three times, and the average was calculated for the statistical analysis.
The outcome measures were analysed and presented as the mean ± standard deviation and percentage. An independent t-test was performed to compare the continuous outcome variables, such as the number of active TrPs, latent TrPs, tenderness points and PPTs of the most painful active TrPs between the CTTH and CON groups and between the number of TrPs in the HNS muscles and the number of TrPs in the UB muscles. To achieve statistical significance, an 80% power and an overall two-sided 5% significance were used.
RESULTS
The active TrP, latent TrP and tenderness point totals per person in CTTH were 4.3 ± 2.1, 0.6 ± 1.0 and 1.9 ± 1.8, respectively. The active TrP, latent TrP and tenderness point totals per person in CON were 0, 0.7 ± 1.5 and 1.9 ± 1.8, respectively. CON did not have any active TrPs, and there were no significant differences in the latent TrPs and tenderness points between the 2 groups. The TrPs and tenderness points were equally distributed on the left and right sides in both groups. In CTTH, the number of TrPs in the HNS muscles was significantly higher than that in the UB muscles (p<0.001). The active TrPs were found most often in the suboccipital (right, n=32; left, n=30), temporalis (right, n=29; left, n=27), splenius/semispinalis (right, n=20; left, n=20) and upper trapezius (right, n=18; left, n=16) muscles. The PPT levels of the active TrPs in HNS and UB were 0.7 ± 0.2 and 1.2 ± 0.6 kg/cm2, respectively. The average PPT of the active TrPs in both areas was 1.0 ± 0.3 kg/cm2.
DISCUSSION
This study showed the characteristics and distributions of the active and latent myofascial TrPs of the HNS and UB muscles in individuals with CTTH. CTTH exhibited a large number of active TrPs in the HNS region; however, the latent TrP and tenderness point totals in the individuals with CTTH were not different from those of the healthy individuals. None of the healthy individuals had any active TrPs. The PPTs of the active TrPs in the individuals with CTTH were found to be very low.
The TrPs in the muscles are critically involved in CTTH based on the characteristics of the referred pain pattern7), and pericranial tissue tenderness has been found in those individuals with CTTH13, 14). However, although the tenderness points are responsible for local pain, they do not provoke referred pain6). Fernandez-de-Las-Penas et al. has suggested that the referred pain from an active TrP is a headache indicator for individuals with CTTH. Individuals with CTTH have greater numbers of both active and latent TrPs than healthy participants9). In line with our results, individuals with CTTH have a significantly greater number of active TrPs in the head and neck muscles, but not latent TrPs, than healthy controls6).
The individuals in CTTH had active TrPs in the HNS and UB muscles, while those in CON had only tenderness points. The average PPT of the active TrPs in these muscles was 1.0 ± 0.3 kg/cm2. It has been suggested that if the PPT was lower than 2 kg/cm2, the pain was at an abnormal level15). The level of PPTs was low in individuals with CTTHs16). The peripheral nociceptive input area may be responsible for the development of central sensitization17), and a certain pain or headache level could result from the sum of the nociceptive inputs from the cranial and extracranial tissues5). These results suggest that both peripheral and central sensitization may be involved in the development of chronic headaches, because central sensitization is driven by prolonged nociceptive inputs from the periphery sensitization of peripheral nociceptors18). An active TrP could play a relevant role in the origin or perpetuation of the sensitization of central pathways19).
This study did have some limitations. Our results cannot be extrapolated to ETTH or migraine cases, and it would be interesting to investigate the TrPs in individuals suffering from these types of headaches. In addition, a therapeutic approach based on TrP management and the role of TrP activation needs to be verified.
In conclusion, this study showed that active TrPs were found in the HNS and UB muscles of individuals with CTTH. The PPTs of the active TrPs were found to be very low in the individuals with CTTH. The results suggested that the low PPTs of the active TrPs in the HNS region could influence the headaches in individuals with CTTH.
Funding
Research Center in BNOJPH, Khon Kaen University, Thailand.
Conflicts of interest
No conflicts of interest.
