Abstract
Background
Through improved understanding of the structure-activity relationship attributes of fluoroquinolones, molecule development has improved efficacy, safety, and tolerability of the class. Adverse events (AEs) associated with the fluoroquinolones are well defined and a prospective part of the development process. However, not all fluoroquinolones have the same AE profile with different substitutions on the core molecule resulting in differences in side effects and spectrum of activity. Unique structural attributes of delafloxacin (DLX) may differentiate its AE profile compared to other fluoroquinolones. This analysis compared the incidence of AEs between DLX and vancomycin/aztreonam across two phase 3 ABSSSI studies in order to provide a broader overview of DLX safety.
Methods
Safety events occurring in all subjects in the pivotal phase 3 trials were pooled to provide a broad overview of DLX safety.
Results
DLX was safe and well-tolerated in the pooled phase 3 ABSSSI trial population of 741 subjects. Treatment-emergent AEs (TEAEs) were seen in the DLX group versus the comparator group at 45.1% and 47.7%, respectively. Most were mild or moderate in severity. Treatment-related TEAEs were reported in the DLX group versus the comparator group at rates of 22.1% and 26.1%, respectively.
Conclusions
Available data show DLX is well tolerated in both intravenous and oral formulation for the treatment of ABSSSI and does not appear to be associated with increased risk of AEs associated with other fluoroquinolones. It remains important to monitor for potential AEs that have been observed with other fluoroquinolones.
Keywords: delafloxacin, ABSSSI, safety, fluoroquinolone
The quinolones have been one of the most widely administered class of antibiotics over the last 30 years and have been used to treat approximately 20 billion patients worldwide. Although fluoroquinolones (FQs) are generally well tolerated, they have certain class side effects, which have been documented.
Reporting of adverse events (AEs) can vary in clinical studies on the basis of time of approval, evolving knowledge of toxicity, and improved diagnostic capabilities. Clinician awareness of potential AEs also plays a part in the clinical setting. In the case of FQs, the class effects have been accounted for in reports of clinical experience, Food and Drug Administration (FDA) advisories, regulatory documents, and clinical studies. Through improved understanding of the structure-activity relationship (SAR) attributes of FQs, molecule development has been directed to improving the efficacy, safety, and tolerability of FQs [1–3].
Delafloxacin has recently been approved in the United States for the treatment of adult patients with acute bacterial skin and skin structure infections (ABSSSIs). The benefits of SAR extend to the potency of delafloxacin relative to other FQs against Gram-positive bacteria, including methicillin resistant Staphylococcus aureus, as well as enhanced potency at acidic pH. Further, the unique structural attributes of delafloxacin may be associated with a differentiated AE profile compared to other FQs [1, 2].
Delafloxacin has been evaluated in 30 phase 1, 2, and 3 clinical trials that included a total of 2658 delafloxacin-treated subjects. Collectively, these trials provide evidence for the favorable safety profile and effectiveness of the intravenous (IV) and oral formulations of delafloxacin at 300-mg IV and 450-mg oral tablet. The phase 2 and phase 3 ABSSSI safety database includes 1840 patients, of which 868 received multiple doses of delafloxacin 300 mg IV/450 mg oral in 4 completed phase 2 and phase 3 studies. Table 1 All 4 studies used the intended marketed dose of delafloxacin of 300 mg IV Q12h. Study 303 [4] included the delafloxacin 450-mg oral dose for oral step-down therapy.
Table 1.
Description of Phase 2 and Phase 3 Clinical Acute Bacterial Skin and Skin Structure Infection Studies
| No. of Subjects (N = 1840)a | ||||
|---|---|---|---|---|
| Study Number | Title | Delafloxacin (n = 868) | Comparator (n = 972) | Dose |
| RX-3341-201[5] | A Randomized, double-blind, multicenter study of the safety and efficacy of RX-3341 compared with tigecycline for the treatment of complicated skin and skin structure infections | 49 | 50 | Delafloxacin: 300 or 450 mg BID IV tigecycline: 100 loading and 50 mg BID IV (Multiple dose: 5–14 d) |
| RX-3341-202[6] | A phase 2 exploratory study of objective endpoints in subjects with acute bacterial skin and skin structure infections treated with delafloxacin, vancomycin, or linezolid | 78 | 171 | Delafloxacin: 300 mg BID IV vancomycin: 15 mg/kg BID or local standard of care linezolid 600 mg BID IV (with concomitant aztreonam in linezolid and vancomycin subjects) (Multiple dose: 5–14 d) |
| RX-3341-302[7] | Phase 3, multicenter, randomized, double-blind, active-controlled study to evaluate the efficacy and safety of delafloxacin compared with vancomycin + aztreonam in patients with acute bacterial skin and skin structure infections | 324 | 326 | Delafloxacin: 300 mg IV in a 1-hour infusion every 12 hrs. Vancomycin: 15 mg/kg every 12 hrs (with concomitant aztreonam) (Multiple dose: 5–14 d) |
| RX-3341-303[4] | Phase 3, multicenter, randomized, double-blind, active-controlled study to evaluate the efficacy and safety of IV and oral delafloxacin compared with vancomycin + aztreonam in patients with acute bacterial skin and skin structure infections | 417 | 425 | Delafloxacin: 300 mg IV in a 1-hour infusion every 12 hrs for 6 doses then 450 mg oral every 12 hrs. Vancomycin: 15 mg/kg every 12 hrs or local standard of care (with concomitant aztreonam) (Multiple dose: 5–14 d) |
RX-3341-201 also enrolled 51 patients who received 450 mg IV Q12h delafloxacin for 5 to 14 days; the data for these patients at the 450-mg IV Q12h dose are not included in the pooled multiple dose analysis set because the 450-mg IV dose provides higher exposure than planned with the marketed 300-mg IV dose.
Abbreviations: BID, two times a day; IV, intravenous.
aSubjects had to receive at least 1 dose in a treatment.
Safety data were collected from the 348 subjects who comprised the safety population in the two phase 2 clinical trials [5, 6]. Based upon gastrointestinal events seen in the phase 2 studies, the 300 mg IV Q12h dose was chosen for further development. These phase 2 studies were reported elsewhere and due to differences in comparators and trial design, the focus of this analysis will be based on pooled data from the two phase 3 trials that were of similar design and used identical comparators [4, 7]. Safety events occurring in all subjects in the pivotal phase 3 trials were pooled to provide a broader overview of delafloxacin safety.
METHODS
The results from the two phase 3 trials with over 1500 subjects established delafloxacin’s noninferiority to the comparator vancomycin ± aztreonam in the treatment of adults with ABSSSIs. In these studies, adult patients with ABSSSI were randomized 1:1 to receive delafloxacin monotherapy or vancomycin plus aztreonam. Patients received between 5 and 14 days of delafloxacin 300 mg IV every 12 hours in study 302 [7] or delafloxacin 300 mg IV every 12 hours for 3 days with a mandatory blinded switch to delafloxacin 450 mg orally every 12 hours in study 303. Vancomycin was dosed 15 mg/kg IV based upon actual body weight with aztreonam 1–2 g every 12 hours. The safety analysis population consisted of all enrolled subjects who received at least 1 dose of study drug. Of the 741 subjects randomized to the delafloxacin arm, 324 subjects were from study 302 and 417 subjects from study 303. The safety outcomes in IV and oral delafloxacin in study 303 are pooled because the 450-mg oral dose of delafloxacin has comparable total systemic exposure (area under the curve [AUC]) to the 300-mg IV dose [4].
Safety was assessed by collection of AE reports, clinical laboratory tests, physical examinations, vital sign measurements, and 12-lead electrocardiograms (ECGs) at baseline and as clinically indicated thereafter. In study 302, blood samples for intensive blood glucose analysis were obtained on day 3 (± 1 day) within 2 hours before the first study drug administration, and at 1, 2, 3, 5, and 12 hours after the start of the first infusion (± 10-minute window after a minimum of 3 consecutive doses of study drug). Treatment-emergent AEs (TEAEs) were defined as events that occurred or worsened following administration of the first dose of the study drug through the 30-day telephone follow-up. The investigator assessed the “relatedness” of the event to the treatments. These events were coded using the Medical Dictionary for Regulatory Activities (MedDRA) 16.1, which allows standardization in classification and coding into organ classes. AE reports potentially related to the AEs of special interest (AESIs) were identified by the use of Standardized MedDRA queries (SMQS) where possible. SMQs are validated, standard sets of MedDRA terms used to support signal detection and evaluation of safety topics of interest, casting a wide net over multiple AE terms potentially associated with a specific medical event of interest.
RESULTS
The pooled phase 3 analysis set included 741 subjects treated with delafloxacin and 751 treated with vancomycin/aztreonam. Patient medical history and demographics were similar between the delafloxacin and comparator groups. (Table 2) Patients ranged from 18 to 94 years of age with a mean of 49.0 years in the delafloxacin group and a mean of 48.0 years in the comparator group. In both treatment groups, approximately 11% of patients had diabetes and 16% baseline renal impairment. A body mass index (BMI) ≥30 kg/m2 was seen in 44.1% of delafloxacin patients and 40.7% in the comparator. Patients with a history of hepatitis B or C made up 29.1% and 28.9% of the delafloxacin and comparator groups, respectively.
Table 2.