REFERENCES
- 1.Ward TN, Levin M, Phillips JM: Evaluation and management of headache in the emergency department. Med Clin North Am, 2001, 85: 971–985 . [DOI] [PubMed] [Google Scholar]
- 2.Jensen R: Pathophysiological mechanisms of tension-type headache: a review of epidemiological and experimental studies. Cephalalgia, 1999, 19: 602–621 . [DOI] [PubMed] [Google Scholar]
- 3.Schwartz BS, Stewart WF, Lipton RB: Lost workdays and decreased work effectiveness associated with headache in the workplace. J Occup Environ Med, 1997, 39: 320–327 . [DOI] [PubMed] [Google Scholar]
- 4.Rasmussen BK: Epidemiology of headache. Cephalalgia, 1995, 15: 45–68 . [DOI] [PubMed] [Google Scholar]
- 5.Olesen J: Clinical and pathophysiological observations in migraine and tension-type headache explained by integration of vascular, supraspinal and myofascial inputs. Pain, 1991, 46: 125–132 . [DOI] [PubMed] [Google Scholar]
- 6.Fernández-de-Las-Peñas C, Alonso-Blanco C, Cuadrado ML, et al. : Myofascial trigger points and their relationship to headache clinical parameters in chronic tension-type headache. Headache, 2006, 46: 1264–1272 . [DOI] [PubMed] [Google Scholar]
- 7.Gerwin RD: Myofascial pain syndromes in the upper extremity. J Hand Ther, 1997, 10: 130–136 . [DOI] [PubMed] [Google Scholar]
- 8.Wolfe F, Smythe HA, Yunus MB, et al. : The American college of rheumatology 1990 criteria for the classification of fibromyalgia. Report of the multicenter criteria committee. Arthritis Rheum, 1990, 33: 160–172 . [DOI] [PubMed] [Google Scholar]
- 9.Marcus DA, Scharff L, Mercer S, et al. : Musculoskeletal abnormalities in chronic headache: a controlled comparison of headache diagnostic groups. Headache, 1999, 39: 21–27 . [DOI] [PubMed] [Google Scholar]
- 10.Olesen J, Steiner TJ: The International classification of headache disorders, 2nd edn (ICDH-II). J Neurol Neurosurg Psychiatry, 2004, 75: 808–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanz DR, Lobo CC, López DL, et al. : Interrater reliability in the clinical evaluation of myofascial trigger points in three ankle muscles. J Manipulative Physiol Ther, 2016, 39: 623–634 . [DOI] [PubMed] [Google Scholar]
- 12.Simons DG, Travell JG, Simons LS: Apropos of all muscles: trigger point release. In: Myofascial pain and dysfunction: the trigger point manual. Upper half of body. Baltimore: Williams & Wilkins, 1999, pp 140–150. [Google Scholar]
- 13.Langemark M, Olesen J: Pericranial tenderness in tension headache. A blind, controlled study. Cephalalgia, 1987, 7: 249–255 . [DOI] [PubMed] [Google Scholar]
- 14.Jensen R, Bendtsen L, Olesen J: Muscular factors are of importance in tension-type headache. Headache, 1998, 38: 10–17 . [DOI] [PubMed] [Google Scholar]
- 15.Fischer AA: Algometry in the daily practice of pain management. J Back Musculoskeletal Rehabil, 1997, 8: 151–163 . [DOI] [PubMed] [Google Scholar]
- 16.Schoenen J, Bottin D, Hardy F, et al. : Cephalic and extracephalic pressure pain thresholds in chronic tension-type headache. Pain, 1991, 47: 145–149 . [DOI] [PubMed] [Google Scholar]
- 17.Bendtsen L: Central sensitization in tension-type headache--possible pathophysiological mechanisms. Cephalalgia, 2000, 20: 486–508 . [DOI] [PubMed] [Google Scholar]
- 18.Mendell LM, Wall PD: Responses of single dorsal cord cells to peripheral cutaneous unmyelinated fibres. Nature, 1965, 206: 97–99 . [DOI] [PubMed] [Google Scholar]
- 19.Shah JP, Phillips TM, Danoff JV, et al. : An in vivo microanalytical technique for measuring the local biochemical milieu of human skeletal muscle. J Appl Physiol 1985, 2005, 99: 1977–1984 . [DOI] [PubMed] [Google Scholar]