Demographics and Baseline Characteristics—Pooled Phase 3 Analysis Set
| Delafloxacin (N = 741) | Vancomycin + Aztreonam (N = 751) | |
|---|---|---|
| Age categories (year), n (%) | ||
| ≤65 | 640 (86.4) | 656 (87.4) |
| >65 | 101 (13.6) | 95 (12.6) |
| Sex, n (%) | ||
| Male | 459 (61.9) | 483 (64.3) |
| Female | 282 (38.1) | 268 (35.7) |
| Race, n (%) | ||
| American Indian or Alaska Native | 16 (2.2) | 9 (1.2) |
| Asian | 12 (1.6) | 16 (2.1) |
| Black or African American | 38 (5.1) | 36 (4.8) |
| Native Hawaiian or Other Pacific Islander | 3 (0.4) | 4 (0.5) |
| White | 636 (85.8) | 656 (87.4) |
| Other | 36 (4.9) | 30 (4.0) |
| Ethnicity, n (%) | ||
| Hispanic or Latino | 231 (31.2) | 201 (26.8) |
| Not Hispanic or Latino | 510 (68.8) | 550 (73.2) |
| Region, n (%) | ||
| Asia | 9 (1.2) | 14 (1.9) |
| Europe | 225 (30.4) | 228 (30.4) |
| Latin America | 46 (6.2) | 43 (5.7) |
| North America | 461 (62.2) | 466 (62.1) |
| Weight (kg) | ||
| n | 741 | 751 |
| Mean (SD) | 85.5 (21.6) | 85.8 (22.1) |
| Median | 82.6 | 83.0 |
| Min, Max | 41.9, 198.5 | 43.8, 185.0 |
| BMI ranges (kg/m2), n (%) | ||
| BMI < 30 | 414 (55.9) | 445 (59.3) |
| BMI ≥ 30 | 327 (44.1) | 306 (40.7) |
| Diabetes, n (%) | 84 (11.3) | 83 (11.1) |
| Baseline renal impairment, n (%) | 121 (16.3) | 121 (16.1) |
| Patients with history of hepatitis B or C, n (%) | 216 (29.1) | 217 (28.9) |
Abbreviations: BMI, body mass index; SD, standard deviation.
Overall, 13.4% of patients treated with delafloxacin withdrew from the studies compared with 14.9% of patients treated with vancomycin/aztreonam. The most common reason for withdrawal from study was patients lost to follow-up (6.6% in the delafloxacin group and 7.3% vancomycin/aztreonam). Overall, 1.5% of patients treated with delafloxacin withdrew from study due to an AE compared with 2.8% of patients treated with comparators.
Delafloxacin exposure ranged from 0.5 to 14.0 days, with a mean of 6.8 days. Most patients (59.2%) received 11 to 28 doses of delafloxacin, with 40.7% receiving ≤ 10 doses. There were no notable differences between delafloxacin and the comparators with regard to exposure (Table 3).
Table 3.
Study Drug Exposure—Pooled Phase 3 Skin Infections
| Delafloxacina (N = 741) |
Vancomycina (Aztreonam) (N = 751) |
|
|---|---|---|
| Duration of exposure (day) | ||
| n | 741 | 751 |
| Mean (SD) | 6.8 (2.94) | 6.6 (2.82) |
| Median | 6.0 | 6.0 |
| Min, Max | 0.5, 14.0 | 0.5, 14.5 |
| Duration of exposure, n (%) | ||
| 0.5 to < 4 days | 48 (6.5) | 54 (7.2) |
| 4 to < 6 days | 301 (40.6) | 291 (38.7) |
| 6 to < 8 days | 180 (24.3) | 211 (28.1) |
| 8 to < 10 days | 88 (11.9) | 89 (11.9) |
| 10 to < 14 days | 104 (14.0) | 88 (11.7) |
| ≥14 days | 20 (2.7) | 18 (2.4) |
| Number of doses, n (%) | ||
| 1 to 10 doses | 293 (39.5) | 293 (39.0) |
| 11 to 28 doses | 447 (60.3) | 457 (60.9) |
| >28 doses | 1 (0.1) | 1 (0.1) |
One day of exposure is 2 doses. Abbreviation: SD, standard deviation.
aSummaries are for delafloxacin and vancomycin: Delafloxacin 300 mg intravenous (IV) / 450 mg oral Q12h; vancomycin 15 mg/kg (actual body weight) ± aztreonam 1–2 g IV q12h.
Delafloxacin was safe and well tolerated in the pooled phase 3 ABSSSI trial population of 741 subjects (Table 4). TEAEs were reported in the delafloxacin group versus the comparator group at rates of 45.1% and 47.7%, respectively. Most TEAEs were mild or moderate in severity, with < 4% of patients in either group experiencing severe TEAEs. Similarly, treatment-related TEAEs in the delafloxacin group versus the comparator group were seen in 22.1% and 26.1% of patients, respectively. Related TEAEs leading to premature study drug discontinuation were lower in the delafloxacin group versus vancomycin/aztreonam (0.8% and 2.4% of patients, respectively).
Table 4.
Overall Summary of Treatment-emergent Adverse Events—Pooled Phase 3
| Pooled Phase 3 Skin | ||
|---|---|---|
| Delafloxacin (N = 741) n (%) |
VAN/AZ (N = 751) n (%) |
|
| Total number of TEAEs | 775 | 879 |
| Patients with any TEAE | 334 (45.1) | 358 (47.7) |
| Patients with any related TEAE | 164 (22.1) | 196 (26.1) |
| Patients with any TEAE leading to premature study drug discontinuation | 13 (1.8) | 26 (3.5) |
| Patients with any related TEAE leading to premature study drug discontinuation | 6 (0.8) | 18 (2.4) |
| Patients with any TEAE of special interest, all cause | 52 (7.0) | 69 (9.2) |
| Patients with any serious TEAE | 27 (3.6) | 26 (3.5) |
| Patient with any related serious TEAE | 2 (0.3) | 4 (0.5) |
| Subjects with at least one related TEAE with incidence of ≥2% | ||
| Gastrointestinal disorders | 81 (10.9) | 45 (6.0) |
| Nausea | 45 (6.1) | 32 (4.3) |
| Diarrhea | 45 (6.1) | 15 (2.0) |
| Skin and subcutaneous tissue disorders (pruritus, urticaria, dermatitis, rash) | 7 (0.9) | 35 (4.7) |
A TEAE was defined as an adverse event with (1) start date/time on or after the date/time of first study drug administration and prior to or on the date/time of last study medication administration + 28 days or (2) start date/time prior to the date/time of first study drug administration and worsening on or after the date/time of first study drug administration and prior to or on the date/time of last study medication administration + 28 days. Percentages are calculated as 100 × (n/N). The total number of TEAEs counts all TEAEs for patients. At each level of patient summarization, a patient with 1 or more reported events was counted only once, and the most severe reported event was used for the maximum severity. Related includes “possibly related,” “probably related,” “related,” and “definitely related.” Adverse events were coded using Medical Dictionary for Regulatory Activities Version 16.1.
Abbreviations: TEAE, treatment-emergent adverse event; VAN/AZ, vancomycin/aztreonam.
Treatment-related TEAEs with an incidence of ≥2% in either treatment arm of the pooled phase 3 data included gastrointestinal disorders comprising nausea at 6.1% versus 4.3%, and diarrhea at 6.1% and 2.0% for delafloxacin and vancomycin/aztreonam, respectively. Gastrointestinal events were reported at comparable rates when delafloxacin was given by IV (17.3%, study 302) or by IV followed by oral doses (16.8%, study 303) [4, 7]. Skin and subcutaneous tissue disorders were seen at 0.9% for delafloxacin and 4.7% for vancomycin/aztreonam.
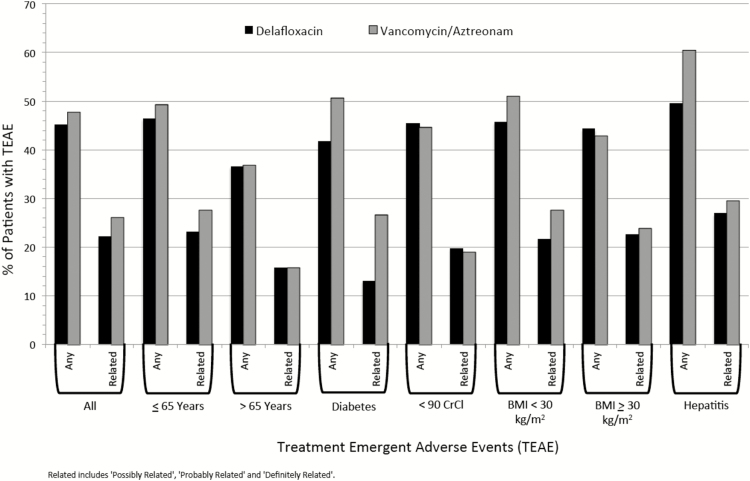
The incidence of TEAEs between treatment groups was similar regardless of age, gender, renal impairment, diabetic status, BMI, or hepatitis medical history (Figure 1).
Figure 1.
Treatment-emergent adverse events by subgroup. Abbreviations: BMI, body mass index; CrCl, creatinine clearance; TEAE, treatment-emergent adverse event.
Fluoroquinolone-related Adverse Events
There was no evidence of an increase in AESIs associated with FQs compared to the vancomycin/aztreonam group [8]. Numerically fewer patients had treatment-related FQ-related AEs in the delafloxacin group versus the comparator group, and there were no premature discontinuations from delafloxacin due to FQ-related AEs.
Hepatic events: Rates of all causality hepatic events (3.2% and 3.4% for delafloxacin and vancomycin/aztreonam, respectively) and treatment-related hepatic events consisting of elevation of transaminases (2.2% and 2.7%, respectively) were similar between patients in the delafloxacin and comparator groups. These events were generally mild or moderate in severity, nonserious, and no patients in either group met Hy’s Law. There were no discontinuations of treatment due to hepatic events.
Glucose events: Rates of treatment-related hyperglycemia and hypoglycemia were similar between patients in the delafloxacin and vancomycin/aztreonam groups (0.3% vs 0.1%) and (0.1% vs 0.3%) respectively. In study 302, glucose was carefully monitored over a period of 12 hours following IV delafloxacin doses in patients who were undergoing pharmacokinetic testing. No notable differences in blood glucose levels were noted at any time point for either treatment group in that analysis [7].
Potential neuromuscular events: There were no cases of peripheral neuropathy or myopathy in the pooled phase 3 data and no cases that met the FDA definition of FQ-associated disability (FQAD) as defined by the FDA [9]. (Patients who were previously healthy, prescribed a FQ, and developed an adverse event in 2 or more of the following body systems: peripheral nervous system, neuropsychiatric, musculoskeletal, senses, cardiovascular, or skin. The events had to last for more than 30 days after the FQ was discontinued and had a reported outcome of disability.)
Clostridium difficile: One patient (0.1%) in the delafloxacin group had C. difficile infection compared with none in the vancomycin/aztreonam group. The patient entered the study as a prior treatment failure with previous treatment with sulfamethoxazole/trimethoprim and clindamycin. The event of C. difficile infection was mild in severity and did not lead to treatment discontinuation.
Other notable class events: There were no patients in the delafloxacin group that had cases of QT prolongation or convulsions. No patients in either treatment group had treatment-related tendon disorder/rupture or phototoxicity.
CLINICAL LABORATORY EVALUATIONS
Changes in laboratory parameters were generally small and similar between treatment groups, and mean values at end of treatment remained within normal ranges. For relevant events that can be associated with a laboratory signal, such as hepatic events and dysglycemias, these laboratory changes generally occurred less frequently among delafloxacin patients than in the comparator group. In evaluation of laboratory testing, regardless of baseline values, only 8 patients in the delafloxacin treatment group reported alanine transaminase (ALT) >5 times the upper limit of normal (ULN) result at any time in the study, whereas 13 patients in the vancomycin/aztreonam treatment groups reported ALT >5 times ULN results at any time in the study. When such laboratory changes were seen, they typically were unlikely to be considered related to delafloxacin treatment, were confounded by other medical history/events/social circumstances, did not require treatment, and were reversible. (Table 5)
Table 5.
Treatment-emergent Adverse Events of Special Interest (All Cause and Treatment-related)—Pooled Phase 3
| AESI (All Cause) |
AESI (Related) |
|||
|---|---|---|---|---|
| Special Interest Preferred Term | Delafloxacin (N = 741) n (%) |
VAN/AZ (N = 751) n (%) |
Delafloxacin (N = 741) n (%) |
VAN/AZ (N = 751) n (%) |
| Subjects with at least one TEAE of special interest | 52 (7.0) | 69 (9.2) | 25 (3.4) | 43 (5.7) |
| Hepatic related events | 23 (3.1) | 30 (4.0) | 16 (2.2) | 20 (2.7) |
| Increased ALT | 14 (1.9) | 14 (1.9) | 10 (1.3) | 10 (1.3) |
| Increased AST | 10 (1.3) | 14 (1.9) | 6 (0.8) | 10 (1.3) |
| Increased transaminases | 3 (0.4) | 5 (0.7) | 3 (0.4) | 2 (0.3) |
| Increased hepatic enzyme | 2 (0.3) | 2 (0.3) | 1 (0.1) | 2 (0.3) |
| Liver function test abnormal | 0 | 2 (0.3) | 0 | 2 (0.3) |
| Hypertransaminasaemia | 2 (0.3) | 1 (0.1) | 1 (0.1) | 1 (0.1) |
| Increased gamma-glutamyltransferase | 1 (0.1) | 1 (0.1) | 0 | 0 |
| Hepatic cirrhosis | 0 | 1 (0.1) | 0 | 0 |
| Potential myopathy | 15 (2.0) | 34 (4.5) | 7 (0.9) | 20 (2.7) |
| Increased blood creatinine Phosphokinase | 8 (1.1) | 15 (2.0) | 3 (0.4) | 7 (0.9) |
| Increased blood creatinine | 2 (0.3) | 4 (0.5) | 1 (0.1) | 4 (0.5) |
| Myalgia | 1 (0.1) | 2 (0.3) | 0 | 1 (0.1) |
| Renal impairment | 2 (0.3) | 1 (0.1) | 2 (0.3) | 0 |
| Renal failure – acute | 1 (0.1) | 7 (0.9) | 1 (0.1) | 3 (0.4) |
| Musculoskeletal pain | 1 (0.1) | 2 (0.3) | 0 | 1 (0.1) |
| Renal failure | 0 | 3 (0.4) | 0 | 3 (0.4) |
| Decreased creatinine renal clearance | 0 | 1 (0.1) | 0 | 1 (0.1) |
| Hyperglycemia | 6 (0.8) | 4 (0.5) | 2 (0.3) | 1 (0.1) |
| Hyperglycemia | 2 (0.3) | 2 (0.3) | 2 (0.3) | 1 (0.1) |
| Diabetes mellitus | 3 (0.4) | 1 (0.1) | 0 | 0 |
| Type 2 diabetes mellitus | 1 (0.1) | 1 (0.1) | 0 | 0 |
| Potential peripheral neuropathy | 4 (0.5) | 3 (0.4) | 1 (0.1) | 2 (0.3) |
| Paraesthesia | 4 (0.5) | 1 (0.1) | 1 (0.1) | 1 (0.1) |
| Hypoaesthesia | 1 (0.1) | 1 (0.1) | 0 | 1 (0.1) |
| Neuropathy peripheral | 0 | 1 (0.1) | 0 | 0 |
| Potential QT prolongation | 2 (0.3) | 1 (0.1) | 0 | 1 (0.1) |
| Syncope | 2 (0.3) | 0 | 0 | 0 |
| Loss of consciousness | 0 | 1 (0.1) | 0 | 1 (0.1) |
| Potential tendon disorder | 3 (0.4) | 1 (0.1) | 0 | 0 |
| Tendonitis | 3 (0.4) | 0 | 0 | 0 |
| Trigger finger | 0 | 1 (0.1) | 0 | 0 |
| Hypoglycemia | 2 (0.3) | 3 (0.4) | 1 (0.1) | 2 (0.3) |
| C. difficile infection | 1 (0.1) | 0 | 1 (0.1) | 0 |
| Convulsions | 0 | 1 (0.1) | 0 | 1 (0.1) |
| Potential phototoxicity | 0 | 0 | 0 | 0 |
Abbreviations: AESI, adverse events of special interest; ALT, alanine transaminase; AST, aspartate transaminase; TEAE, treatment-emergent adverse event; VAN/AZ, vancomycin/aztreonam.
DISCUSSION
Despite some challenges with the class, persistent efforts have been made over the years to produce quinolones with superior antimicrobial properties enabled by the understanding of how molecular modification of the core quinolone structure affect the antimicrobial agent’s activity progressed rapidly [10, 11]. With the advent of newer agents, FQ prescribing increased 3-fold in the United States from 1995 to 2002 as they became the most commonly prescribed class of antibiotics to adults by 2002 [12].
With such widespread use and overuse came the recognition of a range of risks and AEs that were associated with the class [13]. In 2008, the FDA issued a black box warning for tendon rupture and most recently, in 2016, required changes to the label for all oral and injectable FQs, including a boxed warning related to disabling and potentially permanent side effects involving the tendons, muscles, joints, nerves, and central nervous system [14].
As experience with the class has grown, AEs associated with the class have been well defined and have become a prospective part of the development process of more current FQs [1, 15]. However, not all FQs have the same AE profile. SAR work has shown that different substitution on the core quinolone molecule results in difference among the class not only in spectrum of activity but also side effects [1, 16]. For example, the introduction of fluorine or chlorine into the molecule, which otherwise provided beneficial effects, was associated with phototoxicity. It was later found that the severity of a side effect might be influenced by moieties attached to other sites on the FQ core structure. For example, the moiety at position 1 appears to influence the phototoxic potential of the 8-halogen FQs [16, 17].
Delafloxacin is an IV/oral FQ approved in the United States for the treatment of adults with ABSSSI and has unique structural attributes that may be associated with a differentiated AE profile compared to other FQs. Although the delafloxacin label carries the FDA class boxed warning, other FQ-associated events are not found in the warnings/precautions section. Those absent from the label include peripheral neuropathy, CNS effects, and myasthenia gravis, QT/torsades, photosensitivity, hepatotoxicity/hepatitis, dysglycemias, and multiple classes of drug interactions [18]. Regardless, as with all drugs including antibiotics, it is sensible to continue to assess and further characterize delafloxacin’s safety profile and the pooling of phase 3 safety data for delafloxacin in ABSSSI provides an opportunity to do so. This analysis compared the incidence of AEs between delafloxacin and vancomycin/aztreonam across two phase 3 registrational ABSSSI studies.
Any related TEAEs were seen in the pooled delafloxacin group versus comparator group (22.1% vs 26.1%, respectively) and delafloxacin had a low rate of discontinuations due to treatment-related AEs (0.8%) compared to vancomycin/aztreonam (2.4%). The most common TEAE reported for delafloxacin have been diarrhea and nausea, both at reported rates of 6.1% and were mild to moderate in nature. Oral delafloxacin doses were not associated with an increase in overall gastrointestinal events in the comparison of these 2 pivotal trials (17.3%, study 302 vs 16.8%, study 303). Serious AEs occurred at similar rates in patients treated with delafloxacin versus vancomycin/aztreonam, suggesting a favorable risk-benefit ratio for delafloxacin in the treatment of ABSSSIs as vancomycin is widely used in this indication. There has been only 1 death in patients with delafloxacin in the phase 3 studies, which was considered unrelated to delafloxacin.
FQs have been associated with a number of well-described risks. The development of new FQs can thus include prospective evaluation and monitoring for these potential class-related AESIs. In these 2 pooled studies, AE reports potentially related to the AESIs were identified by medical review and use of SMQS where possible. SMQs are validated, standard sets of MedDRA terms used to support signal detection, which are used to assess and evaluate a variety of safety topics of regulatory interest [19]. The SMQs provide a framework for broad consideration of multiple AE terms that could potentially be associated with a medical event of interest. For example, the SMQ for potential QT prolongation includes events as specific as Torsade de pointes as well as events as general as syncope. This conservative approach allows a view of many events across body systems, in order to evaluate signals that may be otherwise difficult to assess. Even with the conservative assessment encompassing any AE that might even remotely be considered, the AESIs in the phase 3 clinical studies associated with FQ use were less frequently seen in the delafloxacin group than in the vancomycin/aztreonam group. This safety analysis indicates that delafloxacin does not appear to have an increased rate of AESIs that have been seen with other FQs. A more detailed analysis is published elsewhere [8].
When selecting an appropriate antibiotic for patients with ABSSSI, the prescribing physician has to consider a number of issues including potential pathogens, patient’s comorbidities, concomitant medications, and challenges for drug administration. Pooling of the phase 3 data allows a more robust analysis of subsets of patients that may offer further insight into various patient types. Fixed dose delafloxacin has comparable safety outcomes regardless of age, sex, renal impairment, diabetic status, BMI, or hepatitis medical history, does not require therapeutic drug monitoring, and does not require weight-based dosing.
Changes in laboratory parameters were generally small and similar between treatment groups, and mean values at end of treatment remained within normal ranges. For AESIs that can be associated with a laboratory signal, such as hepatic events and dysglycemias, these laboratory changes generally occurred less frequently among delafloxacin patients than in the vancomycin/aztreonam group. When such laboratory changes were seen, they typically were unlikely to be considered related to delafloxacin treatment, were confounded by other medical history/events/social circumstances, did not require treatment, and were reversible.
Patients that may require an antibiotic with a profile like delafloxacin often have comorbidities that are associated with polypharmacy, and so potential drug-drug-interaction is an important consideration. No known drug interactions were observed in the clinical studies. In vitro studies conducted with human liver microsomes showed that delafloxacin is not an inhibitor of CYP1A2 nor any of the other isozymes (CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1, or CYP3A4/5). However, delafloxacin is a mild inducer of CYP3A in cultures of human hepatocytes. A study using midazolam as an established CYP3A probe showed no clinically meaningful induction of CYP3a by delafloxacin [20].
There are limitations to this analysis. The database is robust but still limited in terms of the number of patients and duration. The 14-day duration of treatment in the phase 3 trials is the longest duration of dosing in a clinical trial to date. Further, the comparator was not an FQ, and so a dependence on a comparison of the AESIs within the basal population rate is necessary. Characterizing the risk of some AESIs in the general population is difficult [2]; however, delafloxacin rates of any TEAEs as well as related TEAEs were lower than those of vancomycin/aztreonam.
The key with all medical treatments is to balance the good and helpful with the risk of potential harm from side effects. If a patient has an invasive serious bacterial infection, antibiotics are potentially life-saving. Available data show delafloxacin is well tolerated in both IV and oral formulation for the treatment of ABSSSI and does not appear to be associated with increased risk of AESIs associated with other FQs. It remains important to monitor for potential AEs that have been observed with other FQs.
Financial support. Editorial support was funded by Melinta Therapeutics and was provided by Strategic Healthcare Communications, Hillsborough, NJ.
Supplement sponsorship. This supplement is sponsored by Melinta Therapeutics, Inc.
Potential conflicts of interest. M. B. has participated in advisory boards and/or received speaker honoraria from Achaogen, Angelini, Astellas, AstraZeneca, Bayer, Basilea, Cidara, Gilead, Menarini, Melinta, Merck Sharp Dohme, Paratek, Pfizer, The Medicine Company, Tetraphase, and Vifor. D. H. has participated in advisory boards for Melinta, Macrolide Pharmaceuticals, The Medicines Company, Selux Diagnostics, and Daner-Cepheid. G. T. has participated in advisory boards and/or consulted for Hennepin Life Sciences, Summit Therapeutics, Novobiotics, Spero Therapeutics, Melinta, and Shionogi. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Van Bambeke F. Delafloxacin, a non-zwitterionic fluoroquinolone in Phase III of clinical development: evaluation of its pharmacology, pharmacokinetics, pharmacodynamics and clinical efficacy. Future Microbiol 2015; 10:1111–23. [DOI] [PubMed] [Google Scholar]
- 2. Bassetti M, Pecori D, Cojutti P, Righi E, Pea F. Clinical and pharmacokinetic drug evaluation of delafloxacin for the treatment of acute bacterial skin and skin structure infections. Expert Opin Drug Metab Toxicol 2017; 13:1193–200. [DOI] [PubMed] [Google Scholar]
- 3. Owens RC Jr, Ambrose PG. Antimicrobial safety: focus on fluoroquinolones. Clin Infect Dis 2005; 41:S144–57. [DOI] [PubMed] [Google Scholar]
- 4. O’Riordan W, McManus A, Teras J, et al. ; PROCEED Study Group A comparison of the efficacy and safety of intravenous followed by oral delafloxacin with vancomycin plus aztreonam for the treatment of acute bacterial skin and skin structure infections: a phase 3, multinational, double-blind, randomized study. Clin Infect Dis 2018; 67:657–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. O’Riordan W, Mehra P, Manos P, Kingsley J, Lawrence L, Cammarata S. A randomized phase 2 study comparing two doses of delafloxacin with tigecycline in adults with complicated skin and skin-structure infections. Int J Infect Dis 2015; 30:67–73. [DOI] [PubMed] [Google Scholar]
- 6. Kingsley J, Mehra P, Lawrence LE, et al. . A randomized, double-blind, phase 2 study to evaluate subjective and objective outcomes in patients with acute bacterial skin and skin structure infections treated with delafloxacin, linezolid or vancomycin. J Antimicrob Chemother 2016; 71:821–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pullman J, Gardovskis J, Farley B, et al. ; PROCEED Study Group Efficacy and safety of delafloxacin compared with vancomycin plus aztreonam for acute bacterial skin and skin structure infections: a phase 3, double-blind, randomized study. J Antimicrob Chemother 2017; 72:3471–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lodise T, Corey R, Hooper D, Cammarata S. Safety of delafloxacin: focus on adverse events of special interest. Open Forum Infect Dis 2018; 5:ofy220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Food and Drug Administration. (2015). FDA briefing document: Joint meeting of the Antimicrobial Drugs Advisory Committee and the Drug Safety and Risk Management Advisory Committee, November 5, 2015 Retrieved on October 27, 2017, from: https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Anti-InfectiveDrugsAdvisoryCommittee/UCM467383.pdf. Accessed 27 October 2017.
- 10. Hoover R, Hunt T, Benedict M, et al. . Safety, tolerability, and pharmacokinetic properties of intravenous delafloxacin after single and multiple doses in healthy volunteers. Clin Ther 2016; 38:53–65. [DOI] [PubMed] [Google Scholar]
- 11. De Sarro A, De Sarro G. Adverse reactions to fluoroquinolones. an overview on mechanistic aspects. Curr Med Chem 2001; 8:371–84. [DOI] [PubMed] [Google Scholar]
- 12. Peterson LR. Quinolone molecular structure-activity relationships: what we have learned about improving antimicrobial activity. Clin Infect Dis 2001; 33:S180–6. [DOI] [PubMed] [Google Scholar]
- 13. Linder JA, Huang ES, Steinman MA, Gonzales R, Stafford RS. Fluoroquinolone prescribing in the United States: 1995 to 2002. Am J Med 2005; 118:259–68. [DOI] [PubMed] [Google Scholar]
- 14. U.S. Food and Drug Administration. FDA drug safety communication: FDA updates warnings for oral and injectable fluoroquinolone antibiotics due to disabling side effects https://www.fda.gov/Drugs/DrugSafety/ucm511530.htm. Published May 10, 2017. Accessed 12 December 2017.
- 15. Douros A, Grabowski K, Stahlmann R. Safety issues and drug-drug interactions with commonly used quinolones. Expert Opin Drug Metab Toxicol 2015; 11:25–39. [DOI] [PubMed] [Google Scholar]
- 16. Hayashi N, Nakata Y, Yazaki A. New findings on the structure-phototoxicity relationship and photostability of fluoroquinolones with various substituents at position 1. Antimicrob Agents Chemother 2004; 48:799–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dawe RS, Ferguson J, Ibbotson S, et al. . Lack of phototoxicity potential with delafloxacin in healthy male and female subjects: comparison to lomefloxacin. Manuscript submitted for publication. Photochem Photobiol Sci. 2018;17:773–780. [DOI] [PubMed]
- 18. BAXDELA™ (delafloxacin) [package insert]. Lincolnshire, IL: Melinta Therapeutics; 2017. [Google Scholar]
- 19. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use Understanding MedDRA: the medical dictionary for regulatory activities: 2013. https://www.meddra.org/sites/default/files/main_page_slideshow/meddra2013.pdf. Accessed 1 September 2019. [Google Scholar]
- 20. Paulson SK, Wood-Horrall RN, Hoover R, Quintas M, Lawrence LE, Cammarata SK. The pharmacokinetics of the CYP3A substrate midazolam after steady-state dosing of delafloxacin. Clin Ther 2017; 39:1182–90. [DOI] [PubMed] [Google Scholar]